Abstract
BACKGROUND & AIMS
Despite advances in critical care medicine, the mortality rate is high among critically ill patients with cirrhosis. We aimed to identify factors that predict early (7 d) mortality among patients with cirrhosis admitted to the intensive care unit (ICU) and to develop a risk-stratification model.
METHODS
We collected data from patients with cirrhosis admitted to the ICU at Indiana University (IU–ICU) from December 1, 2006, through December 31, 2009 (n = 185), or at the University of Pennsylvania (Penn–ICU) from May 1, 2005, through December 31, 2010 (n = 206). Factors associated with mortality within 7 days of admission (7-d mortality) were determined by logistic regression analyses. A model was constructed based on the predictive parameters available on the first day of ICU admission in the IU–ICU cohort and then validated in the Penn–ICU cohort.
RESULTS
Median Model for End-stage Liver Disease (MELD) scores at ICU admission were 25 in the IU–ICU cohort (interquartile range, 23–34) and 32 in the Penn–ICU cohort (interquartile range, 26–41); corresponding 7-day mortalities were 28.3% and 53.6%, respectively. MELD score (odds ratio, 1.13; 95% confidence interval [CI], 1.07–1.2) and mechanical ventilation (odds ratio, 5.7; 95% CI, 2.3–14.1) were associated independently with 7-day mortality in the IU–ICU. A model based on these 2 variables separated IU–ICU patients into low-, medium-, and high-risk groups; these groups had 7-day mortalities of 9%, 27%, and 74%, respectively (concordance index, 0.80; 95% CI, 0.72– 0.87; P < 10−8). The model was applied to the Penn–ICU cohort; the low-, medium-, and high-risk groups had 7-day mortalities of 33%, 56%, and 71%, respectively (concordance index, 0.67; 95% CI, 0.59–0.74; P < 10−4).
CONCLUSIONS
A model based on MELD score and mechanical ventilation on day 1 can stratify risk of early mortality in patients with cirrhosis admitted to the ICU. More studies are needed to validate this model and to enhance its clinical utility.
Keywords: Cirrhosis, Predictors, Mortality, Intensive Care, MELD
Liver disease is the 12th most common cause of death in the United States, and the second most common cause of death among digestive diseases.1 Decompensation in patients with chronic liver disease leading to hospitalization usually develops after an acute insult such as variceal bleeding, hepatorenal syndrome, or an infection such as spontaneous bacterial peritonitis, and it occurs in up to 40% of patients with advanced cirrhosis at 5 years.2 Between 1999 and 2004, the rate of hospitalization of these patients in the United States increased by 33%,1 with a continued increase in 2009.3 This trend is associated with an inflation-adjusted increase in economic burden from $1.15 billion to $2.1 billion.4
Hospitalized patients with decompensated cirrhosis are at a substantially increased risk for sepsis, which can precipitate renal dysfunction, respiratory failure, encephalopathy, and coagulopathy, leading to multiorgan failure and increased mortality.5,6 Liver transplantation is the only definitive intervention to improve survival in these patients; however, they frequently become critically ill and require intensive care unit (ICU) admission.
Despite advances in critical care that have improved ICU outcomes in cirrhosis over the past decade, these patients still have a poor short-term prognosis.7,8 Reports from European and Asian centers have indicated in-hospital mortality rates of 43% to 87% in ICU patients with decompensated cirrhosis.9–11 There have been scarce data on outcomes of critically ill patients with end-stage liver disease in the United States over the past decade. Given the high use of increasingly limited resources for this population, if outcomes are similarly poor for cirrhotic patients in the United States, it would underscore the futility of aggressive interventions that currently are being used in a large proportion of these patients. Furthermore, the ability to reliably risk stratify critically ill patients with end-stage liver disease would be extremely useful in discussing goals of critical care with patients and their families.
In this study, we describe in tandem the demographics, clinical characteristics, and outcomes of 2 cohorts of critically ill patients with end-stage liver disease from 2 university hospitals with high-volume liver transplant programs. These cohorts were studied independently for ICU outcomes and predictors of mortality. We developed and validated a model to predict short-term mortality in critically ill patients with end-stage liver disease. The predictive model was developed from the experience of one center (Indiana University [IU]–ICU) using clinical parameters on the first day of ICU care, and then subsequently was validated by assessing the accuracy of the model in the cohort from the second center (University of Pennsylvania [Penn]–ICU).
Materials and Methods
Two cohorts of patients with cirrhosis receiving ICU care (IU–ICU and Penn–ICU) were studied retrospectively, after Institutional Review Board approval at both centers.
A total of 185 patients with cirrhosis admitted to the IU–ICU between December 1, 2006, and December 31, 2009, and 206 patients admitted to the Penn–ICU between May 1, 2005, and December 1, 2010, were evaluated. Patients with fulminant hepatic failure, prior liver transplantation, or without evidence of cirrhosis were excluded. Patient demographic and clinical data, indication for ICU admission (eg, shock or acute kidney injury based on review of the clinical narratives on ICU admission), and use of ICU-specific interventions (mechanical ventilation, vasopressors, and renal replacement therapy [dialysis or continuous venous–venous hemodialysis]) were collected. Gastrointestinal bleeding at the time of ICU admission was classified as an indication for ICU admission. The Model for End-stage Liver Disease (MELD) and Child–Turcotte–Pugh scores were determined on ICU admission. The primary outcome for this model was mortality within 7 days of ICU admission (7-d mortality). The secondary outcome was mortality within 30 days after ICU admission (30-d mortality).
Statistical Analysis
Descriptive data were described as means with standard deviation for normally distributed continuous variables, medians with interquartile range (IQR) for non-normally distributed continuous variables, and percentages for categoric variables. Variables were compared between groups using the Mann–Whitney test or the Student t test for continuous variables, and the chi-square test for categoric variables. Simple logistic regression was conducted to identify predictors associated with 7-day mortality after ICU admission (7-d mortality event was coded as follows: 0, alive; 1, dead). The output from the simple logistic regression yielded unadjusted odds ratios. The odds ratio is a measure of association that approximates how much more likely an outcome is to occur among those with an exposure of interest compared with those without the same exposure. If an exposure of interest is modeled in a continuous rather than binary fashion, the odds ratio then represents the likelihood of the outcome associated with a 1-unit change in the covariate of interest. Variables with a P value less than .1 were considered for inclusion in the final model. Multiple logistic regression subsequently was performed with purposeful variable selection to determine predictors of 7-day mortality. A P value less than .05 was considered statistically significant.
Prediction Model
A model to predict 7-day ICU mortality was constructed using the results of the multiple logistic regression analysis of clinical parameters available on day 1 of ICU care in the IU cohort. Continuous variables were converted to categoric variables. The final model’s performance in predicting 7-day mortality was assessed by the concordance index (c-index), also known as the area under the receiver operator curve. The c-index provides a summary of the model’s discriminatory ability that ranges from 0.0 (all incorrect predictions) through 0.5 (chance prediction) to 1.0 (all correct predictions). The c-index and its 95% confidence intervals (CIs) have to be greater than 0.5 to prove that the model predicts better than chance alone. The “goodness of fit” of our model was assessed with the Hosmer–Lemeshow test. Our prediction model was validated externally by assessing its performance to predict 7-day mortality in the Penn–ICU cohort according to the method described by Braitman and Davidoff.12
Results
Clinical Characteristics and Patient Outcomes
Indiana University intensive care unit cohort
There were 185 patients who met the predefined eligibility criteria and their baseline demographics and clinical characteristics are described in Table 1. Complications of liver disease included ascites in 57%, hepatic encephalopathy in 64%, and hepatocellular carcinoma in 7%. Medical comorbidities included diabetes mellitus in 42%, hypertension in 41%, chronic renal disease in 25%, and cardiac disease in 24%. Sepsis was present in 23% of patients at ICU admission. Mechanical ventilation, renal replacement therapy, and/or vasopressors were used in 77% of patients during their ICU stay. However, mechanical ventilation in 51%, vasopressors in 31%, and renal replacement therapy in 18% were used on the first day of ICU care. Mortality within 7 and 30 days after ICU admission was 28.3% and 53.6%, respectively. The median interval from ICU admission to death was 8 days (IQR, 3–21 d).
Table 1.
Selected Demographics and Clinical Characteristics of 2 ICU Cohorts
| IU–ICU cohort (N = 185) | Penn–ICU cohort (N = 206) | P value | |
|---|---|---|---|
| Mean age (± SD), y | 55 ± 10 | 56.1 ± 10 | .11 |
| Male, % | 67 | 61 | .2 |
| Race, % | <.001 | ||
| White | 89 | 59 | |
| Black | 9 | 24 | |
| Hispanic | 2 | 2 | |
| Cirrhosis etiology, % | |||
| Alcohol | 43 | 36 | .2 |
| Viral | 38 | 47 | .1 |
| NAFLD | 18 | 11 | .04 |
| Autoimmune | 5 | 8 | .2 |
| Othersa | 8 | 13 | .2 |
| Presence of hepatocellular carcinoma, % | 7 | 8 | .8 |
| Child–Pugh class, A/B/C, % | 4/19/77 | 1/5/94 | <.001 |
| MELD score, median (IQR) | 25 (23–34) | 32 (26–41) | <.001 |
| Total bilirubin level, mg/dL (IQR) | 9.8 (2.8–21) | 7.5 (3.5–18.1) | .007 |
| INR (IQR) | 1.9 (1.6–2.5) | 2.4 (1.8–3.2) | <.001 |
| Creatinine level, mg/dL (IQR) | 1.9 (1–3.3) | 2.4 (1.5–4.1) | <.001 |
| Serum albumin level, g/dL (IQR) | 2.7 (2.3–3.4) | 2.2 (1.8–2.6) | .10 |
| Serum sodium level, mmol/L (IQR) | 137 (134–139) | 135 (130–139) | .16 |
| White blood count, 1000/mL (IQR) | 11.3 (7.4–13.9) | 12.5 (7.7–19.9) | .03b |
| Indication for ICU care, % | |||
| Gastrointestinal bleed | 26 | 22 | |
| Renal failure | 15 | 9 | |
| Infection/sepsis | 23 | 25 | |
| Admitted to ICU directly or transferred within 3 d of hospitalization, % | 83 | 75 | .07 |
| Mechanical ventilation, % | 64 | 77 | .01 |
| Renal replacement therapy, % | 32 | 42 | .035 |
| Vasopressor use, % | 43 | 78 | <.001 |
| Length of stay, d, median (IQR) | |||
| Total hospitalization | 11 (8–19) | 8 (4–16) | .6 |
| ICU | 4 (2–9) | 3 (2–9) | .4 |
INR, international normalized ratio; NAFLD, nonalcoholic fatty liver disease; SD, standard deviation.
Others include Budd Chiari syndrome, nodular regenerative hyperplasia, α-1 antitrypsin deficiency, and secondary biliary cirrhosis.
There were no predefined criteria for establishing indications for ICU admission at both centers, limiting this to a strictly descriptive comparison.
Overall mortality increased by length of stay in the ICU with 33% mortality in patients in the ICU up to 3 days, and more than 60% mortality in patients requiring more than 3 days of ICU care. Overall mortality also increased according to the number of ICU-specific interventions used (mechanical ventilation, vasopressors, and renal replacement therapy). Overall mortality in those requiring 0, 1, 2, and 3 interventions was 16%, 38%, 74%, and 91%, respectively (P < 10−10).
Eighteen patients (9.7%) were listed for liver transplantation with a similar distribution between survivors (9%) and nonsurvivors (10.4%) (P = .5). None of the patients underwent liver transplantation while in the ICU. However, 15 survivors ultimately underwent liver transplantation after a median interval of 163 days (IQR, 12–398 d). Resuscitation status was limited according to patient and family wishes immediately before death or discharge from the ICU in 83.3% of nonsurvivors and palliated/hospice patients.
Variables collected on the first ICU day that predicted 7-day mortality by simple logistic regression analysis are described in Table 2. The predictors of 7-day mortality by multiple logistic regression were MELD score (odds ratio [OR], 1.13; 95% CI, 1.07–1.2; P < .001) and mechanical ventilation (OR, 5.7; 95% CI, 2.3–14.1; P < .001). MELD score was categorized using cut-off values that were associated with a stable stepwise increase in 7-day mortality risk with each incremental step in MELD category. These corresponded to MELD categories of 15 or less, 16 to 32, 33 to 39, and 40 or more. When MELD as a categoric variable was entered into the multiple logistic regression, the OR for a MELD of 16 to 32 was 2.5 (95% CI, 0.6–11.3; P = .3), for a MELD of 33 to 39 was 11.2 (95% CI, 2.0–62; P = .006), for a MELD of 40 or greater was 40.8 (95% CI, 7–237; P < .0001), and for mechanical ventilation was 3.9 (95% CI, 1.7– 8.6; P = .001).
Table 2.
Predictors of 7-Day Mortality on Simple and Multiple Logistic Regression in the IU–ICU and Penn–ICU Cohorts
| Variables from ICU day 1 | OR | 95% CI | P |
|---|---|---|---|
| IU–ICU cohorta | |||
| Simple logistic regression | |||
| MELDb | 1.13 | 1.09–1.18 | <.001 |
| Mechanical ventilationb | 3.3 | 1.7–6.7 | .001 |
| Renal replacement therapyb | 2.4 | 1.1–5.2 | .03 |
| Vasopressor requirementb | 4.7 | 2.4–9.3 | <.001 |
| Mean arterial pressure | 0.95 | 0.92–0.95 | <.001 |
| Serum sodium | 0.94 | 0.9–0.99 | .02 |
| Serum bicarbonate | 0.93 | 0.87–0.99 | .03 |
| White blood cell count | 1.06 | 1.01–1.11 | .01 |
| ICU admission within 3 d of hospitalizationb | 0.3 | 0.14–0.68 | .004 |
| ICU admission for gastrointestinal bleedingb | 0.5 | 0.22–1.12 | .09 |
| Multiple logistic regression | |||
| MELDb | 1.13 | 1.07–1.2 | <.001 |
| Mechanical ventilation | 5.7 | 2.3–14.1 | <.001 |
| Penn–ICU cohortc | |||
| Simple logistic regression | |||
| MELDb | 1.06 | 1.03–1.09 | <.001 |
| Mechanical ventilationb | 3.16 | 1.78–5.59 | <.001 |
| Shock | 4.35 | 1.91–9.90 | <.001 |
| Vasopressor requirementb | 2.63 | 1.33–5.17 | .005 |
| Serum lactate | 1.17 | 1.09–1.26 | <.001 |
| APACHE III | 1.03 | 1.02–1.04 | <.001 |
| Acute kidney injury | 2.60 | 1.17–5.76 | .02 |
| Renal replacement therapyb | 0.52 | 0.30–0.91 | .02 |
| ICU admission within 3 d of hospitalizationb | 0.42 | 0.21–0.83 | .013 |
| Receipt of blood products | 0.86 | 0.81–0.91 | <.001 |
| ICU admission for gastrointestinal bleedingb | 0.33 | 0.18–0.59 | <.001 |
| Multiple logistic regression | |||
| MELDb | 1.08 | 1.03–1.13 | .001 |
| Shock | 4.81 | 1.56–14.89 | .006 |
| Serum lactate | 1.14 | 1.04–1.25 | .005 |
| Receipt of blood products | 0.83 | 0.77–0.90 | <.001 |
| Renal replacement therapy | 0.17 | 0.06–0.43 | <.001 |
NOTE. Variables achieving a P value less than 0.1 on simple logistic regression were included in the multiple logistic regression.
Nonpredictive factors in the IU–ICU cohort included the following: age, sex, etiology of liver disease, presence of ascites, hepatic encephalopathy or underlying malignancy, serum albumin, sepsis, and being listed for liver transplant.
Common predictors.
Nonpredictive factors in the Penn–ICU cohort included the following: age, sex, race, etiology of liver disease, underlying malignancy, presence of sepsis, being listed for liver transplant, hepatic encephalopathy, ascites, serum sodium, white blood cell count, and serum albumin.
The independent predictors of 30-day mortality were MELD (OR, 3.5; 95% CI, 2.0–6.2; P < .0001), mechanical ventilation (OR, 3.4; 95% CI, 1.56–7.41; P = .001), and ICU admission/transfer within 3 days of hospitalization (OR, 0.28; 95% CI, 0.09–0.85; P = .03) (Supplementary Table 1).
University of Pennsylvania intensive care unit cohort
There were 206 patients who met the predefined eligibility criteria and their baseline demographics and clinical characteristics are described in Table 1. Thirty-seven percent of patients were listed for liver transplantation. Reasons for ICU admission were as follows: 52 patients (25%) were admitted with an initial diagnosis of sepsis (a total of 170 patients [83%] developed sepsis while in the ICU), and 45 patients (22%) were admitted with a gastrointestinal bleed (80 patients overall [39%] had a gastrointestinal bleed in the ICU). Of the patients with sepsis in the ICU, the most common source of infection was respiratory (34%), and spontaneous bacterial peritonitis accounted for 19% of infections. A total of 172 patients (84%) had evidence of shock and 160 patients (78%) required vasopressor support (Table 1).
A total of 175 patients (85%) had evidence of acute kidney injury and 42% required renal replacement therapy. A total of 158 patients (77%) required mechanical ventilation during their ICU stay and 108 patients (52%) were intubated within 24 hours of ICU admission. Of the 80 patients with evidence of gastrointestinal bleeding, the median number of packed red blood cell transfusions received was 8.5 U (IQR, 5–15 U), and 12.5% underwent transjugular intrahepatic portosystemic shunt placement for control of bleeding. The median MELD score at ICU admission was 32 (IQR, 26–41), and the median peak MELD score during ICU admission was 40 (IQR, 33– 46). The median Child-Pugh score was 13 (IQR, 12–14). The median serum lactate within 24 hours of ICU admission (measured in 179 patients) was 5.1 mmol/L (IQR, 2.7–9.9 mmol/L) and the median Acute Physiology and Chronic Health Evaluation (APACHE) III score was 112 (IQR, 91–143). A total of 188 patients (92%) died at a median of 5 days (IQR, 2–12 d) after ICU admission. Mortality within 7 and 30 days after ICU admission was 55.3% and 87%, respectively.
The variables that predicted 7-day mortality by simple logistic regression analysis are described in Table 2. In the multiple logistic regression, the predictors of 7-day mortality were MELD score (OR, 1.08; 95% CI, 1.03–1.13; P = .001), shock (OR, 4.8; 95% CI, 1.56–14.9; P = .006), and serum lactate level (OR, 1.14; 95% CI, 1.04–1.25; P = .005). Presentation with gastrointestinal bleeding and receipt of renal replacement therapy were protective in multiple logistic regression (Table 2). Predictors of 30-day mortality on multiple logistic regression included MELD score (OR, 1.18; 95% CI, 1.08–1.29; P < .001) and shock (OR, 6.57; 95% CI, 1.33–32.4; P = .02); receipt of blood products was associated with a lower 30-day mortality (OR, 0.87; 95% CI, 0.82– 0.94; P < .001) (Supplementary Table 1).
Prediction model
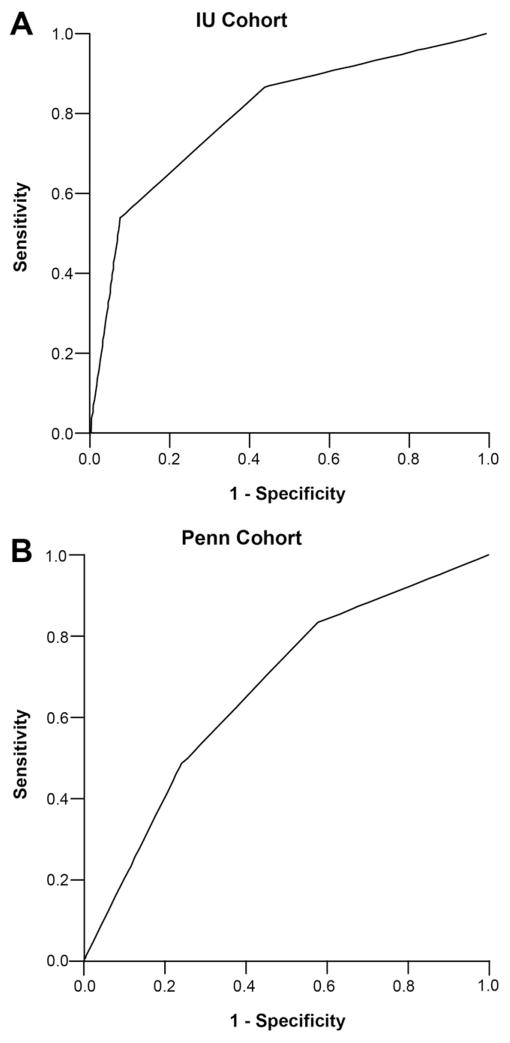
A model to risk stratify 7-day mortality was developed using predictors on multiple logistic regression from the IU–ICU cohort (MELD categories and mechanical ventilation on day 1) (Table 3). There were 80 patients in the low-risk group, 64 patients in the medium-risk group, and 38 patients in the high-risk group. The 7-day mortality rates for the low-, medium-, and high-risk groups were 9%, 27%, and 74%, respectively (P < 10−8) (Table 4). The c-index for this model to predict 7-day mortality in the IU–ICU cohort was 0.80 (95% CI, 0.72–0.87) (Figure 1, Table 4). The Hosmer–Lemeshow goodness-of-fit test resulted in a P value of .4, and this indicated that our prediction model fit the actual data well.
Table 3.
Scoring System for Predicting 7-Day ICU Mortality Based on Variables Obtained on the First ICU Day
| Points | Ventilation by day 1 | Points | |
|---|---|---|---|
| MELD score | |||
| ≤15 | 0 | No | 0 |
| 16–32 | 1 | Yes | 1 |
| 33–39 | 2 | ||
| ≥40 | 3 | ||
| Points | Risk group |
|---|---|
| 0–1 | Low |
| 2 | Intermediate |
| 3–4 | High |
Table 4.
Seven-Day Mortality Predicted by the Risk Stratification Model in the IU–ICU and Penn–ICU Cohorts
| IU–ICU cohort (N = 185)
|
Penn–ICU cohort (N = 206)
|
|||
|---|---|---|---|---|
| 7-d mortalitya | C-index (95% CI) | 7-d mortalitya | C-index (95% CI) | |
| Risk groups | ||||
| Low | 9% | 0.80 (0.72–0.87) | 33% | 0.67 (0.59–0.74) |
| Medium | 27% | 56% | ||
| High | 74% | 71% | ||
| Risk score | ||||
| 0 | 7% | 0.81 (0.73–0.88) | 13% | 0.69 (0.62–0.76) |
| 1 | 9% | 36% | ||
| 2 | 27% | 56% | ||
| 3 | 64% | 61% | ||
| 4 | 88% | 89% | ||
P value for analyses of mortality risk stratification was less than 10−4 in both cohorts.
Figure 1.
The area under the receiver operator curve for the model in prediction of 7-day mortality in the (A) IU–ICU and (B) Penn–ICU cohorts, with the respective c-index and sensitivity and specificity according to low-risk (0–1 points), medium-risk (2 points), and high-risk (3–4 points) scores. (A) The c-index was 0.80 (95% CI, 0.72–0.87). The sensitivity and specificity for the low score were 100% and 0%, for a medium score were 87% and 56%, and for a high score were 51% and 91%, respectively. (B) The c-index was 0.67 (95% CI, 0.59–0.74). The sensitivity and specificity for a low score were 100% and 0%, for a medium score were 83% and 42%, and for a high score were 48% and 76%, respectively.
When this prediction model was applied to the Penn–ICU cohort, 58 patients were classified in the low-risk group, 71 patients were classified in the medium-risk group, and 77 patients were classified in the high-risk group. The 7-day mortality rates for low-, medium-, and high-risk groups were 33%, 56%, and 71%, respectively (P < 10−4). The c-index for the model in predicting 7-day mortality in the Penn–ICU cohort was 0.67 (95% CI, 0.59–0.74) (Figure 1, Table 4). The Hosmer–Lemeshow goodness-of-fit test resulted in a P value of .6. The positive predictive value for 7-day mortality with a high risk score was 0.7 (95% CI, 0.53– 0.83) in the IU–ICU cohort and 0.71 (95% CI, 0.60–0.81) in the Penn–ICU cohort (Table 5).
Table 5.
Performance of High-Risk Score (>2) in Predicting 7-Day Mortality in IU–ICU and Penn–ICU Cohorts
| 7-d mortality | Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|---|
| IU–ICU cohort | 0.28 (0.23–0.37) | 0.51 (0.37–0.64) | 0.91 (0.84–0.95) | 0.7 (0.53–0.83) | 0.82 (0.74–0.87) |
| Penn–ICU cohort | 0.55 (0.48–0.62) | 0.48 (0.39–0.58) | 0.76 (0.66–0.84) | 0.71 (0.60–0.81) | 0.54 (0.45–0.63) |
With respect to the prediction of the secondary end point of 30-day mortality, the c-index of the model was 0.75 (95% CI, 0.68–0.83) in the IU–ICU cohort and 0.74 (95% CI, 0.64–0.83) in the Penn–ICU cohort (Supplementary Table 2). The 30-day mortality associated with a low-, medium-, and high-risk score was 30%, 60%, and 89% in the IU–ICU cohort (P < 10−9) and 72%, 87%, and 97% in the Penn–ICU cohort (P < 10−4), respectively (Supplementary Table 2). The positive predictive value of a high-risk score for 30-day mortality was 0.89 in the IU–ICU cohort and 0.97 in the Penn–ICU cohort (Supplementary Table 3).
Discussion
We performed a large contemporary study of ICU outcomes in US patients with decompensated cirrhosis. The high 30-day mortality ranging from 54% to 87% in the study cohorts emphasizes the need for risk stratification in this patient population. We describe a novel and validated model to stratify short-term mortality risk based on clinical parameters including MELD score at ICU admission and an early need for mechanical ventilation. The advantage of the 2 parameters used in our model is in their simplicity and reproducibility. The model provides a format to discuss risk and expectations with patients or their families at the outset of ICU care. It may be particularly useful in nonmechanically ventilated patients with high MELD scores (≥33) and progressive respiratory failure to inform discussions about limiting advanced measures such as mechanical ventilation.
It is not surprising that MELD was the main risk stratification variable in our study. MELD as a continuous variable has been a significant predictor of overall mortality in multiple ICU cohorts of cirrhotic patients with a c-index ranging from 0.75 to 0.92,11,13–15 and MELD scores higher than 32 in patients with cirrhosis in the ICU have been associated with increased mortality.16 The higher mortality rates in the Penn–ICU cohort compared with the IU–ICU cohort likely is attributable to the higher median MELD scores on ICU admission of 32 vs 25, respectively.
Mechanical ventilation also has been identified as a predictor of mortality in numerous studies.11,15,17–19 Differences in baseline variables used in the independent logistic regression analyses in the 2 cohorts may explain the differences in the independent predictors identified, such as Child–Pugh class, vasopressor use, and renal replacement therapy. ICU-specific predictors of mortality such as the APACHE II and III scores, Sequential Organ Failure Assessment scores, and the Simplified Acute Physiology Score II have been consistent predictors of mortality9,11,14,15; however, they have never been used in a validated risk-stratification model that identifies patients at high risk of mortality. The c-index of the model in the Penn–ICU cohort was relatively low (0.67) compared with the performance of other scoring systems including MELD in published studies.10,11,13–15,19 This was expected and likely explained by our use of an early primary end point of 7-day mortality compared with overall ICU/hospital mortality used in those studies. Our model does not discredit these scores as predictors of mortality; indeed, APACHE III score was a predictor of mortality in the Penn–ICU cohort on simple logistic regression. All the parameters needed to calculate the APACHE III or Sequential Organ Failure Assessment scores were not available retrospectively in the IU–ICU cohort to perform a comparative study or to incorporate those scores in the model. Factors not included in the model also could explain differences in mortality between the 2 study cohorts, with the higher frequency of sepsis and vasopressor use in the Penn–ICU group. Our model is by no means an exclusive predictor of early ICU mortality risk; however, with a high positive predictive value for early mortality, it is most useful in patients with a high risk score. In addition, many low-to intermediate-risk patients do not survive beyond a few weeks after ICU admission and are missed by this scoring system.
The lead time benefit of ICU admission was a novel finding identified in both our study cohorts, with time to ICU admission/transfer within 3 days of hospitalization being protective against early mortality. This finding may suggest a benefit to early ICU intervention, and/or reflect the more refractory nature of physiologic deterioration that fails to respond to 3 days of medical care; however, the increase in ICU mortality in patients not improving within the first few days of intensive care favors the latter.
Interestingly, in both study cohorts, presentation with a gastrointestinal bleed and receipt of blood products (studied only in the Penn–ICU cohort) were associated with a lower ICU mortality. This is consistent with recent data showing a changing trend in the prognosis of upper gastrointestinal hemorrhage and a lower mortality associated with both variceal and nonvariceal bleeding.20 Perhaps this is reflective of improvements in overall bleeding management in patients in the ICU, including timely resuscitation, use of proton pump inhibitors, somatostatin analogues, antibiotics, as well as early transjugular intrahepatic portosystemic shunt placement in patients determined to be at high risk for recurrent variceal bleeding. On the other hand, renal failure was associated with worse outcomes in both our study groups. Acute kidney injury, including hepatorenal syndrome, is an ominous sign in patients with end-stage liver disease, and the need for renal replacement therapy in the ICU in patients with decompensated cirrhosis has been shown to be associated with a mortality rate of more than 90%.21
This study had several limitations. First, our findings may not be generalizable to institutions that are not tertiary care, high-volume transplant centers. Further validation of our MELD-based model is needed in other centers with different referral patterns. Second, there were no defined criteria for ICU admission or use of renal replacement therapy in the study cohorts, which may introduce an element of bias in patient selection. Third, the impact of early mortality risk stratification on ICU care of cirrhotic patients is undefined and requires prospective study. In addition, this was a retrospective study, which carries the risk of inadequacy of data captured and other qualitative issues.
In summary, these 2 large contemporary cohorts indicate a high rate of short-term mortality in patients with cirrhosis requiring intensive care, despite the support of dedicated intensivists, hepatologists, and the resources of high-volume US transplant centers. We describe a novel and validated model of early mortality risk stratification, based on simple and easily defined parameters of MELD and mechanical ventilation. We believe that this prediction model needs to be validated by other investigators and needs to be strengthened further to enhance its clinical utility.
Supplementary Material
Acknowledgments
The authors thank Allison Fullenkamp, BS, Haripriya Maddur, MD, and Sarika Tiwari, MD, for their assistance in data collection.
R.B. and M.G. are co-primary authors.
Funding
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases, K24 DK069290 (N.C.).
Abbreviations used in this paper
- APACHE
Acute Physiology and Chronic Health Evaluation
- c-index
concordance index
- CI
confidence interval
- ICU
intensive care unit
- IQR
interquartile range
- IU
Indiana University
- MELD
Model for End-stage Liver Disease
- OR
odds ratio
- Penn
University of Pennsylvania
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2013.03.035.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Escorsell Mañosa A, Ordeig A. Acute on chronic liver failure. Gastroenterol Hepatol. 2010;33:126–134. doi: 10.1016/j.gastrohep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen GC, Segev DL, Thuluvath PJ. Nationwide increase in hospitalizations and hepatitis C among inpatients with cirrhosis and sequelae of portal hypertension. Clin Gastroenterol Hepatol. 2007;5:1092–1099. doi: 10.1016/j.cgh.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj JS, O’Leary JG, Wong F, et al. Bacterial infections in end-stage liver disease: current challenges and future directions. Gut. 2012;61:1219–1225. doi: 10.1136/gutjnl-2012-302339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multi-organ failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 7.Galbois A, Trompette ML, Das V, et al. Improvement in the prognosis of cirrhotic patients admitted to an intensive care unit, a retrospective study. Eur J Gastroenterol Hepatol. 2012;24:897–904. doi: 10.1097/MEG.0b013e3283544816. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien AJ, Welch CA, Singer M, et al. Prevalence and outcome of cirrhosis patients admitted to UK intensive care: a comparison against dialysis-dependent chronic renal failure patients. Intensive Care Med. 2012;38:991–1000. doi: 10.1007/s00134-012-2523-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen YC, Tsai MH, Ho YP, et al. Comparison of the severity of illness scoring systems for critically ill cirrhotic patients with renal failure. Clin Nephrol. 2004;61:111–118. doi: 10.5414/cnp61111. [DOI] [PubMed] [Google Scholar]
- 10.Cholongitas E, Calvaruso V, Senzolo M, et al. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol. 2009;24:1639–1647. doi: 10.1111/j.1440-1746.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- 11.Levesque E, Hoti E, Azoulay D, et al. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95–102. doi: 10.1016/j.jhep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Braitman LE, Davidoff F. Predicting clinical states in individual patients. Ann Intern Med. 1996;125:406–412. doi: 10.7326/0003-4819-125-5-199609010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Chen YC, Tian YC, Liu NJ, et al. Prospective cohort study comparing sequential organ failure assessment and acute physiology, age, chronic health evaluation III scoring systems for hospital mortality prediction in critically ill cirrhotic patients. Int J Clin Pract. 2006;60:160–166. doi: 10.1111/j.1742-1241.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 14.Cholongitas E, Senzolo M, Patch D, et al. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883–893. doi: 10.1111/j.1365-2036.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 15.Jenq CC, Tsai MH, Tian YC, et al. Serum sodium predicts prognosis in critically ill cirrhotic patients. J Clin Gastroenterol. 2010;44:220–226. doi: 10.1097/MCG.0b013e3181aabbcd. [DOI] [PubMed] [Google Scholar]
- 16.Lehner S, Stemmler HJ, Mück A, et al. Prognostic parameters and risk stratification in intensive care patients with severe liver diseases. J Gastrointest Liver Dis. 2010;19:399–404. [PubMed] [Google Scholar]
- 17.Arabi Y, Ahmed QA, Haddad S, et al. Outcome predictors of cirrhosis patients admitted to the intensive care unit. Eur J Gastroenterol Hepatol. 2004;16:333–339. doi: 10.1097/00042737-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Benhaddouch Z, Abidi K, Naoufel M, et al. Mortality and prognostic factors of the cirrhotic patients with hepatic encephalopathy admitted to medical intensive care unit. Ann Fr Anesth Reanim. 2007;26:490–495. doi: 10.1016/j.annfar.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Filloux B, Chagneau-Derrode C, Ragot S, et al. Short-term and long-term vital outcomes of cirrhotic patients admitted to an intensive care unit. Eur J Gastroenterol Hepatol. 2010;22:1474–1480. doi: 10.1097/MEG.0b013e32834059cd. [DOI] [PubMed] [Google Scholar]
- 20.Crooks C, Card T, West J. Reductions in 28-day mortality following hospital admission for upper gastrointestinal hemorrhage. Gastroenterology. 2011;141:62–70. doi: 10.1053/j.gastro.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackle IJ, Swann DG, Cook B. One year outcome of intensive care patients with decompensated alcoholic liver disease. Br J Anaesth. 2006;97:496–498. doi: 10.1093/bja/ael177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.