Abstract
Children exposed to methamphetamine during brain development as a result of maternal drug use have long-term hippocampus-dependent cognitive impairments, but the mechanisms underlying these impairments are not understood. The acetylcholine system plays an important role in cognitive function and potential methamphetamine-induced acetylcholine alterations may be related to methamphetamine-induced cognitive impairments. In this study, we investigated the potential long-term effects of methamphetamine exposure during hippocampal development on the acetylcholine system in adolescence mice on postnatal day 30 and in adult mice on postnatal day 90. Methamphetamine exposure increased the density of acetylcholine neurons in regions of the basal forebrain and the area occupied by acetylcholine axons in the hippocampus in adolescent female mice. In contrast, methamphetamine exposure did not affect the density of GABA cells or total neurons in the basal forebrain. Methamphetamine exposure also increased the number of muscarinic acetylcholine receptors in the hippocampus of adolescent male and female mice. Our results demonstrate for the first time that methamphetamine exposure during hippocampal development affects the acetylcholine system in adolescent mice and that these changes are more profound in females than males.
Keywords: acetylcholine, adolescence, basal forebrain, development, hippocampus, methamphetamine
Methamphetamine (MA) use among women is high compared with other drugs of abuse. Recent data show that women make up 45% of individuals admitted to drug abuse treatment centers for MA abuse whereas women make up only 26% of individuals admitted to drug abuse treatment centers for alcohol or marijuana abuse in the United States (NSDUH 2007; DASIS 2008). Furthermore, MA users tend to be of child-bearing age (DAWN 2010) and MA use increases the likelihood of engaging in risky sexual behavior, thereby increasing the chance of a female MA user becoming pregnant (Zule et al. 2007). MA use during pregnancy endangers mothers and babies (Good et al. 2010). Maternal MA use is associated with increased risk of preterm birth, complications associated with hypertension, and placental abruption (Good et al. 2010). Furthermore, a recent study found that MA abuse has become the most common reason for pregnant women to seek drug counseling (Terplan et al. 2009).
MA exposure during brain development causes physical abnormalities at birth (Little et al. 1988; Smith et al. 2008) and cognitive impairments, including hippocampus-dependent cognitive impairments later in life (Chang et al. 2004, 2009; Piper et al. 2011). Animal studies examining the effects of MA exposure during hippocampal development also show cognitive impairments later in life. The granule cells of the hippocampus develop during the first three postnatal weeks in rodents, modeling human hippocampal development during the third trimester (Bayer et al. 1993; Clancy et al. 2007a,b). Exposure to MA during hippocampal development impairs spatial learning and memory (Vorhees et al. 2000; Acevedo et al. 2007) and novel location and novel object recognition memory (Acevedo et al. 2007; Siegel et al. 2010) in adult rodents. These cognitive impairments are more severe in adult female than male mice (Acevedo et al. 2007; Siegel et al. 2010). Although animal models of MA exposure during postnatal brain development have typically focused on the long-term effects of MA in adulthood, recent data from our laboratory (Siegel et al. 2011b) and others (Vorhees et al. 2007) do support cognitive impairments in adolescent rodents following neonatal MA exposure (Vorhees et al. 2007; Siegel et al. 2011b).
Exposure to MA during brain development affects various neurotransmitter systems in adulthood in rodents, including the basal forebrain acetylcholine (ACh) system (Lehmann et al. 2004; Busche et al. 2006; Siegel et al. 2010). The basal forebrain is comprised of the medial septum (MS), vertical limb nucleus of the diagonal band (VDB), horizontal limb nucleus of the diagonal band (HDB), and the nucleus basalis (NB). Each nucleus contains ACh, GABA, and glutamate neurons (Mesulam et al. 1983; Gritti et al. 2003). ACh projections from the basal forebrain innervate the hippocampus and cortex (Mesulam et al. 1983) and the basal forebrain ACh system plays a unique and important role in cognition (Frick et al. 2004; Murai et al. 2007; Sambeth et al. 2007). Furthermore, choline acetyltransferase (ChAT; the enzyme that synthesizes ACh) and muscarinic ACh receptors (mAChRs) develop during the first three postnatal weeks in rodents and model the late development of the mAChRs in humans during the third trimester (Ravikumar and Sastry 1985; Kiss and Patel 1992; Aubert et al. 1996). Thus, MA may affect the ACh system during hippompal development and this in turn might contribute to MA-induced cognitive impairments. In the only studies to examine the effects of postnatal MA exposure on the ACh system, it was found that MA decreases cholinergic axons in the hippocampus and cortex in adult male gerbils (Lehmann et al. 2004; Busche et al. 2006) and increases M1 mAChRs in adult male and female mice (Siegel et al. 2010).
Therefore, in this study the potential effects of MA exposure during hippocampal development on the density of ACh neurons in the basal forebrain and area occupied by cholinergic axons in the hippocampus and cortex using ChAT as a marker were investigated (Oda 1999). The cholinergic axons were also examined in adult mice. The effects of MA on the GABAergic neurons and total neuronal density in the basal forebrain were examined using parvalbumin (PVA; Gritti et al. 2003) and NeuN (Mullen et al. 1992) as markers, respectively. As previous data from our laboratory showed increased mAChRs in adult mice following MA exposure during hippocampal development, the current study also examined the mAChRs in adolescent mice.
Materials and methods
Mice
Three-month-old C57BL/6J mice were bred in our colony using breeding cages containing one male and two female mice. Female mice were singly housed from the first sign of pregnancy. Laboratory chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St Louis, MO, USA), water, and soft foods were given ad libitum. On postnatal day (PND) 21, the pups were weaned and group housed with five mice per cage. All pups from all litters were used in these studies. The mice were kept on a 12 h light/dark schedule (lights on at 06 : 00). All procedures conformed to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University. When possible, tissues from the same animals were used for multiple experiments to minimize the number of animals used.
Injections
(d)-MA hydrochloride (5 mg/kg), obtained from the Research Triangle Institute (Research Triangle Park, NC, USA), through the National Institutes on Drug Abuse drug supply program, was diluted with 0.9% sodium chloride (saline) to the appropriate concentration. This dose was selected based on our previous findings that it causes long-term changes in the mAChR system and cognition (Acevedo et al. 2007; Siegel et al. 2010, 2011b). Male and female pups from a total of 23 separate litters were given a single intra-peritoneal injection (0.1 mL) of MA (n = 49) or saline (n = 50) daily at 10 : 00 from PND 11–20. A within-litter injection design was used to balance the number of MA and saline injections within a litter and across sexes.
Choline acetyltransferase immunohistochemistry
On PND 30 (n = 24 for basal forebrain and n = 19 for hippocampus/cortex) or PND 90 (n = 18 for hippocampus/cortex), mice were deeply anesthetized with a cocktail containing 100 mg/kg ketamine, 10 mg/kg xylazine, and 2 mg/kg acepromazine and were transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (pH = 7.4). Brains were removed and post-fixed overnight in 4% paraformaldehyde at 4°C, transferred to 30% sucrose solution, and embedded in cryoprotectant. Serial coronal sections (50 μm) were collected onto Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA, USA) through the entire MS/VDB/HDB, NB, or hippocampus/cortex for each brain with a 200 μm inter-section distance (Franklin and Paxinos 1997). Because of a lack of clear anatomical boundaries, the MS/VDB/HDB was considered as one region. Six sections per mouse were collected for the MS/VDB/HDB (+ 1.18 to 0.00 mm Bregma), four sections for the NB (−0.25 to −1.00 mm Bregma), and 12 sections for the hippocampus/cortex (−1.00 mm to −4.00 mm Bregma). Following an antigen retrieval step (H-3000; Vector Laboratories, Burlingame, CA, USA), sections were incubated for 2 h at 22°C in a blocking solution [5% normal donkey serum in PBS containing 0.2% Triton X-100 and 0.2% bovine serum albumen (PBT)], followed by incubation overnight at 4°C in goat-anti-ChAT (1 : 400; Millipore, Billerica, MA, USA) primary antibody. Following four washes with PBT (10 min each) and one wash with PBS (15 min), sections were incubated for 2 h at 22°C in donkey-anti-goat IgG antibody (1 : 50) conjugated to Texas Red for fluorescent visualization (Jackson Immunoresearch, West Grove, PA, USA). Following four washes with PBT (10 min each) and one wash with PBS (15 min), the sections were covered with anti-fade solution containing a DAPI counter-stain (Vectashield, Vector Laboratories) and were covers-lipped (Fisher Scientific).
Parvalbumin and NeuN immunohistochemistry
All procedures for the PVA and NeuN immunohistochemistry were identical to those for ChAT, except that imaging was performed only on sections from the MS/VDB/HDB. The same PND 30 mice used for the ChAT-positive cell counting were used for the current studies (n = 20) and the sections used were directly adjacent to those used in the same mouse for the ChAT immunohistochemistry. Following an antigen retrieval step, sections were incubated for 2 h at 22°C in a blocking solution [5% normal donkey serum in PBS containing 0.05% Tween-20 (PT)], followed by incubation overnight at 4°C with guinea pig-anti-PVA (1 : 300; Chemicon International, Billerica, MA, USA) and mouse-anti-NeuN primary (1 : 400; Millipore) antibodies. Following four washes with PT (10 min each) and one wash with PBS (15 min), sections were incubated with donkey-anti- guinea pig and donkey-anti-mouse IgG antibody (1 : 50 for each) conjugated to fluorescein isothiocyanate (PVA) or texas red (NeuN) for fluorescent visualization (Jackson Immunoresearch) for 2 h at 22°C. Following four washes with PT (10 min each) and one wash with PBS (15 min), the sections were covered with anti-fade solution containing a DAPI counter-stain and were coverslipped.
Confocal microscopy and unbiased stereology analysis
The density of ChAT-positive cells in the MS/VDB/HDB and NB was calculated by counting the total number of cells using an unbiased rare event stereological procedure using the 60× objective lens (N.A. = 1.42, W.D. = 0.15, F.N. = 26.5) and an Olympus spinning disc confocal microscope (IX81, Olympus Imaging Corp., Center Valley, PA, USA) equipped with Slidebook software (Intelligent Imaging Solutions, Denver, CO, USA). The density of ChAT-positive cells within each section was calculated by dividing the number of counted cells by the sample volume. The average density was calculated by averaging the sample densities for each mouse.
The density of PVA- and NeuN-positive cells in the MS/VDB/HDB was estimated using the non-biased stereological physical volume fractionators technique (West et al. 1991) using the 60× objective lens and a confocal microscope. Six sections spanning the MS/VDB/HDB were imaged for each mouse using 15 dissectors for each section. Each dissector (50 × 50 μm) consisted of a stack of 10 images with a 2 μm inter-image distance. PVA- and NeuN-positive cells were counted when they appeared within the dissector in one image of the 10-image stack but not in the preceding image. Cells were counted only when the staining appeared with a distinctly labeled DAPI-stained nucleus. Thus, a total of approximately 90 individual dissectors were counted to obtain an unbiased and accurate average density of PVA- and NeuN-positive cells per cubic centimeter within the MS/VDB/HDB of each mouse. The density of PVA- and NeuN-positive cells within each dissector was averaged for each mouse across all sections.
Densitometry analysis
Densitometry analysis was used to quantify the area occupied by ChAT-immunoreactive axons innervating the hippocampus and cortex. Immunofluorescence was imaged in stacks of 10 images with a 1 lm inter-image range in selected sub-regions of the hippocampus and cortex using the 60× objective lens and a confocal microscope. Stacks of images were vertically collapsed into a single image using a summation algorithm. An intensity threshold was chosen for each group of brains stained together, containing mice from each treatment group, based on the average intensities of immunoreactivity. The total number of pixels with ChAT immunofluorescence intensity above the threshold was determined within each image and converted to area. Twelve sections per mouse were used.
Muscarinic receptor binding
Mice were killed by cervical dislocation on PND 30 and receptor saturation binding experiments were performed using cortical (n = 27) and hippocampal (n = 40) membrane preparations and radioligands specific for M1 ([3H] Pirenzepine) or M2 ([3H] AF-DX-384) mAChRs (Siegel et al. 2011a). The receptor saturation-binding procedure has been described elsewhere (Siegel et al. 2011a). Briefly, cortices and hippocampi were dissected, membrane pellets prepared, and protein concentrations measured (BCA protein assay kit; Pierce, Rockford, IL, USA). Tissue samples were incubated in eight different concentrations of [3H] pirenzepine (330 pmol–46 nmol) or [3H] AF-DX-384 (252 pmol–46 nmol) for cortical binding. Single-point saturation binding was performed for hippocampal preparations because of the limited amount of tissue (98 nmol M1 and 85 nmol M2). Radioactivity was counted using a beta counter (LS 6000SC, Beta Counter, Beckman, Fullerton, CA, USA). The maximal number of binding sites (Bmax) and the equilibrium dissociation constant (Kd, for cortical binding only) were determined according to the Hill equation (Whiteaker et al. 2000) using non-linear regression analysis and Graphpad Prism 4.0 software (Graphpad, San Diego, CA, USA).
Statistical analysis
Two-way analysis of variance (ANOVA) was used to assess the effects of treatment and sex on the density of ChAT-, PVA-, and NeuN-positive cells, and the Bmax and Kd of the mAChRs. Duncan’s post hoc tests were performed to compare between groups when necessary. A linear mixed model with unstructured covariance as an optimal correlation was used to assess potential treatment and sex differences in the proportion of the percent of ChAT-positive, PVA-positive, and other (ChAT- and PVA-negative) cells out of total NeuN-positive neurons in the MS/VDB/HDB after accounting for the structural independency of the data (% PVA + % Chat + % other = 100%). A Bayesian Information Criteria was used to determine an optimal covariance structure. As six separate regions of hippocampus and cortex were measured in each mouse, a mixed linear model was used to assess the area occupied by cholinergic axons with animal nested within treatment and sex as a random effects and region as a within group factor. Data from four mice were removed from the cortical M1 mAChR binding study as a result of technical errors. Only significant interactions are reported. All statistical tests were conducted with a two-tailed significance alpha level of 0.05.
Results
Weight gains and general health
Death rates were very low and there were no significant differences in mortality rates between MA- and saline-exposed mice during the injection period (data not shown). While MA-exposed mice show lower weight gain over the injection period compared with saline-exposed mice (Acevedo et al. 2007; Siegel et al. 2010, 2011b), weight differences were not detected anymore by adolescence and adulthood (E. Eastwood and J. Raber, unpublished observations). We recognize that by maximizing the number of sex-and treatment-matched mice per litter and not culling the litters we might have increased error variance as litter size affects the weight of the pups and this could have masked potential group differences in body weight in adolescence or adulthood.
Effects of MA exposure during brain development on cells in the basal forebrain of adolescent mice
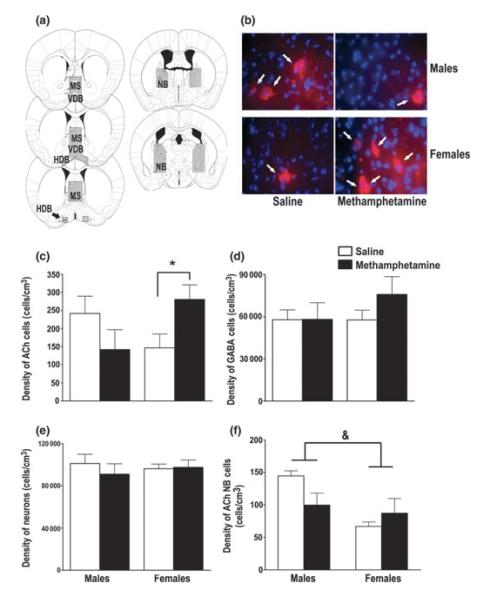
The volumes of the MS/VDB/HDB and the NB did not differ between MA- and saline-exposed or male and female mice. Representative images of these areas are shown in Fig. 1a. For the density of ChAT-positive cells in the MS/VDB/HDB, there was an interaction between treatment and sex [F(1, 16) = 6.53, p = 0.02]. Post hoc tests revealed that female MA-exposed mice had a higher density of ChAT-positive cells than female saline-exposed mice. There were no significant differences among the males (Fig. 1b and c). There was no effect of treatment on the density of ChAT-positive cells in the NB (Fig. 1f). Male mice, however, had a higher density of ChAT-positive cells in the NB compared with female mice [main effect of sex; F(1, 18) = 8.15, p = 0.01].
Fig. 1.
MA exposure during hippocampal development increased the density of ACh cells in the basal forebrain of adolescent female mice. (a) Representative images of the area defined as the MS/VDB/HDB and NB. (b) Representative images of ACh cells, indicated by the white arrows (red; ChAT-positive), in the MS/VDB/HDB in MA- and saline-exposed male and female adolescent mice. The blue is a DAPI stain for cell bodies. (c) MA exposure increased the density of ACh cells (ChAT-positive) in the MS/VDB/HDB in female mice compared saline-exposed female mice. This effect was not observed in adolescent male mice. (d) MA exposure did not alter the density of GABAergic cells (PVA-positive) or (e) the density of total neurons (NeuN-positive) in the MS/VDB/HDB. (f) MA exposure did not alter the density of ACh cells in the NB. Male mice had a higher density of ACh cells in the NB compared with female mice, regardless of treatment. Data expressed as mean ± SEM. n = 5–6 mice per treatment per sex. *p < 0.05 female MA-exposed versus female saline-exposed mice. &p < 0.05 male versus female mice. MS, medial septum; VDB, vertical limb nucleus of the diagonal band; HDB, horizontal limb nucleus of the diagonal band; NB, nucleus basalis; ACh, acetylcholine.
Because there was a difference in ACh cell density in the MS/VDB/HDB between MA- and saline-exposed female mice, we predicted that MA might also affect other cell populations in this region of the basal forebrain. Therefore, the density of GABAergic cells and the total neuronal density in the MS/VDB/HDB were assessed. However, MA exposure during brain development had no effect the density of PVA-positive GABA neurons (Fig. 1d) or NeuN-positive neurons (Fig. 1e) in the MS/VDB/HDB. There was also no effect of sex on the density of PVA- or NeuN-positive neurons.
Finally, the proportion of the percent of ACh, GABAergic, and other (non-ACh or GABAergic) neurons was analyzed. There was no difference in the percent of ACh, GABAergic, or other neurons between the MA- and saline-exposed mice or the male and female mice (Table 1). However, similar to the findings for ACh cell density, MA exposure increased the percent of ACh cells in adolescent female mice [t(1, 20) = −2.17, p = 0.04; Table 1].
Table 1.
The percent of acetylcholine, GABA, and other neurons in the MS/VDB/HDB of adolescent mice
| Treatment | Sex | Acetylcholine | GABA | Other |
|---|---|---|---|---|
| Saline | Males | 0.25 ± 0.05 | 58.12 ± 7.61 | 41.64 ± 7.64 |
| Methamphetamine | Males | 0.14 ± 0.05 | 61.48 ± 7.61 | 38.38 ± 7.64 |
| Saline | Females | 0.15 ± 0.05 | 59.54 ± 7.61 | 40.31 ± 7.64 |
| Methamphetamine | Females | 0.29 ± 0.05* | 75.86 ± 7.61 | 23.85 ± 7.64 |
p < 0.05 methamphetamine female mice higher than saline female mice.
Data expressed as mean percentages ± estimated SEM. n = 5 mice per treatment per sex.
MS, medial septum; VDB, vertical limb nucleus of the diagonal band; HDB, horizontal limb nucleus of the diagonal band.
Effects of MA exposure during brain development on the area occupied by ACh axons in the hippocampus and cortex of adolescent mice
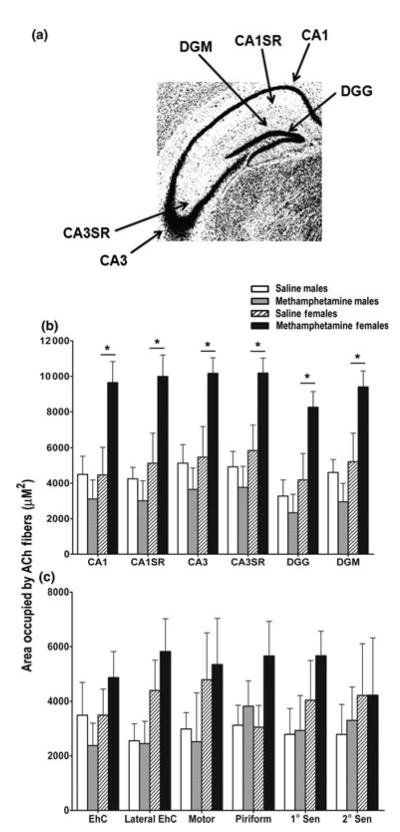
As MA increased the density of ACh basal forebrain neurons in adolescent female mice, the effects of MA exposure on ACh axons in the hippocampus and cortex were also examined. Six separate sub-regions of the hippocampus (CA1, CA1 stratum radiatum, CA3 CA3SR, dentate gyrus granule and molecular layer; Fig. 2a) and cortex (motor, primary and secondary sensory, entorhinal, lateral entorhinal, and piriform) were assessed. In the hippocampus, there was a treatment × sex interaction [F(1, 15) = 7.53, p = 0.02]. Post hoc tests showed that MA exposure increased the area occupied by cholinergic axons in all regions of the hippocampus compared with saline exposure in female mice (Fig. 2b). There were no significant differences among the male mice. Unlike the hippocampus, there was no effect of treatment or sex on the area occupied by ChAT-positive axons in the cortex (Fig. 2c).
Fig. 2.
MA exposure during hippocampal development increased the area occupied by acetylcholine axons in the hippocampus of adolescent female mice. (a) Sub-regions of the hippocampus that were examined for ACh axons. (b) MA exposure during brain development increased the area occupied by ACh axons (ChAT-positive) in all regions of the hippocampus in adolescent female mice compared with female mice exposed to saline. This effect of MA was not observed in adolescent male mice. (c) MA exposure during brain development did not alter the area occupied by ACh axons in any region of the cortex. Data expressed as mean ± SEM. n = 4–5 mice per treatment per sex. *p < 0.05 female MA-exposed mice versus female saline-exposed mice. ACh, acetylcholine; SR, stratum radiatum; DGG, dentate gyrus granule layer; DGM, dentate gyrus molecular layer; EhC, entorhinal cortex.
Effects of MA exposure during brain development on the mAChRs in the hippocampus and cortex of adolescent mice
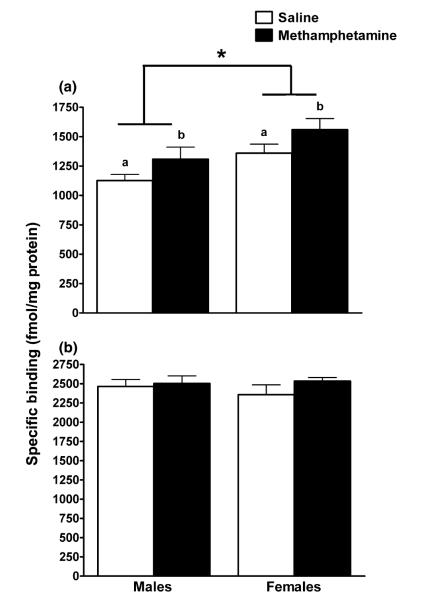
There are two types of ACh receptors, nicotinic and muscarinic (Cummings 2000). Both receptor subtypes are important for cognitive function, but the mAChRs are more commonly expressed in the mammalian brain and show very high levels in both the hippocampus and cortex (Schwab et al. 1992). Furthermore, previous data from our laboratory show increased hippocampal M1 mAChRs in adult mice following exposure to MA during brain development (Siegel et al. 2010). Therefore, we examined the M1 and M2 mAChRs in the hippocampus and cortex of adolescent mice exposed to MA or saline during brain development. Similar to our previous findings in adults, MA-exposed adolescent mice had a higher number (Bmax) of M1 mAChRs compared with saline-exposed adolescent mice in the hippocampus [main effect of treatment; F(1, 15) = 4.63, p = 0.04; Fig. 3a]. Female mice had a higher number of M1 mAChRs compared with male mice in the hippocampus [main effect of sex; F(1, 16) = 7.45, p = 0.02]. There was no effect of treatment or sex on the number of M2 mAChRs in the hippocampus (Fig. 3b). In the cortex, there was no effect of treatment or sex on the number or dissociation constant (Kd) of either the M1 or M2 mAChRs (Table 2).
Fig. 3.
MA exposure during hippocampal development increased the number of M1 mAChRs in the hippocampus in adolescent mice. (a) MA-exposed miceb had a higher number (Bmax) of M1 muscarinic receptors compared with saline-exposed micea. Female mice also had a higher number of M1 muscarinic receptors compared with male mice. (b) There was no difference between MA- and saline-exposed or male and female adolescent mice in the number of M2 mAChRs in the hippocampus. Data expressed as mean ± SEM. n = 4–6 mice per treatment per sex. aSaline-exposed mice; bMA-exposed mice. *p < 0.05 female versus male mice.
Table 2.
The number and dissociation constant of cortical muscarinic acetylcholine receptors in adolescent mice
| M1 muscarinic receptors |
M2 muscarinic receptors |
||||
|---|---|---|---|---|---|
| Treatment | Sex | B max | K d | B max | K d |
| Saline | Males | 1,028.0 ± 131.8 | 5,954.0 ± 582.4 | 2,047.7 ± 96.0 | 10,927.6 ± 2,021.5 |
| Methamphetamine | Males | 1,045.2 ± 56.2 | 6,527.6 ± 543.5 | 1,835.0 ± 106.8 | 8,348.6 ± 747.1 |
| Saline | Females | 1,302.0 ± 96.9 | 8,621.0 ± 1,053.7 | 1,819.1 ± 145.1 | 10,434.3 ± 749.3 |
| Methamphetamine | Females | 1,045.1 ± 167.1 | 7,527.7 ± 1,532.9 | 2,051.4 ± 62.2 | 10,827.0 ± 1,498.2 |
Bmax = fmol/mg protein, Kd = pM. Data expressed as mean ± SEM. n = 5–7 mice per treatment per sex.
Effects of MA exposure during brain development on the area occupied by ACh axons in the hippocampus and cortex of adult mice
Next, we assessed whether the effect of MA exposure on the adolescent ACh system were present in adulthood. As previous studies in our laboratory examined the mAChRs in adulthood (Siegel et al. 2010), the current study examined the area occupied by ACh axons in the hippocampus and cortex in adult mice exposed to MA or saline during brain development. Unlike the effects observed in adolescent mice, there were no effects of treatment or sex on the area occupied by ACh axons in any region of the hippocampus or cortex in adult male or female mice (Table 3).
Table 3.
The area occupied by acetylcholine axons in the hippocampus and cortex of adult mice
| Hippocampus |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Sex | CA1 | CA1SR | CA3 | CA3SR | DGG | DGM |
| Saline | Males | 2,624.8 ± 1,502.2 | 2,026.0 ± 798.0 | 2,780.2 ± 1,287.5 | 2,409.3 ± 905.3 | 2,292.4 ± 1,025.5 | 3,074.0 ± 1,213.9 |
| Methamphetamine | Males | 1,951.2 ± 544.8 | 2,184.6 ± 290.5 | 2,513.1 ± 516.7 | 2,521.4 ± 454.2 | 2,521.2 ± 489.8 | 3,861.7 ± 403.1 |
| Saline | Females | 3,454.1 ± 1,524.7 | 3,360.1 ± 1,496.9 | 4,594.2 ± 1,675.6 | 4,139.3 ± 1,557.1 | 3,802.9 ± 1,328.5 | 4,067.6 ± 1,428.6 |
| Methamphetamine | Females | 3,301.0 ± 587.1 | 2,942.0 ± 592.0 | 3,833.2 ± 715.5 | 3,464.2 ± 708.2 | 2,763.7 ± 755.8 | 3,689.8 ± 536.8 |
| Cortex |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Sex | EhC | Lateral EhC | Motor | Piriform | 1° Sensory | 2° Sensory |
| Saline | Males | 2,253.7 ± 1,069.1 | 3,196.7 ± 1,476.4 | 289.7 ± 286.8 | 3,377.1 ± 1,395.5 | 790.4 ± 286.6 | 261.9 ± 247.2 |
| Methamphetamine | Males | 4,781.4 ± 1,963.6 | 5,814.0 ± 1,839.6 | 1,946.8 ± 599.6 | 4,971.8 ± 1,367.8 | 2,141.9 ± 748.8 | 4,718.3 ± 2,367.4 |
| Saline | Females | 3,561.8 ± 1,297.3 | 3,599.2 ± 1,441.9 | 1,426.6 ± 747.3 | 4,404.9 ± 1,248.8 | 1,889.7 ± 1,140.4 | 2,635.4 ± 1,556.7 |
| Methamphetamine | Females | 3,709.0 ± 684.3 | 4,876.1 ± 742.5 | 1,744.1 ± 695.8 | 5,638.3 ± 670.1 | 2,255.9 ± 904.9 | 1,784.2 ± 547.1 |
Data expressed as mean area occupied by choline acetyltransferase immunoreactivity (μM2) ± SEM. n = 4–5 mice per treatment per sex. SR, stratum radiatum; DGG, dentate gyrus granule layer; DGM, dentate gyrus molecular layer; EhC, entorhinal cortex.
Discussion
The number of children exposed to MA in utero increased over the past two decades because of the increased prevalence of MA use amongst pregnant women (Terplan et al. 2009). Children exposed to MA in utero have long-term cognitive problems, yet the neurobiological mechanisms underlying these problems are unknown. The findings from this study show that MA exposure during a time period of hippocampal development affects the ACh system during adolescence. Female MA-exposed mice show increased density of ACh neurons in the MS/VDB/HDB and increased area occupied by ACh axons in the hippocampus whereas both male and female MA-exposed mice show an increased number of M1 mAChRs in the hippocampus compared with saline-exposed mice. As the ACh system plays a unique and important role in cognitive function (Murai et al. 2007; Sambeth et al. 2007), these data might be relevant for children exposed to MA in utero who experience cognitive impairments as a result of drug exposure during hippocampal development (Chang et al. 2004, 2009; Piper et al. 2011).
The effects of MA on the basal forebrain were specific for the ACh cells, as there were no MA-induced alterations in the GABAergic cell density or the total neuronal density in the MS/VDB/HDB. The basal forebrain also contains glutamatergic neurons and these neurons are important for cognitive function and for regulating the output of the basal forebrain (Fremeau et al. 2001; Sotty et al. 2003; Danik et al. 2005). Thus, any potential MA-induced alterations in the basal forebrain glutamate system may be associated with the alterations in the ACh system observed in this study. Future studies are needed to assess the potential effects of MA exposure during brain development on the density of glutamatergic neurons in the basal forebrain.
The mechanism by which MA alters the ACh system in adolescent mice is unknown. MA exposure during brain development may enhance the basal forebrain ACh system by increasing dopamine release into the basal forebrain (Gaykema and Zaborszky 1996) and exciting ACh neurons, resulting in an up-regulation of ACh cells, processes, and receptors. The basal forebrain ACh system is also modulated by the histaminergic system (for a review, see Bacciottini et al. 2001) and histamine release from the tuberomammillary nucleus to the MS increases ACh release from the MS to the hippocampus (Bacciottini et al. 2001). MA exposure during hippocampal development increases brain histamine levels in neonates and this increase might mediate MA-induced cognitive impairments in adulthood, as blockade of histamine activity during MA exposure blocks the effects of MA on cognition (Acevedo et al. 2007, 2008). Thus, MA-induced alterations in the histamine system may contribute to the effects on the ACh system observed in this study. Alternatively, MA exposure during hippocampal development may reduce ACh synthesis and release in the basal forebrain via activation of basal forebrain GABAergic neurons and subsequent inhibition of ACh neurons. As a result of decreased ACh cell activation and ACh release, there could be compensatory up-regulations in ChAT expression in the basal forebrain and hippocampus and mAChR expression in the hippocampus.
Another way in which MA exposure might alter the basal forebrain ACh system is by increasing levels of nerve growth factor (NGF) in the hippocampus. In cultures of mouse septum, NGF increases ChAT activity, ACh production, and the number of ACh cells (Hartikka and Hefti 1988; Yuhara et al. 2003). Intraventricular injections of NGF during postnatal development in rats increase ChAT activity in the MS, NB, cortex, and hippocampus in pre- adolescent and adolescent rats (Mobley et al. 1986; Tian et al. 1996). Furthermore, MA exposure from PND 11–20 increases NGF levels in the rat hippocampus on PND 20 (Skelton et al. 2007), suggesting that MA may have increased NGF in the hippocampus of female mice in the current study, thus increasing ACh markers in the basal forebrain and hippocampus.
The current findings suggest that female mice are more sensitive to the effects of MA on the ACh system. This may be due to slower metabolism of MA in neonatal female than male mice (Acevedo et al. 2008). Alternatively, androgens may protect against MA-induced impairments on the ACh system, as male sex hormones are protective against other brain challenges (Pike 2001; Raber et al. 2002) and in the context of aging (Benice and Raber 2009). However, androgen levels have not reached maturity by PND 30 (Selmanoff et al. 1977), suggesting that sex hormones cannot account for the relative protection observed in the male mice. The sex-dependent effects of MA on the ACh system may be due to sex differences in basal forebrain development. ChAT activity levels in the MS peak around PND 18 in female rats but peak later in development in male rats (Loy and Sheldon 1987). Furthermore, basal forebrain NGF receptor mRNA levels are consistently higher during the first two postnatal weeks in female rats compared with male rats (Kornack et al. 1991). Thus, MA exposure from PND 11–20 could target a more sensitive period of ChAT development and NGF regulation of the ACh system in female mice.
The increased susceptibility of female mice to the effects of developmental MA exposure on the ACh system is associated with more severe cognitive impairments in adolescent female than male mice in spatial memory retention (E. Eastwood and J. Raber, unpublished observations) and adult female than male mice in object location recognition and spatial memory retention (Acevedo et al. 2007; Siegel et al. 2010). Therefore, in female mice, the changes in the cholinergic system might be part of a compensatory response to reduce the impact of the MA insult.
The cholinergic system plays a unique and important role in cognitive function (Sarter and Bruno 1998; McKinney and Jacksonville 2005). Increases in aspects of the cholinergic system following MA exposure may at first seem at odds with the impaired cognitive function following MA exposure (Acevedo et al. 2007; Siegel et al. 2010, 2011b). However, increases in distinct cholinergic measures observed in the current study may be coupled with decreases in other functional measures of the cholinergic system and thus might not necessarily reflect an overall increased cholinergic function.
Alternatively, MA-induced increases in cholinergic function may disrupt the balance of cholinergic signaling that is essential for cognitive function. For example, MA-induced increases in cholinergic function may disrupt hippocampal signaling and thus cognition by disrupting cholinergic regulation of the hippocampal theta rhythm. Hippocampal theta rhythm is an oscillatory EEG pattern that is important for encoding and retrieval of learned information (O’Keefe and Burgess 1999; Hasselmo et al. 2002; Buzsaki 2005) and is modulated by input from the MS/VDB (Lee et al. 1994). MA-induced increases in cholinergic basal forebrain activity may serve to disrupt the theta rhythm. High concentrations of carbachol, a general mAChR agonist, can induce gamma EEG in the hippocampus (Fellous and Sejnowski 2000) and high doses of the acetylcholinesterase inhibitor physostigmine, which increase ACh levels, suppress theta activity at the higher frequencies and shift the peak of the theta to a lower frequency (Podol’skii et al. 2001). Thus, an increase in ACh release into the hippocampus that may result from increases in the density of cholinergic basal forebrain cells and axons innervating the hippocampus can distort hippocampal theta wave activity in a fashion that may disrupt cognition. The greater cognitive impairments following developmental MA exposure in adult female than male mice (Acevedo et al. 2007; Siegel et al. 2010) may be associated with greater cholinergic alterations and potential disruption of hippocampal theta in adult female than male mice. Although both male and female adolescent mice show impaired object recognition following developmental MA exposure (Siegel et al. 2011b), female, but not male, mice show impaired spatial memory retention in the water maze following MA exposure (E. Eastwood and J. Raber, unpublished findings). The impaired cognitive function in adolescent female mice may be due to increases in cholinergic function and a disruption of hippocampal theta rhythms. The impaired cognitive function in adolescent male mice, however, is likely caused by other mechanisms as well, as the male mice in the current study did not show the increases in cholinergic cells and axons seen in female mice and only showed the increase in muscarinic receptors seen in female mice. The other mechanisms underlying impaired cognitive function following MA exposure in adolescent male mice are currently not understood and warrant further investigation.
Adolescent female mice showed MA-induced increases in the area occupied by ACh processes in the hippocampus, but this effect was no longer present in the adult female mice. Estrogens are generally beneficial for ACh function. For example, there is an estrogen response element on the ChAT gene where estrogen receptor α can translocate to the nucleus and enhance transcription of ChAT (Miller et al. 1999). Ovariectomy reduces ChAT activity in the cortex and hippocampus, the number of ChAT-positive cells in the MS, and ACh release in the hippocampus in adult rats, and these effects are reversed by estradiol treatment (Yamamoto et al. 2007; Ping et al. 2008; Mitsushima et al. 2009). Thus, the adult female mice may be relatively protected and partially recover from the MA insult because of mature hormone levels. In contrast, the adolescent female mice may not be producing adult levels of circulating estrogens on PND 30 (Drickamer 1984) and therefore be more susceptible to the MA challenge. Future research is warranted to examine the intricate interactions between sex hormones, the ACh system, and MA exposure.
The effects of MA in the current study were specific for the MS and the corresponding ACh projections to the hippocampus. In contrast, MA did not affect the NB or the corresponding ACh projections to the cortex. The mechanism underlying this regional specificity is unknown. MA increases synaptic levels of dopamine, norepinephrine, and serotonin (Sulzer et al. 2005). It may be the case that the dopaminergic, noradrenergic, and serotonergic projections from the ventral tegmental area, locus coeruleus, and raphe nucleus, respectively, have greater innervation in the MS/VDB/HDB compared with the NB and that MA increases levels of these neurotransmitters in the MS/VDB/HDB to a greater extent than in the NB (Zilles et al. 1991; Smiley et al. 1999; Berlanga et al. 2005). More research is required to determine this possibility. However, our results corroborate previous findings showing effects of postnatal MA exposure on the hippocampus. For example, MA-exposed rodents show reduced levels of the dendritic marker microtubule-associated protein-2 (Acevedo et al. 2008), altered expression of brain derived neurotrophic factor (Skelton et al. 2007), and decreased dendritic spine density in the hippocampus (Williams et al. 2004). Furthermore, MA-exposed children show reduced hippocampal volumes (Chang et al. 2004). Thus, MA exposure during brain development affects the integrity of the hippocampus, a region of the brain that is important for higher cognitive function (Morris et al. 1982; Deacon et al. 2002). The current findings show for the first time that MA exposure during hippocampal development affects the basal forebrain ACh system in adolescent mice and these effects are more severe in females than males. Future studies are warranted to determine whether the effects of MA on the ACh system may be related to MA-induced cognitive impairments.
Acknowledgements
The authors would like to thank Catherine Dayger for her help with breeding and injecting the mice used in this study. This work was supported by NIDA F31DA026243, Methamphetamine Abuse Research Center Grant 1P50DA018165, and the development account of Dr Raber.
Abbreviations used
- ACh
acetylcholine
- ANOVA
analysis of variance
- ChAT
choline acetyltransferase
- DGG
dentate gyrus granule layer
- DGM
dentate gyrus molecular layer
- EhC
entorhinal cortex
- HDB
horizontal limb nucleus of the diagonal band
- MS
medial septum
- MA
methamphetamine
- mAChR
muscarinic acetylcholine receptor
- NGF
nerve growth factor
- NB
nucleus basalis
- PVA
parvalbumin
- PBS
phosphate-buffered saline
- PND
postnatal day
- VDB
vertical limb nucleus of the diagonal band
Footnotes
The authors have no competing interests or disclosures to report.
References
- Acevedo SF, de Esch IJ, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychopharmacology. 2007;32:665–672. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Pfankuch T, van Meer P, Raber J. Role of histamine in short- and long-term effects of methamphetamine on the developing mouse brain. J. Neurochem. 2008;107:976–986. doi: 10.1111/j.1471-4159.2008.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert I, Cecyre D, Gauthier S, Quirion R. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J. Comp. Neurol. 1996;369:31–55. doi: 10.1002/(SICI)1096-9861(19960520)369:1<31::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bacciottini L, Passani MB, Mannaioni PF, Blandina P. Interactions between histaminergic and cholinergic systems in learning and memory. Behav. Brain Res. 2001;124:183–194. doi: 10.1016/s0166-4328(01)00230-3. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Benice TS, Raber J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn. Mem. 2009;16:479–485. doi: 10.1101/lm.1428209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga ML, Simpson TK, Alcantara AA. Dopamine D5 receptor localization on cholinergic neurons of the rat forebrain and diencephalon: a potential neuroanatomical substrate involved in mediating dopaminergic influences on acetylcholine release. J. Comp. Neurol. 2005;492:34–49. doi: 10.1002/cne.20684. [DOI] [PubMed] [Google Scholar]
- Busche A, Bagorda A, Lehmann K, Neddens J, Teuchert-Noodt G. The maturation of the acetylcholine system in the dentate gyrus of gerbils (Meriones unguiculatus) is affected by epigenetic factors. J. Neural Transm. 2006;113:113–124. doi: 10.1007/s00702-005-0317-1. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am. J. Psychiatry. 2000;157:4–15. doi: 10.1176/ajp.157.1.4. [DOI] [PubMed] [Google Scholar]
- Danik M, Cassoly E, Manseau F, Sotty F, Mouginot D, Williams S. Frequent coexpression of the vesicular glutamate transporter 1 and 2 genes, as well as coexpression with genes for choline acetyltransferase or glutamic acid decarboxylase in neurons of rat brain. J. Neurosci. Res. 2005;81:506–521. doi: 10.1002/jnr.20500. [DOI] [PubMed] [Google Scholar]
- DASIS The Drug and Alcohol Services Information System Report: Primary Methamphetamine/Amphetamine Admissions to Substance Abuse Treatment. 2008:1–4. [Google Scholar]
- DAWN The Drug Abuse Warning Network Report: Emergency Department Visits Involving Methamphetamine. 2010 [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav. Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Acceleration of puberty in female mice by a urinary chemosignal from pregnant or lactating females: timing and duration of stimulation. Dev. Psychobiol. 1984;17:451–455. doi: 10.1002/dev.420170503. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz) bands. Hippocampus. 2000;10:187–197. doi: 10.1002/(SICI)1098-1063(2000)10:2<187::AID-HIPO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim JJ, Baxter MG. Effects of complete immunotoxin lesions of the cholinergic basal forebrain on fear conditioning and spatial learning. Hippocampus. 2004;14:244–254. doi: 10.1002/hipo.10169. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Zaborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain. II. Substantia nigraventral tegmental area projections to cholinergic neurons. J. Comp. Neurol. 1996;374:555–577. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Good MM, Solt I, Acuna JG, Rotmensch S, Kim MJ. Methamphetamine use during pregnancy: maternal and neonatal implications. Obstet. Gynecol. 2010;116:330–334. doi: 10.1097/AOG.0b013e3181e67094. [DOI] [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J. Comp. Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Hartikka J, Hefti F. Development of septal cholinergic neurons in culture: plating density and glial cells modulate effects of NGF on survival, fiber growth, and expression of transmitter-specific enzymes. J. Neurosci. 1988;8:2967–2985. doi: 10.1523/JNEUROSCI.08-08-02967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ. Development of the cholinergic fibres innervating the cerebral cortex of the rat. Int. J. Dev. Neurosci. 1992;10:153–170. doi: 10.1016/0736-5748(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Lu B, Black IB. Sexually dimorphic expression of the NGF receptor gene in the developing rat brain. Brain Res. 1991;542:171–174. doi: 10.1016/0006-8993(91)91015-s. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Hundsdorfer B, Hartmann T, Teuchert-Noodt G. The acetylcholine fiber density of the neocortex is altered by isolated rearing and early methamphetamine intoxication in rodents. Exp. Neurol. 2004;189:131–140. doi: 10.1016/j.expneurol.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC., III Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet. Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- Loy R, Sheldon RA. Sexually dimorphic development of cholinergic enzymes in the rat septohippocampal system. Brain Res. 1987;431:156–160. doi: 10.1016/0165-3806(87)90205-7. [DOI] [PubMed] [Google Scholar]
- McKinney M, Jacksonville MC. Brain cholinergic vulnrability: relevance to behavior and disease. Biochem. Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KB. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Takase K, Funabashi T, Kimura F. Gonadal steroids maintain 24 h acetylcholine release in the hippocampus: organizational and activational effects in behaving rats. J. Neurosci. 2009;29:3808–3815. doi: 10.1523/JNEUROSCI.5301-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley WC, Rutkowski JL, Tennekoon GI, Gemski J, Buchanan K, Johnston MV. Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res. 1986;387:53–62. doi: 10.1016/0169-328x(86)90020-3. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Murai T, Okuda S, Tanaka T, Ohta H. Characteristics of object location memory in mice: Behavioral and pharmacological studies. Physiol. Behav. 2007;90:116–124. doi: 10.1016/j.physbeh.2006.09.013. [DOI] [PubMed] [Google Scholar]
- NSDUH The National Survey on Drug Use and Health Report: Methamphetamine Use. 2007:1–4. [Google Scholar]
- Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol. Int. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. Theta activity, virtual navigation and the human hippocampus. Trends Cogn. Sci. 1999;3:403–406. doi: 10.1016/s1364-6613(99)01396-0. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Ping SE, Trieu J, Wlodek ME, Barrett GL. Effects of estrogen on basal forebrain cholinergic neurons and spatial learning. J. Neurosci. Res. 2008;86:1588–1598. doi: 10.1002/jnr.21609. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Kolchugina GK, Butler RW, Corbett SM, Honeycutt EB, Craytor MJ, Raber J. Abnormalities in parentally rated executive function in methamphetamine/polysubstance exposed children. Pharmacol. Biochem. Behav. 2011;98:432–439. doi: 10.1016/j.pbb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podol’skii IY, Vorob’ev VV, Belova NA. Long-term changes in hippocampus and neocortex EEG spectra in response to pharmacological treatments affecting the cholinergic system. Neurosci. Behav. Physiol. 2001;31:589–595. doi: 10.1023/a:1012312926021. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J. Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar BV, Sastry PS. Muscarinic cholinergic receptors in human foetal brain: characterization and ontogeny of [3H]quinuclidinyl benzilate binding sites in frontal cortex. J. Neurochem. 1985;44:240–246. doi: 10.1111/j.1471-4159.1985.tb07136.x. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Riedel WJ, Smits LT, Blokland A. Cholinergic drugs affect novel object recognition in rats: relation with hippocampal EEG? Eur. J. Pharmacol. 2007;572:151–159. doi: 10.1016/j.ejphar.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Age-related changes in rodent cortical acetylcholine and cognition: main effects of age versus age as an intervening variable. Brain Res. Rev. 1998;27:143–156. doi: 10.1016/s0165-0173(98)00003-4. [DOI] [PubMed] [Google Scholar]
- Schwab C, Bruckner G, Rothe T, Castellano C, Oliverio A. Autoradiography of muscarinic cholinergic receptors in cortical and subcortical brain regions of C57BL/6 and DBA/2 mice. Neurochem. Res. 1992;17:1057–1062. doi: 10.1007/BF00967281. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Goldman BD, Ginsburg BE. Developmental changes in serum luteinizing hormone, follicle stimulating hormone and androgen levels in males of two inbred mouse strains. Endocrinology. 1977;100:122–127. doi: 10.1210/endo-100-1-122. [DOI] [PubMed] [Google Scholar]
- Siegel JA, Craytor MJ, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav. Pharmacol. 2010;21:602–614. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JA, Benice TS, Van Meer P, Park BS, Raber J. Acetylcholine receptor and behavioral deficits in mice lacking apolipoprotein E. Neurobiol. Aging. 2011a;32:75–84. doi: 10.1016/j.neurobiolaging.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JA, Park BS, Raber J. Long-term effects of neonatal methamphetamine exposure on cognitive function in adolescent mice. Behav. Brain Res. 2011b;219:159–164. doi: 10.1016/j.bbr.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Subramanian M, Mesulam MM. Monoaminergic–cholinergic interactions in the primate basal forebrain. Neuroscience. 1999;93:817–829. doi: 10.1016/s0306-4522(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Smith LM, Lagasse LL, Derauf C, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol. Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J. Physiol. 2003;551:927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet. Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Tian X, Sun X, Suszkiw JB. Developmental age-dependent upregulation of choline acetyltransferase and vesicular acetylcholine transporter mRNA expression in neonatal rat septum by nerve growth factor. Neurosci. Lett. 1996;209:134–136. doi: 10.1016/0304-3940(96)12629-x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J. Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav. Pharmacol. 2007;18:549–562. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. Identification of a novel nicotinic binding site in mouse brain using [(125)I]-epibatidine. Br. J. Pharmacol. 2000;131:729–739. doi: 10.1038/sj.bjp.0703616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific longterm neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur. J. Neurosci. 2004;19:3165–3170. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kitawaki J, Kikuchi N, Okubo T, Iwasa K, Kawata M, Honjo H. Effects of estrogens on cholinergic neurons in the rat basal nucleus. J. Steroid Biochem. Mol. Biol. 2007;107:70–79. doi: 10.1016/j.jsbmb.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Yuhara A, Ishii K, Nishio C, Abiru Y, Yamada M, Nawa H, Hatanaka H, Takei N. PACAP and NGF cooperatively enhance choline acetyltransferase activity in postnatal basal forebrain neurons by complementary induction of its different mRNA species. Biochem. Biophys. Res. Commun. 2003;301:344–349. doi: 10.1016/s0006-291x(02)03037-1. [DOI] [PubMed] [Google Scholar]
- Zilles K, Werner L, Qu M, Schleicher A, Gross G. Quantitative autoradiography of 11 different transmitter binding sites in the basal forebrain region of the rat – evidence of heterogeneity in distribution patterns. Neuroscience. 1991;42:473–481. doi: 10.1016/0306-4522(91)90390-a. [DOI] [PubMed] [Google Scholar]
- Zule WA, Costenbader EC, Meyer WJ, Jr, Wechsberg WM. Methamphetamine use and risky sexual behaviors during heterosexual encounters. Sex. Transm. Dis. 2007;34:689–694. doi: 10.1097/01.olq.0000260949.35304.22. [DOI] [PubMed] [Google Scholar]