Abstract
Neurulation, the process of neural tube formation, is a complex morphogenetic event. In the mammalian embryo, an understanding of the dynamic nature of neurulation has been hampered due to its in utero development. Here we use laser point scanning confocal microscopy of a membrane expressed fluorescent protein to visualize the dynamic cell behaviors comprising neural tube closure in the cultured mouse embryo. In particular, we have focused on the final step wherein the neural folds approach one another and seal to form the closed neural tube. Our unexpected findings reveal a mechanism of closure in the midbrain different from the zipper-like process thought to occur more generally. Individual non-neural ectoderm cells on opposing sides of the neural folds undergo a dramatic change in shape to protrude from the epithelial layer and then form intermediate closure points to “button-up” the folds. Cells from the juxtaposed neural folds extend long and short flexible extensions and form bridges across the physical gap of the closing folds. Thus, the combination of live embryo culture with dynamic imaging provides intriguing insight into the cell biological processes that mold embryonic tissues in mammals.
Keywords: Imaging, neural tube closure, mouse embryo
Introduction
Neurulation is a complex, highly dynamic morphogenetic process during which the flat neural plate rolls up and closes to form the neural tube, the precursor of the central nervous system. This process in mammals occurs in at least four distinct steps: Thickening of the ectoderm to form a flat neural plate, elevation of the lateral edges of the neural plate to form the neural folds, apposition of the neural folds, and finally, joining of the neural folds in the mid-line, more commonly referred to as fusion. This last step includes separation of the neural ectoderm from the non-neural ectoderm and closing of these tissues to form the neural tube covered by a single layer of ectoderm.
Developmental biologists have long been interested in the events of neural tube closure and many histological studies have described these events in embryos including human, mouse, amphibian, chick and fish. These studies have revealed the similarities of neurulation among different species and highlighted the usefulness of exploiting the advantages of each system to decipher the molecular mechanisms that control neurulation. These studies have also highlighted some of the differences in neurulation in different organisms such as the multilayered (deep and superficial) neural plate in Xenopus and zebrafish or the adherent nature of the zebrafish neural cells which results in formation of an almost solid lumen-less structure, called the neural keel rather than discrete neural folds seen in mouse, human, Xenopus and chick (Harrington et al., 2009). Moreover, there are known regional differences in neurulation within an organism including primary versus secondary neurulation (tube formation resulting from elevation and closure of neural folds versus hollowing out from a mass of mesenchyme cells); differences in the extent and position of hinge points; and the extent of neural fold movements in the spinal cord region versus the cranial region with its larger tissue mass and structure.
Descriptive studies and molecular analyses have revealed the intricate coordination of cell proliferation, differentiation, cell shape changes and tissue architecture. These studies have provided a rich source of information on the normal events of neural tube closure. Moreover, this knowledge has provided the framework to understand how the process of neural tube closure is disrupted in cases of neural tube defects. For example, lengthening and narrowing of the neural plate is critical to bring the neural folds close together in the midline. Genetic mutations discovered in mice and humans and molecular genetic studies in Xenopus embryos have shown the importance of the non-canonical Wnt/planar cell polarity pathway in controlling the convergence and extension movements of the neural plate cells to bring the neural folds together (Davidson and Keller, 1999; Greene et al., 1998; Hamblet et al., 2002; Kibar et al., 2007; Lu et al., 2004; Murdoch et al., 2001; Reynolds et al., 2010; Wallingford, 2006; Wallingford and Harland, 2002; Ybot-Gonzalez et al., 2007). Genetic studies in mice have uncovered over two hundred genes that when mutated can lead to neural tube defects. Histological and molecular analyses of these mutant embryos have provided a wealth of insight into how they regulate various steps in the complex process of neural tube development (reviewed in (Harris and Juriloff, 2007).
Dynamic imaging is another important tool to aid in the study of neural tube closure. The dynamic cell behaviors during neurulation have been studied in easily accessible embryos such as chick, fish and amphibian (Bancroft and Bellairs, 1975; Davidson and Keller, 1999; Ezin et al., 2009; Graeden and Sive, 2009; Jaskoll et al., 1991; Kieserman and Wallingford, 2009; Schroeder, 1970; Van Straaten et al., 1996; Wallingford and Harland, 2002). These studies have expanded upon the data obtained from static images and revealed new insights into the tissue and cell movements as the neural folds elevate and close including cell intercalation, the convergence-extension movements of the neural plate, cell migration trajectories and the orientation of cell divisions. In a developing mammalian embryo, however, relatively little is known about the dynamics of neurulation as a consequence of inaccessibility due to its in utero development. Culture of whole mouse embryos (Jones et al., 2002; Martin and Cockroft, 1999; Tam, 1998) offers the means to study the cell behaviors of neural tube development. Jones et al. (2002) visualized closure in the hindbrain at a tissue level, although the magnification did not allow individual cell behaviors to be followed. Here we use a modified mouse embryo culture method combined with fluorescent reporters and laser point scanning confocal microscopy to visualize at high magnification the dynamic behaviors of cells of the neural folds as they meet and connect.
The last step of neural tube closure, wherein the neural ectoderm cells come together to form a closed tube and the non-neural ectoderm cells also join to form a contiguous layer, is the least well understood step in any experimental system and is the focus of this study. We also focus on neural fold fusion within the cranial region, versus the more commonly studied spinal cord region. Fusion of the neural folds is thought to require changes in cell adhesion, including N-cadherin and E-cadherin although their exact roles in this process are still unclear (Lawson and England, 1998). Neural fold fusion in mouse and humans has been hypothesized to start from three distinct points on the neural tube, known as initial closure sites (Supplemental Figure 1B), and then to proceed in a zipper-like manner both rostrally and caudally until the neural tube is closed along its length, (for review see (Fleming et al., 1997). Past studies have supported the idea that neural tube closure in avian embryos occurs in a zipper-like manner (Jaskoll et al., 1991). Here we have used live imaging to study mouse cranial neural tube closure and to observe the cell-cell interactions during the joining of the neural folds. Our studies indicate the hindbrain closes by the hypothesized zippering mechanism, but in the midbrain, a different mechanism functions to join the neural folds wherein multiple secondary closure sites form to “button-up” the midbrain neural folds.
Failure to close the neural tube results in one of the most common birth defects in humans, with neural tube defects occurring in ∼1 of every 1000 live births. The mouse embryo is thought to represent an excellent model of human neurulation to understand the cellular and genetic mechanisms underlying neural tube formation. There are many similarities between human and mouse neurulation from the steps of neural tube closure, to the position of the initial closure sites, to the position and type of neural tube defects. Moreover, genetic studies in the mouse have highlighted candidate genes for analysis in human neural tube defects and provided the opportunity to understand the embryological basis for these defects. The studies here add a new dimension to our understanding by identifying the cell behaviors that participate in closure of the neural tube. Combining live mouse embryo culture with dynamic imaging provides a unique viewpoint and highlights unknown aspects of mammalian neural tube closure and opens the path to better understand this complex developmental phenomenon that often goes awry leading to severe birth defects.
Materials and Methods
Mice and whole embryo culture
Males and female mice transgenic for CAG::myr-venus (Rhee et al., 2006) were mated and embryos dissected at E8.5 in freshly prepared Tyrodes solution removing Reichert's membrane but leaving the yolk sac intact. The media for embryo culture consisted of two parts rat serum, which was extracted from the dorsal aorta of isoflorane anesthetized male rats, and one part culture media (no phenol red DMEM-F12 containing 55U/ml penicillin/streptomycin, 2.2mM glutamax and 11mM Hepes buffer). The mixture was filtered through a 0.2μm filter and equilibrated at 37°C with a 5% O2, 5% CO2, and 90% N2 gas mixture for approximately two hours before embryos were added to roller culture (BT Bioscience roller culture system, England). The embryos were cultured until initial neural closure points 1 and 2 were formed.
Imaging of neural tube closure
The embryos were removed from the roller culture, the yolk sac opened in a small region and the embryo gently pushed out so that the head was exposed. The embryo was placed on a glass bottomed dish (MatTek glass bottomed dishes P35G-1.5-20-C) coated with a thin layer of 1% agar in H2O into a 0.5mm diameter well created using a glass Pasteur pipette and positioned using minutien pins with the appropriate region of the neural folds lightly touching the glass. The dish was transferred to an environmental chamber (under the same culture conditions as described above) fitted on the stage of an inverted ZEISS LSM510 META confocal microscope. For fast imaging, the same environmental chamber was fitted on the stage of a Zeiss 5LIVE confocal microscope. All the images were acquired with the use of a 40X water immersion lens c-Apochromat NA 1.2. The depth of imaging varied from 100 to 150 μm to focus on the non-neural ectoderm as it wraps around the neural ectoderm. The time intervals for live image acquisition are denoted in the legends to the figures and movies. Photobleaching and photodamage is not significant due to the very low laser power used (excitation wavelength 514, power 5%) and images were obtained for more than 12 hours without an alteration in the intensity of fluorescence and without the need to increase the detection gain. However, prolonged imaging had a significant effect on the fine cellular extensions, both the dynamically moving extensions and those connecting the two folds. The bridge-like extensions connecting the two folds are particularly prone to photodamage as even brief imaging for a few minutes leads to severance of these structures.
Results
Whole embryo culture system to image neural tube closure in the living mouse embryo
In this study we have imaged neural tube closure in a living mammalian embryo. We used whole embryo culture system in combination with live imaging of a genetically-encoded reporter to visualize neural tube formation in the developing mouse embryo, with emphasis on the last step of the process, neural fold fusion. We used a strain of transgenic mice exhibiting widespread expression of a myristoylated Venus fluorescent fusion reporter to highlight the cell membranes (Rhee et al., 2006). Hemizygous transgenic embryos at embryonic day (E) 8.5 (8-10 somites) were removed with the yolk sac intact (Supplemental Figure 1A) and grown in media and a gas mixture of CO2 and O2 that supports the development of the embryo in culture and continued developmental progression (Supplemental Figure 1C). When the embryo had turned and formed closure points 1 and 2, it was positioned head down in a glass bottom dish (Supplemental Figure 2 and as shown schematically in the Figures) and placed in an environmental incubator for confocal imaging.
A new mechanism of neural tube closure in the mouse midbrain
We first focused on closure of the neural folds rostral to closure point 1, within the hindbrain. Dynamic imaging of the hindbrain showed a zipper-like closure (Figure 1A, B, Movie 1, n=3), supporting the current zippering model for neural tube closure. Closure in the hindbrain proceeds at a rate of ∼1um/min when the folds are at least 120 um apart through closure (Figure 1C).
Figure 1. Zipper-like closure within the mouse hindbrain.
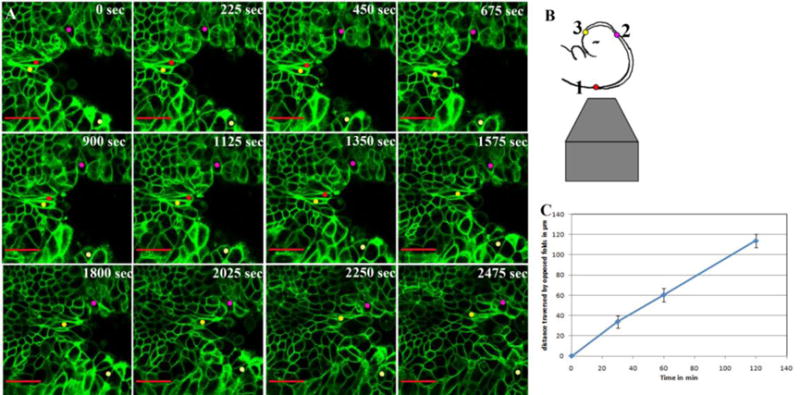
A. Still images from movie 1 captured from time lapse imaging of the hindbrain with the time points in seconds indicated in each panel. The folds come together in a zipper-like manner in a region of the hindbrain rostral to closure point 1. Colored dots track individual cells throughout the time period of imaging. Scale bar 20μm.
B. The schematic on the right represents embryo orientation relative to the objective lens and the red dot represents closure point 1.
C. Closure in the hindbrain proceeds at a rate of ∼1um/min when the folds are at least 120 um apart through closure. Error bars denote the standard deviation for the distance crossed for each time point in three different embryos.
By contrast, in the midbrain, closure was characterized by a distinct series of cell behaviors suggesting a different mechanism of closure. As closure of the neural tube proceeded rostrally, the transition from the hindbrain to the midbrain region was marked by the presence of cellular extensions that originated from cells on the edges of the neural folds (Figure 2A, white arrowheads). These cell extensions and the cell behaviors described below were not observed in the hindbrain region, which consistently closed in the zippering manner. Live imaging in the embryonic midbrain (n=10) showed that as the folds approached each other and came to lie 40-60 microns apart, a small number of cells (1-3) from opposing sides of the neural folds underwent a dramatic change in shape such that they protruded from the epithelial layer, they extended long cellular processes (Figure 2 and described further below), and then formed contacts between the juxtaposed folds (Figure 2B, C and Movie 2, 3). This contact across the gap of the opposing neural folds resulted in the formation of new intermediate or secondary closure points, between the initial closure points 1 and 2 (Supplemental Figure 1B). The protruding cells that form the secondary closure sites in the mouse midbrain are spaced at more than 150 μm apart. Even though the protruding cells appear to be stochastically positioned, there is an intriguing correspondence between the cells on opposing sides of the folds with the cell contacts occurring between cells in close apposition (directly or within a few cells apart across the gap).
Figure 2. Secondary closure points in the midbrain.
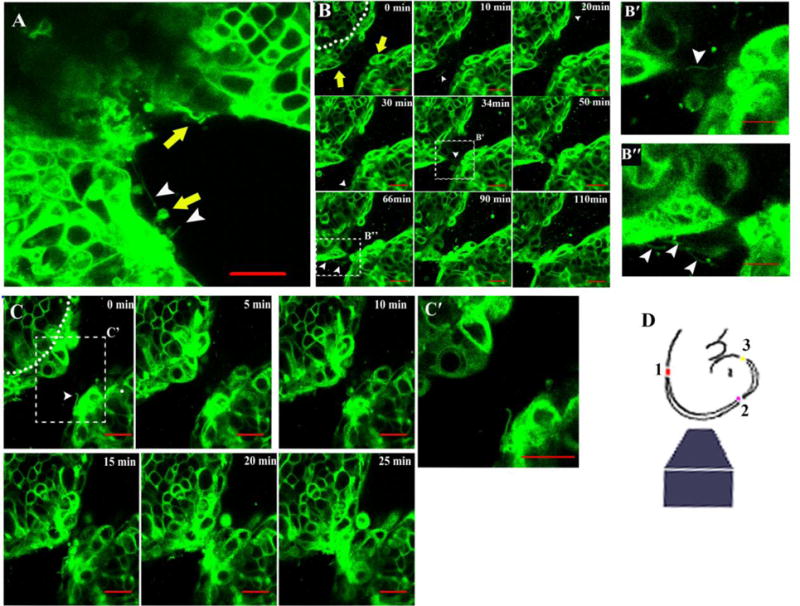
(A) Transition between zippering in the hindbrain to buttoning in the midbrain. White arrowheads indicate thin cell extensions, yellow arrows indicate protruding cells (three neighboring cells protrude from the fold on lower left as observed over time and in a different z plane; not shown). Scale bar 20μm.
(B, C) and movies 2 and 3. Imaging of the ∼E9.25 midbrain region schematically shown between closure points 1 and 2 (D, red and purple dots, respectively). Time points in minutes are indicated in each panel. Note a single or a few cells protrude from the non-neural ectoderm layer (demarcated from neural ectoderm by dotted line) on both sides of the neural folds (yellow arrows) and interact with protruding cells on the opposing neural fold. Boxes highlight magnified views showing thin cell extensions in the gap between neural folds (white arrowheads) and which appear to connect the opposing folds (panels B′ and B′) at the position that the secondary closure will form. Scale bar in all panels is 20 μm.
The persistence of secondary closure points leads us to conclude that neural tube closure in the midbrain occurs not in a zipper-like manner as it does for the hindbrain, but rather in a buttoning up-like manner that leaves small openings between intermediate closure points that will eventually close to generate a continuous midline. Although zippering does not initiate the process of neural fold closure in the mammalian midbrain, zippering does appear to be involved in closing the gap between the “buttons”.
Non-neural ectoderm initiates neural fold closure in the midbrain
The next question we addressed is whether neural or non-neural ectoderm initiates neural fold closure in the midbrain. First we analyzed wildtype embryos that were immediately fixed upon dissection from the pregnant mother, embedded and sectioned. As shown in Figures 3A and 3B, the non-neural ectoderm (as seen by their flattened cell shape and by staining with E-cadherin) wraps around and covers much of the neural ectoderm right before and as the neural folds meet. This indicates that the non-neural ectoderm faces the gap between the closing folds and comes to lie between the folds at the onset of closure. Similarly, an optical cross section near the tip of the folds in the midbrain of a cultured embryo also highlights that the non-neural ectoderm (large flattened cells in Figure 3C) wraps around the neuroectoderm (hexagonal honeycomb-like cells: an representative plane of section is indicated in 3A). This image also suggests that the membrane of the non-neural ectoderm cells facing the gap is highly dynamic due to its fuzzy or ruffled appearance. As shown in Figure 2 and as discussed later, there is extensive activity within the cell membrane of the non-neural ectoderm including thin filopodia-like extensions across the gap between the non-neural ectoderm cells. These data together indicate that the non-neural ectoderm is the first cell type to establish direct cell-cell contact and initiate neural tube closure.
Figure 3. Non-neural ectoderm wraps around neural ectoderm.
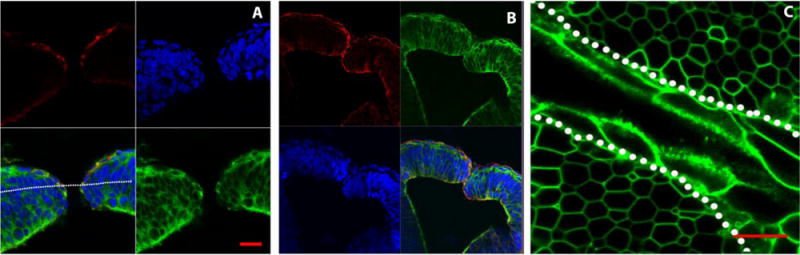
(A, B) Frontal sections in the midbrain region of an E9.0 embryo fixed immediately after dissection. The image in A shows the two neural folds just preceding neural fold fusion whereas the image in B shows a location where the neural folds have met in the midline. Cryosections in the midbrain region were processed with an anti-E-cadherin antibody (red) to highlight the non-neural ectoderm, phalloidin (green) which marks filamentous actin, and Hoescht (blue) that labels the nuclei. The plane marked by the dotted line denotes the approximate optical plane for the image shown in (C).
(C) An embryo that had been cultured and an optical section taken when the neural folds were closely apposed but not yet closed. This optical section was taken on a Zeiss LSM510 META microscope through the single cell layer of non-neural ectoderm (large flattened cells) that wraps around the neural ectoderm (hexagon shaped cells) at the edge of the closing neural folds (the dotted line separates the non-neural from the neural ectoderm). The cell surface facing the physical gap between the folds has a ruffled appearance. Scale bar 20μm.
Highly dynamic cell extensions and cellular bridges
Next, we extended these observations using the same experimental setup but with a high-speed laser slit scanning confocal microscope to acquire images at a faster frame rate (1 frame every 3 seconds). Additional novel observations included multiple short and long flexible cell extensions that emanated from the non-neural ectoderm cells and that reside in the gap between the juxtaposed folds (Figure 4A, B and Movie 4). Short cell extensions with bulbous ends were present on the folds and they were highly dynamic, moving rapidly and non-directionally (Figure 4B, Movie 4). Long (over 50um) thin cell extensions also arose from non-neural ectoderm cells (Figure 4A).
Figure 4. Flexible, motile extensions and cellular bridges from the non-neural ectoderm.
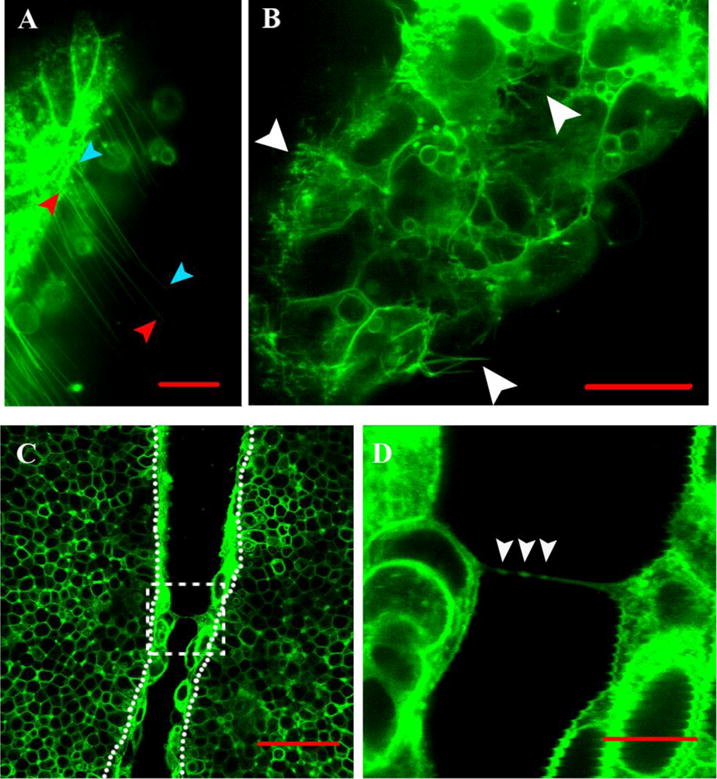
Images of the neural folds in the midbrain region. (A) Numerous long (over 50um) thin cell extensions from the non-neural ectoderm of one fold that extend across the gap towards the converging fold (not visible in this view). Scale bar 20μm.
(B) Still image from Movie 4 of the non-neural ectoderm on one fold in the midbrain region that highlights a few of the long flexible cell extensions and short bulbous flexible extensions. Scale bar 20μm.
(C) Cellular bridges connect the two juxtaposed folds when they are 20μm apart. In the boxed area, two cellular bridges connect opposing non-neural ectoderm cells. Dotted lines separate the non-neural from neural ectoderm. Scale bar 50μm.
(D) Magnification of one of the cell bridges from Figure 4C. Three structures ∼0.5 μm in diameter that are highlighted with the myristoylated Venus fluorescence reporter are present within the cellular bridge (white arrowheads). Scale bar 10μm.
As the neural folds in the midbrain region come close to each other (∼20um), cellular “bridges” are observed spanning the gap between the two folds (Figure 4C, n=5). The cellular bridges appear continuous with the membrane of the cells that they connect. These cellular bridges which are less than 1 μm in width contain structures ∼0.5μm in diameter that are highlighted by the myristoylated Venus reporter (Figure 4D). Similar fine cell extensions and bulging structures, referred to as beaded threads and midbodies, respectively, were described in the chick embryo and thought to be remnants of the cell division apparatus (Bancroft and Bellairs, 1975). The cellular bridges observed here extend over the physical gap prior to the folds coming together and hence are not remnants of cell division. It is still a formal possibility that these cellular bridges are remnants left by pulling apart of the two folds during a transient separation. However, in no case (>40 movies) have we observed the wildtype neural folds to contact each other and then transiently separate or move apart.
Discussion
Neural tube defects are the second most common birth defect in humans. However, the cell behaviors underlying neural tube closure are largely unknown and the dynamics of these cell behaviors have not been studied in a developing mammalian embryo. By visualizing neural tube formation in the living mouse embryo we have discovered unanticipated cellular interactions as the neural folds close. First, the long-standing idea in the field is that the neural tube closes by a “zipper-like” process. We observe “zippering” in the hindbrain but see a different mechanism of closure in the midbrain that we term buttoning-up. Second, we observe highly dynamic cellular activity including long filopodial-like extensions and membrane bridge-like structures that form across the gap between the closing folds. Third, it has been debated for decades as to whether it is neural or non-neural ectoderm that initiates closure. Our results indicate that the non-neural ectoderm initiates neural tube closure in the midbrain.
Our dynamic imaging shows a distinctly different process of neural tube closure in the midbrain, in contrast to the zippering mechanism of the hindbrain and spinal cord in the mammalian embryo. In the midbrain, a small number of non-neural ectoderm cells protrude from the epithelial layer on opposing sides of the neural folds, extend long cellular processes toward each other and then form contacts between the juxtaposed folds. This “buttoning-up” of the neural folds results in secondary closure sites that are later resolved into a contiguous midline. The idea that neural fold closure may occur in different manners in different parts of the neural tube was introduced as a possibility from observations of the chick embryo (Jaskoll et al., 1991; Van Straaten et al., 1996) and these authors also suggested a buttoning-up process, although the resolution did not allow analysis at a cellular level and this mechanism was invoked, not in the brain regions, but in the spinal cord. Multisite closure has been suggested in the human embryo based on various examples of a small region of incomplete closure at a distance from the initial closure points (Van Allen et al., 1993), although it is not possible to distinguish whether this represents evidence of secondary closure sites or failure to complete the progression of closure from initial sites. While unexpected due to the widely accepted zippering model that has prevailed for several decades, it is known that the midbrain and forebrain in chordates arise from developmental cues distinct from those of hindbrain and spinal cord (for review see (Prakash and Wurst, 2004; Schilling and Knight, 2001). These early molecular differences may serve to establish different mechanisms to achieve closure of the mammalian midbrain relative to the hindbrain and spinal cord, i.e. buttoning versus zippering.
Another unexpected finding was the observation of long thin and flexible cell extensions that extend across the physical gap of the closing neural folds. These structures were reminiscent of cytonemes. Cytonemes are long thin cellular extensions observed in imaginal disks of the Drosophila embryo (Ramirez-Weber and Kornberg, 1999). It was hypothesized that cytonemes serve in the communication of cells that are at a distance from each other. Although there is not yet a specific definition of cytonemes or their function, we speculate that the long thin cellular structures we observe in vertebrate embryos may be similar to cytonemes and may facilitate communication between cells that in this case are separated by a physical gap.
We also observed fine cellular bridges between the closing folds. To the best of our knowledge, cellular bridges have not been previously described in the mammalian neural tube or any other tissue. However, these cellular bridges are reminiscent of structures to date only described between cells in culture, called nanotubes (Eugenin et al., 2009; Gurke et al., 2008; Rustom et al., 2004). Intriguingly with respect to our tissue imaging studies, the cell culture data shows that the cells first extend elongated, fast-moving membrane extensions. After contact with a neighboring cell, one structure is stabilized to establish a connection between the two cells while the remaining extensions retract and subsequently disappear (Rustom et al., 2004). During mammalian neural tube closure, we suggest that the numerous cellular extensions seen as the two folds come close to one another provide transient bridges. We speculate that “transient filopodial bridging” helps to promote the formation of stable intermediate closure points by establishing connections between the two opposing sides of the folds. Studies by others using cultured cells indicate that the nanotube connections can serve as trafficking avenues for viruses (Eugenin et al., 2009) or endocytotic organelles (Gurke et al., 2008). This intriguing cell culture data raises a further conjecture that the cellular bridges might serve as communication avenues between the closing folds and, in this respect, it is of interest that small structures highlighted by the membrane-Venus reporter are seen in the thin cellular extensions. Future studies, perhaps using other imaging approaches to help prevent photodamage to these fine filopodia, are needed to determine the nature of these structures, whether the structures may be trafficked from one neural fold to the other and, if so, what kind of information may be transferred through these potential cellular communication avenues and the effect of that information on the process of neural tube closure.
In conclusion, our studies provide a new way to think about the cellular basis of mammalian neural tube closure beyond the static histological data that has been obtained to date. It is intriguing to speculate that there may be genetic mutations that disrupt neural tube closure due to a defect in the cellular behaviors described here or in the hypothesized information transfer between the two folds. Thus, live embryo culture combined with dynamic imaging of mammalian neural tube closure provides the framework to better understand this complex developmental process that often goes awry leading to severe birth defects.
Supplementary Material
Movie 1 is made from images taken in a single z plane every 2min on a Zeiss LSM510 META confocal microscope. Scale bar 20μm.
Movies 2 and 3 are images taken in a single z plane every 3 minutes (movie 2) or every 5 minutes (movie 3) on a Zeiss LSM510 META.
Movies 2 and 3 are images taken in a single z plane every 3 minutes (movie 2) or every 5 minutes (movie 3) on a Zeiss LSM510 META.
Movie 4 demonstrates two different viewpoints of the closing neural fold (the opposing converging neural fold is not visible in this movie). The first viewpoint in the first half of the movie is of two edges of one neural fold (separated by ∼50 μm) from a superficial optical cross section through the non-neural ectoderm with a few slightly deeper neuroectoderm cells in the middle of the tissue. This viewpoint highlights the long flexible cell extensions emanating from the non-neural ectoderm. The second viewpoint in the last half of the movie is of a surface view of the non-neural ectoderm which highlights the short bulbous flexible extensions. Movie 4 is made from images taken every 3 seconds in a single z plane on a Zeiss 5Live.
Acknowledgments
We thank B. Appel, K. Artinger, L. Barlow, R. Massarwa, D. McKean and D. Restrepo for comments on the manuscript and L. Bulwith for help with the mouse colony. LN is an investigator of the Howard Hughes Medical Institute and the work was also supported by NINDS 48154-05 award.
Footnotes
Author Contributions: C.P. designed and performed experiments, analysed data and wrote the paper; P.T. provided training and advice for embryo culture; A-K.H. developed the transgenic mouse; and L.N. designed experiments, analysed data and wrote the paper.
The authors state there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bancroft M, Bellairs R. Differentiation of the neural plate and neural tube in the young chick embryo. A study by scanning and transmission electron microscopy. Anat Embryol (Berl) 1975;147:309–35. doi: 10.1007/BF00315078. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126:4547–56. doi: 10.1242/dev.126.20.4547. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–8. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezin AM, Fraser SE, Bronner-Fraser M. Fate map and morphogenesis of presumptive neural crest and dorsal neural tube. Dev Biol. 2009;330:221–36. doi: 10.1016/j.ydbio.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Gerrelli D, Greene ND, Copp AJ. Mechanisms of normal and abnormal neurulation: evidence from embryo culture studies. Int J Dev Biol. 1997;41:199–212. [PubMed] [Google Scholar]
- Graeden E, Sive H. Live imaging of the zebrafish embryonic brain by confocal microscopy. J Vis Exp. 2009 doi: 10.3791/1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene ND, Gerrelli D, Van Straaten HW, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res. 2008;314:3669–83. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Harrington MJ, Hong E, Brewster R. Comparative analysis of neurulation: first impressions do not count. Mol Reprod Dev. 2009;76:954–65. doi: 10.1002/mrd.21085. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Greenberg G, Melnick M. Neural tube and neural crest: a new view with time-lapse high-definition photomicroscopy. Am J Med Genet. 1991;41:333–45. doi: 10.1002/ajmg.1320410315. [DOI] [PubMed] [Google Scholar]
- Jones EA, Crotty D, Kulesa PM, Waters CW, Baron MH, Fraser SE, Dickinson ME. Dynamic in vivo imaging of postimplantation mammalian embryos using whole embryo culture. Genesis. 2002;34:228–35. doi: 10.1002/gene.10162. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, Wallingford JB, Drapeau P, Capra V, Gros P. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007;356:1432–7. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- Kieserman EK, Wallingford JB. In vivo imaging reveals a role for Cdc42 in spindle positioning and planar orientation of cell divisions during vertebrate neural tube closure. J Cell Sci. 2009;122:2481–90. doi: 10.1242/jcs.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson A, England MA. Neural fold fusion in the cranial region of the chick embryo. Dev Dyn. 1998;212:473–81. doi: 10.1002/(SICI)1097-0177(199808)212:4<473::AID-AJA1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Martin P, Cockroft DL. Culture of postimplantation mouse embryos. Methods Mol Biol. 1999;97:7–22. doi: 10.1385/1-59259-270-8:7. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Rachel RA, Shah S, Beermann F, Stanier P, Mason CA, Copp AJ. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics. 2001;78:55–63. doi: 10.1006/geno.2001.6638. [DOI] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Specification of midbrain territory. Cell Tissue Res. 2004;318:5–14. doi: 10.1007/s00441-004-0955-x. [DOI] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Reynolds A, McDearmid JR, Lachance S, Marco PD, Merello E, Capra V, Gros P, Drapeau P, Kibar Z. VANGL1 rare variants associated with neural tube defects affect convergent extension in zebrafish. Mech Dev. 2010 doi: 10.1016/j.mod.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee JM, Pirity MK, Lackan CS, Long JZ, Kondoh G, Takeda J, Hadjantonakis AK. In vivo imaging and differential localization of lipid-modified GFP-variant fusions in embryonic stem cells and mice. Genesis. 2006;44:202–18. doi: 10.1002/dvg.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Knight RD. Origins of anteroposterior patterning and Hox gene regulation during chordate evolution. Philos Trans R Soc Lond B Biol Sci. 2001;356:1599–613. doi: 10.1098/rstb.2001.0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TE. Neurulation in Xenopus laevis. An analysis and model based upon light and electron microscopy. J Embryol Exp Morphol. 1970;23:427–62. [PubMed] [Google Scholar]
- Tam PP. Postimplantation mouse development: whole embryo culture and micro-manipulation. Int J Dev Biol. 1998;42:895–902. [PubMed] [Google Scholar]
- Van Allen MI, Kalousek DK, Chernoff GF, Juriloff D, Harris M, McGillivray BC, Yong SL, Langlois S, MacLeod PM, Chitayat D, et al. Evidence for multi-site closure of the neural tube in humans. Am J Med Genet. 1993;47:723–43. doi: 10.1002/ajmg.1320470528. [DOI] [PubMed] [Google Scholar]
- Van Straaten HW, Janssen HC, Peeters MC, Copp AJ, Hekking JW. Neural tube closure in the chick embryo is multiphasic. Dev Dyn. 1996;207:309–18. doi: 10.1002/(SICI)1097-0177(199611)207:3<309::AID-AJA8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006:R227–34. doi: 10.1093/hmg/ddl216. 15 Spec No 2. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–11. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1 is made from images taken in a single z plane every 2min on a Zeiss LSM510 META confocal microscope. Scale bar 20μm.
Movies 2 and 3 are images taken in a single z plane every 3 minutes (movie 2) or every 5 minutes (movie 3) on a Zeiss LSM510 META.
Movies 2 and 3 are images taken in a single z plane every 3 minutes (movie 2) or every 5 minutes (movie 3) on a Zeiss LSM510 META.
Movie 4 demonstrates two different viewpoints of the closing neural fold (the opposing converging neural fold is not visible in this movie). The first viewpoint in the first half of the movie is of two edges of one neural fold (separated by ∼50 μm) from a superficial optical cross section through the non-neural ectoderm with a few slightly deeper neuroectoderm cells in the middle of the tissue. This viewpoint highlights the long flexible cell extensions emanating from the non-neural ectoderm. The second viewpoint in the last half of the movie is of a surface view of the non-neural ectoderm which highlights the short bulbous flexible extensions. Movie 4 is made from images taken every 3 seconds in a single z plane on a Zeiss 5Live.


