Abstract
Stearoyl-acyl-carrier-protein-desaturase-mediated conversion of stearic acid (18:0) to oleic acid (18:1) is a key step, which regulates levels of unsaturated fatty acids in cells. We previously showed that stearoyl-acyl-carrier-protein-desaturase mutants ssi2/fab2 carrying a loss-of-function mutation in the plastidial glycerol-3-phosphate (G3P) acyltransferase (act1) have elevated 18:1 levels and are restored in their altered defense signaling. Because G3P is required for the acylation of 18:1 by G3P acyltransferase, it was predicted that reduction of G3P levels should increase 18:1 levels and thereby revert ssi2-triggered phenotypes. Here we show that a mutation in G3P dehydrogenase restores both salicylic acid- and jasmonic acid-mediated phenotypes of ssi2 plants. The G3P dehydrogenase gene was identified by map-based cloning of the ssi2 suppressor mutant rdc8 (gly1-3) and confirmed by epistatic analysis of ssi2 with gly1-1. Restoration of ssi2-triggered phenotypes by the gly1-3 mutation was age-dependent and correlated with the levels of 18:1. Regeneration of G3P pools by glycerol application in ssi2 and ssi2 gly1-3 plants caused a marked reduction in the 18:1 levels, which rendered these plants hypersensitive to glycerol. This hypersensitivity in ssi2 was rescued by the act1 mutation. Furthermore, overexpression of the ACT1 gene resulted in enhanced sensitivity to glycerol. Glycerol application also lowered the 18:1 content in SSI2 plants and converted these into ssi2-mimics. Our results show that 18:1 levels in plastids are regulated by means of acylation with G3P, and a balance between G3P and 18:1 is critical for the regulation of salicylic acid- and jasmonic acid-mediated signaling pathways.
Keywords: salicylic acid, fatty acid, stearoyl-acyl-carrier-protein desaturase, jasmonic acid, glycerol 3-phosphate
De novo fatty acid (FA) synthesis occurs exclusively in the plastids of all plant cells and leads to the synthesis of palmitic acid (16:0)-acyl carrier protein (ACP) and oleic acid (18:1)-ACP (1). These FAs either enter glycerolipid synthesis via the prokaryotic pathway in the inner envelope of chloroplasts or are exported from plastids as CoA thioesters to enter the eukaryotic glycerolipid synthesis pathway. Desaturation of stearic acid (18:0)-ACP to 18:1-ACP catalyzed by the SSI2/ FAB2-encoded stearoyl-ACP desaturase (S-ACP-DES) is one of the key steps in the FA biosynthesis pathway that regulates levels of unsaturated FAs in the cell. The 18:1-ACP generated in this reaction enters the prokaryotic pathway through acylation of glycerol 3-phosphate (G3P), and this reaction is catalyzed by the ACT1-encoded G3P acyltransferase.
G3P is an obligatory component and precursor for the biosynthesis of all plant glycerolipids, including storage lipids. Plants appear to generate G3P either by means of the G3P dehydrogenase (G3Pdh)-catalyzed reduction of dihydroxyacetone phosphate (DHAP) or by means of the glycerokinase-catalyzed phosphorylation of glycerol. However, the relative contributions of these enzymes to generation of G3P pools and overall glycerolipid biosynthesis are unclear. This situation is further complicated by the presence of several cytosolic, mitochondrial, and plastidial isoforms of the G3Pdh (2, 3).
A mutation in GLY1-encoded G3Pdh results in reduced carbon flux through the prokaryotic pathway, which leads to a reduction in the hexadecatrienoic acid (16:3) levels (4, 5). The gly1-1 plants continue to show normal growth characteristics, suggesting that contribution from the other G3Pdh and increased flux through the eukaryotic pathway compensates for their defect. The gly1-1 phenotype can be complemented by glycerol application, which suggests that the gly1-1 plants have a reduced pool of plastidial G3P (4).
Our previous results have shown that a reduction of 18:1 levels in ssi2/fab2 plants results in constitutive activation of the salicylic acid (SA)-dependent pathway and repression of the jasmonic acid (JA)-dependent pathway (6-8). The altered morphology and defense phenotypes in the ssi2/fab2 plants are restored by a loss-of-function mutation in the ACT1-encoded G3P acyltransferase, which elevates 18:1 levels in ssi2/fab2 plants (7). Because both 18:1 and G3P are required for the acyltransferase-catalyzed reaction, a reduction in either is likely to reduce the carbon flux through ACT1. To investigate the importance of 18:1 levels and the G3P pools in defense signaling, we assessed the role of G3Pdh in SSI2 (WT) and ssi2/fab2 plants. Our results show that a loss-of-function mutation in the GLY1-encoded G3Pdh can restore ssi2/fab2 phenotypes in an age-dependent manner, which coincides with plastidial 18:1 levels. This work establishes that 18:1 acylation of G3P is an important step in plastidial glycerolipid biosynthesis and acts to regulate plant defense signaling pathways.
Materials and Methods
Plant Growth Conditions and Genetic Analysis. Plants were grown in MTPS 144 Conviron (Winnipeg, MB, Canada) walk-inchambers at 22°C, 65% relative humidity, and 14-h photoperiod. Map-based-cloning of gly1-3 (rdc8) was carried out by crossing ssi2 gly1-3 plants with fab2 and scoring the F2 plants for WT-like morphology. Eight additional cleaved amplified polymorphic sequence (CAPS) markers were generated, which mapped within the region between nga168 and AthB102 on chromosome 2. These, and the existing markers from Arabidopsis thaliana Database, were used to map rdc8 to a region between the T16B24 and T6D20 bacterial artificial chromosome (BAC) contigs. This step was followed by sequence analysis of all of the putative ORFs within this region that indicated a function in FA or lipid metabolism. Sequence analysis of At2g40690, which is present on BAC T7D17, from ssi2 gly1-3 showed a point mutation that resulted in a stop codon at base pair 1492. Genomic CAPS for the gly1-3 mutation was performed by amplifying a 1.2-kb fragment by using primers GGTCTGGAGCTTAATACTCTT and AAGAGTATTAAGCTCCAGACC and digesting with BccI. Sequence analysis of At2g40690 amplified from fab2 tw2-1 and gly1-1 indicated that these plants contain a G-to-A point mutation at base pair 258. This result was further confirmed by generating derived-CAPS and digesting the 100-bp amplified product with BstNI. The derived-CAPS primers used for amplification were A ACCGATGT TCT TGAGCGTACTCGCCAG and CA ACA ACCTAAAA ACCCCCAGATTC. Complementation of ssi2 gly1-3 and gly1-1 plants was carried out by transforming these with a genomic sequence of At2g40690 cloned into the pBar1 plasmid and selecting the transgenic plants on soil sprayed with the herbicide Basta (Farnam Companies, Phoenix, AZ). The complementation was confirmed by FA analysis of transgenic plants.
Crosses were performed by pollinating flowers of ssi2 or ssi2 gly1-3 plants with pollen from fab2 tw2-1, gly1-1, or fab2 plants. The fab2 gly1-1 double mutant was isolated by crossing gly1-1 with fab2 and screening the F2 plants with gly1-1 and fab2 CAPS (7). The fab2 fad6 tw2-1 triple-mutant plants were obtained by pollinating fab2 tw2-1 flowers with pollen from fad6 plants. The genotypes at the ssi2, fad6, and act1 loci were determined as described earlier (6, 8).
G3Pdh Enzyme Assays. The G3Pdh gene from SSI2 and ssi2 gly1-3 plants was amplified as NcoI-BamHI-linkered PCR fragments and cloned into the Escherichia coli expression vector pET28a (Novagen). Recombinant protein was synthesized as a soluble protein in E. coli strain BL21. G3Pdh activity was determined by measuring the changes in A340 at 25°C caused by NAD+ formation from NADH and DHAP by using a spectrophotometer. The reaction mixture contained 100 mM Hepes buffer at pH 6.9, 0.2 mM NADH, 5 mM DHAP, and an appropriate amount of enzyme in a total volume of 1 ml.
RNA Extraction and Northern Analyses. Small-scale extraction of RNA from one or two leaves was performed with the TRIzol reagent (Invitrogen), following the manufacturer's instructions. Northern blot analysis and synthesis of random-primed probes for PR-1 and PDF1.2 were carried out as described (9).
Glycerol, JA, and Benzo[1,2,3]thiadiazole-7-carbothioic Acid S-Methyl Ester (BTH) Treatment. WT and mutant plants grown in a 14-h photoperiod were used for glycerol and BTH treatments. Glycerol solutions (50 mM) were prepared in sterile water and sprayed every 24 h as described (4). Control plants were sprayed with water. Plate assays were carried out by growing seeds on Murashige and Skoog medium containing 1% sucrose and supplemented with or without 0.2-0.3% glycerol. JA and BTH treatments were carried out as described (6, 9).
Chloroplast Isolation, FA Analysis, and Trypan Blue Staining. For chloroplast isolation, leaves from WT and mutant plants were harvested at the end of the night period. Five grams (fresh weight) of leaves were homogenized, and the chloroplasts were isolated as described (10). FA analyses of isolated chloroplasts and leaf tissue were carried out as described (11, 12). Trypan blue staining was performed as described (13).
Pathogen Infection. Infections with Peronospora parasitica Emco5 were performed by spraying asexual inoculum suspension as described (6). Pathogen inoculation on glycerol-treated plants was carried out 3 days after glycerol application and pathogen growth was scored 7 days after inoculation.
Results
RDC8 Is Allelic to GLY1 and Encodes G3Pdh. We used map-based cloning to isolate the RDC8 gene (see Materials and Methods) and found it to encode a 420-aa-long protein with a high level of amino acid identity to both plant and bacterial G3Pdh. The rdc8 mutant (Nössen ecotype) harbored a point mutation that generates a stop codon and results in carboxyl-terminal truncation of 109 aa from the mature protein (Fig. 1 A and B). Noting that the rdc8 mutant was allelic to a previously characterized fab2 suppressor, designated as tw2-1 (Col-0 ecotype, ref. 14), we sequenced the RDC8 gene in the tw2-1 mutant and found it to contain a G-to-A point mutation at the first exon-intron junction. Because the map location of RDC8 was the same as that of GLY1, we also sequenced the RDC8 gene from the gly1-1 mutant and found it to contain a mutation identical to that in tw2-1 (Fig. 1C). To further confirm the suppressive effect of the gly1 mutation on fab2 phenotypes, we crossed gly1-1 and fab2 mutants and scored F2 plants for tw2-1-like phenotypes. As expected, the F2 plants containing both gly1-1 and fab2 mutations were phenotypically similar to tw2-1 plants. A final confirmation was obtained by complementing gly1-1 plants with a genomic clone of RDC8. Thus, rdc8, tw2-1, and gly1-1 are mutations in the same gene, also called sfd1 (15). Because gly1 has two alleles (5), the tw2-1 and rdc8 mutations were renamed gly1-1 and gly1-3, respectively.
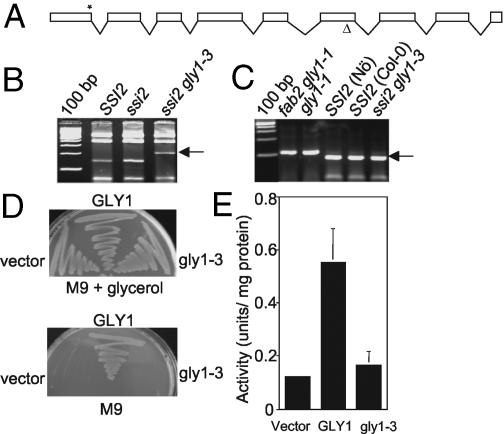
Fig. 1.
Genomic and biochemical characterization of G3Pdh. (A) The structure of the G3Pdh gene. Boxes indicate exons, and the open triangle indicates an intron. The triangle and asterisk indicate the position of the mutation in gly1-3 and gly1-1 (tw2-1), respectively. (B and C) BccI (B) and BstNI (C) restriction polymorphism generated by the gly1-3 and the gly1-1 mutations, respectively. PCR-amplified products from SSI2, ssi2 (Nössen ecotype), gly1-1 (Col-0 ecotype), fab2 gly1-1 (Col-0), and ssi2 gly1-3 (Nössen) were digested with BccI or BstNI and resolved on an agarose gel. The arrows indicate the polymorphic band. (D) Functional complementation of E. coli gpsA strain auxotrophic for G3P. E. coli strain BB20-14 cells were transformed with the vector control or the vector containing the GLY1 or gly1-3 gene, and the transformed cells were cultured on M9 medium with or without 0.1% glycerol. (E) G3Pdh activity of the recombinant enzyme.
To confirm that the GLY1 gene encodes G3Pdh, we performed functional complementation experiments with the E. coli mutant strain BB20-14 (16). BB20-14 carries a loss-of-function mutation in the G3Pdh-encoding gpsA gene and requires glycerol or G3P for growth, because it is unable to synthesize G3P. As expected, the mutant strain harboring the control vector failed to grow without glycerol supplementation. The mutant strain transformed with GLY1 grew well on M9 basal medium in the absence of glycerol, whereas the gly1-3-transformed strain did not (Fig. 1D). These results confirmed the function of the GLY1 protein to be a G3P-generating enzyme.
To further ascertain that GLY1-encoded protein possesses G3Pdh activity, we overexpressed the protein in E. coli and assayed for G3Pdh activity by monitoring conversion of DHAP to G3P. In comparison with the basal activity seen in protein extracts from vector-transformed E. coli cells, the GLY1 protein showed ≈4-fold higher G3Pdh activity (Fig. 1E). By contrast, gly1-3 showed basal level activity, similar to the vector control.
Exogenous Application of Glycerol Restores ssi2-Like Phenotypes in ssi2 gly1-3 Plants. Given that GLY1 encodes a G3Pdh, a mutation in gly1 should render the plants deficient in G3P levels, thus affecting plastidial FA synthesis. A deficiency in the G3P pool would also account for the reduction in the hexadecatrienoic acid levels in gly1 plants. These reduced levels of hexadecatrienoic acid in gly1-1 plants can be restored by glycerol application (4), suggesting that these plants are defective in their G3P supply within the chloroplasts. To determine whether increasing the G3P pool restores ssi2-like phenotypes in the ssi2 gly1-3 plants, we sprayed 2-week-old plants with water or glycerol and analyzed these for PR-1 gene expression and cell-death phenotypes 36 h after treatments. In comparison with the glycerol-treated SSI2 and gly1-1 single-mutant plants, ssi2 gly1-3 plants showed hypersensitive response (HR)-like cell death and induced a high level of PR-1 gene expression (Fig. 2 A and B). These phenotypes are similar to those seen in the ssi2 plants and suggest that glycerol treatment is sufficient to complement the deficiency of GLY1-encoded G3Pdh in the ssi2 gly1-3 plants.
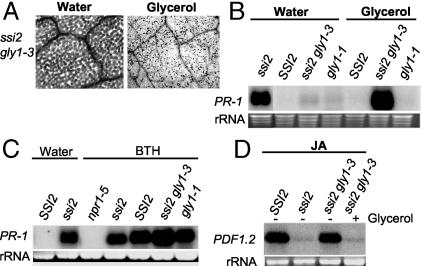
Fig. 2.
Effect of glycerol application on ssi2 gly1-3 leaves. (A) Microscopy of trypan blue-stained leaves from water- or glycerol-treated ssi2 gly1-3 plants. All of the treatments were carried out for 36 h before removing the samples. (B) Expression of the PR-1 gene in SSI2, gly1-1, ssi2, and ssi2 gly1-3 plants treated with water or glycerol for 36 h. Total RNA extracted from 2-week-old soil-grown plants was used for RNA gel-blot analysis, and ethidium bromide staining of rRNA was used as a loading control. (C) SA responsiveness of gly1-1 and ssi2 gly1-3 plants. SSI2, gly1-1, ssi2, npr1-5, and ssi2 gly1-3 plants were treated with water or 100 μM BTH and analyzed for PR-1 gene expression 48 h after treatment. Ethidium bromide staining of rRNA was used as a loading control. (D) Expression of PDF1.2 in water- or glycerol-treated plants. Two week-old plants were treated with water or glycerol for 72 h followed by application of 50 μM JA for 48 h. Ethidium bromide staining of rRNA was used as a loading control.
To determine whether, in addition to glycerol, ssi2 gly1-3 plants are responsive to exogenous application of SA, we treated SSI2, gly1-1, and ssi2 gly1-3 plants with BTH and analyzed PR-1 gene expression 48 h after treatment. Both gly1-1 and ssi2 gly1-3 induced high levels of PR-1 in response to BTH, which suggests that the basal level expression of PR-1 in ssi2 gly1-3 plants is not due to a defect in SA responsiveness (Fig. 2C).
Because ssi2 plants are repressed in JA-induced expression of the PDF1.2 gene, we also analyzed glycerol-treated plants for their ability to respond to JA and induce PDF1.2 gene expression. Interestingly, glycerol application resulted in a marked suppression of JA-induced expression of PDF1.2 in ssi2 gly1-3 plants (Fig. 2D). These phenotypes are similar to those seen in ssi2 plants and thus argue that glycerol application mimics a mutation in S-ACP-DES by lowering the levels of 18:1.
Glycerol Application Converts WT Plants into ssi2-Mimics. Glycerolipid biosynthesis within plastids is primarily initiated upon acylation of G3P with 18:1. It is therefore conceivable that an increase in G3P levels may have a quenching effect and result in a reduction of 18:1 levels. Thus, exogenous application of glycerol should cause a reduction in the 18:1 levels and render SSI2 plants defective in SA signaling, a result similar to that of ssi2 plants. Because 18:1 levels are highest at the cotyledon stage (Fig. 3A and see Fig. 5D), we chose 10-day-old plants to test the effects of glycerol and monitored changes in the leaf FA profile at 24-h intervals for 5 days. The 18:1 levels in 10-day-old plants were highest in SSI2 plants followed by ssi2 gly1-3 and ssi2. These differences are likely due to lack of a functional S-ACP-DES in ssi2 gly1-3 and ssi2 plants. A higher level of 18:1 in ssi2 gly1-3 as opposed to ssi2 is likely due to reduced amounts of G3P in ssi2 gly1-3, which would allow 18:1 to accumulate in these plants. Glycerol application led to a gradual and significant decline in the 18:1 levels in SSI2, ssi2 gly1-3, and ssi2 plants (Fig. 3A). Even though all genotypes showed a similar pattern of reduction in 18:1 levels, the glycerol-treated ssi2 and ssi2 gly1-3 plants showed a severe decline as compared with SSI2 plants.
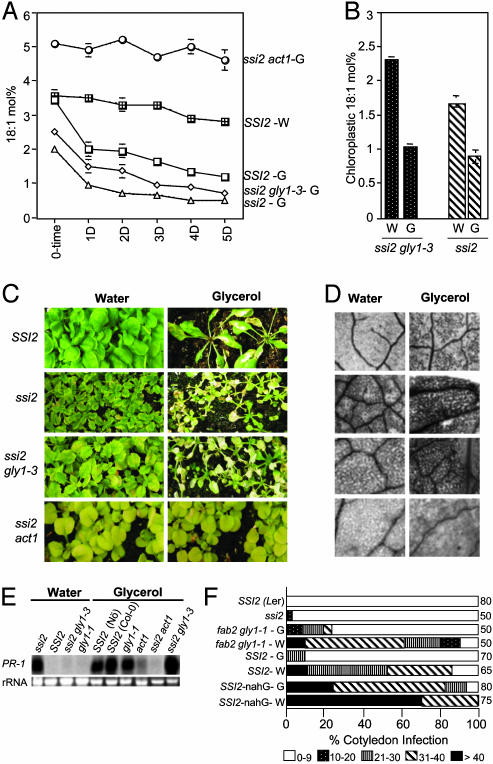
Fig. 3.
Glycerol application (50 mM) and its effects on 18:1 levels, morphological phenotypes, cell death, PR gene expression, and pathogen resistance. (A) Glycerol-induced changes in the 18:1 levels in leaf tissue of 10-day-old plants. Plants were treated with glycerol (G) or water (W), and samples taken every 24 h were analyzed for FAs by using GC. The values shown are averages of six to eight independent experiments. (B) Glycerol-induced changes in plastidial 18:1 levels. Fifteen-day-old plants were treated with water (W) or glycerol (G), and chloroplasts isolated 3 days after treatment were analyzed for their FA content by using GC. (C) Morphological phenotypes of SSI2, ssi2, ssi2 gly1-3, and ssi2 act1 plants treated with water or glycerol. Twelve-day-old plants were subjected to various treatments and photographed 5 days after treatment. Fewer glycerol-treated SSI2 plants are shown to highlight their symptoms. (D) Microscopy of trypan blue-stained leaves from plants shown in C.(E) Expression of the PR-1 gene in SSI2, ssi2, ssi2 gly1-3, gly1-1, act1, and ssi2 act1 treated with water or glycerol for 4 days. Ethidium bromide staining of rRNA was used as a loading control. (F) Growth of P. parasitica biotype Emco5 on various plant genotypes listed at the left. The Landsberg erecta (Ler) ecotype and ssi2 were used as resistant controls. The Nössen ecotype with or without nahG transgene was used as a susceptible background to assess effects of glycerol. The shade of each box indicates the severity of infection, based on the number of sporangiophores per cotyledon (see key at the bottom). Numbers to the right of the sample boxes indicate the number of cotyledons assayed.
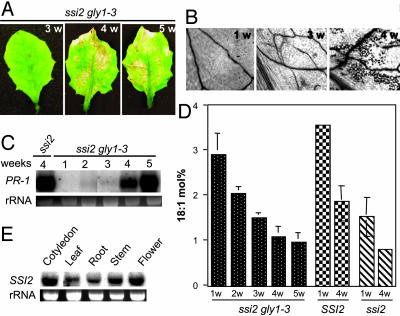
Fig. 5.
Morphological, molecular, and biochemical phenotypes of ssi2 gly1-3 plants. (A) Comparison of the morphological phenotypes displayed by the leaves from ssi2 gly1-3 plants grown on soil for 3, 4, and 5 weeks (w). (B) Microscopy of trypan blue-stained leaves from ssi2 gly1-3 leaves. (C) Age-dependent expression of the PR-1 gene in ssi2 gly1-3 plants. Ethidium bromide staining of rRNA was used as a loading control. (D) Age-dependent decline in the levels of 18:1 in leaf tissue of ssi2 gly1-3, SSI2, and ssi2 plants. The values shown are an average of 6-8 independent experiments. (E) Analysis of tissue-specific expression of the SSI2 gene. Ethidium bromide staining of rRNA was used as a loading control.
To verify whether the drop in 18:1 seen in glycerol-treated ssi2 gly1-3 and ssi2 plants is plastidial, we conducted FA analysis from ssi2 and ssi2 gly1-3 chloroplasts. Both ssi2 and ssi2 gly1-3 plants showed an approximately 2-fold reduction in their chloroplastic 18:1 content 3 days after glycerol treatment (Fig. 3B). This result confirmed that glycerol application impacts plastidial 18:1 levels.
To determine whether the reduction in 18:1, caused by the glycerol application, has an effect similar to the one seen in ssi2, we monitored various genotypes for morphological and molecular phenotypes. Both ssi2 gly1-3 and ssi2 plants were hypersensitive to glycerol and were decimated within 5 days of glycerol treatment (Fig. 3C). By comparison, water-treated plants grew normally and did not show any visible symptoms. Glycerol treatment of SSI2 and gly1-1 plants also led to chlorosis and HR-like lesion formation, although these plants developed these symptoms more slowly than did ssi2 and ssi2 gly1-3 plants. Trypan blue staining of randomly selected leaves from SSI2, gly1-1, ssi2, and ssi2 gly1-3 showed cell death after glycerol treatment, and this phenotype was much more pronounced in ssi2 and ssi2 gly1-3 leaves (Fig. 3D). The induction of a HR-like cell death in SSI2 and gly1 plants also correlated with higher expression of the PR-1 gene, although it was delayed compared with that of ssi2 gly1-3 plants (Figs. 2B and 3E). The glycerol-treated SSI2 plants also showed enhanced resistance to the oomycete pathogen P. parasitica, and these plants supported very little or no pathogen growth (Fig. 3F). By comparison, water-treated SSI2 plants showed extensive sporulation of the pathogen (Fig. 3F). The presence of the salicylate-degrading enzyme, nahG, abolished the glycerol-mediated enhanced resistance to P. parasitica, which suggests that glycerol activates the SA signaling pathway in SSI2 plants. However, susceptibility of glycerol-treated nahG plants could also be attributed to increased catechol production (17, 18).
Glycerol Hypersensitivity of ssi2 Can Be Rescued by the act1 Mutation.To provide further evidence that the glycerol-related phenotypes are mediated specifically by means of quenching of 18:1 levels caused by increased G3P, we analyzed the effects of glycerol in the act1 background. ACT1 catalyzes the acylation of G3P with 18:1; thus, glycerol treatment of act1 plants should not change the 18:1 levels in these plants. Indeed, both act1 and ssi2 act1 plants did not exhibit a drop in the levels of 18:1 on glycerol treatment (Fig. 3A and data not shown), strongly suggesting that the glycerol-mediated effect involves quenching of 18:1 by G3P. Both act1 and ssi2 act1 plants showed a high degree of tolerance to glycerol application, exhibiting fewer or no cell death lesions as compared to the SSI2, gly1-1, ssi2 gly1-3, and ssi2/fab2 plants (Fig. 3D). Furthermore, both act1 and ssi2 act1 showed basal level PR-1 (Fig. 3E) and PR-2 (data not shown) gene expression as compared with the high levels of PR-1/PR-2 gene expression observed in SSI2, gly1-1, and ssi2 gly1-3 plants. Taken together, these results suggest that glycerol treatment specifically affects the acylation reaction between G3P and 18:1. Furthermore, inhibiting this acylation reaction abolishes the glycerol-triggered reduction of 18:1 and thereby the ssi2-related phenotypes.
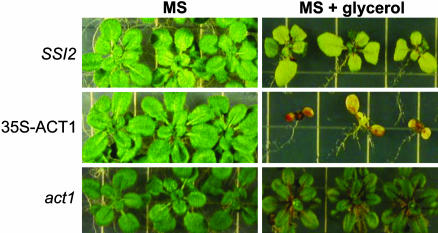
Overexpression of the ACT1-Encoded G3P Acyltransferase Renders Plants Hypersensitive to Glycerol. One plausible reason why SSI2 plants are less sensitive to glycerol in comparison with ssi2 or ssi2 gly1-3 plants is that the G3P acyltransferase-catalyzed acylation can be a rate-limiting step and, therefore, unable to deplete the levels of 18:1 in SSI2 plants. By comparison, it would be easier to deplete the 18:1 levels in ssi2 or ssi2 gly1-3 plants, because these plants lack a functional S-ACP-DES and are not able to synthesize 18:1 as efficiently as the SSI2 plants. To test this hypothesis, we overexpressed ACT1 in act1 plants and assayed the T2 plants for sensitivity to glycerol. Overexpression of ACT1 did not result in any morphological phenotype, and these plants grew normally on Murashige and Skoog medium (Fig. 4). However, it did result in hypersensitivity toward glycerol and these plants showed severe retardation of growth on medium containing glycerol. By comparison, SSI2 plants showed slower growth rate and chlorosis, whereas act1 plants had a normal phenotype on the glycerol-containing medium. These results suggest that the ACT1-catalyzed reaction is a rate-limiting step, and that increased levels of ACT1 are required to acylate the increased levels of G3P triggered by glycerol application.
Fig. 4.
Enhanced sensitivity to glycerol conferred by overexpression of ACT1. Seeds were grown on Murashige and Skoog (MS) medium for 7 days and subsequently transferred to Murashige and Skoog medium with or without 0.25% glycerol. The 35S-ACT1 plants overexpress ACT1 because the expression is driven by the cauliflower mosaic virus 35S promoter.
The gly1-3-Mediated Reversion of ssi2 Phenotypes Correlates with an Age-Dependent Decrease in the 18:1 Levels. The altered defense signaling of the SA and JA pathways in the ssi2 plants can be restored by a second-site mutation in the gly1 gene. Unlike the ssi2 plants, the ssi2 gly1-3 plants have WT-like morphology, show basal level expression of the PR-1 gene, and induce high levels of PDF1.2 in response to exogenous application of JA (7). Interestingly, the morphological phenotype of ssi2 gly1-3 plants undergoes a noticeable change after 3 weeks of growth and is associated with stunting, chlorosis, and formation of HR-like visible lesions on the leaves (Fig. 5 A and B). These visual phenotypes coincided with increased expression of the PR-1 gene in ssi2 gly1-3 plants (Fig. 5C). To determine whether the reappearance of ssi2-like phenotypes in ssi2 gly1-3 plants is associated with a reduction in 18:1 levels, we analyzed total FA levels of ssi2 gly1-3 plants at weekly intervals, for 5 weeks. The highest levels of 18:1 were found at the cotyledon stage in SSI2, ssi2, and ssi2 gly1-3 plants, and this correlates with increased expression of the SSI2 gene at this stage (Fig. 5 D and E). The levels of 18:1 decline gradually in all genotypes; however, the 18:1 levels in 4-week-old ssi2 gly1-3 were significantly reduced as compared with those in SSI2 plants. The 18:1 levels in 4-week-old ssi2 gly1-3 plants were only slightly higher compared with those in ssi2 plants of the same age. The reduction in 18:1 levels in older leaf tissues also coincided with reduced expression of GLY1 in mature leaves as compared with young leaves (data not shown). The levels of other FAs, including hexadecatrienoic acid, did not show any significant alteration between various growth periods. These results suggest that an age-dependent decrease in 18:1 levels in ssi2 gly1-3 plants is the likely cause for the reappearance of ssi2-like phenotypes in these plants.
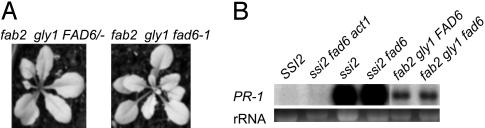
The gly1 Mutation Is Epistatic to fad6 and Restores WT-Like Phenotypes in fab2 fad6 Plants. Earlier (8), we had shown that, whereas the mutation in the plastidial membrane-localized ω6 desaturase (encoded by fad6) mediates a partial restoration of the ssi2 morphological phenotype, it is not sufficient to suppress PR-1 gene expression or allow JA-mediated induction of PDF1.2. Because the act1 mutation restores normal signaling of SA and JA pathways in ssi2 fad6 act1 plants, it was concluded that the act1 mutation is epistatic to the fad6 mutation and is likely to mediate its effect by allowing increased accumulation of 18:1 in the ssi2 background. Interestingly, although the ssi2 gly1 plants accumulate ≈5- to 6-fold lower levels of 18:1 in comparison with the ssi2 act1 plants, they are still restored in the various ssi2 phenotypes. To determine whether the lower levels of 18:1 in ssi2 gly1 plants are sufficient to suppress constitutive PR-1 gene expression in ssi2 fad6 plants, we generated fab2 gly1 fad6 triple-mutant plants and assessed them for various SA- and JA-induced phenotypes. Because ssi2 gly1 and fab2 gly1 plants showed age-dependent phenotypes, the double- and triple-mutant plants were analyzed for various phenotypes at the 2- to 3-week stage. The fab2 gly1 fad6 plants (10.65 ± 1.4 mol % 18:1) were indistinguishable from fab2 gly1 FAD6 plants (2.55 ± 0.4 mol % 18:1) and did not show any visible or microscopic cell death (Fig. 6A and data not shown). In comparison with the ssi2 fad6 plants, the fab2 gly1 fad6 plants showed suppression of PR-1 gene expression, which suggests that levels of 18:1 generated in the ssi2/fab2 background by a mutation in gly1-1 may restore normal SA signaling in these plants (Fig. 6B). A low level expression of PR-1 seen in the fab2 gly1 fad6 and fab2 gly1 FAD6 plants is age-dependent and is induced as these plants get older. Furthermore, unlike ssi2 fad6 plants, the fab2 gly1 fad6 plants were responsive to JA and induced high levels of the PDF1.2 gene (data not shown). These results suggest that, although restoration of signaling in the ssi2 mutant requires an increase in 18:1 levels beyond a certain threshold, these levels need not be as high as those in ssi2 act1 plants.
Fig. 6.
Morphological and molecular phenotypes of fab2 gly1-1 FAD6 and fab2 gly1-1 fad6 plants. (A) Comparison of the morphological phenotypes displayed by soil-grown, 3-week-old fab2 gly1-1 FAD6 and fab2 gly1-1 fad6 plants. (B) Expression of the PR-1 gene in SSI2, ssi2 act1 fad6, ssi2, ssi2 fad6, fab2 gly1-1 FAD6, and fab2 gly1-1 fad6 plants. Ethidium bromide staining of rRNA was used as a loading control.
Discussion
Our results demonstrate that the acylation reaction of G3P with 18:1 is one of the critical steps in the regulation of SA and JA defense-signaling pathways. We were able to reduce 18:1 levels by glycerol application in SSI2, gly1, ssi2, and ssi2 gly1-3 plants. Glycerol application, likely resulting in increased levels of G3P (19), eventually quenched 18:1 in the chloroplast. Most interestingly, reduced 18:1 levels in SSI2 plants were able to trigger SA-mediated expression of the PR-1 gene and resulted in enhanced resistance to oomycete pathogens. These phenotypes were similar to the ones seen in ssi2 plants, which contain reduced 18:1 levels as a result of a defective S-ACP-DES. Moreover, this quenching of 18:1 by glycerol application is inhibited in plants carrying a mutation in the G3P acyltransferase (act1) gene, further underscoring that glycerol affected the defense-related pathways by impacting the acylation reaction between G3P and 18:1.
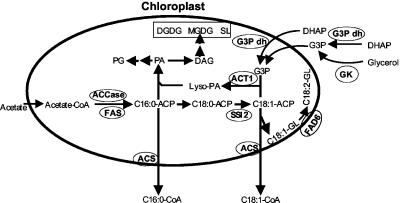
G3P, an essential metabolite derived from glycerol metabolism, is required in several primary biochemical pathways. It is present in both the cytoplasm and chloroplasts of leaf cells and is formed by means of either the G3Pdh-catalyzed reduction of DHAP or the glycerokinase-catalyzed phosphorylation of glycerol. It is very likely that the G3P generated on glycerol application in G3Pdh-defective plants is formed by means of the glycerokinase-catalyzed reaction (Fig. 7). This leads to the possibility that the glycerol-triggered phenotypes may be a result of phosphate deprivation as opposed to a reduction in 18:1 levels. However, exogenous application of phosphate did not alter these glycerol-triggered phenotypes, suggesting that a sufficient phosphate pool is available in the cell to make G3P. Moreover, if phosphate deprivation were the cause of these phenotypic changes, they would be evident in act1 and ssi2 act1 plants as well.
Fig. 7.
A condensed scheme for lipid biosynthesis in the chloroplasts of Arabidopsis leaves. Acetyl-CoA carboxylase (ACCase) and FA synthase (FAS) complex are key enzymes involved in the biosynthesis of palmitic acid (C16:0). On elongation to stearic acid (C18:0), this FA undergoes desaturation to oleic acid (C18:1). This step is catalyzed by the SSI2-encoded S-ACP-DES. The product of this reaction (C18:1-ACP) either enters the prokaryotic pathway of lipid biosynthesis through acylation of G3P or is exported from plastids as a CoA-thioester to enter the eukaryotic pathway. Acylation of G3P is catalyzed by an ACT1-encoded G3P acyltransferase. G3P can be made in both cytosol and plastids and can be transported between the cytoplasm and the chloroplast stroma. G3P can also be made by means of a cytosolic enzyme glycerokinase (GK), which appears to be the likely route of generating glycerol-induced G3P in gly1 plants. Lyso-PA, acyl-G3P; PG, phosphatidylglycerol; MGDG, monogalactosyldiacylglycerol; DGDG, digalactosyldiacylglycerol; SL, sulfolipid; DAG, diacylglycerol.
We also observed a significant reduction of 18:1 levels in older ssi2 gly1-3 plants, and this coincided with the reappearance of ssi2-like phenotypes. One plausible explanation is that younger tissue undergoing active chloroplast biogenesis has an increased demand for G3P. A mutation in gly1-1 causes G3P levels to drop, resulting in inefficient incorporation of 18:1 to form lysophosphatidic acid (LPA), and this causes 18:1 levels to rise (Fig. 7). During later stages of growth chloroplast biogenesis slows down, reducing the demand for G3P and thus allowing efficient acylation with 18:1. This in turn results in the depletion of free 18:1-ACP levels causing the reappearance of ssi2-related phenotypes in the older ssi2 gly1-3 plants. The fact that young tissue has elevated levels of the SSI2 and GLY1 transcripts further supports the notion that 18:1 and G3P levels are developmentally regulated.
What is the trigger for the defense-related phenotypes in ssi2? The altered defense-signaling phenotypes in ssi2 plants could also be a result of reduced levels of phosphatidic acid (PA) and LPA arising from 18:1 deficiency (Fig. 7). However, act1 plants, which also accumulate lower levels of PA (20), do not show any of the ssi2-like phenotypes, arguing against this possibility. Furthermore, LPA or PA levels are also likely reduced in gly1 plants, because these plants very likely contain a reduced amount of G3P. However, gly1 plants are morphologically similar to SSI2 plants and do not show any ssi2-like phenotypes.
Glycerol application lowers 18:1 levels by elevating G3P content and increasing the acylation of G3P with 18:1. This could also result in an increase in the LPA and PA contents, which could be responsible for ssi2-like phenotypes in the SSI2 plants. However, several observations argue against this possibility. First, although SSI2 plants contain higher levels of 18:1 and thus possibly make higher levels of PA, they do not exhibit any of the ssi2-related phenotypes. Second, although both SSI2 and ssi2/ ssi2 gly1-3 plants are unaltered in their G3P-18:1 acylation step, glycerol application resulting in an increase in G3P levels has a less pronounced effect in SSI2 plants. Third, PA has recently been shown to decrease H2O2-induced cell death (21); thus, it is unlikely that increased PA levels result in the induction of defense responses.
An alternative scenario would be that glycerol induces high levels of G3P, and this alone causes altered defense signaling. This possibility can be easily ruled out because, unlike other genotypes, act1 and ssi2 act1 plants do not respond to glycerol. Furthermore, the increased sensitivity of 35S-ACT1 plants to glycerol suggests that the step leading to the use of G3P is a key mediator of the various defense phenotypes.
Our results argue that the glycerol-triggered phenotypes are a result of decreased 18:1 content in the plastids. This conclusion accounts for the altered defense-signaling phenotypes seen in ssi2, the age-dependent reappearance of phenotypes in ssi2 gly1-3 plants, the suppression of ssi2-triggered phenotypes by mutations in act1 and gly1 genes, and the hypersensitivity of ssi2, ssi2 gly1-3, and 35S-ACT1 plants to glycerol.
Similar to the act1 mutation, the gly1 mutation also causes an increased flux of FAs through the eukaryotic pathway. Owing to the direct effect by the act1 mutation and the indirect effect by the gly1 mutation, acylation of G3P with 18:1 is reduced and likely leads to increased accumulation of 18:1-ACP within plastids. Thus, both mutations result in the restoration of WT-like phenotypes in ssi2/fab2 plants by means of an increase in 18:1 levels. In conclusion, our results with the ssi2 gly1-3 plants show that 18:1 levels in plastids are regulated by means of the acylation step with G3P and is an important mediator of the SA- and JA-mediated signaling pathways. Further efforts to understand how these molecules mediate their effects will help unravel the biochemical mysteries of this signaling pathway.
Acknowledgments
We thank John Browse and Martine Miquel for useful discussions, John Browse for providing tw2-1 and gly1-1 seeds, Chris Somerville for providing useful resources, Jyoti Shah for sharing unpublished results, Keiko Yoshioka and Daniel Klessig for providing oomycete isolate Emco5, and the E. coli Genetic Stock Center for providing the BB20-14 strain. We are grateful to John Johnson for help with GC-MS analysis and David Smith for comments on the manuscript. This work was supported by Kentucky Science and Engineering Foundation Grant 4-67441 (to P.K.), U.S. Department of Agriculture/National Research Initiative Grant 2002-01661 (to D.H.), and Kentucky Science and Engineering Foundation Grant 148-502-02-11 (to D.H.). This study is publication No. 04-12-045 of the Kentucky Agricultural Experiment Station.
Abbreviations: 18:1, oleic acid; ACP, acyl carrier protein; BTH, benzo[1,2,3]thiadiazole-7-carbothioic acid S-methyl ester; CAPS, cleaved amplified polymorphic sequence; DHAP, dihydroxyacetone phosphate; FA, fatty acid; G3P, glycerol 3-phosphate; G3Pdh, G3P dehydrogenase; HR, hypersensitive response; JA, jasmonic acid; PA, phosphatidic acid; LPA, lysophosphatidic acid; SA, salicylic acid; S-ACP-DES, stearoyl-ACP desaturase.
References
- 1.Ohlrogge, J. & Browse, J. (1995) Plant Cell 7, 957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen, W., Wei, Y., Dauk, M., Zheng, Z. & Zou, J. (2003) FEBS Lett. 536, 92-96. [DOI] [PubMed] [Google Scholar]
- 3.Wei, Y., Periappuram, C., Datla, R., Selvaraj, G. & Zou, J. (2001) Plant Physiol. Biochem. 39, 841-848. [Google Scholar]
- 4.Miquel, M., Cassagne, C. & Browse, J. (1998) Plant Physiol. 117, 923-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miquel, M. (2003) in Advanced Research on Plant Lipids, eds. Murata, N., Yamada, N., Nishida, I., Okuyama, H., Sekiya, J. & Hajime, W. (Kluwer, Dordrecht, The Netherlands), pp. 45-47.
- 6.Kachroo, P., Shanklin, J., Shah, J., Whittle, E. J. & Klessig, D. F. (2001) Proc. Natl. Acad. Sci. USA 98, 9448-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kachroo, P., Kachroo, A., Lapchyk, L., Hildebrand, D. & Klessig, D. F. (2003) Mol. Plant-Microbe Interact. 11, 1022-1029. [DOI] [PubMed] [Google Scholar]
- 8.Kachroo, A., Lapchyk, L., Fukushige, H., Hildebrand, D., Klessig, D. F. & Kachroo, P. (2003) Plant Cell 15, 2952-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kachroo, P., Yoshioka, K., Shah, J., Dooner, H. K. & Klessig, D. F. (2000) Plant Cell 12, 677-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronsson, H. & Jarvis, P. (2002) FEBS Lett. 529, 215-220. [DOI] [PubMed] [Google Scholar]
- 11.Dahmer, M. L., Fleming, P. D., Collins, G. B. & Hildebrand, D. F. (1989) J. Am. Oil Chem. Soc. 66, 534-538. [Google Scholar]
- 12.He, Y., Fukushige, H., Hildebrand, D. F. & Gan, S. (2002) Plant Physiol. 128, 876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowling, S. A., Clarke, J. D., Liu, Y., Klessig, D. F. & Dong, X. (1997) Plant Cell 9, 1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lightner, J., James, D., Lark, E. & Browse, J. (1997) Theor. Appl. Genet. 94, 975-981. [Google Scholar]
- 15.Nandi, A., Welti, R. & Shah, J. (2004) Plant Cell 16, 465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronan, J. E. & Bell, R. M. (1974) J. Bacteriol. 118, 598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Wees, S. C. & Glazebrook, J. (2003) Plant J. 33, 733-742. [DOI] [PubMed] [Google Scholar]
- 18.Heck, S., Grau, T., Buchala, A., Metraux, J. P. & Nawrath, C. (2003) Plant J. 36, 342-352. [DOI] [PubMed] [Google Scholar]
- 19.Leegood, R., Labate, C. A., Huber, S. C., Neuhaus, H. E. & Stitt, M. (1988) Planta 176, 117-126. [DOI] [PubMed] [Google Scholar]
- 20.Kunst, L., Browse, J. & Somerville, C. (1988) Proc. Natl. Acad. Sci. USA 85, 4143-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, W., Wang, C., Qin, C., Wood, T., Olafsdottir, G., Welti, R. & Wang, X. (2003) Plant Cell 15, 2285-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]