Abstract
Human ability to resolve temporal variation, or flicker, in the luminance (brightness) or chromaticity (color) of an image declines with increasing frequency and is limited, within the central visual field, to a critical flicker frequency of ≈50 and 25 Hz, respectively. Much remains unknown about the neural filtering that underlies this frequency-dependent attenuation of flicker sensitivity, most notably the number of filtering stages involved and their neural loci. Here we use the process of flicker adaptation, by which an observer's flicker sensitivity is attenuated after prolonged exposure to flickering lights, as a functional landmark. We show that flicker adaptation is more sensitive to high temporal frequencies than is conscious perception and that prolonged exposure to invisible flicker of either luminance or chromaticity, at frequencies above the respective critical flicker frequency, can compromise our visual sensitivity. This suggests that multiple filtering stages, distributed across retinal and cortical loci that straddle the locus for flicker adaptation, are involved in the neural filtering of high temporal frequencies by the human visual system.
The ability of the human visual system to resolve temporal modulation, or flicker, in the luminance or chromaticity of the light incident onto the retina (the light-sensitive neural structure at the back of the eye) is limited. Within the central retina, where spatial resolution is optimal, the temporal resolution of a typical observer is limited to a critical flicker frequency (CFF) of ≈50 and 25 Hz for luminance and chromatic flicker, respectively; higher-frequency flicker is invisible (1-4). Below the CFF, our flicker sensitivity is strongly frequency-dependent. The modulation transfer function (MTF) for human flicker perception, which traces modulation sensitivity as a function of flicker frequency, peaks around 8 Hz (4 Hz for chromatic flicker) and falls precipitously, by >100-fold, with increasing flicker frequency, out to the resolution limit (1-4). The neural processing that mediates this sharply low-pass nature of the MTF for flicker perception remains little understood (5-9). Specifically, the questions of what the number and anatomical loci of the neural filtering stages involved are remain open.
Here we pursue these questions through an experimental design that exploits, as a functional landmark, the known process of flicker adaptation, by which flicker sensitivity of an observer is attenuated after prolonged exposure to flickering lights (10-22). The attenuation of human flicker sensitivity, consequent to adaptation, is evident in both behavioral (10-22) and neural (9, 23, 24) response measures, but its frequency dependence has never been assessed systematically. Here we measure the reduction in flicker sensitivity after prolonged exposure, or adaptation, to both luminance and chromatic flicker across a wide range of frequencies. From these measurements, we derive the MTF for flicker adaptation and compare it with that for flicker perception. The comparison allows us to reach conclusions regarding the number and neural loci of temporal filtering stages in the human visual system by using the neural locus of flicker adaptation as a reference.
Methods
Stimulation. In all experiments, the stimulus was a small, spatially uniform spot subtending ≈3° of visual angle at the retina and presented to the central retina (fovea), with the image of the source light focused in the plane of the pupil (in “Maxwellian view”). The light sources were red (λpeak = 632 nm) and green (λpeak = 532 nm) light-emitting diodes. Stimulus control was via a 12-bit digital-to-analog converter (At-AO-6/10, National Instruments, Austin, TX), with a 1-kHz sampling resolution, in an IBM-compatible personal computer. For luminance flicker, only the red light source was used; modulation was defined as the proportional variation in stimulus luminance above and below the time-average level. Chromatic flicker was produced through equal, but counterphase modulation of spatially superimposed, isoluminant (see below) red and green sources. Modulation for chromatic flicker was defined as the proportional luminance variation of the individual chromatic (red/green) components. The time-average luminance was maintained at 2,000 Troland (Td) throughout the experimental session and across all experiments. Depending on the phase of the experiment (see below for details), the stimulus was either steady at a luminance of 2,000 Td or flickered symmetrically above and below that time-average level.
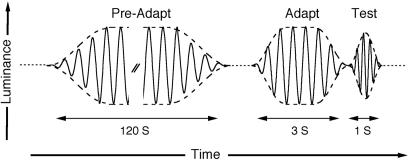
Flicker Adaptation. For a period of 2 min, the stimulus flickered in luminance or chromaticity (but not both) at each of a range of frequencies (2-60 Hz) and modulation levels (10-100%). After this initial exposure to the adapting flicker at a given frequency and modulation level, the modulation threshold (the minimum modulation required for flicker detection) was determined for either a 10- or 30-Hz test flicker; test frequency was selected to ensure that both adaptation and detection were mediated by a single temporal channel (25, 26). To maintain the observers' level of flicker adaptation throughout the experiment, test presentations were interleaved with repeated 3-s presentations of the adapting flicker (Fig. 1). Results of this experiment were used to derive the MTF for flicker adaptation (see Results).
Fig. 1.
Stimulus profile. For each condition (a given adapting frequency and modulation), 2 min of preadaptation were followed by interleaved presentations of the test (duration of 1 s) and adapting (duration of 3 s) flicker. Both the adapting and test flicker were presented within a temporal window defined by half-cosine edges (dashed curves) in order to avoid high-frequency artifacts at flicker onset and offset.
Flicker Perception. The MTF for flicker perception was obtained directly by measuring the modulation thresholds for a range of test frequencies (2-50 Hz), presented upon a steady field, with no prior flicker exposure. The stimuli and experiment design were the same as in the flicker-adaptation experiment (see above and Fig. 1) but with two critical modifications. First, during the initial 2-min adaptation period, instead of flicker, observers viewed a steady light, with luminance set equal to the time-average luminance (2,000 Td) of the flicker presented in the adaptation experiment. Second, the brief flicker presentations interleaved between test presentations in the adaptation experiment were replaced with presentations of steady light (at 2,000 Td). It is well known that the slope of the human MTF, our primary focus in this work, varies considerably with time-average luminance (1, 4). Maintaining the same time-average luminance across both the flicker-detection and flicker-adaptation experiments was thus a critical control.
Flicker Photometry. The point of red-green isoluminance (the ratio of physical intensities at which a red and a green light are of equal luminance) depends on the spectral composition of the light sources as well as on the spectral sensitivities of the individual observers. Therefore, to produce purely chromatic flicker, the red-green isoluminant point was determined experimentally by using the technique of flicker photometry. In particular, observers viewed the same 3° spot (as described above), within which the spatially superimposed red and green lights were counterphase-modulated at a frequency of 30 Hz. At this frequency (above the chromatic CFF but below the luminance CFF), the flicker was too fast for observers to track the alternation in chromaticity, and any appearance of flicker could be attributed entirely to residual luminance perturbations. Thus, with the red modulation fixed, the isoluminance point for each observer was determined by asking the observer to adjust the green modulation to eliminate the appearance of flicker.
Data Acquisition. Modulation thresholds for test flicker detection were determined by using an adaptive, yes-no staircase and a 50% threshold criterion. Each staircase consisted of 30 trials. Within each trial, the 1-s test presentation was demarcated by two audible tones; after the second tone, the observer indicated whether the spot appeared to flicker during the test interval. Modulation thresholds were estimated with a 95% confidence interval of ±0.1 log unit. For chromatic flicker, the red and green modulations were always equal. A two-alternative forced-choice procedure was not used because of practical limitations. However, our goal was to compare the relative positions of the MTFs for perception and adaptation (see Results); a two-alternative forced-choice procedure would have offered no advantages, because it would have led to proportionately lower threshold estimates in both cases, leaving the relative positions (and slopes) of the two functions unchanged.
Results
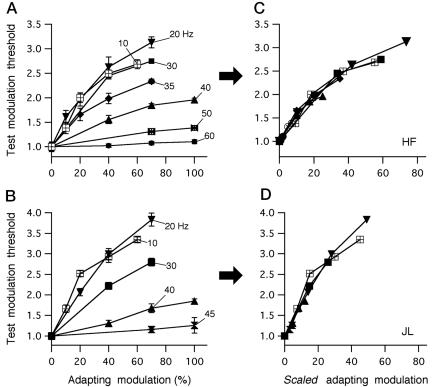
Luminance Flicker Adaptation. Fig. 2 A and B shows, for each of two observers, modulation thresholds for the test flicker, plotted as a function of the modulation of the adapting flicker; each curve traces that function for a different adapting frequency, as labeled. Test modulation thresholds were largest (modulation sensitivity was minimal) after adaptation to frequencies in the 10- to 20-Hz range, with higher adapting frequencies, out to 60 Hz, producing progressively lower test thresholds (Fig. 2 A and B). Scaling the curves relating test threshold to adapting modulation along the horizontal (modulation) axis by a single multiplicative factor for each frequency brought the curves into near-perfect register (Fig. 2 C and D). This confirmed that the effect of adaptation on luminance flicker sensitivity followed a single function of the adapting flicker modulation, scaled by the effectiveness of the adapting frequency. The scaling factors used in Fig. 2 C and D were thus directly proportional to modulation sensitivity as a function of adapting frequency and could be used to plot the MTF at the neural site for luminance flicker adaptation (see below).
Fig. 2.
Data analysis (luminance flicker). (A and B) For each of two observers, test modulation threshold, expressed as a multiple of the unadapted threshold (without prior flicker exposure), is plotted vs. modulation of the adapting flicker; adapting frequency is the curve parameter. Symbols and error bars are the average across four to eight independent measurements ±1SE(C and D). The curves from A and B are shown scaled along the horizontal (modulation) axis to bring all curves into register.
Chromatic Flicker Adaptation. The data for chromatic flicker (not shown) followed a very similar pattern as for luminance flicker, but, given the lower perceptual limit for chromatic flicker (1), spanned a more limited frequency range. Test modulation thresholds were largest after adaptation to a 4-Hz flicker, with higher adapting frequencies, out to 30 Hz, producing progressively lower test thresholds. A simple multiplicative scaling of the curves relating test threshold to adapting modulation along the horizontal (modulation) axis was sufficient, as it was for the luminance flicker data, to bring all curves into register. These scaling factors, plotted as a function of adapting frequency, thus were used to trace the MTF at the neural site for chromatic flicker adaptation.
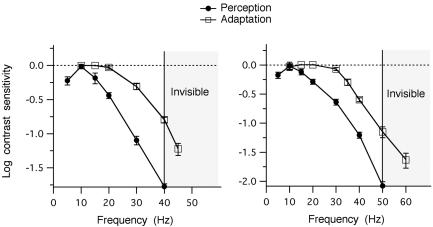
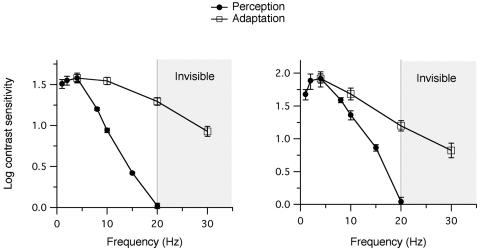
The MTF. Figs. 3 and 4 show the MTF for flicker adaptation (open squares) and flicker perception (filled circles) for luminance and chromatic flicker, respectively. Flicker adaptation proved to be much more sensitive to high temporal frequencies than was conscious perception. For both luminance and chromatic flicker, the MTF for adaptation traced a notably shallower slope than that for perception.
Fig. 3.
Luminance MTF. The MTF for luminance flicker adaptation (open squares), derived by plotting the modulation scaling factor (from Fig. 2 C and D) vs. adapting frequency, and for the conscious perception of luminance flicker (filled circles), derived by plotting the reciprocal of the unadapted modulation threshold vs. test frequency. Both functions are shown normalized to the value at 10 Hz. Each graph shows the data from one observer. Symbols and error bars represent the average of four to eight independent measurements ±1 SE. The vertical line in each graph marks the CFF value; higher frequencies (hatched regions) were invisible to the observers.
Fig. 4.
Chromatic MTF. Same as described for Fig. 3 but for chromatic flicker. The MTFs for adaptation (open squares) and perception (filled circles) are shown normalized to the value at 4 Hz. Each graph shows the data from one observer. Symbols and error bars represent the average of four to eight independent measurements ±1 SE. The vertical line in each graph marks the CFF value; higher frequencies (hatched regions) were invisible to the observers.
At the CFF for luminance flicker, the normalized modulation sensitivity for flicker adaptation was 10 times (1 log unit) greater than that for conscious perception (Fig. 3). Additionally, the adaptation MTF extended into the invisible range (hatched region), out to frequencies 20% above the luminance CFF (Fig. 3). It is worth noting that despite eye movements, which under certain conditions can render visible flicker that would be invisible otherwise (or, indeed, lead to the perception of flicker when none at all is physically present), observers reported that, whenever the adapting flicker frequency was above the independently measured CFF, the f licker remained invisible throughout the period of exposure.
The same held true for chromatic flicker (Fig. 4), for which the divergence between the MTF for adaptation and perception was even more striking. At the chromatic CFF, normalized modulation sensitivity was 20-30 times (1.3-1.5 log unit) greater for adaptation than for conscious perception, and the adaptation MTF extended well into the invisible range, out to frequencies 50% above the chromatic CFF.
Discussion
The results of Figs. 3 and 4 show that ambient flicker at imperceptibly high frequencies can penetrate to the neural site for flicker adaptation, which is presumed to be in primary visual cortex (23). Indeed, earlier physiological studies have demonstrated activity in human visual cortex in response to imperceptibly high flicker frequencies (5, 6), but these studies suggested no impact on perception as a result of this cortical activity. Our present findings show a clear, deleterious impact of this visuo-cortical response, as elicited by invisible flicker, on our ability to see subsequent perturbations in luminance or chromaticity.
The key implication of our findings is that the substantial neural attenuation of high temporal frequencies by the human visual system is accomplished through a series of filtering stages, distributed across multiple retinal and cortical loci, with at least one filtering stage peripheral and one central to the neural locus for flicker adaptation. This runs counter to the long-held belief in an early (likely retinal), single-stage temporal filter.
Our findings also have an interesting potential implication on the applied front for the design and use of computer displays, now in ubiquitous use by the general population. The use of refresh rates at frequencies as low as 60 Hz has long been considered adequate for use in these displays, because that frequency falls outside the visible frequency range of most observers. Our findings, however, suggest that an observer's visual sensitivity can be compromised by prolonged exposure to the image flicker (refresh rate) on these displays even when that flicker is invisible and that a rather sizeable safety margin above the perceptual limit is advisable.
Acknowledgments
This work was supported by National Institutes of Health Grant EY-01711.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CFF, critical flicker frequency; MTF, modulation transfer function; Td, Troland.
References
- 1.de Lange, H. (1958) J. Opt. Soc. Am. 48, 771-783. [Google Scholar]
- 2.Brown, J. L. (1965) in Vision and Visual Perception, ed. Graham, C. H. (Wiley, New York).
- 3.Matin, L. (1968) J. Opt. Soc. Am. 58, 404-415. [DOI] [PubMed] [Google Scholar]
- 4.Kelly, D. H. (1971) J. Opt. Soc. Am. 61, 537-546. [DOI] [PubMed] [Google Scholar]
- 5.Van der Tweel, L. H. (1963) Doc. Ophthalmol. 18, 287-304. [DOI] [PubMed] [Google Scholar]
- 6.Regan, D. (1968) Electroencephalogr. Clin. Neurophysiol. 25, 231-237. [DOI] [PubMed] [Google Scholar]
- 7.Regan, D. & Beverley, K. I. (1973) Perception 2, 61-65. [DOI] [PubMed] [Google Scholar]
- 8.Burns, S. A., Elsner, A. E. & Kreitz, M. R. (1992) Optom. Vis. Sci. 69, 95-105. [DOI] [PubMed] [Google Scholar]
- 9.Wu, S., Burns, S. A. & Elsner, A. E. (1995) Vision Res. 35, 2943-2953. [DOI] [PubMed] [Google Scholar]
- 10.Granit, R. A. & von Ammon, W. (1930) Am. J. Physiol. 95, 229-241. [Google Scholar]
- 11.Ginsburg, N. (1966) Am. J. Psychol. 79, 296-300. [PubMed] [Google Scholar]
- 12.Pantle, A. (1971) Vision Res. 11, 943-952. [DOI] [PubMed] [Google Scholar]
- 13.Smith, R. A., Jr. (1971) J. Physiol. (London) 216, 531-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly, D. H. (1972) Vision Res. 12, 89-101. [DOI] [PubMed] [Google Scholar]
- 15.Krauskopf, J. (1976) in Information Processes in Visual System: Proceedings of the IVth Symposium on Sensory System Physiology, ed. Gilezer, V.D. (Leningrad, Russia), p. 234.
- 16.Jameson, D., Hurvich, L. M. & Varner, F. D. (1979) Proc. Natl. Acad. Sci USA. 76, 3034-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauskopf, J., Williams, D. R. & Heeley, D. W. (1982) Vision Res. 22, 1123-1131. [DOI] [PubMed] [Google Scholar]
- 18.Benzschawel, T. & Guth, S. L. (1982) Vision Res. 22, 69-76. [DOI] [PubMed] [Google Scholar]
- 19.Lorenceau, J. (1987) Vision Res. 27, 2185-2191. [DOI] [PubMed] [Google Scholar]
- 20.Schieting, S. & Spillmann, L. (1987) Vision Res. 27, 277-284. [DOI] [PubMed] [Google Scholar]
- 21.Webster, M. A. & Mollon, J. D. (1994) Vision Res. 34, 1993-2020. [DOI] [PubMed] [Google Scholar]
- 22.Anstis, S. (1996) Vision Res. 36, 3479-3485. [DOI] [PubMed] [Google Scholar]
- 23.Movshon, J. A. & Lennie, P. (1979) Nature 278, 850-852. [DOI] [PubMed] [Google Scholar]
- 24.Chander, D. & Chichilnisky, E. J. (2001) J. Neurosci. 21, 9904-9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandler, M. B. & Makous, W. (1984) Vision Res. 24, 1881-1887. [DOI] [PubMed] [Google Scholar]
- 26.Hammett, S. T. & Smith, A. T. (1992) Vision Res. 32, 285-291. [DOI] [PubMed] [Google Scholar]