Abstract
Brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4) are two neurotrophins that play distinct roles in geniculate (taste) neuron survival, target innervation, and taste bud formation. These two neurotrophins both activate the tropomyosin-related kinase B (TrkB) receptor and the pan-neurotrophin receptor p75. Although the roles of these neurotrophins have been well studied, the degree to which BDNF and NT-4 act via TrkB to regulate taste development in vivo remains unclear. In this study, we compared taste development in TrkB−/− and Bdnf−/−/Ntf4−/− mice to determine if these deficits were similar. If so, this would indicate that the functions of both BDNF and NT-4 can be accounted for by TrkB-signaling. We found that TrkB−/− and Bdnf−/−/Ntf4−/− mice lose a similar number of geniculate neurons by E13.5, which indicates that both BDNF and NT-4 act primarily via TrkB to regulate geniculate neuron survival. Surprisingly, the few geniculate neurons that remain in TrkB−/− mice are more successful at innervating the tongue and taste buds compared with those neurons that remain in Bdnf−/−/Ntf4−/− mice. The remaining neurons in TrkB−/− mice support a significant number of taste buds. In addition, these remaining neurons do not express the TrkB receptor, which indicates that either BDNF or NT-4 must act via additional receptors to influence tongue innervation and/or targeting.
Introduction
The cell bodies of taste neurons are located in the geniculate ganglion. During development, taste neurons innervate specific regions of the gustatory epithelium with a precise number of neurons. The development of the geniculate ganglion and the formation of peripheral connections are highly regulated by two neurotrophins, brain-derived neurotrophic factor (BDNF) and Neurotrophin-4 (NT-4). Bdnf−/− and Ntf4−/− mice each lose approximately 50% of their geniculate neurons by birth [1], [2]. However, NT-4 regulates the survival of geniculate neurons at an earlier stage of development compared with BDNF via a different mechanism [3]. BDNF in the tongue epithelium also directs gustatory axons to their correct targets, while NT-4 does not [4], [5]. Bdnf−/− mice, which have a deficit in targeting, have a more severe loss of innervation to taste buds than Ntf4−/− mice [3], [6]–[8].
BDNF and NT-4 function via the TrkB and p75 receptors [9], [10]. Mice that lack TrkB lose more nodose-petrosal ganglion neurons than BDNF or NT-4 mutants [1], which indicates that both BDNF and NT-4 may act via TrkB to influence neuron development. Consistently, both Bdnf−/−/Ntf4−/− and TrkB−/− mice lose approximately 95% of their geniculate neurons [2], [11]. The maintenance of taste buds and fungiform papillae requires innervation by birth, and a lack of innervation results in taste bud loss in both Bdnf −/− and Ntf4 −/− mice at birth [7], [12]. Surprisingly, a substantial number of fungiform papillae and taste buds remain at birth in TrkB−/− mice [11], while Bdnf−/−/Ntf4−/− mice exhibit a substantial loss of fungiform papillae and taste buds at birth [13]. The reasons for the differences in the developing taste system in Bdnf−/−/Ntf4−/− and TrkB−/− are unclear. Furthermore, the results are difficult to compare because the two studies used different quantification methods. Therefore, two open questions remain: 1) How much do BDNF and NT-4 act via TrkB to regulate geniculate neuron survival and taste bud innervation during development?; and 2) Why do taste buds develop in TrkB−/− mice despite the substantial loss of taste neurons?
To address these questions, we directly compared the development of the gustatory system in Bdnf−/−/Ntf4−/− and TrkB−/− mice. These data provide direct evidence that BDNF and NT-4 act primarily via TrkB to regulate the survival of taste neurons in vivo. However, a small subpopulation of these geniculate neurons does not require or express TrkB, and these neurons can innervate and support the development of a substantial number of taste buds.
Materials and Methods
Animals
Heterozygous TrkB+/− (stock no. 002544), Bdnf+/− (stock no. 002266), and Ntf4+/− (stock no. 002497) mice were acquired from Jackson Laboratories (Bar Harbor, Maine, USA). TrkBtauEGFP mice were a generous gift from Dr. David Ginty [14]. TrkB−/− embryos were obtained by breeding heterozygous mice with a target mutation of the TrkB gene. TrkBtauEGFP/− embryos were obtained by breeding TrkBtauEGFP mice with TrkB+/− mice. Bdnf−/−/Ntf4−/− mice were obtained by breeding Bdnf+/−/Ntf4−/− mice. Animals were genotyped using polymerase chain reaction. Embryonic mice were identified from female mice that were bred and examined for plugs the following morning. The day that a plug was positively identified was designated as embryonic day 0.5. The care, handling, and use of all animals followed the guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals. All procedures used in this study were approved by the University of Louisville Institutional Animal Care and Use Committee (IACUC#10074).
Quantification of Geniculate Ganglion Volume and Neuron Number
Staining
Embryos aged E11.5 (TrkB−/− n = 3, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 3), E12.5 (TrkB−/− n = 3, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 3), and E13.5 (TrkB−/− n = 3, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 3) were transcardially perfused with ice-cold 4% phosphate-buffered paraformaldehyde (PFA). Following perfusion, embryos were post-fixed overnight in 4% PFA. Following fixation, embryo heads were dissected, moved to 70% ethanol, and processed for paraffin embedding. Geniculate ganglion neurons were visualized by class III β-tubulin (TUJ-1) antibody as previously described [15]. Briefly, serial sections (5 µm) of paraffin-embedded embryos were collected on SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA). Paraffin was removed by immersion in Citrisolv overnight. Following rehydration and endogenous peroxidase blocking, slides were treated for antigen retrieval in a citrate buffer (0.1 M citric acid, 0.1 M sodium citrate, dH2O; pH 6). Sections were washed in phosphate-buffered saline (PBS), blocked for 1 h in a blocking solution (PBS, 5% goat serum, 0.25% Triton X-100), and incubated overnight in mouse anti-β-III tubulin antibody (1∶500, Covance, Princeton, NJ, USA; catalog #MMS-435P) in a blocking solution. On the following day, sections were washed and incubated for 1.5 h in biotinylated anti-mouse secondary antibody (1∶250, Vector Laboratories, Burlingame, CA, USA; #BA-2000) in a blocking solution, and visualized with an ABC diaminobenzidine reaction kit (Vector Laboratories, Burlingame, CA, USA; #PK-6200).
Quantification
The area of the geniculate ganglion was measured in each section and multiplied by the section thickness (5 µm) to compute the volume of the geniculate ganglion within a single section; these volumes were added to compute the total volume of the entire ganglion. The TUJ-1 antibody was used to identify and count neuronal profiles in sections in which the nucleus was visible to quantify the number of geniculate neurons. Neuronal profiles were counted in six representative sections per ganglion. The geniculate ganglion volume in each of these six sections was also measured. The total number of neuronal profiles of the entire ganglion was estimated as the product of the number of profiles per volume of the counted section multiplied by the total volume of the entire ganglion. The total number of neurons per ganglion was estimated by multiplying the number of total neuron profiles by a correction factor to compensate for the presence of a nucleus in multiple sections [16]. The correction factor was calculated from the formula: N = n×[T/(T×D)], where N is the estimated total number of neurons, n is the number of nuclear profiles, T is the measured section thickness, and D is the average diameter of the nuclei [15].
Quantification of Fungiform Papilla Number using a Scanning Electron Microscope (SEM)
Mice (TrkB−/− n = 3, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 5) were anesthetized on the day of birth and transcardially perfused with ice-cold 4% PFA. The tongues were dissected and post-fixed in 1% aqueous OsO4 for 2.0 to 2.5 h, washed in buffer, and successively dehydrated in a graded series of ethanol and hexamethyldisilazane (HMDS). The tongues were housed in a desiccator overnight to evaporate the HMDS. On the following day, the tongues were mounted onto stubs, sputter-coated with gold, and examined by SEM (Phillips 505). Digital SEM images were captured at 130×magnification to distinguish the fungiform and filiform papillae. Individual fungiform papillae were imaged at 1770×magnification.
Quantification of Taste Bud Number
Mice (TrkB−/− n = 5, Bdnf−/−/Ntf4−/− n = 5, and wild-type n = 4) were anesthetized on the day of birth and transcardially perfused with ice-cold 4% PFA. The front of the tongue, which contains the fungiform field, was separated and post-fixed in 4% PFA for 2 h. The tongues were placed overnight in 30% sucrose as a cryoprotectant. On the following day, the tongues were embedded in OCT (Sakura Finetek USA, Torrance, CA, USA, #4583). Serial sagittal sections of the tongue (25 µm) were collected onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA). Sections were heat-dried overnight, rehydrated, placed into citrate buffer (pH 6.0), heated for 15 min in a boiling water bath, and incubated for 10 min at room temperature (RT) to retrieve antigens. The slides were washed in PBS and incubated overnight in 1∶50 rat anti-TROMA1 antibodies (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) in PBS. On the following day, the slides were rinsed in PBS (3 times for 5 min each) and incubated in anti-rat Alexa 555 secondary antibodies (1∶500, Molecular Probes, Eugene, OR, USA) for 2 h. After washing in PBS (3 times for 5 min each), the slides were dehydrated, cleared in Citrisolv, and cover-slipped using a DPX mounting medium (Fluka, St. Louis, MO, USA). The sections were examined in order, and the taste buds were followed across sections to ensure that each taste bud was counted only once.
Quantification of the Amount of P2X3 Labeled Innervation within a Taste Bud
The same procedures as described above were used to identify the taste buds in another set of mice (TrkB−/− n = 4, Bdnf−/−/Ntf4−/− n = 4, and wild-type n = 4). Following the procedures for antigen retrieval, the tongues were incubated overnight with both rat anti-Troma1 antibody and rabbit anti-P2X3 antibody (1∶500, Millipore, Billerica, MA, USA, #AB5895) as primary antibodies to label the taste fibers. Secondary anti-rat Alexa 488 (green) and anti-rabbit Alexa 555 (red) antibodies (1∶500, Molecular Probes, Eugene, OR, USA) were also used to visualize the taste buds and taste fibers, respectively.
Confocal stacks of optical sections with a Z step of 0.5 were imaged for 3 to 5 taste buds from every mouse within each genotype and analyzed by the ImageJ software (Version 1.43, 22 April 2010, http://rsbweb.nih.gov/ij/). The area occupied by the taste bud in each image section was measured, and areas were summed and multiplied by the section thickness (0.5 µm) to calculate the taste bud volume. The area occupied by P2X3-positive staining within the outlined taste bud was also measured in each optical section. These areas were summed and multiplied by the section thickness (0.5 µm) to measure the volume of innervation within the taste bud. The percentage of the taste bud that was occupied by the innervation was determined by dividing the volume of the P2X3 label by the volume of the Troma1 label.
Geniculate Ganglion Labeling using DiI
Embryos at ages E14.5, E15.5, E16.5, and E18.5 were anesthetized and transcardially perfused in ice-cold 4% PFA. The tongues were post-fixed in 4% PFA overnight. On the following day, DiI labeling was performed as described previously [17]. Embryos were incubated at 37°C for 2 to 8 weeks depending on the age of the embryo. The tongue was then dissected, examined, and photographed using a fluorescent dissecting microscope (Leica MZFL) equipped with a camera (QImaging CE). Images were collected from the tongues of TrkB−/−, Bdnf−/−/Ntf4−/−, and wild-type mice at the following ages: E14.5 (TrkB−/− n = 4, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 3), E15.5 (TrkB−/− n = 4, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 5), E16.5 (TrkB−/− n = 6, Bdnf−/−/Ntf4−/− n = 4, and wild-type n = 3), and E18.5 (TrkB−/− n = 4, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 5).
Quantification of the Innervation to the Lingual Epithelium
Embryos at E16.5 (TrkB−/− n = 3, Bdnf−/−/Ntf4−/− n = 3, and wild-type n = 3) were anesthetized and transcardially perfused with ice-cold 4% PFA. The front of the tongue, which contains the fungiform field, was separated and post-fixed for 2 h. The tongues were placed overnight in 30% sucrose and embedded in OCT (Sakura Finetek USA, Torrance, CA, USA, #4583) the following day. Serial sagittal sections of the tongue (25 µm) were collected onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA). Sections were heat-dried overnight, rehydrated, placed into citrate buffer (pH 6.0), heated for 15 min in a boiling water bath, and incubated for 10 min at RT to retrieve antigens. The slides were washed in PBS and incubated overnight in mouse anti-2H3 antibody (1∶100, Developmental Studies Hybridoma Bank, Iowa City, IA, USA) and rabbit anti-P2X3 antibody (1∶500, Millipore, Billerica, MA, USA, #AB5895) in PBS. The slides were rinsed in PBS (3 times for 5 min each) and incubated in anti-rabbit Alexa 488 and anti-mice Alexa 555 secondary antibodies (1∶500, Molecular Probes, Eugene, OR, USA) for 2 h. After washing in PBS (3 times for 5 min each), the slides were dehydrated, cleared in Citrisolv, and cover-slipped using a DPX mounting medium (Fluka, St. Louis, MO, USA). The sections were examined in order. Each instance in which the nerve fibers invaded the epithelium was quantified. Each location was examined in serial sections to ensure that each invading fiber bundle was counted only once.
Quantification of TrkB-GFP Expression in the Geniculate Ganglion
Embryos at E13.5 (TrkB tauEGFP/− n = 3 and wild-type n = 3) were anesthetized and transcardially perfused in ice-cold 4% PFA. The head was dissected and post-fixed overnight in 4% PFA. The head was then placed during the following overnight period in 30% sucrose as a cryoprotectant. On the following day, the tissue was embedded in OCT (Finetek USA, Torrance, CA, USA, #4583) and the heads were sectioned at 25 µm and collected onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA). The slides were allowed to dry at 40°C for 1 h. The slides were rinsed 3 times for 5 min each in PBST (PBS with 2.5% triton), blocked in 5% normal serum in PBST for 1 h, and incubated overnight at RT in chicken anti-GFP (1∶1000, Invitrogen, Grand Island, NY, USA #A11122) and rabbit anti-P2X3 (1∶500, Millipore, Billerica, MA, USA, #AB5895) antibodies in a blocking solution. On the following day, the slides were rinsed in PBST (3 times for 5 min each) and incubated in anti-chicken Alexa 488 and anti-rabbit Alexa 555 (1∶500, Molecular Probes, Eugene, OR, USA) secondary antibodies for 1 h. After washing in PBST (4×5 min), the slides were mounted with fluoromount-G (SouthernBiotech, Birmingham, Alabama, USA #0100-01). The number of GFP- and/or P2X3-positive neurons were counted. The ratio of the number of neurons that expressed both TrkB and P2X3 compared with the number of neurons that expressed P2X3 only was calculated.
Data Analysis
The total neuron number and total volumes were compared between genotypes on embryonic days E11.5, E12.5 and E13.5 using a two-way analysis of variance (ANOVA, IBM SPSS version 20). The fungiform papillae number and area, the taste bud number and volume, and the taste bud innervation data were compared using a one-way ANOVA. The alpha levels were set at p<0.05 for all statistical comparisons. The data are reported as the mean ± S.E.M.
Results
BDNF and NT-4 Both Act Primarily via TrkB to Support Geniculate Ganglion Neuron Survival during Embryonic Development
BDNF and NT-4 have been shown to regulate gustatory neuron development at different embryonic stages. Ntf4−/− mice start to lose geniculate neurons at E11.5, but Bdnf−/− mice do not start to lose geniculate neurons until E13.5 [3], [15]. Both Bdnf−/− and Ntf4−/− mice lose approximately half of their geniculate neurons by birth. To understand whether the regulation of geniculate neuron loss by these two neurotrophins acts via the same receptor, TrkB, we quantified the number of geniculate neurons in wild-type, Bdnf−/−/Ntf4−/−, and TrkB−/− mice from E11.5 to E13.5. We reasoned that if TrkB mediates the effects of these two ligands, then Bdnf−/−/Ntf4−/− and TrkB−/− mice would lose a similar number of geniculate neurons over the same timeframe.
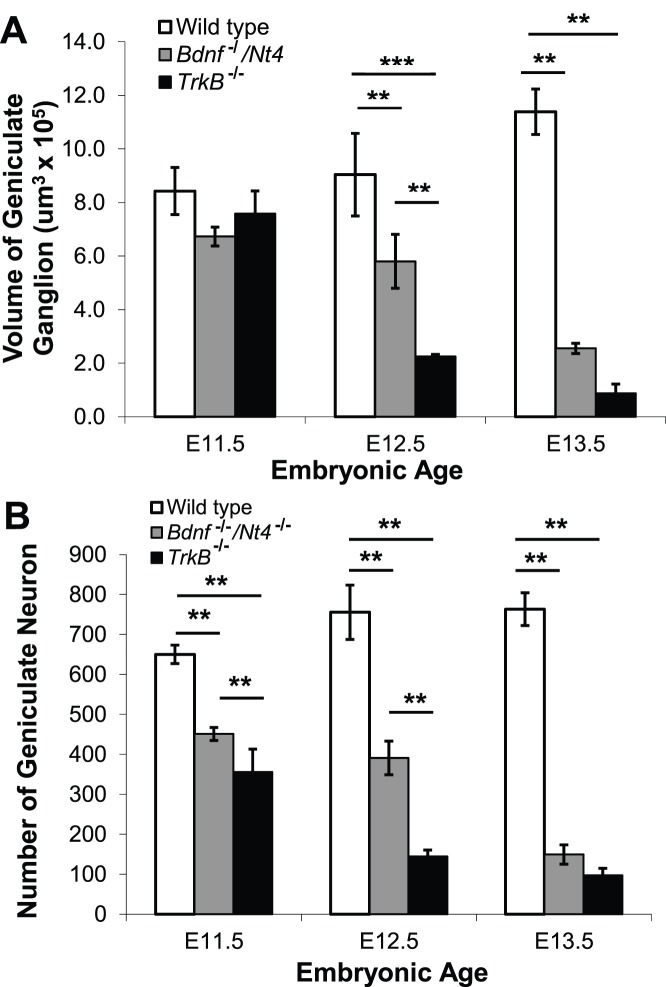
First, we compared geniculate ganglion volumes in wild-type, Bdnf−/−/Ntf4−/− , and TrkB−/− mice. At E11.5, there were no differences in geniculate ganglion volume between genotypes: wild-type (8.4±0.88×105 µm3), Bdnf−/−/Ntf4−/− (6.7±0.35×105 µm3), and TrkB−/− mice (7.6±0.85×105 µm3) (Figure 1A, B, C; Figure 2A). At E12.5, geniculate ganglion volume was reduced by 36% (p<0.01) in Bdnf−/−/Ntf4−/− (5.8±1.01×105 µm3) and 76% (p<0.001) in TrkB−/− (2.2±0.08×105 µm3) compared with wild-type mice (9.0±1.54×105 µm3). The geniculate ganglion volume was significantly smaller in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice (p<0.01) (Figure 1D, E, F; Figure 2A). At E13.5, the geniculate ganglion volume increased in wild-type mice (p<0.05) and continued to decrease in both Bdnf−/−/Ntf4−/− and TrkB−/− mice (p<0.01). There was no significant difference between the geniculate ganglion volumes in Bdnf−/−/Ntf4−/− and TrkB−/− mice at E13.5. These results show that geniculate ganglion volume increases in wild-type mice during early development, but is significantly reduced in Bdnf−/−/Ntf4−/− and TrkB−/− mice. Furthermore, the geniculate ganglion volume reduction is slightly delayed in Bdnf−/−/Ntf4−/− mice compared with TrkB−/− mice.
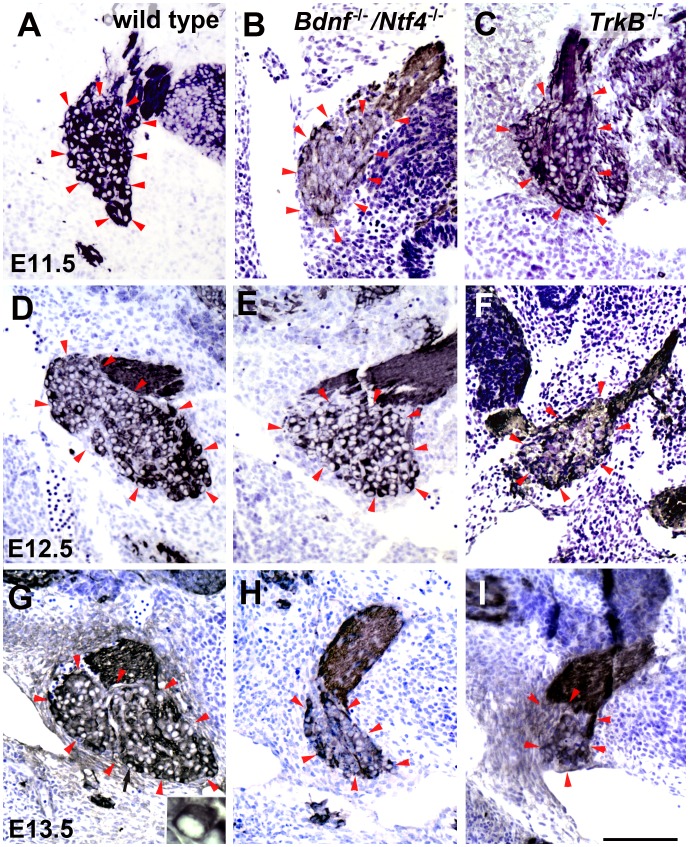
Figure 1. TUJ-1 labeled geniculate ganglia from Bdnf−/−/Ntf4−/− and TrkB−/− mice decrease in size compared with wild-type mice between E11.5 and E13.5.
Paraffin-embedded sections were stained with TUJ-1 antibody. This antibody is against β-tubulin and labels the cytoplasm of neurons because of the large number of microtubules present. The sections were counterstained with nissl stain which also stains the cytoplasm of neuron cell bodies. At E11.5, the geniculate ganglion was similar in size in wild-type (A), Bdnf−/−/Ntf4−/− (B), and TrkB−/− mice (C). Red arrow heads define the borders of the geniculate ganglion. At E12.5, the size of the geniculate ganglion was reduced in Bdnf−/−/Ntf4−/− (E) and TrkB−/− (F) mice compared with wild-type mice (D). The size of the geniculate ganglion appeared larger at E13.5 compared with E11.5 in wild-type mice (G compared with A). However, the size of the geniculate ganglion continued to decrease in Bdnf−/−/Ntf4−/− (H) and TrkB−/− mice (I) at E13.5. The inset in panel G shows the geniculate ganglion at a higher magnification to illustrate a cell (indicated by black arrow) that was positively labeled for the neuronal marker TUJ-1 with a dark cytoplasm and a clear nucleus. The scale bar in I = 100 µm and applies to A–I. The inset is 5X larger than the original image.
Figure 2. The volume and number of geniculate ganglion neurons decrease in Bdnf−/−/Ntf4−/− and TrkB−/− mice between E11.5 and E13.5.
(A) At E11.5, there was no difference between the geniculate ganglion volumes in wild-type, Bdnf−/−/Ntf4−/−, and TrkB−/− mice. At E12.5, the geniculate ganglion volume was reduced by 36% and 76% in Bdnf−/−/Ntf4−/− and TrkB−/− mice, respectively, compared with wild-type mice. The geniculate ganglion volume was significantly smaller in the TrkB−/− compared with the Bdnf−/−/Ntf4−/− mice. There was a significant increase in the geniculate ganglion volume in wild-type mice at E13.5 compared with E11.5. There were significant reductions in the geniculate ganglion volumes of Bdnf−/−/Ntf4−/− and TrkB−/− mice at E13.5, but no significant difference between the two mutant genotypes at this stage. (B) At E11.5, the number of geniculate neurons was reduced by 31% and 45% in the Bdnf−/−/Ntf4−/− and TrkB−/− mice compared with the wild-type mice. At E12.5, the number of geniculate neurons was reduced by 48% and 81% in the Bdnf−/−/Ntf4−/− and TrkB−/− mice, respectively, compared with the wild-type mice. There were also significantly fewer neurons in the TrkB−/− mice than the Bdnf−/−/Ntf4−/− mice at E12.5. At E13.5, the number of geniculate neurons was reduced by 80% and 87% in Bdnf−/−/Ntf4−/− and TrkB−/− mice, respectively, compared with the wild-type mice. There was no significant difference in the number of neurons between the two mutant genotypes at E13.5. *p<0.05, **p<0.01, and ***p<0.001.
Next, we quantified the number of neurons present in the geniculate ganglion. Geniculate neurons were easy to identify because they have a clear nucleus and dark cytoplasm when stained with TUJ-1 antibody (Figure 1G). There was a slight increase (17%; p<0.01) in the number of geniculate neurons in wild-type mice from E11.5 to E13.5. However, there was a continuous neuronal loss in Bdnf−/−/Ntf4−/− and TrkB−/− littermates over this same embryonic period (p<0.01; Figure 2B). Specifically, at E11.5, the number of geniculate neurons in Bdnf−/−/Ntf4−/− (451±16) and TrkB−/− mice (355±58) was reduced by 31% (p<0.01) and 45% (p<0.01), respectively, compared with wild-type mice (650±23) (Figure 2B). There were 21% fewer geniculate neurons in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice (p<0.01) at this age. This finding is consistent with earlier studies, which found that neuron number is a more sensitive measure of cell loss than geniculate volume during early development when neurons are small [3]. At E12.5, there was a 16% increase in the number of geniculate neurons in the wild-type mice (756±68) compared with the E11.5 littermates (p<0.01, Figure 2B). Although there was no significant change in the number of geniculate neurons in the Bdnf−/−/Ntf4−/− mice at E12.5 compared with E11.5, Bdnf−/−/Ntf4−/− mice had 48% fewer geniculate neurons compared with the wild-type mice (p<0.01), which is similar to the neuron loss that has been observed in Ntf4−/− mice [3]. However, the number of geniculate neurons in TrkB−/− mice (144±16) decreased 59% between ages E11.5 and E12.5 (p<0.01) and was reduced by 81% compared with wild-type littermates (p<0.01), which is consistent with an earlier study that showed a substantial loss of geniculate neurons in TrkB−/− mice by E12.5 [12]. There were also 64% fewer neurons in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice at E12.5 (p<0.01). One explanation for this finding is that other neurotrophins, such as NT-3, may also act via TrkB to regulate neuronal survival during early development. At E13.5, the number of geniculate neurons was reduced by 80% and 87% in Bdnf−/−/Ntf4−/− (149±24; p<0.01) and TrkB−/− mice (97±18; p<0.01), respectively, compared with wild-type mice (763±41, Figure 2B). Therefore, the bulk of the ganglion has disappeared in both genotypes by E13.5. Together, these data suggest a continuous neuron loss in both BDNF/NT-4 and TrkB mutant genotypes between E11.5 and E13.5. This loss is equivalent by E13.5, which indicates that BDNF and NT-4 act primarily via TrkB to regulate neuron survival during early development.
TrkB−/− Mice Lose the Same Number of Fungiform Papillae but have More Taste Buds than Bdnf−/−/Ntf4−/− Mice by Birth
Post-natal taste buds are supported by innervation from neuronal fibers. If the chorda tympani nerve is severed during development, then the taste buds and fungiform papillae are lost [18]–[20]. Many neurotrophin knockout studies have demonstrated a loss of taste buds at birth due to the earlier loss of geniculate neurons [3], [6], [7], [12], [13], [15], [21]. However, minimal taste bud loss was observed in TrkB −/− mice despite a significant loss of geniculate neurons [11], but the number of taste buds was not quantified. Therefore, in this study, both the fungiform papilla and taste buds were quantified in Bdnf−/−/Ntf4−/− and TrkB−/− mice at day of birth (Figure 3). There was a significant reduction in the number of fungiform papillae at P0 in Bdnf−/−/Ntf4−/− (61±5) and TrkB−/− (53±2) compared with wild-type (84±2, p<0.01) mice, but there was no significant difference between the two mutant genotypes. There was also a reduction of 32% and 35% in the surface area of the fungiform papillae in Bdnf−/−/Ntf4−/− (132±6 µm2) and TrkB−/− (126±8 µm2) mice, respectively, compared with wild-type mice (195±12 µm2) (Figure 3). There was no difference in the size of the fungiform papillae between Bdnf−/−/Ntf4−/− and TrkB−/− mice.
Figure 3. Bdnf−/−/Ntf4−/− and TrkB−/− mice have fewer and smaller fungiform papillae than wild-type mice at P0.

The number and size of the fungiform papillae were quantified with scanning electron microscopy (SEM) images of the tongue from wild-type (A), Bdnf−/−/Ntf4−/− (B), and TrkB−/− mice (C). Two insets in each panel illustrate individual fungiform papillae at higher magnification from the tip (arrow) and back (arrowhead) of the tongue of each genotype. There were significantly fewer fungiform papillae at P0 in Bdnf−/−/Ntf4−/− (61±5) and TrkB−/− (53±2) compared with wild-type mice (84±2, p<0.01), but there was no significant difference in the number and size of the fungiform papillae between the two mutant genotypes. The scale bar in C = 300 µm and applies to A–C; the scale bar in the inset panels = 40 µm and applies to the three inset panels in A–C.
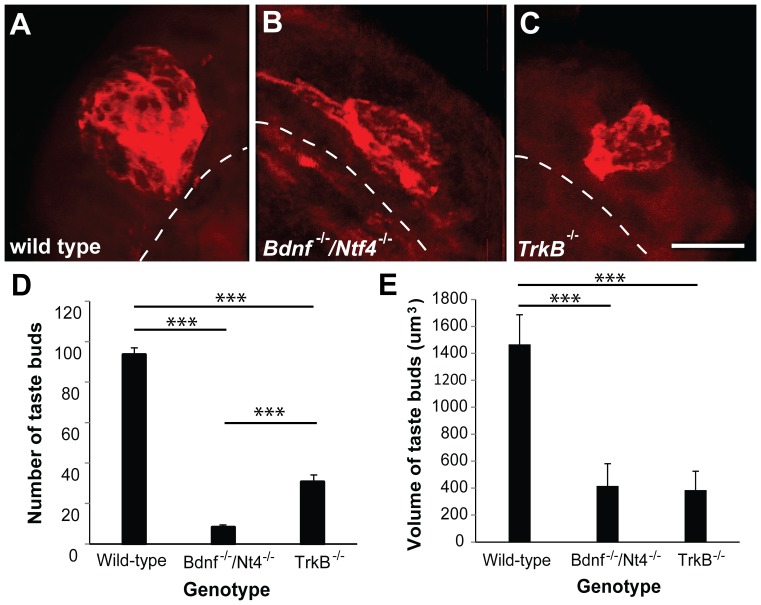
Although the number and size of fungiform papillae were reduced in Bdnf−/−/Ntf4−/− and TrkB−/− mice compared with wild-type mice, many fungiform papillae remained despite the severe neuron loss in these mutant genotypes. We tested the possibility that the smaller fungiform papillae lacked taste buds by examining the fungiform taste buds from Bdnf−/−/Ntf4−/−, TrkB−/−, and wild-type mice at P0. Taste buds were visualized using anti-Troma1 (Figure.4 A, B, C). There was a substantial loss of taste buds in both Bdnf−/−/Ntf4−/− (8±1) and TrkB−/− (31±3) compared with wild-type (93±3, p<0.001) mice (Figure 4D), although more taste buds were observed in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice (p<0.001). Taste bud volume was also smaller in both mutant genotypes compared with wild-type mice (p<0.001); however, no difference in taste bud volume was observed between the mutant genotypes (Figure 4E).
Figure 4. Bdnf−/−/Ntf4−/− mice lose more taste buds than TrkB−/− mice at P0.
The taste buds were visualized by anti-Troma1 (red) staining in wild-type (A), Bdnf−/−/Ntf4−/− (B), and TrkB−/− mice (C). A dashed line separates the papilla epithelium from the papilla core. The number of taste buds was reduced by 87% and 67% in Bdnf−/−/Ntf4−/− and TrkB−/− mice, respectively; there were significantly more taste buds in the TrkB−/− mice than the Bdnf−/−/Ntf4−/− mice (D). The taste bud volume was smaller in the Bdnf−/−/Ntf4−/− and TrkB−/− mice compared with the wild-type mice; however, there was no significant difference between the two mutant genotypes (E). The scale bar in C = 10 µm and applies to A–C. **p<0.01 and ***p<0.001.
More P2X3-positive Label of Fungiform Taste Buds was Observed in TrkB−/− Compared with Bdnf−/−/Ntf4−/− Mice at P0
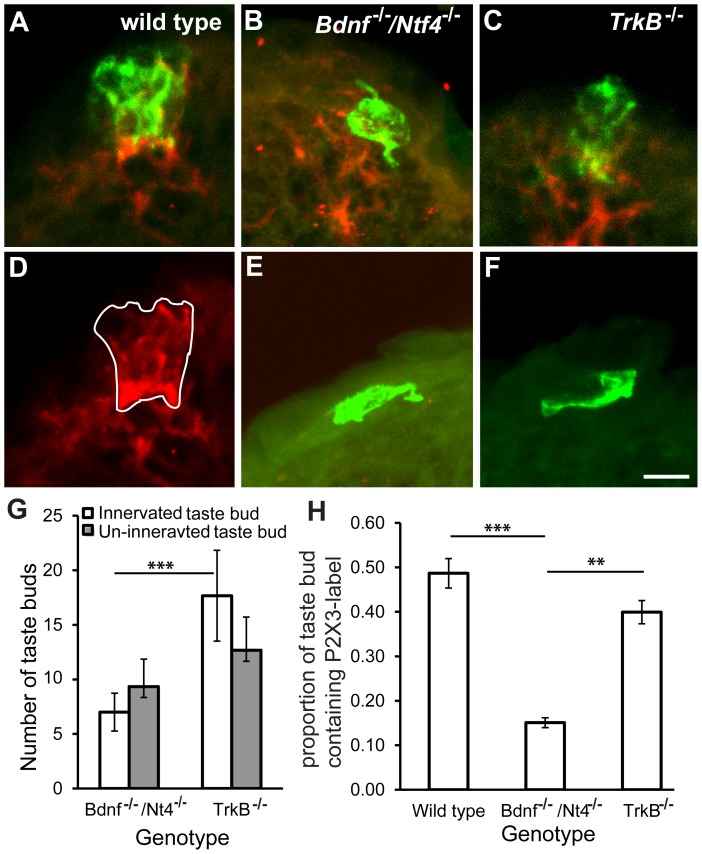
Taste buds are supported by gustatory innervation that must be present by birth [8], [22]–[24]. Because more taste buds were observed in the TrkB−/− compared with Bdnf−/−/Ntf4−/− mice at P0, we tested the possibility that the taste buds in the TrkB−/− mice had more innervation. Taste buds and taste nerves were visualized with anti-Troma1 and anti-P2X3, respectively. P2X3 is an ATP receptor that is required for gustatory nerves to respond to taste stimuli [25]. Anti-P2X3 appears to label most gustatory innervation and few trigeminal fibers [25], [26]. Taste buds that were innervated by P2X3-positive fibers (Figure 5 A, B, C) and those that were not innervated (Figure 5 E, F) were counted in Bdnf −/−/Ntf4−/− and TrkB−/− mice (Figure 5G). There were significantly more taste buds innervated by P2X3-positive fibers in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice (p<0.05). There were no differences in the number of non-P2X3-innervated taste buds in TrkB−/− and Bdnf−/−/Ntf4−/− mice. These results suggest that the extra taste buds that are observed in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice are also innervated, which indicates that more taste innervation to the tongue surface exists in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice.
Figure 5. The remaining taste buds had more innervation in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice at P0.
Anti-Troma1 (green) and anti-P2X3 (red) were used to visualize the taste buds and taste innervation, respectively, in wild-type (A), Bdnf−/−/Ntf4−/− (B), and TrkB−/− mice (C). One optical section (D) shows the area that was occupied by the P2X3-positive nerve fibers within the taste bud. Some non-P2X3-innervated taste buds were observed in the Bdnf−/−/Ntf4−/− (E) and TrkB−/− mice, as defined by a cytokeratin 8-positive label (F). These taste buds appear to lack the normal taste bud morphology. (G) A comparison of the number of P2X3-innervated and P2X3-non-innervated taste buds in Bdnf−/−/Ntf4−/− and TrkB−/− mice indicates that there are more P2X3-innervated taste buds in TrkB−/− mice. (H) For those taste buds that were innervated, the percentage of the taste bud that is occupied by P2X3-positive nerve fibers was compared. There is more innervation with in TrkB−/− taste buds than Bdnf−/−/Ntf4−/−taste buds. The scale bar in F = 10 µm and applies to A–F. **p<0.01.
Innervated taste buds also appeared to have more innervation in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice. To measure the amount of innervation within individual taste buds, the volumes occupied by the taste bud (Troma1) and taste nerve fibers (P2X3) within each taste bud were measured for each optical section (Figure 5D). In wild-type mice, the taste bud volume was 1441±227.3 µm3 and the volume of P2X3-positive nerves was 678±146.7 µm3, which indicates that approximately 50% of the taste bud is innervated in wild-type mice at birth (Figure 5H). The proportion of the taste bud that was innervated was substantially reduced in Bdnf−/−/Ntf4−/− mice (p<0.001, Figure 5H): the taste bud volume was 313±37.7 µm3 and the volume of P2X3-positive nerves was 46±5.2 µm3. The proportion of the taste bud that was innervated in TrkB−/− mice (taste bud volume = 321±35 µm3, volume of P2X3-positive nerves = 129±9.2 µm3) was not significantly different from the wild-type mice, but higher than the proportion of the taste bud that was innervated in Bdnf−/−/Ntf4−/− mice (p<0.01) (Figure. 5H). Together, these data suggest that the remaining taste buds in TrkB−/− mice are more heavily innervated than the taste buds in Bdnf−/−/Ntf4−/− mice at birth.
More Taste Fibers Reach their Targets in TrkB−/− Mice Compared with Bdnf−/−/Ntf4−/− Mice during Development
To determine whether TrkB−/− mice have more innervation to the tongue compared with Bdnf−/−/Ntf4−/− mice, we labeled chorda tympani axons with the lipophilic tracer, DiI, to examine the innervation pattern in the tongue. At E14.5, chorda tympani fibers branch from the base of the tongue toward the surface in wild-type mice. We have previously shown that chorda tympani fibers defasciculate and form a structure called a “neural bud” as the fibers invade the epithelium (Figure 6A, [5], [27]) At E14.5, there were no visible fibers on the dorsal surface of the tongue in Bdnf−/−/Ntf4−/− or TrkB−/− mice (Figure 6B, C), which suggests that either too few chorda tympani axons remain to innervate the tongue or the growth of the axons is delayed in these mutant genotypes.
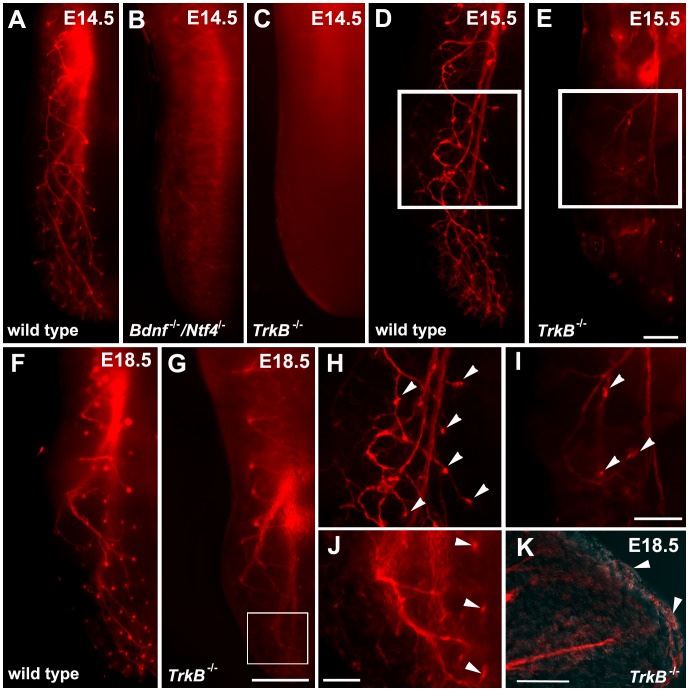
Figure 6. Innervation to the tongue surface remained in TrkB−/− mice.
Half tongues following DiI-labeling of the geniculate ganglion in wild-type (A, D, F), Bdnf−/−/Ntf4−/− (B), and TrkB−/− mice (C, E, G) at E14.5, E15.5, and E18.5. A high magnification view of the tongue at E15.5 (H and I, which correspond to the boxed areas in D and E, respectively) and E18.5 (J, which corresponds to the boxed area in G) illustrates that some DiI-labeled innervation is visible to the tongue surface at E15.5 in TrkB −/− mice. (K) A sagittal section of the innervation to the tip of the tongue in E18.5 TrkB−/− mice also illustrates innervation reaching the tongue surface. The scale bar in E = 200 µm and applies to A–E. The scale bar in G = 500 µm and applies to F and G. The scale bar in I = 200 µm and applies to H and I. The scale bar in J = 300 µm. The scale bar in K = 500 µm.
We examined the innervation patterns of the chorda tympani fibers at ages E15.5 and E18.5 to determine whether the growth of chorda tympani fibers is delayed in the Bdnf−/−/Ntf4−/− and TrkB−/− mice. We also examined whether more fibers reach the target in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice. At later embryonic stages, wild-type mice had similar innervation patterns to those observed at E14.5 (Figure 6 A, D, F). At E15.5, fiber bundles branched from the base of the tongue toward the lingual surface to form a neural bud in wild-type mice (Figure 6H). However, in Bdnf−/−/Ntf4−/− mice, fibers did not branch to the surface of the tongue at E15.5. Furthermore, in rare cases in which innervation was observed at the epithelial surface in Bdnf−/−/Ntf4−/− mice, only thin wispy branches that lacked neural buds were observed. These findings are similar to previous observations in Bdnf−/−/Ntf4−/− mice [5]. By E18.5, there was virtually no innervation to the tongue in Bdnf−/−/Ntf4−/− mice (images not shown). There was clear innervation to the tongue in TrkB−/− mice at E15.5 (Figure 6 E, G), but the number of branches was fewer compared with wild-type littermates due to the loss of geniculate neurons. From E15.5 to E18.5, a few chorda tympani fibers appeared to reach the tongue surface in TrkB−/− mice (Figure 6 I, J, K arrowheads). The fiber bundles were so thin that it was difficult to determine whether the fibers invaded the epithelial surface.
To determine whether the additional innervation that was observed in the tongues of TrkB−/− compared with Bdnf−/−/Ntf4−/− mice also invaded the tongue epithelium, tongues at E16.5 were double-stained with anti-P2X3 (green) and anti-Neurofilament (red) (Figure 7). While many fibers in the tongue were double-labeled with these two markers, P2X3 was more effective at labeling the neural bud ending (Figure 7A). There were some thin P2X3-negative but neurofilament positive fibers in the lamina propria of all genotypes (Figure 7 D, E, F); these fibers rarely invaded the epithelium and were likely from the trigeminal nerve. Although all three genotypes had some P2X3-positive nerve fibers that invaded the epithelium, only the wild type mice had a clear P2X3-positive neural bud (Figure 7 B, C compared with Figure 7A), innervation in both knockout genotypes did not have an expanded ending. Also, the endings do not appear to differ in size between TrkB−/− and Bdnf−/−/Ntf4−/−. This was a bit surprising since a larger proportion of the taste bud will be occupied by P2X3 label in TrkB−/− mice compared with Bdnf−/−/Ntf4−/− mice by birth. This finding implies a slightly greater expansion of the neural bud between E16.5 and birth in TrkB−/− mice compared with Bdnf−/−/Ntf4−/− mice. However it should be noted that remaining taste buds in TrkB −/− mice are considerably smaller than wild type mice, consistent with the observation that both TrkB−/− and Bdnf−/−/Ntf4−/− have substantially smaller neural endings than wild type mice.
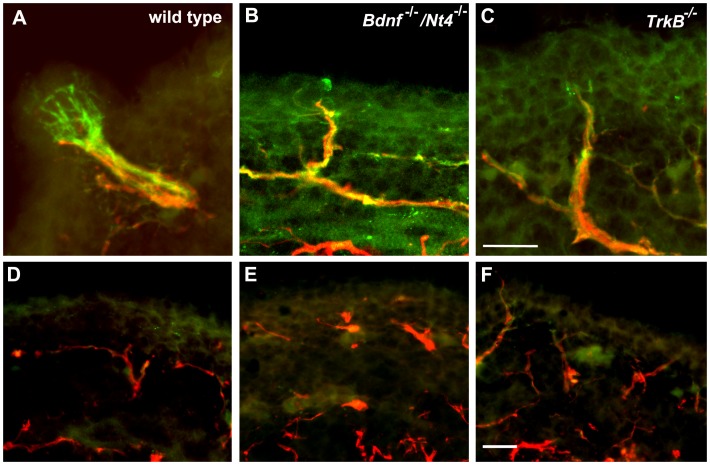
Figure 7. A comparison of taste nerve fibers at E16.5.
Anti-P2X3 (green) and anti-neurofilament (red) antibodies were used to label taste nerve fibers in wild-type (A, D), Bdnf−/−/Ntf4−/− (B, E), and TrkB−/− mice (C, F). While only wild type mice had P2X3-positive neural buds innervating fungiform placodes (A), both hybrid Bdnf −/−/Nt4 −/− mice (B) and TrkB −/− mice (C) had P2X3 positive fibers penetrating the epithelium of fungiform placodes. Neurofilament-positive and P2X3-negative innervation was present in the epithelium of all three genotypes (D, E, F). The scale bar in C and F = 20 µm and applies to A–C or D–F, respectively.
The number of locations at which the fibers penetrated the epithelium was quantified in serial sections. There were fewer locations where the nerve fibers penetrated the epithelium within the tongues of the TrkB−/− (21±3) and Bdnf−/−/Ntf4−/− mice (8±2) compared with the wild-type mice (107±6). However, there were significantly more locations with innervation in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice despite the significant loss of innervation in both mutant genotypes, which indicates that TrkB−/− mice have more gustatory innervation than Bdnf−/−/Ntf4−/− mice. This greater amount of taste innervation may account for the higher number of taste buds that were observed in TrkB−/− mice.
The Geniculate Neurons that Remain in TrkB−/− Mice do not Express TrkB
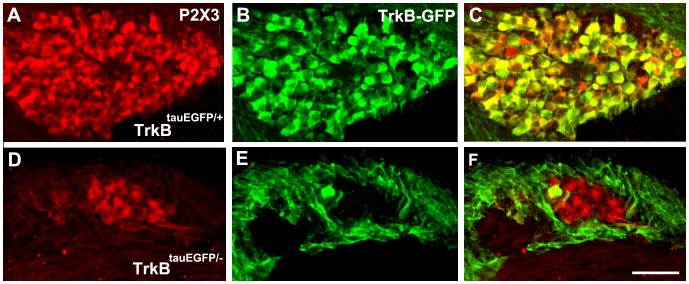
The reason why gustatory neurons remain and provide greater innervation to the tongue in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice is unclear. Because the remaining neurons provide more innervation to the taste bud in TrkB−/− mice than Bdnf−/−/Ntf4−/− mice, the implication is that these neurons can respond to either BDNF or NT-4. The TrkB receptor has a full-length signaling form as well as truncated forms that may or may not signal [28], [29]. The TrkB−/− mice used in this study still express truncated forms of TrkB [29], [30]. One possibility is that the innervation observed in TrkB−/− mice is maintained by a truncated form of the TrkB receptor that binds BDNF and/or NT-4 [31]. Alternatively, the subpopulation of neurons that remain in the absence of the full-length TrkB receptor may lack all forms of TrkB and be maintained by an alternate mechanism. To determine whether the remaining neurons in TrkB−/− mice express any form of the TrkB receptor, TrkBtauEGFP/+ mice were bred with TrkB+/− mice to obtain TrkBtauEGFP/− littermates. In TrkBtauEGFP/+ mice, GFP is expressed in all cells that would normally express either truncated or full-length forms of the TrkB receptor [14]. Because TrkB−/− mice at E13.5 have already lost most of the geniculate neurons, we examined TrkB−/− mice at this age to determine whether the remaining neurons in TrkB−/− mice express TrkB. Geniculate neurons were double-stained with anti-P2X3 (red) and anti-GFP (green) (Figure 8). In the wild-type mice, 94±1% of the geniculate neurons that expressed P2X3 also expressed TrkB. In contrast, only 11±2% of the P2X3-positive geniculate neurons expressed GFP in the TrkBtauEGFP/− mice. This result indicates that most of the neurons that remain in TrkB mutants belong to the 6% of neurons that are negative for all forms of the TrkB receptor. Together with our other findings, this result suggests that there exists a small population of TrkB-independent neurons in the geniculate ganglion that do not express TrkB, but need BDNF and/or NT-4 to regulate innervation patterns and support post-natal taste bud development.
Figure 8. The geniculate neurons that remain in TrkB−/− mice do not express TrkB.
Anti-P2X3 (red) was used to label most neurons in the geniculate ganglion of E13.5 embryos (A, D) and anti-GFP (green) was used to label TrkB-positive geniculate neurons (B, E). The connective tissue surrounding the ganglion was TrkB-GFP positive in both genotypes. The geniculate ganglion from the TrkBtauEGFP/− mouse is smaller and contains few GFP positive neurons then the geniculate ganglion from the TrkBtauEGFP/− mouse. Merged pictures are shown (C, F). The scale bar = 50 µm and applies to A–F.
Discussion
The neurotrophins BDNF and NT-4 play fundamental roles in the regulation of gustatory neuron survival and the ability of gustatory neurons to innervate their peripheral targets during development [1], [3], [5]–[8], [12], [13], [15], [17], [32]–[36]. However, the roles of the TrkB and p75 receptors are less clear. Here, we compared taste neuron survival, taste bud development, and target innervation in Bdnf−/−/Ntf4−/− and TrkB−/− mice to determine the extent to which BDNF and NT-4 act via TrkB to regulate taste development. Our data show that Bdnf−/−/Ntf4−/− and TrkB−/− mice lose a similar number of geniculate ganglion neurons by E13.5. In addition, Bdnf−/−/Ntf4−/− and TrkB −/− mice also lose most innervation to the tongue as well as most of their taste buds. These findings indicate that BDNF and NT-4 act primarily via TrkB to regulate the survival of taste neurons.
Subtle differences between the Bdnf−/−/Ntf4−/− and TrkB−/− mice provide insight into the possible role of other neurotrophins and receptors in the regulation of taste development. For example, the loss of geniculate neurons was slightly delayed in Bdnf−/−/Ntf4−/− compared with TrkB−/− mice at E12.5. One possible explanation is that other neurotrophins act via the TrkB receptor as survival factors at this stage. The most likely candidate is neurotrophin-3 (NT-3), which acts via TrkB to support DRG neuron survival [37]. NT-3 is robustly expressed in the lingual epithelium, the fungiform papillae core, and the tongue mesenchyme [8], [32], [38]–[40], and has been shown to regulate gustatory neuron functional development [32]. Approximately one-third of the geniculate and nodose-petrosal ganglion depend on NT-3 for survival [41]–[43], while only 11% of geniculate neurons depend on TrkC [44], which indicates that NT3 might also function through TrkB to support geniculate neuron survival. Another possibility is that in the absence of TrkB, BDNF binds p75 to increase cell death of geniculate neurons; BDNF causes cell death of sympathetic neurons by binding p75 [45], [46]. Lastly, the delayed neuron loss in Bdnf−/−/Ntf4 −/− mice compared with TrkB −/− mice may also be ligand independent. Several growth factors receptors, including TrkB, can function at low levels independent from ligand binding [47]–[49]. Regardless of the underlying mechanism, TrkB signaling in the absence of BDNF and NT-4 can maintain the number of geniculate neurons only temporarily: by E13.5, the effects of TrkB removal and TrkB ligand removal on the number of geniculate neurons are similar.
We observed that more taste buds are present in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice on the day of birth. This finding is consistent with previous reports that TrkB−/− mice [11], but not Bdnf−/−/Ntf4−/− mice [13], retain a substantial number of taste buds. A previous examination of TrkB −/− mice claimed that these mice lose most of their geniculate neurons and have no remaining innervation to the tongue [11]. We quantified innervation to the tongue to re-examine whether taste buds survive to birth in the absence of innervation in TrkB −/− mice and to determine why there were more taste buds in TrkB −/− compared with Bdnf−/−/Ntf4−/− mice. We found that neurons innervated more of the remaining taste buds with larger amounts of innervation in TrkB−/− mice than in Bdnf−/−/Ntf4−/− mice. We also found significantly more taste fibers in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice. Based on these experiments, we concluded that the increase in taste bud survival in TrkB−/− mice compared with Bdnf−/−/Ntf4−/− mice is due to the significant innervation that remains in the tongues of TrkB−/− mice. How NT4 and BDNF function to maintain more innervation to the taste bud in the absence of TrkB is unclear. However, one obvious explanation is that these BDNF and/or NT4 can function via p75 to support targeting and taste bud innervation.
We observed similar numbers of non-innervated taste buds, which do not occur in wild-type mice, in both mutant genotypes. These taste bud remnants were smaller than normal taste buds. These results demonstrate that some keratin 8-stained profiles do not require innervation to be maintained until birth in mice. Neither Bdnf−/−/Ntf4−/− or TrkB −/− survive much past birth, so it is not possible to track changes in postnatal taste bud numbers in TrkB−/− compared with Bdnf−/−/Ntf4−/− mice. Taste buds are more dependent on innervation for their survival in postnatal development than they are in adulthood [18], [19]. Therefore, we could predict that uninnervated taste buds would be lost during this time, while innervated taste buds could remain. Alternatively, the presence of taste bud remnants independent of nerve fibers is consistent with mouse studies in which some keratin 8-positive groups of cells remain following nerve sectioning [50]. The non-innervated remnants observed in our study do not appear to have normal taste bud morphology and may or may not differentiate into functional taste cell types. Therefore, the question as to whether taste buds remain in the absence of innervation depends largely on the definition of taste buds.
Our findings showed that the tongues of TrkB−/− mice retained approximately one-third of the normal number of taste buds compared with wild-type mice, although only one-tenth of the normal number of geniculate neurons remained to innervate the tongue. Although the remaining number of taste buds is large compared with the loss of geniculate neurons, these results are consistent with studies that demonstrate that taste bud loss cannot be predicted from neuron loss [12]. For example, Ntf3−/− mice lose geniculate neurons [42], [43] but do not lose taste buds [7], and Ntf4−/− mice lose approximately half of the geniculate neurons but lose only a few taste buds by birth [3], [12]. The reason that taste buds remain despite a significant loss of geniculate neurons is because each taste bud is innervated by multiple geniculate neurons [51]. In TrkB−/− mice, the 194 (97/side) remaining geniculate neurons are more than sufficient to innervate and maintain the 31±3 remaining taste buds. Because similar numbers of neurons remain in the geniculate ganglion of Bdnf −/−/Ntf4 −/− and TrkB −/− mice, it follows that the number of neurons available in Bdnf −/−/Ntf4 −/− mice should be sufficient to maintain 31 taste buds. However they do not; this finding indicates that Bdnf −/−/Ntf4 −/− mice exhibit a disruption in the target innervation of the remaining afferents, which is consistent with observations in Bdnf −/− mice [5]. Therefore, the failure of these remaining neurons to reach their targets successfully contributes to the loss of taste bud innervation in Bdnf −/−/Ntf4 −/− mice.
If TrkB−/− and Bdnf−/−/Ntf4−/− mice lose approximately the same number of geniculate ganglion neurons during development and the reduced innervation in Bdnf−/−/Ntf4−/− mice is due to disrupted targeting, then these findings suggest that target innervation is not disrupted in TrkB−/− mice. We observed that the small number of geniculate ganglion neurons that remain in TrkB−/− mice were able to innervate the tongue surface despite lacking all forms of the TrkB receptor. While we did find that a few TrkB-positive geniculate neurons remain in TrkB−/− mice, this number would not be sufficient to support the remaining taste buds. Together these results suggest that TrkB-negative geniculate neurons are responsive to BDNF, NT-4 or both. Other neurotrophins and their receptors may be responsible for the survival of the remaining geniculate neurons [52], [53], such as the NT-3 receptor TrkC and the receptor for the Gdnf family of ligands, Ret [54]–[56]. However, BDNF and NT-4 must somehow be responsible for the ability of these neurons to innervate the lingual epithelium, since innervation is completely disrupted in Bdnf−/−/Ntf4−/− mice. One possible mechanism for this innervation is the p75 receptor, which binds BDNF and NT-4 in addition to other neurotrophins. NT-4 may also function through another Trk receptor. For example, NT-4 may stimulate TrkA in some circumstances [57]. Regardless of the mechanism, our results indicate that there exists a small subpopulation of TrkB-negative geniculate neurons that depend on either BDNF or NT-4 to innervate taste buds.
The presence of a TrkB-negative gustatory subpopulation is intriguing, because it demonstrates that gustatory neuron subpopulations exist in the ganglion and perhaps can be identified based on the expression of factors that regulate taste neuron development. In the developing dorsal root ganglion (DRG), neuron diversity is established through a step-wise program that includes both transcription factors and Trk receptors [58] that define different functional classes or groups of DRG neurons. Prior to the current study, all taste neurons were presumed to depend on TrkB [11]. In the current study, we show that a small TrkB-negative subpopulation exists in the geniculate ganglion and supports taste buds. This TrkB-independent subpopulation may have a specific functional taste role. This result raises the possibility that TrkB-expressing neurons that co-express other growth factor receptors, like Ret [54] or TrkC [55], [59] could also define functional subpopulations. In addition, some gustatory neurons may lose TrkB receptor expression during post-natal development [53], [60] to define another subpopulation of taste neurons. The presence of gustatory neuron subpopulations that are defined by the expression of developmental factors opens several future directions of research.
Acknowledgments
We would like to thank Ms. Darlene Burke for statistical support.
Funding Statement
This work was supported by National Institute on Deafness and Communication Disorders grant DC009418 to RFK. The project made use of a statistical core facility supported by National Institutes of Health grant 8P30GM103507. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, et al. (1995) Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature 375: 235–238. [DOI] [PubMed] [Google Scholar]
- 2. Liu X, Ernfors P, Wu H, Jaenisch R (1995) Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature 375: 238–241. [DOI] [PubMed] [Google Scholar]
- 3. Patel AV, Krimm RF (2012) Neurotrophin-4 regulates the survival of gustatory neurons earlier in development using a different mechanism than brain-derived neurotrophic factor. Dev Biol 365: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoshino N, Vatterott P, Egwiekhor A, Rochlin MW (2010) Brain-derived neurotrophic factor attracts geniculate ganglion neurites during embryonic targeting. Dev Neurosci 32: 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma L, Lopez GF, Krimm RF (2009) Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. J Neurosci 29: 3354–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mistretta CM, Goosens KA, Farinas I, Reichardt LF (1999) Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol 409: 13–24. [PMC free article] [PubMed] [Google Scholar]
- 7. Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L (1997) Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development 124: 1333–1342. [DOI] [PubMed] [Google Scholar]
- 8. Oakley B, Brandemihl A, Cooper D, Lau D, Lawton A, et al. (1998) The morphogenesis of mouse vallate gustatory epithelium and taste buds requires BDNF-dependent taste neurons. Brain Res Dev Brain Res 105: 85–96. [PubMed] [Google Scholar]
- 9. Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299–309. [DOI] [PubMed] [Google Scholar]
- 10.Huang EJ, Reichardt LF (2003) TRK Receptors: Roles in Neuronal Signal Transduction. Annu Rev Biochem. [DOI] [PubMed]
- 11. Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I (1997) Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Neurosci 15: 563–576. [DOI] [PubMed] [Google Scholar]
- 12. Patel AV, Huang T, Krimm RF (2010) Lingual and palatal gustatory afferents each depend on both BDNF and NT-4, but the dependence is greater for lingual than palatal afferents. J Comp Neurol 518: 3290–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ito A, Nosrat CA (2009) Gustatory papillae and taste bud development and maintenance in the absence of TrkB ligands BDNF and NT-4. Cell Tissue Res 337: 349–359. [DOI] [PubMed] [Google Scholar]
- 14. Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, et al. (2011) The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147: 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel AV, Krimm RF (2010) BDNF is required for the survival of differentiated geniculate ganglion neurons. Dev Biol 340: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247. [DOI] [PubMed] [Google Scholar]
- 17. Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM (2001) Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol 232: 508–521. [DOI] [PubMed] [Google Scholar]
- 18. Nagato T, Matsumoto K, Tanioka H, Kodama J, Toh H (1995) Effect of denervation on morphogenesis of the rat fungiform papilla. Acta Anat (Basel) 153: 301–309. [DOI] [PubMed] [Google Scholar]
- 19. Sollars SI (2005) Chorda tympani nerve transection at different developmental ages produces differential effects on taste bud volume and papillae morphology in the rat. J Neurobiol 64: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sollars SI, Smith PC, Hill DL (2002) Time course of morphological alterations of fungiform papillae and taste buds following chorda tympani transection in neonatal rats. J Neurobiol 51: 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nosrat IV, Agerman K, Marinescu A, Ernfors P, Nosrat CA (2004) Lingual deficits in neurotrophin double knockout mice. J Neurocytol 33: 607–615. [DOI] [PubMed] [Google Scholar]
- 22. Hosley MA, Hughes SE, Morton LL, Oakley B (1987) A sensitive period for the neural induction of taste buds. J Neurosci 7: 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hosley MA, Hughes SE, Oakley B (1987) Neural induction of taste buds. J Comp Neurol 260: 224–232. [DOI] [PubMed] [Google Scholar]
- 24. Hosley MA, Oakley B (1987) Postnatal development of the vallate papilla and taste buds in rats. Anat Rec 218: 216–222. [DOI] [PubMed] [Google Scholar]
- 25. Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, et al. (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 26. Ishida Y, Ugawa S, Ueda T, Yamada T, Shibata Y, et al. (2009) P2X(2)- and P2X(3)-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. J Comp Neurol 514: 131–144. [DOI] [PubMed] [Google Scholar]
- 27. Lopez GF, Krimm RF (2006) Refinement of innervation accuracy following initial targeting of peripheral gustatory fibers. J Neurobiol 66: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ninkina N, Adu J, Fischer A, Pinon LG, Buchman VL, et al. (1996) Expression and function of TrkB variants in developing sensory neurons. Embo J 15: 6385–6393. [PMC free article] [PubMed] [Google Scholar]
- 29. Luikart BW, Nef S, Shipman T, Parada LF (2003) In vivo role of truncated trkb receptors during sensory ganglion neurogenesis. Neuroscience 117: 847–858. [DOI] [PubMed] [Google Scholar]
- 30. Escandon E, Soppet D, Rosenthal A, Mendoza-Ramirez JL, Szonyi E, et al. (1994) Regulation of neurotrophin receptor expression during embryonic and postnatal development. J Neurosci 14: 2054–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, et al. (1993) Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 75: 113–122. [PubMed] [Google Scholar]
- 32. Al-Hadlaq SM, Bradley RM, MacCallum DK, Mistretta CM (2003) Embryonic geniculate ganglion neurons in culture have neurotrophin-specific electrophysiological properties. Neuroscience 118: 145–159. [DOI] [PubMed] [Google Scholar]
- 33. Harlow DE, Yang H, Williams T, Barlow LA (2011) Epibranchial placode-derived neurons produce BDNF required for early sensory neuron development. Dev Dyn 240: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun H, Oakley B (2002) Development of anterior gustatory epithelia in the palate and tongue requires epidermal growth factor receptor. Dev Biol 242: 31–43. [DOI] [PubMed] [Google Scholar]
- 35. Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA (2009) Fate mapping of mammalian embryonic taste bud progenitors. Development 136: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zaidi FN, Krimm RF, Whitehead MC (2007) Exuberant neuronal convergence onto reduced taste bud targets with preservation of neural specificity in mice overexpressing neurotrophin in the tongue epithelium. J Neurosci 27: 13875–13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farinas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A (1998) Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron 21: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan L, Girnius S, Oakley B (2004) Support of trigeminal sensory neurons by nonneuronal p75 neurotrophin receptors. Brain Res Dev Brain Res 150: 23–39. [DOI] [PubMed] [Google Scholar]
- 39. Nosrat CA, Ebendal T, Olson L (1996) Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J Comp Neurol 376: 587–602. [DOI] [PubMed] [Google Scholar]
- 40. Nosrat CA, Olson L (1995) Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J Comp Neurol 360: 698–704. [DOI] [PubMed] [Google Scholar]
- 41. ElShamy WM, Ernfors P (1997) Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 complement and cooperate with each other sequentially during visceral neuron development. J Neurosci 17: 8667–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF (1994) Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature 369: 658–661. [DOI] [PubMed] [Google Scholar]
- 43. Liebl DJ, Tessarollo L, Palko ME, Parada LF (1997) Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci 17: 9113–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, et al. (1997) Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci U S A 94: 14776–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, et al. (1998) The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol 140: 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, et al. (2008) A model for neuronal competition during development. Science 320: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klau M, Hartmann M, Erdmann KS, Heumann R, Lessmann V (2001) Reduced number of functional glutamatergic synapses in hippocampal neurons overexpressing full-length TrkB receptors. J Neurosci Res 66: 327–336. [DOI] [PubMed] [Google Scholar]
- 48. Leoni C, Valtorta F (2002) Constitutive TrkA activity in receptor-overexpressing PC12 clones. Biochem Biophys Res Commun 291: 972–978. [DOI] [PubMed] [Google Scholar]
- 49. Tan PK, Wang J, Littler PL, Wong KK, Sweetnam TA, et al. (2007) Monitoring interactions between receptor tyrosine kinases and their downstream effector proteins in living cells using bioluminescence resonance energy transfer. Mol Pharmacol 72: 1440–1446. [DOI] [PubMed] [Google Scholar]
- 50. Guagliardo NA, Hill DL (2007) Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J Comp Neurol 504: 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zaidi FN, Whitehead MC (2006) Discrete innervation of murine taste buds by peripheral taste neurons. J Neurosci 26: 8243–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cho TT, Farbman AI (1999) Neurotrophin receptors in the geniculate ganglion. Brain Res Mol Brain Res 68: 1–13. [DOI] [PubMed] [Google Scholar]
- 53. Farbman AI, Brann JH, Rozenblat A, Rochlin MW, Weiler E, et al. (2004) Developmental expression of neurotrophin receptor genes in rat geniculate ganglion neurons. J Neurocytol 33: 331–343. [DOI] [PubMed] [Google Scholar]
- 54. D'Autreaux F, Coppola E, Hirsch MR, Birchmeier C, Brunet JF (2011) Homeoprotein Phox2b commands a somatic-to-visceral switch in cranial sensory pathways. Proc Natl Acad Sci U S A 108: 20018–20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matsumoto I, Emori Y, Ninomiya Y, Abe K (2001) A comparative study of three cranial sensory ganglia projecting into the oral cavity: in situ hybridization analyses of neurotrophin receptors and thermosensitive cation channels. Brain Res Mol Brain Res 93: 105–112. [DOI] [PubMed] [Google Scholar]
- 56. Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M (1998) Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci 18: 7040–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, et al. (1993) Tissue-specific alternative splicing generates two isoforms of the trkA receptor. J Biol Chem 268: 15150–15157. [PubMed] [Google Scholar]
- 58. Lallemend F, Ernfors P (2012) Molecular interactions underlying the specification of sensory neurons. Trends Neurosci 35: 373–381. [DOI] [PubMed] [Google Scholar]
- 59. Yamout A, Spec A, Cosmano J, Kashyap M, Rochlin MW (2005) Neurotrophic factor receptor expression and in vitro nerve growth of geniculate ganglion neurons that supply divergent nerves. Dev Neurosci 27: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farbman AI, Guagliardo N, Sollars SI, Hill DL (2004) Each sensory nerve arising from the geniculate ganglion expresses a unique fingerprint of neurotrophin and neurotrophin receptor genes. J Neurosci Res 78: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]