Abstract
The results of 5484 submissions from cats to the Canadian Veterinary Urolith Centre between February 1998 and February 2003 are presented. Of the submissions, 618 were urethral plugs and 4866 were bladder uroliths. The majority of the urethral plugs were from male domestic shorthair and longhair cats. Approximately 50% of the urolith submissions were oxalate, 44% were struvite. Oxalate uroliths were the most common mineral type in males and in Himalayan, Persian, and Siamese cats. Struvite uroliths were the most common mineral type in domestic shorthair and longhair cats. Females outnumbered males by 1.4:1 in struvite urolith submissions. A review of risk factors for urethral plugs and bladder uroliths is presented.
Introduction
The Canadian Veterinary Urolith Centre (CVUC), located in Guelph, Ontario, opened in February 1998. More than 22 000 submissions have been quantitatively analyzed over the past 5 y. Of these, 4866 were feline bladder uroliths and 618 were feline urethral plugs. Submissions to the CVUC have been received from all parts of Canada, including 17% from western Canada (British Columbia, Alberta, Saskatchewan, and Manitoba), 52% from Ontario, 21% from Québec, and 10% from eastern Canada (Nova Scotia, New Brunswick, Prince Edward Island, and Newfoundland).
The purpose of this paper is to report on the number and mineral composition of urethral plugs and bladder stones either passed by or surgically removed from cats in Canada over a 5-year period.
Materials and methods
A computer-assisted search of questionnaires returned to the CVUC was used to compile information from all feline urinary calculi and urethral plugs that were analyzed between February 1, 1998, and February 1, 2003. The age, sex, and breed of affected cats were recorded.
The uroliths or urethral plugs that were analyzed had been surgically removed or voided (the majority of urethral plugs were removed manually). To determine the mineral composition, each layer of each specimen was analyzed by optical crystallography, using polarized light microscopy. If additional clarification was needed, an additional quantitative technique was used (X-ray microanalysis, Fourier transform infrared spectroscopy, or scanning electron microscopy).
As previously described, uroliths containing at least 70% of a single mineral were classified as being of that type 1). For purposes of this paper, the terms “calcium oxalate” or “oxalate” include calcium oxalate monohydrate, calcium oxalate dihydrate, or both; the term “urate” includes the salts of uric acid (ammonium, potassium, and sodium acid urate).
Results
Urethral plugs
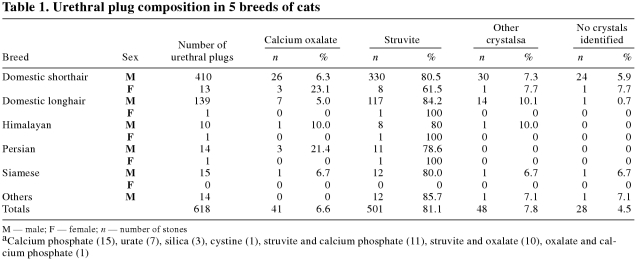
A total of 618 urethral plugs were submitted to the CVUC. The majority (501;81.1%) contained magnesium ammonium phosphate or struvite (Table 1). The majority (447;89.2%) were from male domestic shorthair (DSH) and domestic longhair (DLH) cats. Only a small number (89;14.4%) contained a different crystal type (oxalate, calcium phosphate, urate, silica, cystine) or a combination of crystals (struvite and calcium phosphate, struvite and oxalate, oxalate and calcium phosphate). Fewer than 10% (28 urethral plugs) contained no crystals (Table 1).
Table 1.
Only 16 (2.6%) urethral plugs were submitted from female cats; 13 (81.3%) of these were from DSH cats (13).
Uroliths
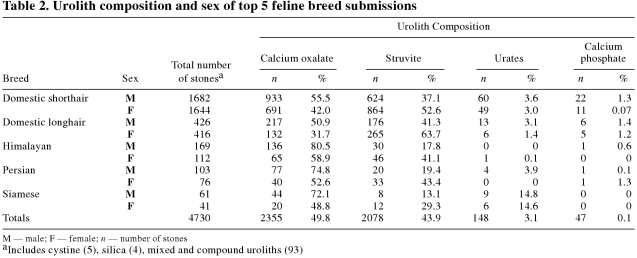
The majority (3326;68.4%) of urolith submissions were from DSH, 842 (18.9%) were from DLH, 281 (5.5%) from Himalayan, 179 (5.5%) from Persian, and 102 (2.4%) from Siamese (Table 2). Approximately 50% of urolith submissions were composed of oxalate, 44% of struvite.
Table 2.
Males outnumbered females 1.5:1 in oxalate submissions (Table 2). Himalayan, Persian, and Siamese cats appeared to be at risk of developing oxalate rather than struvite uroliths, especially if they were male (Table 2).
The majority of cats with struvite uroliths were DSH and DLH (Table 2). More females (1220) than males (858) had struvite uroliths (1.4:1).
Less commonly submitted uroliths included ammonium urate, cystine, xanthine, silica, and calcium phosphate. Although the numbers are small, Siamese cats appeared to be over-represented, compared with other breeds, in the proportion of urate submissions.
Discussion
The prevalence of feline lower urinary tract disease (FLUTD), irrespective of cause, has been reported to be approximately 1.5% to 8% (2,3). The majority (> 60%) of these cases in cats ≪≤≪λτ≦ 10 y of age were diagnosed as idiopathic cystitis (3,4). It has been estimated that 10% to 20% of cats with FLUTD have urethral plugs or urolithiasis (1,3,4,5,6).
Most urethral plugs contain large quantities of matrix (mucoprotein, consisting of mucus and inflammatory debris) with varying quantities of minerals. Any crystal type may become trapped in the plug matrix, but in most cases, as in the current study, struvite predominates (7). The mucus is secreted in excess by mucosal cells within the bladder and urethra in response to a mucosal irritant or inflammatory stimulus. The matrix of the plug may also be composed of sloughed tissue, blood, inflammatory cells, prostatic secretions, and Tamm-Horsfall mucoprotein from renal tubular cells. Mucus and crystal plugs usually occur at the tip of the penis, where the urethra has a small luminal diameter, but they can occur anywhere in the urethra, including where the urethra narrows just caudal to the bulbourethral gland and between the bladder and the area of the prostate gland (7).
As previously reported, struvite and calcium oxalate are found in ≪≤≪γτ≦ 80% of uroliths (1,3,4,5,6). Ammonium urate and uric acid, calcium phosphate, cystine, xanthine, and silica are less commonly reported types of uroliths in cats.
It is important to note that the presence or absence of struvite or oxalate crystals identified in the urine on routine urinalysis does not necessarily signify a problem. Many normal cats can be expected to have some degree of crystalluria, especially in highly concentrated urine (8,9).
When urine becomes supersaturated with minerals (increased urinary excretion of minerals, high urine specific gravity, urine retention of crystalloids, decreased presence of natural inhibitors of crystallization) and the urine pH is conducive to crystallization, minerals precipitate out of solution to form crystals. These crystals can congregate to form uroliths. The duration and degree of urine supersaturation with crystalloids, the urine pH, and the physical characteristics of the urolith (size, shape, porosity, composition) all contribute to the ability of the urolith to remain and grow in the urinary system (8,9).
Some cats with bladder uroliths do not have crystals in their urine and, although uncommon, cats with bladder uroliths may have crystals that are different from those of the stone (10).
Urinary crystals (both struvite and oxalate) can form in-vitro as urine is refrigerated or allowed to sit for prolonged periods. Urine samples should be analyzed within 60 min of collection to minimize temperature- and time-dependent effects on in-vitro crystal formation. Presence of crystals observed in stored samples should be validated by reevaluation of fresh urine (11). If crystal morphology is questioned, submission to a laboratory offering polarized light microscopy is highly recommended.
Struvite bladder uroliths appear to be more common in young to mid-age cats (1 to 7 y) (1). Cats ≪≤≪λτ≦ 1 y of age or ≪≤≪γτ≦ 10 y of age appear to be at greater risk for infection-induced struvite urolithiasis. Although urinary tract infections are not common in healthy cats, infection with urease-producing organisms (Staphylococcus spp. Proteus spp.) may contribute to the formation of struvite urolithiasis in cats (12).
Compared with other mineral types, struvite uroliths can occur in any breed but are reported most often in DSH and DLH cats (1,5). In our study, female cats were at greater risk than male cats of struvite urolith development; this is similar to what has been reported in previous studies (1), although results vary with age, with one study reporting predominance in male cats ≪≤≪λτ≦ 2 y of age and in female cats ≪≤≪γτ≦ 2 y of age (5). In one study, risk factors for FLUTD, including struvite urolithiasis, included inactivity and prolonged restriction indoors, coupled with the use of a litter tray (13). Obesity may also be a risk factor (8).
During the 5 y that the CVUC has operated, the yearly number of calcium oxalate submissions has been rising. This has been reported in other studies (1,12,14) to be a trend over the past decade or so. The widespread use of manufactured magnesium-restricted, urine-acidifying diets to control struvite crystalluria in cats at risk for calcium oxalate crystalluria is implicated. It is thought that urine acidification and hypomagnesemia may be risk factors for oxalate development in cats at risk (1). Oxalate urolithiasis may develop at any age, but middle age to older cats (7 to 9 y) are reported to be at greater risk (1,14).
The explanation for the increased risk of having calcium oxalate uroliths in older cats is unknown (1), although, again, speculation exists about the widespread use of manufactured magnesium-restricted diets, urine-acidifying diets, or both, to control struvite crystals in cats as a predisposing factor (1,14). In one study (14), cats with calcium oxalate urolithiasis were more than 3 times as likely as hospital control cats to have been fed diets that typically promote a urine pH of 6.29 or less (P ≪≤≪λτ≦ 0.0001). In this study, because of the diverse methods used by manufacturers and laboratories to determine urine pH, a pH score was assigned to each cat food (for example, a score of 1 was assigned to a diet producing an average urine pH of 5.8 to 6.29). A statistical difference was found between cats consuming diets with high urine acidification scores versus diets with low urine acidification scores. Highly acidifying diets and metabolic acidosis promote hypercalciuria by increasing the quantity of calcium filtered by the kidneys, by decreasing tubular calcium reabsorption, and by promoting skeletal mobilization of calcium (14). As in other species, acidic urine appears to alter the effect or concentration of certain inhibitors of calcium oxalate crystallization, including citrate, magnesium, pyrophosphate, Tamm-Horsfall mucoprotein, and nephrocalcin (14). Diets that contain reduced concentrations of magnesium and citrate to prevent struvite urolith formation may predispose an animal to calcium oxalate stone formation (15).
Males in our data base appear to have an increased risk for calcium oxalate urolithiasis, as has been previously reported (1). The role of sex hormones is unclear at this time.
Oxalate crystals and stones are reported more often than are other mineral types in Himalyan, Persian, and Burmese cats (1,3,14). Other investigators report the Russian blue as a breed with a higher incidence of oxalate crystals and uroliths compared with other mineral types (3). Genetic factors may contribute to increased risk of calcium oxalate urolith formation; familial patterns of calcium oxalate urolithiasis have been reported in other species, including the dog (16).
Source of water has been implicated as contributing to calcium oxalate development. The mineral content of water is expressed as parts per million, and the mineral content of food is expressed as parts per hundred, a 10 000 fold difference. Even hard water (water high in mineral content) would not contribute substantially to the amount of available dietary minerals compared with the contribution supplied by the diet. Thus, water source is unlikely to contribute to the development of calcium oxalate uroliths (14).
Administration of urinary acidifiers or glucocorticoids, and diseases that cause hypercalcemia (primary hyperparathyroidism, cancer, idiopathic) may also be contribut ing factors (8). Serum hypercalcemia has been reported in 35% of cats with calcium oxalate uroliths (12), and the cause is rarely found. Risk may be increased by high urine specific gravity. In one study, maintaining cats only in an indoor environment was a risk factor for calcium oxalate urolithiaisis (14).
Metabolic uroliths (urate, cystine, xanthine) often appear in mid-age cats (4 to 6 y) with no obvious gender predisposition (5,12). In our study, Siamese cats were over-represented in urate submissions compared with other breeds; in another study, Siamese cats appeared to be at risk for cystine uroliths (5). Urate uroliths may occur in cats with portosystemic shunts, when the urine is highly acidic, and when cats are fed diets high in purines, such as liver or other organ meats (12). CVJ
Footnotes
Address all correspondence and reprint requests to Dr. Doreen M. Houston; e-mail address: dhouston@medi-cal.ca
References
- 1.Thumchai R, Lulich JP, Osborne CA, et al. Epizootiologic evaluation of urolithiasis in cats: 3498 cases (1982-1992). J Am Vet Med Assoc 1996;208:547–551. [PubMed]
- 2.Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999;214: 1336–1341. [PubMed]
- 3.Lekcharoensuk C, Osborne CA, Lulich JP. Epidemiologic study of risk factors for lower urinary tract diseases in cats. J Am Vet Med Assoc 2001;218:1429–1435. [DOI] [PubMed]
- 4.Buffington CAT, Chew DJ, Kendall MS, et al. Clinical evaluation of cats with nonobstructive urinary tract diseases. J Am Vet Med Assoc 1997;210:46–50. [PubMed]
- 5.Ling GV, Franti CE, Ruby AL, Johnson DL. Epizootiologic evaluation and quantitative analysis of urinary calculi from 150 cats. J Am Vet Med Assoc 1990;196:1459–1462. [PubMed]
- 6.Osborne CA, Lulich JP, Bartges JW, et al. Canine and feline urolithiasis: relationship of etiopathogenesis to treatment and prevention. In: Osborne CA, Finco DR, eds. Canine and Feline Urology. Baltimore: Williams and Wilkins 1995:851–865.
- 7.Osborne CA, Lulich JP, Kruger JM, et al. Feline urethral plugs-etiology and pathophysiology. Vet Clin North Am Small Anim Pract 1996;26:233–253. [PubMed]
- 8.Laboto MA. Managing urolithiaisis in cats. Vet Med 2001:708–718.
- 9.Houston DM. Diagnosis and management of feline lower urinary tract disease. Standards of Care: Emerg and Crit Care Med 2002; 4:5–10.
- 10.Buffington CAT, Chew DJ. Diet therapy in cats with lower urinary tract disorders. Vet Med 1999;94:626–630.
- 11.Albasan H, Lulich JP, Osborne CA, et al. Effects of storage time and temperature on pH, specific gravity, and crystal formation in urine samples from dogs and cats. J Am Vet Med Assoc 2003;222:176–179. [DOI] [PubMed]
- 12.Osborne CA, Lulich JP, Thumchai R, et al. Feline urolithiasis: Etiology and pathophysiology. In: Osborne CA, Kruger JM, Lulich JP, eds. Vet Clin North Am Small Anim Pract 1996;26:217–232. [PubMed]
- 13.Jones BR, Sanson RL, Morris RS. Elucidating the risk factors of feline lower urinary tract disease. NZ Vet J 1997;45:100–108. [DOI] [PubMed]
- 14.Kirk CA, Ling GV, Franti CE, Scarlett JM. Evaluation of factors associated with development of calcium oxalate urolithiasis in cats. J Am Vet Med Assoc 1995;207:1429–1434. [PubMed]
- 15.McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of 5 cases. J Am Anim Hosp Assoc 1999;35:297–301. [DOI] [PubMed]
- 16.Lulich JP, Osborne CA, Unger IK, et al. Prevalence of calcium oxalate uroliths in miniature schnauzers. Am J Vet Res 1991;52:1579–1582. [PubMed]