Abstract
This article reviews advances in the field of human immunodeficiency virus type 1 (HIV-1) and AIDS vaccine development over the last decade, with an emphasis on the DNA vaccination approach. Despite the discovery of HIV-1 and AIDS in humans nearly 20 years ago, there is no vaccine yet that can prevent HIV-1 infection. The focus has shifted toward developing vaccines that can control virus replication and disease progression by eliciting broadly cross-reactive T-cell responses. Among several approaches evaluated, the DNA-based modality has shown considerable promise in terms of its ability to elicit cellular immune responses in primate studies. Of great importance are efforts aimed at improvement of the potency of this modality in the clinic. The review discusses principles of DNA vaccine design and the various mechanisms of plasmid-encoded antigen presentation. The review also outlines current DNA-based vaccine strategies and vectors that have successfully been shown to control virus replication and slow disease progression in animal models. Finally, it lists recent strategies that have been developed as well as novel approaches under consideration to enhance the immunogenicity of plasmid-encoded HIV-1 antigen in various animal models.
INTRODUCTION
Obstacles to Vaccine Development
There are currently 40 million individuals in the world infected with human immunodeficiency virus (HIV), and nearly 16,000 new infections occur worldwide each day based on World Health Organization estimates. The search for an effective vaccine to control the AIDS pandemic is still continuing long after the discovery and isolation of HIV some 20 years ago (22, 156). This has been due to several unique challenges that HIV-1 has presented which have confounded vaccine development.
Attempts to develop a safe and effective AIDS vaccine have been slowed, in part, by the difficulty in clearly defining specific immune responses that can prevent infection and limit disease progression. The complex structure and life cycle, as well as the high mutation rate, of HIV-1 have provided further obstacles to the development of an effective vaccine. The conserved receptor- and coreceptor-binding sites on the viral envelope (Env) glycoproteins that engage in virus attachment and fusion to host membrane receptors are camouflaged by variable residues that are glycosylated (108). The conserved sites are also thermostably concealed (143). These features enable the virus to evade detection by cross-reactive antibody (Ab)-producing B cells that recognize the conserved sites. The conformational flexibility of gp120 may further decrease the efficiency of presentation of receptor-binding sites to the immune system by creating an entropic barrier that must be overcome or bypassed by Abs targeting receptor-binding regions (107). As a part of its life cycle, HIV-1 integrates into the genome of its host to form a latent provirus and escapes immune recognition by the absence of significant protein expression (63). The high error rate of the reverse transcriptase (158), combined with the rapid turnover of plasma virions (87), has further hindered the development of an effective vaccine by supplying a broad range of variants for selection and escape from both cellular and humoral immune responses (57, 153). Another consequence of its high mutation rate is the unusual degree of diversity of the virus. Presently at least 12 known genetic subtypes of HIV-1 which are rapidly diversified to yield intersubtype recombinants exist in humans (105, 135) and present an even greater challenge for development of a universal AIDS vaccine.
Attributes of an Ideal Vaccine
As with most prophylactic and/or therapeutic antiviral vaccines, two potential sites of action for an HIV vaccine are viral entry and viral replication. Viral entry can be inhibited by neutralizing Abs that specifically target epitopes on the viral envelope, while viral replication can be hindered by cell-mediated immune responses that can potentially target any of the epitopes derived from viral proteins produced during the viral life cycle, as indicated in Fig. 1. An ideal HIV vaccine should ultimately confer sterilizing immunity by eliciting the production of broadly cross-reactive neutralizing Abs that block virus entry and aid in clearance of the infection. To date, it has proved difficult to generate vaccines capable of eliciting these specific Abs (153), for reasons discussed previously such as poor accessibility of conserved receptor-binding sites, extensive glycosylation, and antigenic variation of gp120 (158). The interactions between the virus membrane and host cell membrane are complex and involve different fusion intermediates, as indicated in Fig. 2. Vaccines need to elicit Abs that target unique complex epitopes as well as trimeric fusion intermediates for efficient inhibition of virus binding and entry. An understanding of the different possible conformations of HIV-1 gp160 in the context of a monomer and a functional envelope glycoprotein complex may enable the development of newer, sophisticated envelope vaccine immunogens to prevent HIV-1 infection. Presently, it seems most likely that HIV-1 vaccines can be developed which contribute to the containment of the virus after infection by priming cellular immune responses. These include induction of cross-reactive antiviral T cells, particularly cytotoxic T lymphocytes (CTLs) that can kill virus-infected cells. Such T-cell-based vaccines would not prevent infection but can control virus replication. Several lines of evidence indicate that a strong CD8 CTL response is critical for the control of HIV-1 infection (74, 170).
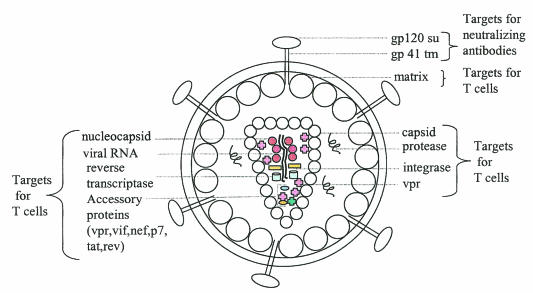
FIG. 1.
Potential targets for an HIV-1 vaccine. HIV-1 vaccines can be designed to prevent virus entry and/or to control virus replication. To block virus entry, an HIV-1 vaccine should elicit neutralizing Ab against surface glycoproteins on the viral envelope, such as gp120 and gp41, that mediate binding and entry into host cells; to control virus replication, an HIV-1 vaccine should elicit cell-mediated (T-cell) immune responses to peptide epitopes derived from other structural components of the virus such as the matrix, capsid, nucleocapsid, viral enzymes (reverse transcriptase, protease, and integrase), and accessory proteins (vpr, vpu, nef, p7, rev, and tat). su, surface; tm, transmembrane.
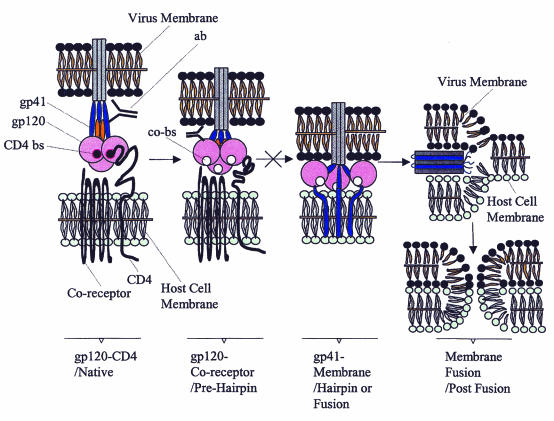
FIG. 2.
Current model for HIV binding and entry into target cells. The native state of viral surface glycoproteins is readily triggered by binding of gp120 to CD4 and coreceptor. This conformational change leads to the prehairpin intermediate and frees the fusion peptide on gp41. The prehairpin intermediate spans the cell and virus membranes, with the transmembrane domain of gp41 anchored in the viral membrane and the fusion peptide inserted into the target cell membrane. N peptides and C peptides or anti-gp41 Ab (ab) are thought to prevent transition to the hairpin/fusion structure. The C-heptad repeat folds back onto the N-heptad repeat to generate the trimer-of-hairpins in the fusion structure. This brings the two membranes into close proximity, driving fusion (postfusion structure). Current strategies targeting inhibition of virus entry aim to design structured envelope vaccines that block the complex virus-host cell membrane fusion intermediates as indicated in the figure. gp, glycoprotein; ab, Ab that binds the C region of gp41; CD4-bs, CD4-binding site; co-bs, coreceptor-binding site. See the text for additional references.
Three different mechanisms of CD8 T-cell-mediated control of HIV infection are currently known: (i) cytolytic killing of infected cells, (ii) secretion of soluble factors that suppress viral replication (114), and (iii) blocking of viral entry by secreting chemokines, such as macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES (regulated on activation, normal T-cell expressed and secreted), that compete with the virus for binding to chemokine coreceptors (69).
Recent T-cell epitope mapping studies have revealed a considerable intra- and intersubtype conservation among the ∼200 specific epitopes mapped for various proteins of the virus, providing an additional incentive to devise T-cell-based vaccines. In fact, the majority of the vaccine approaches currently under development are aimed at generating high levels of antiviral T cells.
ANIMAL MODELS FOR EVALUATION OF VARIOUS VACCINES
Vaccine efficacy studies are routinely assessed in animal models following natural or experimental infection of animals with animal lentiviruses such as HIV-1 and HIV-2 or following inoculation of recombinants derived from these viruses or in the context of HIV-1 transgenes (133). Each of these approaches carries certain advantages and disadvantages, as indicated below (Table 1).
TABLE 1.
Comparison of various models
| Model | Advantages | Disadvantages |
|---|---|---|
| Transgenic mouse | Good correlates of immunity | Mice naturally resistant to HIV-1 infections |
| SCID mouse | Humanized mouse model to permit testing of vaccine formulations | Variability of reconstitution and small numbers of animals available at any given time |
| Macaques and SIV | Causes AIDS-like disease in Asian macaques | Differences from HIV-1 in viral sequences and envelope epitopes; disease progression is faster |
| Macaques and SHIV | Conserves the necessary envelope sequences from HIV and can infect macaques | Kinetics and extent of CD4 loss in macaques much higher than HIV-1-induced effect in humans |
| Chimpanzees | Naturally susceptible to HIV; complete protection in vaccine models | Scarcity of animals, expense, limited viral replication, and absence of disease when infected with patient isolates |
Transgenic Mouse Models
Transgenic mouse models may be used to test the mechanism of action of certain antiviral compounds in vivo, although to date these studies have not been pursued. The dissimilarities between the immune systems of transgenic mouse models and humans limit their use in vaccine development.
SCID Mouse Models
SCID mice (mice with severe combined immune deficiency) effectively lack an adaptive immune system (i.e., they have no functioning T or B lymphocytes) and as such are permissive, when reconstituted with human tissues such as liver and thymus and peripheral blood leukocytes, for infection with HIV. To date, this model has been utilized, with some degree of success, for testing anti-HIV-1 agents as well as assessing the potential in vivo HIV-1 neutralization efficacy of some humanized and human monoclonal Abs (139). In principle, since human peripheral blood leukocyte-reconstituted SCID mice synthesize human Abs, this model could be used to assess the efficacy of different HIV-1 vaccine preparations and delivery platforms. One problem with the use of this model for vaccine testing, however, is the variability of reconstitution and the small numbers of animals that can be developed for study at any time. Nevertheless, this mouse model could be potentially useful for assessing the efficacy of certain vaccines. The utility of this model needs to be further explored.
Animal Lentivirus Models
The HIV-1 and HIV-2 isolates are members of the lentivirus family that endemically infect nonhuman primate species in Africa. These nonhuman primate viruses, known as simian immunodeficiency viruses (SIVs), do not cause disease in their natural host species. Depending on the SIV isolate used and the host macaque monkey employed, they do cause AIDS to various degrees when inoculated experimentally in Asian or cynomologous macaques (71, 86, 160).
SIV-rhesus macaque models recapitulate several pathologic features of HIV-1 disease in humans. However, there is some dissimilarity in the genomic organization of SIV and HIV-1, as well as in important features of pathogenesis, that could affect vaccine efficacy. The SIV viral accessory protein Vpx is not found in HIV-1, while the vpu gene product in HIV-1 is not found in SIV and the functions of Vpr also differ between the two viruses. Furthermore, there are antigenic and structural differences between viral envelopes of SIV and HIV-1. While macrophage-tropic SIV and HIV strains utilize CCR5 as the chemokine coreceptor to facilitate viral entry, T-cell-tropic SIV strains do not always utilize CXCR4 as do T-cell-tropic HIV strains. These differences have limited the utility of the SIV-macaque model for evaluating HIV-1 envelope-based vaccine strategies. Progression to disease in macaques following SIV infection is a lot faster than in humans infected with HIV. Furthermore, the challenge doses of the virus used in these models are several orders of magnitude higher than the doses of HIV encountered normally by humans. It is important to consider these factors in making meaningful interpretations of the protective effects of vaccine strategies tested in this model.
Chimeric viruses that express HIV-1 envelope glycoproteins on SIV backbones, known as simian human immunodeficiency viruses (SHIVs) (92, 115, 116, 124), have been developed. Importantly, some of these viruses like SHIV-89.6P following passage in macaques have been shown to cause rapidly progressive AIDS-like disease in macaques (163). Some concern about using SHIV-89.6P to evaluate AIDS vaccines is that this virus has evolved to use only CXCR4 whereas most HIV-1 strains transmitted between humans use CCR5 as the principal coreceptor (62, 134, 171, 193). Additionally, macaques infected with pathogenic SHIV display a more rapid and higher peripheral CD4 T-lymphocyte loss in comparison to the CD4 T-cell loss induced by HIV-1 in humans. Interpretations regarding vaccine efficacy in SHIV-macaque models may not be relevant or applicable to humans in the context of HIV-1 infection due to these differences in host-virus interactions and disease patterns. Nonetheless, this remains an important and highly defined challenge model.
An effective HIV-1 vaccine for humans should be able to prevent the spread and expansion of R5 viruses early after virus encounter or infection. To this end, alternate models that use R 5 SHIVs such as SHIV-162P-macaque models are being developed (81). Macaque monkeys infected with SHIV-162P do not exhibit a rapid and precipitous loss of CD4 T cells (163). A recent study (194) using a SHIV-162P-macaque model has demonstrated the prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal Ab to HIV-1 gp120. These models may also be useful to retest vaccine concepts that were shown to be effective in earlier SHIV-89.6 macaque models. A recent problem uncovered in these studies relates to the major histocompatibility complex (MHC) haplotype and spontaneous control of infection. (144, 186, 216). Some haplotypes can spontaneously control SIV and SHIV challenge. Therefore, haplotypes must be considered when designing vaccine studies in macaques.
HIV-1 Infection Models
Apart from humans, HIV-1 can also infect great apes such as chimpanzees and bonobos. One HIV-1 infection model that has been studied is the HIV-1-infected chimpanzee. This model facilitates a direct preclinical analysis of candidate antiviral compounds and vaccines. However, it has several weaknesses. Although vaccine strategies have protected chimpanzees from infection by weakly replicating HIV-1 isolates (30), there is no or very little detectable viral RNA in the plasma of animals infected chronically with primary patient HIV-1 isolates. Additionally, these isolates replicate poorly in chimpanzees and do not induce disease. To date, only one chimpanzee has developed AIDS following HIV-1 infection (149). There are species differences in the host response to virus between primates and humans, as indicated by resistance of chimpanzees to infection by CCR5-tropic isolates that cause infection and disease in most humans (149). Thus, the relevance of vaccine protection in the chimpanzee model has been the subject of considerable controversy. Questions arise regarding extrapolation of the model to human infections, where disease is one outcome of infection. In contrast, chimpanzee models of hepatitis B that have had similar limitations have been effective for supporting the development of vaccines against this viral agent. A pathogenic HIV-1 isolate in chimpanzees that causes CD4 T-cell loss and persistent viral loads has been recently characterized (148) and may yet provide an opportunity to assess vaccine efficacy in a chimpanzee model of a pathogenic HIV-1 infection. Unfortunately, major drawbacks of the chimpanzee model include its high cost as well as the extreme scarcity of animals and the ethical issues involved in using this animal species in lethal challenges. At present, the weaknesses of using this model appear to outweigh the potential benefits.
Another important limitation shared among the primate animal models is that the animals are not inbred. This generates diversity in challenge take and poses issues for immune monitoring. Further, the supply of animals for large-scale testing is limited. Nevertheless, these models have proven to be particularly powerful systems for study of AIDS models by providing the means of assessing the prophylactic and/or therapeutic efficacy of a variety of vaccines. The collective features of these systems teach us something important about each potential vaccine candidate.
EARLY VACCINE APPROACHES
Conventional vaccine approaches that have proven effective in preventing disease from a variety of pathogens, such as live attenuated virus (Sabin polio vaccine, measles vaccine, and chickenpox vaccine), inactivated or killed vaccines (Salk polio vaccine and hepatitis A vaccine), recombinant proteins (hepatitis B vaccine), and toxoids (diphtheria vaccine), have been utilized to various degrees in potential HIV vaccine strategies. However, these strategies have significant risk that counters their success in protecting against HIV-1 infections, as indicated (Table 2). Preliminary studies with the SIV-macaque model suggested that genetically modified viruses that were infective but pathogenically attenuated (nef-deleted SIV) could serve as vaccines that prevented subsequent infections with wild-type virus (50). However, later studies revealed that many newborn or adult monkeys infected for long durations with such vaccine strains of virus may eventually succumb to disease and die (12). A similar occurrence was reported for a group of Australian blood recipients infected with mutant HIV lacking the same regulatory nef gene that would be removed from the vaccine virus (109). None of these individuals developed symptoms, and all maintained normal CD4 T-cell counts for more than 10 years. However, follow-up data from these patients have revealed T-cell declines remarkably similar to those heralding AIDS in vaccinated monkeys infected with a pathogenically attenuated, replication-limited HIV strain and that subsequently developed an AIDS like disease (89, 110). An AIDS vaccine must, ultimately, be safe, and at present it seems that the genetically attenuated live-vaccine approach is unlikely to uncouple the level of infectivity needed to elicit protective immune responses from the pathogenicity of infection. Therefore, efforts at developing live attenuated HIV-1 vaccines are now minimal.
TABLE 2.
Comparison of various traditional approaches
| Approach | Advantages | Disadvantages |
|---|---|---|
| Live attenuated virus | Prolonged CTL responses | Eventual pathogenicity |
| Inactivated virus with adjuvants | Safe | Limited specificity of neutralizing Ab; absence of CTLs; protective response to cells in which the virus was prepared and not the virus itself |
| Recombinant envelope protein | Safe, complete protection | Need for identical glycoprotein sequence in the challenge virus as the immunizing protein; no neutralizing Ab for patient isolates; no CTLs |
Inactivated viral vaccines assessed in the SIV-macaque model have also yielded disappointing outcomes. These vaccines failed to elicit broad and vigorous protective responses (140). Moreover, protection in this model was not demonstrated when the vaccine and challenge virus strains were even slightly different genetically or when the vaccinated monkeys were challenged shortly after peak immunity was reached. Some studies have suggested that the protection in this model may have reflected an experimental artifact (184). Inactivated vaccines evaluated in limited early-phase human immunogenicity trials (113) also have proven to be disappointing, eliciting neither neutralizing antibodies nor cytotoxic T-lymphocyte (CTL) responses, since they retained very little viral envelope glycoprotein and additionally did not initiate protein synthesis in cells. Therefore, as is the case for live vaccines, more research is needed before inactivated HIV-1 immunogens can be developed as vaccines.
The use of highly purified recombinant proteins or peptides as vaccines was tested in a nonhuman primate model using recombinant HIV envelope glycoprotein as an immunogen. Vaccinated animals were only modestly protected and only when the challenge virus and immunizing virus had the identical envelope glycoprotein sequence (25). Ab responses elicited in early-phase human trials were similarly modest in titer, and the Abs exhibited a restricted ability to neutralize the range of HIV isolates tested. Furthermore, as expected, these subunit immunogens did not elicit CTL responses. The results of a phase III study of the envelope immunogen, recombinant gp120 (rgp120), have been recently announced and showed that there was not a statistically significant reduction of HIV infection within the study population (93, 200). Another phase III study using the same immunogen is nearing unblinding. These results were suggested by the prior clinical studies. Despite their failure to demonstrate the desirable level of protection in vaccinees, these trials have greatly advanced HIV vaccine research and development by proving that a true phase III trial can be effectively designed, implemented, and completed. It is likely that the outcome of these studies will significantly influence the concept of envelope subunit immunogens and their implementation in HIV vaccine development studies. More recent studies attempting to develop designed envelope subunit immunogens are testing many exciting concepts and, while only in the early stages, are likely to influence vaccine design well beyond HIV vaccines (Fig. 2).
NOVEL VACCINE APPROACHES
The inability of traditional vaccine approaches to generate a suitable vaccine for HIV-1 prompted the quest for the development of novel vaccine strategies. Among the most promising of these approaches is the use of live recombinant vectors and plasmid DNA immunogens.
Live Vaccine Vectors
In the live vaccine vector technology, genes of HIV are molecularly cloned into live, replication-competent or incompetent microorganisms and immune responses develop to both the vector and to the HIV open reading frame product carried by that vector. Such immunogens have proven particularly useful for eliciting CTLs, since the replicating vector produces HIV proteins intracellularly, allowing them to enter the MHC class I processing pathway. In certain cases, the vectors have been shown to directly target antigen (Ag) to dendritic cells (DCs) (6, 7, 8, 176, 217). Some live viral vectors that are being developed for use as HIV vaccines include several avian and mammalian poxviruses (9, 19), rhabdoviruses (167), alphaviruses (52), replication-defective adenoviruses (176), herpesviruses (141), picornaviruses (11), and adeno-associated viruses (118).
Recombinant vaccinia virus, tested as a vaccine candidate in nonhuman primates, was shown to elicit CTL responses to HIV and SIV proteins (173); however vaccinia virus may disseminate in immunocompromised humans, causing fatal encephalitis (162). Attenuated poxviruses such as modified vaccinia Ankara (MVA) and NYVAC, obtained by genetic deletions following serial passages of the parental strain, have been shown to elicit reasonable immune responses in nonhuman primate models and are soon to be evaluated as HIV vaccine vectors in early-phase human testing. Avian poxviruses such as the recombinant canarypox virus constructs have also undergone extensive human testing (58). These vectors have proven to be safe and immunogenic, and an efficacy trial for a recombinant canarypox virus immunogen in Southeast Asia is currently under consideration. In SHIV challenge models, impressive levels of virus containment and protection from disease have been achieved by both the adenovirus-based approach and a vesicular stomatitis virus (VSV)-based approach. These results have propelled the recombinant virus vector field to a higher level of promise and scrutiny. Furthermore, the use of adenovirus vectors to elicit cellular immunity in small phase II human studies appears encouraging. The E1- and E3-deleted replication-incompetent serotype 5 adenovirus (Ad5), has demonstrated impressive immunogenicity in both murine and nonhuman primate studies (176).
The presence of preexisting immunity to the vector presents a major challenge in using this approach. Nearly 45% of the U.S. population have neutralizing Abs that are specific for Ad5 (61). Individuals who are now receiving smallpox vaccinations will develop preexisting immunity to MVA. Utilization of higher vaccine doses, as well as heterologous prime-boost protocols, can overcome the problem of preexisting immunity. The preferred approach to overcome this problem is to immunize a vector-naive population. However, the existence of a vector-naive population would be practically improbable, given the high likelihood for exposure of an individual to the range of microorganisms in a lifetime. An alternate approach would be to immunize with an agent for which preexisting immunity does not exist, such as DNA. This establishes a memory T-cell pool for the antigen of interest. The live recombinant booster vector would then have to achieve only the minimum level of infection required to enhance the primed immune response. One could also use adenovirus isolates of unusual serotypes to which most humans have not been previously exposed or adenovirus isolates from nonhuman primate species to construct similar vaccines with comparable immunogenicity. It is likely that vectors derived from such viruses may not be immunogenic in humans since they do not naturally infect humans.
Alphaviruses employed in the construction of replicase-based DNA vaccines to increase Ag production from nucleic acid vaccines (85, 218) express Ag under the control of an alphavirus replicase enzyme that is used by the alphavirus to produce a very large number of viral copies. It was recently demonstrated (111) that replicase-based DNA vaccines could activate the innate antiviral host mechanism such as double-stranded RNA-dependent pathways (RNA-dependent protein kinase and 2′-5′-A synthetase or RNase L pathways), accounting for their heightened immunogenicity. Activation of these pathways in alphavirus-transfected cells leads to cellular apoptosis and cross-presentation of Ag, yet another mechanism that has previously been shown to stimulate the immune system (5, 43). Therefore, the use of replication-incompetent virus particles (replicons) or vectors to deliver either DNA or RNA vaccines has potential for facilitating immune priming in two ways: cross-priming and direct targeting of DCs. Furthermore, the induction of apoptosis represents an additional safety feature of replicase-based vaccines. Hence, construction of such DNA vaccines that deliver stronger “adjuvant-like” signals to the innate immune system would enable the development of more powerful vaccines while circumventing the side effects of strong adjuvants.
Bacterial vectors that can be administered orally to facilitate worldwide administration are also undergoing development. For example, Salmonella vectors have been developed for oral delivery of HIV antigens and have generated some interesting preclinical results (172). Recombinant Listeria monocytogenes has been evaluated as a vector to express the HIV Gag protein in murine models (65, 130, 131) and has successfully been shown to elicit Gag-specific CTL and CD4 T-cell mucosal responses following a systemic immunization (117). Macaque safety and immunogenicity studies using a highly attenuated L. monocytogenes vector expressing HIV gag produced by genetically disrupting genes required for the biosynthesis of the bacterial cell wall are about to begin. A recent study with mice (211) demonstrated that recombinant Shigella encoding p55 Gag can elicit Gag-specific immune responses to a comparable degree to naked DNA Gag-encoding plasmid when used as a priming agent or as a booster following an initial DNA plasmid prime. In these mice, intranasal delivery of recombinant Shigella elicited immune responses in the lungs in addition to the spleen. Additional studies with nonhuman primates are needed to address the utility of recombinant Shigella vectors for immunization against HIV-1. Bacille Calmette-Guérin (BCG), an attenuated preparation of Mycobacterium bovis, is also being explored as a vaccine vector candidate because it establishes a chronic, persistent infection like HIV and has proven to be safe in worldwide use. Limited studies with rhesus monkeys have indicated that infection with recombinant BCG can elicit AIDS virus-specific CTL responses (214). Overall, most of these live-vector approaches alone may elicit only the minimal level of cellular immunity thought by many AIDS researchers to be necessary to support clinical evaluation of these approaches. Accordingly, combination approaches may be very important.
Nucleic Acid-Based Vaccines
DNA-based vaccines and, to a lesser extent, RNA-based vaccines have been investigated as potential HIV vaccine candidates. mRNA transfection for Ag delivery to DCs has been used to induce potent T-cell-based antitumor immunity in vivo and in vitro (28, 46, 47, 72, 145). Transfection of DCs with mRNA encoding the HIV core protein Gag resulted in potent primary CD4 and CD8 T-cell immune responses in an in vitro system (201), with the generation of frequencies of Ag-specific cells similar to that observed in in vivo model systems (130). This study demonstrated an extremely efficient ex vivo delivery of encoded Ag to DCs since Gag mRNA-pulsed DCs had expressed the encoded protein in 90% of the transfected DCs. The mRNA transfection was also shown to induce a DC maturation signal. This study represents one of the few addressing the use of RNA plasmids as potential vaccines. Further studies to evaluate the potential of RNA-based vaccines are under way. The use of RNA vaccines circumvents the requirement for entry of a plasmid into the nucleus prior to being expressed, a challenge often encountered with DNA vaccine strategies. However, one major drawback of RNA vaccines is the inherent instability of RNA compared to DNA, particularly for in vivo delivery. This instability is yet to be addressed. Hence, the more clinically advanced strategy of the two nucleic acid-based vaccine approaches and the more extensively applied in HIV vaccine development is the DNA-based vaccine modality.
WHAT ARE DNA VACCINES AND WHY DO WE NEED THEM AGAINST HIV-1?
DNA-based immunization refers to the induction of an immune response to a protein Ag expressed in vivo following the introduction of vector-carried DNA encoding the polypeptide sequence. In most cases, these vaccines can be designed simply. The transcription unit in a plasmid DNA vaccine encodes the antigen of interest that is expressed by a promoter such as the cytomegalovirus or β-actin promoter. The viral cytomegalovirus promoter currently appears to be an excellent choice for attaining reasonable Ag expression (186), as well as with limiting safety concerns. A poly(A) tract is incorporated into the 3′ end of the sequence to ensure mRNA stability and proper translation of the transcribed gene product. The first reported use of this vaccine modality for HIV was by Wang et al. (195). Plasmid DNA can be injected into skeletal muscle or inoculated as plasmid-coated gold beads by a gene gun into the epidermis. The protein is expressed in transfected mammalian cells, including macrophages and DCs, and enters into both the MHC class I and II processing pathways, where it can support the expansion of both humoral and cellular immune responses (40, 54, 136, 186, 205). The use of plasmid DNA offers many potential vaccine advantages, including quick and easy manufacturing, better quality control, and nonintegration of the plasmid DNA. The rates of integration-induced mutation in animal models were found to be much lower than the rates of spontaneous mutation for a mammalian genome (129, 147). Thus, DNA vaccine vectors, in addition to being safer, retain the advantages associated with using live vectors such as immunogenicity and ensuring delivery of the Ag protein in its correct and native conformation. Additionally, these vaccines are heat stable and easily transportable when stored in lyophilized form and can be engineered to express artificial immunogens and coexpress immunomodulatory proteins. Their simplicity of design and development of DNA vaccines and the power they bring to the development of subunit vaccines that are expressed in cells have made them extremely popular over the past decade. This, accompanied by their ability to prime both CD4 and CD8 responses, has made DNA vaccines a particularly valuable modality in the design of HIV-1 vaccines. Most important, however, for HIV vaccine development has been their ability to modulate viral replication in primate challenge systems.
MECHANISMS OF ANTIGEN PRESENTATION
There are currently three proposed mechanisms for antigen presentation and immune cell activation following DNA plasmid administration, as shown in Fig. 3.
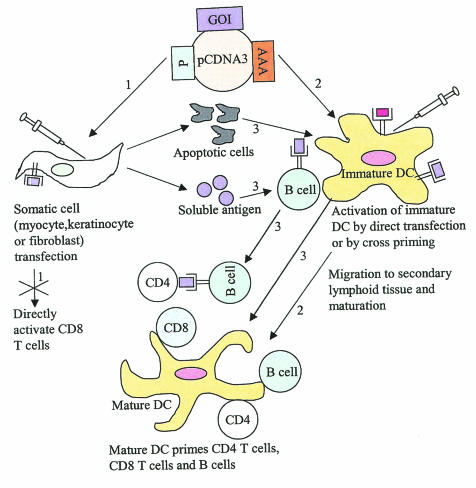
FIG. 3.
Proposed mechanisms of Ag presentation. Step 1. Somatic cells following plasmid transfection can serve as Ag factories expressing the protein Ag encoded by the gene of interest (GOI). Somatic cells cannot serve as APCs since they lack costimulatory molecules; however, they may regulate immune responses by providing Ag for uptake by professional APCs. Step 2. Immature DCs can be transfected directly and, on maturation, can activate CD4 T cells, CD8 T cells, and B cells. Step 3. Immature DCs can acquire Ag, following uptake of transfected apoptotic muscle cells, and present the Ag to CD4 T cells and CD8 T cells. Alternatively, immature DCs and B cells, following uptake of secreted soluble Ag, can present Ag to CD4 T cells and CD8 T cells. P, promoter; AAA, polyadenylation signal; pcDNA3, plasmid vector.
Lymphocyte Priming by Transfected Somatic Cells
Somatic cells such as myocytes or keratinocytes express MHC class I constitutively, and myocytes express protein (147) and elicit CTL responses (192) after being directly transfected with DNA plasmids. These studies suggested that myocytes could activate CD8 T cells directly. However, these cells do not express conventional costimulatory molecules, and subsequent studies to elucidate the mechanism of immune priming by muscle cells, using bone marrow chimera experiments (1, 189), provided clear evidence that myocytes do not directly activate CTLs. Both keratinocytes in the skin and myocytes may play a role in regulating the magnitude and duration of Ag-specific responses by serving as Ag reservoirs.
Lymphocyte Priming by Transfected APCs
Ag-presenting cells (APCs) at the sites of immunization have been proposed to prime immune cells, including CD4 T cells, CD8 T cells, and B cells, following direct transfection (16, 152). Professional APCs such as epidermal Langerhans' cells, macrophages, and interstitial DCs have been shown to contain the plasmid following either intramuscular DNA immunization (35, 42) or gene gun delivery of plasmid (45). Transfected DCs that primed immune responses in these studies (3, 34, 157) constituted a mere fraction (0.4%) of the bone marrow cells, indicating their high efficiency as well as their critical role in stimulating antigen-specific immune responses following Ag expression in them.
Cross-Presentation
A final mechanism that is now receiving considerable research attention is cross-priming. The basis of cross-priming stemmed from the observation that professional APCs could present Ag or peptides derived exogenously rather than from the classical endogenous pathway via the MHC class I pathway of Ag presentation (59, 80, 183, 190). For DNA vaccines, there is considerable evidence (3, 48, 66) to suggest that cross-priming may be another mechanism of Ag presentation and priming of immune responses following DNA delivery to cells. A test Ag expressed by transfected myoblasts of one haplotype was able to cross-prime a CTL restricted to another MHC haplotype when transferred to F1 recipient mice (191). In fact, recent reports have elegantly demonstrated immune activation via cross-priming by APCs that process secreted peptides or proteins from phagocytosed apoptotic bodies (4, 5, 169). The cellular mechanisms by which cross-priming occurs are still undefined, but they involve the internalization and intracellular processing of exogenous protein Ags by APCs for presentation by MHC class I molecules and priming of CTL responses (26). The source of Ag may be inoculated protein (derived from a vaccine), proteins from a circulating pathogen, or proteins released by cells (during an infection). This process may involve whole protein, peptide fragments of these proteins, complexes with heat shock proteins (182), or apoptotic bodies from dying cells (4). A specialized subset of APCs containing functional transporter-associated peptide-encoding genes (90) have the ability to internalize and process these Ags in a manner distinct from the classic pathway of MHC class I presentation of newly synthesized proteins. A DNA vaccine vector driven by a muscle-specific promoter was capable of inducing a full range of immune responses, including CTLs, in mice (120). The fact that CTLs were primed by a DNA vaccine that expressed Ag only in a non-APC (i.e., muscle cells), regardless of which cells internalized the plasmid, indicated that cross-priming was involved. Corr et al. (48) used an expression-suppressible plasmid system (a tetracycline-responsive promoter system to regulate plasmid gene expression) to separate transport of the plasmid and protein from the site of injection. Mice lacking B and T cells (RAG−/− [recombinase-activating genes]) were immunized with and without the suppressing drug. Splenocytes (APCs) from these mice were injected into recipient mice and assessed for their ability to prime a CTL response. Recipient mice that received splenocytes from mice without suppressive treatment mounted a greater CTL response, suggesting that exogenous transfer was important for the magnitude of the response. Additionally, a CTL response was not induced when the site of plasmid injection was ablated by amputation and the suppressing drug was subsequently removed, indicating that the magnitude of the immune response is dependent on Ag produced by transfected nonlymphoid cells. Additionally, the immunogenic protein was expressed predominantly locally at the site of injection and not in the draining lymph nodes or spleen. These investigators also used bone marrow chimera experiments to elegantly demonstrate that cross-priming played a major role in eliciting the immune response to the Ag following Ag-encoding DNA plasmid injection. In these experiments, they generated chimeric mice that had a transgenic transcriptional transactivator in their resident tissues or in their adoptively transferred bone marrow. The transactivator was required for high levels of plasmid DNA expression. They observed that wild-type mice with transgenic bone marrow had weaker CTL and humoral responses than did transgenic mice with wild-type bone marrow after injection of plasmid DNA, supporting the importance of cross-presentation wherein nonlymphoid tissues predominantly expressed Ag peptides encoded by plasmid DNA, which were then transferred to APCs to stimulate the bulk of the immune response. Furthermore, B7.2 as a part of a DNA vaccine can allow for muscle-specific T-cell priming (1).
Thus, both direct transfection of APCs and cross-presentation have been shown to contribute to immune responses following plasmid transfection. The degree to which any of these mechanisms contribute to this DNA vaccine-driven immune expansion is still very much under investigation. However, it is clear that DNA vaccines, under the right circumstances, can produce immune responses mimicking live responses.
METHOD AND ROUTE OF ADMINISTRATION
The nature of immune responses to DNA vaccines can be influenced by the transfection method used (and therefore the types of cells transfected) and the route of immunization (192, 68, 51, 132) (Table 3). The most widely used strategies for application of DNA vaccine vectors are intramuscular needle injections and intradermal inoculation using a gene gun (186). Noninvasive methods of plasmid delivery shown to induce antigen-specific immune responses involve topical application of the plasmid to the skin or mucosa either orally (56), intranasally (78, 104, 106), or intravaginally (14, 197). Delivery methods such as intradermal injection, gene gun bombardment, and topical application that targets the skin elicit a humoral response associated with the production of Th2-type immunoglobulin A (IgA) and IgG1 Ab isotypes (31, 60, 67, 154). Intramuscular injections, on the other hand, induce cell-mediated responses that prime CTLs and elicit the production of Th1-type IgG2a Ab (177). Typically, intramuscular immunization via saline needle injections requires 100- to 1,000-fold more DNA than does gene gun immunization in order to generate an equivalent antibody response (154). One explanation for this difference in the efficiency of DNA-elicited immunity is that cells take up DNA from extracellular spaces following saline-DNA immunization (51, 204, 205) while gene gun DNA immunization directly transfects cells by depositing DNA-coated gold beads within the cell cytoplasm (55). Furthermore, DNA inoculation into the skin and muscle transfects different types of cells. Intramuscular delivery of DNA leads to the expression of protein in skeletal muscle cells (205). Professional APCs such as macrophages or tissue DCs within skeletal muscle may also be transfected (42, 152). Intradermal saline injections and gene gun delivery of DNA result in Ag expression primarily from keratinocytes, although there have been reports of expression from occasional dermal fibroblasts (84, 161). Subcutaneous or intradermal delivery of Ag can generate a strong cellular and humoral response, while oral or intravenous delivery of the same Ag can induce tolerance, resulting in unresponsiveness. For instance, the influenza virus hemagglutinin (HA) protein injected intramuscularly was shown to elicit a different anti-HA Ab isotype profile from the one elicited when it was given intranasally (88).
TABLE 3.
Comparison of various plasmid delivery systems
| Approach | Nature of immune response | Types of cells transfected |
|---|---|---|
| Topical, intradermal injection, gene gun delivery | Humoral response; IgA, IgG1 | Skin fibroblasts, keratinocytes |
| Intramuscular | Cell-mediated response; CTL and IgG2a | Myocytes |
| Electroporation | Both humoral and cell-mediated responses | Most probably myocytes (under investigation) |
The use of these different routes and methods of delivery of DNA vaccines in general has been more potent in smaller animals and not as effective in primates. Thus far, there have been only a few cases where immune responses were noted in humans, and the magnitude of these responses has not been substantial (33, 125, 199). One reason for this lack of efficiency in responses may be a low uptake of DNA. To overcome this limitation, other methods are being pursued in vivo.
The electroporation method is a physical method for delivery of compounds as well as genes to tissues in vivo. Confined short electrical pulses are delivered to target cells and tissues at levels which increase cell permeability without killing the cells, enabling hydrophilic drugs as well as DNA to pass through the cell membrane. Electroporation has been used to effectively deliver chemotherapeutic agents to tumors in animals and in humans. The first report that electroporation could transfect cells in vivo with plasmid DNA came when Titomirov et al. showed reporter gene expression in skin cells of mice (187). Since then, this technique has been applied to many other tissues in mice and rats, such as skin, liver, testis, brain, and skeletal muscle as well as tumor tissue such as melanoma and hepatocellular carcinoma (2, 83, 138), and clinical trials have been performed to test this therapy on melanoma, squamous cell carcinoma, and basal cell carcinoma (23). Animal and human studies have reported response rates of 45 to 99%. Due to its ability to express plasmid DNA for long periods, skeletal muscle has been frequently used in electroporation experiments for purposes ranging from reporter gene expression and cytokine expression to Ag expression for vaccines. Studies have also shown that electroporation can be used to deliver plasmid DNA in multiple treatments without causing adverse side effects. Although pain elicitation on electroporation has been reported by a segment of the patient population (166), experimental studies examining the pain response to electroporation in animals have not been done.
In vivo electroporation is capable of delivering plasmid DNA as an antitumor agent. This approach has been used for treatment of hepatocellular carcinomas, adenocarcinoma, breast tumors, and B16-F10 melanoma in rodent models. In the B16-F10 melanoma model, cures of established tumors with resistance to challenge has been demonstrated (82). Lohr et al. compared delivery by electroporation with the use of adenovirus vectors and found that electroporation was effective in delivering plasmid coding for interleukin-12 (IL-112) and, unlike adenovirus delivery, did not result in toxic side effects (119).
Recent studies have shown that electroporation is capable of efficiently delivering plasmid DNA to the skin. These studies demonstrated the feasibility of using this approach for DNA vaccination or for increasing the levels of a specific protein in serum. Delivery was successful in several models including rodents, pigs, and primates. Expression in these models was enhanced as much as 100-fold.
Delivery of plasmids by electroporation was found to enhance Ag expression in mice, guinea pigs, and rabbits (188, 203) and, more recently, in pigs (13). The immunogenicity of a potent HIV gag DNA vaccine was increased in mice, as seen by higher Ab titers, a substantial reduction in the dose of DNA required to induce an Ab response, and an increase in CD8 T-cell responses (203). The precise mechanism for the observed enhancement in the potency of DNA vaccines using electroporation is not yet known. One explanation could be the availability of higher levels of Ag for priming immune responses as a consequence of increased expression of encoded Ag in transfected myocytes. Electroporation appears to be well tolerated by the animals and is a simple technique that takes only a few seconds after inoculation.
These reports show the feasibility of electroporation for the delivery of plasmid DNA encoding therapeutic molecules. Therefore, such delivery systems may have utility for the delivery of potential HIV-1 vaccines. However, there has been some concern that electroporation could result in the integration of DNA from the plasmid into the host genome. If this were the case, it would effectively limit the utility of electroporation for the delivery of prophylactic DNA vaccines and could limit its utility for therapeutic vaccine purposes as well. It will be important to specifically address the question of integration.
EARLY TRIALS OF DNA-BASED VACCINES
The first DNA vaccines to be tested in humans were HIV-1 vaccines (125). Several groups have subsequently been directing their efforts toward developing DNA vaccines for HIV-1. DNA vaccination with HIV-1 Env-encoding plasmids was first shown to elicit Env-specific humoral and cellular immune responses in mice (122, 195, 198) and macaques (196). The immune responses in these studies were dose dependent, could be boosted, and were long-lived (greater than 6 months) (174, 175). Chimpanzees vaccinated with DNA plasmids containing HIV-1 env and gag/pol were completely protected against high-dose challenge with the SF2 strain of HIV-1 (30). However, since HIV-1 SF2 replication in chimpanzees occurs at very low levels and is nonpathogenic, the significance of this study remains uncertain (95). Rhesus macaques, immunized six times with DNA encoding SIV Env, still remained unprotected against intravenous challenge with the virulent SIVmac251 isolate (121, 125). However, the viral load was reduced and pathogenicity was attenuated in the vaccinated animals. Another study showed that DNA vaccination of pigtail macaques decreased viral loads following intrarectal challenge with SIVmne, a viral isolate of intermediate pathogenicity (77). Hence, while DNA vaccination with HIV-1 or SIV antigens elicited humoral and cellular immune responses in nonhuman primates, the immune responses did not protect primates against infection by a pathogenic viral challenge. However, these vaccines could protect in a nonpathogenic primate model and could affect the viral load to various degrees following challenge.
One limitation of DNA vaccines in most of these early studies was a lack of induction of a robust immune response due to insufficient uptake and expression of DNA. For HIV DNA vaccines, the problem of insufficient gene expression has been further exacerbated due to the inefficient expression of HIV-1 mRNAs outside of the context of the HIV-1 genome. New-generation DNA vaccines have circumvented this problem by using Rev and the Rev-responsive elements to facilitate the expression of HIV-1 mRNAs. In fact, Rev-dependent subgenomic splicing can now be used to express multiple HIV-1 proteins from single transcripts (9) and has the potential for expressing noninfectious virus-like particles (122). Still higher levels of HIV-1 gene expression have been achieved by optimizing the HIV-1 genes for the codons that are used most frequently in human cells (219). Such codon-optimized sequences can achieve exceptionally high levels of gene expression by designing transcripts as a single, efficiently translated mRNA. Codon-optimized sequences are designed to express either single-gene products (20) or fusion proteins (91). The majority of the codon-optimized vaccines under development have used the group-specific Ag (Gag) protein that is a major target for CD8 T cells in long-term nonprogressors (155). Other approaches tested to improve the potency of DNA vaccines have involved the use of use genetic adjuvants (44), conventional adjuvants (176), microspheres that increase DNA transfer to APCs (181), in vivo electroporation (203), immunostimulatory sequences such as CpG in the plasmid or vector modification to enhance Ag expression (91), peptides that target the Ag to sites of immune response induction (53), and codelivery of plasmids activating the death pathway (41, 168). Conventional adjuvants, such as alum and block copolymers, have not markedly increased the efficacy of DNA-based vaccines for HIV (176). However, a new adjuvant, IL-2/Ig, containing IL-2 fused to the heavy chain of immunoglobulin to increase the half-life of IL-2 activity, has been developed (21). When delivered either as a protein or as a genetic adjuvant in monkeys, IL-2/Ig enhanced DNA vaccine-elicited HIV-1 Env- and Gag-specific Ab and CTL responses (20).
Immunomodulators
One strategy that has been extensively applied to augment DNA vaccine-elicited immune responses to a broad range of Ag including hepatitis B virus, hepatitis C virus, HIV-1, influenza virus, Plasmodium, and Leishmania is the coadministration of plasmids encoding immunomodulator molecules such as cytokines, chemokines, costimulatory molecules, and adhesion molecules. Coinoculation of granulocyte-macrophage colony-stimulating factor (207, 150), IL-2 (208), IL-12 (96), and IL-15 (18, 100, 209) along with HIV DNA vaccines was shown to enhance the cellular immune responses to HIV-1 Ags in mice. Intramuscular delivery of IL-12 cDNA significantly increased the percentage of activated lymphocytes (expressing Ly6, a T-cell activation marker) in a herpesvirus-infected mouse model system (178). The same group also demonstrated enhanced cellular immune responses to herpes simplex virus gD DNA vaccine on coadministration of IL-2, IL-12, IL-15, or IL-18 cytokines (179). IL-2 coinjections in nonhuman primate studies were shown to modulate HIV Ag-specific immune responses (102). Other studies (97, 98, 99, 101, 123, 210) have indicated an augmentation of HIV-1-specific responses in mice after coimmunization with plasmids expressing the costimulatory molecule B7-2, the adhesion molecules ICAM-1 and LFA-3, and the chemokines RANTES, MIP-1α, and monocyte chemotactic protein 1 along with HIV DNA vaccines. It is clear from this brief discussion that there are many interesting options for improving the potency of DNA vaccines.
Prime-Boost Strategies
The combination of vaccination modalities is now widely used to enhance specific protective immune responses in nonhuman primates. DNA-primed responses can be boosted with live recombinant vectors or proteins in various prime-boost strategies. DNA priming followed by Env IIIB protein boosting increased antibody responses and successfully protected rhesus macaques against challenge with the nonpathogenic SHIV-IIIB (112); however, in a similar study, cynomolgous monkeys boosted with protein following DNA immunization were not protected against a nonpathogenic SHIV-Lai challenge (159). Protein boosting has not been able to generate broadly reactive neutralizing-Ab responses or provide protection against diverse pathogenic viruses, although it appears to augment the neutralizing-Ab responses to T-cell-line-adapted nonpathogenic viruses. The first finding of increased immunogenicity following the consecutive use of DNA and an attenuated virus was with mice when vectors carrying the HA gene of influenza virus were used. The efficiency of priming with DNA followed by boosting with a live vector is due to the DNA focusing the immune response on the vaccine Ags. Since the DNA is nonimmunogenic itself, immunity is elicited primarily to the encoded Ags. This is in contrast to approaches such as the live vector system, where immunity is elicited to both the vector and the vaccine Ag, leading to an overall dilution of vaccine-elicited protective immunity. The use of a live vector as the booster enhances the DNA-primed immune response both by expressing larger amounts of Ag and by stimulating a proinflammatory response that augments immunity. Live-virus vectors that have the ability to infect professional Ag-presenting DCs (217) such as MVA (6) or rAd5 (8, 10, 176) are now used to boost DNA-primed responses against HIV Ags with higher efficiency (36).
CURRENT EXCITEMENT ABOUT DNA VACCINE STRATEGIES
Some DNA-based approaches have generated significant interest due to their results, particularly in nonhuman primate models of infection. Adjuvanted DNA vaccines (20) and DNA-primed live-virus vector-boosted vaccines (prime-boost) (9, 176) designed to raise high levels of CD8 T cells have been shown to control the viral load following challenge in rhesus macaques from pathogenic SHIV hybrids (164). Most of these vaccines have used SIV Gag-Pol and HIV-1 Env 89.6 or 89.6P as immunogens, and the animals were challenged with the pathogenic SHIV 89.6P, which usually kills the majority of nonvaccinated control animals within 6 months (94). Challenges have been performed between 6 weeks (176) and 7 months (7, 9, 10) after the final immunization and have been administered by both intravenous (20, 176) and intrarectal routes (9, 10). All of the vaccinated animals were infected but rapidly controlled their plasma viral loads at or below the level of detection (500 to 1,000 copies per ml of plasma) by 8 to 12 weeks. More than 80% of the control animals at close to 2 years after the SHIV challenge lost their CD4 cells and succumbed to AIDS, whereas a majority of the vaccinated animals maintained their CD4 cells, controlled their levels of virus, and have not yet progressed toward AIDS. Vaccinated animals registered an early and rapid expansion of antiviral T cells and a delayed appearance of neutralizing Abs, suggesting a crucial role for CD8 T cells early in the viral control (9, 20). However, a recent follow-up study (17) showed that there could be escape from this protection. An additional 3-year study of macaques immunized with DNA encoding SIV Gag and challenged with heterologous SIV demonstrated waning immune responses and serial breakthroughs in viral replication in study animals after an initial control of the plasma viral load for 1 to 2 years (18). These studies collectively suggest that more work is needed for a DNA approach alone or a DNA prime followed by a viral vector boost to completely control the pathogenic challenge in these model systems. However, these studies (9, 20, 30) have clearly brought us closer to the dream of a potent DNA vaccine approach to HIV-1.
DNA VACCINE CLINICAL STUDY
The first DNA vaccine to be tested in the clinic was for HIV-1 in the context of immune therapy (125). Since that time, there have been several clinical studies for both prophylaxis and immune therapy of HIV. Structural genes for subtype B have been tested, as have cocktails of the regulatory genes tat, rev, and nef, in immune therapy (103). In addition, in the area of prophylaxis, both structural genes (126) and epitope-based approaches (79) have been tested. Importantly, these collective studies involving several hundred persons are establishing that DNA approaches appear very well tolerated in humans. No study has reported a single significant adverse event. Of relevance is that these vaccines have been immunogenic in humans to various degrees. In the therapy situation, some evidence for increases in cellular immunity have been reported (103); however, effects on viral load are still awaiting confirmation. In the prophylaxis trial, Ab responses have been very low to nonexistent. CD4 T-cell responses have been observed with reasonable frequency, but overall the CD8 T-cell responses have not been as strong as is probably necessary to effectively control the infection. It is clear that the most important area of focus for the further clinical development of this technology is to improve the immune potency of the next generation of plasmid vaccines. In this regard, there is much interest in testing newer prime-boost studies, where DNA priming is followed by a live MVA vector boost (8) or a live adenovirus vector boost (176, 213). Many of these studies will include optimized DNA vectors. Preliminary results from a phase I study that tested HIV-1 gag-specific inmmune responses in uninfected humans immunized with nonadjuvanted HIV-1 gag DNA vaccines or HIV-1 gag-expressing Ad5 were recently announced. Both vaccines appear to be well tolerated by volunteers and also are able to successfully elicit cross-clade anti-gag responses. The Ad5 vaccine was more immunogenic than the DNA vaccine at all doses tested since even at high doses of DNA vaccine (5 mg) administered, anti-gag immune responses were observed in fewer than half of the individuals in the study group even at week 30 following immunization. Recent studies have shown consistent and strong CTL responses to gag in macaques immunized with DNA-CRL 1005-adjuvanted gag plasmids and boosted with rAd5 encoding gag (36). These studies do establish that a significant percentage of humans respond to both the DNA and adenovirus approaches, and more evaluation of more potent DNA vaccines is clearly warranted. In this regard, novel approaches testing the codelivery of gene adjuvants such as the IL-2/Ig vectors (20) along with the HIV Ag vectors, should be mentioned. Additionally, there are plans to use more potent Th1 cytokine adjuvants such as IL-12 or IL-15 for clinical evaluation (96, 100). The goal for use of these new HIV vaccines should be to drive greater CD8 T-cell responses in a broad segment of vaccine recipients. It is hoped that ultimately the frequency of CD8 T-cell responses in vaccine recipients can break the 50% response rate barrier and move closer to a more comfortable range around 80%. These exciting studies will closely be watched over the next few years.
FUTURE DIRECTIONS
Multiple Epitopes
Approximately 200 T-cell epitopes specific for various genes of HIV have been mapped in humans. The use of viral peptide pools to enumerate antigen-specific CD4 and CD8 T-cell responses in vitro regardless of the HLA type has helped to assess the contribution of various HIV-1 proteins in disease control in long-term nonprogressors (individuals who have controlled virus replication and maintained plasma virus loads at 3,000 copies or less per ml of plasma for several years). Gag, Nef, and Env proteins were demonstrated through these studies to account for the majority of the T-cell response. A recent study designed to evaluate the requirement for Env in a DNA-rMVA vaccine revealed that Gag-Pol-Env immunization was far superior in controlling subsequent infection than was Gag-Pol immunization alone (7). Following Gag-Pol immunization, only 7 of 12 animals managed to control the challenge infection, whereas following Gag-Pol-Env immunization, 23 of 24 animals controlled virus replication, suggesting a crucial role for Env. Another recent study (142) with a pathogenic macaque SIV challenge model used a multiplasmid DNA vaccine consisting of Gag-Pol/Env-Rev to demonstrate a greater protection from CD4 loss as well as lower viral loads in immunized macaques as opposed to control animals. This study further highlights the importance of including multiple Ags of HIV-1 in designing DNA vaccines to enhance vaccine-conferred protection in a challenge context (Fig. 4). Given the high diversity of HIV-1 isolates and their frequent escape from immune control (17), it would be advantageous to include multiple HIV genes to elicit a broad and a more universal immune response. Furthermore, the use of “epitope string” approaches is also under investigation (146).
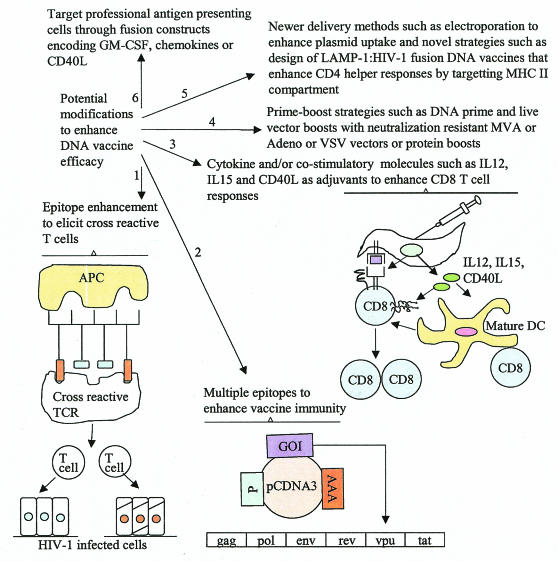
FIG. 4.
Recent strategies to enhance DNA vaccine-elicited immune responses. Strategy 1. Construction of chimeric epitopes derived from different HIV-1 strains to elicit cross-reactive T-cell responses. Such cross-reactive T cells can target HIV-1-infected cells that express different Ag epitopes. Strategy 2. Inclusion of multiple HIV-1 genes in the plasmid vaccine to enhance vaccine-elicited immune responses and to control the rate of generation of escape mutants. Strategy 3. Enhancement of CD8 T-cell responses by coinjecting cytokine genes such as IL-12 and IL-15 along with HIV-1 vaccine Ag. IL-12 enhances Th1-type responses, and IL-15 enhances CD8 T-cell memory proliferation. Inclusion of CD40L can activate and deliver maturation signals to DCs, enhancing their Ag presentation ability and associated functions. See the text for explanation of the other strategies (strategies 4, 5, and 6) illustrated. GOI, gene of interest; P, promoter; AAA, polyadenylation signal; pcDNA3, plasmid vector; TCR, T-cell receptor.
Enhancing Memory Responses
The mechanisms of T-cell priming with DNA vaccination are not well understood, whether by direct transfection, indirectly through cross-presentation or both. Also, the types of effector and memory T cells that are formed and the duration of Ag expression following DNA vaccination are not known. The efficacy of vaccines can be enhanced by selectively modulating a particular stage of a T-cell response. For instance, increased levels of IL-4, IL-7, or IL-15 in vivo can increase the numbers of Ag-specific CD4 and CD8 T cells, and IL-15 can enhance protective immunity (179, 127, 212). Furthermore, IL-15 has been shown to increase the proliferation of CD8 memory T cells without having a comparable effect on CD4 cells (215). Thus inclusion of IL-15 along with the vaccine Ag in a DNA vaccine to specifically magnify CD8 memory is now being actively pursued in the design of several HIV vaccines (Fig. 4). Whether coadministration of these cytokines can have lasting effects on the generation or maintenance of Ag-specific memory T cells needs to be investigated further. It might be possible to increase the proliferation of effector T-cell populations by modulating the factors that regulate Ag-independent cell division. This would be particularly helpful in cases where Ag distribution by a vaccine is limited or short-lived. Alternatively, it might be possible to reduce effector cell death, thereby increasing the number of memory T cells that are formed. In CD154 (CD40L)-deficient mice, effector CD8 T-cell death is enhanced and the mice display a nearly 10-fold reduction in Ag-specific memory T cells after lymphocytic choriomeningitis virus infection. Interestingly, however, the lack of CD154 has no effect on CD8 T-cell clonal expansion (29, 202). Therefore, coadministration of CD40L may enhance CD40-CD154 interactions and specifically modulate the effector contraction phase (180) (Fig. 4). Several candidate approaches to enhance T-cell memory are currently under trial and show promise. These strategies will, however, be challenging because of a lack of understanding of the precise signals and mechanisms that regulate the various stages of T-cell differentiation.
Targeting DCs
DCs are the key professional APC needed to initiate a cellular immune response by naive T cells. Fusion of Ag to CTLA-4, which binds CD80/CD86 on APCs, directs the fusion Ag to the APCs. This strategy was successfully employed to confer protection in sheep rechallenged with Corynebacterium pseudotropicali (39) and recently in enhancing Ab responses to an Ag derived from Taenia ovis in mice (53). The kinetics of the Ab response generated following CTLA-4-targeted DNA vaccination was also significantly greater than that achieved with nontargeted DNA vaccination or with adjuvanted protein vaccination in this study (53). The application of CTLA-4 targeting of HIV DNA vaccines requires further investigation. Strategies are being developed to expand DCs in vivo with cytokines and other agents such as Flt-3 ligand. A recent study (27) demonstrated for the first time that mice immunized with DNA encoding gp120 fused with proinflammatory chemoattractants of immature DCs, such as β-defensin 2, MCP-3/CCL7, or macrophage-derived chemokine (MDC/CCL22), elicited high titers of both neutralizing and nonneutralizing anti-Env antibodies. Immune sera inhibited HIV-1 Env-mediated cell fusion and infection by pseudotype virus expressing the same Env as well as infection by pseudotype viruses expressing various other Env proteins (89.6;R5X4, NL4-3;R5, and JRFL;R5), despite different coreceptor usage. Responses required the physical linkage of gp120 with the chemokine, since there was a lack of immune response following immunization of mice with DNA encoding a free mixture of chemokine and gp120; this result is in contrast to an earlier study that reported an adjuvant effect of the chemokine moiety in the absence of physical linkage with the Ag (100). Although the DNA vaccine in this study was delivered into skin by gene gun immunization, significant CD8 CTL activity was detected in both the spleen and Peyer's patches, suggesting induction of both systemic and mucosal immune responses. In addition to targeting gp120 to chemokine receptors on professional APCs, it has been proposed that chemokine or defensin fusion proteins may stimulate surface expression of costimulatory molecules and synthesis of proinflammatory cytokines by subsets of immature DCs in vivo. Additionally, chemokines could differentially attract Th1 or Th2 cells, thus modulating immunity. An understanding of the mechanism of immunity elicited by these gp120-chemokine or gp-120 defensin fusion proteins is required. Another strategy under consideration to enhance APC function is to administer HIV DNA-based vaccines with IL-12 as a genetic adjuvant (96) and use an appropriate recombinant live vector as the booster. Additionally, coadministration of appropriate costimulatory molecules could enhance and sustain CTL responses (97).
Newer Plasmid Delivery Systems
The recombinant VSV (rVSV), a minus-strand RNA virus, is undergoing substantial modification to increase its utility as a vaccine vector. rVSVs were shown to elicit a high level of primary CD8 responses to HIV Ags when used as vaccine vectors (75) and could also protect rhesus macaques from a pathogenic SHIV infection (167). rVSVs in which different serotypes of VSV-G are exchanged for the standard Indiana serotype G (glycoprotein exchange vectors) have now been developed to eliminate neutralization of the VSV vector when used as boosters (76). Gag-specific CTL responses were increased on immunization with plasmids encoding HIV-1 Gag particles pseudotyped with VSV-G (76). Following an in vitro observation that uptake of HIV-1 virions bearing either HIV-1 or VSV-G could result in presentation of HIV-1 Gag epitopes on MHC class I molecules in the absence of viral protein synthesis in primary human DCs and macrophages (exogenous presentation by cross-priming) (32), VSV-G envelope pseudotyped Gag particles were developed and used as DNA vaccines to increase Ag uptake after DNA-based immunization by taking advantage of the fusogenic activity and receptor-mediated entry into cells in vivo. Such VSV-G-pseudotyped Gag particles entered both the MHC class I and II processing pathways. In contrast, naked Gag particles entered only the MHC class II processing pathway. Hence, one could combine DNA-based immunization and nonreplicating pseudotyped virus when designing vaccines to deliver HIV-1 Ag to the immune system in vivo. Additionally, one could potentially enhance the primary responses elicited by VSV-G pseudotyped HIV-1 DNA vaccines by using the modified VSV-G booster that is resistant to neutralization. Gag-LAMP-1 fusion proteins have been administered intramuscularly as DNA vaccines in mice in a recent study (128). Unmodified Gag was shown to be highly expressed as a Gag-LAMP-1 fusion protein in immunized animals, and the vaccine was able to stimulate Gag-specific CD4 helper responses by being targeted to the endosomal MHC class II pathway of processing and Ag presentation. Another recent study demonstrated that boosting the Gag-encoding DNA plasmid with Gag protein adsorbed to polylactide coglycolide particles (PLG) enhanced the immunogenicity of the priming vaccine as well as eliciting broad and sustained CTL CD4 helper responses and anti-Gag Ab responses in rhesus macaques (151). This synergy with Gag DNA priming and Gag-PLG boosting provides yet another strategy to elicit protective immune responses while circumventing the need for a live vector boost. Further optimization of these strategies in nonhuman primate models is eagerly awaited and will open up exciting possibilities for the development of strategies for producing HIV-1 DNA vaccines for humans.
Novel Adjuvants
The influence of adjuvants on the dynamics and durability of T-cell responses to candidate HIV vaccines was assessed in a recent study (37). Codon-optimized sequences from the HIV gag gene were inserted into DNA vaccine vectors in a manner so as to express the coding sequence with or without the tissue plasminogen activator leader sequence. The vaccines were delivered as plasmid DNA without adjuvant or as plasmid DNA formulated with a novel block copolymer adjuvant (CRL8623). This study reported a persistent cell-mediated immune response in rhesus macaques for at least 18 months following a four-dose vaccination regimen. Both CRL8623 adjuvanted and nonadjuvanted plasmid vaccines were immunogenic; however, the adjuvanted formulation elicited enhanced T-cell responses, with a bias toward more Ag-specific CD8 T cells. This approach has now been moved to clinical evaluation. A more recent study (15) with mice demonstrated a higher level of Ab and CTL responses to HIV-1 gp120 when the plasmid was administered along with the catalytic domain of the cholera toxin as an adjuvant than that obtained when the gp120 plasmid was administered alone. Concomitant with the development of conformationally constrained envelope immunogens (see below), the development of such novel adjuvants provide a means of enhancing and sustaining immune responses elicited against these plasmid immunogens.
Structured Envelope Vaccines
Since envelope glycoproteins exist as oligomers on the native virus, attempts are being made to develop a variety of stable oligomeric envelope proteins for evaluation as immunogens (64). The HIV-1 envelope undergoes a series of conformational changes during the process of viral fusion to the cell membrane (107), and attempts are being made to develop subunit immunogens that mimic the fusion intermediate forms of the envelope (Fig. 2). Elegant mutagenic and crystal structure studies of the HIV-1 envelope gp120 are increasing our understanding of the range of confirmations available to these glycoproteins and have also demonstrated that designing mutated envelopes with restricted conformational flexibility may be one way to achieve an efficient response to these immunogens (137, 206).
Plasmid expression vectors can be easily modified to express these various forms of HIV envelope proteins, allowing a rapid assessment of these vaccine candidates. Some innovative studies with mice have used mutated envelopes as plasmid immunogens to elicit both neutralizing-antibody responses to the envelope as well as CTL responses to the immunogen (38). This is clearly an important and an exciting area. If a structural immunogen is developed that does induce cross-reactive neutralizing responses, its inclusion in current DNA approaches should be evaluated.
Contending with Diversity and Enhancing Cross-Clade Protection
While most current HIV vaccines have shown the ability to control homologous challenges, their ability to control a heterologous challenge is yet to be demonstrated. Based on the data available from the Los Alamos database for known T-cell epitopes, perfect conservation varies from 88% for the least variable protein (p24) to 46% for the most variable protein (gp120) (24) within clade B. This level of variation poses a potential problem for these vaccines even as they progress through human efficacy trials. Currently, candidate gp120 subunit vaccines derived from isolates (164) are now in phase III efficacy trials (49), and it is hoped that they will be sufficiently cross-reactive to protect against circulating viruses. Given that HIV-1 envelope proteins can differ in more than 30% of their amino acids, this may be exceedingly optimistic. One way to overcome the problem of strain variability would be to use vaccines that represent several different subtypes. This approach that is now under active development (70, 73).
Epitope enhancement via generation of a chimeric peptide sequence between different strains of the virus can be yet another strategy to elicit more broadly cross-reactive T cells. This strategy was effectively applied in the generation of a broadly cross-reactive CTL that recognized multiple strains of HIV for a CTL epitope from a variable segment of the HIV envelope protein, by substituting one T-cell receptor-interacting residue from one strain with that from another (185). Furthermore, such cross-reactive components of the repertoire can be preferentially induced by an appropriate manipulation of the amino acid sequence of the epitope, thus allowing a better representation of all possible clones in the repertoire that may otherwise be dominated by type-specific clones (Fig. 4). Finally, since an effective HIV vaccine must elicit Abs that bind to neutralizing determinants from a variety of HIV envelopes, it has been suggested that polyvalent envelopes should be assessed as potential immunogens.
Expression of several MHC class I alleles has been demonstrated to confer a protective effect against disease progression in both HIV-1 and SIV infection. A very recent study (216) with a SHIV-macaque model indicated a significant attenuation of disease progression in Mamu-A(*)01-positive rhesus monkeys infected with the highly pathogenic SHIV 89.6P. This correlated with a Mamu-A(*)01-restricted dominant CTL response, a lower viral load in lymph nodes, and preservation of lymph node structure during early infection. In contrast, Mamu-A(*)01-negative monkeys exhibited massive destruction of lymphoid tissue and rapid disease progression. These findings additionally support the requirement for an effective AIDS vaccine to elicit CTL responses that protect lymphoid tissue from HIV-mediated destruction early after infection and show that appropriate vaccine studies should be designed to segregate rather than concentrate MHC genotypes in order to prevent study bias. However, replication of this study by other laboratories will be important to generalize this study.
CONCLUSIONS
With accumulating evidence for the importance of CTLs in containing HIV spread in infected individuals, a number of vaccine strategies are being pursued for the elicitation of these immune effector cells. DNA-based strategies, in particular, have shown great promise and potential for further development as HIV vaccines. The new vectors and vaccination modalities including DNA-rMVA, DNA-rAd5, DNA/IL-2/Ig, and DNA with other cytokines are currently entering phase I clinical trials. These vaccination modalities have been chosen for their ability to raise reasonable titers of immune responses in animal models and for their impact on protection outcomes in nonhuman primate models. There is a growing conviction now that vaccine modalities such as DNA-based immunization will result in control and slower disease progression, if not clean prevention of infection. The simplest approach to plasmid vaccination originally reported over 10 years (165, 186, 192, 198) ago has grown in scope and in complexity of design. The current generation of DNA vaccines includes many very exciting ones that have captured the scientific imagination. As we move forward through the next decade of advances, we can hope that the 20-year review will see the description of many phase III studies of DNA-based approaches to HIV prophylaxis with, hopefully, at least some significant efficacy.
REFERENCES
- 1.Agadjanyan, M. G., J. J. Kim, N. Trivedi, D. M. Wilson, B. Monzavi-Karbassi, L. D. Morrison, L. K. Nottingham, T. Dentchev, A. Tsai, K. Dang, A. A. Chalian, M. A. Maldonado, W. V. Williams, and D. B. Weiner. 1999. CD86 (B7-2) can function to drive MHC-restricted antigen-specific CTL responses in vivo. J. Immunol. 162:3417-3427. [PubMed] [Google Scholar]
- 2.Aihara, H., and J. Miyazaki. 1998. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16:867-870. [DOI] [PubMed] [Google Scholar]
- 3.Akbari, O., N. Panjwani, S. Garcia, R. Tascon, D. Lowrie, and B. Stockinger. 1999. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J. Exp. Med. 189:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert, M. L., S. F. Pearce, L. M. Francisco, B. Sauter, P. Roy, R. L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 6.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 7.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2002. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine 20:1949-1955. [DOI] [PubMed] [Google Scholar]
- 9.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 10.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andino, R., D. Silvera, S. D. Suggett, P. L. Achacoso, C. J. Miller, D. Baltimore, and M. B. Feinberg. 1994. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science 265:1448-1451. [DOI] [PubMed] [Google Scholar]
- 12.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 13.Babiuk, S., M. E. Baca-Estrada, M. Foldvari, M. Storms, D. Rabussay, G. Widera, and L. A. Babiuk. 2002. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine 20:3399-3408. [DOI] [PubMed] [Google Scholar]
- 14.Bagarazzi, M. L., J. D. Boyer, K. E. Ugen, M. A. Javadian, M. Chattergoon, A. Shah, M. Bennett, R. Ciccarelli, R. Carrano, L. Coney, and D. B. Weiner. 1998. Safety and immunogenicity of HIV-1 DNA constructs in chimpanzees. Vaccine 16:1836-1841. [DOI] [PubMed] [Google Scholar]
- 15.Bagley, K. C., M. T. Shata, D. Y. Onyabe, A. L. DeVico, T. R. Fouts, G. K. Lewis, and D. M. Hone. 2003. Immunogenicity of DNA vaccines that direct the coincident expression of the 120 kDa glycoprotein of human immunodeficiency virus and the catalytic domain of cholera toxin. Vaccine 21:3335-3341. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 17.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 18.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 21.Barouch, D. H., S. Santra, T. D. Steenbeke, X. X. Zheng, H. C. Perry, M. E. Davies, D. C. Freed, A. Craiu, T. B. Strom, J. W. Shiver, and N. L. Letvin. 1998. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 161:1875-1882. [PubMed] [Google Scholar]
- 22.Barré-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 23.Belehradek, M., C. Domenge, B. Luboinski, S. Orlowski, J. Belehradek, Jr., and L. M. Mir. 1993. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 72:3694-3700. [DOI] [PubMed] [Google Scholar]
- 24.Berman, P. W., A. M. Gray, T. Wrin, J. C. Vennari, D. J. Eastman, G. R. Nakamura, D. P. Francis, G. Gorse, and D. H. Schwartz. 1997. Genetic and immunologic characterization of viruses infecting MN-rgp120-vaccinated volunteers. J. Infect. Dis. 176:384-397. [DOI] [PubMed] [Google Scholar]
- 25.Berman, P. W., T. J. Gregory, L. Riddle, G. R. Nakamura, M. A. Champe, J. P. Porter, F. M. Wurm, R. D. Hershberg, E. K. Cobb, and J. W. Eichberg. 1990. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345:622-625. [DOI] [PubMed] [Google Scholar]
- 26.Bevan, M. J. 1976. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 117:2233-2238. [PubMed] [Google Scholar]
- 27.Biragyn, A., I. M. Belyakov, Y. H. Chow, D. S. Dimitrov, J. A. Berzofsky, and L. W. Kwak. 2002. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood 100:1153-1159. [DOI] [PubMed] [Google Scholar]
- 28.Boczkowski, D., S. K. Nair, D. Snyder, and E. Gilboa. 1996. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 184:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrow, P., A. Tishon, S. Lee, J. Xu, I. S. Grewal, M. B. Oldstone, and R. A. Flavell. 1996. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 183:2129-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyer, J. D., K. E. Ugen, B. Wang, M. Agadjanyan, L. Gilbert, M. L. Bagarazzi, M. Chattergoon, P. Frost, A. Javadian, W. V. Williams, Y. Refaeli, R. B. Ciccarelli, D. McCallus, L. Coney, and D. B. Weiner. 1997. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat. Med. 3:526-532. [DOI] [PubMed] [Google Scholar]
- 31.Boyle, J. S., A. Silva, J. L. Brady, and A. M. Lew. 1997. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc. Natl. Acad. Sci. USA 94:14626-14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buseyne, F., S. Le Gall, C. Boccaccio, J. P. Abastado, J. D. Lifson, L. O. Arthur, Y. Riviere, J. M. Heard, and O. Schwartz. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 7:344-349. [DOI] [PubMed] [Google Scholar]
- 33.Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandstrom, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351:1320-1325. [DOI] [PubMed] [Google Scholar]
- 34.Casares, S., C. A. Bona, and T. D. Brumeanu. 1997. Engineering and characterization of a murine MHC class II-immunoglobulin chimera expressing an immunodominant CD4 T viral epitope. Protein Eng. 10:1295-1301. [DOI] [PubMed] [Google Scholar]
- 35.Casares, S., K. Inaba, T. D. Brumeanu, R. M. Steinman, and C. A. Bona. 1997. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J. Exp. Med. 186:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caulfield, M. J., S. Wang, J. G. Smith, T. W. Tobery, X. Liu, M. E. Davies, D. R. Casimiro, T. M. Fu, A. Simon, R. K. Evans, E. A. Emini, and J. Shiver. 2002. Sustained peptide-specific gamma interferon T-cell response in rhesus macaques immunized with human immunodeficiency virus gag DNA vaccines. J. Virol. 76:10038-10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaplin, P. J., R. De Rose, J. S. Boyle, P. McWaters, J. Kelly, J. M. Tennent, A. M. Lew, and J. P. Scheerlinck. 1999. Targeting improves the efficacy of a DNA vaccine against Corynebacterium pseudotuberculosis in sheep. Infect. Immun. 67:6434-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chattergoon, M., J. Boyer, and D. B. Weiner. 1997. Genetic immunization: a new era in vaccines and immune therapeutics. FASEB J. 11:753-763. [DOI] [PubMed] [Google Scholar]
- 41.Chattergoon, M. A., J. J. Kim, J. S. Yang, T. M. Robinson, D. J. Lee, T. Dentchev, D. M. Wilson, V. Ayyavoo, and D. B. Weiner. 2000. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nat. Biotechnol. 18:974-979. [DOI] [PubMed] [Google Scholar]
- 42.Chattergoon, M. A., T. M. Robinson, J. D. Boyer, and D. B. Weiner. 1998. Specific immune induction following DNA-based immunization through in vivo transfection and activation of macrophages/antigen-presenting cells. J. Immunol. 160:5707-5718. [PubMed] [Google Scholar]
- 43.Chen, Z., T. Moyana, A. Saxena, R. Warrington, Z. Jia, and J. Xiang. 2001. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int. J. Cancer 93:539-548. [DOI] [PubMed] [Google Scholar]
- 44.Cohen, A. D., J. D. Boyer, and D. B. Weiner. 1998. Modulating the immune response to genetic immunization. FASEB J. 12:1611-1626. [PubMed] [Google Scholar]
- 45.Condon, C., S. C. Watkins, C. M. Celluzzi, K. Thompson, and L. D. Falo, Jr. 1996. DNA-based immunization by in vivo transfection of dendritic cells. Nat. Med. 2:1122-1128. [DOI] [PubMed] [Google Scholar]
- 46.Conry, R. M., A. F. LoBuglio, and D. T. Curiel. 1996. Polynucleotide-mediated immunization therapy of cancer. Semin. Oncol. 23:135-147. [PubMed] [Google Scholar]
- 47.Conry, R. M., A. F. LoBuglio, M. Wright, L. Sumerel, M. J. Pike, F. Johanning, R. Benjamin, D. Lu, and D. T. Curiel. 1995. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 55:1397-1400. [PubMed] [Google Scholar]
- 48.Corr, M., A. von Damm, D. J. Lee, and H. Tighe. 1999. In vivo priming by DNA injection occurs predominantly by antigen transfer. J. Immunol. 163:4721-4727. [PubMed] [Google Scholar]
- 49.Cunningham, C. K., D. W. Wara, M. Kang, T. Fenton, E. Hawkins, J. McNamara, L. Mofenson, A. M. Duliege, D. Francis, E. J. McFarland, and W. Borkowsky. 2001. Safety of 2 recombinant human immunodeficiency virus type 1 (HIV-1) envelope vaccines in neonates born to HIV-1-infected women. Clin. Infect. Dis. 32:801-807. [DOI] [PubMed] [Google Scholar]
- 50.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 51.Davis, H. L., R. G. Whalen, and B. A. Demeneix. 1993. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum. Gene Ther. 4:151-159. [DOI] [PubMed] [Google Scholar]
- 52.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deliyannis, G., J. S. Boyle, J. L. Brady, L. E. Brown, and A. M. Lew. 2000. A fusion DNA vaccine that targets antigen-presenting cells increases protection from viral challenge. Proc. Natl. Acad. Sci. USA 97:6676-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 55.Eisenbraun, M. D., D. H. Fuller, and J. R. Haynes. 1993. Examination of parameters affecting the elicitation of humoral immune responses by particle bombardment-mediated genetic immunization. DNA Cell Biol. 12:791-797. [DOI] [PubMed] [Google Scholar]
- 56.Etchart, N., R. Buckland, M. A. Liu, T. F. Wild, and D. Kaiserlian. 1997. Class I-restricted CTL induction by mucosal immunization with naked DNA encoding measles virus haemagglutinin. J. Gen. Virol. 78:1577-1580. [DOI] [PubMed] [Google Scholar]
- 57.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 58.Evans, T. G., M. C. Keefer, K. J. Weinhold, M. Wolff, D. Montefiori, G. J. Gorse, B. S. Graham, M. J. McElrath, M. L. Clements-Mann, M. J. Mulligan, P. Fast, M. C. Walker, J. L. Excler, A. M. Duliege, and J. Tartaglia. 1999. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp 120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J. Infect. Dis. 180:290-298. [DOI] [PubMed] [Google Scholar]
- 59.Falo, L. D., Jr., M. Kovacsovics-Bankowski, K. Thompson, and K. L. Rock. 1995. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat. Med. 1:649-653. [DOI] [PubMed] [Google Scholar]
- 60.Fan, H., Q. Lin, G. R. Morrissey, and P. A. Khavari. 1999. Immunization via hair follicles by topical application of naked DNA to normal skin. Nat. Biotechnol. 17:870-872. [DOI] [PubMed] [Google Scholar]
- 61.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 63.Finzi, D., and R. F. Siliciano. 1998. Viral dynamics in HIV-1 infection. Cell 93:665-671. [DOI] [PubMed] [Google Scholar]
- 64.Fouts, T., K. Godfrey, K. Bobb, D. Montefiori, C. V. Hanson, V. S. Kalyanaraman, A. DeVico, and R. Pal. 2002. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc. Natl. Acad. Sci. USA 99:11842-11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frankel, F. R., S. Hegde, J. Lieberman, and Y. Paterson. 1995. Induction of cell-mediated immune responses to human immunodeficiency virus type 1 Gag protein by using Listeria monocytogenes as a live vaccine vector. J. Immunol. 155:4775-47782. [PubMed] [Google Scholar]
- 66.Fu, T. M., J. B. Ulmer, M. J. Caulfield, R. R. Deck, A. Friedman, S. Wang, X. Liu, J. J. Donnelly, and M. A. Liu. 1997. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol. Med. 3:362-371. [PMC free article] [PubMed] [Google Scholar]
- 67.Fuller, D. H., and J. R. Haynes. 1994. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res. Hum. Retroviruses 10:1433-1441. [DOI] [PubMed] [Google Scholar]
- 68.Fynan, E. F., R. G. Webster, D. H. Fuller, J. R. Haynes, J. C. Santoro, and H. L. Robinson. 1993. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. USA 90:11478-11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garzino-Demo, A., A. L. DeVico, F. Cocchi, and R. C. Gallo. 1998. Beta-chemokines and protection from HIV type 1 disease. AIDS Res. Hum. Retroviruses 14(Suppl. 2):S177-S184. [PubMed] [Google Scholar]
- 70.Gaschen, B., J. Taylor, K. Yusim, B. Foley, F. Gao, D. Lang, V. Novitsky, B. Haynes, B. H. Hahn, T. Bhattacharya, and B. Korber. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354-2360. [DOI] [PubMed] [Google Scholar]
- 71.Georges-Courbot, M. C., C. Y. Lu, M. Makuwa, P. Telfer, R. Onanga, G. Dubreuil, Z. Chen, S. M. Smith, A. Georges, F. Gao, B. H. Hahn, and P. A. Marx. 1998. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J. Virol. 72:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilboa, E., S. K. Nair, and H. K. Lyerly. 1998. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol. Immunother. 46:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goulder, P. J., C. Brander, Y. Tang, C. Tremblay, R. A. Colbert, M. M. Addo, E. S. Rosenberg, T. Nguyen, R. Allen, A. Trocha, M. Altfeld, S. He, M. Bunce, R. Funkhouser, S. I. Pelton, S. K. Burchett, K. McIntosh, B. T. Korber, and B. D. Walker. 2001. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412:334-338. [DOI] [PubMed] [Google Scholar]
- 74.Goulder, P. J., S. L. Rowland-Jones, A. J. McMichael, and B. D. Walker. 1999. Anti-HIV cellular immunity: recent advances towards vaccine design. AIDS 13(Suppl. A):S121-S136. [PubMed] [Google Scholar]
- 75.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8+ T-cell response to human immunodeficiency virus type 1 gag and env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. Robust recall and long-term memory T-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing human immunodeficiency virus type 1 Gag and Env proteins. J. Virol. 76:7506-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haigwood, N. L., C. C. Pierce, M. N. Robertson, A. J. Watson, D. C. Montefiori, M. Rabin, J. B. Lynch, L. Kuller, J. Thompson, W. R. Morton, R. E. Benveniste, S. L. Hu, P. Greenberg, and S. P. Mossman. 1999. Protection from pathogenic SIV challenge using multigenic DNA vaccines. Immunol. Lett. 66:183-188. [DOI] [PubMed] [Google Scholar]
- 78.Hamajima, K., S. Sasaki, J. Fukushima, T. Kaneko, K. Q. Xin, I. Kudoh, and K. Okuda. 1998. Intranasal administration of HIV-DNA vaccine formulated with a polymer, carboxymethylcellulose, augments mucosal antibody production and cell-mediated immune response. Clin. Immunol. Immunopathol. 88:205-210. [DOI] [PubMed] [Google Scholar]
- 79.Hanke, T., and A. J. McMichael. 2000. Design and construction of an experimental HIV-1 vaccine for a year-2000 clinical trial in Kenya. Nat. Med. 6:951-955. [DOI] [PubMed] [Google Scholar]
- 80.Harding, C. V., and R. Song. 1994. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J. Immunol. 153:4925-4933. [PubMed] [Google Scholar]
- 81.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 82.Heller, L. C., and D. Coppola. 2002. Electrically mediated delivery of vector plasmid DNA elicits an antitumor effect. Gene Ther. 9:1321-1325. [DOI] [PubMed] [Google Scholar]
- 83.Heller, R., M. J. Jaroszeski, L. F. Glass, J. L. Messina, D. P. Rapaport, R. C. DeConti, N. A. Fenske, R. A. Gilbert, L. M. Mir, and D. S. Reintgen. 1996. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer 77:964-971. [DOI] [PubMed] [Google Scholar]
- 84.Hengge, U. R., E. F. Chan, R. A. Foster, P. S. Walker, and J. C. Vogel. 1995. Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat. Genet. 10:161-166. [DOI] [PubMed] [Google Scholar]
- 85.Herweijer, H., J. S. Latendresse, P. Williams, G. Zhang, I. Danko, S. Schlesinger, and J. A. Wolff. 1995. A plasmid-based self-amplifying Sindbis virus vector. Hum Gene Ther. 6:1161-1167. [DOI] [PubMed] [Google Scholar]
- 86.Hirsch, V. M., and P. R. Johnson. 1994. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 32:183-203. [DOI] [PubMed] [Google Scholar]
- 87.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 88.Hocart, M. J., J. S. Mackenzie, and G. A. Stewart. 1989. The IgG subclass responses to influenza virus haemagglutinin in the mouse: effect of route of inoculation. J. Gen. Virol. 70:809-818. [DOI] [PubMed] [Google Scholar]
- 89.Hofmann-Lehmann, R., J. Vlasak, A. L. Williams, A. L. Chenine, H. M. McClure, D. C. Anderson, S. O'Neil, and R. M. Ruprecht. 2003. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS 17:157-166. [DOI] [PubMed] [Google Scholar]
- 90.Huang, A. Y., A. T. Bruce, D. M. Pardoll, and H. I. Levitsky. 1996. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity 4:349-355. [DOI] [PubMed] [Google Scholar]
- 91.Huang, Y., W. P. Kong, and G. J. Nabel. 2001. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J. Virol. 75:4947-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Igarashi, T., R. Shibata, F. Hasebe, Y. Ami, K. Shinohara, T. Komatsu, C. Stahl-Hennig, H. Petry, G. Hunsmann, T. Kuwata, et al. 1994. Persistent infection with SIVmac chimeric virus having tat, rev, vpu, env and nef of HIV type 1 in macaque monkeys. AIDS Res. Hum. Retroviruses 10:1021-1029. [DOI] [PubMed] [Google Scholar]
- 93.Johnston, R. 2003. AIDSVAX results: an answer, or just more questions? AIDS Patient Care STDS 17:47-51. [DOI] [PubMed] [Google Scholar]
- 94.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, K. A. Reimann, M. K. Axthelm, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 71:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kennedy, R. C. 1997. DNA vaccination for HIV. Nat. Med. 3:501-502. [DOI] [PubMed] [Google Scholar]
- 96.Kim, J. J., V. Ayyavoo, M. L. Bagarazzi, M. A. Chattergoon, K. Dang, B. Wang, J. D. Boyer, and D. B. Weiner. 1997. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J. Immunol. 158:816-826. [PubMed] [Google Scholar]
- 97.Kim, J. J., M. L. Bagarazzi, N. Trivedi, Y. Hu, K. Kazahaya, D. M. Wilson, R. Ciccarelli, M. A. Chattergoon, K. Dang, S. Mahalingam, A. A. Chalian, M. G. Agadjanyan, J. D. Boyer, B. Wang, and D. B. Weiner. 1997. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat. Biotechnol. 15:641-646. [DOI] [PubMed] [Google Scholar]
- 98.Kim, J. J., L. K. Nottingham, J. I. Sin, A. Tsai, L. Morrison, J. Oh, K. Dang, Y. Hu, K. Kazahaya, M. Bennett, T. Dentchev, D. M. Wilson, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. CD8-positive T cells influence antigen-specific immune responses through the expression of chemokines. J. Clin. Investig. 102:1112-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim, J. J., L. K. Nottingham, D. M. Wilson, M. L. Bagarazzi, A. Tsai, L. D. Morrison, A. Javadian, A. A. Chalian, M. G. Agadjanyan, and D. B. Weiner. 1998. Engineering DNA vaccines via co-delivery of co-stimulatory molecule genes. Vaccine 16:1828-1835. [DOI] [PubMed] [Google Scholar]
- 100.Kim, J. J., N. N. Trivedi, L. K. Nottingham, L. Morrison, A. Tsai, Y. Hu, S. Mahalingam, K. Dang, L. Ahn, N. K. Doyle, D. M. Wilson, M. A. Chattergoon, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur. J. Immunol. 28:1089-1103. [DOI] [PubMed] [Google Scholar]
- 101.Kim, J. J., A. Tsai, L. K. Nottingham, L. Morrison, D. M. Cunning, J. Oh, D. J. Lee, K. Dang, T. Dentchev, A. A. Chalian, M. G. Agadjanyan, and D. B. Weiner. 1999. Intracellular adhesion molecule-1 modulates beta-chemokines and directly costimulates T cells in vivo. J. Clin. Investig. 103:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim, J. J., J. S. Yang, T. C. VanCott, D. J. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2000. Modulation of antigen-specific humoral responses in rhesus macaques by using cytokine cDNAs as DNA vaccine adjuvants. J. Virol. 74:3427-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kjerrstrom, A., J. Hinkula, G. Engstrom, V. Ovod, K. Krohn, R. Benthin, and B. Wahren. 2001. Interactions of single and combined human immunodeficiency virus type 1 (HIV-1) DNA vaccines. Virology 284:46-61. [DOI] [PubMed] [Google Scholar]
- 104.Klavinskis, L. S., C. Barnfield, L. Gao, and S. Parker. 1999. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J. Immunol. 162:254-262. [PubMed] [Google Scholar]
- 105.Korber, B., B. Gaschen, K. Yusim, R. Thakallapally, C. Kesmir, and V. Detours. 2001. Evolutionary and immunological implications of contemporary HIV-1 variation. Br. Med. Bull. 58:19-42. [DOI] [PubMed] [Google Scholar]
- 106.Kuklin, N., M. Daheshia, K. Karem, E. Manickan, and B. T. Rouse. 1997. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J. Virol. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kwong, P. D., M. L. Doyle, D. L. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 108.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Learmont, J., B. Tindall, L. Evans, A. Cunningham, P. Cunningham, J. Wells, R. Penny, J. Kaldor, and D. A. Cooper. 1992. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet 340:863-867. [DOI] [PubMed] [Google Scholar]
- 110.Learmont, J. C., A. F. Geezy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 111.Leitner, W. W., L. N. Hwang, M. J. DeVeer, A. Zhou, R. H. Silverman, B. R. Williams, T. W. Dubensky, H. Ying, and N. P. Restifo. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 9:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Letvin, N. L., D. C. Montefiori, Y. Yasutomi, H. C. Perry, M. E. Davies, C. Lekutis, M. Alroy, D. C. Freed, C. I. Lord, L. K. Handt, M. A. Liu, and J. W. Shiver. 1997. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 94:9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levine, A. M., S. Groshen, J. Allen, K. M. Munson, D. J. Carlo, A. E. Daigle, F. Ferre, F. C. Jensen, S. P. Richieri, R. J. Trauger, J. W. Parker, P. L. Salk, and J. Salk. 1996. Initial studies on active immunization of HIV-infected subjects using a gp120-depleted HIV-1 Immunogen: long-term follow-up. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11:351-364. [DOI] [PubMed] [Google Scholar]
- 114.Levy, J. A., C. E. Mackewicz, and E. Barker. 1996. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today 17:217-224. [DOI] [PubMed] [Google Scholar]
- 115.Li, J., C. I. Lord, W. Haseltine, N. L. Letvin, and J. Sodroski. 1992. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J. Acquir. Immune Defic. Syndr. 5:639-646. [PubMed] [Google Scholar]
- 116.Li, J. T., M. Halloran, C. I. Lord, A. Watson, J. Ranchalis, M. Fung, N. L. Letvin, and J. G. Sodroski. 1995. Persistent infection of macaques with simian-human immunodeficiency viruses. J. Virol. 69:7061-7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lieberman, J., and F. R. Frankel. 2002. Engineered Listeria monocytogenes as an AIDS vaccine. Vaccine 20:2007-2010. [DOI] [PubMed] [Google Scholar]
- 118.Liu, X. L., K. R. Clark, and P. R. Johnson. 1999. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene Ther. 6:293-299. [DOI] [PubMed] [Google Scholar]
- 119.Lohr, F., D. Y. Lo, D. A. Zaharoff, K. Hu, X. Zhang, Y. Li, Y. Zhao, M. W. Dewhirst, F. Yuan, and C. Y. Li. 2001. Effective tumor therapy with plasmid-encoded cytokines combined with in vivo electroporation. Cancer Res 61:3281-3284. [PubMed] [Google Scholar]
- 120.Loirat, D., Z. Li, M. Mancini, P. Tiollais, D. Paulin, and M. L. Michel. 1999. Muscle-specific expression of hepatitis B surface antigen: no effect on DNA-raised immune responses. Virology 260:74-83. [DOI] [PubMed] [Google Scholar]
- 121.Lu, S., J. Arthos, D. C. Montefiori, Y. Yasutomi, K. Manson, F. Mustafa, E. Johnson, J. C. Santoro, J. Wissink, J. I. Mullins, J. R. Haynes, N. L. Letvin, M. Wyand, and H. L. Robinson. 1996. Simian immunodeficiency virus DNA vaccine trial in macaques. J. Virol. 70:3978-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu, S., J. C. Santoro, D. H. Fuller, J. R. Haynes, and H. L. Robinson. 1995. Use of DNAs expressing HIV-1 Env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology 209:147-154. [DOI] [PubMed] [Google Scholar]
- 123.Lu, Y., K. Q. Xin, K. Hamajima, T. Tsuji, I. Aoki, J. Yang, S. Sasaki, J. Fukushima, T. Yoshimura, S. Toda, E. Okada, and K. Okuda. 1999. Macrophage inflammatory protein-1 alpha (MIP-1α) expression plasmid enhances DNA vaccine-induced immune response against HIV-1. Clin. Exp. Immunol. 115:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luciw, P. A., E. Pratt-Lowe, K. E. Shaw, J. A. Levy, and C. Cheng-Mayer. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. USA 92:7490-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.MacGregor, R. R., J. D. Boyer, K. E. Ugen, K. E. Lacy, S. J. Gluckman, M. L. Bagarazzi, M. A. Chattergoon, Y. Baine, T. J. Higgins, R. B. Ciccarelli, L. R. Coney, R. S. Ginsberg, and D. B. Weiner. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 178:92-100. [DOI] [PubMed] [Google Scholar]
- 126.MacGregor, R. R., R. Ginsberg, K. E. Ugen, Y. Baine, C. U. Kang, X. M. Tu, T. Higgins, D. B. Weiner, and J. D. Boyer. 2002. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. AIDS 16:2137-2143. [DOI] [PubMed] [Google Scholar]
- 127.Maeurer, M. J., P. Trinder, G. Hommel, W. Walter, K. Freitag, D. Atkins, and S. Storkel. 2000. Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect. Immun. 68:2962-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marques, E. T., Jr., P. Chikhlikar, L. B. De Arruda, I. C. Leao, Y. Lu, J. Wong, J. S. Chen, B. Byrne, and J. T. August. 2003. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 (LAMP-1) as a DNA plasmid vaccine chimera is highly expressed, traffics to MHC II compartment, and elicits enhanced immune responses. J. Biol. Chem. 278:37926-37936. (First published 24 June 2003; 10.1074/jbc.M303336200.) [DOI] [PubMed] [Google Scholar]
- 129.Martin, T., S. E. Parker, R. Hedstrom, T. Le, S. L. Hoffman, J. Norman, P. Hobart, and D. Lew. 1999. Plasmid DNA malaria vaccine: the potential for genomic integration after intramuscular injection. Hum. Gene Ther. 10:759-768. [DOI] [PubMed] [Google Scholar]
- 130.Mata, M., and Y. Paterson. 1999. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogenous listerial antigens. J. Immunol. 163:1449-1456. [PubMed] [Google Scholar]
- 131.Mata, M., Z. J. Yao, A. Zubair, K. Syres, and Y. Paterson. 2001. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine 19:1435-1445. [DOI] [PubMed] [Google Scholar]
- 132.McCluskie, M. J., C. L. Brazolot Millan, R. A. Gramzinski, H. L. Robinson, J. C. Santoro, J. T. Fuller, G. Widera, J. R. Haynes, R. H. Purcell, and H. L. Davis. 1999. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 5:287-300. [PMC free article] [PubMed] [Google Scholar]
- 133.McCune, J. M. 1997. Animal models of HIV-1 disease. Science 278:2141-2142. [DOI] [PubMed] [Google Scholar]
- 134.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 135.McCutchan, F. E. 2000. Understanding the genetic diversity of HIV-1. AIDS 14(Suppl. 3):S31-S44. [PubMed] [Google Scholar]
- 136.McDonnell, W. M., and F. K. Askari. 1996. DNA vaccines. N. Engl. J. Med. 334:42-45. [DOI] [PubMed] [Google Scholar]
- 137.McGaughey, G. B., M. Citron, R. C. Danzeisen, F. R. M., V. M. Garsky, W. M. Hurni, J. G. Joyce, X. Liang, M. Miller, J. Shiver, and M. J. Bogusky. 2003. HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemistry 25:3214-3223. [DOI] [PubMed] [Google Scholar]
- 138.Mir, L. M., P. Devauchelle, F. Quintin-Colonna, F. Delisle, S. Doliger, D. Fradelizi, J. Belehradek, Jr., and S. Orlowski. 1997. First clinical trial of cat soft-tissue sarcomas treatment by electrochemotherapy. Br. J. Cancer 76:1617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mosier, D. E., R. J. Gulizia, S. M. Baird, D. B. Wilson, D. H. Spector, and S. A. Spector. 1991. Human immunodeficiency virus infection of human-PBL-SCID mice. Science 251:791-794. [DOI] [PubMed] [Google Scholar]
- 140.Murphey-Corb, M., L. N. Martin, B. Davison-Fairburn, R. C. Montelaro, M. Miller, M. West, S. Ohkawa, G. B. Baskin, J. Y. Zhang, S. D. Putney, et al. 1989. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science 246:1293-1297. [DOI] [PubMed] [Google Scholar]
- 141.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muthumani, K., M. Bagarazzi, D. Conway, D. S. Hwang, K. Manson, R. Ciccarelli, Z. Israel, D. C. Montefiori, K. Ugen, N. Miller, J. Kim, J. Boyer, and D. B. Weiner. 2003. A Gag-Pol/Env-Rev SIV239 DNA vaccine improves CD4 counts, and reduce viral loads after pathogenic intrarectal SIV(mac)251 challenge in rhesus macaques. Vaccine 21:629-637. [DOI] [PubMed] [Google Scholar]
- 143.Myszka, D. G., R. W. Sweet, P. Hensley, M. Brigham-Burke, P. D. Kwong, W. A. Hendrickson, R. Wyatt, J. Sodroski, and M. L. Doyle. 2000. Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. USA 97:9026-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nacsa, J., J. Stanton, K. J. Kunstman, W. P. Tsai, D. I. Watkins, S. M. Wolinsky, and G. Franchini. 2003. Emergence of cytotoxic T lymphocyte escape mutants following antiretroviral treatment suspension in rhesus macaques infected with SIVmac251. Virology 305:210-218. [DOI] [PubMed] [Google Scholar]
- 145.Nair, S. K., D. Boczkowski, M. Morse, R. I. Cumming, H. K. Lyerly, and E. Gilboa. 1998. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat. Biotechnol. 16:364-369. [DOI] [PubMed] [Google Scholar]
- 146.Newberg, M. H., M. J. Kuroda, W. A. Charini, A. Miura, C. I. Lord, J. E. Schmitz, D. A. Gorgone, M. A. Lifton, K. Kuus-Reichel, and N. L. Letvin. 2002. A simian immunodeficiency virus nef peptide is a dominant cytotoxic T lymphocyte epitope in Indian-origin rhesus monkeys expressing the common MHC class I allele mamu-A*02. Virology 301:365-373. [DOI] [PubMed] [Google Scholar]
- 147.Nichols, W. W., B. J. Ledwith, S. V. Manam, and P. J. Troilo. 1995. Potential DNA vaccine integration into host cell genome. Ann. N. Y. Acad. Sci. 772:30-39. [DOI] [PubMed] [Google Scholar]
- 148.Novembre, F. J., J. de Rosayro, S. Nidtha, S. P. O'Neil, T. R. Gibson, T. Evans-Strickfaden, C. E. Hart, and H. M. McClure. 2001. Rapid CD4(+) T-cell loss induced by human immunodeficiency virus type 1(NC) in uninfected and previously infected chimpanzees. J. Virol. 75:1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Novembre, F. J., M. Saucier, D. C. Anderson, S. A. Klumpp, S. P. O'Neil, C. R. Brown, Jr., C. E. Hart, P. C. Guenthner, R. B. Swenson, and H. M. McClure. 1997. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J. Virol. 71:4086-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Okada, E., S. Sasaki, N. Ishii, I. Aoki, T. Yasuda, K. Nishioka, J. Fukushima, J. Miyazaki, B. Wahren, and K. Okuda. 1997. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J. Immunol. 159:3638-3647. [PubMed] [Google Scholar]
- 151.Otten, G., M. Schaefer, C. Greer, M. Calderon-Cacia, D. Coit, J. Kazzaz, A. Medina-Selby, M. Selby, M. Singh, M. Ugozzoli, J. zur Megede, S. W. Barnett, D. O'Hagan, J. Donnelly, and J. Ulmer. 2003. Induction of broad and potent anti-human immunodeficiency virus immune responses in rhesus macaques by priming with a DNA vaccine and boosting with protein-adsorbed polylactide coglycolide microparticles. J. Virol. 77:6087-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pardoll, D. M., and A. M. Beckerleg. 1995. Exposing the immunology of naked DNA vaccines. Immunity 3:165-169. [DOI] [PubMed] [Google Scholar]
- 153.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau, 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl A):S137-S162. [PubMed] [Google Scholar]
- 154.Pertmer, T. M., M. D. Eisenbraun, D. McCabe, S. K. Prayaga, D. H. Fuller, and J. R. Haynes. 1995. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine 13:1427-1430. [DOI] [PubMed] [Google Scholar]
- 155.Pontesilli, O., M. R. Klein, S. R. Kerkhof-Garde, N. G. Pakker, F. de Wolf, H. Schuitemaker, and F. Miedema. 1998. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J. Infect. Dis 178:1008-1018. [DOI] [PubMed] [Google Scholar]
- 156.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 157.Porgador, A., K. R. Irvine, A. Iwasaki, B. H. Barber, N. P. Restifo, and R. N. Germain. 1998. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 188:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Preston, B. D. 1997. Reverse transcriptase fidelity and HIV-1 variation. Science 275:228-229. [DOI] [PubMed] [Google Scholar]
- 159.Putkonen, P., M. Quesada-Rolander, A. C. Leandersson, S. Schwartz, R. Thorstensson, K. Okuda, B. Wahren, and J. Hinkula. 1998. Immune responses but no protection against SHIV by gene-gun delivery of HIV-1 DNA followed by recombinant subunit protein boosts. Virology 250:293-301. [DOI] [PubMed] [Google Scholar]
- 160.Putkonen, P., K. Warstedt, R. Thorstensson, R. Benthin, J. Albert, B. Lundgren, B. Oberg, E. Norrby, and G. Biberfeld. 1989. Experimental infection of cynomolgus monkeys (Macaca fascicularis) with simian immunodeficiency virus (SIVsm). J. Acquir. Immune Defic. Syndr. 2:359-365. [PubMed] [Google Scholar]
- 161.Raz, E., D. A. Carson, S. E. Parker, T. B. Parr, A. M. Abai, G. Aichinger, S. H. Gromkowski, M. Singh, D. Lew, M. A. Yankauckas, et al. 1994. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc. Natl. Acad. Sci. USA 91:9519-9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Redfield, R. R., D. C. Wright, W. D. James, T. S. Jones, C. Brown, and D. S. Burke. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316:673-676. [DOI] [PubMed] [Google Scholar]
- 163.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Robinson, H. L. 2002. New hope for an AIDS vaccine. Nat. Rev. Immunol. 2:239-250. [DOI] [PubMed] [Google Scholar]
- 165.Robinson, H. L., L. A. Hunt, and R. G. Webster. 1993. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine 11:957-960. [DOI] [PubMed] [Google Scholar]
- 166.Rodriguez-Cuevas, S., S. Barroso-Bravo, J. Almanza-Estrada, L. Cristobal-Martinez, and E. Gonzalez-Rodriguez. 2001. Electrochemotherapy in primary and metastatic skin tumors: phase II trial using intralesional bleomycin. Arch. Med. Res. 32:273-276. [DOI] [PubMed] [Google Scholar]
- 167.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 168.Sasaki, S., R. R. Amara, A. E. Oran, J. M. Smith, and H. L. Robinson. 2001. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat. Biotechnol. 19:543-547. [DOI] [PubMed] [Google Scholar]
- 169.Sauter, B., M. L. Albert, L. Francisco, M. Larsson, S. Somersan, and N. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 171.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shata, M. T., M. S. Reitz, Jr., A. L. DeVico, G. K. Lewis, and D. M. Hone. 2001. Mucosal and systemic HIV-1 Env-specific CD8(+) T-cells develop after intragastric vaccination with a Salmonella Env DNA vaccine vector. Vaccine 20:623-629. [DOI] [PubMed] [Google Scholar]
- 173.Shen, L., Z. W. Chen, M. D. Miller, V. Stallard, G. P. Mazzara, D. L. Panicali, and N. L. Letvin. 1991. Recombinant virus vaccine-induced SIV-specific CD8+ cytotoxic T lymphocytes. Science 252:440-443. [DOI] [PubMed] [Google Scholar]
- 174.Shiver, J. W., M. E. Davies, H. C. Perry, D. C. Freed, and M. A. Liu. 1996. Humoral and cellular immunities elicited by HIV-1 vaccination. J. Pharm. Sci. 85:1317-1324. [DOI] [PubMed] [Google Scholar]
- 175.Shiver, J. W., M. E. Davies, Y. Yasutomi, H. C. Perry, D. C. Freed, N. L. Letvin, and M. A. Liu. 1997. Anti-HIV env immunities elicited by nucleic acid vaccines. Vaccine 15:884-887. [DOI] [PubMed] [Google Scholar]
- 176.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 177.Sin, J. I., M. Bagarazzi, C. Pachuk, and D. B. Weiner. 1999. DNA priming-protein boosting enhances both antigen-specific antibody and Th1-type cellular immune responses in a murine herpes simplex virus-2 gD vaccine model. DNA Cell Biol. 18:771-779. [DOI] [PubMed] [Google Scholar]
- 178.Sin, J. I., J. J. Kim, R. L. Arnold, K. E. Shroff, D. McCallus, C. Pachuk, S. P. McElhiney, M. W. Wolf, S. J. Pompa-de Bruin, T. J. Higgins, R. B. Ciccarelli, and D. B. Weiner. 1999. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 162:2912-2921. [PubMed] [Google Scholar]
- 179.Sin, J. I., J. J. Kim, J. D. Boyer, R. B. Ciccarelli, T. J. Higgins, and D. B. Weiner. 1999. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J. Virol. 73:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sin, J. I., J. J. Kim, D. Zhang, and D. B. Weiner. 2001. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum. Gene Ther. 12:1091-1102. [DOI] [PubMed] [Google Scholar]
- 181.Singh, M., M. Briones, G. Ott, and D. O'Hagan. 2000. Cationic microparticles: a potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 97:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Srivastava, P. K., H. Udono, N. E. Blachere, and Z. Li. 1994. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics 39:93-98. [DOI] [PubMed] [Google Scholar]
- 183.Staerz, U. D., H. Karasuyama, and A. M. Garner. 1987. Cytotoxic T lymphocytes against a soluble protein. Nature 329:449-451. [DOI] [PubMed] [Google Scholar]
- 184.Stott, E. J. 1991. Anti-cell antibody in macaques. Nature 353:393. [DOI] [PubMed] [Google Scholar]
- 185.Takahashi, H., Y. Nakagawa, C. D. Pendleton, R. A. Houghten, K. Yokomuro, R. N. Germain, and J. A. Berzofsky. 1992. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science 255:333-336. [DOI] [PubMed] [Google Scholar]
- 186.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 187.Titomirov, A. V., S. Sukharev, and E. Kistanova. 1991. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta 1088:131-134. [DOI] [PubMed] [Google Scholar]
- 188.Tollefsen, S., T. Tjelle, J. Schneider, M. Harboe, H. Wiker, G. Hewinson, K. Huygen, and I. Mathiesen. 2002. Improved cellular and humoral immune responses against Mycobacterium tuberculosis antigens after intramuscular DNA immunisation combined with muscle electroporation. Vaccine 20:3370-3378. [DOI] [PubMed] [Google Scholar]
- 189.Torres, C. A., A. Iwasaki, B. H. Barber, and H. L. Robinson. 1997. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J. Immunol. 158:4529-4532. [PubMed] [Google Scholar]
- 190.Udono, H., and P. K. Srivastava. 1993. Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med. 178:1391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ulmer, J. B., R. R. Deck, C. M. Dewitt, J. I. Donnhly, and M. A. Liu. 1996. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology 89:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, et al. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 193.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626-633. [DOI] [PubMed] [Google Scholar]
- 194.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343-346. [DOI] [PubMed] [Google Scholar]
- 195.Wang, B., J. Boyer, V. Srikantan, L. Coney, R. Carrano, C. Phan, M. Merva, K. Dang, M. Agadjanan, L. Gilbert, et al. 1993. DNA inoculation induces neutralizing immune responses against human immunodeficiency virus type 1 in mice and nonhuman primates. DNA Cell Biol. 12:799-805. [DOI] [PubMed] [Google Scholar]
- 196.Wang, B., J. Boyer, V. Srikantan, K. Ugen, L. Gilbert, C. Phan, K. Dang, M. Merva, M. G. Agadjanyan, M. Newman, et al. 1995. Induction of humoral and cellular immune responses to the human immunodeficiency type 1 virus in nonhuman primates by in vivo DNA inoculation. Virology 211:102-112. [DOI] [PubMed] [Google Scholar]
- 197.Wang, B., K. Dang, M. G. Agadjanyan, V. Srikantan, F. Li, K. E. Ugen, J. Boyer, M. Merva, W. V. Williams, and D. B. Weiner. 1997. Mucosal immunization with a DNA vaccine induces immune responses against HIV-1 at a mucosal site. Vaccine 15:821-825. [DOI] [PubMed] [Google Scholar]
- 198.Wang, B., K. E. Ugen, V. Srikantan, M. G. Agadjanyan, K. Dang, Y. Refaeli, A. I. Sato, J. Boyer, W. V. Williams, and D. B. Weiner. 1993. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 90:4156-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 200.Watanabe, M. E. 2003. Skeptical scientists skewer VaxGen statistics. Nat. Med. 9:376. [DOI] [PubMed] [Google Scholar]
- 201.Weissman, D., H. Ni, D. Scales, A. Dude, J. Capodici, K. McGibney, A. Abdool, S. N. Isaacs, G. Cannon, and K. Kariko. 2000. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J. Immunol. 165:4710-4717. [DOI] [PubMed] [Google Scholar]
- 202.Whitmire, J. K., and R. Ahmed. 2000. Costimulation in antiviral immunity: differential requirements for CD4+ and CD8+ T cell responses. Curr. Opin. Immunol. 12:448-455. [DOI] [PubMed] [Google Scholar]
- 203.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164:4635-4640. [DOI] [PubMed] [Google Scholar]
- 204.Wolff, J. A., J. J. Ludtke, G. Acsadi, P. Williams, and A. Jani. 1992. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1:363-369. [DOI] [PubMed] [Google Scholar]
- 205.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Aesadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 206.Xiang, S. H., P. D. Kwong, R. Gupta, C. D. Rizzuto, D. J. Casper, R. Wyatt, L. Wang, W. A. Hendrickson, M. L. Doyle, and J. Sodroski. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct confromation of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76:9888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xiang, Z., and H. C. Ertl. 1995. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity 2:129-135. [DOI] [PubMed] [Google Scholar]
- 208.Xin, K. Q., K. Hamajima, S. Sasaki, A. Honsho, T. Tsuji, N. Ishii, X. R. Cao, Y. Lu, J. Fukushima, P. Shapshak, S. Kawamoto, and K. Okuda. 1998. Intranasal administration of human immunodeficiency virus type-1 (HIV-1) DNA vaccine with interleukin-2 expression plasmid enhances cell-mediated immunity against HIV-1. Immunology 94:438-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xin, K. Q., K. Hamajima, S. Sasaki, T. Tsuji, S. Watabe, E. Okada, and K. Okuda. 1999. IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine 17:858-866. [DOI] [PubMed] [Google Scholar]
- 210.Xin, K. Q., Y. Lu, K. Hamajima, J. Fukushima, J. Yang, K. Inamura, and K. Okuda. 1999. Immunization of RANTES expression plasmid with a DNA vaccine enhances HIV-1-specific immunity. Clin. Immunol. 92:90-96. [DOI] [PubMed] [Google Scholar]
- 211.Xu, F., M. Hong, and J. B. Ulmer. 2003. Immunogenicity of an HIV-1 gag DNA vaccine carried by attenuated Shigella. Vaccine 21:644-648. [DOI] [PubMed] [Google Scholar]
- 212.Yajima, T., H. Nishimura, R. Ishimitsu, T. Watase, D. H. Busch, E. G. Pamer, H. Kuwano, and Y. Yoshikai. 2002. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J. Immunol. 168:1198-1203. [DOI] [PubMed] [Google Scholar]
- 213.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yasutomi, Y., S. Koenig, S. S. Haun, C. K. Stover, R. K. Jackson, P. Conard, A. J. Conley, E. A. Emini, T. R. Fuerst, and N. L. Letvin. 1993. Immunization with recombinant BCG-SIV elicits SIV-specific cytotoxic T lymphocytes in rhesus monkeys. J. Immunol. 150:3101-3107. [PubMed] [Google Scholar]
- 215.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591-599. [DOI] [PubMed] [Google Scholar]
- 216.Zhang, Z. Q., T. M. Fu, D. R. Casimiro, M. E. Davies, X. Liang, W. A. Schleif, L. Handt, L. Tussey, M. Chen, A. Tang, K. A. Wilson, W. L. Trigona, D. C. Freed, C. Y. Tan, M. Horton, E. A. Emini, and J. W. Shiver. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76:12845-12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhong, L., A. Granelli-Piperno, Y. Choi, and R. M. Steinman. 1999. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 29:964-972. [DOI] [PubMed] [Google Scholar]
- 218.Zhou, X., P. Berglund, G. Rhodes, S. E. Parker, M. Jondal, and P. Liljestrom. 1994. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine 12:1510-1514. [DOI] [PubMed] [Google Scholar]
- 219.zur Megede, J., M. C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett. 2000. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J. Virol. 74:2628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]