Abstract
Purpose
To determine the cytokine response to ocular lysates of peripheral blood mononuclear cells (PBMCs) from patients with birdshot chorioretinopathy (BSCR).
Methods
In the PBMCs of 19 patients with BSCR, T cell cytokine production in response to human retina and choroid lysates was analyzed with flow cytometry and compared to the responses against skin lysates. Five patients had active disease and had not yet been treated (naïve to systemic therapy); 14 patients had either immunomodulatory therapy (IMT) or inactive disease (referred as inactive/IMT). The PBMCs of 11 HLA-A29-positive healthy individuals were used as controls.
Results
The levels of interleukin-17 (IL-17) in supernatant of cultures stimulated with retina lysate were higher in patients with active BSCR compared to the HLA-A29 positive controls. The levels of other T cell cytokines (IL-10 and interferon-γ [IFN-γ]) in PBMC cultures did not change significantly after stimulation with ocular lysate. The frequency of CD4+ IL-17+ (T helper 17 [Th17]) T cells but not of CD4+ IFN-γ (Th1) T cells was elevated in the PBMCs of patients with active BSCR stimulated by retina lysates compared to skin lysates.
Conclusions
Our data demonstrate that PBMCs exhibit an IL-17-mediated immune response to retina lysate in patients with active disease naïve to systemic therapy. This is accompanied by the enrichment of IL-17-producing CD4+ T cells. These findings support the current concept of chronic Th17-cell mediated inflammation and provide evidence that links the Th17 signatures to ocular-specific immune responses in BSCR.
Introduction
Birdshot chorioretinopathy (BSCR) is an organ-specific and potentially blinding chronic inflammation of the posterior eye segment [1]. Disease hallmarks manifest as distinct multiple hypopigmented chorioretinal lesions [2]. BSCR affects predominantly middle-aged and elderly individuals of European descent [2] who almost all, if not all, carry the HLA-A29 allele [3,4]. The pathogenesis of the disease is poorly understood. The increased peripheral immune response to the retinal S-antigen [3] and the presence of T cell infiltration of the chorioretinal lesions [5,6] support the current view of T cell–mediated autoimmune inflammation in BSCR. T helper 17 (Th17) cells, a subset of CD4+ T lymphocytes that typically produce interleukin (IL)-17, play an important role in the pathogenesis of noninfectious uveitis and autoimmune diseases [7-10]. Previously, we reported on elevated levels of IL-17 in the aqueous humor of patients with BSCR [11], indicating a role for Th17 cells in BSCR pathogenesis. Yang and associates [12] recently showed elevated serum levels of immune mediators that promote Th17 responses in patients with active BSCR. To better understand the potential role of Th17 cells in BSCR, we investigated the cytokine secretion profile in response to human ocular lysate in the peripheral blood of patients with BSCR and healthy controls.
Methods
Subjects
This case-control study included 19 patients with BSCR, diagnosed based on research criteria established by an international consensus conference [13]. All patients were HLA-A29 positive and had bilateral chronic uveitis with the characteristic hypopigmented chorioretinal lesions and associated vitritis. Whole blood was drawn into Vacutainer tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque density centrifugation and cryopreserved. The isolation conditions were identical for all blood samples. Our group consisted of five patients with active disease that were naïve to systemic therapy and 14 patients with inactive disease and/or a current or past history (<3 months) of immunomodulatory therapy (see Table 1 for more information). Peripheral blood mononuclear cells (PBMCs) from 11 HLA-A29 positive anonymous donors who had no history of intraocular inflammation and/or vitreoretinal disease were isolated from buffy coats obtained from the national blood bank (Sanquin, Amsterdam, the Netherlands). Informed consent for the post-mortem donation of ocular tissue was received before the authors acquired the tissue under the auspices of the head of the department of Anatomy, University Medical Center Utrecht, the Netherlands. All tissues were acquired in compliance with Dutch law (“Wet op de lijkbezorging,” Art 18, lid 1/ geldigheidsdatum_18–06–2013) and the institutional guidelines of the University Medical Center Utrecht. Blood samples from patients were collected after written informed consent and were obtained in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of the University Medical Centre Utrecht, the Netherlands.
Table 1. Demographic information of patients in this study.
|
Patient |
Age |
Gender |
Year of Diagnosis |
Disease status |
Prior Treatment |
Current
Treatment |
Cataract or aphakic/ pseudophakic |
Comorbidity |
|
|---|---|---|---|---|---|---|---|---|---|
| Patients with active disease naïve to immunomodulatory therapy | |||||||||
| 1 |
63 |
f |
2011 |
active |
- |
- |
- |
||
| 2 |
68 |
f |
2009 |
active |
- |
- |
- |
hypothyroidism |
|
| 3 |
54 |
f |
2011 |
active |
- |
- |
- |
||
| 4 |
74 |
m |
2009 |
active |
- |
- |
- |
Diabetes |
|
| 5 |
57 |
f |
2011 |
active |
- |
- |
- |
||
| Patients with inactive disease and/or current or past history (<6 months) of immunomodulatory therapy | |||||||||
| 6 |
68 |
f |
2004 |
inactive |
Corticosteroids
Methotrexate |
Methotrexate |
+ |
||
| 7 |
63 |
f |
2000 |
inactive |
Corticosteroids
Methotrexate |
Methotrexate |
+ |
hypothyroidism |
|
| 8 |
56 |
f |
2006 |
active |
Corticosteroids
Methotrexate |
Mycophenolate mofetil |
- |
hypothyroidism |
|
| 9 |
61 |
f |
1994 |
active |
Corticosteroids
Methotrexate
Cyclosporine |
Mycophenolate mofetil |
+ |
||
| 10 |
52 |
f |
2008 |
inactive |
Methotrexate |
Methotrexate |
+ |
||
| 11 |
61 |
m |
2010 |
inactive |
Methotrexate |
Methotrexate |
- |
||
| 12 |
71 |
f |
1993 |
inactive |
Corticosteroids
Methotrexate |
Methotrexate |
+ |
||
| 13 |
53 |
f |
2000 |
inactive |
Methotrexate |
- |
+ |
||
| 14 |
68 |
f |
2004 |
inactive |
Corticosteroids
Methotrexate
azathioprine |
Methotrexate |
+ |
||
| 15 |
66 |
f |
1992 |
inactive |
Corticosteroids |
Methotrexate |
+ |
Multiple-sclerosis |
|
| 16 |
58 |
f |
2006 |
inactive |
Corticosteroids
Methotrexate |
Methotrexate |
+ |
||
| 17 |
58 |
f |
2007 |
inactive |
Methotrexate |
Adalimumab
Corticosteroids |
- |
||
| 18 |
50 |
f |
2007 |
inactive |
Corticosteroids
Methotrexate Mycophenolate mofetil |
Methotrexate |
+ |
||
| 19 | 66 | f | 2011 | inactive | Mycophenolate mofetil | - | + | Mamacarcinoma hypothyroidism | |
Retina, choroid, and skin extracts
Retina and choroid were prepared from post-mortem tissue within 6–9 h. Retina extracts and choroid extracts were prepared from eyes from two unrelated Caucasian donors who had no history of intraocular inflammation or vitreoretinal disease. Retinas were carefully dissected from the underlying pigment epithelium and choroid by rinsing with 0.5 ml PBS (140 mM NaCl, 21 mM Na2HPO4, 2.7 mM NaH2PO4, pH 7.4, with 0.02% NaN3). Retinas were placed in the same 0.5mL PBS (0.02% NaN3) to preserve soluble antigens in the protein lysate. The choroid/pigment epithelium was dissected from the sclera and kept in 0.5 ml PBS (0.02% NaN3). Skin obtained during reduction mammoplasty in two unrelated anonymous healthy Caucasian patients served as the control and was prepared within 6 h after surgery. The skin was briefly washed in PBS (0.02% NaN3) to remove blood contamination. Fatty tissue was removed, and the sample was cut into small pieces (0.3 cm2) using a scalpel. About 0.5 ml PBS (0.02% NaN3) was added to the dissected retina, choroid, and skin and homogenized with 1.4 mm ceramic beads in a Bench Top bead-based Homogenizer (MO BIO, Solana Beach, CA). After centrifugation at 10,000 ×g for 30 min (4 °C), supernatant was carefully collected and heat-inactivated at 100 °C for 7 min to neutralize endogenous tissue protease activity. Protein yields were assessed with the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Extracts were aliquoted and stored at −80 °C. All extracts were used at a final concentration of 100 µg/ml to stimulate the PBMCs.
Cytokine analysis of peripheral blood mononuclear cells
The T cell–associated cytokine production by PBMCs was analyzed after they had been exposed to the retina and choroid lysate (100 µg/ml final concentration). Staphylococcal enterotoxin B (SEB) from Staphylococcus aureus (1 µg/ml; Sigma-Aldrich, St. Louis, MO) served as a positive control, and lysate derived from a skin biopsy (100 µg/ml) served as an irrelevant lysate control because the cytokine production of PBMCs in response to skin lysate was similar to cytokine release in medium only (data not shown). About 2.5×105 PBMCs were cultured in serum-free AIM-V medium (Invitrogen, Carlsbad, CA) in 96-well flat-bottom culture plates for 6 days in the presence of lysate. Cell-free supernatant was collected and centrifuged at 1,000 ×g for 5 min. Cytokines (IL-10, IFN-γ, and IL-17A) in the supernatant were measured using the DIAplex multiplex fluorescent bead-based immunoassay (Diaclone, Besançon, France) according to the manufacturer’s specifications. The limit for detection of cytokines (pg/ml) was >1.7 for IL-10, >8.7 for IL-17, and >0.8 for IFN-γ.
Intracellular staining of interleukin-17 and interferon-γ in CD4+ T cells
PBMCs were stimulated as described above. Intracellular staining of cytokines in PBMCs was done after the cells were treated with phorbol myristate acetate (12.5 ng/ml) and ionomycin (0.5 µg/ml) for 5 h, the last 4 of which were in the presence of 5 µg/ml brefeldin A (all from Sigma-Aldrich). Cells were then stained for anti-CD3-allophycocyanin, CD4-PerCP, IL-17-PE(SCPL1362 ); IFN-γ- fluorescein isothiocyanate (4S.B3; BD, Franklin Lakes, NJ; Beckman Coulter, Fullerton, CA) and analyzed with a FACSCanto II flow cytometer (BD). Acquired data were analyzed with FACSDiva software.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego CA). We used the Kruskal–Wallis test with Dunn's multiple-comparison post-hoc test in GraphPad Prism 5 software to test group differences. Statistical significance was accepted at a significance level of adjusted p<0.05.
Results
The mean age of the patients with BSCR was 61.4 years, ranging from 50 to 74 years, and the female-to-male ratio was 17:2 (Table 1). The mean age of the five patients with active BSCR naïve to systemic treatment was 63.2 (range 54–74 years), the mean age of the 14 patients with inactive BSCR and/or current therapy was 60.7 (range 51–70), and the mean age of the 11 HLA-A29 positive controls was 49.7 (range 28–63 years).
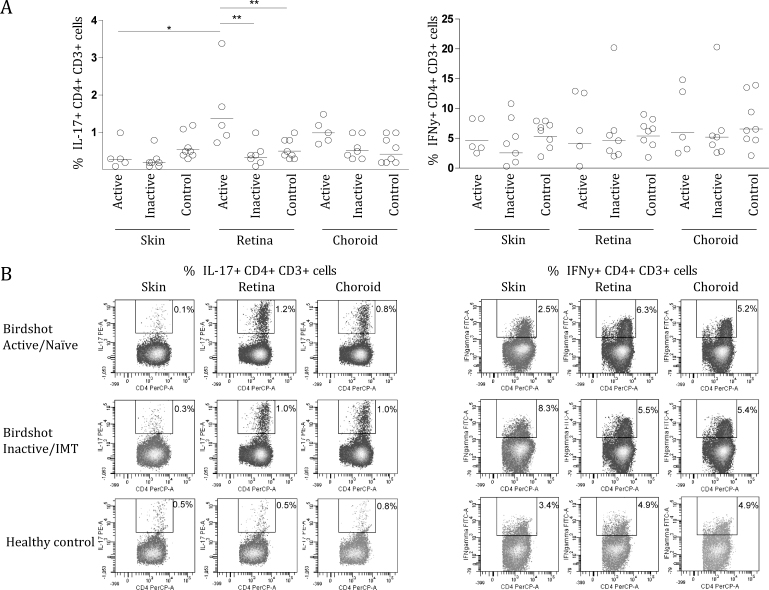
The production of all cytokines to SEB, a positive control antigen, was statistically similar for all groups (Table 2). The PBMCs of patients with active BSCR displayed an enhanced yet not significant IFN- γ response to all lysates compared to patients with inactive BSCR and the healthy controls. In patients with inactive BSCR, the IFN-γ levels in response to retina and choroid lysates were higher, but the levels were not significantly different from the response to skin lysate. In contrast, the PBMCs of the patients with active BSCR secreted significantly increased levels of IL-17 in response to retina lysate compared to skin lysate (Figure 1). The levels of IL-17 in cultures exposed to retina lysate were significantly higher compared to those of the healthy controls (Figure 1 and Table 2). IL-17 and IFN-γ production in response to choroid lysate increased in patients with active BSCR and patients with inactive BSCR/IMT, but did not reach statistical significance (Figure 1). Next, we investigated the induction of CD17+, CD4+, CD3+ (Th17) T cells and IFN-γ+ CD4+, CD3+ (Th1) in PBMCs stimulated under the same conditions in five patients with active BSCR, seven patients with inactive BSCR/IMT, and eight controls. Only the PBMCs from the patients with active BSCR demonstrated induction of CD17+, CD4+, CD3+ T cells after stimulation with retina lysate (Figure 2), but not IFN-γ+ CD4+ CD3+ (Th1). The levels of IL-10 in the PBMC cultures were heterogeneous and did not change significantly after stimulation with SEB or ocular lysates (Figure 1).
Table 2. Comparison of the cytokines levels secreted by the different groups.
| Cytokines | IL-10 | IL-17 | IFNy |
|---|---|---|---|
|
Differences in Skin response? | |||
| Skin Active versus Skin inactive |
ns |
ns |
ns |
| Skin Active versus Skin control |
ns |
ns |
ns |
| Skin inactive versus Skin control |
ns |
ns |
ns |
|
Differences in SEB response? |
|||
| SEB Active versus SEB inactive |
ns |
ns |
ns |
| SEB Active versus SEB control |
ns |
ns |
ns |
| SEB inactive versus SEB control |
ns |
ns |
ns |
|
Antigen specific responses? |
|||
| Skin Active versus SEB Active |
ns |
*** |
ns |
| Skin inactive versus SEB inactive |
ns |
*** |
*** |
| Skin control versus SEB control |
ns |
*** |
*** |
|
Ocular specific response? |
|||
| Skin Active versus Retina Active |
ns |
** |
ns |
| Skin Active versus Choroid Active |
ns |
ns |
ns |
| Skin inactive versus Retina inactive |
ns |
ns |
ns |
| Skin inactive versus Choroid inactive |
ns |
ns |
ns |
| Skin control versus Retina control |
ns |
ns |
ns |
| Skin control versus Choroid control |
ns |
ns |
ns |
|
Differences in Retinal responses? |
|||
| Retina Active versus Retina inactive |
ns |
ns |
ns |
| Retina Active versus Retina control |
ns |
** |
ns |
| Retina inactive versus Retina control |
ns |
ns |
ns |
|
Differences in Choroid responses? |
|||
| Choroid Active versus Choroid inactive |
ns |
ns |
ns |
| Choroid Active versus Choroid control |
ns |
ns |
ns |
| Choroid inactive versus Choroid control | ns | ns | ns |
P values were calculated by Kruskal–Wallis with post-hoc Dunn's multiple comparison test in Graphpad Prism v5 *=p<0.05, **=p<0.01, ***=p<0.001).
Figure 1.
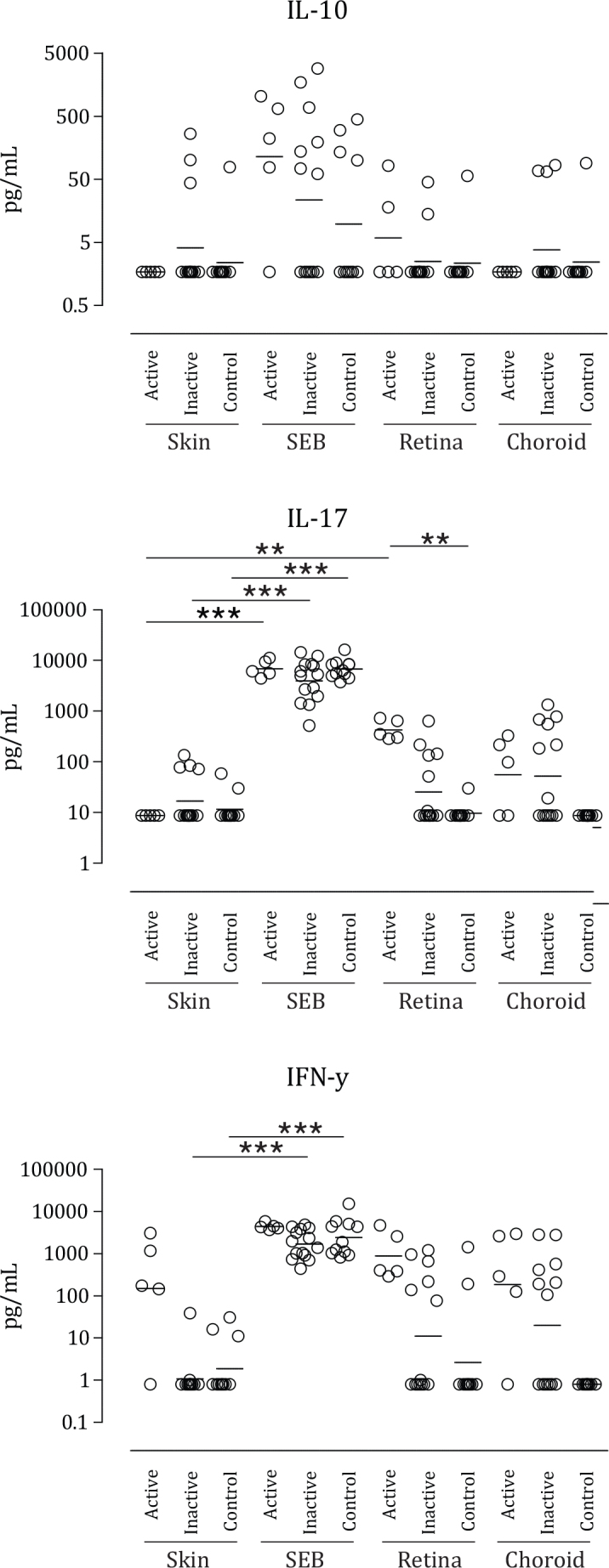
Scatterplots showing the levels of interleukin (IL)-10, IL-17, and interferon (IFN)-γ in supernatant (pg/ml) of peripheral blood mononuclear cell cultures stimulated for 6 days with ocular and control lysates, for each birdshot chorioretinopathy subgroup and the control group. Each circle represents a patient or control as indicated in the upper right legend. Horizontal lines indicate the geometric mean per group. Active=patients with active disease naïve to immunomodulatory therapy (IMT). Inactive=patients with a current or past history of IMT or inactive disease. Skin=protein lysate derived from a skin biopsy of two healthy controls. SEB=Staphylococcal enterotoxin B. Retina=protein lysate derived from retinas of two healthy controls. Choroid=protein lysate derived from choroid of two healthy controls. Limit for detection of cytokines (pg/ml); interleukin (IL)-10, >1.7; IL-17, >8.7; interferon (IFN)-γ, >0.8. Statistical analysis was performed using Kruskal-Wallis test with Dunn's multiple-comparison post-hoc test. *=p<0.05, **=p<0.01, ***=p<0.001.
Figure 2.
Th1 and Th17 cells in peripheral blood mononuclear cells stimulated with ocular and control lysates. A: Scatterplots showing the percentages of interleukin (IL)-17 CD4+, CD3+, and interferon (IFN)-γ+ CD4+, CD3+ T cells in peripheral blood monocytes cultures stimulated for 6 days with ocular and control lysates, for each birdshot chorioretinopathy (BSCR) subgroup and the control group. Each circle represents a patient or control as indicated in the upper right legend. Horizontal lines indicate the geometric mean. B: Representative flow cytometric plots of a healthy control, a patient with a current or past history of immunomodulatory therapy (IMT), or inactive disease (birdshot inactive/IMT) and a patient naïve to immunomodulatory therapy (birdshot active/naïve). Cells were gated on CD3+, CD4+ lymphocytes. Skin=protein lysate derived from a skin biopsy of two healthy unrelated controls. Retina=protein lysate derived from retinas of two healthy unrelated controls. Choroid=protein lysate derived from choroid of two healthy unrelated controls. Statistical analysis was performed using Kruskal-Wallis test with Dunn's multiple-comparison post-hoc test. *=p<0.05, **=p<0.01, ***=p<0.001.
Discussion
The current study demonstrates that PBMCs from patients with active BSCR naïve to systemic therapy specifically produce IL-17 in response to retina lysate. This was accompanied by the observation of a modest, but significant, induction of CD17+, CD4+, CD3+ T cells (Th17), but not IFN-γ+ CD4+, CD3+ T cells (Th1).
BSCR is an autoimmune disease [2], strongly associated with the HLA-A29 antigen [3] and mediated by autoreactive T lymphocytes [11] that specifically target the retina and/or choroid. Although the number of available samples of this rare disease is limited, we investigated differences in cytokine production in response to human retina and choroid lysate in 19 patients with BSCR and 11 HLA A-29-positive controls, and the induction of Th1 and Th17 cells in 12 patients and eight controls. IL-17 can be produced by various cell types, including natural killer (NK) cells, neutrophils, and other innate cells [7,14,15]. However, the predominant source of IL-17 is Th17 cells. In the last decade, experimental and clinical evidence has revealed unequivocal evidence for a common mechanism involving a pathogenic T helper 17 cell subset that predominantly produces the cytokine IL-17, which sustains chronic inflammation in T cell–associated autoimmune diseases, including rheumatoid arthritis [16], Crohn’s disease [8], ankylosing spondylitis [17], and noninfectious uveitis [10]. Evidence for a prominent role for Th17 cells in BSCR was previously demonstrated by the presence of increased intraocular levels of IL-17 and elevated intraocular [11] and serum levels [12] of immune mediators that promote Th17 responses in patients with BSCR. Interestingly, both studies suggested organ-specific Th17 cell–mediated inflammation [11,12]. Our data in patients with BSCR with active disease are consistent with and extend this hypothesis, since we observed IL-17 secretion and the induction of CD17+, CD4+, CD3+ (Th17) cells only after the PBMCs of patients with BSCR were stimulated with ocular lysates. Thus, we provide evidence that retina lysate contains antigens that initiate IL-17 production and link the Th17 signatures to ocular-specific immune responses rather than, for example, nonspecific constitutive Th17 activation. Identification of the exact antigen is highly valuable for understanding the exact disease mechanism of BSCR. Patients with BSCR frequently demonstrate positive proliferation in vitro after stimulation with the retinal S-antigen [3] or interphotoreceptor retinoid-binding protein (IRBP) [18,19]. These highly uveitogenic proteins cause uveitis in animal models via Th1 (IFN-γ) and Th17 (IL-17) responses [20,21]. In agreement with these observations, patients with BSCR display Th17 responses when stimulated with retina lysate. However, since many highly immunogenic autoantigens are present in the retina [20,22], a systematic strategy for identifying BSCR-related antigens using biochemical fractionation of the crude extracts, followed by testing overlapping peptides that span the entire amino acid sequence of each potential antigen is necessary to identify the exact antigens recognized in the crude extracts. Although we did not detect significant IL-17 responses to choroid lysates in all patients, several clinical [23] and experimental [5,6] observations suggest the choroid is the primary affected site. Thus, the responses against choroid antigens may still be relevant and should be addressed in a larger group of patients.
Several remarks must be made regarding the results of this study. For instance, some patients with inactive BSCR/IMT appeared to produce IL-17 and IFN-γ in response to the lysates, while others did not produce any cytokine. This heterogeneity may be due to the effectiveness of the treatment. However, probably due to the small size of our cohort, we were not able to link this heterogeneity to any therapy-related data. Therefore, this issue needs further investigation in larger cohorts. In addition, it remains difficult to determine if the observed cytokine responses are the cause or result of BSCR. Retina-specific CD4+ T cells in noninfectious uveitis may initiate the disease or may just be the result of the loss of the blood–retinal barrier and exposure of the highly uveitogenic retinal antigens to circulating peripheral retina-specific T cells. In any case, this “secondary” autoimmune response may be essential for significantly amplifying inflammation in the eye and could explain why BSCR shares Th17 signatures [11,12] and peripheral immune response to the retinal S-antigen [3] with other clinically and genetically distinct forms of uveitis [10,18]. Other cell types such as dendritic cells have been shown to play an important role in many ocular pathologies [24,25]. Furthermore, we observed that the PBMCs of the patients with active BSCR displayed an overall enhanced IFN- γ response to all lysates compared to the patients with inactive BSCR and the healthy controls. It is tempting to speculate that these results reflect overall enhanced T cell activity in patients with active BSCR [19] as similar observations have been reported in experimental autoimmune uveitis [23]. The exact mechanisms and contribution of this overall elevated IFN- γ require further investigation, however. Finally, the control group was younger than both groups of patients. Age-dependent changes in the innate and adaptive immune system were reported to affect the individual susceptibility to autoimmunity [26-28]. However, the difference in the mean age of the patient groups was only about 10 years. Moreover, antigen-specific cytokine responses by T cells (including IL-17) do not differ between various age groups [29]. Therefore, it is unlikely that the ocular lysate-specific IL-17 production in our study is associated with age-related differences.
In conclusion, our results underscore the importance of IL-17 in BSCR and suggest that targeting Th17 cells may be of particular interest in controlling inflammation in BSCR. Recently, anti-IL17 therapy (secukinumab) [30] did not demonstrate significant efficacy in noninfectious uveitis [31]. However, the authors noted that the results may have been confounded by the use of other relatively high doses of immunosuppressive medications in the studied patients [31]. Although the efficacy of anti-IL-17 therapy has not been evaluated in patients with BSCR, a better strategy could be targeting IL-23, because this cytokine is crucial for maintaining Th17 cells and can be targeted by the human monoclonal antibody ustekinumab [32]. Thus, our results warrant further studies on the manipulation of the Th17 cell axis, which hopefully reduces ocular inflammation and preserves visual function in patients with BSCR.
Acknowledgments
The authors declare no conflicts of interest. We would like to thank dr. Barabara Giovannone (department of Dermatology, University Medical Center Utrecht, The netherlands) for generously providing the skin biopsy samples. This study was supported by the combined grants from the Dr F.P. Fischer Stichting, Amersfoort; the Algemene Nederlandse Vereniging Ter Voorkoming Van Blindheid, Doorn; the Landelijke Stichting Voor Blinden en Slechtzienden, Utrecht; the Blindenpenning Stichting, Amsterdam, the Netherlands.
References
- 1.Monnet D, Brezin AP. Birdshot chorioretinopathy. Curr Opin Ophthalmol. 2006;17:545–50. doi: 10.1097/ICU.0b013e3280109479. [DOI] [PubMed] [Google Scholar]
- 2.Shah KH, Levinson RD, Yu F, Goldhardt R, Gordon LK, Gonzales CR, Heckenlively JR, Kappel PJ, Holland GN. Birdshot chorioretinopathy. Surv Ophthalmol. 2005;50:519–41. doi: 10.1016/j.survophthal.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982;94:147–58. doi: 10.1016/0002-9394(82)90069-1. [DOI] [PubMed] [Google Scholar]
- 4.Priem HA, Kijlstra A, Noens L, Baarsma GS, De Laey JJ, Oosterhuis JA. HLA typing in birdshot chorioretinopathy. Am J Ophthalmol. 1988;105:182–5. doi: 10.1016/0002-9394(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 5.Gaudio PA, Kaye DB, Crawford JB. Histopathology of birdshot retinochoroidopathy. Br J Ophthalmol. 2002;86:1439–41. doi: 10.1136/bjo.86.12.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulido JS, Canal I, Salomao D, Kravitz D, Bradley E, Vile R. Histological findings of birdshot chorioretinopathy in an eye with ciliochoroidal melanoma. Eye (Lond) 2012;26:862–5. doi: 10.1038/eye.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 8.Hölttä V, Klemetti P, Sipponen T, Westerholm-Ormio M, Kociubinski G, Salo H, Rasanen L, Kolho KL, Farkkila M, Savilahti E, Vaarala O. IL-23/IL-17 immunity as a hallmark of Crohn's disease. Inflamm Bowel Dis. 2008;14:1175–84. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- 9.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 10.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper JJ, Mutis T. de Jager W, de Groot-Mijnes JD, Rothova A. Intraocular interleukin-17 and proinflammatory cytokines in HLA-A29-associated birdshot chorioretinopathy. Am J Ophthalmol. 2011;152:177–82. doi: 10.1016/j.ajo.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Foster CS. Interleukin 21, Interleukin 23, and Transforming Growth Factor beta1 in HLA-A29-Associated Birdshot Retinochoroidopathy. Am J Ophthalmol. 2013;156:400–6. doi: 10.1016/j.ajo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Levinson RD, Brezin A, Rothova A, Accorinti M, Holland GN. Research criteria for the diagnosis of birdshot chorioretinopathy: results of an international consensus conference. Am J Ophthalmol. 2006;141:185–7. doi: 10.1016/j.ajo.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–42. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passos ST, Silver JS, O'Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184:1776–83. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 17.Brown MA. Genetics of ankylosing spondylitis. Curr Opin Rheumatol. 2010;22:126–32. doi: 10.1097/BOR.0b013e3283364483. [DOI] [PubMed] [Google Scholar]
- 18.de Smet MD, Yamamoto JH, Mochizuki M, Gery I, Singh VK, Shinohara T, Wiggert B, Chader GJ, Nussenblatt RB. Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am J Ophthalmol. 1990;110:135–42. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- 19.Jobin D, Thillaye B, de Kozak Y, Sainte-Laudy J, Faure JP, Le Hoang P. Severe retinochoroidopathy: variations of humoral and cellular immunity to S-antigen in a longitudinal study. Curr Eye Res. 1990;9:91–6. doi: 10.3109/02713689008999426. [DOI] [PubMed] [Google Scholar]
- 20.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–83. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattapallil MJ, Silver PB, Mattapallil JJ, Horai R, Karabekian Z, McDowell JH, Chan CC, James EA, Kwok WW, Sen HN, Nussenblatt RB, David CS, Caspi RR. Uveitis-associated epitopes of retinal antigens are pathogenic in the humanized mouse model of uveitis and identify autoaggressive T cells. J Immunol. 2011;187:1977–85. doi: 10.4049/jimmunol.1101247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspi RR, Silver PB, Luger D, Tang J, Cortes LM, Pennesi G, Mattapallil MJ, Chan CC. Mouse models of experimental autoimmune Uveitis. Ophthalmic Res. 2008;40:169–74. doi: 10.1159/000119871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbort CP, Probst K, Cimino L, Tran VT. Differential inflammatory involvement in retina and choroid in birdshot chorioretinopathy. Klin Monatsbl Augenheilkd. 2004;221:351–6. doi: 10.1055/s-2004-812827. [DOI] [PubMed] [Google Scholar]
- 24.Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234:282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 25.Liang D, Zuo A, Shao H, Born WK, O'Brien RL, Kaplan HJ, Sun D. Role of CD25+ dendritic cells in the generation of Th17 autoreactive T cells in autoimmune experimental uveitis. J Immunol. 2012;188:5785–91. doi: 10.4049/jimmunol.1200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes L. Le febvre JS. Age-related Deficiencies in Antigen-Specific CD4 T cell Responses: Lessons from Mouse Models. Aging Dis. 2011;2:374–81. [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;12:875–87. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinke KH, Calzavara B, Faria PF, do Nascimento MP, Venturini J, Lara VS. Proinflammatory profile of in vitro monocytes in the ageing is affected by lymphocytes presence. Immun Ageing. 2013;10:10. doi: 10.1186/1742-4933-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid P, Selak S, Keller M, Luhan B, Magyarics Z, Seidel S, Schlick P, Reinisch C, Lingnau K, Nagy E, Grubeck-Loebenstein B. th17/Th1 biased immunity to the pneumococcal proteins PcsB, StkP and PsaA in adults of different age. Vaccine. 2011;29:3982–9. doi: 10.1016/j.vaccine.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 30.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, Tak PP, Gomez-Reino JJ, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Rose K, Haider A, Di PF. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 31.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, Androudi S. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120:777–87. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Benson JM, Peritt D, Scallon BJ, Heavner GA, Shealy DJ, Giles-Komar JM, Mascelli MA. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3:535–45. doi: 10.4161/mabs.3.6.17815. [DOI] [PMC free article] [PubMed] [Google Scholar]