Abstract
Cell lytic peptides are a class of drugs that can be used to selectively kill invading organisms or diseased cells. Several of these peptides have been identified as potential therapeutics. Herein, we report a novel process for purifying recombinant melittin, a cell lytic peptide that inserts into the membranes of cells causing cell lysis, from Escherichia coli. The process involves surfactant and low pH to solubilize melittin fusion proteins from the insoluble fraction of bacterial lysates. We are able to significantly improve purity of the final product and confirm the activity of the peptide. The process yields recombinant melittin that is effective when used to treat U-87 MG glioma cells and inhibits growth of the Gram-positive pathogenic bacterium Streptococcus pyogenes. We demonstrate a method of repeated extraction of the insoluble protein fraction with mild detergent at a low pH that is able to generate a yield of pure, soluble melittin of approximately 0.5 to 1 mg/L of E. coli culture.
Keywords: Recombinant protein, fusion protein, protein extraction, surfactant, melittin
Introduction
Melittin is a cell lytic peptide originally identified in honeybee venom.1 It has the potential to act as an anti-bacterial,2 anti-tumor,3 and anti-inflammatory agent.4 This small peptide is a versatile drug due to its surfactant-like mechanism of action.5 This amphipathic, cell-penetrating peptide works by inserting itself into the cytoplasmic membrane of cells. At a critical density, melittin multimers form pores in the membrane, collapsing the electrochemical and ion gradients, and causing cell death.6 Until recently, melittin was either isolated from honeybee venom extract or was synthesized using solid phase synthesis.
Expression of recombinant melittin has been recently reported in the literature.7, 8 In both of these cases, recombinant melittin tagged with glutathione-S-transferase (GST) was purified from the soluble fraction of Escherichia coli lysates by affinity chromatography. In our own studies with melittin containing the dual tag GST-6xHis and purified using nickel agarose, we noticed that the majority of the protein in the soluble fraction, binding to nickel agarose was a truncated product containing only GST-6xHis and not the GST-6xHis-melittin fusion protein. We hypothesized that this was a result of fusion-protein degradation during the process of expression in E. coli. Consequently, we sought to improve the purity and yield of soluble melittin from E. coli extracts. In doing this, we developed an unusual process of repeated extraction from the insoluble fraction with increasing concentrations of mild, non-ionic detergents. The process enriched the GST-6xHis-melittin fusion protein relative to GST-6xHis. We were able to further enhance this purification using acidic solubilization of the GST degradation product. We were able to achieve a remarkably pure yield of GST-6xHis-melittin without any further purification using this technique. Detergents were removed with a final purification on nickel agarose to achieve a final protein yield of 5 to 10 mg/L of E. coli culture. This corresponds to a pure melittin yield of 0.5 to 1.0 mg/liter of E. coli culture after removal of the GST tag.
Further, we confirm that recombinant melittin is similar to synthetic melittin in terms of cell lysis using in two very different organisms: U-87 MG human cancer cells and S. pyogenes bacteria. We show, in these studies, that recombinant melittin is effective at inhibiting growth of both U-87 MG cells and pathogenic S. pyogenes bacteria. We propose that this relatively high yield method of purifying functional melittin will make the potential drug more accessible for study and formulation.
Methods
Cloning of melittin gene into expression vector
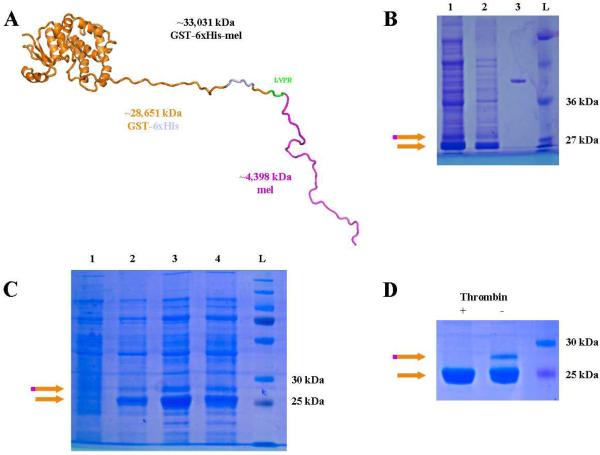
Melittin was cloned using standard cloning procedures. All restriction enzymes were purchased from New England Biolabs, MA. The melittin peptide was designed as reported (GIGAVLKVLTTGLPALISWIKRKRQ).1 The rDNA was codon optimized by the JCAT codon optimization tool:9 AGC GGA TCC GGT ATC GGT GCT GTT CTG AAA GTT CTG ACC ACC GGT CTG CCG GCT CTG ATC TCT TGG ATC AAA CGT AAA CGT CAG TAG GAA TTC TCA CG. Restriction sites BamHI (double underlined) and EcoRI (underlined) were engineered to the 5' and 3' ends respectively, and an amber stop codon (TAG, italicized and underlined) was engineered at the 3' end. This double-stranded fragment was synthesized by Integrated DNA technologies (Skokie, IL) and was cloned into the pJB-HTS variant of the pGEX6p-1 expression vector (GE Healthcare Biosciences, Pittsburgh, PA)10 generating pJB-HTS-melittin. Positive clones were screened by direct sequencing (ACGT, Wheeling, IL). The layout of the expected protein is N-GST-6xHis-thrombin cleavage site-melittin-C thus allowing dual purification with glutathione or nickel columns (Figure 1A).
Figure 1. Purification of GST-6xHis-melittin from soluble protein fraction.
(A) Predicted structure of GST-6xHis-melittin fusion protein based upon template matching.11 Approximate molecular weights are denoted below fragments as they would be generated by thrombin cleavage following the sequence LVPR. (B) GST-6xHis-melittin (orange and magenta arrow) was induced with 1 mM IPTG for 16 hours (lane 1) or 3 hours (lane 2) and compared with 200 ng of bovine serum albumin (BSA; lane 3), and a protein ladder for size (L). (C) GST-6xHis-melittin was also induced for 16 hours at 37°C (lanes 1 and 2) or 25°C (lanes 3 and 4) with 0.1 mM (lane 1 and 3) or 0.01 mM IPTG (lane 2 and 4). (D) The induced GST-6xHis-melittin has two purified products of the expected sizes for the fusion protein and GST-6xHis (orange arrow). Upon addition of 2U thrombin (+), the GST-6xHis-melittin was cleaved and only the GST-6xHis band was observed. In each panel, the orange and magenta arrow indicates GST-6xHis-melittin and the orange arrow indicates GST-6xHis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Expression and purification of Melittin
GST-6xHis-melittin containing plasmid (pJB-HTS-melittin) was transformed into competent E. coli Rosetta 2 cells (Novagen, Darmstadt, Germany) in order to negate the truncating effects of underrepresented codons within restriction sites on the pJB-HTS vector, upstream of the melittin insertion (CTC, AGA, GGA) and to eliminate reduced expression effects of the outer membrane protease T and Lon protease.12, 13 Cells from saturated, overnight starter cultures were incubated at 37°C at 180 revolutions per minute (RPM) until the desired cell density (A600 ~ 0.4) before addition of IPTG, 1 mM , 0.1 mM, or 0.01 mM, and removal to the appropriate induction temperature, 37°C, 25°C, or 4°C. After induction for varying times, 3 or 16 hours, cells were collected by centrifugation at 3600g and 4°C for 20 minutes and were resuspended in lysis buffer (50 mM NaHPO4, 300 mM NaCl, 10 mM imidazole, buffered at pH=8.0). Lysozyme (1 mg, Sigma-Aldrich, St. Louis, MO, L7651) was added to the resuspended bacteria, and the suspension was subjected to 3 rounds of freezing on dry ice and thawing in cold water. Samples were sonicated at 40% intensity (Misonix sonicator, model XL2015, Newtown, CT) three times for 15 seconds each followed by 15 seconds on ice, or until they were no longer viscous. The lysate (1 mL) was aliquoted to microcentrifuge (eppendorf) tubes and centrifuged for 30 minutes at 11,300g at 4°C to pellet the insoluble fraction. The soluble fraction was removed and analyzed by SDS-PAGE. The insoluble fraction was resuspended in 500 μL of detergent-containing buffer.
To determine the effect of different surfactants on the extraction from the insoluble fraction, non-ionic detergents, sorbitin monooleate 80 (Span-80), polysorbate 80 (Tween-80), and polysorbate 20 (Tween-20), in buffer at varying concentrations, 0.1%, 0.5%, 1.0%, and 2.0%, were used to extract the insoluble fraction. In the first experiment, a single pellet was resuspended in the lowest concentration of the surfactant with each successive extraction of that pellet using the next higher concentration of surfactant; this is referred to as sequential extraction. In a second experiment to determine the efficiency of repeated extraction with a given concentration of surfactant, the same concentration of surfactant was used to extract an insoluble protein fraction several times.
To determine the influence of acid on the solubilization of the protein, two different pH buffers were used. A pH of 2.3 is designated as “low pH” and a pH of 7.4 is designated as “high pH.” Low pH groups were attained by either the addition of 70 mM TCEP•HCl (+TCEP; Thermo Scientific, Hanover Park, IL) or 70 mM glycine (−TCEP) buffered at pH 2.3 to PBS. High pH groups were attained by adding 10 N sodium hydroxide to PBS with 70 mM TCEP•HCl until the pH was 7.4 or by using PBS alone.
For all experiments, the fractions were centrifuged at 11,300g at 4°C for ten minutes to repellet the remaining insoluble material. GST-melittin containing fractions were further purified by Nickel-NTA agarose (Qiagen, Germantown, MD) or agarose immobilized GSH (GoldBio, St. Louis, MO) affinity according to the manufacturer's recommendations and after raising the pH of the protein solution to approximately 7.5 with 1 M sodium hydroxide. Protein was eluted from beads in appropriate elution buffer recommended by the manufacturer but was also supplemented with 10% glycerol to increase protein stability during storage at −80°C. Total protein was determined by the Bradford Assay (Thermo Scientific, Hanover Park, IL)14 used according to the manufacturer recommendations and measured with a Beckman model DU640 spectrophotometer (Brea, CA). Recombinant melittin was purified as described, then digested with 2 units (U) of thrombin for 2 hours at 37°C. Protein molecular weights were confirmed with matrix-assisted laser desorption-ionization-time of flight (MALDI-TOF) mass spectroscopy (Applied Biosystems, Grand Island, NY).
The surfactant-containing insoluble fraction extracts were analyzed by SDS-PAGE. All SDS-PAGE gels were between 12% and 15% acrylamide crosslinked at a 1:37.5 ratio with N,N'-methylene bisacrylamide (Thermo Scientific, Hanover Park, IL ).15 Samples were prepared in Laemmli buffer and boiled for one minute before loading. Gels were run in a Biorad (Hercules, CA) mini-protean II apparatus between 100 and 200 V. After completion of the run, gels were stained with Coomassie brilliant blue R-250 (Thermo Scientific, Hanover Park, IL) according to manufacturer recommendations.
Tumor Cell Growth Inhibition
All proteins were incubated with thrombin at 37°C for two hours prior to cellular assays. Malignant glioma cells, U-87 MG, (ATCC, Manassas, VA, 3,000 cells/cm2) were seeded in a 96 well plate together with appropriate treatments in 100 μL DMEM and 10% FCS (Sigma-Aldrich, St. Louis, MO). Treatments included a cell only control (untreated), thrombin (2 U), GST (10 μM), GST-6xHis-melittin (10 μM), GST-6xHis-melittin (10 μM) simultaneously with thrombin (2 U), or synthetic melittin (10 μM; GenScript RP20415, Sigma-Aldrich, St. Louis, MO). The cells with their respective treatments were incubated at 37°C in 5% CO2 overnight, approximately 16 hours. The MTS assay (Promega, Madison, WI, CellTitre96) was performed according to the manufacturer recommendation and incubated for two hours before reading absorbance at 495 nm on a Labsystems Multiskan plus plate reader (Thermo Scientific, Hanover Park, IL).
Bacterial growth inhibition
Following digestion with thrombin as described above, GSH-agarose beads (GoldBio, St. Louis, MO) were used to separate GST from recombinant melittin. Synthetic melittin and GST-6xHis-melittin underwent a similar process without thrombin or being subjected to GSH-agarose treatment. Overnight cultures of S. pyogenes (ATCC, Manassas, VA, BAA-1633) cells were diluted in chemically defined medium16, 17 were diluted to approximately 108 colony forming units (CFUs), corresponding to an absorbance at 600 nm (A600) of 0.1, and 150 μL distributed into 96-well, optical-bottom plates (Greiner BioOne, Frickenhausen, Germany) for treatment. GST-6xHis-melittin, recombinant melittin, or synthetic melittin (10 μM) were then added and the plate was incubated at 37°C with shaking using a Biotek Synergy 2 plate reader (Winooski, VT). Absorbance (A600) measurements were obtained every ten minutes for 12 hours.
To measure growth of IPTG induced or non-induced E. coli, the bacteria was diluted to approximately 108 colony forming units (CFUs) in LB medium, corresponding to an absorbance at 600 nm (A600) of approximately 0.1, and 150 μL was distributed into 96-well, optical-bottom plates (Greiner BioOne, Frickenhausen, Germany). Antibiotics and IPTG were added to the appropriate groups, and the plate was incubated at 37°C with shaking using a Biotek Synergy 2 plate reader (Winooski, VT). Absorbance (A600) measurements were obtained every ten minutes for 12 hours. Growth curves were fit to the linear growth equation18, 19 and compared by two-way ANOVA analysis.
Statistical Analysis
Purification conditions were examined one time, but subsequent density analysis on gels was done in triplicate and blinded in order to reduce bias. U-87 MG and S. pyogenes growth assays were repeated with three biological replicates. ANOVA followed by post-hoc Tukey tests were used to determine significance at α ≤ 0.05.
Results & Discussion
Expression of recombinant GST-6xHis-melittin
The plasmid, pJB-HTS-melittin, was designed, cloned, and confirmed to express a fusion protein of GST followed by a hexa-histidine tag, a thrombin cleavage site, and the melittin fragment (Figure 1A). Two variations of induction time, three hours and sixteen hours, were assessed for protein expression (Figure 1B). Total protein and GST-6xHis-melittin were produced at a greater level during the sixteen-hour induction compared with the three-hour induction. Therefore, all future expression studies used the sixteen-hour condition as the induction time.
To determine if the protein band at approximately 25 kDa could be diminished by induction under varying conditions, bacteria were induced at three temperatures, 37°C, 25°C, and 4°C (4°C data not shown), and 2 IPTG induction concentrations, 0.1 mM and 0.01 mM IPTG, to optimize the production of protein (Figure 1C). Thrombin cleavage reduced the size of a 29 kDa band to 25 kDa, which correspond to the calculated sizes of GST-6xHis-melittin and GST-6xHis (Figure 1D). The 0.1 mM IPTG induction at 25°C for 16 hours was the best of the conditions tested based solely on ratio of GST-6xHis-melittin to GST-6xHis from SDS gels; however, in all cases tested, greater than 90% of the dominantly expressed protein was GST-6xHis and not the GST-6xHis-melittin fusion protein. In previous reports of recombinant melittin expression7, 8 there is limited discussion of the presence of a mixture of GST with the fusion protein, yield of active melittin, or confirmation of the lytic activity of the melittin recovered. Based upon the clear identification that the predominantly expressed protein, when analyzed by mass spectrometry, was confirmed to have the predicted mass of GST-6xHis-melittin, we sought to improve the conditions for GST-6xHis-melittin induction and purification.
Induction of GST-6xHis-melittin
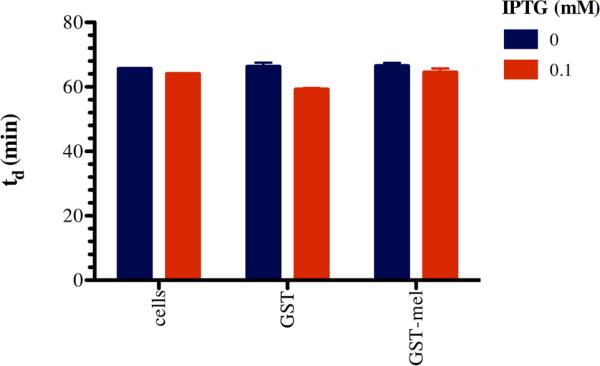
The optimization steps for IPTG concentration, induction time, and temperature were all executed using soluble GST-6xHis-melittin (denoted as a magenta and orange arrow in figures) as a marker of efficiency. However, even at the most efficient condition, more than 90% of protein that was purified by nickel or GSH affinity chromatography did not contain active melittin fusion (Figure 1B & 1C), i.e. the predominant protein produced was GST-6xHis (denoted as a magenta arrow in figures). We hypothesized that the melittin fused to the C-terminal of GST-6xHis was being degraded or simply cleaved from the fusion protein. Melittin actively incorporates into membranes of cells as a multimeric protein,6 so we hypothesized that enriched GST-6xHis-melittin may be in association with the inner membrane of E. coli. We measured the rate of E. coli growth cultures with or without addition of IPTG to assess whether GST-6xHis-melittin affected E. coli growth. IPTG induction of other recombinant proteins in E. coli has been reported to have a minimal affect the logarithmic phase growth rate,20, 21 which agrees with our observations. The produced fusion proteins do cause statistically significant, but biologically insignificant, growth rate inhibition of the E. coli (Figure 2). The fact that there is not significant growth inhibition suggests that the GST-6xHis-melittin cannot form pores, i.e. there is no observable cell death or the protein is not soluble as will be discussed later.
Figure 2. Doubling time (td) of E. coli and E. coli harboring GST-6xHis (GST) or GST-6xHis-melittin (GST-mel) expression plasmid vectors.
E. coli were grown under antibiotic selection to maintain expression vectors for GST-6xHis, GST-6xHis-melittin, or no expression vector control and were grown in the presence and absence of 0.1 mM IPTG. Doubling times were found to be dependent upon the plasmid and IPTG treatment (2-way ANOVA, p-value < 0.05 for the interaction, [IPTG], and plasmid). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Detergent extraction from insoluble fraction
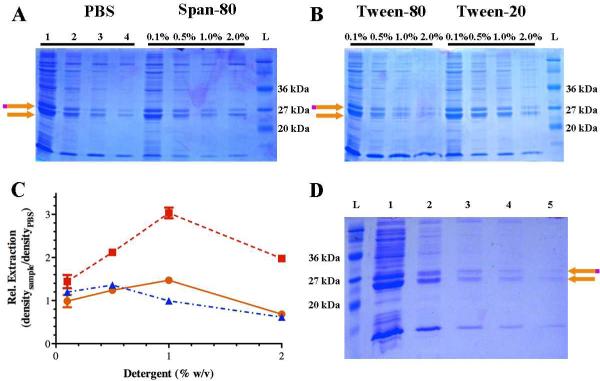
To extract what we believed to be a poorly soluble GST-6xHis-melittin protein, we compared extraction with buffer to 3 non-ionic detergents representing different hydrophilic-lipophilic balances (HLB): Span-80 (HLB 4.3), Tween-80 (HLB 15.0), and Tween-20 (HLB 16.7) (Figure 3). We chose non-ionic detergents because of their non-denaturing nature and capacity for removal by salt addition or by ion exchange chromatography.22 Interestingly, increasing HLB values (increasing hydropilicity) correlates with increased ability to purify melittin (Figure 3C). PBS was able to extract melittin upon repeated extraction more effectively than sequential extraction with Span-80 (Figure 3A), but not as well as sequential extraction with either Tween-20 or Tween-80 (Figure 3B). It is remarkable how pure the GST-6xHis-melittin and GST-6xHis fractions became upon sequential extraction with either Tween-20 or Tween-80 without any further affinity purification. When 1% Tween-20 is used for sequential extraction (Figure 3B), there is clear increase in relative protein extracted compared to the previous extraction as indicated by greater density of the GST-6xHis-melittin and GST-6x bands relative to the same serial PBS extraction (Figure 3C). Comparing the 1% Tween-20 extraction with other surfactants at the same concentration (Figure 3A & Figure 3B), significantly more protein is also extracted relative to the same serial PBS extraction. Based upon these experiments, it is clear that the higher concentration of hydrophilic surfactant has the greatest ability to extract GST-6xHis-melittin from the insoluble protein fraction.
Figure 3. Extraction of GST-6xHis-melittin from the insoluble protein fraction.
Representative SDS-PAGE electrophoresis gels are shown to represent the protein recovered from extraction from the insoluble protein fraction. (A) Protein recovered from repeated extraction of the same insoluble fraction pellet with PBS (lanes 1-4 indicated the first through fourth PBS extraction) or sequential extraction of the same insoluble fraction pellet with 0.1% Span-80 followed by 0.5%, 1%, and finally 2% Span-80. (B) Protein recovered sequential extraction of the insoluble fraction with 0.1% Tween-90 or Tween-20 followed by 0.5%, 1%, and finally 2% Tween-80 or Tween-20. (C) Semi-quantitative density of extracted GST-6xHis melittin using Tween-80 ( ), Tween-20 (
), Tween-20 ( ), or Span-80 (
), or Span-80 ( ) relative to PBS extraction where the density of the protein band is divided by the density of the protein band from the serial PBS extraction. Three technical replicates were examined and the mean plus or minus (±) the standard deviation is presented. (D) Repeated extraction of same insoluble pellet using 1% Tween-20 where each lane indicates the order of extraction. The orange and magenta arrow indicates GST-6xHis-melittin and the orange arrow indicates GST-6xHis. A molecular weight ladder (L) is also included in each gel. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
) relative to PBS extraction where the density of the protein band is divided by the density of the protein band from the serial PBS extraction. Three technical replicates were examined and the mean plus or minus (±) the standard deviation is presented. (D) Repeated extraction of same insoluble pellet using 1% Tween-20 where each lane indicates the order of extraction. The orange and magenta arrow indicates GST-6xHis-melittin and the orange arrow indicates GST-6xHis. A molecular weight ladder (L) is also included in each gel. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Repeated extraction with 1% Tween-20 was performed on the same insoluble pellet in order to maximize yield (Figure 3D). Most of the extractable protein was removed after the first of the repeated extractions. Nearly 50% of the GST-6xHis protein extracted from the insoluble fraction contained a melittin fusion, while the other 50% did not. These conditions resulted in the purification of soluble melittin of approximately 0.5 to 1 mg/L of E. coli culture. In comparison, we were able to achieve a pure melittin yield of less than 0.1 mg/L of E. coli from the soluble protein fraction, and less than 10% of what we purified from the soluble fraction contained an active melittin fusion.
Acidic solubilization of GST-6xHis-Mel
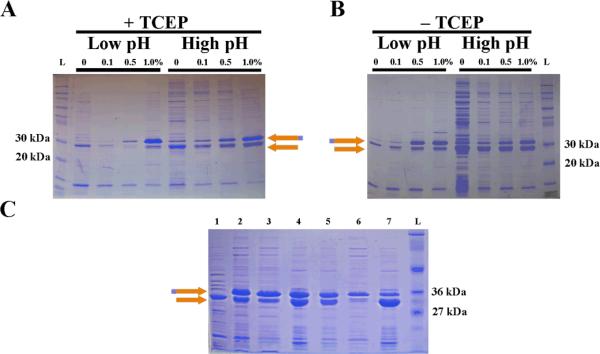
The isoelectric point of GST is known to be between 5.8 and 6.8, depending upon the specific sequence23 and was estimated to be 6.31 using a web-based calculation.24 The isoelectric point of melittin is considerably higher25 with an estimated pI of 12.01 for the recombinant, thrombin cleaved product. The predicted isoelectric point of a GST-6xHis-melittin fusion by primary sequence prediction is 8.2. We hypothesized that detergent extraction of the insoluble lysate at low pH would extract GST-6xHis-melittin by selectively solubilizing the more charged molecule. Further, because GST is known to form aggregates by disulfide bonding between four cysteine residues exposed on the surface,26 we sought to reduce these disulfide bonds using reducing agents. Extraction in the presence (Figure 4A) or absence (Figure 4B) of the reducing agent TCEP at low pH resulted in the selective purification of GST-6xHis-melittin. Interestingly, at lower detergent concentrations and at low pH, little to no GST-6xHis-melittin was extracted. As the detergent concentration exceeded 0.5%, selective extraction of GST-6xHis-melittin was achieved regardless of whether a reducing agent was present (Figure 4). Likewise, at high pH, the reducing agent made little difference (Figure 4) allowing us to conclude that the selective solubilization of GST-6xHis-melittin was not due to a simple process of reduction GST-inclusions.
Figure 4. Acidic solubilization of GST during detergent extraction.
(A) Sequential extraction of the same insoluble fraction pellet in the presence of 70 mM TCEP and 0% Tween-20 followed by 0.1%, 0.5%, and finally 1.0% Tween-20 in PBS (each with 70 mM TCEP) adjusted to pH 2.3 (low pH) or 7.4 (high pH). (B) Sequential extraction of the same insoluble fraction pellet in the presence 0% Tween-20 followed by 0.1%, 0.5%, and finally 1.0% Tween-20 in PBS adjusted to pH 2.3 (low pH) or 7.4 (high pH) in the absence of TCEP. (C) Extraction and nickel purification of GST-6xHis-melittin from 500 mL E. coli induction. The pellet was treated with acidic (pH of 2.3) buffer with no detergent (lane 1) followed by a first (lane 2) and second (lane 3) extraction of the pellet with 1% Tween-20 at a pH of 2.3 and eluted Ni-purified protein from the first (lane 4) and second (lane 5) extractions, respectively. The flow through from this nickel purification is also included (lane 6). Thrombin cleaved, nickel-purified protein from lane 4 (lane 7) shows a marked decrease in GST-6xHis-melittin band. The orange and magenta arrow indicates GST-6xHis-melittin and the orange arrow indicates GST-6xHis. A molecular weight ladder (L) is also included in each gel. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The insoluble pellet from 500 mL of induced culture was extracted with low pH buffer without detergent (Figure 4C, lane 1) then extracted twice with low pH buffer containing 1% Tween-20 (Figure 4C, lanes 2 & 3). To remove the detergent from the GST-6xHis-melittin, the extract was purified by nickel affinity chromatography (Figure 4C, lanes 4 and 5). Unexpectedly, a re-enrichment of the GST-6xHis degradation product occurred through this process. In fact, the purest GST-6xHis-melittin was found in the flow-through fraction of this process (Figure 4C, lane 6). We digested GST-6xHis-melittin purified by metal affinity chromatography with thrombin to further verify that the product was, in fact, GST-6xHis-melittin (Figure 4C, lane 7). The loss of the 37 kDa band and increase in intensity of the 29 kDa band suggested that the band was GST-6xHis-melittin.
GST has long been known to act as a homodimer.27 GST dimers occur only between members of the same GST class.28 Even in fusion proteins, this quaternary structure is maintained.29 Three possible combinations of dimers from GST-6xHis and GST-6xHis-melittin are possible: two homodimers consisting of either GST-6xHis:GST-6xHis or GST-6xHis-melittin:GST-6xHis-melittin, and one heterodimer consisting of GST-6xHis:GST-6xHis-melittin. GST-6xHis:GST-6xHis homodimers were thought to be diminished by acid solubilization; so only the homodimer of GST-6xHis-melittin:GST-6xHis-melittin, and the heterodimer of GST-6xHis:GST-6xHis-melittin are thought to remain. Melittin is a highly basic peptide and when engineered with a hexa-histadine tag, the melittin peptide may change the electrostatic interactions of the hexa-histadine sufficiently to disrupt formation of complexes between histidine and nickel. If this is the case, homodimeric GST-6xHis-melittin:GST-6xHis-melittin may have reduced affinity for nickel (Figure 4C, lane 6), while what is retained on the resin would consist of heterodimeric GST-6xHis:GST-6xHis-melittin (Figure 4C, lanes 4 & 5). Though untested, this hypothesis would explain why only 50% enrichment of GST-6xHis-melittin is possible by nickel chromatography.
Growth inhibition effect of recombinant melittin on U-87 MG and S. pyogenes
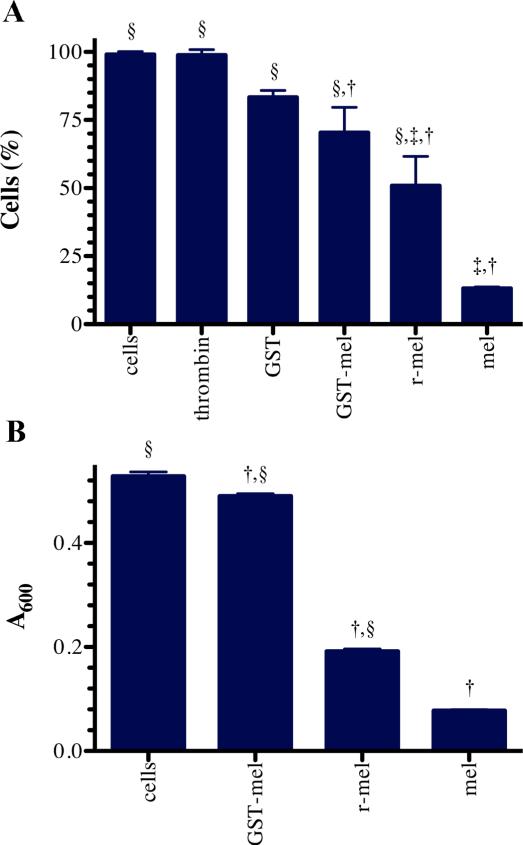
To verify the activity of nickel-purified, recombinant melittin, the effects of 10 μM recombinant GST-6xHis-melittin and synthetic melittin were compared on U-87 MG cells (Figure 5A). As controls, the effects of thrombin, and GST-6xHis were also considered in this experiment. Under these conditions at least 85% survival was observed in the GST-6xHis and thrombin treatment groups (Figure 5A). Cells treated with GST-6xHis-melittin without thrombin retained 70% survival, whereas thrombin-treated GST-6xHis-melittin let to 50% cellular survival. Under similar conditions and concentrations, synthetic melittin allowed 15% survival of U-87 MG cells.
Figure 5. Functional comparison of recombinant melittin to synthetic melittin.
(A) Survival of U-87 MG cells 3 hours after no treatment (cells), treatment with 2U of thrombin (thrombin), 20 μM synthetic melittin (mel), recombinant GST-6xHis-melittin fusion (GST-mel), recombinant purified melittin (r-mel), or GST-6xHis (GST). (B) Growth, as measured by the absorbance at 600 nm, of S. pyogenes cells (cells) five hours after treatment with 10 μM recombinant melittin (r-mel), synthetic melittin (mel), or recombinant GST-6xHis-melittin (GST-mel). In each, three biologic replicates were examined and the mean plus or minus the standard deviation is presented. The symbols indicate significant difference (p-value less than 0.05) from the cells without treatment (†), treatment with GST (‡), or treatment with synthetic melittin (§).
We further examined the potential of nickel-purified, recombinant melittin to inhibit growth of the Gram-positive bacterium, S. pyogenes. GST-6xHis-melittin fusion protein (10 μM), purified recombinant melittin (10 μM), or synthetic melittin (10 μM) were incubated with S. pyogenes cells. Synthetic and recombinant melittin almost completely inhibited the growth of S. pyogenes at 5 hours. GST-6xHis-melittin, however, exhibited minimal growth inhibition, similar to no treatment (Figure 5B).
We believe that differences seen between synthetic and recombinant melittin were due to the overestimation of recombinant protein concentration. Measurements were based on concentration of GST-6xHis-melittin, and assumed that melittin in the 29 kDa band was intact and that thrombin cleavage was 100% effective at releasing melittin from its fusion partner. Neither of these assumptions was likely to be completely true.
Conclusion
In conclusion, we have shown an unusual process for the purification of a GST-6xHis-melittin recombinant protein by repeated extraction of the insoluble protein fraction with detergent. High levels of purification can be achieved simply by surfactant extraction at a low pH. For this particular protein, hydrophilic surfactant, Tween-20, achieved superior quantity and purity of protein extracted. We have shown that usable quantities (0.5 to 1 mg/L) of melittin can be purified by this method, and that the resulting recombinant melittin is functional compared synthetic melittin.
Acknowledgements
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant (C06 RR015482, RAG) from the National Centre for Research Resources of the National Institutes of Health (NIH). This research has been funded, in part, by the University of Illinois at Chicago Center for Clinical and Translational Science (CCTS) award supported by the NCRR (UL1 TR000050, RAG), and the National Institute for Neurologic Disorders and Stroke (R01 NS055095, RAG) and the National Institute of Allergy and Infectious Diseases (AI091779, MJF). The authors also thank Dr. Debra A. Tonetti for use of instruments. In addition, the authors thank anonymous reviewers for careful and meticulous corrections in the review of this manuscript.
References
- 1.Habermann E. Bee and wasp venoms. Science. 1972;177:314–22. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 2.Katsu T, Kuroko M, Morikawa T, Sanchika K, Fujita Y, Yamamura H, Uda M. Mechanism of membrane damage induced by the amphipathic peptides gramicidin S and melittin. Biochim Biophys Acta. 1989;983:135–41. doi: 10.1016/0005-2736(89)90226-5. [DOI] [PubMed] [Google Scholar]
- 3.Holle L, Song W, Holle E, Wei Y, Wagner T, Yu X. A matrix metalloproteinase 2 cleavable melittin/avidin conjugate specifically targets tumor cells in vitro and in vivo. Int J Oncol. 2003;22:93–8. [PubMed] [Google Scholar]
- 4.Kwon YB, Lee HJ, Han HJ, Mar WC, Kang SK, Yoon OB, Beitz AJ, Lee JH. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002;71:191–204. doi: 10.1016/s0024-3205(02)01617-x. [DOI] [PubMed] [Google Scholar]
- 5.van den Bogaart G, Guzman JV, Mika JT, Poolman B. On the mechanism of pore formation by melittin. J Biol Chem. 2008;283:33854–7. doi: 10.1074/jbc.M805171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu N, Yang K, Yuan B, Ma Y. Molecular response and cooperative behavior during the interactions of melittin with a membrane: dissipative quartz crystal microbalance experiments and simulations. J Phys Chem B. 2012;116:9432–8. doi: 10.1021/jp305141r. [DOI] [PubMed] [Google Scholar]
- 7.Gui-Mei Kong X-RZ, Ke-Yan Wu, Yue-Xiao Laio, Ping Bo. Anti-proliferative activity of recombinant melittin expressed in Eschericia coli toward U937 cells. African Journal of Biotechnology. 2012;11:3026–3030. [Google Scholar]
- 8.Shi WJ, Xu HJ, Cheng JA, Zhang CX. Expression of the melittin gene of Apis cerana cerana in Escherichia coli. Protein Expr Purif. 2004;37:213–9. doi: 10.1016/j.pep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Grote A, Hiller K, Scheer M, Munch R, Nortemann B, Hempel DC, Jahn D. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005;33:W526–31. doi: 10.1093/nar/gki376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buhrman JS, Rayahin JE, Kollmer M, Gemeinhart RA. In-house preparation of hydrogels for batch affinity purification of glutathione S-transferase tagged recombinant proteins. BMC Biotechnol. 2012;12:63. doi: 10.1186/1472-6750-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. Template-based protein structure modeling using the RaptorX web server. Nature protocols. 2012;7:1511–22. doi: 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grodberg J, Dunn JJ. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988;170:1245–53. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips TA, VanBogelen RA, Neidhardt FC. lon gene product of Escherichia coli is a heat-shock protein. J Bacteriol. 1984;159:283–7. doi: 10.1128/jb.159.1.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 15.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning : a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1982. p. x.p. 545. [Google Scholar]
- 16.van de Rijn I, Kessler RE. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–8. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ. Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS pathogens. 2011;7:e1002190. doi: 10.1371/journal.ppat.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gompertz B. On the Nature of the Function Expressive of the Law of Human Mortality on a New Method of Determining the Value of Life Contingencies. Phil. Trans. Roy. Soc. 1825:513–585. [Google Scholar]
- 19.Winsor CP. The Compertz Curve as a Growth Curve. Proc. Natl. Acad. Sci. U.S.A. 1932;18:1–8. doi: 10.1073/pnas.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn U, Strittmatter W, Krebber A, Knupfer U, Kujau M, Wenderoth R, Muller K, Matzku S, Pluckthun A, Riesenberg D. High volumetric yields of functional dimeric miniantibodies in Escherichia coli, using an optimized expression vector and high-cell-density fermentation under non-limited growth conditions. Appl Microbiol Biotechnol. 1996;46:524–32. doi: 10.1007/s002530050855. [DOI] [PubMed] [Google Scholar]
- 21.Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–98. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 22.Fricke B. Phase separation of nonionic detergents by salt addition and its application to membrane proteins. Anal Biochem. 1993;212:154–9. doi: 10.1006/abio.1993.1306. [DOI] [PubMed] [Google Scholar]
- 23.Chen HM, Luo SL, Chen KT, Lii CK. Affinity purification of Schistosoma japonicum glutathione-S-transferase and its site-directed mutants with glutathione affinity chromatography and immobilized metal affinity chromatography. J Chromatogr A. 1999;852:151–9. doi: 10.1016/s0021-9673(99)00490-2. [DOI] [PubMed] [Google Scholar]
- 24.Bjellqvist B, Hughes GJ, Pasquali C, Paquet N, Ravier F, Sanchez JC, Frutiger S, Hochstrasser D. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis. 1993;14:1023–31. doi: 10.1002/elps.11501401163. [DOI] [PubMed] [Google Scholar]
- 25.Habermann E, Jentsch J. [Sequence analysis of melittin from tryptic and peptic degradation products]. Hoppe Seylers Z Physiol Chem. 1967;348:37–50. [PubMed] [Google Scholar]
- 26.Abeliovich H, Shlomai J. Reversible oxidative aggregation obstructs specific proteolytic cleavage of glutathione S-transferase fusion proteins. Anal Biochem. 1995;228:351–4. doi: 10.1006/abio.1995.1363. [DOI] [PubMed] [Google Scholar]
- 27.Fabrini R, De Luca A, Stella L, Mei G, Orioni B, Ciccone S, Federici G, Lo Bello M, Ricci G. Monomer-dimer equilibrium in glutathione transferases: a critical re-examination. Biochemistry. 2009;48:10473–82. doi: 10.1021/bi901238t. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Rivera A, Landa A. Glutathione transferases from parasites: a biochemical view. Acta Trop. 2008;105:99–112. doi: 10.1016/j.actatropica.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Lim K, Ho JX, Keeling K, Gilliland GL, Ji X, Ruker F, Carter DC. Three-dimensional structure of Schistosoma japonicum glutathione S-transferase fused with a six-amino acid conserved neutralizing epitope of gp41 from HIV. Protein Sci. 1994;3:2233–44. doi: 10.1002/pro.5560031209. [DOI] [PMC free article] [PubMed] [Google Scholar]