Abstract
The number of indwelling medical devices is escalating, and an increasing proportion of device-related infections are being caused by Candida spp. Candida spp. produce biofilms on synthetic materials, which facilitates adhesion of the organisms to devices and renders them relatively refractory to medical therapy. Management of device-related Candida infections can be challenging. Removal of the infected device is generally needed to establish cure of Candida infections of medical devices. However, since the pathogenesis of Candida bloodstream infection is complicated, more studies are necessary to determine the role of catheter exchange in patients with both gastrointestinal tract mucositis and indwelling catheters. The medical and economic impact of these infections is enormous.
INTRODUCTION
Ever since Elek and Conen demonstrated in 1957 that the presence of a foreign body significantly reduces the number of bacteria required to produce infection (31), the inherent susceptibility of medical devices to infection has been increasingly appreciated. Modern technology has allowed the use of a wider and newer variety of medical devices. The combination of an increasingly aging population and consistently growing number of inserted devices is likely to escalate the occurrence of infectious complications related to medical devices.
At least half of all cases of nosocomial infections are associated with medical devices (97). The medical consequences of device-related infections can be disastrous; they include potentially life-threatening systemic infections and device malfunction that may require device removal, often complicated by tissue destruction. Management of device-related infections can be difficult and is costly. An increasing proportion of device-related infections, particularly those involving the bloodstream and urinary tract, are being caused by Candida spp. (48, 97). A number of published reviews have focused on infections associated with a specific device or a particular bacterial organism. Here, we review Candida infections of commonly used medical devices.
The objectives of this comprehensive review are to (i) discuss the formation of Candida biofilms around medical devices and compare them to bacterial biofilms and (ii) review, in a systematic fashion, the impact of Candida infections on commonly used medical devices.
CANDIDA BIOFILMS
A significant proportion of human infections involve biofilms (88). Microbial biofilms develop when organisms adhere to a surface and produce extracellular polymers that provide a structural matrix and facilitate further adhesion (28). Organisms in biofilms behave differently from freely suspended microbes and have been shown to be relatively refractory to medical therapy (28, 29, 90). Therefore, biofilm-associated infections of retained devices may recur after cessation of antibiotic therapy and hence may necessitate device removal. The formation of bacterial biofilms around devices has been comprehensively investigated (97), but until recently, less focus has been placed on the formation of fungal biofilms. Candida species are emerging as important nosocomial pathogens, and an implanted device with a detectable biofilm is frequently associated with these infections (30). The evidence linking Candida biofilms to device-related infections is growing as more standardized methods for evaluating Candida biofilms in vitro emerge. Here, we review the role of biofilm production in the pathogenicity of Candida-related device infection and the antifungal drug susceptibility of Candida biofilms.
Formation and Structure
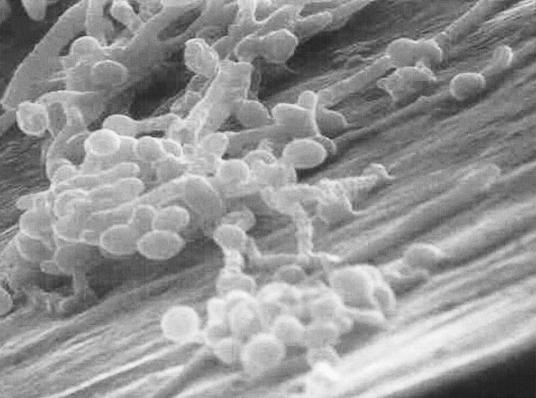
Fungal biofilm formation is a complex and diverse phenomenon. Candida albicans biofilm formation has been studied more extensively than biofilms of other Candida species. C. albicans biofilm formation has three developmental phases: adherence of yeast cells to the device surface (early phase), formation of a matrix with dimorphic switching from yeast to hyphal forms (intermediate phase), and increase in the matrix material taking on a three-dimensional architecture (maturation phase) (19, 43). Fully mature Candida biofilms have a mixture of morphological forms and consist of a dense network of yeasts, hyphae, and pseudohyphae in a matrix of polysaccharides (19), carbohydrate, protein, and unknown components (Fig. 1). The formation and structure of Candida biofilms is influenced by the nature of the contact surface, environmental factors, Candida morphogenesis, and the Candida species involved. These factors are discussed in detail the following paragraphs.
FIG. 1.
Scanning electron micrograph of a C. albicans biofilm that has formed in vitro on the surface of a vascular catheter. Reprinted from reference 61a.
(i) The chemical nature of the contact surface has been shown to influence the magnitude of biofilm formation (43), which is increased on latex compared with polyvinyl chloride but substantially decreased on polyurethane and 100% silicone (43). The architecture of C. albicans biofilm is different when it is formed on cellulose filters from when it is formed on catheter disks (7), indicating that it may depend on highly specific contact-induced gene expression (30).
(ii) High-glucose medium promotes the formation of biofilms (43), particularly of C. parapsilosis, reflecting its potential to cause device-related infections in patients receiving parenteral nutrition (103). Cell surface hydrophobicity correlates positively with Candida biofilm formation (62), and gentle shaking (42) also enhances biofilm formation. These conditions are also encountered in vivo (like in the circulation and urinary system), favoring biofilm formation when devices are inserted.
(iii) The different morphological forms are important in biofilm formation, as evidenced by a study that compared biofilms formed by wild-type strains of C. albicans and two mutants incapable of yeast and hyphal growth, respectively. The wild-type mutant produced a distinct two-layer biofilm as described above, the hypha-negative mutant produced only the basal layer, and the yeast-negative mutant produced only the outer layer, which was more easily detached from the catheter disks. This suggests that dimorphism might be necessary for biofilm architecture and structure (7) and is a pivotal factor for the pathogenic potential of C. albicans.
(iv) Most Candida spp. have been shown to produce biofilm in vitro to various degrees. An in vitro study showed that C. parapsilosis, C. pseudotropicalis, and C. glabrata produced significantly less biofilm on polyvinyl chloride disks than did the more pathogenic C. albicans, as determined by dry-weight, colorimetric, or radioisotope assays (43). Another study confirmed this finding (56) when measured by dry weight and also found that, on microscopy, C. albicans biofilms had a more complex morphology than C. parapsilosis biofilms, which were composed only of clumped blastospores. The interstrain variability in biofilm production differs between Candida spp., but studies have been inconsistent. Some studies have shown little variability in biofilm production in vitro between C. albicans isolates from active infections (invasive isolates) and carrier sites (noninvasive isolates) (43, 62, 103), whereas others have found that invasive C. albicans isolates have an increased ability to form biofilms compared with noninvasive isolates when measured by dry weight but not by biochemical assay (56). Additionally, a study that examined the variation of biofilm formation in 115 C. albicans strains from three different sources (the oral cavity, the vagina, and the environment) found significant differences in biofilm formation within clones and clonal lineages of C. albicans from each source but not between the three different sources by biochemical assay and absorbance following staining (62). This underscores the importance of methodology in evaluating biofilms as well as the importance of fact that Candida exhibits wide phenotypic diversity, which may correlate with pathogenicity.
Antifungal Drug Susceptibility
Organisms in biofilms behave differently from freely suspended cells with respect to antimicrobial resistance (29). Both bacteria and Candida cells within biofilms are markedly resistant to antimicrobial agents. C. albicans in biofilms on polyvinyl chloride disks has been reported to be 30 to 2,000 times more resistant to fluconazole, amphotericin B, flucytosine, itraconazole, and ketoconazole than planktonic cells (44), and the biofilm structure remained intact at an amphotericin B concentration of 11 times the MIC. Non-albicans Candida species were also resistant. In vitro, the newer triazoles were also found to be ineffective against C. albicans and C. parapsilosis biofilms (57); however, caspofungin has been shown to be effective against C. albicans biofilms (91). Glucan synthesis may thus prove to be an effective target for biofilms. Suggested mechanisms of biofilm resistance include restricted penetration of drugs through the matrix, slow growth of organisms in biofilms accompanied by changes in cell surface composition affecting their susceptibility to drugs, and unique biofilm-associated patterns of gene expression (30, 58).
In summary, Candida spp. produce biofilms on medical devices to various degrees and the difference in biofilm production and architecture by Candida spp. on different device materials is reflected in the different epidemiological trends in Candida device-related infections. In vitro, Candida biofilms are highly resistant to most antifungal agents except caspofungin, thereby posing a therapeutic challenge in managing device-associated Candida infections.
CLINICAL FACTORS RELATED TO THE PATHOGENESIS OF CANDIDA INFECTIONS
Candida organisms are commensals, and to act as pathogens, interruption of normal host defenses is necessary. Therefore, general risk factors for Candida infections include immunocompromised states, diabetes mellitus, and iatrogenic factors like antibiotic use, indwelling devices, intravenous drug use, and hyperalimentation fluids. There are several specific risk factors for particular non-albicans species: C. parapsilosis is related to foreign-body insertion, neonates, and hyperalimentation; C. krusei is related to azole prophylaxis and, along with C. tropicalis, to neutropenia and bone marrow transplantation; C. glabrata is related to azole prophylaxis, surgery, and urinary or vascular catheters; and C. lusitaniae is related to previous polyene use (55).
The source of Candida infections has been the subject of considerable debate. An endogenous source has been shown by using DNA typing of paired Candida samples from colonized sites and subsequent bloodstream infection in patients with hematological abnormalities (93) and in nonneutropenic patients (116). A recent review of published studies on potential sources of candidemia found support for a gastrointestinal origin of candidemia based on experimental, clinical, and molecular similarity studies (84). However, a cutaneous origin is suggested for C. parapsilosis since it is frequently recovered from skin samples and since it occurs more frequently in patients with venous catheters in place. Furthermore, the skin may be the origin of candidemia in patients with burns. The authors note that the data used to support skin as the source of candidemia are rather incomplete. The gastrointestinal tract is the primary source of candidemia in neutropenic patients, probably due to the gastrointestinal mucositis and subsequent gut invasion by Candida in this patient population.
IMPACT OF CANDIDA INFECTIONS OF MEDICAL DEVICES
Table 1 summarizes the annual use of commonly used medical devices, the overall rate of infection, the proportion and mortality of infections due to Candida, and the device-specific risk factors predisposing to Candida infections. Additionally, it lists the most common Candida species and whether removal of the device is generally needed for cure. Additional risk factors that have been associated with specific devices are also summarized.
TABLE 1.
Impact of Candida infections of medical devices
| Device | Annual use in the United States | Overall rate of infection (%) | Proportion of infections due to Candida (%) | Mortality due to Candida infections (%) | Risk factors for infection in general | Risk factors for Candida infections | Most common Candida species | Removal needed to achieve cure |
|---|---|---|---|---|---|---|---|---|
| Vascular catheters | 5 × 106 (24) | 3-8 (24) | 10 (94, 96) | 26-38 (118) | Neutropenia for >8 days; Hematologic malignancy; total parenteral nutrition; duration of site use; frequent manipulation of catheter; improper insertion and maintenance of catheter; high APACHE II score (89, 109) | C. parapsilosis in blood; positive quantitative or differential time to positivity of cultures; candidemia without other source; Hyperalimentation; persistent candidemia on antifungal drugs | C. albicans, C. glabrata (32, 96) | Yes, in most casesb |
| Joint prostheses | 6 × 105 (24) | 1-3 (108) | <1 | NKa | Prior surgery; rheumatoid arthritis; immunocompromise; diabetes mellitus; poor nutrition; obesity; psoriasis; advanced age (13) | NK | C. albicans, C. parapsilosis (25) | Yes |
| Dialysis access Hemodialysis fistulas | 2.4 × 105 (patients treated) (24) | 1-4 (20, 61) | ≪1 | 25-50 (83) | PTFE grafts; number of graft revisions; nursing home residents; poor hygiene; bacterial infection at a distant site; hospitalization; duration of graft use; femoral site; diabetes mellitus; S. aureus nasal carriage (5, 61, 71) | NK | C. albicans | Yes (83) |
| Hemodialysis grafts | 10-35 (5, 20, 61) | ≪1 (5) | 25-50 (83) | NK | C. albicans | Yes | ||
| Peritoneal dialysis catheters | 23 (61) | 2.4-7 (39, 61, 69, 114, 117) | 5-25 (69, 117) | Prior hospitalization; recent bacterial peritonitis; gastrointestinal diseases; prior antibiotics; lupus (39, 71) | C. albicans, C. parapsilosis (69) | Yes (39, 54) | ||
| Cardiac devices | ||||||||
| Prosthetic valves | 8.5 × 104 (24) | 2.9 (66) | 2-10 (45, 66) | 33g (112) | Native valve endocarditis; black race; mechanic prosthesis (versus bioprosthesis); male sex; longer cardiopulmonary bypass time; receipt of multiple valves (16, 45) | Intravenous catheters; intravenous drug use; prosthetic valve recipients; fungemia; immunosuppression; total parenteral nutrition; prior bacterial endocarditis; prolonged antibiotic use (8, 66, 78) | C. albicans, C. glabrata plus C. parapsilosis (66) | Yes (80, 122) |
| Pacemakers | 4 × 105 (24)f | 0.5-7.0 (36, 50, 120) | 4.5 (6, 50, 120) | NK | Malnutrition; malignancy; diabetes mellitus; skin disorders; steroid or anticoagulant use (50) | NK | C. glabrata, C. albicans | Yesc (11, 52) |
| ICDs | 2.2-7.2 (60) | <1 | NK | Median sternotomy; longer duration of surgery; generator replacement; depressed immunity; diabetes mellitus; advanced age; another nidus of infection (107) | NK | C. albicans | Yesc (60) | |
| VADs | 700 (24) | 28-66 (77) | 25-39 (38) | 100 (patients with other complications affecting mortality) | Postoperative bleeding necessitating reoperation; chronic underlying disease; receipt of broad-spectrum antibiotics; presence of indwelling tubes (77) | NK, but possibly same as for fungal infections in ICUs (38) | C. albicans, C. tropicalis (40) | Yesd (38) |
| Central nervous system devices | ||||||||
| VPSs | 4 × 104 (24) | 6-15 (73, 99) | 1 (99) | 9-30 (99) | Yong age; other risk factors not well documented (74) | Broad-spectrum antibiotics; prior or concurrent bacterial meningitis; cerebrospinal fluid leakage; bowel perforation; abdominal surgery; steroids; indwelling catheters (35, 73, 82, 99) | albicans tropicalis (35) | Yese (76, 99, 104) |
| Urinary catheters | 3 × 107 (24) | 10-30 (24) | 21 (97) | 19.8-39 (46, 53, 105) | Duration of catheterization; lack of drainage; microbial colonization of the drainage bag; diabetes mellitus; absence of antibiotic use; female sex; abnormal serum creatinine level; errors in catheter care (87) | Diabetes mellitus; urinary tract infection; malignancy; antibiotic use; female sex; ICU patient (41, 53) | C. albicans, C. glabrata (97, 105) | Yesf |
| Penile implants | 1.5 × 104-2 × 104 (24) | 1-9 (17, 47) | 5-9.2 (75) | NK | Urinary tract infection; Spinal cord injury; Insertion of an inflatable device; Neurogenic bladder; Diabetes mellitus; reimplantation; revisions (18, 47) | NK | C. albicans | Yesc |
NK, not known.
Catheter removal may not be necessary in neutropenic patients in whom the infecting fungal organisms originate primarily from the gastrointestinal tract (see the text).
Based on case reports.
Little evidence in the literature to guid treatment.
Not well documented in the literature.
If symptomatic. Includes both pacemakers and ICDs.
Mortality with combined antifungal and surgical therapy.
CENTRAL VENOUS CATHETERS
Over 5 million central venous catheters (CVC) are inserted annually in the United States, accounting for 0.28 to 0.8 central-line days/patient-day (24, 79a). It is estimated that 2 to 12% of CVCs result in sepsis (109). Analysis of the National Nosocomial Infections Surveillance (NNIS) data shows that 87% of primary bloodstream infections occurred in patients with a central line. In U.S. intensive care units (ICUs), approximately 80,000 catheter-related bloodstream infections occur each year and result in up to 20,000 deaths (68). The impact and cost of such infections is thus enormous. Before 1992, there was a steady increase in the incidence of candidemia in combined medical-surgical ICUs (79a), but the contribution of Candida to bloodstream infections stabilized between 1992 and 1998 at about 11.5% (96). A total of 72 to 87% of bloodstream infections, including candidemia, are considered to be catheter related in ICU patients (94, 96). The role of catheters in neutropenic patients is less clear than that in ICU patients because gastrointestinal mucositis is a probable source of candidemia in these patients. Candidemia is an independent risk determinant for predicting death in patients with nosocomial bloodstream infections (70).
The crude mortality due to candidemia has been estimated to be as high as 57%, but the attributable mortality is reported to be 38% (118). The attributable mortality of candidemia was correlated, in a multivariate logistic analysis, with the APACHE II score, the duration of candidemia, and rapidly fatal underlying illnesses (29% of study patients were neutropenic) (32). Other predictors of adverse outcome included evidence of neutropenia and visceral dissemination (4). In general, the likelihood of developing CVC-related infections depends on the type of catheter, the hospital service, the site of insertion, and the duration of catheter placement (68). Risk factors for CVC-related-infections include neutropenia for >8 days, hematologic malignancy, total parenteral nutrition, duration of site use, frequent manipulation of the catheter, improper insertion and maintenance of the catheter, and high APACHE II score (89, 109).
Candidemia most frequently occurs in immunocompromised patients with underlying malignancy, hematologic disorder, gastrointestinal disease, burns, or critical illness (119). Independent risk factors for candidemia include Candida isolated from other sites than blood, hemodialysis, prior insertion of a Hickman catheter, and previous exposure to antibiotics (118, 119). One study, however, found that neutrophil count rather than underlying disease was the most important risk factor (3). Catheter-related infection should be suspected in the presence of the following findings: growth of C. parapsilosis from blood cultures, positive quantitative or differential time to positivity of central versus peripheral blood cultures, receipt of hyperalimentation through the catheter, lack of other potential sources of Candida infection, and persistent candidemia despite appropriate systemic antifungal therapy.
Diagnosis of CVC-related infections can be difficult because the clinical findings for both fungal and bacterial infections are often unreliable. Laboratory methods include semiquantitative (roll plate, the most widely used method) and quantitative (vortex or sonication method) methods for culturing the catheter. The quantitative culturing method retrieves organisms from both the external and internal surfaces of the catheter, whereas the semiquantitative method retrieves organisms from only the external surface. Although the quantitative culturing method can be 20% more sensitive than the semiquantitative method, it is more time-consuming. Other diagnostic approaches include paired blood cultures, with one drawn from the CVC and the other obtained percutaneously. When using quantitative blood cultures, the growth of 5- to 10-fold-greater colony counts from blood samples obtained via the CVC than from peripherally drawn blood samples can accurately relate the episode of bloodstream infection to the indwelling catheter (68), particularly a tunneled catheter. The differential time to positivity constitutes another potentially helpful method for associating a bout of bloodstream infection with an indwelling catheter, if cultures of blood obtained through a CVC become positive at least 2 h earlier than cultures of peripherally obtained blood, the infection is concluded to be associated with the catheter.
C. albicans accounts for up to 63% of all cases of candidemias (32, 96), followed by C. glabrata or, in some hospitals, C. tropicalis (32). However, in recent years, non-albicans species have been isolated more frequently (23, 81). For instance, one prospective study of 427 patients with candidemia (81) found that non-albicans species accounted for 48% of all cases of candidemia, a disturbing fact since the non-albicans spp. can be associated with higher mortality and complication rates than C. albicans, including breakthrough candidemia during antifungal therapy (32, 81). A similar shift in Candida epidemiology was also reported in a study of 474 patients with malignancies (3), presumably due to antifungal prophylaxis use.
Whereas the prevention of CVC-related infections by using an antimicrobial coating has been shown to be beneficial in preventing bacterial infections (26, 67), this approach has not been specifically studied for preventing fungal infections of CVCs. Optimal treatment of CVC-related infections depends on the kind of catheter used (tunneled versus nontunneled), the severity of illness, and the type of causative organism. For instance, coagulase-negative staphylococcal infections of nontunneled catheters may be cured without catheter removal. Such patients are usually treated with intravenous antibiotics, often in combination with antibiotic lock therapy, which involves exposing the catheter lumen to pharmacological concentrations of antibiotics for hours or even days. The potential ability of this therapeutic approach to increase the likelihood of catheter salvage has been reported only in a noncomparative fashion. This antibiotic lock technique, however, has not been studied for the treatment of documented CVC-related candidemia. Recently published guidelines by the Infectious Disease Society of America recommended treatment of both complicated and uncomplicated Candida infections of tunneled and nontunneled CVCs by catheter removal in addition to systemic antifungal therapy, generally for 14 days after the last positive blood culture (95). However, catheter removal may not be necessary for cure of candidemia in neutropenic patients in whom the infecting fungal organisms originate primarily from the gastrointestinal tract (85). A review of published data on the effect of CVC removal on the outcome of patients with candidemia found that studies were somewhat conflicting, and the authors of that review suggested that removal of infected CVCs may not have an impact on the outcome in neutropenic patients (85).
JOINT PROSTHESES
In modern medicine, the most commonly implanted joint prostheses are hip and knee prostheses. The incidence of infection is low, 1% in primary cases and up to 3% in secondary procedures (108). Candida accounts for less than 1% of all cases of infections of joint prostheses. Nevertheless, the magnitude of this infectious complication is quite remarkable, considering that in 1995 about 216,000 total knee replacements were performed, and this number is expected to more than double by the year 2030 (108). This increase in the number of implanted joint prostheses is stimulated by the growing size of the patient population, particularly of older persons, who are most likely to require the implantation and revision of joint prostheses (108). Candida infections of prosthetic joints mostly involves hip and knee prostheses, with only a case report involving other joint prostheses (63). Implantation of knee and hip prostheses carries a higher risk for infection than smaller joint prostheses due to the longer duration of these operations, the inherently low blood flow to cortical bone, and the formation of a hematoma in a larger dead space around such larger devices. These hematomas can devascularize the surrounding tissue and prevent the entry of antibiotics (108). In general, the mean cost of management of an episode of joint infection is estimated to exceed $50,000. The cost is probably even higher for Candida infection because of frequent delays in diagnosis and more prolonged treatment of this fungal infection compared with bacterial infection. The mortality due to prosthetic joint infections is low (24); mortality due to Candida infections is not known in this setting.
Risk factors for infection of prosthetic joints include prior surgery at the site of the prosthesis, rheumatoid arthritis, immunocompromised state, diabetes mellitus, poor nutritional status, obesity, psoriasis, and advanced age (13). A large case-control study of patients at high risk for prosthetic joint infection identified four independent risk factors: surgical site infection, NNIS System surgical patient risk index score of ≥1, history of malignancy, and history of prior total joint arthroplasty (9). In that study (9), rheumatoid arthritis was not found to be an independent risk factor after adjustment for other potentially confounding variables associated with that condition such as steroid therapy, immunologic abnormalities, higher prevalence of revisions, and skin ulcers. Because there are no comprehensive reviews of Candida infections of prosthetic joint infections, it is unclear if the above-mentioned risk factors apply. A review of 10 cases of Candida prosthetic joint infections concluded that such patients, unlike those with Candida infections of natural bone and joints, are not inherently predisposed to Candida infections and are less likely to have evidence of extra-articular candidiasis (25).
The clinical presentation of prosthetic joint infections depends on early (<3 months after surgery) versus late (3 months to 2 years after surgery) manifestations (123). Early infections manifest with pain, erythema, edema, wound healing disturbances, and fever, whereas late manifestations include persistent pain and early loosening with or without fever (123). In the review of Candida prosthetic joint infections, 2 of 10 patients presented late (25). Although hematogenously disseminated fungi other than Candida most often cause fungal infections of natural joints, fungal prosthetic joint infections are usually caused by Candida spp., including C. albicans, C. parapsilosis, and C. glabrata (25). Candida prosthetic infections can occur up to 4 years after surgery (25, 92).
Because joint infections constitute a relatively rare complication and because confirmation of successful treatment requires a long follow-up (up to 2 years), treatment strategies have not been standardized (37, 108). This is particularly true for Candida prosthetic infections, where the relevant medical literature contains only case reports. In general, treatment of Candida prosthetic infection consists of both prosthesis removal and systemic antifungal therapy. Few reports (33) have described cure of Candida infection without removal of the infected prosthesis. Removal of the implant is associated with large skeletal defects, shortening of the extremity, and severe functional impairment. The impact of the type of causative organism on salvage rates is not clear (51). For bacterial infections, it has been suggested that in a selected group of patients, prosthesis salvage may be achieved with prolonged suppressive treatment (101). So far, this strategy has not been successfully applied to Candida infections. Factors that reportedly contribute to failure of therapy for infection of hip prostheses include previous operations, immunocompromised status, early postoperative infection, and retention of bone cement (111).
DIALYSIS ACCESS
The number of persons with a dialysis access continues to rise. In the 1999 United States Renal Data System report (http://www.usrds.org), about 243,000 persons required either hemodialysis or peritoneal dialysis, as compared with 128,000 persons in 1990. Infection is the second most common cause of death in patients with end-stage renal disease. It is the most frequent cause of hospitalizations and is a leading cause of morbidity and mortality in patients requiring dialysis (5, 20). Hemodialysis access can be an arteriovenous prosthetic graft, an arteriovenous fistula, or a catheter. Associated infection rates per patient-day are the lowest with arteriovenous fistulae, followed by grafts, tunneled or cuffed catheters, and nontunneled or noncuffed catheters (39, 61). Bacteria account for the majority of cases of hemodialysis-related infections. Fungal infections of hemodialysis access sites are rare; Candida accounts for 2.6 to 7% of peritoneal dialysis-related infections (39, 61, 69, 114). Dialysis patients are more prone to infection than are the general population, both because of the uremia and because of the dialysis itself. This predisposition is multifactorial and is attributed to impairments in lymphocyte and granulocyte function, circulating inhibitors to chemotactic factors, frequent violation of skin and mucosal barriers, baseline hypothermia and iron overload, underlying disorders, low albumin levels, and metabolic acidosis (20, 71). In addition, in patients undergoing peritoneal dialysis the high glucose concentration, the low pH, and the osmolarity of the dialysis solution further affect the ability of the immune system to handle microbes. Risk factors for all infections in patients receiving dialysis include polytetrafluoroethylene (PTFE) grafts, number of graft revisions, residence in a nursing home, poor hygiene, bacterial infection at a distant site, hospitalization, duration of graft use, femoral site, diabetis mellitus, and S. aureus nasal carriage (5, 10, 61, 71).
Permanent Hemodialysis
Permanent hemodialysis access is preferable secured with an arteriovenous fistula or, if not feasible, with arteriovenous grafts that are made mostly of PTFE (5). Whereas the overall infection rate of these grafts can be as high as 35%, fungal etiology is extremely rare, with only case reports published in the literature (5). Use of PTFE grafts and a larger number of graft revisions are independently associated with hemoaccess site infections (10). Most patients with Candida infection of PTFE grafts received antecedent antibacterial agents (83). The onset of Candida infection can be acute or subacute and most commonly manifests with drainage at the fistula site (20, 83). Whereas graft salvage may be successfully accomplished in selected patients with bacterial infections (5), treatment of Candida infections usually requires graft removal to achieve long-term cure (83).
Peritoneal dialysis
In 1999, a total of 22,797 persons received peritoneal dialysis. In general, peritoneal dialysis is associated with higher rates of infection than hemodialysis (61) because the hyperosmolar peritoneal dialysis fluid with high glucose and low pH can act as an irritant, provide a good growth medium for pathogens, and suppress the host response (61). Infection can affect the exit site, the catheter tunnel, and the peritoneal space. We focus our discussion in this section on peritonitis related to the dialysis catheter. The currently used permanent catheters are made of silicone rubber and are affixed with one or two Dacron cuffs to stabilize the catheter (114). In general, infection can be transluminal, periluminal, transmural, hematogenous, or ascending. The diagnosis is made on the basis of the presence of two of the following criteria: symptoms of peritoneal inflammation, cloudy fluid (>100 white blood cells/mm3), and/or organisms on Gram stain or in culture of the peritoneal dialysis fluid (114).
As many as 23% of peritoneal dialysis catheters become infected. Fungi reportedly cause up to 15% of peritonitis cases, and Candida spp. are the most common fungal isolates (54). The incidence of fungal peritonitis over the past decade appears to have stabilized, whereas the incidence of bacterial peritonitis seems to be decreasing (39). This finding is quite interesting given that fungal peritonitis is most often preceded by bacterial peritonitis (54). Recent reports indicate that about 2.6 to 7% of patients undergoing peritoneal dialysis develop Candida peritonitis. Non-albicans Candida spp. account for up to two-thirds of Candida isolates (39, 69, 117). In these reports, C. parapsillosis seems to be the most prevalent non-albicans sp. This infectious complication is associated with a high mortality (5 to 25 %) and morbidity, including prolonged hospital stay and recourse to hemodialysis (69, 117).
Risk factors for fungal peritonitis include prior hospitalization, recent episodes of bacterial peritonitis, gastrointestinal disease, and treatment with antibiotics (39, 69, 114). There is often a diagnostic delay in diagnosing fungal peritonitis, and failure to respond to treatment with antibacterial agents can be a helpful clue. Treatment of fungal peritonitis can be difficult due to poor penetration of older antifungal agents into the peritoneal cavity and their irritant nature (54). The role of newer antifungal agents for treatment of fungal peritonitis has not been thoroughly investigated. Although there is no real consensus on the choice and duration of systemic antifungal therapy for fungal peritonitis, most authors agree that catheter removal is usually needed to achieve cure (39, 54, 69).
CARDIOVASCULAR DEVICES
Heart Valves
Prosthetic valve endocarditis (PVE) is an infrequent but serious complication of cardiac valvular replacement, with a reported rate of 2.9 to 4.4% (15, 66). The incidence changes over time, and the actuarial risk has been estimated at 3.1% at 1 year and increases to 5.7% at 5 years (16). Fungi are responsible for 2 to 10% of all cases of PVE, and Candida accounts for up to 90% of these fungal infections (45, 66). Patients with prosthetic heart valves who develop nosocomial candidemia have a notable risk of developing Candida PVE, often months or years later (up to 690 days later). In a review of 44 cases of candidemia in patients with prosthetic heart valves, Candida PVE developed in 25% of such patients (78).
Fungal PVE is associated with a higher mortality rate than bacterial PVE. A review (112) of fungal endocarditis concluded that untreated fungal PVE was uniformly fatal, antifungal agents alone or surgery alone reduced the mortality rate to 82%, whereas combined surgery and antifungal therapy decreased the mortality rate to 33%. Others (66) found a 33% overall mortality rate due to fungal PVE with a mean follow-up of 4.5 years. Patients with complicated PVE (congestive heart failure or persistent fungemia) had higher mortality rates regardless of the mode of therapy (80). Surprisingly, one study reported that patients with Candida PVE had a better survival than those with candidemia alone (mortality rates of 25 and 83%, respectively, at 1 year) (78).
Risk factors for PVE generally include receipt of multiple heart valves (16), male gender, prolonged cardiopulmonary bypass time, antecedent native valve endocarditis, mechanic prosthetic valve (as opposed to bioprosthetic valve), and black race (45). Mechanical prostheses seem to be associated with a higher risk of early PVE; however, one study found that porcine valve recipients have a higher risk of late PVE (16). There seems to be a discrepancy between studies regarding the difference in incidence of infection of aortic versus mitral prostheses. Specific risk factors for fungal PVE include the presence of intravascular catheters, prior bacterial endocarditis, prolonged (more than 4 weeks) antibiotic treatment, total parenteral nutrition, intravenous drug use, disseminated fungal infection, prosthetic valve recipient, and immunosuppression (8, 66).
Diagnosis of fungal PVE can be challenging because up to one-third of patients may not have any of the classic signs of endocarditis (100). Making a correct diagnosis carries therapeutic implications because fungal PVE usually requires surgical intervention whereas fungemia without PVE may be treated with antifungal agents alone (78). Fungal PVE should be suspected in the presence of negative bacterial blood cultures, bulky vegetations, metastatic infection, perivalvular invasion, embolization to large blood vessels, and disseminated fungal infection (66, 80, 100). C. albicans is the most common pathogen, accounting for 56 to 66% of all cases of Candida PVE. Less common causes include C. glabrata and C. parapsilosis (66, 78).
Combined medical-surgical therapy is the current standard of therapy of fungal PVE (80, 122). Although one study reported that the mortality in patients with uncomplicated PVE was rather similar in those who received medical therapy alone and in those who underwent combined medical-surgical therapy (40 and 33%, respectively) (80), the authors of that study concluded that medical therapy alone should be considered only for patients for whom surgery is regarded as unduly hazardous.
Pacemakers
It has been estimated that about 400,000 implantable electrophysiologic cardiac devices are placed annually in the United States (24). Infectious complications involving pacemakers have decreased in frequency as a result of improvements in surgical techniques and device technology, but they remain in the range of 0.5 to 7% (36, 50, 120). Candida spp. account for up to 4.5% of these infections (6, 49, 120). One study of more than 8,000 procedures noted pacemaker infections in 5.6% and endocarditis in 0.5% of patients, using careful definitions (6). Candida pacemaker infections have been described mostly in case reports or as single cases in series (14, 49, 59, 98, 115, 120), and so the mortality due to these infections is not well assessed. Risk factors for pacemaker infections include malnutrition, malignancy, diabetes mellitus, skin disorders, and steroid or anticoagulant use (52). Specific risk factors for Candida pacemaker infections have not been identified. The reported cases have been caused by C. glabrata, C. albicans, and, less frequently, C. tropicalis.
Pacemaker infections can be categorized into two groups: (i) Infections of the pulse generator pocket and/or the subcutaneous portion of the lead (pocket infections), and (ii) infections of the transvenous intravascular electrode component (pacemaker-related endocarditis). Pacemaker pocket infections occur either within a month of the pacemaker placement or later as a consequence of the device eroding through the skin (36, 52). These infections are mostly caused by skin organisms; C. albicans has been implicated in a single case report (22). Early infections usually present with local erythema, pain, wound breakdown, and drainage (21, 52). Fever is often absent. In one series of 87 pacemaker pocket infections, fever was documented in only 19% of the patients (21). The optimal management is under debate in the literature. In general, complete device removal and antimicrobial therapy has been most successful (11, 21), although lead-preserving procedures have been shown to be successful in certain cases (121). Conservative treatment should be limited to patients presenting with skin erosions or low-grade pocket infection (11). In the single report of C. albicans pacemaker pocket infection, cure required both device removal and antifungal therapy (22).
Pacemaker-related endocarditis represents about 10% of pacemaker infections (49) and most commonly arises from infection of the subcutaneous portion that has tracked intravascularly, seeding the intracardiac electrode. Therefore, pacemaker endocarditis and early PVE have similar microbiological profiles (49). Most infections are caused by Staphylococcus spp. By 1997, only six cases of pacemaker endocarditis due to Candida had been reported (49). Since then, we have found five additional cases: one due to C. albicans in a polymicrobial infection (14), one due to C. glabrata and C. albicans (98), two caused by C. glabrata (one of these patients had polymicrobial infection) (115, 120), and one caused by C. tropicalis (59). A review of published cases of Candida pacemaker endocarditis revealed a delay between pacemaker placement and diagnosis from <2 months to 8 years (49). Most patients with Candida pacemaker-related endocarditis presented with fever; other symptoms included lethargy, chills, and dyspnea. As with pocket infection, there generally appears to be an advantage for combining surgical and medical treatment of pacemaker-related endocarditis over using medical treatment alone (11, 52). Total removal of the pacemaker system is the most reliable way of eradicating pacemaker-related infection (11). Reports of success with conservative management alone have been based on small numbers of patients (52). The literature does not specify whether the nature of the pathogen affects the need for surgery, and so it is likely that the same surgical recommendations apply to bacterial and candidal pacemaker infections.
Implantable Cardioverter Defibrillators
The use of implantable cardioverter defibrillators (ICD) constitutes an important modality in the management of cardiac arrhythmias. These devices can be implanted either through thoracotomies or via the more recently utilized transvenous approach. One of the most serious complications of the use of ICDs is infection, with an incidence rate as high as 7.2% (60). Risk factors for infection of ICDs placed through thoracotomy include median sternotomy, long duration of surgery, generator replacement, depressed immunity, diabetes mellitus, advanced age, and the presence of another nidus of infection. A retrospective study of 202 ICDs implanted using the thoracotomy approach found that diabetes mellitus was the only variable associated with the development of infection (107). Of the 171 implants analyzed, 9 (4.5%) became infected, and most infections occurred in the first 3 months after implantation. The clinical presentation included pain, fever, and fluid collection around the generator. All nine cases of infection were bacterial, and all required device removal in addition to antimicrobial therapy. Transvenously placed ICDs are associated with a significantly lower incidence of infection than are those implanted through thoracotomy (110). A study of ICD infections from 1992 to 1995 related the escalating use of the transvenous approach to the decrease in the overall incidence of infection from 16.7 to 6.9% (60). Of the seven infected patients, only one had Candida infection, and that occurred in conjunction with bacterial infection. In conclusion, fungal ICD infections are extremely rare and usually co-occur with bacterial infections.
Ventricular Assist Devices
Ventricular assist devices (VADs) are either implantable or extracorporeal. Implantable VADs are usually used to provide temporary support, sometimes for up to 1 year, until patients receive a heart transplant (113). Such implantable VADs are currently being evaluated as a permanent therapy for end-stage heart failure, and so their use is expected to rise in the near future. Extracorporeal VADs are usually used to support patients with severe heart failure when cardiac function is expected to improve within days to weeks. Infectious complications affect both types of devices, affecting about 50% of all VAD recipients (77). However, due to different definitions and duration of VAD support, the reported incidence rates vary greatly, being anywhere from 28 to 66% (77). Fungal colonization or infection has been detected in 35 to 39% of patients with VADs (38). A recent study found that 49% patients with VADs develop bloodstream infections and that 38% of such infections were associated with the device. Candida spp. were found in 19 of these patients and had the highest hazard ratio of 10.9 (40).
Mortality rates are hard to assess in the literature because patients needing VADs most often have other serious complications affecting both mortality and morbidity. Nevertheless, the impact of infection of VADs is enormous because persistent infection may require VAD removal, which can be done only if a heart donor is available or if heart function significantly improves (77).
Risk factors for VAD infections include postoperative bleeding necessitating reoperation, chronic underlying diseases, receipt of broad-spectrum antibiotics, and presence of indwelling tubes (77). Additionally, the VAD itself affects the immune system. The interaction of the VAD with blood seems to activate T cells, causing their death, and causes B-cell hyperreactivity with dysregulated immunoglobulin synthesis. This progressive defect in cellular immunity increases the risk of fungal infections (38). Specific risk factors for fungal VAD infections have not been defined. It has been suggested that the same risks apply for fungal infections of VAD recipients and for patients in ICUs: fungal colonization, prior antibiotics, total parenteral nutrition, prior hemodialysis, indwelling catheters, abdominal surgery, and prolonged ICU stay (38).
Treatment of fungal infections of VADs usually consists of systemic antifungal therapy and device removal. So far, there have been no reports in the literature of long-term survival after a fungal VAD infection (38).
CENTRAL NERVOUS SYSTEM DEVICES
Ventriculoperitoneal Shunts
Most currently used ventriculoperitoneal shunts (VPS) are made of silicone polymers. Obstruction and infections are the two most common complications, with infection occurring in 6 to 15% patients with these devices (73, 99). Candida is the causative agent in 1% of these infections (99). However, this reported incidence may be underestimated, considering that some of the culture-negative shunt infections may be fungal in etiology (65). The mortality of Candida VPS infections is estimated to be 9% (99).
Risk factors for Candida shunt infections and meningitis include the use of broad-spectrum antibiotics, prior or concurrent bacterial meningitis, cerebrospinal fluid leakage (35, 82, 99), bowel perforation and/or abdominal surgery (99), steroids, and indwelling catheters (73).
In one review (99), 77% of Candida infections developed within 3 months of shunt manipulation, suggesting inoculation of the organism during the surgery. Transient candidemia and secondary colonization of the VPS have been suggested by other investigators as possible sources of the infecting Candida organisms (102). The most common species are C. albicans and C. tropicalis. The clinical presentation of Candida shunt infection depends on the location of the infection. Distal shunt infection refers to an infection at the site of shunt drainage, which is either a vascular site or the peritoneum. If the shunt drains into a vascular site like the right atrium, the symptoms are usually nonspecific and include fever and malaise. An infected shunt draining into the peritoneum causes mesothelial inflammation and subsequently decreases drainage. A “shuntoma” can develop when the peritoneum encysts the fluid. Proximal Candida shunt infections are manifested by symptoms of shunt malfunction associated with high intracranial pressure, including headaches, nausea, vomiting, and altered mental status. The most common symptoms of VPS infections include fever (31%), hydrocephalus (36%), and meningoencephalitis (21%) (99). Compared to patients with Candida meningitis without shunt infection, patients with Candida infection of VPSs had a lower frequency of hypoglycorrhagia and lower white blood cell count (73 cells/mm3) in the cerebrospinal fluid. Other rarer clinical manifestations of Candida shunt infections include basal ganglion granuloma surrounding a nonfunctional shunt in a previously healthy person (106).
Candida shunt infections present some therapeutic dilemmas, which are far from being universally approached (76). Most authors agree that removal of the device is necessary to clear the infection (99). So far, the most commonly used antifungal agent is intravenous amphotericin B with or without flucytosine (76, 104). Several factors limit the use of amphotericin B in central nervous system fungal infections. This drug crosses the blood-brain barrier poorly, adverse events limit the amounts that can be given systemically, and its intrathecal use is associated with significant toxicity such as arachnoiditis (27). Delayed sterilization does not seem to worsen the prognosis of Candida meningitis (104), and so most authors recommend shunt removal and only intravenous amphotericin B. Some authors (99) recommend intrathecal use of antifungal therapy only if the patient's clinical condition is poor. The role of the newer antifungal agents has not been established yet. Although the lipid formulations do not cross the blood-brain barrier significantly better than native amphotericin B, several of the new azole agents do.
URINARY CATHETERS
Over 30 million bladder catheters are inserted annually in the United States, with a 10 to 30% overall rate of infection (24). In a NNIS report of nosocomial infections between 1992 and 1997, the urinary tract was the most common site of infection in medical ICUs (31%) and most cases of urinary tract infections were catheter related. Candida species accounted for 31% of urinary isolates in the same study, compared to 22.1% reported between 1986 and 1989 (97).
Candida infections of the urinary tract are strongly associated with the presence of a urinary catheter. The NNIS data indicated that C. albicans caused 21% of catheter-associated urinary tract infections, in contrast to 13% of non-catheter-associated infections (97). In a multicenter surveillance study of 861 patients with funguria, 83% of patients had some form of urinary tract drainage systems, the majority of which were urethral catheters (53). Although the overall mortality among patients with candiduria is reported to be as high as 39% (46, 53, 105), this high mortality rate is mostly attributed to the multiple serious underlying illnesses found in patients with funguria.
Candida growth in urine represents a spectrum of states, ranging from external perineal colonization, catheter infection, cystitis, or even secondary seeding from undetected bloodstream infection. Risk factors for funguria include diabetes mellitus, urinary tract abnormalities, malignancy, and antibiotic use (53). In one study (41), female gender and ICU stay were most strongly associated with funguria due to C albicans and C. glabrata whereas fluconazole and quinolone exposure were specific risk factors for funguria due to C. glabrata.
C. albicans is the fungal species isolated most frequently from urine specimens. According to the NNIS findings, C. albicans accounts for 21% of all urinary isolates and other Candida spp. account for only 10% of isolates (97, 105). In another study (53), C. albicans was found in 51.8% of patients with candiduria, followed by C. glabrata (15.6%).
There are no universally established criteria for assessing the clinical significance and therefore guiding the management of asymptomatic candiduria. Some authors think that asymptomatic candiduria is an early marker of disseminated infection in critically ill patients and may require treatment (79), whereas others have not found asymptomatic candiduria to be a predictor for candidemia or dissemination but, rather, a benign and self-limiting condition (53, 105).
Controversy also exists about whether to treat asymptomatic candiduria. In a surveillance of 861 patients, funguria cleared in 75% of patients without any therapy (53), in 35.5% of patients who had the catheter removed as the only intervention, and in 50.2% of patients given antifungal therapy. Treatment regimens vary. Bladder irrigation with amphotericin B and with oral fluconazole were equally efficient in eliminating candiduria, but recurrences were common with both approaches (46). A prospective study that compared fluconazole with placebo in eradicating candiduria in asymptomatic or minimally symptomatic patients found that fluconazole initially cleared candiduria in 50% of patients, in contrast to 29% in the placebo group. However, cultures at 2 weeks revealed similar rates of candiduria among treated and untreated groups (105). Debate also exists about the threshold of organism concentration (104 versus 105 CFU/ml) in urine that may potentially be used as a criterion for treating candiduria (46, 53). Symptomatic Candida infection of the catheterized urinary tract is treated with antifungal therapy, usually for at least 5 to 7 days in patients with cystitis and 14 days in those with pyelonephritis.
PENILE IMPLANTS
About 15,000 to 20,000 silicone penile prostheses are inserted each year in the United States (24). A pseudocapsule forms around the device, and infections usually occur in the periprosthetic space between the pseudocapsule and the device surface (72). The reported overall rate of infection ranges from 1 to 9% and is higher (18%) in patients with reconstructive procedures or surgical revisions (17, 47). Bacteria account for the vast majority of cases of penile implant-related infections, whereas yeast infections are relatively rare. C. albicans has been reported to cause 5 to 9.2% of infections of penile implants (75).
The mortality specifically related to Candida infections of penile implants remains unknown. However, these infections result in major morbidity with potentially disastrous complications. Cure of infection usually requires removal of the infected device, which can be complicated by subsequent fibrosis formation and loss of tissue. This can make later reimplantation of another penile prosthesis rather difficult or even impossible.
Known risk factors for infections of penile prostheses include urinary tract infection, spinal cord injury, insertion of an inflatable device, neurogenic bladder, diabetes mellitus, reimplantation, and revisions (18, 47). Whether the rate of penile prosthesis-related infection is higher in diabetic and spinal cord-injured patients than in the general population remains controversial (47). Some authors found a statistically insignificantly higher rate of infection in diabetics than nondiabetics, with reported rates of 2.8 and 0.9% (34) and 22 and 6.7% (64), respectively. However, in other reports, diabetics did not appear to be at a greater risk for infection than nondiabetics (47, 72).
In one study (47), the majority of bacterial infections occurred within 3 months after surgery, whereas others observed more than half of the cases manifesting more than 7 months after either implantation or revision. Hematogenous infections occurred anywhere from 8 to 54 months after implantation. Due to the paucity of reported cases of Candida infections of penile prostheses, it is unclear whether the clinical profiles in such cases are similar to those of bacterial infections. C. albicans abscess following penile prosthesis placement reportedly formed 3 months postoperatively (86). The type of infecting Candida species is often not specified in the case reports. One small study of 11 patients undergoing salvage operation reported two Candida infections, one C. albicans infection, and one C. glabrata infection (12).
REFERENCES
- 1.Reference deleted.
- 2.Reference deleted.
- 3.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie, E. J., J. H. Rex, O. Uzun, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, J. E., A. S. Chang, and M. P. Anstadt. 2000. Polytetrafluoroethylene hemoaccess site infections. Asaio J. 46:S18-S21. [DOI] [PubMed] [Google Scholar]
- 6.Arber, N., E. Pras, Y. Copperman, J. Schapiro, V. Meiner, I. Lossos, A. Militianu, D. Hassin, E. Pras, S. A., M. Moshkowitz, and Y. Sidi. 1994. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine 73:299-305. [DOI] [PubMed] [Google Scholar]
- 7.Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671-679. [DOI] [PubMed] [Google Scholar]
- 8.Bayer, A. S., A. F. Bolger, K. A. Taubert, W. Wilson, J. Steckelberg, A. W. Karchmer, M. Levison, H. F. Chambers, A. S. Dajani, M. H. Gewitz, J. W. Newburger, M. A. Gerber, S. T. Shulman, T. J. Pallasch, T. W. Gage, and P. Ferrieri. 1998. Diagnosis and management of infective endocarditis and its complications. Circulation 98:2936-2948. [DOI] [PubMed] [Google Scholar]
- 9.Berbari, E. F., A. D. Hanssen, M. C. Duffy, J. M. Steckelberg, D. M. Ilstrup, W. S. Harmsen, and D. R. Osmon. 1998. Risk factors for prosthetic joint infection: case-control study. Clin. Infect. Dis. 27:1247-1254. [DOI] [PubMed] [Google Scholar]
- 10.Bonomo, R. A., D. Rice, C. Whalen, D. Linn, E. Eckstein, and D. M. Shlaes. 1997. Risk factors associated with permanent access-site infections in chronic hemodialysis patients. Infect. Control Hosp. Epidemiol. 18:757-761. [DOI] [PubMed] [Google Scholar]
- 11.Bracke, F., A. Meijer, and L. van Gelder. 2000. Pacemaker lead complications: when is extraction appropriate and what can we learn from published data? Heart 85:254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brant, M. D., J. K. Ludlow, and J. J. Mulcahy. 1996. The prosthesis salvage operation: immediate replacement of the infected penile prosthesis. J. Urol. 155:155-157. [DOI] [PubMed] [Google Scholar]
- 13.Brause, B. D. 1989. Prosthetic joint infections. Curr. Opin. Rheumatol. 1:194-198. [DOI] [PubMed] [Google Scholar]
- 14.Cacoub, P., P. Leprince, P. Nataf, P. Hausfater, R. Dorent, B. Wechsler, V. Bors, A. Pavie, J. Piette, and I. Gandjbakhch. 1998. Paacemaker infective endocarditis. Am. J. Cardiol. 82:480-484. [DOI] [PubMed] [Google Scholar]
- 15.Calderwood, S. B., L. A. Swinski, A. W. Karchmer, C. M. Waternaux, and M. J. Buckley. 1986. Prosthetic valve endocarditis. Analysis of factors affecting outcome of therapy. J. Thorac. Cardiovasc. Surg. 92:776-783. [PubMed] [Google Scholar]
- 16.Calderwood, S. B., L. A. Swinski, C. M. Waternaux, A. W. Karchmer, and M. J. Buckley. 1985. Risk factors for the development of prosthetic valve endocarditis. Circulation 72:31-37. [DOI] [PubMed] [Google Scholar]
- 17.Carson, C. C., III. 1999. Management of prosthesis infections in urologic surgery. Urol. Clin. North Am. 26:829-839, x. [DOI] [PubMed] [Google Scholar]
- 18.Carson, C. C., III, and C. N. Robertson. 1988. Late hematogenous infection of penile prostheses. J. Urol. 139:50-52. [DOI] [PubMed] [Google Scholar]
- 19.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung, A. H., and L. M. Wong. 2001. Surgical infections in patients with chronic renal failure. Infect. Dis. Clin. North Am. 15:775-796. [DOI] [PubMed] [Google Scholar]
- 21.Chua, J., B. Wilkoff, I. Lee, N. Juratli, D. Longworth, and S. Gordon. 2000. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann. Intern. Med. 133:604-608. [DOI] [PubMed] [Google Scholar]
- 22.Cohen, T., V. Pons, J. Schwartz, and J. Griffin. 1991. Candida albicans pacemaker site infection. Pacing Clin. Electrophysiol. 14:146-148. [DOI] [PubMed] [Google Scholar]
- 23.Coleman, D. C., M. G. Rinaldi, K. A. Haynes, J. H. Rex, R. C. Summerbell, E. J. Anaissie, A. Li, and D. J. Sullivan. 1998. Importance of Candida species other than Candida albicans as opportunistic pathogens. Med. Mycol. 36:156-165. [PubMed] [Google Scholar]
- 24.Darouiche, R. O. 2001. Device-associated infections: a macroproblem that starts with microadherence. Clin. Infect. Dis. 33:1567-1572. [DOI] [PubMed] [Google Scholar]
- 25.Darouiche, R. O., R. J. Hamill, D. M. Musher, E. J. Young, and R. L. Harris. 1989. Periprosthetic candidal infections following arthroplasty. Rev. Infect. Dis. 11:89-96. [DOI] [PubMed] [Google Scholar]
- 26.Darouiche, R. O., I. I. Raad, S. O. Heard, J. I. Thornby, O. C. Wenker, A. Gabrielli, J. Berg, N. Khardori, H. Hanna, R. Hachem, R. L. Harris, G. Mayhall, and the Catheter Study Group. 1999. A comparison of two antimicrobial-impregnated central venous catheters. N. Engl. J. Med. 340:1-8. [DOI] [PubMed] [Google Scholar]
- 27.Davis, L. E. 1999. Fungal infections of the central nervous system. Neurol. Clin. 17:761-781. [DOI] [PubMed] [Google Scholar]
- 28.Donlan, R. M. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donlan, R. M. 2000. Role of biofilms in antimicrobial resistance. Asaio J. 46:S47-S52. [DOI] [PubMed] [Google Scholar]
- 30.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 31.Elek, S. D., and P. E. Conen. 1957. The virulence of Staphylococcus pyogenes for man. A study of the problems of wound infection. Br. J. Exp. Pathol. 38:573-586. [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser, V. J., M. Jones, J. Dunkel, S. Storfer, G. Medoff, and W. C. Dunagan. 1992. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 33.Fukasawa, N., and K. Shirakura. 1997. Candida arthritis after total knee arthroplasty—a case of successful treatment without prosthesis removal. Acta Orthop. Scand. 68:306-307. [DOI] [PubMed] [Google Scholar]
- 34.Garber, B. B., and S. M. Marcus. 1998. Does surgical approach affect the incidence of inflatable penile prosthesis infection? Urology 52:291-293. [DOI] [PubMed] [Google Scholar]
- 35.Geers, T. A., and S. M. Gordon. 1999. Clinical significance of Candida species isolated from cerebrospinal fluid following neurosurgery. Clin. Infect. Dis. 28:1139-1147. [DOI] [PubMed] [Google Scholar]
- 36.Giamarellou, H. 2002. Nosocomial cardiac infections. J. Hosp. Infect. 50:91-105. [DOI] [PubMed] [Google Scholar]
- 37.Gillespie, W. J. 1997. Prevention and management of infection after total joint replacement. Clin. Infect. Dis. 25:1310-1317. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg, S. P., J. W. Baddley, M. F. Aaron, P. G. Pappas, and W. L. Holman. 2000. Fungal infections in ventricular assist devices. Asaio J. 46:S37-S40. [DOI] [PubMed] [Google Scholar]
- 39.Goldie, S. J., L. Kiernan-Tridle, C. Torres, N. Gorban-Brennan, D. Dunne, A. S. Kliger, and F. O. Finkelstein. 1996. Fungal peritonitis in a large chronic peritoneal dialysis population: a report of 55 episodes. Am. J. Kidney Dis. 28:86-91. [DOI] [PubMed] [Google Scholar]
- 40.Gordon, S. M., S. K. Schmitt, M. Jacobs, N. M. Smedira, M. Goormastic, M. K. Banbury, M. Yeager, J. Serkey, K. Hoercher, and P. M. McCarthy. 2001. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann. Thorac. Surg. 72:725-730. [DOI] [PubMed] [Google Scholar]
- 41.Harris, A. D., J. Castro, D. C. Sheppard, Y. Carmeli, and M. H. Samore. 1999. Risk factors for nosocomial candiduria due to Candida glabrata and Candida albicans. Clin. Infect. Dis. 29:926-928. [DOI] [PubMed] [Google Scholar]
- 42.Hawser, S. P., G. S. Baillie, and L. J. Douglas. 1998. Production of extracellular matrix by Candida albicans biofilms. J. Med. Microbiol. 47:253-256. [DOI] [PubMed] [Google Scholar]
- 43.Hawser, S. P., and L. J. Douglas. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawser, S. P., and L. J. Douglas. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivert, T. S., W. E. Dismukes, C. G. Cobbs, E. H. Blackstone, J. W. Kirklin, and L. A. Bergdahl. 1984. Prosthetic valve endocarditis. Circulation 69:223-232. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs, L. G., E. A. Skidmore, K. Freeman, D. Lipschultz, and N. Fox. 1996. Oral fluconazole compared with bladder irrigation with amphotericin B for treatment of fungal urinary tract infections in elderly patients. Clin. Infect. Dis. 22:30-35. [DOI] [PubMed] [Google Scholar]
- 47.Jarow, J. P. 1996. Risk factors for penile prosthetic infection. J. Urol. 156:402-404. [DOI] [PubMed] [Google Scholar]
- 48.Jarvis, W. R. 1995. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 20:1526-1530. [DOI] [PubMed] [Google Scholar]
- 49.Joly, V., N. Belmatoug, A. Leperre, J. Robert, F. Jault, C. Carbon, and P. Yeni. 1997. Pacemaker endocarditis due to Candida albicans: case report and review. Clin. Infect. Dis. 25:1359-1362. [DOI] [PubMed] [Google Scholar]
- 50.Karchmer, A. W. 2000. Infections of prosthetic valves and intravascular devices, p. 912-913. In G. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, vol. 1. Churchill Livingstone, Inc., New York, N.Y. [Google Scholar]
- 51.Karchmer, A. W. 1998. Salvage of infected orthopedic devices. Clin. Infect. Dis. 27:714-716. [DOI] [PubMed] [Google Scholar]
- 52.Karchmer, A. W., and D. L. Longworth. 2003. Infections of intracardiac devices. Cardiol. Clin. 21:253-271, vii. [DOI] [PubMed] [Google Scholar]
- 53.Kauffman, C. A., J. A. Vazquez, J. D. Sobei, H. A. Gallis, D. S. McKinsey, A. W. Karchmer, A. M. Sugar, P. K. Sharkey, G. J. Wise, R. Mangi, A. Mosher, J. Y. Lee, W. E. Dismukes, and the National Institute for Allergy and Infectious Diseases (NIAID) Mycoses Study Group. 2000. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin. Infect. Dis. 30:14-18. [DOI] [PubMed] [Google Scholar]
- 54.Kerr, C. M., J. R. Perfect, P. C. Craven, J. H. Jorgensen, D. J. Drutz, J. D. Shelburne, H. A. Gallis, and R. A. Gutman. 1983. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Ann. Intern. Med. 99:334-336. [DOI] [PubMed] [Google Scholar]
- 55.Kremery, V., and A. J. Barnes. 2002. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243-260. [DOI] [PubMed] [Google Scholar]
- 56.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumamoto, C. A. 2002. Candida biofilms. Curr. Opin. Microbiol. 5:608-611. [DOI] [PubMed] [Google Scholar]
- 59.Kurup, A., M. Janardhan, and T. Seng. 2000. Candida tropicalis pacemaker endocarditis. J. Infect. 41:275-276. [DOI] [PubMed] [Google Scholar]
- 60.Lai, K. K., and S. A. Fontecchio. 1998. Infections associated with implantable cardioverter defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin. Infect. Dis. 27:265-269. [DOI] [PubMed] [Google Scholar]
- 61.Lew, S. Q., and K. Kaveh. 2000. Dialysis access related infections. Asaio J. 46:S6-S12. [DOI] [PubMed] [Google Scholar]
- 61a.Lewis, R. E. 2002. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob. Agents Chemother. 46:3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li, X., Z. Yan, and J. Xu. 2003. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 149:353-362. [DOI] [PubMed] [Google Scholar]
- 63.Lim, E. V., and P. J. Stern. 1986. Candida infection after implant arthroplasty. A case report. J Bone Joint Surg. Am. 68:143-145. [PubMed] [Google Scholar]
- 64.Lynch, M. J., G. M. Scott, J. A. Inglis, and J. P. Pryor. 1994. Reducing the loss of implants following penile prosthetic surgery. Br. J. Urol. 73:423-427. [DOI] [PubMed] [Google Scholar]
- 65.McGinnis, M. R. 1983. Detection of fungi in cerebrospinal fluid. Am. J. Med. 75:129-138. [DOI] [PubMed] [Google Scholar]
- 66.Melgar, G. R., R. M. Nasser, S. M. Gordon, B. W. Lytle, T. F. Keys, and D. L. Longworth. 1997. Fungal prosthetic valve endocarditis in 16 patients. An 11-year experience in a tertiary care hospital. Medicine (Baltimore) 76:94-103. [DOI] [PubMed] [Google Scholar]
- 67.Mermel, L. A. 2001. New technologies to prevent intravascular catheter-related bloodstream infections. Emerg. Infect. Dis. 7:197-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Infect. Control Hosp. Epidemiol. 22:222-242. [DOI] [PubMed] [Google Scholar]
- 69.Michel, C., L. Courdavault, R. al Khayat, B. Viron, P. Roux, and F. Mignon. 1994. Fungal peritonitis in patients on peritoneal dialysis. Am. J. Nephrol. 14:113-120. [DOI] [PubMed] [Google Scholar]
- 70.Miller, P. J., and R. P. Wenzel. 1987. Etiologic organisms as independent predictors of death and morbidity associated with bloodstream infections. J. Infect. Dis. 156:471-477. [DOI] [PubMed] [Google Scholar]
- 71.Minnaganti, V. R., and B. A. Cunha. 2001. Infections associated with uremia and dialysis. Infect. Dis. Clin. North Am. 15:385-406, viii. [DOI] [PubMed] [Google Scholar]
- 72.Montague, D. K. 1987. Periprosthetic infections. J. Urol. 138:68-69. [DOI] [PubMed] [Google Scholar]
- 73.Montero, A., J. Romero, J. A. Vargas, C. A. Regueiro, G. Sanchez-Aloz, F. De Prados, A. De la Torre, and G. Aragon. 2000. Candida infection of cerebrospinal fluid shunt devices: report of two cases and review of the literature. Acta Neurochir. 142:67-74. [DOI] [PubMed] [Google Scholar]
- 74.Morris, A., and D. E. Low. 1999. Nosocomial bacterial meningitis, including central nervous system shunt infections. Infect. Dis. Clin. North Am. 13:735-750. [DOI] [PubMed] [Google Scholar]
- 75.Mulcahy, J. J. 2000. Long-term experience with salvage of infected penile implants. J. Urol. 163:481-482. [PubMed] [Google Scholar]
- 76.Murphy, K., J. Bradley, and H. E. James. 2000. The treatment of Candida albicans shunt infections. Childs Nerv. Syst. 16:4-7. [DOI] [PubMed] [Google Scholar]
- 77.Myers, T. J., T. Khan, and O. H. Frazier. 2000. Infectious complications associated with ventricular assist systems. Asaio J. 46:S28-S36. [DOI] [PubMed] [Google Scholar]
- 78.Nasser, R. M., G. R. Melgar, D. L. Longworth, and S. M. Gordon. 1997. Incidence and risk of developing fungal prosthetic valve endocarditis after nosocomial candidemia. Am. J. Med. 103:25-32. [DOI] [PubMed] [Google Scholar]
- 79.Nassoura, Z., R. R. Ivatury, R. J. Simon, N. Jabbour, and W. M. Stahl. 1993. Candiduria as an early marker of disseminated infection in critically ill surgical patients: the role of fluconazole therapy. J. Trauma 35:290-294, discussion 294-295. [DOI] [PubMed] [Google Scholar]
- 79a.National Nosocomial Infections Surveillance System. 2001. NNIS System report. Data summary from January 1992-June 2001, issue August 2001. J. Infect. Control 29:404-420. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen, M. H., M. L. Nguyen, V. L. Yu, D. McMahon, T. F. Keys, and M. Amidi. 1996. Candida prosthetic valve endocarditis: prospective study of six cases and review of the literature. Clin. Infect. Dis. 22:262-267. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen, M. H., J. E. Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen, M. H., and V. L. Yu. 1995. Meningitis caused by Candida species: an emerging problem in neurosurgical patients. Clin. Infect. Dis. 21:323-327. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen, M. H., V. L. Yu, and A. J. Morris. 1996. Candida infection of the arteriovenous fistula used for hemodialysis. Am. J. Kidney Dis. 27:596-598. [DOI] [PubMed] [Google Scholar]
- 84.Nucci, M., and E. Anaissie. 2001. Revisiting the source of candidemia: skin or gut? Clin. Infect. Dis. 33:1959-1967. [DOI] [PubMed] [Google Scholar]
- 85.Nucci, M., and E. Anaissie. 2002. Should vascular catheters be removed from all patients with candidemia? An evidence-based review. Clin. Infect. Dis. 34:591-599. [DOI] [PubMed] [Google Scholar]
- 86.Peppas, D. S., J. W. Moul, and D. G. McLeod. 1988. Candida albicans corpora abscess following penile prosthesis placement. J. Urol. 140:1541-1542. [DOI] [PubMed] [Google Scholar]
- 87.Platt, R., B. F. Polk, B. Murdoch, and B. Rosner. 1986. Risk factors for nosocomial urinary tract infection. Am. J. Epidemiol. 124:977-985. [DOI] [PubMed] [Google Scholar]
- 88.Potera, C. 1999. Forging a link between biofilms and disease. Science 283:1837, 1839. [DOI] [PubMed] [Google Scholar]
- 89.Raad, I., and G. P. Bodey. 1992. Infectious complications of indwelling vascular catheters. Clin. Infect. Dis. 15:197-210. [DOI] [PubMed] [Google Scholar]
- 90.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramage, G., K. Vande Walle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramamohan, N., N. Zeineh, P. Grigoris, and I. Butcher. 2001. Candida glabrata infection after total hip arthroplasty. J. Infect. 42:74-76. [DOI] [PubMed] [Google Scholar]
- 93.Reagan, D. R., M. A. Pfaller, R. J. Hollis, and R. P. Wenzel. 1990. Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. J. Clin. Microbiol. 28:2733-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, et al. for the Candidemia Study Group and the National Institute. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 95.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 96.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 97.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 98.Roger, P., C. Boissy, M. Gary-Toussaint, R. Foucher, V. Mondain, F. Vandenbos, Y. Le Fichoux, J. Michels, and P. Dellamonica. 2000. Medical treatment of a pacemaker endocarditis due to Candida albicans and to Candida glabrata. J. Infect. 41:176-194. [DOI] [PubMed] [Google Scholar]
- 99.Sanchez-Portocarrero, J., P. Martin-Rabadan, C. J. Saldana, and E. Perez-Cecilia. 1994. Candida cerebrospinal fluid shunt infection. Report of two new cases and review of the literature. Diagn. Microbiol. Infect. Dis. 20:33-40. [DOI] [PubMed] [Google Scholar]
- 100.Seelig, M. S., C. P. Speth, P. J. Kozinn, C. L. Taschdjian, E. F. Toni, and P. Goldberg. 1974. Patterns of Candida endocarditis following cardiac surgery: importance of early diagnosis and therapy (an analysis of 91 cases). Prog. Cardiovasc. Dis. 17:125-160. [DOI] [PubMed] [Google Scholar]
- 101.Segreti, J., J. A. Nelson, and G. M. Trenholme. 1998. Prolonged suppressive antibiotic therapy for infected orthopedic prostheses. Clin. Infect. Dis. 27:711-713. [DOI] [PubMed] [Google Scholar]
- 102.Shapiro, S., T. Javed, and J. Mealey, Jr. 1989. Candida albicans shunt infection. Pediatr. Neurosci. 15:125-130. [PubMed] [Google Scholar]
- 103.Shin, J. H., S. J. Kee, M. G. Shin, S. H. Kim, D. H. Shin, S. K. Lee, S. P. Suh, and D. W. Ryang. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 40:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smego, R. A., Jr., J. R. Perfect, and D. T. Durack. 1984. Combined therapy with amphotericin B and 5-fluorocytosine for Candida meningitis. Rev. Infect. Dis. 6:791-801. [DOI] [PubMed] [Google Scholar]
- 105.Sobel, J. D., C. A. Kauffman, D. McKinsey, M. Zervos, J. A. Vazquez, A. W. Karchmer, J. Lee, C. Thomas, H. Panzer, W. E. Dismukes, and The National Institute of Allergy and Infectious Diseases (NIAID) Mycoses Study Group. 2000. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. Clin. Infect. Dis. 30:19-24. [DOI] [PubMed] [Google Scholar]
- 106.Soto-Hernandez, J. L., M. A. Ramirez-Crescencio, V. M. Moreno Estrada, and R. del Valle Robles. 2000. Candida albicans cerebral granulomas associated with a nonfunctional cerebrospinal fluid shunt: case report. Neurosurgery 47:973-976, discussion 976-977. [DOI] [PubMed] [Google Scholar]
- 107.Spinler, S. A., J. J. Nawarskas, E. F. Foote, D. Sabapathi, J. E. Connors, and F. E. Marchlinski. 1998. Clinical presentation and analysis of risk factors for infectious complications of implantable cardioverter-defibrillator implantations at a university medical center. Clin. Infect. Dis. 26:1111-1116. [DOI] [PubMed] [Google Scholar]
- 108.Stocks, G., and H. F. Janssen. 2000. Infection in patients after implantation of an orthopedic device. Asaio J. 46:S41-S46. [DOI] [PubMed] [Google Scholar]
- 109.Tacconelli, E., M. Tumbarello, M. Pittiruti, F. Leone, M. B. Lucia, R. Cauda, and L. Ortona. 1997. Central venous catheter-related sepsis in a cohort of 366 hospitalised patients. Eur. J. Clin. Microbiol. Infect. Dis. 16:203-209. [DOI] [PubMed] [Google Scholar]
- 110.Trappe, H. J., P. Pfitzner, H. Klein, and P. Wenzlaff. 1995. Infections after cardioverter-defibrillator implantation: observations in 335 patients over 10 years. Br. Heart J. 73:20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsukayama, D. T., R. Estrada, and R. B. Gustilo. 1996. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Joint Surg. Am. 78:512-523. [DOI] [PubMed] [Google Scholar]
- 112.Turnier, E., J. H. Kay, S. Bernstein, A. M. Mendez, and P. Zubiate. 1975. Surgical treatment of Candida endocarditis. Chest 67:262-268. [DOI] [PubMed] [Google Scholar]
- 113.Turowski, G. A., D. P. Orgill, J. J. Pribaz, E. Eriksson, and G. S. Couper. 1998. Salvage of externally exposed ventricular assist devices. Plast. Reconstr. Surg. 102:2425-2430. [DOI] [PubMed] [Google Scholar]
- 114.Vas, S., and D. G. Oreopoulos. 2001. Infections in patients undergoing peritoneal dialysis. Infect. Dis. Clin. North Am. 15:743-774. [DOI] [PubMed] [Google Scholar]
- 115.Victor, F., C. De Place, C. Camus, H. Le Breton, C. Leclercq, D. Pavin, P. Mabo, and C. Daubert. 1999. Pacemaker lead infection: echocardiographic features, management, and outcome. Heart 81:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Voss, A., R. J. Hollis, M. A. Pfaller, R. P. Wenzel, and B. N. Doebbeling. 1994. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J. Clin. Microbiol. 32:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang, A. Y., A. W. Yu, P. K. Li, P. K. Lam, C. B. Leung, K. N. Lai, and S. F. Lui. 2000. Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am. J. Kidney Dis. 36:1183-1192. [DOI] [PubMed] [Google Scholar]
- 118.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 119.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1989. Risk factors for hospital-acquired candidemia. A matched case-control study. Arch. Intern. Med. 149:2349-2353. [PubMed] [Google Scholar]
- 120.Wilhelm, M., C. Schmid, D. Hammel, S. Kerber, H. Loick, M. Herrmann, and H. Scheld. 1997. Cardiac pacemaker infection: surgical management with and without extracorporeal circulation. Ann. Thorac. Surg. 64:1707-1712. [DOI] [PubMed] [Google Scholar]
- 121.Yamada, M., S. Takeuchi, Y. Shiojiri, K. Maruta, A. Oki, K. Iyano, and T. Takaba. 2002. Surgical lead-preserving procedures for pacemaker pocket infection. Ann. Thorac. Surg. 74:1494-1499. [DOI] [PubMed] [Google Scholar]
- 122.Yu, V. L., G. D. Fang, T. F. Keys, A. A. Harris, L. O. Gentry, P. C. Fuchs, M. M. Wagener, and E. S. Wong. 1994. Prosthetic valve endocarditis: superiority of surgical valve replacement versus medical therapy only. Ann. Thorac. Surg. 58:1073-1077. [DOI] [PubMed] [Google Scholar]
- 123.Zimmerli, W., and P. E. Ochsner. 2002. Management of infection associated with prosthetic joints. Infection 31:99-108. [DOI] [PubMed] [Google Scholar]