Abstract
Fragile X Syndrome (FXS) is caused by suppressed expression of fragile X mental retardation protein (FMRP), which results in intellectual disability accompanied by many variably manifested characteristics, such as hyperactivity, seizures, and autistic-like behaviors. Treatment of mice that lack FMRP, Fmr1 knockout (KO) mice, with lithium has been reported to ameliorate locomotor hyperactivity, prevent hypersensitivity to audiogenic seizures, improve passive avoidance behavior, and attenuate sociability deficits. To focus on the defining characteristic of FXS, which is cognitive impairment, we tested if lithium treatment ameliorated impairments in four cognitive tasks in Fmr1 KO mice, tested if the response to lithium differed in adolescent and adult mice, and tested if therapeutic effects persisted after discontinuation of lithium administration. Fmr1 KO mice displayed impaired cognition in the novel object detection task, temporal ordering for objects task, and coordinate and categorical spatial processing tasks. Chronic lithium treatment of adolescent (from 4–8 weeks of age) and adult (from 8–12 weeks of age) mice abolished cognitive impairments in all four cognitive tasks. Cognitive deficits returned after lithium treatment was discontinued for 4 weeks. These results demonstrate that Fmr1 KO mice exhibit severe impairments in these cognitive tasks, that lithium is equally effective in normalizing cognition in these tasks whether it is administered to young or adult mice, and that lithium administration must be continued for the cognitive improvements to be sustained. These findings provide further evidence that lithium administration may be beneficial for individuals with FXS.
Keywords: cognition, fragile X syndrome, lithium, learning, novel object detection, spatial processing
Introduction
Fragile X syndrome (FXS) is the most common cause of inherited intellectual disability and is the most prevalent monogenetic cause of autism spectrum disorders. FXS is caused by a trinucleotide CGG repeat expansion on the X chromosome that suppresses expression of Fragile X Mental Retardation Protein (FMRP) (Verkerk et al. 1991; Pierreti et al. 1991), which is thought to cause the intellectual, behavioral, and physical abnormalities characteristic of FXS. In mouse hippocampus, FMRP expression is highest at postnatal day 7 (Lu et al. 2004), and FMRP is important for establishing functional neuronal networks (Gatto & Broadie 2009). Since individuals with FXS lack FMRP during postnatal development, crucial questions are whether cognitive deficits can be ameliorated pharmacologically, and if improvements depend on early intervention.
FXS is modeled in Fmr1 knockout (KO) mice (Bakker et al. 1994) that display several characteristics of FXS, including impaired social interactions, locomotor hyperactivity, and decreased passive avoidance learning (Kooy et al. 1996; Mineur et al. 2002; Yan et al. 2004). Remarkably, all of these behavioral phenotypes are normalized in Fmr1 KO mice by lithium treatment (Min et al. 2009; Mines et al. 2010; Yuskaitis et al. 2010a; Liu et al. 2011). Furthermore, lithium is the only agent that has improved performance on a cognitive task in FXS patients in a formal trial setting (Berry-Kravis et al. 2008). Since lithium is safely used in patients with bipolar disorder, including children and adolescents (Alessi et al. 1994; Ryan et al. 1999; Findling et al. 2011), these findings suggest that lithium is a promising therapeutic agent for FXS.
Here we tested if lithium treatment can reverse several cognitive deficits in Fmr1 KO mice. We also tested if the beneficial effects of lithium treatment on cognitive tasks in Fmr1 KO mice differ between young and adult mice, since the lack of FMRP during development may have established irreversible morphological and neuronal abnormalities that preclude effective intervention in adults. Administration of lithium to lactating mothers or to pups immediately upon weaning at 3 weeks of age results in retarded growth of the pups (Min et al., 2009), therefore lithium administration was initiated when mice reached 4 weeks of age to compare its effects on performance in cognitive tasks in young (from 4–8 weeks of age) mice with adult-treated (from 8–12 weeks of age) mice. Additionally, the effects of lithium withdrawal from mice treated during adolescence were examined to test if lithium-induced improvements in cognitive task performance by Fmr1 KO mice required continual lithium treatment or if they remained stable once repaired, which may occur if lithium treatment resulted in long-lasting repairs of deficits in neural circuitry or neurogenesis in Fmr1 KO mice. Significant deficits in Fmr1 KO mice were found in object novelty detection, temporal order memory, and spatial learning tests, and each of these was improved by chronic lithium treatment of adolescent and adult mice, whereas the cognitive deficits were reinstated after four weeks of lithium withdrawal. These results further support the potential benefits of lithium treatment in FXS.
Materials and methods
Mice
This study used male C57Bl/6J littermates, with or without a disruption of the Fmr1 gene (originally kindly provided by Dr. W. Greenough, University of Illinois). Mice were weaned 3 weeks after birth, group housed, tested between 1000 and 1400, and 7–20 mice were used in each experiment as described in the figure legends. The Fmr1 KO mice were generated by breeding male C57BL/6J hemizygous Fmr1 KO mice and female C57Bl/6J heterozygous Fmr1 KO mice to generate male homozygous Fmr1 KO mice and wild-type (WT) littermates. Genotypes were determined by PCR using the Jackson Laboratory protocol for genotyping Fmr1 mice. To test chronic lithium treatment, Fmr1 KO mice and WT mice were given water ad libitum, and were fed either normal 18% protein rodent diet or the same diet with 0.2% lithium carbonate (both from Teklad, Madison, WI) with provision of an additional bottle containing saline to prevent hyponatremia. This is a therapeutically relevant treatment regimen that produces serum lithium concentrations of 0.6–0.8 mM (Chen et al. 2000; O'Brien et al. 2004; Shaltiel et al. 2008; Jope 2011; Contestabile et al. 2013), within the 0.5–1.2 mM range that is therapeutic in human patients. Adult mice were treated with lithium for 4 weeks and throughout the behavioral tests. For lithium treatment during adolescence, mice were treated with lithium from 4 until 8 weeks of age and throughout the behavioral tests, then lithium was discontinued for 4 weeks, and the mice were retested. Mice were housed in light and temperature controlled rooms and treated in accordance with NIH and University of Miami Institutional Animal Care and Use Committee regulations.
Object novelty detection task
Recognition memory for a novel object compared to a familiar object was assessed by the object novelty detection task (Hoge & Kesner 2007; Hunsaker & Kesner 2008; Hunsaker et al. 2012). For this task, a Plexiglas box (26 cm long × 20 cm wide × 16 cm tall) and four objects in duplicate (4–6 cm diameter × 2–6 cm height) were used. During the first session, two copies of Object 1 were placed at each end of the box, and the mouse was allowed to explore the objects for 5 min. The mouse was then removed to an opaque holding container for 5 min, and the objects were replaced with two copies of Object 2 for the next session. After 5 min exploring during session 2, the mouse was placed in the holding container and the copies of Object 2 were replaced with duplicates of Object 3. Following exploration during session 3, the mouse was removed and the objects were replaced by an unused copy of Object 1 and a novel Object 4 for the mouse to explore during the 5 min test session. More time exploring the novel Object 4 compared to the familiar Object 1 indicates that the mouse remembered previously exploring Object 1, but equal exploration time between the two objects indicates that the mouse has impaired recognition memory. Object exploration was defined as the mouse sniffing or touching the object with its nose, vibrissa, mouth, or forepaws, and time spent near or standing on top of the objects without interacting with the object was not counted as exploration. Exploration time of the novel and familiar object is presented, and changes in object exploration ratio were calculated as: (exploration time of Object 4 − exploration time of Object 1)/ (exploration time of Object 1 + exploration time of Object 4). This calculation constrains the ratios to be between −1 and 1, and a ratio approaching 1 indicates an intact memory of Object 1.
For this and all other behavioral assessments, the sessions were filmed, a white noise generator (55 dB) was used, and each apparatus and object was cleaned with 70% ethanol between each test session.
Temporal ordering for objects task
Temporal order memory was assessed using the temporal ordering for objects task (Mitchell & Laiacono, 1998; Hannesson et al.; 2004; Hoge & Kesner 2007; Hunsaker et al. 2012). Similar to the object novelty detection task, the same box was used and a mouse received three sessions to explore two copies of a new set of objects (Objects 5, 6, 7). For the 5 min test session, an unused copy of Object 5 and an unused copy of Object 7 were placed in the box and the mouse was allowed to explore. A mouse with normal temporal order memory spends more time exploring the first object (Object 5) presented compared to the most recent object (Object 7). Time exploring Object 5 and Object 7 are presented, and changes in object exploration ratio were calculated as: (exploration time of Object 5 − exploration time of Object 7)/ (exploration time of Object 5 + exploration time of Object 7).
Coordinate spatial processing task
Spatial memory was assessed in mice using the coordinate and categorical spatial processing tasks (Goodrich-Hunsaker et al. 2005; Goodrich-Hunsaker et al. 2008; Hunsaker et al. 2009; Hunsaker et al. 2012). The coordinate spatial processing task consisted of a 15 min habituation session, a 5 min holding time, and a 5 min test session. For the habituation session a mouse was placed at the edge of the table facing 2 different objects spaced 45 cm apart, and the mouse was allowed to explore the table and the objects for 15 min. Then the mouse was placed in an opaque holding container for 5 min. For the test session, the objects were moved closer together so that they were 30 cm apart, and the mouse was allowed to explore the objects for 5 min. Mice that have intact spatial memory display increased exploration of the objects during the test session compared with the last 5 min of the habituation session. For the coordinate spatial processing task, the exploration ratio was calculated as: (exploration time during the 5 min test session)/ (exploration time during the 5 min test session + exploration time during the last 5 min of the habituation session). Increased exploration during the 5 min test session compared to the last 5 min of the habituation session is indicated by a ratio >0.5.
Categorical spatial processing task
Like the coordinate spatial processing task, the categorical spatial processing task (Goodrich-Hunsaker et al. 2005; Goodrich-Hunsaker et al. 2008; Hunsaker et al. 2009; Hunsaker et al. 2012), is used to assess spatial memory with 2 novel objects, which are different from the objects used in the coordinate spatial processing task. For the habituation session, a mouse was placed on the edge of the table facing 2 different objects that were spaced 45 cm apart and allowed to explore the table and objects for 15 min. Then the mouse was placed in an opaque container for 5 min, and the position of the objects was interchanged, while the distance was maintained. For the test session, the mouse was allowed to explore the objects for 5 min. Increased exploration of the objects during the test session compared with the last 5 min of the habituation phase indicates that the mice remember the object positions. The same exploration ratio was calculated for the categorical spatial processing task as in the coordinate spatial processing task.
Statistical analysis
Statistical significance was assessed by two factor ANOVA with genotype and treatment as factors followed by Bonferroni's multiple comparison tests (for lithium treatment in WT and Fmr1 KO adult and adolescent mice), or by one factor ANOVA (for discontinued lithium treatment in WT and Fmr1 KO mice), or Student's t-test (for time spent with each object in the object novelty detection task and the temporal ordering for objects task).
Results
Chronic lithium treatment significantly improves object novelty detection in Fmr1 KO mice
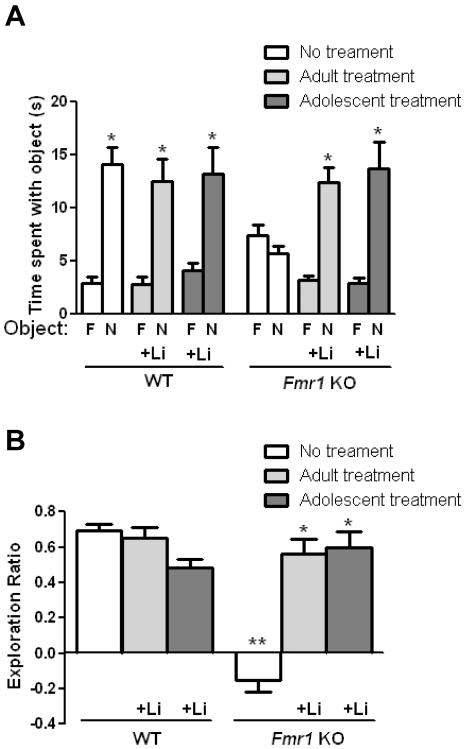
We tested if cognition was impaired in Fmr1 KO mice in four hippocampus-dependent learning tasks, if chronic lithium treatment repaired cognitive deficits in Fmr1 KO mice and if there were different outcomes after lithium was administered to adolescent mice (from 4–8 weeks of age) or adult mice (from 8–12 weeks of age). The object novelty detection task is a dentate gyrus-dependent task that assesses the ability to discriminate between familiar and novel objects, indicated by more time spent exploring a novel object than a familiar object (Otto & Eichenbaum 1992; Knight 1996; Dolan & Fletcher 1997; Lisman 1999; Hunsaker & Kesner 2008). Previous reports show that novel object recognition is impaired in Fmr1 KO mice (Ventura et al. 2004; Pacey et al. 2011; Bhattacharya & Klann 2012). WT mice spent significantly more time exploring the novel object than the familiar object, whereas Fmr1 KO mice spent equivalent amounts of time exploring each object (Figure 1A). Thus, there was a significant interaction between genotype and treatment and the object exploration ratio differed between Fmr1 KO and WT mice (Figure 1B). The impairment in object novelty detection in Fmr1 KO mice was corrected by lithium treatment, and lithium was equally effective after administration to adult or adolescent mice (Figure 1A). Lithium treatment of adolescent or adult WT mice did not alter performance in the object novelty detection task. In either adolescent or adult Fmr1 KO mice that were treated with lithium, the exploration ratio was significantly increased to a level equivalent to that of WT mice (Figure 1B). These results demonstrate that object novelty detection is impaired in Fmr1 KO mice, and that lithium treatment of adolescent or adult Fmr1 KO mice corrects this impairment.
Figure 1. Chronic lithium treatment of adult or adolescent Fmr1 KO mice reverses impaired discrimination in the object novelty detection task.
Lithium was administered for four weeks to adult (from 8 to 12 weeks of age) and adolescent (from 4 to 8 weeks of age) male Fmr1 knockout (KO) and wild-type (WT) mice prior to testing. (A) Times spent exploring the novel (N) and familiar (F) object. (Student's t-test; *p<0.05 compared to time spent with familiar object; WT no treatment: n=20, t(46)=6.51, p<0.05; WT adult lithium treatment: n=10, t(18)=4.29, p<0.05; WT adolescent lithium treatment: n=9, t(10)=3.47, p<0.05; Fmr1 KO no treatment: n=20, t(48)=1.42, p>0.05; Fmr1 KO adult lithium treatment: n=10, t(18)=6.20, p<0.05; Fmr1 KO adolescent lithium treatment: n=9, t(16)=4.19, p<0.05). (B) Exploration ratio. (two-way ANOVA (genotype x treatment) followed by post hoc Bonferroni's multiple comparison test; F(2,72)=33.02, p<0.05; **p<0.05 compared to untreated WT mice; *p<0.05 compared to same genotype without treatment).
Lithium treatment normalizes temporal order memory in Fmr1 KO mice
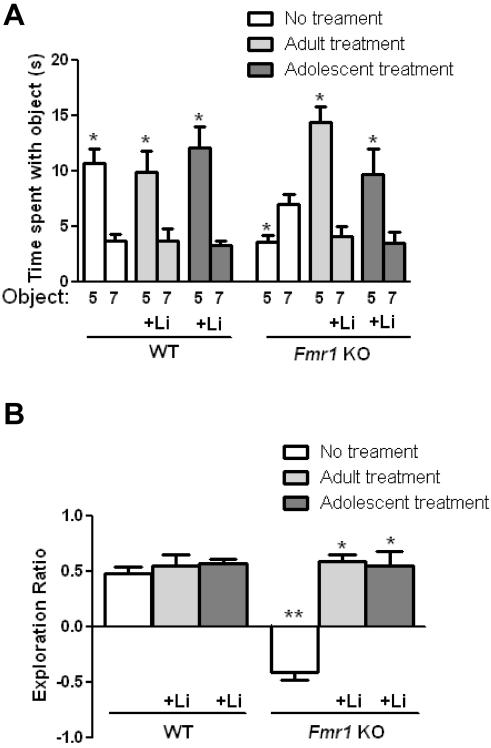
The temporal ordering for objects is a hippocampal CA1-dependent task that is exhibited by mice spending less time with an object most recently presented in the previous habituation session (Honey et al. 1998; Wallenstein et al. 1998; Lisman 1999; Rolls & Kesner 2006; Hoge & Kesner 2007; Hunsaker et al. 2008; Hunsaker et al. 2012). WT mice, but not Fmr1 KO mice, spent more time exploring the first object presented than the most recent object presented (Figure 2A). There was a significant interaction between genotype and treatment in the temporal ordering task, and the object exploration ratio differed significantly between Fmr1 KO and WT mice (Figure 2B). The impairment in temporal order memory in Fmr1 KO mice was corrected by lithium treatment, and lithium was similarly effective after administration to adult mice or adolescent mice (Figure 2A). Lithium treatment of adolescents or adults significantly increased the exploration ratio in Fmr1 KO mice, whereas lithium treatment did not affect the performance of WT mice in this task (Figure 2B). Thus, temporal order memory is impaired in Fmr1 KO mice, and is repaired by lithium treatment of adolescent or adult Fmr1 KO mice.
Figure 2. Chronic lithium treatment of adult or adolescent Fmr1 KO mice ameliorates temporal order memory deficits.
Adult and adolescent male Fmr1 KO and WT mice were treated with lithium for 4 weeks prior to testing. (A) Times spent exploring the first object presented (Object 5) and the object most recently explored (Object 7). (Student's t-test; *p<0.05 compared to time spent with Object 7; WT no treatment: n=20, t(38)=4.82, p<0.05; WT adult lithium treatment: n=10, t(18)=2.74, p<0.05; WT adolescent lithium treatment: n=9, t(12)=4.56, p<0.05; Fmr1 KO no treatment: n=20, t(38)=3.16, p<0.05; Fmr1 KO adult lithium treatment: n=9, t(16)=6.21, p<0.05; Fmr1 KO adolescent lithium treatment: n=9, t(16)=2.38, p<0.05). (B) Exploration ratio. (two-way ANOVA (genotype x treatment) followed by post hoc Bonferroni's multiple comparison test; F(2,75)=27.48, p<0.05; **p<0.05 compared to untreated WT mice; *p<0.05 compared to same genotype without treatment).
Treatment with lithium repairs spatial memory impairment in Fmr1 KO mice
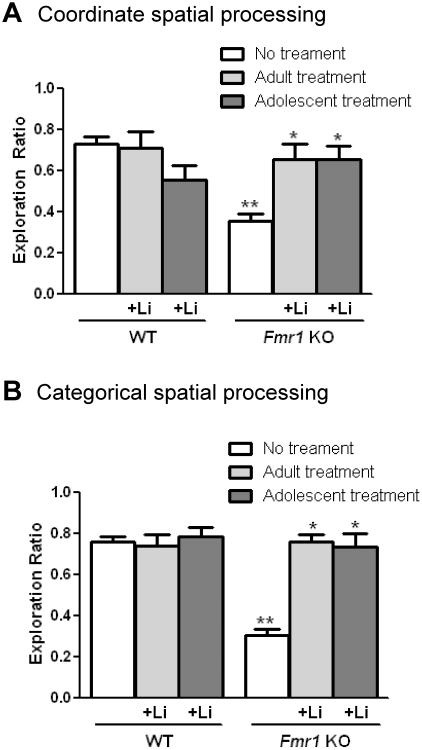
The coordinate and categorical spatial learning tasks assess metrical and topological spatial pattern separation, respectively, in similar spaces (Save et al. 1992; Tsien et al. 1996; Long & Kesner 1996; Lisman 1999; Goodrich-Hunsaker et al. 2005; Hunsaker et al. 2009; Goodrich-Hunsaker et al. 2008; Hunsaker et al. 2009; Hunsaker et al. 2012). The coordinate spatial learning task involves measuring the time spent exploring two objects after the objects have been moved closer together compared to the last 5 min of the habituation period. There was a significant interaction between genotype and treatment in the coordinate spatial learning task indicating that Fmr1 KO mice exhibited an impaired object exploration ratio compared to WT mice, demonstrating a deficit in coordinate spatial memory in Fmr1 KO mice (Figure 3A). Lithium treatment of adult or adolescent Fmr1 KO mice normalized coordinate spatial memory to that of WT mice, but did not affect the performance of WT mice.
Figure 3. Chronic lithium treatment of adult or adolescent Fmr1 KO mice alleviates spatial processing impairments in Fmr1 KO mice.
Adult and adolescent male Fmr1 KO and WT mice were treated with lithium for 4 weeks prior to testing. (A) Exploration ratio in the coordinate spatial processing task (two-way ANOVA (genotype x treatment) followed by post hoc Bonferroni's multiple comparison test; F(2,68)=10.68, p<0.05). (B) Exploration ratio in the categorical spatial processing task (two-way ANOVA (genotype x treatment) followed by post hoc Bonferroni's multiple comparison test; F(2,69)=24.93, p<0.05). **p<0.05 compared to untreated WT mice; *p<0.05 compared to same genotype without treatment; n=20 WT no treatment; n=10 WT adult treatment; n=9 WT adolescent treatment; n=20 Fmr1 KO no treatment; n=10 Fmr1 KO adult treatment; n=9 Fmr1 KO adolescent treatment.
The categorical spatial learning task assesses the time spent exploring two objects after the position of the objects is transposed, with the distance unchanged, following the habituation phase. Fmr1 KO mice spent significantly less time than WT mice exploring the objects after the objects had been transposed, and there was a significant interaction between genotype and treatment (Figure 3B), revealing impaired categorical spatial memory in Fmr1 KO mice. Administration of lithium did not alter the amount of time that WT mice spent exploring the objects that were transposed, but lithium treatment of adult or adolescent Fmr1 KO mice significantly increased the exploration ratio. Thus, the results of the coordinate and categorical spatial learning tests reveal impaired spatial pattern learning in Fmr1 KO mice, and that this is significantly improved by lithium treatment of either adolescents or adults.
Learning deficits in Fmr1 KO mice are reinstated following lithium withdrawal
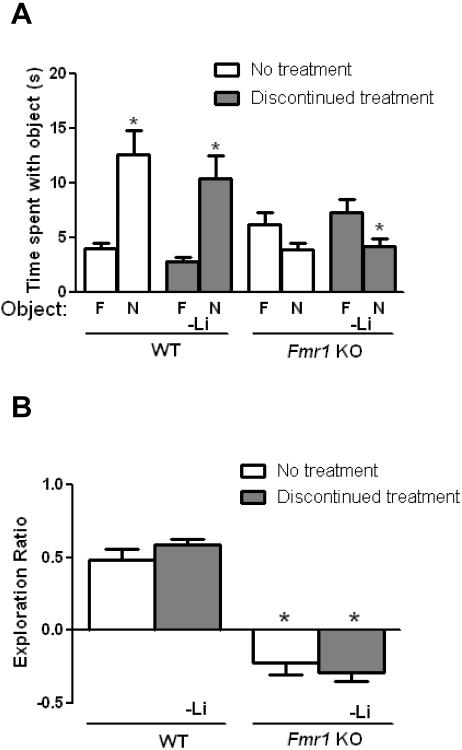
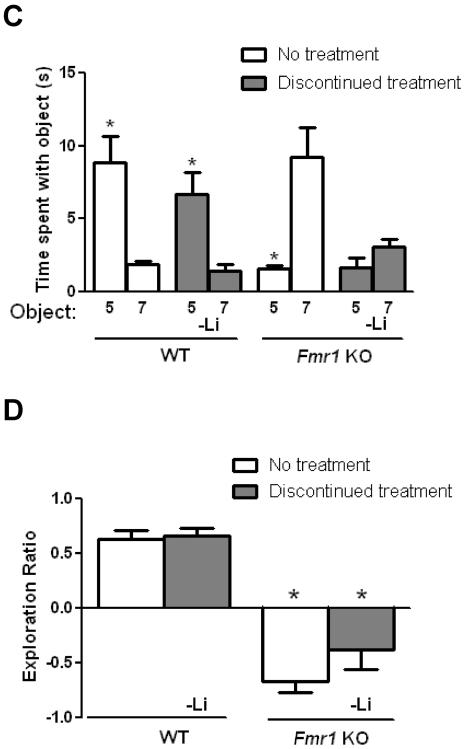
To determine if lithium's enhancing effects on cognition in Fmr1 KO mice are sustained following lithium withdrawal, chronic lithium treatment was discontinued after the behavior tests in mice treated from 4 weeks until 8 weeks of age. Four weeks later the Fmr1 KO and WT mice were retested in all cognitive tasks. Prior testing in the same paradigms had no effect on re-test performance in the WT mice or Fmr1 KO mice that were not treated with lithium (Figures 4 and 5). Following lithium withdrawal, WT mice exhibited normal object novelty detection, indicating that there was no effect of lithium withdrawal in WT mice (Figure 4A). However, Fmr1 KO mice that were withdrawn from lithium treatment spent significantly less time exploring the novel object, revealing that impaired object novelty detection returned following lithium withdrawal in Fmr1 KO mice. Fmr1 KO mice that had been withdrawn from lithium demonstrated a significantly reduced object exploration ratio compared to WT mice that had been withdrawn from lithium (Figure 4B). The results show that the impairment in object novelty detection in Fmr1 KO mice returned following lithium withdrawal.
Figure 4. Impaired cognitive deficits are reinstated in Fmr1 KO mice following discontinuation of lithium in the object novelty task and temporal ordering for objects task.
Adolescent male Fmr1 KO and WT mice were treated with lithium for 4 weeks. After testing, lithium treatment was discontinued for 4 weeks and the mice were retested. Prior testing in the same paradigms had no effect on re-test performance in untreated WT mice or Fmr1 KO mice (Student's t-test, p>0.05 compared to retest; object novelty detection task: WT no treatment: n=7, t(12)=0.78, p>0.05; FX no treatment: n=9, t(16)=0.29, p>0.05; temporal ordering for objects task: WT no treatment: n=7, t(12)=0.68, p>0.05; FX no treatment: n=9, t(16)=1.42, p>0.05). (A,B) Performance in the object novelty detection task. (A) Times spent exploring the novel (N) and familiar (F) object. (Student's t-test; *p<0.05 compared to time spent with familiar object; WT no treatment: n=7, t(12)=3.78, p<0.05; WT discontinued lithium treatment: n=7, t(12)=3.57, p<0.05; Fmr1 KO no treatment: n=9, t(16)=1.87, p>0.05; Fmr1 KO discontinued lithium treatment: n=9, t(16)=2.28, p<0.05) (B) Exploration ratio. (one-way ANOVA followed by post hoc Bonferroni's multiple comparison test; F(3,28)=47.41). *p<0.05 compared to matched WT mice. (C,D) Performance in the temporal ordering for objects task. (C) Times spent exploring the first object presented (Object 5) and the object most recently explored (Object 7). (Student's t-test; *p<0.05 compared to time spent with Object 7; WT no treatment: n=7, t(12)=3.87, p<0.05; WT discontinued lithium treatment: n=9, t(12)=3.48, p<0.05; Fmr1 KO no treatment: n=9, t(16)=3.75, p<0.05; Fmr1 KO discontinued lithium treatment: n=9, t(16)=1.75, p>0.05). (D) Exploration ratio. (one-way ANOVA followed by post hoc Bonferroni's multiple comparison test; F(3,28)=28.80, p<0.05). *p<0.05 compared to matched WT mice.
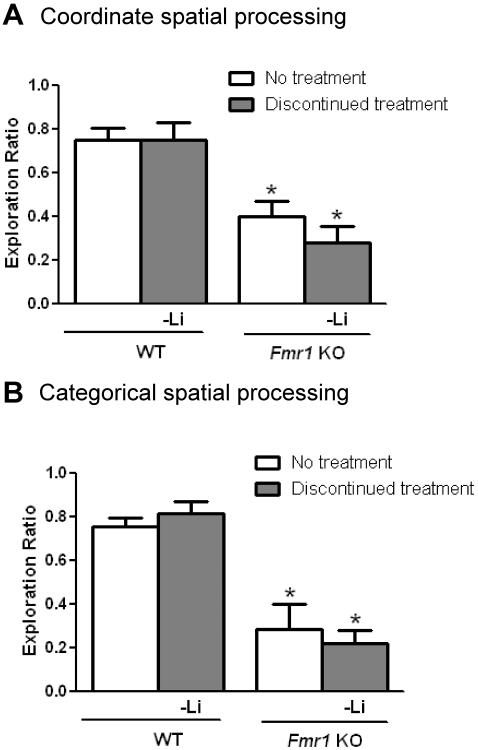
Figure 5. Spatial processing impairments return in Fmr1 KO mice following discontinuation of lithium.
Adolescent male Fmr1 KO and WT mice were treated with lithium for 4 weeks and then following testing, lithium treatment was discontinued for 4 weeks and the mice were retested. Prior cognitive testing in the same paradigms had no effect on re-test performance in untreated WT mice or Fmr1 KO mice (Student's t-test, p>0.05 compared to retest; coordinate spatial processing task: WT no treatment: n=7, t(12)=0.32, p>0.05; FX no treatment: n=9, t(16)=0.27, p>0.05; categorical spatial processing task: WT no treatment: n=7, t(12)=0.24, p>0.05; FX no treatment: n=9, t(16)=0.28, p>0.05). (A) Exploration ratio in the coordinate spatial processing task. (one-way ANOVA followed by post hoc Bonferroni's multiple comparison test; F(3,29)=11.34, p<0.05). (B) Exploration ratio in the categorical spatial processing task. (one-way ANOVA followed by post hoc Bonferroni's multiple comparison test; F(3,27)=17.29, p<0.05). *p<0.05 compared to matched WT mice. n=7 WT no treatment; n=7 discontinued lithium treatment; n=9 Fmr1 KO no treatment; n=9 Fmr1 KO discontinued lithium treatment.
Discontinuation of lithium treatment also reinstated the temporal order memory deficit in Fmr1 KO mice without affecting WT mice. Fmr1 KO mice that were discontinued from lithium treatment demonstrated deficient temporal order memory, whereas temporal order memory was unaltered by lithium withdrawal in WT mice (Figure 4C). The object exploration ratio was significantly reduced in Fmr1 KO, compared with WT, mice withdrawn from lithium (Figure 4D). Thus the effect of lithium in the temporal order task was not sustained in Fmr1 KO mice following four weeks of lithium withdrawal.
Coordinate and categorical spatial memory impairments also returned in Fmr1 KO mice after lithium was withdrawn. Although WT mice maintained intact spatial memory, untreated and previously treated Fmr1 KO mice displayed significantly reduced object exploration ratios compared to WT mice in the coordinate spatial task (Figure 5A) and in the categorical spatial task (Figure 5B). Thus, the improvements in coordinate and categorical spatial processing in Fmr1 KO mice induced by lithium treatment were reversed when lithium treatment was discontinued.
Discussion
Here we report impaired cognition in adult Fmr1 KO mice in four hippocampus-dependent learning and memory tasks, and that each of these was significantly improved by chronic lithium treatment. Furthermore, lithium treatment was equally effective in ameliorating cognitive deficits when administered to adult or adolescent Fmr1 KO mice. Importantly, lithium administration did not affect the performance of adult or adolescent WT mice in these cognitive tasks, demonstrating an Fmr1 KO-specific improvement in learning and memory. Discontinuation of lithium treatment caused cognitive impairments to return in Fmr1 KO mice, but lithium withdrawal did not alter the performance of WT mice.
Although the predominant characteristic of FXS is intellectual disability, severe cognitive deficits were initially difficult to identify in the Fmr1 KO mouse model. Fmr1 KO mice display modest cognitive deficits in several hippocampus-dependent tasks, such as the Morris water maze, radial arm maze, and operant conditioning paradigms (Bakker 1994; Kooy et al. 1996; D'Hooge et al. 1997; Fisch et al. 1999; Paradee et al. 1999; Peier et al. 2000; Mineur et al. 2002). Fmr1 KO mice also exhibit deficits in fear motivated learning tasks, including passive and active avoidance behaviors, and contextual, conditioned and trace fear memory (Yan et al. 2004; Qin et al. 2005; Zhao et al. 2005; Brennan et al. 2006; Hayashi et al. 2007; Baker et al. 2010; Guo et al. 2011). Recently, severe deficits in non-aversive learning and memory tasks, including novel object recognition and context discrimination, have been identified in Fmr1 KO mice (Pacey et al. 2011; Eadie et al. 2012; Bhattacharya & Klann 2012). In the present study, Fmr1 KO mice exhibited significant impairments in recognition memory, working memory, and short-term memory that are assessed in the object novelty detection task and the temporal ordering for objects task, and spatial memory measured in the coordinate and categorical spatial processing tasks (Goodrich-Hunsaker et al. 2005; Hoge & Kesner 2007; Hunsaker & Kesner 2008; Goodrich-Hunsaker et al. 2008; Hunsaker et al. 2009; Hunsaker et al. 2012). As previously discussed (Mineur et al. 2002; Ventura et al. 2004; Spencer et al. 2008; Hagerman et al. 2009; Baker et al. 2010; Bhogal & Jongens 2010; Guo et al. 2011; Bagni et al. 2012), similar deficits have been identified in patients with FXS, such as impaired recognition memory, working memory, short-term memory, and spatial memory (Kemper et al. 1988; Cornish et al. 1999; Orstein et al. 2008; Gatto & Broadie 2009). Thus, the cognitive deficits displayed by Fmr1 KO mice may model some of the impairments in nonverbal measures of cognitive functions in FXS patients.
Chronic lithium treatment proved to be remarkably effective in essentially normalizing severe deficits in Fmr1 KO mice in novel object detection, temporal ordering for objects, and coordinate and categorical spatial processing tasks. The lithium treatments were designed to test the hypothesis that treatment of younger Fmr1 KO mice would be more effective than treatment of adult Fmr1 KO mice. This was based on the finding that FMRP is more highly expressed in young than adult mouse brain (Lu et al. 2004), raising the possibility that its absence may produce irreversible deficits in adult Fmr1 KO mice. However, lithium treatment was equally effective in adolescent and adult Fmr1 KO mice in reversing cognitive deficits. This is an encouraging finding that suggests some cognitive impairments may be pharmacologically reversible even with post-adolescent administration in FXS, although caution must be exercised in translating results from Fmr1 KO mice. However, it is encouraging that lithium administration improved performance on a cognitive task in a small trial in FXS patients (Berry-Kravis et al. 2008).
The improvements in cognitive tasks reported here add to an extensive number of abnormal phenotypes that are improved by lithium treatment of Fmr1 KO mice. Phenotypes in Fmr1 KO mice that have been reported to be improved by lithium treatment include locomotor hyperactivity, audiogenic seizure hypersensitivity, increased spine density, macroorchidism, excess protein synthesis, social behavior deficits, deficient passive avoidance learning, and synaptic plasticity (Min et al. 2009; Yuskaitis et al. 2010a, Yuskaitis et al. 2010b; Mines et al. 2010; Liu et al. 2011; Choi et al. 2011; Liu et al. 2012). Improvement of cognition by lithium treatment correlates well with previous findings in Fmr1 KO mice of altered synaptic plasticity, measured as long-term potentiation (LTP) and long-term depression (LTD). Fmr1 KO mice display enhanced metabotropic glutamate receptor (mGluR)-dependent LTD at hippocampal CA1 synapses (Huber et al. 2002; Hou et al. 2006; Nosyreva & Huber 2006) and deficient LTP at medial perforant path synapses in the dentate gyrus (Eadie et al. 2012). Lithium treatment in adolescent Fmr1 KO mice (from 5–6 weeks of age until 9–11 months of age) or adult Fmr1 KO mice (from 8 weeks of age to 4–5 months of age) normalized mGluR-dependent LTD in the hippocampus, without affecting WT mice (Choi et al. 2011). Lithium inhibits glycogen synthase kinase-3 (GSK3) (Klein & Melton 1996), lithium treatment reduces abnormally hyperactive GSK3 in Fmr1 KO mice (Min et al. 2009; Yuskaitis et al. 2010a), and hyperactive GSK3 impairs LTP and promotes LTD (Hooper et al. 2007; Zhu et al. 2007). Taken together, these findings suggest that inhibition of GSK3 by lithium contributes to the normalization of synaptic plasticity and cognition in Fmr1 KO mice, although this conjecture will require further examination. The cognitive-enhancing actions of lithium in Fmr1 KO mice are clearly dependent on the continued presence of lithium, since cognitive deficits were equivalent in Fmr1 KO mice withdrawn from lithium and Fmr1 KO mice that had never been given lithium. Thus, lithium treatment must be sustained in Fmr1 KO mice for cognitive benefits to persist.
In summary, lithium treatment of adolescent or adult Fmr1 KO mice is safe, and effectively remediates performance in several cognitive tasks, as well as providing many previously reported beneficial effects in Fmr1 KO mice. These results extend previous findings that lithium ameliorates synaptic plasticity and/or cognitive deficits in Fmr1 KO flies (McBride et al. 2005), mice (Yuskaitis et al. 2010a; Liu et al. 2011; Choi et al. 2011) and FXS patients (Berry-Kravis et al. 2008). Thus, there is increasing evidence that lithium may provide therapeutic benefits in FXS.
Acknowledgements
This research was supported by grants from the NIMH (MH038752) and from the FRAXA Foundation. The authors would like to thank Marjelo A. Mines, Eleonore Beurel, Marta Pardo, and Elyse Barrett for their contributions to this project.
Footnotes
Conflict of interest The authors have no financial interests or conflicts of interest.
References
- Alessi N, Baylor MW, Ghaziuddin M, Zubieta JK. Update on lithium carbonate therapy in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994;33:291–304. doi: 10.1097/00004583-199403000-00001. [DOI] [PubMed] [Google Scholar]
- Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012;122:4314–4322. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010;9:562–574. doi: 10.1111/j.1601-183X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- Bakker CE, et al. Consortium Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Dölen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses) Annu Rev Neurosci. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal B, Jongens TA. Fragile X syndrome and model organisms: identifying potential routes of therapeutic intervention. Dis Model Mech. 2010;3:693–700. doi: 10.1242/dmm.002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Klann E. Fragile X syndrome therapeutics S(C)TEP through the developmental window. Neuron. 2012;74:1–3. doi: 10.1016/j.neuron.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, Paylor R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 2006;5:467–471. doi: 10.1111/j.1601-183X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, Gertner MJ, Woo NH, Tranfaglia MR, Bear MF, Zukin RS, McDonald TV, Jongens TA, McBride SM. Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Greco B, Ghezzi D, Tucci V, Benfenati F, Gasparini L. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Invest. 2013;123:348–361. doi: 10.1172/JCI64650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Spatial cognition in males with Fragile-X syndrome: evidence for a neuropsychological phenotype. Cortex. 1999;35:263–271. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Cornish K, Munir F, Wilding J. A neuropsychological and behavioural profile of attention deficits in fragile X syndrome. Rev Neurol. 2001;33(Suppl 1):S24–29. [PubMed] [Google Scholar]
- D'Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, Kooy RF, Oostra BA, Willems PJ, De Deyn PP. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997;76:367–376. doi: 10.1016/s0306-4522(96)00224-2. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2012;22:241–254. doi: 10.1002/hipo.20890. [DOI] [PubMed] [Google Scholar]
- Findling RL, Kafantaris V, Pavuluri M, McNamara NK, McClellan J, Frazier JA, Sikich L, Kowatch R, Lingler J, Faber J, Rowles BM, Clemons TE, Taylor-Zapata P. Dosing strategies for lithium monotherapy in children and adolescents with bipolar I disorder. J Child Adolesc Psychopharmacol. 2011;21:195–205. doi: 10.1089/cap.2010.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch GS, Hao HK, Bakker C, Oostra BA. Learning and memory in the FMR1 knockout mouse. Am J Med Genet. 1999;84:277–282. doi: 10.1002/(sici)1096-8628(19990528)84:3<277::aid-ajmg22>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. The fragile X mental retardation protein in circadian rhythmicity and memory consolidation. Mol Neurobiol. 2009;39:107–129. doi: 10.1007/s12035-009-8057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behav Neurosci. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra BA, Nelson DL, Jin P, Zhao X. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat Med. 2011;17:559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson DK, Vacca G, Howland JG, Phillips AG. Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behav Brain Res. 2004;153:273–285. doi: 10.1016/j.bbr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RC, Watt A, Good M. Hippocampal lesions disrupt an associative mismatch process. J Neurosci. 1998;18:2226–2230. doi: 10.1523/JNEUROSCI.18-06-02226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18:955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009;123:1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kim K, Willemsen R, Berman RF. CGG trinucleotide repeat length modulates neural plasticity and spatiotemporal processing in a mouse model of the fragile X premutation. Hippocampus. 2012;22:2260–2275. doi: 10.1002/hipo.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Frontiers in Molecular Neuroscience. 2011;4:16–26. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper MB, Hagerman RJ, Altshul-Stark D. Cognitive profiles of boys with the fragile X syndrome. Am J Med Genet. 1988;30:191–200. doi: 10.1002/ajmg.1320300118. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kooy RF, D'Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, Willems PJ. Transgenic mouse model for the fragile X syndrome. Am J Med Genet. 1996;64:241–245. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacology. 2011;14:618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Huang T, Smith CB. Lithium reverses increased rates of cerebral protein synthesis in a mouse model of fragile X syndrome. Neurobiol Dis. 2012;45:1145–1152. doi: 10.1016/j.nbd.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, McDonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, Bauchwitz RP. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56:463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mines MA, Yuskaitis CJ, King MK, Beurel E, Jope RS. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of Fragile X syndrome and autism. PLoS. 2010;5:e9706. doi: 10.1371/journal.pone.0009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Laiacona J. The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behav Brain Res. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- O'Brien WT, Harper AD, Jové F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3β haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein PA, Schaaf JM, Hooper SR, Hatton DD, Mirrett P, Bailey DB., Jr. Memory skills of boys with fragile X syndrome. Am J Ment Retard. 2008;113:453–465. doi: 10.1352/2008.113:453-465. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–334. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- Pacey LK, Doss L, Cifelli C, van der Kooy D, Heximer SP, Hampson DR. Genetic deletion of regulator of G-protein signaling 4 (RGS4) rescues a subset of fragile X related phenotypes in the FMR1 knockout mouse. Mol Cell Neurosci. 2011;46:563–572. doi: 10.1016/j.mcn.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet. 2000;9:1145–1159. doi: 10.1093/hmg/9.8.1145. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005;135:999–1009. doi: 10.1016/j.neuroscience.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Ryan ND, Bhatara VS, Perel JM. Mood stabilizers in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:529–536. doi: 10.1097/00004583-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, Rogawski M, Gasior M, Luckenbaugh D, Chen G, Manji HK. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Graham DF, Yuva-Paylor LA, Nelson DL, Paylor R. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122:710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- Tassone F, Long KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, Nguyen D, Mu LY, Laffin J, Bailey DB, Hagerman RJ. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4:100. doi: 10.1186/gm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Ventura R, Pascucci T, Catania MV, Musumeci SA, Puglisi-Allegra S. Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: involvement of dopamine in the medial prefrontal cortex. Behav Pharmacol. 2004;15:433–442. doi: 10.1097/00008877-200409000-00018. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, van Ommen GB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004;3:337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of Fragile X Syndrome. Biochemical Pharmacology. 2010a;79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuskaitis C, Beurel E, Jope RS. Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of Fragile X syndrome. Biochim Biophys Acta. 2010b;1802:1006–1012. doi: 10.1016/j.bbadis.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–7892. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, Wang Q, Chen JG, Wang JZ. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27:12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]