Abstract
Background
Adiponectin, particularly high-molecular weight adiponectin, and resistin are recently discovered adipokines that may provide a molecular link between adiposity and type 2 diabetes.
Objective
To evaluate whether total and high-molecular weight adiponectin and resistin are associated with the future risk for type 2 diabetes, independent of obesity and other known diabetes risk factors.
Design
Prospective, nested case-control study.
Settings
United States.
Participants
1,038 initially healthy women of the Nurses’ Health Study who developed type 2 diabetes after blood sampling (1989–1990) through 2002 and 1,136 matched control subjects.
Measurements
Plasma concentrations of adipokines were measured by enzyme-linked immunosorbent assays.
Results
In multivariate models adjusting for diabetes risk factors including body-mass index (BMI), both higher total and high-molecular weight adiponectin levels were associated with a substantially lower risk for type 2 diabetes. The odds ratios comparing the highest with the lowest quintiles were 0.17 (95% CI, 0.12–0.25) for total adiponectin and 0.10 (CI, 0.06–0.15) for high-molecular weight adiponectin. In addition, a higher high-molecular weight-to-total adiponectin ratio was associated with a significantly lower risk even after adjusting for total adiponectin (odds ratio, 0.45 [CI, 0.31–0.65]). In the multivariate model adjusting for diabetes risk factors except BMI, high resistin levels were significantly associated with an increased diabetes risk for (odds ratio, 1.68 [CI, 1.25–2.25]). However, after additional adjustment for BMI, this association was no longer statistically significant (odds ratio, 1.28 [CI, 0.93–1.76]).
Limitations
Residual confounding by imperfectly or unmeasured confounders cannot be excluded.
Conclusions
These results indicate that adiponectin, but not resistin is strongly associated with diabetes risk independent of BMI. The high-molecular weight-to-total adiponectin ratio is related to diabetes risk independent of total adiponectin, suggesting an important role of the relative proportion of high-molecular weight adiponectin in diabetes pathogenesis.
Introduction
Excess adiposity is a major modifiable risk factor for the development of type 2 diabetes (1). It is now established that the adipose tissue is an important metabolically active endocrine organ that secretes a variety of hormones and cytokines, termed adipokines (2). These adipokines, particularly adiponectin and resistin, have been proposed to provide a molecular link between adiposity and type 2 diabetes (3, 4).
Adiponectin is a collagen-like protein that is exclusively synthesized by adipocytes (5) and circulates at relatively high concentrations of 2–30 μg/mL in blood (3, 6). Unlike other adipokines, concentrations of adiponectin are paradoxically reduced in obese compared with lean subjects (3, 6). There is no clear explanation for this inverse association, but in vitro and in vivo studies suggest endogenous inflammatory cytokines such as interleukin-6, interleukin-8, and tumor necrosis factor-α, which are elevated in obesity, may inhibit the production of adiponectin (7). In addition, prospective studies have consistently found a decreased risk for type 2 diabetes with increasing concentrations of total adiponectin (8–18). However, adiponectin exists in plasma as a trimer, a hexamer, and a high-molecular weight form (2, 6). Recent evidence suggests that high-molecular weight adiponectin is the most biologically active form of the hormone (6), but few epidemiologic studies have investigated high-molecular weight adiponectin seperately from total adiponectin (18–20).
Resistin is a cysteine-rich polypeptide that is expressed at relatively lower levels in human adipocytes, but higher levels in macrophages (4, 21). Initial experimental studies in rodents pointed to an important role of resistin as a mediator of obesity-associated insulin resistance (22). While the majority of recent animal studies further supports this hypothesis, the physiological relevance of resistin for obesity-related conditions in humans remains controversial (4), and inference from most human studies was limited by cross-sectional design and small sample size (23–33).
Therefore, we conducted a large, propsective case-control study nested within the Nurses’ Health Study to evaluate whether total and high-molecular weight adiponectin and resistin are associated with the future risk for type 2 diabetes, independent of obesity and other known diabetes risk factors.
Methods
Study population
The Nurses’ Health Study was initiated in 1976, when 121,700 female U.S. nurses aged 30–55 years responded to a questionnaire requesting health-related information. Since 1976, this cohort has been followed using a biennially mailed questionnaire to obtain updated information. Between 1989 and 1990, a subset of 32,826 consenting women provided blood samples. For the present study, we included those 1,059 women who were free of diabetes, coronary heart disease, stroke, and cancer at blood collection and developed type 2 diabetes at least one year after their blood sampling through June 2002. Control subjects had similar exclusion criteria at baseline and were matched to diabetes cases by age at blood draw (±1 year), date of blood draw (±3 month), fasting status (≥8 hours vs. <8 hours since last meal), and race. To improve statistical control for obesity, additional control subjects were matched by these factors and by body-mass index (BMI; ±1 unit) to diabetes cases in the upper decile of the BMI distribution, providing 1,164 control subjects (1,059 non-BMI matched and 105 BMI-matched control subjects). From this sample, we excluded all women, who had missing information on BMI (n=4) and missing values (n=44) or values below the detection limit (n=1) for the adipokines. The final analytical sample comprised 1,038 diabetes cases and 1,136 control subjects (1,032 non-BMI matched and 104 BMI-matched control subjects). The study was approved by the Institutional Review Board of the Brigham and Women’s Hospital, Boston, Massachusetts.
Ascertainment of type 2 diabetes
Cases of type 2 diabetes were identified by the biennially mailed questionnaires and confirmed by a validated supplementary questionnaire regarding symptoms, diagnostic laboratory test results, and diabetes treatment as described previously (34). Validation studies in subsamples of the Nurses’ Health Study revealed a high validity of the self-reported diagnosis of diabetes through the supplementary questionnaire (98.4% were comfirmed by medical records) and a low percentage of false negative self-reports (0.5%) (35).
Assessment of lifestyle and dietary information
Body weight was reported at the time of blood collection. BMI was calculated as the ratio of weight (in kg) to squared height (in m2). The presence or abence of a family history of diabetes in a first-degree relative was reported in 1982 and 1988. Information on cigarette smoking, menopausal status, use of hormone replacement therapy, and physical activity was obtained from the questionnaire in 1988. Physical activity was computed as metabolic equivalents per week based on the average time spent per week on various leisure time activities, weighted by their intensity level (36). Dietary intake was expressed as avarage of the intakes obtained from semiquantitative food frequency questionnaires administered in 1986 and 1990. Values for nutrients were energy adjusted by the residual method (37). The validity of self-reported body weight, physical activity, and dietary intake in this cohort of women has been previously reported (38).
Laboratory procedures
The blood collection and processing procedure has been described in detail previously (34). Briefly, women consenting to provide blood samples were sent a phlebotomy kit. Blood samples were returned by overnight mail in a frozen water bottle, processed upon arrival, and stored in liquid nitrogen −130 °C or lower until laboratory analysis.
Study samples were analyzed in randomly ordered case-control pairs and the laboratory personnel was blinded to the case-control status to reduce interassay variation and systematic bias. Resistin concentration was measured by an enzyme-linked immunosorbent assay (ELISA) (Linco Research, Inc., St. Charles, Missouri) with a minimum detectable limit of 0.16 ng/mL. The intracclass correlation for the reproducibility of resistin over one year was 0.75 (n=35). Total and high-molecular weight adiponectin were determined by ELISA (ALPCO Diagnostics, Salem, New Hampshire) with a sensitivity of 0.04 ng/mL. The recovery rate was 99–103% for total adiponectin and 97–105% for high-molecular weight adiponectin (20). In our study, the coefficient of variation for total adiponectin, high-molecular weight adiponectin, and resistin based on blinded quality control samples was 8.9%, 9.9%, and 2.5% respectively. Assays used for the measurement of C-reactive protein, interleukin-6, and soluble tumor necrosis factor-α receptor 2 and of fasting insulin, proinsulin, C-peptide, and HbA1c were described elsewhere (34, 39).
Statistical analysis
Based on their distribution among control subjects, adipokine concentrations were divided into quintiles. One exception was the categorization into tertiles for the joint classification of adipokines due to low numbers of subjects in extreme joint categories. Category-specific odds ratios of type 2 diabetes were estimated using unconditional logistic regression adjusted for matching factors (age [years], date of blood draw [month], fasting status [≥8 hours vs. <8 hours since last meal], and ethnicity). We repeated the main analyses using conditional logistic regression and observed essentially the same results. Thus, we presented the results from unconditional regression for both main effects and subgroup analyses. In multivariate models, odds ratios were further adjusted for known and suggested diabetes risk factors (1) including physical activity (quintiles of metabolic equivalents/week), smoking (never, past, current 1–14 cigarettes/d, and current ≥15 cigarettes/d), family history of diabetes (yes/no), hormone replacement therapy (premenopause, never, past, current, and missing information), alcohol consumption (nondrinkers, 0.1–4.9, 5–10, and >10 g/d), glycemic load (quintiles), ratio of polyunsaturated to saturated fatty acids (quintiles), intakes (quintiles) of cereal fiber, trans-fatty acids, magnesium, caffeine, and total energy intake, and BMI (<23, 23–24.9, 25–26.9, 27–29.9, 30–34.9, and ≥35 kg/m2). Adjustment for BMI as a continuous instead of a categorized variable yielded nearly identical results. Trend tests were conducted by including the median adipokine concentration of each quintile as a continuous variable into the models. Potential interactions between adipokines and covariates were tested by using the likelihood ratio test (40). Akaike’s information criterion was used to compare the goodness of fit for non-nested models (41).
We used restricted cubic spline regression with 4 knots according to the 5th, 35th, 65th, and 95th percentiles of the adipokine concentrations to examine a possible non-linear relation of the biomarkers to diabetes risk (42). Tests for non-linearity were conducted using the likelihood ratio test (40).
All reported P values are two-sided. Statistical analyses were performed using SAS software 9.1 (SAS Institute Inc., Cary, NC).
Role of the funding sources
The funding sources had no role in the collection, analysis, and interpretation of the data and in the decision to submit the manuscript for publication.
Results
Women who developed type 2 diabetes during the follow-up had higher mean baseline values for BMI and waist circumference, were less physically active, and were more likely to have a family history of diabetes than those who remained free of diabetes (Table 1). In addition, women developing type 2 diabetes had significantly lower baseline concentrations of total and high-molecular weight adiponectin, a lower high-molecular weight-to-total adiponectin ratio, and higher concentrations of resistin than control subjects.
Table 1.
Comparison of adipokine concentrations and risk factors for type 2 diabetes between case and control subjects at baseline. *
| Risk factor | Cases (n=1,038) | Controls (n=1,136) | P Value |
|---|---|---|---|
| Age, y (matching factor) | 56.1 (7.0) | 55.9 (7.0) | 0.49 |
| Body-mass index, kg/m2 | 30.1 (5.4) | 26.4 (6.1) | <0.001 |
| Waist circumference, in | 35.1 (4.8) | 31.2 (4.7) | <0.001 |
| Physical activity, metabolic equivalents/week | 12.2 (14.9) | 15.5 (24.7) | <0.001 |
| Family history of diabetes, % | 45.2 | 23.0 | <0.001 |
| Current smoking, % | 13.4 | 12.2 | 0.42 |
| Postmenopausal, % | 62.7 | 60.7 | 0.32 |
| Current postmenopausal hormone use, % | 18.9 | 22.0 | 0.07 |
| Dietary intake | |||
| Cereal fiber, g/day | 4.6 (2.2) | 5.0 (2.6) | <0.001 |
| Glycemic load | 102 (16) | 102 (18) | 0.82 |
| Trans fatty acids, g/d | 2.6 (0.8) | 2.5 (0.8) | 0.003 |
| Polyunsaturated/saturated fatty acids ratio | 0.56 (0.15) | 0.57 (0.1)8 | 0.04 |
| Magnesium, mg/d | 297 (62) | 307 (70) | <0.001 |
| Caffeine, mg/d | 237 (185) | 257 (197) | 0.02 |
| Alcohol, g/day | 3.4 (7.3) | 5.7 (9.1) | <0.001 |
| Total energy, kcal/day | 1832 (510) | 1775 (472) | 0.007 |
| Total adiponectin, μg/mL (median [IQR]) | 11.4 [7.6–16.3] | 17.8 [12.9–22.7] | <0.001 |
| High-molecular weight adiponectin, μg/mL (median [IQR]) | 3.6 [2.2–5.8] | 6.6 [4.4–9.8] | <0.001 |
| High-molecular weight/total adiponectin ratio (median [IQR]) | 0.33 [0.27–0.40] | 0.39 [0.32–0.47] | <0.001 |
| Resistin, ng/mL (median [IQR]) | 16.4 [12.4–23.6] | 15.1 [11.5–21.2] | <0.001 |
All data are presented as mean (SD) or percentage unless otherwise specified. Characteristics of case and control subjects were compared by χ2 test (for percentages), t test (for means), or Wilcoxon rank-sum test (for medians). For waist circumference: 799 controls, 635 cases; for physical activity: 1,118 controls, 1,024 cases; and for dietary intake: 1,125 controls, 1,027 cases.
IQR = interquartile range.
Among control subjects, both total and high-molecular weight adiponectin showed moderate inverse correlations with markers of obesity, inflammation, insulin secretion, and hyperglycemia, with the correlations being slightly stronger for high-molecular weight adiponectin (Appendix Table 1). Adjustment for BMI attenuated these correlations, although the correlations of total and high-molecular weight adiponectin with fasting insulin, proinsulin, and C-peptide remained significant. The high-molecular weight-to-total adiponectin ratio showed similar patterns of correlations; however, after adjustment for BMI, correlations with fasting proinsulin and C-peptide were no longer significant. Resistin also showed modest positive associations with BMI and with inflammatory and insulin markers. Adjustment for BMI nearly abolished the correlations between resistin and the markers of insulin secretion, although resistin remained significantly correlated with the inflammatory markers interleukin-6 and soluble tumor necrosis factor-α receptor 2.
In multivariate models adjusting for diabetes risk factors except BMI, both total and high-molecular weight adiponectin levels were strongly and inversely associated with the risk for type 2 diabetes (Table 2). These associations were only slightly attenuated after further adjusting for BMI; the odds ratios comparing the highest with the lowest quintiles were 0.17 (95% CI, 0.12–0.25) for total adiponectin and 0.10 (CI, 0.06–0.15) for high-molecular weight adiponectin. Results from conditional logistic regression were essentially the same; the odds ratios comparing extreme quintiles were 0.12 (95% CI, 0.08–0.20) for total adiponectin and 0.06 (CI, 0.04–0.12) for high-molecular weight adiponectin. Additional adjustment for weight and physical activity change between 1986 and baseline did not alter the results; the odds ratios comparing extreme quintiles were identical to those in the analysis without these potential confounder variables.
Table 2.
Odds ratio (95% CI) of type 2 diabetes according to quintiles of adipokine concentration.
| Variable | Quintile of adipokine concentration
|
P for Trend | ||||
|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | 5 (highest) | ||
| Total adiponectin, μg/mL | ||||||
| Median | 8.1 | 13.9 | 17.9 | 21.7 | 28.4 | |
| Range | 0.05–11.6 | 11.7–16.0 | 16.1–20.0 | 20.1–24.3 | 24.4–42.1 | |
| Cases, n/controls, n | 548/227 | 221/228 | 139/227 | 73/227 | 57/227 | |
| Crude odds ratio (95% CI) * | 1.0 | 0.39 (0.30–0.49) | 0.24 (0.19–0.32) | 0.13 (0.09–0.17) | 0.10 (0.07–0.13) | <0.001 |
| Multivariate odds ratio (95% CI) † | 1.0 | 0.42 (0.32–0.54) | 0.26 (0.20–0.35) | 0.15 (0.11–0.21) | 0.12 (0.08–0.17) | <0.001 |
| Multivariate odds ratio + BMI (95% CI) ‡ | 1.0 | 0.46 (0.35–0.60) | 0.31 (0.23–0.42) | 0.21 (0.15–0.30) | 0.17 (0.12–0.25) | <0.001 |
| High-molecular weight adiponectin, μg/mL | ||||||
| Median | 2.4 | 4.7 | 6.5 | 9.1 | 14.7 | |
| Range | 0.2–3.9 | 4.0–5.5 | 5.6–7.7 | 7.8–11.0 | 11.1–43.7 | |
| Cases, n/controls, n | 556/227 | 203/227 | 150/228 | 97/227 | 32/227 | |
| Crude odds ratio (95% CI) * | 1.0 | 0.36 (0.28–0.45) | 0.26 (0.20–0.34) | 0.16 (0.12–0.22) | 0.05 (0.04–0.08) | <0.001 |
| Multivariate odds ratio (95% CI) † | 1.0 | 0.37 (0.28–0.48) | 0.28 (0.22–0.37) | 0.19 (0.14–0.26) | 0.07 (0.04–0.10) | <0.001 |
| Multivariate odds ratio + BMI (95% CI) ‡ | 1.0 | 0.41 (0.31–0.53) | 0.35 (0.26–0.47) | 0.26 (0.19–0.36) | 0.10 (0.06–0.15) | <0.001 |
| High-molecular weight/total adiponectin ratio | ||||||
| Median | 0.25 | 0.33 | 0.39 | 0.45 | 0.55 | |
| Range | 0.02–0.30 | 0.31–0.36 | 0.37–0.41 | 0.42–0.49 | 0.50–94.0 | |
| Cases, n/controls, n | 390/227 | 243/226 | 189/228 | 139/228 | 77/227 | |
| Crude odds ratio (95% CI) * | 1.0 | 0.63 (0.49–0.80) | 0.48 (0.37–0.62) | 0.35 (0.27–0.46) | 0.20 (0.14–0.27) | <0.001 |
| Multivariate odds ratio (95% CI) † | 1.0 | 0.63 (0.49–0.82) | 0.53 (0.40–0.69) | 0.39 (0.29–0.52) | 0.23 (0.16–0.31) | <0.001 |
| Multivariate odds ratio + BMI (95% CI) ‡ | 1.0 | 0.73 (0.56–0.97) | 0.61 (0.46–0.82) | 0.47 (0.35–0.64) | 0.33 (0.23–0.47) | <0.001 |
| Multivariate odds ratio + BMI + total adiponectin (95% CI) § | 1.0 | 0.84 (0.63–1.12) | 0.76 (0.56–1.03) | 0.62 (0.45–0.85) | 0.45 (0.31–0.65) | <0.001 |
| Resistin, ng/mL | ||||||
| Median | 9.0 | 12.2 | 15.0 | 19.2 | 30.3 | |
| Range | 2.3–10.7 | 10.8–13.4 | 13.5–16.7 | 16.8–22.7 | 22.8–183.7 | |
| Cases, n/controls, n | 147/228 | 191/226 | 205/229 | 220/226 | 275/227 | |
| Crude odds ratio (95% CI) * | 1.0 | 1.31 (0.99–1.74) | 1.40 (1.06–1.85) | 1.52 (1.15–2.00) | 1.91 (1.45–2.50) | <0.001 |
| Multivariate odds ratio (95% CI) † | 1.0 | 1.24 (0.92–1.69) | 1.36 (1.00–1.83) | 1.35 (1.00–1.82) | 1.68 (1.25–2.25) | 0.001 |
| Multivariate odds ratio + BMI (95% CI) ‡ | 1.0 | 1.08 (0.78–1.50) | 1.13 (0.82–1.57) | 1.04 (0.75–1.44) | 1.28 (0.93–1.76) | 0.15 |
Adjusted for matching factors (age, date of blood draw, fasting status, and ethnicity).
Additionally adjusted for physical activity (quintiles of metabolic equivalents/week), smoking (never, past, current 1–14 cigarettes/d, and current ≥15 cigarettes/d), family history of diabetes (yes/no), hormone replacement therapy (premenopause, never, past, current, and missing information), alcohol consumption (nondrinkers, 0.1–4.9, 5–10, and >10 g/d), glycemic load (quintiles), ratio of polyunsaturated fat to saturated fat (quintiles), and intake (quintiles) of cereal fiber, trans-fat, magnesium, and total energy.
Additionally adjusted for BMI (<23, 23–24.9, 25–26.9, 27–29.9, 30–34.9, and ≥35 kg/m2).
Additionally adjusted for total adiponectin (quintiles).
BMI = body-mass index.
When both total and high-molecular weight adiponectin were included in the same multivariate model including BMI, the inverse associations with type 2 diabetes remained significant (P for trend=0.006 or <0.001 for total or high-molecular weight adiponectin, respectively). However, because of the colinearity between total and high-molecular weight adiponectin, odds ratios were attenuated and less precise (as indicated by wider confidence intervals): odds ratios across quintiles were 1.0, 0.63 (CI, 0.46–0.88), 0.48 (0.31–0.73), 0.38 (0.23–0.64), and 0.55 (0.29–1.04) for total adiponectin and 1.0, 0.56 (CI, 0.40–0.78), 0.63 (0.41–0.96), 0.52 (0.31–0.86), and 0.17 (0.09–0.35) for high-molecular weight adiponectin. The multivariate models with total and high-molecular weight adiponectin yielded values of 2423 and 2407 for the Akaike’s information criterion, respectively, suggesting a slightly better model fit with high-molecular weight than total adiponectin. The hypothesis that adiponectin might be a molecular link between adiposity and type 2 diabetes was supported by results showing attenuated risk estimates of BMI after adjusting for adiponectin (odds ratio 1.11 [CI, 1.09–1.13] and 1.07 [1.05–1.09] for BMI as continuous variable in the multivariate model with and without total adiponectin, respectively).
The high-molecular weight-to-total adiponectin ratio also showed a significantly inverse association with diabetes risk even after adjusting for BMI and other diabetes risk factors (Table 2). This association was attenuated but remained significant after further adjustment for total adiponectin (odds ratio comparing extreme quintiles 0.45, [CI, 0.31–0.65]).
In a multivariate model adjusting for diabetes risk factors except BMI, resistin levels were significantly and positively associated with diabetes risk (Table 2). However, additional adjustment for BMI attenuated the risk estimates and the association was no longer significant; the odds ratio comparing extreme quintiles was 1.28 (CI, 0.93–1.76). Further adjustment for total or high-molecular weight adiponectin did not alter these results appreciably.
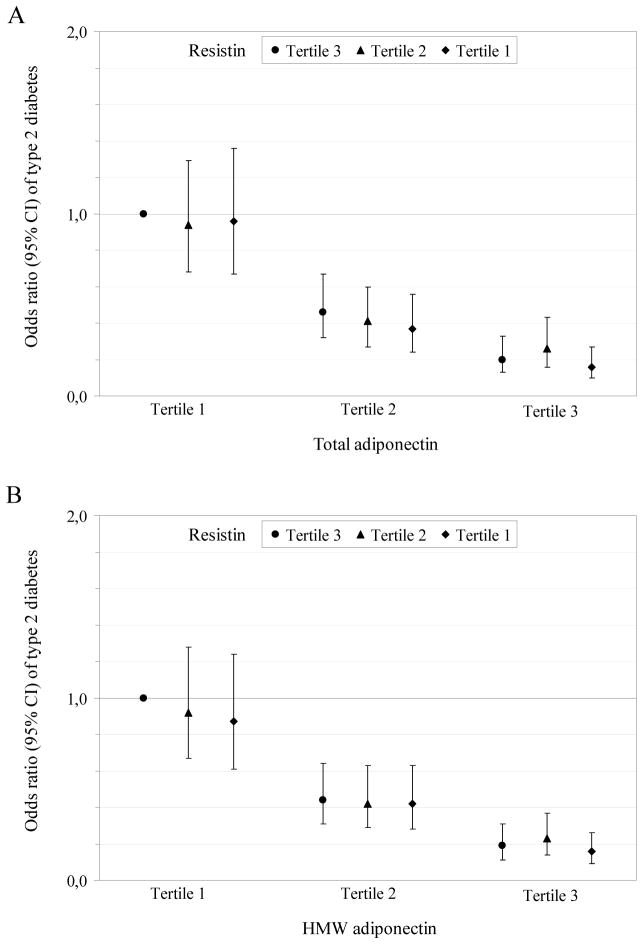
Joint classification of the adipokines indicated no additional role of resistin above total or high-molecular weight adiponectin in risk estimation (Figure 1A–B). In addition, joint analysis of total adiponectin and the high-molecular weight-to-total adiponectin ratio pointed to an independent association of the ratio with type 2 diabetes at both low and high total adiponectin levels (Figure 1C).
Figure 1. Multivariate-adjusted odds ratio (95% CI) of type 2 diabetes according to the joint classification of adipokines.
Joint analysis of total adiponectin and resistin (A), high-molecular weight adiponectin and resistin (B), and total adiponectin and the high-molecular weight-to-total adiponectin ratio (C). Odds ratios are adjusted for the same variables as in the multivariate model including BMI of table 3. Tertiles were calculated among control subjects and defined as ≤12.55, 12.56–18.36, and >18.36 ng/mL for resistin, ≤14.67, 14.68–21.19, and >21.19 μg/mL for total adiponectin, ≤4.97, 4.98–8.58, and >8.58 μg/mL for high-molecular weight adiponectin, and ≤0.342, 0.343–0.442, and >0.442 for the high-molecular weight-to-total adiponectin ratio. HMW = high-molecular weight.
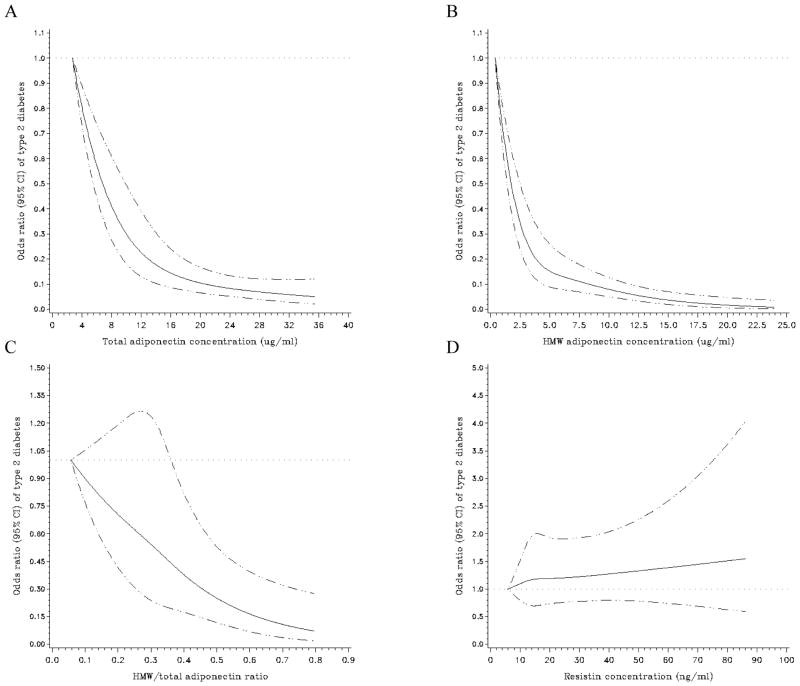
Spline regression models illustrated a deviation from linearity for the relation between total (P for non-linearity =0.005) and high-molecular weight adiponectin (P for non-linearity <0.001) and risk for type 2 diabetes, with a stronger decrease in risk within the lower than the higher range of adiponectin concentrations (Figure 2A–B). However, spline regression showed a strong linear association between the high-molecular weight-to-total adiponectin ratio and diabetes risk (P for non-linearity =0.71) (Figure 2C). Moreover, spline regression further confirmed the non-significant assocition between resistin and diabetes risk after accounting for confounder variables including BMI (Figure 2D).
Figure 2. Multivariate-adjusted odds ratio (95% CI) of type 2 diabetes according to continuous adipokine concentrations.
Spline regression models to examine the possible non-linear relation of total adiponectin (A), high-molecular weight adiponectin (B), the high-molecular weight-to-total adiponectin ratio (C), and resistin (D) to type 2 diabetes (adjusted for same variables as in the multivariate model including BMI of table 3). The solid black line represents odds ratios and the dotted lines represent 95% CIs. Women with extreme low or high adipokine concentrations (<1st or >99th percentile) were excluded from these analyses (n=41 for total adiponectin, n=42 for high-molecular weight adiponectin, n=43 for the high-molecular weight-to-total adiponectin ratio, and n=42 for resistin). HMW = high-molecular weight.
Stratified and sensitivity analysis
To explore whether overweight status, physical activity level, family history of diabetes, or the C-reactive protein level modify the observed associations, we performed stratified analyses (Appendix Table 2). The observed associations were largely consistent across all subgroups and most interaction tests were not statistically significant. There was some evidence suggesting that the association between resistin and diabetes was stronger among normal weight than overweight women, but the P value for interaction was not significant.
To address the possibility of undiagnosed diabetes or of subclinical cardiovascular disease at baseline, we repeated the analysis after excluding women with baseline HbA1c values >6.5%; the first 6 years of follow-up; or women who developed an incident myocardial infarction or stroke during the follow-up, respectively. The results from these sensitivity analyses remained essentially the same (Appendix Table 3). In addition, adjustment for waist circumference, history of hypertension and hyperlipdemia, or C-reactive protein did not appreciably change the results. Adjustment for fasting insulin (as a measure of insulin resistance) and the proinsulin-to-insulin ratio (as a measure of β-cell dysfunction) did not materially alter results. Sensitivity analyses were performed in subsamples of the study popualtion with available waist or biomarker measurements.
Discussion
In this large, nested case-control study of initially healthy women, high baseline concentrations of total and high-molecular weight adiponectin were associated with a substantially reduced risk for developing type 2 diabetes over 12 years of follow-up. These associations were independent of several known diabetes risk factors including BMI and not explained by markers of insulin resistance or inflammation. A high ratio of high-molecular weight to total adiponectin was also related to a decreased risk for type 2 diabetes, independently of total adiponectin. In contrast, higher concentrations of resistin were associated with a significantly increased diabetes risk, but this association was attenuated after adjusting for BMI.
Our results of the inverse association between total adiponectin and diabetes risk are in accordance with previous prospective studies among other populations such as Pima Indians (8), Europeans (9–13), Asians (14–17), and Japanese-Americans (18). However, inferences from some of the previous studies were hindered by highly selected study populations such as patients with impaired glucose tolerance or impaired fasting glucose (14, 17), coronary artery disease (14), and kidney transplantation (10) or a relatively short period of follow-up. Further, most of the previous studies did not account for lifestyle factors such as smoking and physical activity or dietary factors (8, 10, 14–18), which might confound the association between adiponectin and diabetes risk. Therefore, the results of the present study, with women free of major chronic diseases at baseline, a long follow-up period, and a comprehensive adjustment for most known diabetes risk factors, extend the evidence linking total adiponectin to the future risk for type 2 diabetes.
Adiponectin circulates in several forms, principally as a low-molecular weight hexamer (~180 kDa) and a high-molecular weight multimer (~360 kDa) (6). Recent evidence suggests that the high-molecular weight adiponectin complex may be the more biologically active form. For example, the proportion of high-molecular weight to total adiponectin was more strongly correlated with 2-hour glucose levels in glucose tolerance testing in Indo-Asian males (43) and was more predictive of insulin resistance or the metablolic syndrom in Japanese patients (44) than total adiponectin. Further, high-molecular weight adiponectin was shown to be more strongly related to multiple characteristics within the metabolic syndrom complex than total adiponectin (19). However, other data do not support a superiority of the high-molecular weight form over total adiponectin in identifying the presence of insulin resistance (20). It must be pointed out that all these studies were small and cross-sectional in nature. The only prospective study that was conducted in Japanese-Americans reported that high-molecular weight adiponectin was more closely related to incident type 2 diabetes than total adiponectin (18). In our study, both higher total and high-molecular weight adiponectin levels were strongly associated with a decreased risk for type 2 diabetes. Our data also suggest a strong association of the high-molecular weight-to-total adiponectin ratio with diabetes risk, independent of total adiponectin levels, providing evidence for an important role of the relative proportion of the high-molecular weight form in the development of type 2 diabetes.
Interestingly, the inverse association with diabetes risk appeared to be particularly pronounced within the lower ranges of total and high-molecular weight adiponectin distributions. This suggests that potentially high risk subjects with very low adiponectin levels could benefit most from interventions that increase adiponectin levels such as weight loss by lifestyle modifications or thiazolidinedione therapy (6). However, further studies are needed to confirm the markedly elevated diabetes risk within the low range of adiponectin levels observed in this study and to elucidate the underlying biological mechanisms.
For resistin, initial experimental studies showed that circulating concentrations are markedly increased in both diet-induced and genetically obese mice and decreased after treatment with insulin-sensitizing peroxisome proliferator-activated receptor (PPAR)-γ agonists (22). Moreover, treatment with recombinant resistin induced insulin resistance in healthy mice, whereas immunoneutralization of resistin improved insulin sensitivity in obese, insulin-resistant mice (22). However, human studies are highly inconsistent. While several studies reported higher resistin concentrations in obese than in leaner persons (23–25, 45), others did not observe significant differences (28–30, 46). Similarly, several studies demonstrated higher resistin concentrations in insulin-resistant or diabetic persons as compared with their healthy counterparts (23–27), whereas other studies did not find such a difference (28–33). These conflicting data may result from lack of adjustment for potential confounding variables, limited power due to small sample sizes, and the cross-sectional design. To our knowledge, the present study is the first prospective analysis on resistin and the risk for type 2 diabetes. Our results indicate that resistin is not significantly related to diabetes risk after adjusting for BMI, although resistin remained positively, albeit moderately, associated with several inflammatory markers. These findings suggest that resistin could be a marker of subclinical inflammation rather than an independent predictor of diabetes risk.
The apparent differences between rodents and humans in the role of resistin in the pathogenesis of type 2 diabetes could in part reflect different expression and regulatory mechanisms of resistin in these species. For example, murine resistin is almost exclusively expressed in adipocytes, whereas human resistin is expressed at significantly lower concentrations in adipocytes and readily detectable in other cell types, especially macrophages (47). Further, mouse and human resistin share only 59% identity at the amino acid level (47).
The strengths of the present study comprise the prospective design with a long follow-up, the large sample size, and detailed information on diet and lifestyle. Several limitations of this study should also be acknowledged. First, the results were based on single measurements of the adipokines and therefore, may not reflect long-term exposure to these hormones. However, single measurements of resistin and adiponectin have been suggested to be reliable for the risk assessment in longitudinal studies based on reported intraclass correlations of 0.95 for resistin and 0.73 for adiponectin over 3 years (48). Second, the present study was limited to predominantly Caucasian women. Because of ethnic and gender differences in the level of adiponectin and resistin (6, 47), the generalizablility of our results to other populations may be limited. Finally, we adjusted for known confounding factors, but residual confounding by imperfectly or unmeasured confounders cannot be excluded.
In conclusion, we observed a strong and inverse association between total and high-molecular weight adiponectin and risk for type 2 diabetes in apparently healthy women independent of BMI and other diabetes risk factors. The high-molecular weight-to-total adiponectin ratio is related to type 2 diabetes risk independent of total adiponectin, suggesting an important role of the relative proportion of high-molecular weight adiponectin in diabetes pathogenesis. In contrast, the positive association between resistin and type 2 diabetes was largely explained by BMI.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the grants CA87969, DK58845, and DK080140 from the National Institutes of Health and the Intramural Research Program of the National Institute of Child Health & Human Development. C.H. was supported by fellowships of the German Academic Exchange Service and the Hans & Eugenia Juetting-Foundation. J.B.M. received a Career Development Award from the American Diabetes Association and research grants from GlaxoSmithKline and sanofi. C.S.M. was supported by discretionary grants from the Tanita corporation and and the Beth Israel Deaconess Medical Center.
Footnotes
Study protocol: Available at www.channing.harvard.edu/nhs.
Statistical code: Available subject to approval by the NHS committees.
Data set: Available subject to approval by the NHS committees
References
- 1.Schulze MB, Hu FB. Primary prevention of diabetes: what can be done and how much can be prevented? Annu Rev Public Health. 2005;26:445–67. doi: 10.1146/annurev.publhealth.26.021304.144532. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14 (Suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 3.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 4.McTernan PG, Kusminski CM, Kumar S. Resistin. Curr Opin Lipidol. 2006;17:170–5. doi: 10.1097/01.mol.0000217899.59820.9a. [DOI] [PubMed] [Google Scholar]
- 5.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 6.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007;9:282–9. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527–33. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Lowe GD, Rumley A, Cherry L, Whincup PH, Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007;30:1200–5. doi: 10.2337/dc06-2416. [DOI] [PubMed] [Google Scholar]
- 10.Bayes B, Granada ML, Pastor MC, Lauzurica R, Salinas I, Sanmarti A, et al. Obesity, adiponectin and inflammation as predictors of new-onset diabetes mellitus after kidney transplantation. Am J Transplant. 2007;7:416–22. doi: 10.1111/j.1600-6143.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- 11.Snijder MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CD, Nijpels G, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the hoorn study. Diabetes Care. 2006;29:2498–503. doi: 10.2337/dc06-0952. [DOI] [PubMed] [Google Scholar]
- 12.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. J Am Coll Cardiol. 2006;48:1369–77. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 14.Knobler H, Benderly M, Boyko V, Behar S, Matas Z, Rubinstein A, et al. Adiponectin and the development of diabetes in patients with coronary artery disease and impaired fasting glucose. Eur J Endocrinol. 2006;154:87–92. doi: 10.1530/eje.1.02054. [DOI] [PubMed] [Google Scholar]
- 15.Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, et al. Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clin Endocrinol (Oxf) 2004;61:75–80. doi: 10.1111/j.1365-2265.2004.02063.x. [DOI] [PubMed] [Google Scholar]
- 16.Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes Care. 2003;26:2015–20. doi: 10.2337/diacare.26.7.2015. [DOI] [PubMed] [Google Scholar]
- 17.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26:3226–9. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima R, Kamei N, Yamane K, Nakanishi S, Nakashima A, Kohno N. Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab. 2006;91:3873–7. doi: 10.1210/jc.2006-1158. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–59. [PubMed] [Google Scholar]
- 20.Bluher M, Brennan AM, Kelesidis T, Kratzsch J, Fasshauer M, Kralisch S, et al. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007;30:280–5. doi: 10.2337/dc06-1362. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005;130:583–97. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- 22.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 23.Al-Harithy RN, Al-Ghamdi S. Serum resistin, adiposity and insulin resistance in Saudi women with type 2 diabetes mellitus. Ann Saudi Med. 2005;25:283–7. doi: 10.5144/0256-4947.2005.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chanchay S, Tungtrongchitr R, Harnroongroj T, Phonrat B, Rungseesakorn O, Paksanont S, et al. Plasma resistin, insulin concentration in non-diabetic and diabetic, overweight/obese Thai. Int J Vitam Nutr Res. 2006;76:125–31. doi: 10.1024/0300-9831.76.3.125. [DOI] [PubMed] [Google Scholar]
- 25.Lu HL, Wang HW, Wen Y, Zhang MX, Lin HH. Roles of adipocyte derived hormone adiponectin and resistin in insulin resistance of type 2 diabetes. World J Gastroenterol. 2006;12:1747–51. doi: 10.3748/wjg.v12.i11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokuyama Y, Osawa H, Ishizuka T, Onuma H, Matsui K, Egashira T, et al. Serum resistin level is associated with insulin sensitivity in Japanese patients with type 2 diabetes mellitus. Metabolism. 2007;56:693–8. doi: 10.1016/j.metabol.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Youn BS, Yu KY, Park HJ, Lee NS, Min SS, Youn MY, et al. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:150–6. doi: 10.1210/jc.2003-031121. [DOI] [PubMed] [Google Scholar]
- 28.Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, et al. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004;89:1844–8. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- 29.Utzschneider KM, Carr DB, Tong J, Wallace TM, Hull RL, Zraika S, et al. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 2005;48:2330–3. doi: 10.1007/s00125-005-1932-y. [DOI] [PubMed] [Google Scholar]
- 30.Norata GD, Ongari M, Garlaschelli K, Raselli S, Grigore L, Catapano AL. Plasma resistin levels correlate with determinants of the metabolic syndrome. Eur J Endocrinol. 2007;156:279–84. doi: 10.1530/eje.1.02338. [DOI] [PubMed] [Google Scholar]
- 31.Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, Tataranni PA. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes. 2004;53:1279–84. doi: 10.2337/diabetes.53.5.1279. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–56. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 33.Yaturu S, Daberry RP, Rains J, Jain S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–23. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 35.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–6. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 36.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 37.Willett WC, Stampfer MJ. Nutritional epidemiology. New York/Oxford: Oxford University Press; 1998. Implications of total energy intake for epidemiologic analysis; pp. 273–301. [Google Scholar]
- 38.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 39.Schulze MB, Solomon CG, Rifai N, Cohen RM, Sparrow J, Hu FB, et al. Hyperproinsulinaemia and risk of Type 2 diabetes mellitus in women. Diabet Med. 2005;22:1178–84. doi: 10.1111/j.1464-5491.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ, Greenland S. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 1998. Fundamentals of epidemiologic data analysis; pp. 201–229. [Google Scholar]
- 41.Akaike H. A new look at the statistical model identification. IEE Trans Autom Control. 1974;19:716–23. [Google Scholar]
- 42.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–7. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 44.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–62. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 45.Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452–5. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- 46.Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149:331–5. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 47.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cell Mol Immunol. 2006;3:29–34. [PubMed] [Google Scholar]
- 48.Kaplan RC, Ho GY, Xue X, Rajpathak S, Cushman M, Rohan TE, et al. Within-individual stability of obesity-related biomarkers among women. Cancer Epidemiol Biomarkers Prev. 2007;16:1291–3. doi: 10.1158/1055-9965.EPI-06-1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.