Abstract
Objective
The purpose of this study was to determine whether metformin promotes weight loss in overweight out-patients with chronic schizophrenia or schizoaffective disorder.
Method
In a double-blind study, 148 clinically stable, overweight (body mass index [BMI] ≥27) outpatients with chronic schizophrenia or schizoaffective disorder were randomly assigned to receive 16 weeks of metformin or placebo. Metformin was titrated up to 1,000 mg twice daily, as tolerated. All patients continued to receive their prestudy medications, and all received weekly diet and exercise counseling. The primary outcome measure was change in body weight from baseline to week 16.
Results
Fifty-eight (77.3%) patients who received metformin and 58 (81.7%) who received placebo completed 16 weeks of treatment. Mean change in body weight was −3.0 kg (95% CI=−4.0 to −2.0) for the metformin group and −1.0 kg (95% CI= −2.0 to 0.0) for the placebo group, with a between-group difference of −2.0 kg (95% CI=−3.4 to −0.6). Metformin also demonstrated a significant between-group advantage for BMI (−0.7; 95% CI=−1.1 to −0.2), triglyceride level (−20.2 mg/dL; 95% CI=−39.2 to −1.3), and hemoglobin A1c level (−0.07%; 95% CI=−0.14 to −0.004). Metformin-associated side effects were mostly gastrointestinal and generally transient, and they rarely led to treatment discontinuation.
Conclusions
Metformin was modestly effective in reducing weight and other risk factors for cardiovascular disease in clinically stable, overweight outpatients with chronic schizophrenia or schizoaffective disorder over 16 weeks. A significant time-by-treatment interaction suggests that benefits of metformin may continue to accrue with longer treatment. Metformin may have an important role in diminishing the adverse consequences of obesity and metabolic impairments in patients with schizophrenia.
The difference in life expectancy for patients with schizophrenia compared with the general population is more than 20 years (1), and cardiovascular disease accounts for at least 50% of this excess mortality (2). While the basis for increased cardiovascular disease in schizophrenia is multifactorial, adverse metabolic side effects of antipsychotic medications include increased risk for weight gain, hyperlipidemia, and impaired glucose metabolism (3–6). Studies suggest that antipsychotic-induced metabolic side effects can increase both the 10-year risk of cardiovascular disease (7) and mortality from cardiovascular disease (8). Given the magnitude of the problem, surprisingly little evidence from randomized trials is available to guide the management of antipsychotic-induced weight gain and related metabolic deficits (9).
Among treatments approved by the U.S. Food and Drug Administration (FDA) for weight loss, options for persons with schizophrenia are very limited. Sympathomimetics, such as diethylpropion, phentermine, and a recently approved combination of phentermine and topiramate, are relatively contraindicated given the potential risk for psychosis exacerbation. In a 16-week study, orlistat, a pancreatic lipase inhibitor, produced no significant weight loss in overweight patients with schizophrenia (10). The safety and efficacy of other weight loss agents, including lorcaserin, a newly approved 5- HT2c agonist, and a combination of naltrexone and bupropion currently under development, are unknown in schizophrenia. Among agents without an FDA indication for weight loss, several recent meta-analyses indicate that metformin, topiramate, sibutramine, fenfluramine, and reboxetine have modest efficacy for antipsychotic-induced weight gain (9, 11), although only metformin and topiramate are currently marketed in the United States.
Metformin is a well-tolerated biguanide antihyperglycemic approved for treatment of type 2 diabetes mellitus. Metformin normalizes blood glucose levels by blocking hepatic gluconeogenesis and increasing peripheral insulin sensitivity (12). Since metformin does not increase insulin production, it is rarely associated with hypoglycemia (13). Metformin is known to cause modest weight loss in people with prediabetes and with type 2 diabetes (14, 15). Contributing mechanisms for weight loss are thought to include appetite suppression and slowing of gastric emptying related to stimulation of glucagon-like peptide–1 secretion (13, 16).
Studies of the use of metformin to produce weight loss or to prevent antipsychotic-induced weight gain in non-diabetic patients with schizophrenia have demonstrated positive (17–21), suggestive (22), and negative (23–25) results. A meta-analysis of available studies (N=7) found weight loss of approximately 3 kg with metformin over 3 to 4 months (9), primarily in narrowly defined populations, including children (19, 24), adults with first-episode schizophrenia (17, 18, 20, 21), and predominantly non-obese individuals (17, 18, 22–25). The present study is the first, to our knowledge, to examine whether metformin is effective for weight loss in a typical sample of overweight adults with a chronic psychotic illness.
Method
Setting and Study Design
The study was conducted between March 2009 and February 2010 at 17 U.S. academic, Veterans Affairs, and private research clinic sites affiliated with the National Institute of Mental Health-funded Schizophrenia Trials Network (see the acknowledgment section for a list of the research sites). In this double-blind randomized study, stable outpatients with schizophrenia or schizoaffective disorder with a body mass index (BMI) ≥27 received 16 weeks of adjunctive metformin or placebo. The primary outcome measure was between-group change in body weight over 16 weeks. It was hypothesized that metformin would produce greater weight loss compared with placebo.
Study treatments were randomly assigned using a central computerized system, and eligible participants received double-blind over-encapsulated metformin or placebo in a 1:1 ratio. Randomization was stratified by site in blocks of four. All participants received a standardized behavioral intervention focused on improving diet and exercise habits. An institutional review board approved the study at each research site.
Participants
Eligible participants were 18 to 65 years old; met criteria for schizophrenia or schizoaffective disorder, as determined by the Structured Clinical Interview for DSM-IV; had a duration of illness ≥1 year; had a BMI ≥27; had outpatient status; and were receiving one or a combination of two FDA-approved antipsychotics with no change in antipsychotic agents for 2 months and no change in dosage for 1 month prior to study entry. Concomitant medications were allowed if dosages were unchanged for 1 month prior to entry. Women of childbearing potential were required to use an acceptable method of birth control. All participants provided written informed consent.
Exclusion criteria included inpatient status; a Clinical Global Impressions scale severity rating (CGI-S) ≥6 (severely or very severely ill); diabetes mellitus; a fasting glucose level >125 mg/dL; current or previous treatment with metformin; current treatment with insulin or an oral hypoglycemic; treatment with more than two antipsychotics; use of any prescription or nonprescription medication for weight loss within the past month; pregnancy or breastfeeding; an uncorrected thyroid disorder; and any serious and unstable medical illness. Rarely, metformin has been associated with lactic acidosis (3 in 100,000 patient-years), a condition that often co-occurs with a serious medical condition and that can be potentiated by excessive alcohol intake (13). To minimize the risk of lactic acidosis, individuals were excluded if they had congestive heart failure, renal impairment (serum creatinine levels ≥1.5 mg/dL for men and ≥1.4 for women; estimated glomerular filtration rate <55 mL/min/ 1.73 m2), hepatic disease (ALT, AST, GGT levels >1.5 times the upper limit of normal; total bilirubin level >1.2 times the upper limit of normal), metabolic acidosis (serum CO2 level below the lower limit of normal), iodinated contrast material in the past month, or alcohol abuse or dependence in the past month.
Intervention
Study treatments were double-blind, with identical capsules containing metformin (500 mg) or placebo. After randomization, participants began treatment with one 500-mg metformin capsule or matching placebo twice daily, to be taken with morning and evening meals for the first week. If well tolerated, the study medication was then increased to one capsule in the morning and two capsules in the evening for the following week and then increased to the final dosage of two capsules twice daily at week 3. The maximum total daily dose for metformin was 2,000 mg. During the titration period, the dosage could be reduced or the drug discontinued for tolerability concerns. Following a reduction or interruption in the dosage, study medication would be retitrated in one-capsule increments per week up to two capsules twice daily or continued at the maximum tolerated dosage.
Behavioral Intervention
All participants received weekly diet and exercise counseling during the study. The intervention was adapted from a weight-reduction program developed for patients with severe mental illnesses (26). The intervention included eight 20- to 30-minute lessons and seven interim telephone calls to reinforce lessons. (For details, see the data supplement that accompanies the online edition of this article.)
Outcome Measures
The primary outcome measure was change in body weight from baseline to week 16. Secondary outcome measures included change from baseline to week 16 in BMI, waist circumference, waist-hip ratio, and levels of fasting total cholesterol, non-high-density lipoprotein (non-HDL) cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, glucose, insulin, and hemoglobin A1c (HbA1c). Additional safety outcomes included the frequency and severity of adverse events (assessed using a side effects scale adapted from the Clinical Antipsychotic Trials of Intervention Effectiveness study [3]), vital signs, laboratory measures (for estimated glomerular filtration rate and creatinine, ALT, AST, and lactate levels), and CGI-S ratings. Weight and vital signs were assessed at screening, at baseline, weekly during the first 2 weeks of treatment, and then every 2 weeks until week 16 or treatment discontinuation. Laboratory blood tests were obtained at screening, baseline, and weeks 4, 8, 12, and 16; CGI-S ratings were determined at screening, at baseline, and monthly until week 16.
Statistical Analysis
Participants who were screened but not randomly assigned were not included in efficacy or safety analyses. Efficacy was assessed for participants who received at least one dose of study medication and had at least one postbaseline weight measure (i.e., the intent-to-treat population). A linear mixed-model analysis was used to compare treatment groups with respect to least-squares-mean estimates of change in body weight from baseline to week 16. The mixed model used data from all visits for which the participant was receiving the study drug and included fixed effects for treatment group, visit, and treatment-by-visit interaction and random effects for participant and site. Only visits that occurred after participants discontinued the study drug and did not restart it were excluded from the analysis. A similar approach was used to compare continuous secondary efficacy and safety outcome variables. Graphical representation of change in primary outcome over the follow-up period was based on least-squares-mean estimates of change for each group and associated 95% confidence intervals.
Safety analyses were performed for participants who received at least one dose of the study drug and included any event occurring between the time of the first dose and within 30 days of discontinuation of the study drug.
The target sample size of 148 participants was estimated to yield 80% power to detect a 2-kg (SD=4) difference in weight change between the treatment groups, assuming a two-tailed test with an alpha level of 0.05 (18, 19, 27).
Results
Randomization and Baseline Characteristics
A total of 193 individuals consented to participate in the study, and 148 were found eligible and randomly assigned to receive metformin or placebo. Of these, 75 received metformin and 71 received placebo. Two participants in the placebo arm withdrew before receiving study medication. Fifty-eight (77.3%) participants who received metformin and 58 (81.7%) who received placebo completed 16 weeks of treatment. (The CONSORT flowchart is presented in the online data supplement.) The baseline demographic and clinical characteristics of the intent-to-treat population are summarized in Table 1. For the baseline distribution of antipsychotic medications and concomitant medications commonly associated with weight gain, see Table S1 in the data supplement.
TABLE 1.
Baseline Demographic and Clinical Characteristics of Overweight Outpatients With Schizophrenia or Schizoaffective Disorder Treated With Metformin or Placebo
| Characteristic | Total (N=146) | Metformin Group (N=75) | Placebo Group (N=71) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | N | % | |
| Sex (female) | 45 | 30.8 | 23 | 30.7 | 22 | 31.0 |
| Race | ||||||
| White | 111 | 76.0 | 57 | 76.0 | 54 | 76.1 |
| Black | 31 | 21.2 | 16 | 21.3 | 15 | 21.1 |
| Other | 4 | 2.7 | 2 | 2.7 | 2 | 2.8 |
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
| Age (years) | 43.2 | 11.06 | 41.4 | 11.53 | 45.0 | 10.31 |
| Clinical Global Impressions, severity score | 3.5 | 0.7 | 3.5 | 0.8 | 3.5 | 0.6 |
|
| ||||||
| N | % | N | % | N | % | |
|
| ||||||
| Tobacco use | 66 | 45.2 | 36 | 48.0 | 30 | 42.3 |
| Alcohol use | ||||||
| Abstinent | 114 | 78.1 | 61 | 81.3 | 53 | 74.6 |
| Not abstinent | 32 | 21.9 | 14 | 18.7 | 18 | 25.4 |
| Illicit drug use | ||||||
| Abstinent | 128 | 87.7 | 65 | 86.7 | 63 | 88.7 |
| Not abstinent | 18 | 12.3 | 10 | 13.3 | 8 | 11.3 |
| Antipsychotic use | ||||||
| Higher-risk agents causing weight gaina | 83 | 56.9 | 40 | 53.3 | 43 | 60.6 |
| Lower-risk agents causing weight gainb | 47 | 32.2 | 26 | 34.7 | 21 | 29.6 |
| Both higher- and lower-risk agentsc | 16 | 11.0 | 9 | 12.0 | 7 | 9.9 |
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
| Weight (kg) | 101.9 | 18.5 | 101.8 | 15.2 | 101.9 | 21.6 |
| Body mass index | 34.6 | 5.9 | 34.4 | 5.1 | 34.7 | 6.6 |
| Waist circumference (cm) | 113.5 | 13.0 | 113.3 | 11.7 | 114.0 | 14.3 |
| Waist-hip ratio | 0.98 | 0.07 | 0.97 | 0.07 | 0.98 | 0.06 |
| Total cholesterol (mg/dL) | 193.1 | 36.8 | 192.0 | 29.5 | 194.2 | 43.4 |
| Non-high-density lipoprotein cholesterol (mg/dL) | 147.4 | 37.0 | 145.7 | 30.5 | 149.2 | 42.9 |
| High-density lipoprotein cholesterol (mg/dL) | 45.7 | 13.6 | 46.3 | 15.1 | 45.1 | 11.9 |
| Low-density lipoprotein cholesterol (mg/dL) | 117.3 | 34.4 | 116.0 | 27.6 | 118.7 | 40.5 |
| Triglycerides (mg/dL) | 150.3 | 76.5 | 148.7 | 72.6 | 152.0 | 80.9 |
| Fasting glucose (mg/dL) | 97.0 | 9.7 | 95.7 | 9.3 | 98.3 | 10.0 |
| Insulin (mU/L) | 18.1 | 15.8 | 18.7 | 17.0 | 17.3 | 14.6 |
| Hemoglobin A1c (%) | 5.5 | 0.4 | 5.5 | 0.5 | 5.6 | 0.4 |
Antipsychotics categorized a priori as higher-risk agents for weight gain are clozapine, olanzapine, paliperidone, quetiapine, and risperidone.
Antipsychotics categorized a priori as lower-risk agents for weight gain are aripiprazole, fluphenazine, haloperidol, loxitane, perphenazine, thiothixene, and ziprasidone.
Data are for patients receiving a combination of one higher- and one lower-risk agent.
Weight and BMI
Least-squares-mean body weight change across the 16-week treatment period was −3.0 kg (95% confidence interval [CI]= −4.0 to −2.0) for the metformin group and −1.0 kg (95% CI=−2.0 to 0.0) for the placebo group, with a between-group difference of −2.0 kg (95% CI=−3.4 to −0.6; p=0.007) (Figure 1, Table 2). The between-group difference demonstrated a significant time-by-treatment interaction (p=0.007). In the active treatment arm, the mean final daily dose of metformin was 1,887 mg (SD=292). Over 16 weeks, participants who received metformin experienced a 2.8% weight reduction, compared with a 1.0% reduction among those who received placebo. Thirteen (17.3%) participants who received metformin, compared with seven (9.8%) who received placebo, lost more than 5% of their baseline weight. BMI changed by −1.0 (95% CI=−1.3 to −0.7) in the metformin group and by −0.3 (95% CI=−0.7 to 0.0) in the placebo group, with a between-group difference of −0.7 (95% CI=−1.1 to −0.1; p=0.006).
FIGURE 1. Weight Change Across 16 Weeks of Treatment With Metformin or Placebo in Overweight Outpatients With Schizophrenia or Schizoaffective Disordera.
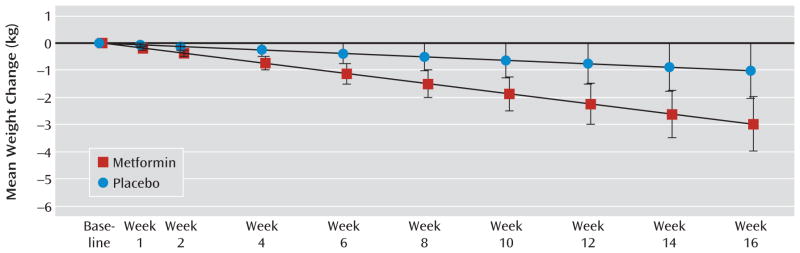
aError bars represent 95% confidence intervals. Significantly different between groups, p=0.007.
TABLE 2.
Mean Change From Baseline to 16 Weeks for Primary and Secondary Outcome Variables Among Overweight Outpatients With Schizophrenia or Schizoaffective Disorder Treated With Metformin or Placebo
| Variable | Metformin Group (N=75)
|
Placebo Group (N=71)
|
Between-Group Difference
|
Metformin Compared With Placebo
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | e | 95% CI | t | p | |
| Weight (kg) | −3.0 | −4.0 to −2.0 | −1.0 | −2.0 to 0.0 | −2.0 | −3.4 to −0.6 | −2.606 | 0.0065 |
| Body mass index | −1.0 | −1.3 to −0.7 | −0.3 | −0.7 to 0.0 | −0.7 | −1.1 to −0.2 | −2.739 | 0.0063 |
| Waist circumference (cm) | −2.8 | −4.1 to −1.3 | −2.3 | −3.6 to −1.0 | −0.5 | −2.3 to 1.5 | −0.423 | 0.673 |
| Waist-hip ratio | 0.002 | −0.009 to 0.013 | −0.006 | −0.017 to 0.005 | 0.008 | −0.008 to 0.023 | 1.011 | 0.314 |
| Total cholesterol (mg/dL) | −8.9 | −15.6 to −2.3 | 0.2 | −6.5 to 6.9 | −9.2 | −18.6 to 0.3 | −1.922 | 0.057 |
| Non-HDL cholesterol (mg/dL) | −8.4 | −14.8 to −1.9 | 0.7 | −5.9 to 7.2 | −9.0 | −18.3 to 0.2 | −1.945 | 0.054 |
| HDL cholesterol (mg/dL) | −0.6 | −2.4 to 1.2 | −0.4 | −2.3 to 1.4 | −0.1 | −2.7 to 2.4 | −0.093 | 0.926 |
| LDL cholesterol (mg/dL) | −7.1 | −12.6 to −1.5 | −2.0 | −7.7 to 3.7 | −5.1 | −13.0 to 2.9 | −1.265 | 0.209 |
| Triglycerides (mg/dL) | −7.0 | −20.4 to 6.3 | 13.2 | −0.3 to 26.7 | −20.2 | −39.2 to −1.3 | −2.112 | 0.037 |
| Glucose (mg/dL) | −2.3 | −5.0 to 0.5 | −1.6 | −4.3 to 1.2 | −0.7 | −4.6 to 3.2 | −0.359 | 0.720 |
| Insulin (mU/L) | 1.6 | −5.5 to 8.6 | 5.5 | −1.7 to 12.6 | −3.9 | −13.9 to 6.1 | −0.771 | 0.443 |
| Hemoglobin A1c (%) | −0.06 | −0.11 to −0.01 | 0.01 | −0.04 to 0.06 | −0.07 | −0.14 to −0.004 | −2.091 | 0.039 |
Lipid Measures
Triglyceride levels changed by −7.0 mg/dL (95% CI= −20.4 to 6.3) in the metformin group and by 13.2 mg/dL (95% CI=−0.3 to 26.7) in the placebo group, with a between-group difference of −20.2 mg/dL (95% CI=−39.2 to −1.3; p=0.037) and a significant time-by-treatment interaction (p=0.04). Between-group differences for met-formin and placebo fell short of statistical significance for levels of non-HDL cholesterol (−9.0 mg/dL, 95%= CI=−18.3 to 0.2; p=0.054) and total cholesterol (−9.2 mg/dL, 95% CI=−18.6 to 0.3; p=0.057). Similarly, no differences emerged for HDL and LDL cholesterol levels (Table 2).
Indices of Glucose Metabolism
Changes in fasting glucose and insulin levels did not differ between the metformin and placebo groups (Table 2). Mean HbA1c levels changed by −0.06% (95% CI=−0.11 to −0.01) in the metformin group and 0.011% (95% CI=−0.04 to 0.06) in the placebo group, with a between-group difference of −0.07% (95% CI=−0.14 to −0.004; p=0.04) and a significant time-by-treatment interaction (p=0.039).
Adherence to Treatment
In the intent-to-treat population, 94.9% of participants in the metformin group and 96.0% in the placebo group demonstrated adherence to study medication across all study visits either all (81%–100%) or most (61%–80%) of the time, based on pill counts, patient report, and any other available information, with no between-group differences. For the behavioral intervention, 84.9% of participants receiving metformin and 89.7% of those receiving placebo exhibited reasonable-to-excellent adherence based on patient report and rater assessment, with no between-group differences.
Adverse Events and Safety Assessments
Ten serious adverse events (metformin group, N=3; placebo group, N=7) were reported. None were attributed to metformin. A systematic inquiry about seven side effects commonly associated with metformin and rated at severity levels of “moderate” or “severe” is summarized in Table 3. Only diarrhea was reported significantly more frequently with metformin (33.3%) than placebo (16.9%). The side effects were generally transient. Eleven of the 75 (14.7%) participants receiving metformin and eight of the 71 (11.3%) participants receiving placebo discontinued the study treatment because of intolerability. Two participants in the metformin group discontinued treatment because of abdominal symptoms. In all, 17 participants discontinued metformin and 13 discontinued placebo before the end of the 16-week treatment period.
TABLE 3.
Adverse Events From Systematic Inquiry in Overweight Outpatients With Schizophrenia or Schizoaffective Disorder Treated With Metformin or Placebo
| Adverse Event | Total (N=146)
|
Metformin Group (N=75)
|
Placebo Group (N=71)
|
p | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Participants with ≥1 adverse event rated at moderate or severe intensity | 73 | 50.0 | 40 | 53.3 | 33 | 46.5 | 0.323 |
| Diarrhea | 37 | 25.3 | 25 | 33.3 | 12 | 16.9 | 0.018 |
| Headache | 28 | 19.2 | 11 | 14.7 | 17 | 23.9 | 0.181 |
| Muscle pain | 23 | 15.8 | 13 | 17.3 | 10 | 14.1 | 0.542 |
| Abdominal discomfort | 21 | 14.4 | 12 | 16.0 | 9 | 12.7 | 0.522 |
| Nausea | 20 | 13.7 | 12 | 16.0 | 8 | 11.3 | 0.370 |
| Weakness | 14 | 9.6 | 8 | 10.7 | 6 | 8.5 | 0.611 |
| Vomiting | 11 | 7.5 | 8 | 10.7 | 3 | 4.2 | 0.128 |
Safety assessments for vital signs, for levels of ALT, AST, and creatinine, and for estimated glomerular filtration rate revealed no change in both groups. Lactate levels demonstrated a small increase in the metformin group (mean=0.240 mmol/L, 95% CI=0.06–0.42) compared with the placebo group (mean=−0.07 mmol/L, 95% CI=−0.24 to 0.11), with a mean group difference of 0.31 mmol/L (95% CI=0.06–0.56; p=0.02).
Recent illicit drug use was reported in at least one study visit by 10 participants in the metformin group (cocaine, opiate, or cannabis use) and eight in the placebo group (cocaine, opiate, or cannabis use). No between-group differences emerged.
Exploratory Analyses
We explored the effect of age and baseline BMI using a median split and the effect of sex, antipsychotic medication, smoking status, and presence or absence of nausea, vomiting, or diarrhea (during the first 2 weeks of study treatment) as categorical variables on metformin-placebo weight change. A priori, antipsychotic medications were categorized as having “higher” or “lower” risk for causing weight gain (see Table 1 footnotes), based on published data (3, 6, 28, 29). Exploratory analyses revealed significant metformin-placebo weight reductions in the following groups: younger-age participants (<44 years old), participants with a baseline BMI <33.38, men, and nonsmokers (Table 4).
TABLE 4.
Exploratory Moderators of Metformin-Placebo Weight Change in Overweight Outpatients With Schizophrenia or Schizoaffective Disorder
| Variable | N | Weight Change (kg) | 95% CI | p |
|---|---|---|---|---|
| Age (years)a | ||||
| <44 | 74 | −3.2 | −5.4 to −1.0 | 0.005 |
| ≥44 | 72 | −1.0 | −2.9 to 0.8 | 0.276 |
| Baseline body mass indexa | ||||
| <33.38 | 73 | −1.9 | −3.6 to −0.2 | 0.028 |
| ≥33.38 | 73 | −2.0 | −4.2 to 0.3 | 0.088 |
| Sex | ||||
| Female | 45 | −1.7 | −4.0 to 0.6 | 0.140 |
| Male | 101 | −2.1 | −3.9 to −0.3 | 0.020 |
| Antipsychotic medicationb | ||||
| Higher risk for weight gain | 83 | −1.5 | −3.2 to 0.1 | 0.074 |
| Lower risk for weight gain | 47 | −2.3 | −5.4 to 0.9 | 0.157 |
| Tobacco use | ||||
| Nonsmokers | 80 | −2.6 | −4.3 to −0.8 | 0.005 |
| Smokers | 66 | −1.2 | −3.5 to 1.2 | 0.323 |
| Nausea, vomiting, or diarrheac | ||||
| Present | 83 | −1.9 | −3.9 to 0.2 | 0.072 |
| Absent | 63 | −1.9 | −4.0 to 0.2 | 0.070 |
Analyzed using a median split.
Participants receiving one higher- and one lower-risk agent (N=16) were not included in this analysis. (For a list of higher- and lower-risk agents, see Table 1 footnotes.)
Data refer to the presence or absence of nausea, vomiting, or diarrhea within the first 2 weeks of study medication.
Discussion
In this study, treatment with metformin over 16 weeks, along with weekly diet and exercise counseling, was associated with significantly more weight loss (2.0 kg) than placebo in overweight adult outpatients with chronic schizophrenia or schizoaffective disorder. The weight change corresponds to a BMI reduction of 1.0 with metformin and an advantage of −0.7 for metformin over placebo. Unlike previous studies, this study placed no restrictions on the timing of weight gain, the chronicity of psychosis, or the presence of comorbid psychiatric conditions. Furthermore, patients could be on a regimen of any single antipsychotic medication or any combination of two antipsychotic medications as well as stable dosages of other concomitant medications. The only medical exclusions were for diabetes and conditions that could increase risk for metformin-induced lactic acidosis. Taken together, these factors suggest that the results are generalizable and applicable to a broad group of patients.
An increase of one BMI unit has previously been identified as an important threshold for triggering an intervention to address weight gain in patients receiving antipsychotic medication (30). While this study suggests that metformin can reasonably be expected to lead to loss of one BMI unit, it is also clear that the magnitude of needed weight loss in this population often exceeds the demonstrated effects of 16 weeks of metformin. It would be important to determine whether metformin-associated weight loss in overweight patients receiving antipsychotics can extend beyond 16 weeks, as suggested by the linear time-by-treatment interaction. Continued weight loss with metformin is also supported by a study of 84 mostly nonobese female patients with first-episode schizophrenia and amenorrhea that demonstrated significant weight loss with 6 months of metformin compared with placebo (20), as well as evidence of modest weight loss for up to 12 months in patients with prediabetes and type 2 diabetes (14, 15).
Other approaches to promote weight loss among people diagnosed with schizophrenia are limited. Topiramate is the only adjunctive agent other than metformin marketed in the United States that has demonstrated significant weight loss (approximately 2.5 kg) in a meta-analysis (9). The other main pharmacological approach to weight loss in this population is to switch to an antipsychotic with lower weight-gain liability. Studies have demonstrated comparable modest weight loss using this method (31, 32). Although switching in these studies was generally safe and did not lead to widespread relapses, switching antipsychotics must be undertaken with considerable care and close clinical monitoring. Taken together, the evidence suggests that metformin represents one of the few safe and modestly effective approaches for overweight patients receiving antipsychotics who are also committed to making healthy lifestyle changes consistent with weight loss.
Among previous studies of metformin for antipsychotic-induced weight gain, many have focused on adults with first-episode schizophrenia. In 128 Chinese adults with first-episode schizophrenia (the largest previous study) who had gained >10% of their baseline weight since starting antipsychotics, metformin was associated with a mean weight loss of 4.7 kg after 12 weeks of treatment together with a daily behavioral intervention, while the behavioral intervention alone produced a mean weight loss of 1.4 kg (18). Comparing those findings with our data suggests that metformin may be somewhat more effective for weight loss in patients with first-episode psychosis. In adults with chronic multiepisode psychosis, previous metformin studies have been smaller with mostly nonobese participants. Two studies found no significant metformin-placebo weight change: the first examined 40 inpatients (mean BMI, approximately 23) starting olanzapine (25), and the second examined 80 inpatients and outpatients (mean BMI, approximately 25.5) who were stable on olanzapine (23). A study of 61 chronic patients receiving clozapine (mean BMI, approximately 28) found a small metformin-placebo difference over 14 weeks, but only in a completer analysis (22). The present study represents the largest study to date of metformin in antipsychotic-treated patients (N=146) and extends previous findings to a more typical group of substantially overweight patients receiving antipsychotics.
Although our cohort was not selected for lipid abnormalities, their mean baseline levels of triglycerides, non-HDL cholesterol, LDL cholesterol, and HDL cholesterol were at or near unhealthy levels (33). For triglycerides, the metformin-placebo difference was −20 mg/mL. While this effect reflected, in part, a triglyceride increase in the placebo arm, the pattern is consistent with the effects of metformin on triglycerides in type 2 diabetes (14, 34), and the significant time-by-treatment interaction suggests the potential for continued improvement. In the largest previous study of metformin in first-episode schizophrenia, lipids were not measured (18). In a study of patients receiving clozapine, no differential effect of metformin was found on lipids (22). Our study provides evidence that metformin may improve cardiovascular disease risk in overweight patients with schizophrenia.
No changes in fasting glucose or insulin levels were demonstrated between the metformin and placebo groups, although a small between-group difference was found for HbA1c levels (−0.07%), which also revealed a significant time-by-treatment interaction. These results contrast with findings from a study of patients with first-episode schizophrenia in which metformin was associated with a 50% reduction of fasting insulin levels compared with placebo (18). This could reflect distinct pathophysiological, pharmacogenetic, and dietary differences between the two populations studied. Although mean glucose and insulin levels did not change in our study, the benefits of metformin in preventing the onset of diabetes in prediabetic individuals (15) suggest that diabetes prevention is another potential long-term benefit for overweight patients with schizophrenia who are receiving metformin.
In exploratory analyses, younger age (<44 years old), lower BMI (<33), male sex, and nonsmoking status were significantly associated with metformin-associated weight loss. The evidence that younger patients who are not morbidly obese appear to exhibit greater metformin-placebo weight loss appears to be consistent with previous studies of metformin in first-episode psychosis that found somewhat more weight loss than we observed in the present study (18, 20). The lack of significant metformin-associated weight loss in female patients is likely confounded by the relatively small percentage of women (approximately 30%). Smoking has been associated with lower body mass and reduced appetite (35), and given that appetite suppression has been linked to metformin (16), the appetite-suppressing effects of smoking may have reduced the potential for metformin-induced weight loss. While these associations could represent important predictors of weight loss response to metformin, they must be interpreted cautiously given that the study was not powered to test these post hoc analyses.
Metformin was generally well tolerated by participants. No serious adverse events were attributed to metformin. While the frequency of adverse events was relatively high, the difference between metformin and placebo was generally small. Gastrointestinal symptoms were more common in the metformin group, but most of these were transient, and only diarrhea was substantially more common in the metformin group compared with the placebo group. Of the 19 participants who discontinued the study medication because of unacceptable side effects, 11 received metformin and eight received placebo.
The study did not follow participants after discontinuation of metformin, and therefore it remains unknown whether and when any weight lost might be regained. Furthermore, because an important goal was to obtain results with broad generalizability, the study did not standardize antipsychotic treatment or exclude concomitant medications with a risk for weight gain. However, weight change in participants receiving antipsychotics associated with higher compared with lower risk for causing weight gain did not differ between the metformin and placebo groups. Another potential confounder was the lack of standardization of caloric intake and physical activity. Analysis of adherence to the behavioral intervention suggested that adherence was high and similar between the groups. However, it is uncertain how well patients in clinical practice can modify their diet and activity level in a manner similar to that achieved in this study and whether less frequent behavioral counseling could also be effective.
Conclusions
This double-blind randomized controlled trial demonstrates the efficacy of metformin to achieve weight loss in overweight outpatients with schizophrenia or schizoaffective disorder. While the benefit of metformin on weight was modest, there are few alternatives for this patient population. For example, switching antipsychotic agents may not be appropriate for patients already receiving an agent with lower risk for causing weight gain or for patients for whom risks to clinical stability are considerable, such as for those receiving clozapine. Metformin represents a safe and reasonable option for overweight patients who are motivated to lose weight. It should be further emphasized that weight loss with any pharmacological intervention is more likely when accompanied by concurrent modifications of diet and physical activity levels.
Supplementary Material
Acknowledgments
Dr. Jarskog has served as a consultant for AstraZeneca, has received research grant support from GlaxoSmithKline, Novartis, Roche, and Sunovion, and has served on a data and safety monitoring board for Janssen. Dr. Hamer has served on an advisory board for, consulted with, served on a data and safety monitoring board/data monitoring committee with, or participated as an expert witness in patent lawsuits involving Abbott, Acadia, Allergan, Alpharma, Anesta, AstraZeneca, Barr, Biolinerx, Caraco, Cenerx, Cephalon, Edo, Epix, Eurand, Fidopharm, Forest, Johnson & Johnson, Lilly, Lundbeck, Marial, Mylan, NeuroPharmaBoost, Novartis, Pfizer, Proctor and Gamble, Roche, Solvay, Sun, Teva, Titan, and Velcera. Dr. LaVange has received an honorarium from GlaxoSmithKline and research grant support from Sanofi-Aventis. Dr. Lieberman serves on the advisory boards of Bioline, Intracellular Therapies, and PsychoGenics; he receives research grant support from Allon, F. Hoffman-La Roche, GlaxoSmithKline, Eli Lilly, Merck, Novartis, Pfizer, PsychoGenics, Sepracor (Sunovion), and Targacept; and he holds a patent with Repligen. Dr. Stroup has served as a consultant for Janssen and Eli Lilly. All other authors report no financial relationships with commercial interests.
Supported by NIMH contract N01MH-90001 to Drs. Stroup and Lieberman.
The Metformin in the Treatment of Antipsychotic-Induced Weight Gain in Schizophrenia (METS) Study investigators and sites were as follows: Lawrence Adler, M.D., Clinical Insights, Glen Burnie, Md.; Michael Barber, M.D., Baylor College of Medicine, Houston; Matthew Byerly, M.D., University of Texas-Southwestern, Dallas; Jose M. Canive, M.D., New Mexico VA Health Care System, Albuquerque, N.Mex.; Ira Glick, M.D., Stanford University, Palo Alto, Calif.; David C. Henderson, M.D., Massachusetts General Hospital/Freedom Trail Clinic, Boston; L. Fredrik Jarskog, M.D. (during subject enrollment period), New York State Psychiatric Institute/Columbia University, New York; J. Steven Lamberti, M.D., University of Rochester Medical Center, Rochester, N.Y.; Ahsan Khan, M.D., Clinical Research Institute, Wichita, Kan.; Joseph P. McEvoy, M.D., Duke University, Durham, N.C.; Herbert Meltzer, M.D., Vanderbilt University, Nashville; Alexander Miller, M.D., University of Texas Health Science Center at San Antonio; Del D. Miller, M.D., University of Iowa, Iowa City; Henry A. Nasrallah, M.D., University of Cincinnati; Stephen Olson, M.D., University of Minnesota, Minneapolis; Jayendra K. Patel, M.D., University of Massachusetts Medical School, Worcester; and Bruce L. Saltz, M.D., Mental Health Advocates, Boca Raton, Fla.
Footnotes
Presented at the 65th annual meeting of the Society of Biological Psychiatry, New Orleans, May 20–22, 2010, and the 49th annual meeting of the American College of Neuropsychopharmacology, Miami Beach, Fla., Dec. 5–9, 2010.
ClinicalTrials.gov identifier: NCT00816907.
References
- 1.Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374:620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 2.Osby U, Correia N, Brandt L, Ekbom A, Sparén P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000;45:21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 4.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Grobbee DE. EUFEST study group: Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 6.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 7.Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Rosenheck R, Davis SM, Hsiao JK, Stroup TS, Lieberman JA. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105:175–187. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson DC, Nguyen DD, Copeland PM, Hayden DL, Borba CP, Louie PM, Freudenreich O, Evins AE, Cather C, Goff DC. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66:1116–1121. doi: 10.4088/jcp.v66n0905. [DOI] [PubMed] [Google Scholar]
- 9.Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35:1520–1530. doi: 10.1038/npp.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joffe G, Takala P, Tchoukhine E, Hakko H, Raidma M, Putkonen H, Eronen M, Räsänen P. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:706–711. doi: 10.4088/jcp.v69n0503. [DOI] [PubMed] [Google Scholar]
- 11.Björkhem-Bergman L, Asplund AB, Lindh JD. Metformin for weight reduction in non-diabetic patients on antipsychotic drugs: a systematic review and meta-analysis. J Psychopharmacol. 2011;25:299–305. doi: 10.1177/0269881109353461. [DOI] [PubMed] [Google Scholar]
- 12.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Goodman AM the Multicenter Metformin Study Group. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Messeri G, Rotella CM. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24:489–494. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- 17.Wu RR, Zhao JP, Guo XF, He YQ, Fang MS, Guo WB, Chen JD, Li LH. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165:352–358. doi: 10.1176/appi.ajp.2007.07010079. [DOI] [PubMed] [Google Scholar]
- 18.Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, He YQ, Liu YJ, Chen JD, Li LH. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185–193. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 19.Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072–2079. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- 20.Wu RR, Jin H, Gao K, Twamley EW, Ou JJ, Shao P, Wang J, Guo XF, Davis JM, Chan PK, Zhao JP. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2012;169:813–821. doi: 10.1176/appi.ajp.2012.11091432. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Tong JH, Zhu G, Liang GM, Yan HF, Wang XZ. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138:54–57. doi: 10.1016/j.schres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Carrizo E, Fernández V, Connell L, Sandia I, Prieto D, Mogollón J, Valbuena D, Fernández I, de Baptista EA, Baptista T. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113:19–26. doi: 10.1016/j.schres.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Baptista T, Rangel N, Fernández V, Carrizo E, El Fakih Y, Uzcátegui E, Galeazzi T, Gutiérrez MA, Servigna M, Dávila A, Uzcátegui M, Serrano A, Connell L, Beaulieu S, de Baptista EA. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophr Res. 2007;93:99–108. doi: 10.1016/j.schres.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double-blind, placebo-controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Med J. 2008;29:1130–1134. [PubMed] [Google Scholar]
- 25.Baptista T, Martínez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, Arapé Y, Martinez M, de Mendoza S, Teneud L, Hernández L. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51:192–196. doi: 10.1177/070674370605100310. [DOI] [PubMed] [Google Scholar]
- 26.Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2005;66:205–212. doi: 10.4088/jcp.v66n0208. [DOI] [PubMed] [Google Scholar]
- 27.Pavo I, Jermendy G, Varkonyi TT, Kerenyi Z, Gyimesi A, Shoustov S, Shestakova M, Herz M, Johns D, Schluchter BJ, Festa A, Tan MH. Effect of pioglitazone compared with metformin on glycemic control and indicators of insulin sensitivity in recently diagnosed patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:1637–1645. doi: 10.1210/jc.2002-021786. [DOI] [PubMed] [Google Scholar]
- 28.Nussbaum AM, Stroup TS. Paliperidone for treatment of schizophrenia. Schizophr Bull. 2008;34:419–422. doi: 10.1093/schbul/sbn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, Kissling W, Davis JM, Leucht S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, Kane JM, Lieberman JA, Schooler NR, Covell N, Stroup S, Weissman EM, Wirshing DA, Hall CS, Pogach L, Pi-Sunyer X, Bigger JT, Jr, Friedman A, Kleinberg D, Yevich SJ, Davis B, Shon S. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- 31.Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, Rosenheck RA, Perkins DO, Nussbaum AM, Lieberman JA. Schizophrenia Trials Network: A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP) Am J Psychiatry. 2011;168:947–956. doi: 10.1176/appi.ajp.2011.10111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newcomer JW, Campos JA, Marcus RN, Breder C, Berman RM, Kerselaers W, L’italien GJ, Nys M, Carson WH, McQuade RD. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry. 2008;69:1046–1056. doi: 10.4088/jcp.v69n0702. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Coordinating Committee of the National Cholesterol Education Program: Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P Quartet Study Group. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89:6068–6076. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 35.Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77:439–444. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


