Abstract
Objective(s): Garlic (Allium sativum L. family Liliaceae) is well known in Iran and its leaves, flowers, and cloves have been used in traditional medicine for a long time. Research in recent decades has shown widespread pharmacological effects of A. sativum and its organosulfur compounds especially Allicin. Studies carried out on the chemical composition of the plant show that the most important constituents of this plant are organosulfur compounds such as allicin, diallyl disulphide, S-allylcysteine, and diallyl trisulfide. Allicin represents one of the most studied among these naturally occurring compounds. In addition to A. sativum, these compounds are also present in A. hirtifolium (shallot) and have been used to treat various diseases. This article reviews the pharmacological effects and traditional uses of A. sativum, A. hirtifolium, and their active constituents to show whether or not they can be further used as potential natural sources for the development of novel drugs.
Materials and Methods: For this purpose, the authors went through a vast number of sources and articles and all needed data was gathered. The findings were reviewed and classified on the basis of relevance to the topic and a summary of all effects were reported as tables.
Conclusion: Garlic and shallots are safe and rich sources of biologically active compounds with low toxicity. Further studies are needed to confirm the safety and quality of the plants to be used by clinicians as therapeutic agents.
Key Words: Allium hirtifolium, Allium sativum, Garlic, Pharmacological effects, Shallot, Traditional uses
Introduction
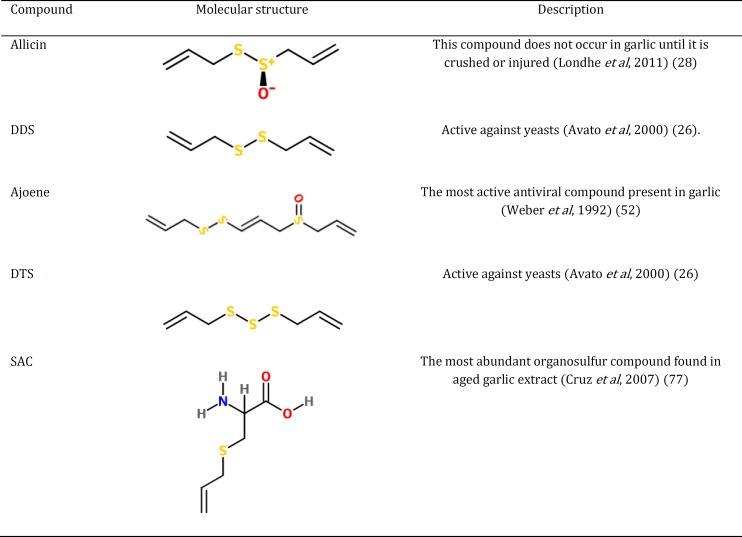
Garlic (Allium sativum L. family Liliaceae) is originally from Asia but it is also cultivated in China, North Africa (Egypt), Europe and Mexico. It is well known in Iran and various parts of this plant have long been used in traditional folk medicines of Iran and some other cultures. It is also used as a spice and food additive (1, 2). The plant is a bulb growing to 25-70 cm with hermaphrodite flowers (3). Leaves and cloves of A. sativum have been used in traditional medicine of Iran and other countries for a long time (4, 5). In pharmacological research, there is a lot of evidence about a wide spectrum of pharmacological effects of A. sativum and its active compounds with low toxicity (Table 1) (1). Studies carried out on the chemical composition of the plant show that sulfur compounds such as Allicin are important constituents of the plant (6). Although allicin (diallyl-dithiosulfinate) is the most important alkaloid that is generally claimed to be responsible for their beneficial effects and numerous studies have been conducted so far (7), it is pointed out that other sulfur compounds such as diallyl disulphide (DDS), S-allylcysteine (SAC) and diallyl trisulfide (DTS) also have some roles in the effects of the plant (Figure 1) (8). In addition to A. sativum, allicin, ajoene and other organosulfides are present in A. hirtifolium and play important pharmacological roles (Table 2) (9). This article reviews the pharmacological effects and traditional uses of A. sativum, A. hirtifolium, and their active constituents.
Table 1.
Pharmacological effects of Allium sativum
| System | Effect | Preparation | Reference |
|---|---|---|---|
| Antibacterial | Staphylococcus aureus | Aqueous, ethanol, chloroform extract | EL-mahmood, 2009 (5) |
| Escherichia coli, Salmonella typhi | Aqueous and ethanolic extract | Ankri and Mirelman, 1999 (10) | |
| Bacillus subtilis, Kelebsiella pneumoniae | Aqueous, methanol and ethanol extract | Meriga et al, 2012; Pundir et al, 2010 (11, 12) | |
| Helicobacter pylori | Extract | Liu et al, 2010 (13) | |
| Sal enteritidis | Extract | Benkeblia, 2004 (14) | |
| Shigella sp, Proteus mirabilis | Extract | Eja et al, 2007 (2) | |
| Actinobacillus pleuro pneumonia serotype 9 | Extract | Becker et al, 2012 (17) | |
| Streptococcus mutan | Extract | Loesche, 1986 (18) | |
| Antiviral |
Human cytomegalo virus(HCMV),
Influenza B, Herpes simplex virus type 1-2, Parainfluenza virus type 3, vaccine virus, Vesicular stomatitis virus, Human rhino virus type 2 |
Not mentioned | Ankri and Mirelman, 1999 (10) |
| Antifungal | Candidia albicans, C. tropicalis, Blastoschizomyces capitatus | Extract | Avato et al., 2000 (26) |
| Botr. cinerea, Trichoderma harzianum | Extract | Lanzotti et al., 2012 (8) | |
| Ascosphaera apis | Essential oil vapors | Kloucek et al., 2012 (27) | |
| Paracoccidioides brasiliensis | Extract | Thomaz et al., 2008 (30) | |
| Aspergillus niger | Extract | Matsuura and Nakagawa, 1987 (31) | |
| Dermatophytes, saprophytes | Ethanol extract | Shamim et al., 2004 (32) | |
|
Cryptococcal
Botr. cinerea, Mycosphaerella arachidicola, Physalospara piricola |
Alcoholic extract Extract |
Khan and Katiyar, 2000 Wang and Ng, 2001 (34) |
|
| Anti-parasitic | Trypanosoma sp, Entamoeba hirtolytica, Giardia lamblia | Extract | Lun et al, 1994 (39) |
|
Trypa. Cruzi
Plasmodium spp, Giardia spp, |
Extract | Gallwitz et al, 1999 (40) | |
| Leishmania spp, Cochlospermum planchomi | Extract | Anthony et al, 2005 (42) | |
| Hymenolepiasis, Giardiasis | Aqueous extract | Soffar and Mokhtar, 1991 (43) | |
| Haemonchus contortus | Ethanol, dichloro methane and water extract | Ahmed et al, 2012 (44) | |
| Cardiovascular | Hypotensive via increasing nitric oxide synthesis | Extract | Al-Qattan et al, 2006 (63) |
| Hypotensive (endothelial dependent and independent) | Not mentioned | Fallon et al, 1998 (72) | |
| Induces vasodilation with H2S | Extract | Ginter and Simko, 2010 (76) | |
| Angiotensin converting enzyme-inhibiting activity | Aqueous extract | Sener et al, 2007 (62) | |
| Stimulation of nitric oxide generation in endothelial cells | Garlic derived polysulfides | Ginter and Simko, 2010 (76) | |
| Bradycardia | Aqueous extract | Nwokocha et al, 2011(59) | |
| Hepatopulmonary syndrome | Garlic powder | Theveno et al, 2009 (81) | |
| Decreases systolic blood pressure | Aged garlic | Harauma and Moriguchi, 2006 (64) | |
| Vasorelaxant | Not mentioned | Zahid Ashraf et al, 2005 (65) | |
| Coronary artery disease | Extract | Verma et al, 2005 (67) | |
| Reduce myocardial infarction, Shoke | Not mentioned | Yang et al, 2011 (68) | |
| Anti-thrombotic | Extract and derived | Choi and Park, 2012 (108) | |
| Anti-atherosclerotic | Volatiles derived | Calvo-Gómez et al, 2004 (90) | |
| Blood | hypolipemic effect | Capsule of garlic preparation | Duda et al, 2008 (61) |
| Hypolipidemia, Hypocholesterolaemic, Hypotriacylglyceride |
Extract | Kuda et al, 2004 (124) | |
| Hypoglycemic | Extract | Sengupta et al, 2004 (128) | |
| Hypolipidemic | Organosulfur compound | Lii et al, 2012 (139) | |
| Immune system | Immunomodulation | Extract | Chandrashekar and Venkatesh, 2012 (160) |
| All | Anti-inflammatory | Extract | Ben et al, 2012 |
| Antioxidant properties | Organo sulfur compound in aged garlic | Cruz et al, 2007 (77) |
Figure 1.
Molecular structure of important organosulfur compounds from Allium sativum and Allium hirtifolium
Table 2.
Pharmacological effects of Allium hirtifolium
| System | Effect | Reference |
|---|---|---|
| Antibacterial | Antibacterial: Staphylococcus spp, Salmonella spp, Nibrio spp, Mycobacteria spp, Proteus spp |
Tariq et al., 1988 (7) |
| Escherichia coli | Ankri and Mirelman, 1999 (10) | |
| Antiviral | Common cold virus | Josling, 2001 (53) |
| Antifungal | Candida albicans (Candidiasis) | Ankri and Mirelman, 1999 (10) |
| Saccharomyces cerevisia | Khodavandi et al., 2010 (21) | |
| Aspergillus fumigatus | An et al., 2009; Ogita et al, 2006 (23) | |
| Antiparasitic |
Schistosoma mansoni (male) Plasmodium falciparum, Trypanosoma brucei brucei |
Lima et al, 2011 (37) |
| Entamoeba histolytica, Giardia lamblia | Ankri and Mirelman, 1999 (10) | |
| Cardiovascular | Hypotensive intraocular pressure | Chu et al, 1993 (70) |
| Hypotensive | Younis et al, 2010 (86) | |
| Anti-Atherosclerotic | Gonen et al, 2005 (92) | |
| Antithrombotic or Anti-aggregatory | Cavagnaro et al, 2007 (112) | |
| Blood | Hypolipidaemic | Sela et al, 2004 (142) |
| Hypoglycaemic | Mathew and Augusti, 1973 (143) | |
| Immune system | Immunomodulatory | Bruck et al, 2005 (164) |
| CNS | Neuro protection | Zhu et al, 2012 (182) |
| Respiratory system | Pulmonary oedema | Krumm et al, 2012 (183) |
| Other | Anticancer | Bar-chen et al, 2010 (152) |
| Anti-inflammatory | Krishna and Yadav, 2012 (158) | |
| Antidiabetic | Younis et al, 2010 (86) |
Materials and Methods
Data sources and data extraction
In order to gather the needed information, systematic literature searches were conducted on MEDLINE, EMBASE, and BIOSIS databases. A vast number of papers (more than 200 original articles and reviews) were studied during the years 2011-2013 and data extraction was performed methodologically based on previously identified keywords including: Allium sativum, garlic, Allium hirtifolium, shallot, organosulfur compounds, allicin, and ajoene. The dates of articles used as references ranged from 1973 to 2012. Discrepancies were settled through discussion for a final period of 3 months.
Data presentation
The findings were interpreted and classified on the basis of relevance to the topic and a summary of all effects were reported as tables. Each topic starts with a brief review of the traditional uses of the plant that suits the topic (if present) and then the information is supported by the results of various pharmacological studies conducted in that field. The molecular structures of every active compound present in the studied plants were drawn using the online service (www.emolecules.com). Finally based on the reviewed information a conclusion was reached.
Anti microbial effects
Antibacterial
Allicin and other sulfur compounds are thought to be the major compounds responsible for the antimicrobial effect of garlic. Garlic is effective against a number of gram-negative, gram-positive, and acid-fast bacteria, including Staphylococcus, Salmonella, Vibrio, Mycobacteria, and Proteus species (7). Aqueous, ethanol and chloroform extracts of garlic inhibited the growth of the pathogenic bacteria, though with varying degrees of susceptibility. The gram positive Staphylococcus aureus was more susceptible to the toxic effects of garlic than its gram negative counterparts. It has been shown that the aqueous extract of garlic can be used alongside conventional antibiotics to fight agents of nosocomial infections that are so prevalent in hospitals (5). An in vitro study on the effects of aqueous and ethanolic extracts of garlic against specific bacteria such as Escherichia coli and sal. typhi showed that the aqueous extract had little or no inhibition while the ethanolic extract had a higher inhibitory effect. Allicin in its pure form was found to exhibit antibacterial activity against multidrug-resistant enterotoxicogenic strains of E. coli (10). In another study, the aqueous extract exhibited antibacterial activity against gram positive (Bacillus subtilis, Staph. aureus) and Gram negative (E. coli and Klebsiella pneumoniae) strains, while methanol extract showed antimicrobial activity against all the tested microorganisms except Stap. aureus (11).
Garlic ethanolic extract showed maximum activity against B. subtilis (12). Allitridi, a proprietary garlic derivative, has been successfully used to treat systemic bacterial infections (such as Helicobacter pylori) in China (13). It was shown in another study that the extract of garlic strongly inhibits Sal. enteritidis; however Staph. aureus showed less sensitivity (14). It has been shown that Gram-negative diarrheagenic pathogens (E. coli, Shigella sp, Salmonella sp, and Pro. mirabilis) from stool samples were highly sensitive to garlic (2). The significant antibacterial activity of garlic extract on streptomycin-resistant strains (Gram-positive Staph. aureus and Gram-negative E. coli) solely and in synergism with streptomycin has also been proved (15). In a study by Lai and Roy, fresh extracts of A. sativum (garlic) and Nigella sativum (black cumin) had more antibacterial activity against the isolates of the urinary tract infection, compared to the individual extract or drugs, such as cefalexin, cotrimoxazole, and nalidixic acid (16). Garlic has antibacterial activity against the pig pathogen Actinobacillus pleuropneumoniae serotype 9. The main compound that is suggested to be responsible for this effect of garlic is volatile allyl methyl sulfide (AMS) as a lead compound of volatile garlic metabolites (17). Garlic extract was also effective against Streptococcus mutans when tested both in vitro and in vivo. As Strep. mutans is one of the primary aetiological organisms in dental caries development (18), garlic extract mouth rinse might be used effectively in the prevention of dental caries (19).
Antifungal
Allicin (diallyl-dithiosulfinate), which is produced by the garlic enzyme alliinase from the alliin, has been shown to have wide-range antifungal specificity. An in vivo study showed that antibody-alliinase conjugates and alliin are effective against murine pulmonary aspergillosis (20). One study showed that allicin from garlic has antifungal activity particularly against Candida albicans (10). Another in vitro study showed both intrinsic antifungal activity of allicin and its synergy with the azoles, in the treatment of candidiasis (21). Studies on the effect of Amphotericin B (AmB) against C. albicans showed that allicin enhances significantly the effect of AmB against Candida albicans, Saccharomyces cerevisiae and against Aspergillus fumigatus in vitro and in vivo (22, 23). It was found in another study that polymyxin B (PMB), is effective against various yeasts and filamentous fungi when used in combination with allicin. This combination increases the plasma membrane permeability in Saccharo cerevisiae. Swollen spherical structure of the yeast disappeared as a result of structural alterations of its vacuole caused by the synergistic activity between PMB and allicin combination (24). A study showed the effects of diallyldisulphide (DADS), one of the components of garlic, on antioxidant systems in Candida species. Changes in antioxidant metabolites and antioxidant activity in the presence of DADS were found in C. albicans and C. tropicalis. DADS caused a decrease in the activity of all antioxidant enzymes except catalase (25). One study showed that six different mixtures of garlic distilled oils containing diallyl disulfide(DDS) and diallyl trisulfide (DTS), are active against a number of yeasts (C. albicans, C. tropicalis and Blastoschizomyces capitatus) (26). Saponins from A. sativum were shown to be effective against Botrytis cinerea and Trichoderma harzianum (8). Essential oil vapors from A. sativum also have inhibitory activity against Ascosphaera apisin in vitro (27). In one study, allicin was shown to be more potent in the growth inhibition of C. albicans and also suppression of HWP1 gene expression in comparison with fluconazole, a commonly used antifungal. This compound does not occur in garlic until it is crushed or injured (21, 28). Ajoene, another constituent of garlic, is responsible for many pharmacological activities of this plant specially its antifungal effect (29). This substance is more effective in association with antifungal drugs (sulfametoxazol/ trimethoprim) in the treatment of mice intratracheally infected with Paracoccidioides brasiliensis (30). In an in vitro study the growth of both Asper. niger and C. albicans were inhibited by ajoene at <20, ug/ml (31). High zones of inhibition were noted with ethanol extracts of A. sativum tested against dermatophytes, saprophytes, and Candida species isolated from infected hospitalized patients (32). It has been proven that the blockage of lipid synthesis by aqueous extracts of garlic plays an important role in the anticandidal activity of this plant (33). Alcoholic extracts also have potential anticryptococcal activity against murine disseminated cryptococcosis (34). Another study also showed the sensitivity of Cryptococcus neoformans against A. sativum (35). A novel antifungal protein, designated allivin, was isolated from A. sativum with antifungal activity against Botrytis cinerea, Mycosphaerella arachidicola and Physalosporapiricola (36).
Anti-parasitic
An ultrastructural study showed that allicin is able to produce morphological changes in the male Schistosoma mansoni (37). Another study indicated that Allicin has antiparasitic activity against Plasmodium falciparum and Trypanosoma brucei brucei (38). It is also effective against some major human intestinal protozoan parasites such as Entamoeba histolytica and Giardia lamblia (10). Diallyl trisulfide is a chemically stable final transformation product of allicin. The activity of diallyl trisulfide was investigated against several important protozoan parasites in vitro. The results indicated that the compound has the potential to be used in treatment of several human and animal parasitic diseases such as Trypanosoma sp, Ent. histolytica and Giar. lamblia (39). Ajoene isolated from A. sativum is an inhibitor of human glutathione reductase and Trypa. cruzi trypanothione reductase. The antiparasitic and cytostatic actions of ajoene may at least in part be due to the multiple effects on key enzymes of the antioxidant thiol metabolism (40). Alchinal is a preparation of three different substances including Echinacea purpurea and A. sativum extracts and cocoa. It has been demonstrated that this preparation significantly decreases the number of adult forms and muscular larvae of Trichinella spiralis. It was demonstrated that after Alchinal administration, the number of adult forms and muscular larvae of this parasite was significantly decreased (41). Garlic oil is effective against a wide range of microorganisms including Plasmodium spp, Trypanosoma spp, Leishmania spp, Giardia spp, and Cochlospermum planchonii (). Its aqueous extract has been shown to be effective against hymenolepiasis and giardiasis also (43). In an in vitro study the ethanol, dichloromethane and water extracts of A. sativum were shown to have anthelmintic activity against Haemonchus contortus from sheep. The ethanol extract was the most effective in decreasing larval count (44). Another study showed that garlic is effective against nematodes. Aqueous extract from garlic has good activity against Trichuris muris and Angiostrongylus cantonensis when followed by chloroform extract (45). Garlic is an ingredient of a mixture (Prepared from the extracts of coconut, onion, garlic, fig, date tree, chicory, ananas, and cistrose) tested in vivo and in vitro for its anthelmintic activity against cestodes (Hymenolepis diminuta, H. microstoma, andTaenia taeniaeformis) and trematodes (Fasciola hepatica, Echinostoma caproni). In all in vitro tests, the target parasites died. In addition, the same composition was effective against the intestinal fluke Echino caproni, but not against the liver fluke F. hepatica in the final host, while both worms were killed in vitro (46). Essential oil of A. sativum has paralytic effect on F. gigantica. The essential oil produced significant reduction in the frequency and the amplitude of the spontaneous muscular activity of whole fluke at 1 and 3 mg/ml concentrations (1). The extract of A. sativum also possesses mosquito larvicidal properties. It is effective against filarial mosquito Culex quinquefasciatus (after 24 hr treatment) (47), Cul. quinquefasciatus and Anopheles stephensi (48). Essential oil from A. sativum has acaricidal activity against Rhipicephalus (Boophilus) microplus (Canestrini) tick larvae (49). The insecticidal activity of A. sativum against larvae of Aedes albopictus (Skuse) (50), Lycoriella ingénue (51), and Spodoptera litura (1000 ppm) has also been shown (11).
Antiviral
A. sativum has been shown to have antiviral activity. In one study the virucidal activity of this plant was attributed to the following contents in this order: ajoene > allicin > allyl methyl > thiosulfinate > methyl allyl thiosulfinate (52). Also Allicin, the main constituent of A. sativum, has a variety of antimicrobial activities both in vitro and in vivo. Among the viruses which are sensitive to garlic extracts are the human Cytomegalovirus (HCMV), influenza B virus, Herpes simplex virus type 1, Herpes simplex virus type 2, Parainfluenza virus type 3, vaccinia virus, vesicular stomatitis virus, and human Rhinovirus type 2 (10). One study showed that Allicin-containing supplements can prevent attacks by the common cold virus (53). The main antimicrobial effect of Allicin is due to its chemical reaction with thiol groups of various enzymes, e.g. alcohol dehydrogenase (10). In an In vivo study the administration of garlic in mice models protected them against intranasal inoculation with influenza viruses and enhanced the production of neutralizing antibodies when given the vaccine (7).
Ajoene, isolated from extracts of garlic may inhibit adhesive interaction and fusion of leukocytes (54). In a study investigating the effect of Allitridin (diallyl trisulfide, a compound from A. sativum extraction) on the replication of HCMV and the expression of viral immediate-early genes, it was revealed that this substance has anti-HCMV efficacy (55). In another study, it was supposed that the antiviral activity of garlic in humans may be secondary to a direct toxic effect on viruses. It also enhanced NK-cell (Natural killer-cell) activity that destroys virus-infected cells (7).
Cardiovascular effects
Antihypertensive
A statistical study showed that individuals whose blood pressures are on the lower side are more likely to consume more garlic in their diets (56). Various epidemiologic studies have indicated an inverse correlation between garlic consumption and progression of cardiovascular disease (57). The authors are of the opinion that garlic is effective in treatment of mean systolic blood pressure but not d-penicillamine (58).
In one study the aqueous garlic extract (AGE) caused a decrease in blood pressure and bradycardia by direct mechanism not involving the cholinergic pathway, suggesting a likely involvement of peripheral mechanism for hypotension (59). Another study showed that AGE prevents oxidative stress, systolic blood pressure, aortic NAD(P)H oxidase activity and vascular remodeling in rats with metabolic syndrome (60).
It has been also shown that preparations of garlic may be tentatively used as an adjunct agent in treatment of arterial hypertension because of its hypolipemic and antioxidant properties (61). In vivo and in vitro ischemia reperfusion studies have shown that prophylactic administration of AGE prior to ischemia reperfusion inhibits lipid peroxidation and prevents depletion in glutathione through its compounds that led to functional recovery. Its ability to inhibit neutrophil migration could suppress fibrosis formation. These preventive effects are seen in studied model organs such as kidney and liver with functional recovery. Organ system specific activity such as angiotensin converting enzyme-inhibiting action contributes to a cardioprotective and blood pressure lowering effect of garlic (62). The authors are of the opinion that the blood pressure lowering effect of garlic in rats (two-kidney one-clip model) may be partly mediated through the nitric oxide (NO) pathway, by enhanced NO synthesis (63).
A study on the effects of two garlic sources has the potential to reduce systolic blood pressure. The effect of aged garlic extract was accompanied by a decrease of pulse pressure (PP), suggesting an improvement of the pliability of the artery, although raw garlic (RG) powder did not affect PP. However, harmful effects were observed in the RG group, including a decrease in erythrocytes, an increase in reticulocytes, and generation of papilloma in the forestomach (64). Another study showed that garlic is a potent vasorelaxant and could reduce the atherogenic properties of cholesterol (65).
A small pilot study indicated the potential ability of aged garlic extract to inhibit the rate of progression of coronary calcification (66). In a study garlic appeared to be a good adaptogen to be utilized in patients with coronary artery disease (67). One study indicated that increased intake of garlic has been associated with reduced mortality in cardiovascular patients or reduced incidence of myocardial infarction, stroke, and hypertension (68). Another study showed that garlic may beneficially affect two risk factors for atherosclerosis--hyperlipidemia and hypertension (69).
One survey suggested that allicin lowered intraocular pressure, in part, by dual actions at the neuroeffector junction (70). Oxidative damage by free radicals has been implicated in the pathogenesis of vascular disease in hypertension. Authors concluded that the total antioxidant status can be significantly improved by treatment with garlic (71). An in vivo study indicated that garlic blocks hypoxic pulmonary hypertension and demonstrated a combination of endothelium-dependent and -independent mechanisms for the effect in pulmonary arterial rings (72).
An in vitro study showed that intravenous administration of garlic extracts produced dose-dependent and reversible hypotensive and bradycardic effects (73).
In One survey the authors are of the opinion that although H2S (hydrogen sulfide) role in blood pressure regulation and interaction with NO is controversial, H2S, through its anti-apoptotic, anti-inflammatory and antioxidant effects, has demonstrated significant cardioprotection. As a result, a number of sulfide-donor drugs, including garlic-derived polysulfides such as diallyl disulfide, diallyl trisulfide and S-ally cysteine, are currently being designed and investigated for the treatment of cardiovascular conditions such as hypertension (74, 75). Stimulation of nitric oxide generation in endothelial cells seems to be the critical preventive mechanism. Cardioprotective effects of dietary garlic are mediated in large part via the generation of H2S. Garlic-derived organic polysulfides are converted by erythrocytes into hydrogen sulfide which relaxes vascular smooth muscle, induces vasodilation of blood vessels, and significantly reduces blood pressure (76).
Progressive renal damage and hypertension are associated with oxidative and nitrosative stress. On the other hand, S-allylcysteine (SAC), the most abundant organosulfur compound in aged garlic (AG) extract, has antioxidant properties. The effects of SAC and AG on blood pressure, renal damage, and oxidative and nitrosative stress were studied. The data suggested that the antihypertensive and renoprotective effects of SAC and AG are associated with their antioxidant properties and that they may be used to ameliorate hypertension and delay the progression of renal damage (77). Daily treatment with 600 mg of Allicor (garlic powder tablets) has decreasing effects on both systolic and diastolic blood pressures. It has been shown that time-released tablets of Allicor are more effective in the treatment of mild and arterial hypertension than regular garlic additives (78). Allicin within garlic tablets was shown to be the possible responsible substance for the anti-hypertensive effect of the tablets. Other organo-sulfur compounds may also have a role in the hypotensive mechanisms of garlic (6). It was shown in a study that administration of garlic extract decreases systolic and diastolic blood pressure only in hypertensive animals with no such effect in normotensive ones (79). Allyl methyl sulphide (AMS) and diallyl sulphide (DAS), two garlic derivatives, are shown to inhibit migration and angiotensin II-stimulated cell-cycle progression in smooth muscle cells of aorta. As a result, AMS and DAS may serve as effective antioxidant compounds in the arterial structural changes caused by hypertension (80). Hepatopulmonary syndrome is characterized by the presence of portal hypertension and dilated pulmonary capillaries. In a study, garlic powder and iloprost inhalation demonstrated clinical improvements in the pre- and in the post-transplant period (81).
Administration of moderate doses of garlic along with propranolol has been shown to have beneficial effects in animals with hypertension and myocardial damage (82). Another study indicated that garlic in moderate doses with added hydrochlorothiazide(HCTZ) possesses synergistic cardioprotective and antihypertensive properties against fructose- and isoproterenol-induced toxicities, by increasing the lactate dehydrogenase, creatinine phosphokinase, superoxide dismutase and catalase activities in heart homogenate when used concurrently or separately (83). The influence of garlic on pharmacokinetics of HCTZ was studied. The administration of HCTZ in garlic homogenate pretreated rats was found to decrease the QRS duration, RR interval, QT segment, systolic blood pressure, heart rate, serum potassium level, serum LDH and serum CK-MB activities significantly. It was concluded that careful addition of garlic in moderate doses might result in beneficial effect during treatment of hypertension in patients with myocardial stress as garlic causes substantial fall in excretion of potassium when compared to HCTZ alone treatment in rats (84). One study represented that combination of garlic or its bioactive constituent, S-allyl cysteine sulphoxide, and captopril exerted super-additive (synergistic) interaction with respect to fall in blood pressure and ACE inhibition (85). Another study showed that S-allyl-mercapto-captopril (CPSSA), a conjugate of captopril with allicin, was effective in attenuating systolic and diastolic blood pressures as well as significantly reducing glucose levels (86). A comparable study between the effects of allicin and enalapril on blood pressure (BP) showed similar effects, both of which reduce BP (87).
Antiatherosclerotic
One study by Wang and Ng (1999) showed that garlic compounds possess anti-atherosclerotic activity (88). Also numerous animal studies have reported that garlic can have protective effect against atherosclerosis (89). Sulfur-containing volatiles from garlic are the principal compounds responsible for such property and the most abundant volatile compound is diallyl disulfide followed by diallyl trisulfide (90). These active constituent(s) of garlic responsible for its anti-atherogenic action are shown to be mostly present in the oily fraction of the plant (91). Among these constituents, allicin is another compound that plays an important role in anti-atherosclerotic activity of garlic. It is produced upon crushing of the garlic clove. A pure allicin preparation may affect atherosclerosis not only by acting as an antioxidant, but also by other mechanisms, such as lipoprotein modification and inhibition of LDL uptake and degradation by macrophages (92). In a study, 112 patients (47 men and 65 women) 40 to 60 years of age were examined. 56 patients had ischemic heart disease and/or equal disorders. Another 56 patients were free of any signs of atherosclerosis, but had one or more cardiovascular pathology risk factor. Six month therapy using allicor results in moderate hypolipidemic and antioxidative effect. A dosage of 600 mg per day decreases ten-year chance of fatal cardiovascular complications in patients with clinical signs of atherosclerosis, whereas in patients who have no signs of atherosclerosis the complications are decreased with dosage of 300 mg per day (93). Another survey indicated that garlic indirectly affects atherosclerosis by reduction of hyperlipidemia. Moreover, in animal models, garlic causes direct antiatherogenic (preventive) and anti-atherosclerotic (causing regression) effects at the level of artery wall. It was suggested in one study that garlic powder also manifests direct anti-atherogenic-related action not only in vitro but also in vivo (94). Garlic's direct effect on atherosclerosis may be explained by its capacity to reduce lipid content in arterial cells and to prevent intracellular lipid accumulation. This effect, in turn, is accompanied by other atherosclerotic manifestations, i.e., stimulation of cell proliferation and extracellular matrix synthesis (95). A study demonstrated that garlic reduces the atherogenic properties of cholesterol (65). As sited above, suppressed LDL oxidation may be one of the powerful mechanisms accounting for the anti-atherosclerotic properties of garlic (96, 97). In one study, intake of high-dose garlic powder dragees significantly reduced the increase in arteriosclerotic plaque volume by 5-18% or even caused a slight regression within the observational period of 48 months (98).
Fish oil and garlic combinations can serve as good dietary supplements with anti-atherosclerotic properties (99). Other possible mechanisms for lipid lowering and anti-atherogenic effects of garlic include inhibition of the hepatic activities of lipogenic and cholesterogenic enzymes that are thought to be the origin for dyslipidemias, increased excretion of cholesterol and suppression of LDL-oxidation (100). In an in vitro study, the potential anti-atherosclerotic property of moderate and high doses of garlic homogenate (GH was significantly attenuated by propranolol and hydrochlorothiazide. However, GH anti-hyperlipidemic activity was augmented by captopril (101). Another study indicated that (egg yolk-enriched garlic powder) EGP inhibits copper-induced LDL oxidation in a dose-dependent manner that might be ascribed, in part, to the biodistribution of garlic compounds and egg yolk interaction. This finding suggests that EGP might be useful in the prevention of atherosclerosis (102).
Antithrombotic
Garlic extracts and several garlic constituents demonstrated significant antithrombotic actions both in vitro and in vivo. Allicin and adenosine are the most potent antiplatelet constituents of garlic (103). A study suggested that odorless garlic not only activates fibrinolytic action by accelerating (tissue-type plasminogen activator) t-PA-mediated plasminogen activation, but also suppresses the coagulation system by down regulating thrombin formation, suggesting a beneficial role in preventing pathological thrombus formation in such cardiovascular disorders (104). A study mentioned that aqueous extract of garlic inhibits platelet aggregation induced by several aggregation agents, including arachidonate in a dose-dependent manner (105).
Another survey indicated that garlic extracts act through inhibition of the ADP (adenosine diphosphate) pathway. Their mechanisms of action are comparable to that of the clinically used drug clopidogrel. The pharmacologically active component of the extracts appears to be lipophilic rather than hydrophilic (106). One study mentioned that the aromatic thiosulfonate derived from garlic is a very effective inhibitor of platelet aggregation (107). Diallyl trisulfide (DATS) is one of the major constituents in garlic oil and has demonstrated various pharmacological activities, such as antithrombotic (108). DAT-rich garlic oil showed anticoagulant action due to inhibition and/or inactivation of thrombin, in an animal study. In addition DAT-rich garlic oil benefits blood anticoagulation factors, which might further prevent the development of thrombus formation. However, the intake of garlic oil at high dose significantly increased plasma fibrinogen concentration (P<0.05) and affected the levels of several hematological parameters such as erythrocyte count, hemoglobin and platelets (P<0.05). Supplementation of garlic oil at 5 mg/kg BW had anticoagulation effect in this study (109). It was shown in a survey that diallyl disulphide (DADS) and DATS - are usual constituents of garlic oil, with antiplatelet activity. They also inhibit platelet thromboxane formation. In this respect DATS is more potent than DADS (110). The antiplatelet activity of methyl allyltrisulfide (MATS), a component commonly present in steam-distilled garlic oil, has also been demonstrated. MATS inhibits arachidonic acid cascade at the reaction site with PGH synthase (111). In a study allicin and thiosulfinates were considered as responsible compounds for the (in-vitro antiaggregatory activity) IVAA response. It was also shown that the loss of activity, and the partial loss of antithrombotic effect in crushed-cooked garlic may be compensated by increasing the amount consumed (112). Authors mentioned that sulfur compounds’ contribution to the health promotion in allium species are produced via enzymic and thermal reactions. Potent antithrombotic agents which have been identified as allyl trisulfides, dithiins, and ajoene in garlic are thermochemically transformed forms of allicin (allyl 2-propenethiosulfinate) (113). A study showed that allicin had the strongest antiplatelet activity at 0.4 mM inhibiting aggregation by 89% (114). Ajoene is another potent antiplatelet compound isolated from alcoholic extracts of garlic. It is suggested that ajoene may be potentially useful for the acute prevention of thrombus formation induced by severe vascular damage, mainly in arterial sites with low local shear rates (115, 116). One study indicated that the antiaggregatory effect of ajoene is causally related to its direct interaction with the putative fibrinogen receptors (117). Another survey demonstrated that the antithrombotic potential of ajoene is substantially increased in the presence of physiologically and pharmacologically active antiplatelet agents (118). In a study, ajoene inhibited platelet aggregation induced by arachidonic acid, adrenaline collagen, adenosine diphosphate and calcium ionophore. The nature of the inhibition was irreversible (119). It has been suggested that supplements of garlic could adversely affect coagulation when taken alone or in combination with antiplatelet medications (120). In a study coadministration of aged garlic extract and cilostazol did not enhance the antiplatelet activity compared with individual drugs (121). Another study suggested that aged garlic extract is relatively safe and poses no serious hemorrhagic risk for closely monitored patients on warfarin oral anticoagulation therapy (122).
Spolarich and Andrews mentioned that patients undergoing routine dental and dental hygiene procedures do not need to discontinue the use of anticoagulant and antiplatelet medications (such as aspirin). However, alterations in drug use may be required for those patients undergoing invasive surgical procedures. It is recommended that herbal supplements, such as garlic, must be discontinued 2 weeks prior to receiving invasive surgical procedures (123).
Blood factors
One survey mentioned that garlic has antihyperlipidemic, hypocholesterolaemic and hypo triacylglyceride activities (124). The hypoglycemic and hypolipidaemic effects of garlic have been shown in sucrose fed rabbits also (125). In one study, raw and boiled garlic improved plasma lipid metabolism and plasma antioxidant activity in rats. Thus, dietary garlic was effective in reducing the oxidant stress, which was indicated by an increase of antioxidant activity and a decrease of lipids in the rats' blood (126).
In another study, garlic powder significantly (P < 0.05) lowered the animal`s blood lipid levels (127). Garlic has been shown to have applications as a hypoglycemic agent (128). A study suggested a new mechanism for the hypolipidemic effect of fresh garlic. Long-term dietary supplementation of fresh garlic may exert a lipid-lowering effect partly through reducing intestinal MTP (microsomal triglyceride transfer protein) gene expression, thus suppressing the assembly and secretion of chylomicrons from intestine to the blood circulation (129). Short-term garlic therapy in adults with mild to moderate hypercholesterolemia does not affect lipid levels (130). In a study the water soluble protein fraction of garlic was investigated for its effect on hyperlipidemia induced by alcohol (3.76 g/kg body wt/day). It showed hypolipidemic action mainly due to an increase in cholesterol degradation to bile acids and neutral sterols and mobilization of triacyl glycerols in treated rats. Garlic protein (500 mg/kg body wt/day) showed significant hypolipidemic action comparable with a standard dose of gugu-lipid (50 mg/kg body wt/day) (131). One study in 1984 showed that garlic oil has hypolipidemic effects in ethanol-fed rats (132). In another study, the water soluble proteins and the essential oil of garlic were investigated for their hypolipidemic effect on hyperlipidemia induced by cholesterol containing diet in albino rats. Both garlic protein (16% of diet) and garlic oil (100 mg/kg body weight/day) exhibited significant lipid lowering effects (133). A survey mentioned that garlic methanol-extracts behave as hypolipidemic drugs, increasing the activity of peroxisomal fatty acyl-coenzyme A oxidase and of total carnitine acetyl-coenzyme A transferase in primary cultures of rat hepatocytes (134). In an in vivo study, garlic demonstrated a reduction of lipid plaques in the arteries of hypercholesterolemic animals. It decreased accumulation of cholesterol in vascular walls, and had other positive interventions (135).
In one study, the glutathione reductase activity that was lowered in hypercholesterolemic conditions, methemoglobin concentration that was significantly increased in hypercholesterolemic rats and significant fall in hepatic total thiols in hypercholesterolemia were partially corrected by garlic. Similarly, the lowered activities of hepatic antioxidant enzymes in hypercholesterolemic rats were effectively countered by this plant (136).
Garlic treatment significantly diminished total-cholesterol, LDL-cholesterol and triglycerides, but not HDL-cholesterol in chronic nephrotic syndrome (NS). These data indicate that garlic treatment ameliorates hyperlipidemia and renal damage in chronic NS which is unrelated to proteinuria or antioxidant enzymes (137). In a survey, hepatic triglyceride content that was significantly higher in high-fat fed rats was effectively countered by inclusion of the hypolipidemic spice agents such as garlic in the diet (138).
One study mentioned that garlic's organosulfur compounds (such as diallyl trisulfide) display hypolipidemic effects by inhibiting fatty acid and cholesterol synthesis (139).
Diallyl disulfide, an active principle of garlic (A. sativum), is known for its antihyperlipidemic properties (140). Water-soluble organosulfur compounds, S-allyl cysteine (SAC), S-propyl cysteine (SPC) and S-ethyl cysteine (SEC), were studied. The results indicated that SAC, SEC, and SPC inhibit lipid biosynthesis in cultured rat hepatocytes, and further suggested that these S-alk(en)yl cysteines of garlic impair triglyceride synthesis in part due to decreased de novo fatty acid synthesis resulting from inhibition of fatty acids (141). Allicin (diallyl disulphide-oxide) exerts various beneficial biological effects such as antihyperlipidemic (142) and hypoglycaemic actions (143). Dietary garlic also reduces the cholesterol gallstone incidence by 15-39 % (144). Action of long-acting garlic powder tablets (Allicor) have been investigated on blood factors. The results show that allicor lowers total cholesterol, LDLP cholesterol, raises HDLP cholesterol and therefore can be recommended for correction of lipid content in patients with moderate hyperlipidemia (145). A comparative study on the beneficial effects of garlic amla (Emblica Officinalis Gaertn) and onion (A. cepa L) on hyperlipidemia showed that the order of the curative effects of the vegetables is as follows: garlic > amla > onion (146).
Anticancer effects
A study mentioned that phytoalexins have been identified in at least 75 plants including garlic. Preclinical evidence has suggested that these compounds possess anticancer properties including an inhibition of cell proliferation, invasion and metastasis, hormonal stimulation, and stimulatory effects on expression of metabolizing enzymes (147). Diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS) derived from garlic have been shown to exhibit anticancer activities (148). The cytotoxicity caused by DATS is mediated by generation of ROS (reactive oxygen species) and subsequent activation of the ROS-dependent caspase pathway in U937 leukemia cells (108). DATS has been shown to induce apoptosis in many human cancer cell lines in vitro and also affords significant protection against cancer in animal tumor models in vivo i.e. colorectal cancer (149). Another suggested that DADS treatment may inhibit tumor cell motility and invasion and therefore, act as a dietary source to decrease the risk of cancer metastasis (150).
Recently, S-allylcysteine (SAC) has been identified as a potent compound derived from garlic. This substance has in vitro chemo-preventive activity. It may also be a promising candidate for prostate cancer treatment (151). Allicin (diallyl thiosulfinate), the best-known biologically active component in freshly crushed garlic extract, is effective on cell proliferation of colon cancer cells (152). A study indicated that the anticancer action of aged black garlic extract may be partly due to its antioxidant and immunomodulative effects (153).
Anti-inflammatory effect
Garlic extracts have been shown to exert anti-inflammatory effects (154). In one study, garlic treatment significantly attenuated inflammation and injury of the liver induced by Eimeria papillata infections (155). The anti-inflammatory activity exhibited by garlic oil is mainly through inhibiting the assembly-disassembly processes of the cytoskeleton (156).
Other authors have shown the preventive effect and possible toxicity of garlic oil and its organosulfur compounds in endotoxin-induced systemic inflammation and intestinal damage (157). A lead compound derived from allicin is shown to be a good starting point for the development of anti-inflammatory drugs with fewer side effects (158).
One study indicated that thiacremonone, a sulfur compound isolated from garlic, inhibits neuroinflammation and amyloidogenesis through inhibition of NF-κB activity, and thus could be applied for intervention in inflammation-related neurodegenerative diseases including Alzheimer's disease (159).
Immunomodulatory effect
Immunomodulation is among innumerable biological activities of A. sativum. Aged garlic extract has been shown to have superior immunomodula-tory properties over raw garlic extract (160). This effect of garlic is attributed to the transformed organosulfur compounds (161). Aged garlic fructans have recently been shown to possess immunomodulatory activities in vitro (160). Garlic extract is concentration-dependently effective on the proliferation of interleukin (IL)-2 and interferon (INF)-γ gene expression of stimulated lymphocytes (162). Garlic extracts reduced macrophage infection through induction of nitric oxide (NO) production In vitro (163).
A study demonstrated that immune-mediated liver damage in mice can be prevented by allicin, probably because of its immunomodulatory effects on T cells and adhesion molecules and inhibition of NF-kappaB activation (164). Another observation indicated that allicin exerts an inhibitory immunomodulatory effect on intestinal epithelial cells and it may have the potential to attenuate intestinal inflammation (165). Allicin exerted an in vitro immunomodulatory effect on certain functions of the peripheral blood cells (166).
Toxicology
Tattelman mentioned that garlic appears to have no effect on drug metabolism, but patients taking anticoagulants should be cautious. It seems prudent to stop taking high dosages of garlic seven to 10 days before surgery because garlic can prolong bleeding time (167).
One study indicated that garlic application usually results in local inflammation, but, if applied under a pressure bandage, or if there is poor wound care or a secondary infection, it can cause a severe dermal reaction and a deep chemical burn (168). Data of a study showed that a high garlic dose induced liver toxicity and a pro-oxidative status characterized by increased malondialdehyde and decreased antioxidant enzyme activities as catalase, peroxidase, and superoxide dismutase (169). Another study suggested that garlic with high dose has the potential ability to induce liver damage (170).
A parallel study also highlighted the potential ability of a high dose of garlic to induce morphological changes in the liver and kidneys (171). Administration of high doses of garlic (500 mg/kg) results in profound changes in lung and liver tissues of rats. Intraperitoneal administration of the high dose of garlic is more damaging to lung and liver tissue of rats than oral administration (172). It is also shown that the adverse effect of high doses of garlic oil might further influence the hemostatic balance (109).
High doses of diallyl disulfide may further complicate the metabolic disturbances in diabetes (173). High dose of garlic oil worsened intestinal mucosal damage accompanied by elevated peripheral proinflammatory cytokines in another study (157).
Active compounds
It has been shown that sulfur compounds such as allicin are important constituents of garlic (6). Although allicin (diallyl-dithiosulfinate) is the most important alkaloid that is generally claimed to be responsible for most of the beneficial effects of the plant (7); however, it is pointed out that other sulfur compounds such as diallyl disulphide (DDS), S-allylcysteine (SAC) and diallyl trisulfide (DTS) also have some roles in the pharmacological effects of the plant (88). SAC is the most abundant organosulfur compound found in aged garlic extract (77). It has also been shown that allicin (diallyl-dithiosulfinate) does not occur in garlic until it is crushed or injured (28). Among the active compounds present in the plant, DTS and DDS are the most active against yeasts (26) and ajoene is the main compound responsible for the antiviral activity of garlic (52).
Drug interaction and pharmacokinetics
One study indicated that those who use traditional/complementary/alternate medicines (TCAMs) in addition to antiretroviral (ARV) treatment may be at risk of experiencing clinically significant pharmacokinetic (PK) interactions, particularly between the TCAMs and the protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Mechanisms of PK interactions include alterations to the normal functioning of drug efflux transporters, such as P-gp and/or CYP isoenzymes, such a CYP3A4 that mediate the absorption and elimination of drugs in the small intestine and liver. Specific mechanisms include inhibition and activation of these proteins and induction via the pregnane X receptor. Garlic exhibited potentially significant interactions, each with a PI or NNRTI (174). In vivo absorption changes are possible between aged garlic extract and cardiovascular, antidiabetic and antiviral drugs, but the magnitude of the changes depends on the most profound process involved (influx, efflux, passive diffusion) in compound’s permeability (175). In a study, pharmacokinetic interaction of garlic and atorvastatin in dyslipidemic rats was shown (176). Another study indicated that the bioavailability and half-life of propranolol was significantly enhanced by 2- and 3-folds, respectively, in animals pretreated with garlic (250 mg/ kg) (82). It has been also shown that herbs such as garlic with the potential to significantly modulate the activity of drug-metabolizing enzymes (notably cytochrome p450 isozymes) and/or the drug transporter P-glycoprotein participate in potential pharmacokinetic interactions with anticancer drugs (177).
Allium hirtifolium
Antimicrobial
It has been shown that the alcoholic and aqueous extracts of shallot (A. hirtifolium) have good antifungal activity against Aspergillus fumigatus, Asper. flavus, Asper. niger, Penicillium gryseogenum, Alternaria, Microsporum canis and Trichophyton mentagrophytes in comparison with the miconazole (178). A comparative study between Persian shallot aqueous extract and chlorhexidine on salivary bacterial counts indicated that shallot extract has more persistent inhibitory action than chlorhexidine mouth rinse lasting up to 24 hr (179).
In One study A. hirtifolium exhibited significant anti-trichomonas activity due to its components such as allicin, ajoene and other organosulfides, comparable to metronidazole (9).
Anticancer
One study showed that components of A. hirtifolium can dose-dependently inhibit proliferation of tumor cell lines. Therefore, A. hirtifolium might be a candidate for tumor suppression (180).
Clinical trials (garlic)
By a simple search in the literature it could be figured out that garlic has been used clinically to elicit desirable pharmacological and therapeutic effects. For instance, the use of garlic along with iloprost improved both the pre and post-transplant period in patients with hepatopulmonary syndrome (81). It has also been shown that daily administration of allicin may decrease the occurrence of fatal cardiovascular complications in atherosclerotic patients (93). Although garlic has the potential to be used clinically in the treatment of some disorders, care should be taken regarding its usage with other medications due to possible drug interactions that might arise as a result (174).
Discussion
Our aim in preparing this paper was to show the traditional usage and previously confirmed pharmacological effects of garlic along with shallots as two of the most well-known medicinal plants in Iran and to illustrate their potential to be used as novel sources for development of new drugs based on the most recent associated studies. As it is shown in this study, garlic has a wide range of pharmacological effects including antimicrobial, cardiovascular, anti-inflammatory, anticancer, and immunomodulatory activity among many other effects. Organosulfur compounds present in garlic and shallots are the most important contents responsible for most of their pharmacological effects. Among these biologically active compounds, allicin, allyl methyl sulfide, DTS, and ajoene have been shown to be the main responsible compounds for the antifungal, antibacterial, antiprotozoal, and antiviral effects of garlic, respectively. It is evident from this study that A. sativum may exert toxicity only at high doses and that there have been few reports of intoxications following the ingestion of garlic. However, care should be taken by scientists and clinicians regarding usage of this plant for therapeutic purposes until adequate studies confirm the safety and quality of the plant.
Conclusion
Finally based on this information, this review provides the evidence for other researchers to introduce garlic and shallots and their sole active compounds as safe and effective therapeutic sources in the future.
References
- 1.Singh TU, Kumar D, Tandan SK, Mishra SK. Inhibitory effect of essential oils of Allium sativum and Piper longum on spontaneous muscular activity of liver fluke, Fasciola gigantica. Exp Parasitol. 2009;123:302–308. doi: 10.1016/j.exppara.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Eja ME, Asikong BE, Abriba C, Arikpo GE, Anwan EE, Enyi-Idoh KH. A comparative assessinent of the antimicrobial effects of Garlic (Allium sativum) and Antibiotics on diarrheagenic organisms. Southeast Asian J Trop Med Public Health. 2007;38:2. [PubMed] [Google Scholar]
- 3.PDR for Herbal Medicines. Medical Economics Company, Inc: Montvale; 2000. [Google Scholar]
- 4.Mikaili P, Mehdioghli R. Garlic Pharmacology. National Library and Archives Organisation of Iran; 2010. ISBN: 9789647780704. (In Persian) [Google Scholar]
- 5.EL-mahmood MA. Efficacy of crude extracts of garlic (Allium sativum Linn) against nosocomial Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniea and Pseudomonas aeruginosa. J Med Plants Res. 2009;3:179–185. [Google Scholar]
- 6.McRae MP. A review of studies of garlic (Allium sativum) on serum lipids and blood pressure before and after 1994: does the amount of allicin released from garlic powder tablets play a role. J Chiropr Med. 2005;4:82–90. doi: 10.1016/S0899-3467(07)60149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tariq HA, Kandil O, Elkadi A, Carter J. Garlic revisited: therapeutic for the major diseases of our times. J Natl Med Assoc. 1988;80:439–445. [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzotti V, Barile E, Antignani V, Bonanomi G, Scala F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera. Phytochemistry. 2012;78:126–34. doi: 10.1016/j.phytochem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Azimi H, Fallah-Tafti M, Karimi-Darmiyan M, Abdollahi M. A comprehensive review of vaginitis phytotherapy. Pak J Biol Sci. 2011;14:960–966. doi: 10.3923/pjbs.2011.960.966. [DOI] [PubMed] [Google Scholar]
- 10.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;2:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 11.Meriga B, Mopuri R, MuraliKrishna T. Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac J Trop Med. 2012;5:391–395. doi: 10.1016/S1995-7645(12)60065-0. [DOI] [PubMed] [Google Scholar]
- 12.Pundir RK, Jain P, sharma CH. Antimicrobial activity of ethanolic extracts of syzygium aromaticum and Allium sativum against food associated bacteria and fungi. Ethnobotan Leaflets. 2010;14:344–360. [Google Scholar]
- 13.Liu S, Sun Y, Li W, Yu H, Li X, Liu Z, et al. The antibacterial mode of action of allitridi for its potential use as a therapeutic agent against Helicobacter pylori infection. FEMS Microbiol Lett. 2010;303:183–189. doi: 10.1111/j.1574-6968.2009.01877.x. [DOI] [PubMed] [Google Scholar]
- 14.Benkeblia N. Antimicrobial activityof essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) Lebensm.-Wiss. u.-Technol. 2004;37:263–268. [Google Scholar]
- 15.Palaksha MN, Ahmed M, Das S. Antibacterial activity of garlic extract on streptomycin-resistant Staphylococcusaureus and Escherichia coli solely and in synergism with streptomycin. J Nat Sci Biol Med. 2010;1:12–5. doi: 10.4103/0976-9668.71666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004;11:1451–1460. doi: 10.2174/0929867043365107. [DOI] [PubMed] [Google Scholar]
- 17.Becker PM, van Wikselaar PG, Mul MF, Pol A, Engel B, Wijdenes JW, et al. Actinobacillus pleuropneumoniae is impaired by the garlic volatile allyl methyl sulfide (AMS) in vitro and in-feed garlic alleviates pleuropneumonia in a pig model. Vet Microbiol. 2012;154:316–324. doi: 10.1016/j.vetmic.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbial Rev. 1986;50:323–580. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavan SD, Shetty NL, Kanuri M. Comparative evaluation of garlic extract mouthwash and chlorhexidine mouthwash on salivary Streptococcus mutans count - an in vitro study. Oral Health Prev Dent. 2010;8:369–374. [PubMed] [Google Scholar]
- 20.Appel E, Vallon-Eberhard A, Rabinkov A, Brenner O, Shin I, Sasson K, et al. Therapy of murine pulmonary aspergillosis with antibody-alliinase conjugates and alliin. Antimicrob Agents Chemother. 2010;54:898–906. doi: 10.1128/AAC.01267-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khodavandi A, Alizadeh F, Aala F, Sekawi Z, Chong PP. In vitro investigation of antifungal activity of allicin alone and in combination with azoles against Candida species. Mycopathologia . 2010;169:287–295. doi: 10.1007/s11046-009-9251-3. [DOI] [PubMed] [Google Scholar]
- 22.An M, Shen H, Cao Y, Zhang J, Cai Y, Wang R, et al. Allicin enhances the oxidative. Int J Antimicrob Agents. 2009;33:258–263. doi: 10.1016/j.ijantimicag.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Ogita A, Fujita K, Taniguchi M, Tanaka T. Enhancement of the fungicidal activity of amphotericin B by allicin, an allyl-sulfur compound from garlic, against the yeast Saccharomyces cerevisiae as a model system. Planta Med. 2006;72:1247–1250. doi: 10.1055/s-2006-947203. [DOI] [PubMed] [Google Scholar]
- 24.Ogita A, Nagao Y, Fujita K, Tanaka T. Amplification of vacuole-targeting fungicidal activity of antibacterial antibiotic polymyxin B by allicin, an allyl sulfur compound from garlic. J Antibiot. 2007;60:511–518. doi: 10.1038/ja.2007.65. [DOI] [PubMed] [Google Scholar]
- 25.Yousuf S, Ahmad A, Khan A, Manzoor N, Khan LA. Effect of diallyldisulphide on an antioxidant enzyme system in Candida species. Can J Microbiol. 2010;56:816–821. doi: 10.1139/w10-066. [DOI] [PubMed] [Google Scholar]
- 26.Avato P, Tursil E, Vitali C, Miccolis V, Candido V. Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine. 2000;7:239–243. doi: 10.1016/s0944-7113(00)80010-0. [DOI] [PubMed] [Google Scholar]
- 27.Kloucek P, Smid J, Flesar J, Havlik J, Titera D, Rada V, et al. In vitro inhibitory activity of essential oil vapors against Ascosphaera apis. Nat Prod Commun. 2012;7:253–256. [PubMed] [Google Scholar]
- 28.Londhe VP, Gavasane AT, Nipate SS, Bandawane DD, Chaudhari PD. Role of garlic (Allium sativum) in various diseases: An overview. J Pharm Res Opin. 2011;1:129–134. [Google Scholar]
- 29.Ledezma E, Apitz-Castro R. Ajoene the main active compound of garlic (Allium sativum): a new antifungal agent. Rev Iberoam Micol. 2006;23:75–80. doi: 10.1016/s1130-1406(06)70017-1. [DOI] [PubMed] [Google Scholar]
- 30.Thomaz L, Apitz-Castro R, Marques AF, Travassos LR, Taborda CP. Experimental paracoccidioidomycosis: alternative therapy with ajoene, compound from Allium sativum, associated with sulfamethoxazole/trimethoprim. Med Mycol. 2008;46:113–118. doi: 10.1080/13693780701651681. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida S, Kasuga Sh, Hayashi N, Ushiroguchi T, Matsuura H, Nakagawa Sh. Antifungal activity of ajoene derived from garlic. Appl Environ Microbiol. 1987;53:615–617. doi: 10.1128/aem.53.3.615-617.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamim S, Ahmed SW, Azhar I. Antifungal activity of Allium, Aloe, and Solanum species. Pharm Biol. 2004;42:491–498. [Google Scholar]
- 33.Adetumbi M, Javor GT, Lau BHS. Allium sativum (Garlic) inhibits lipid synthesis by candida albicans. Anti'microb Agents Chemother. 1986;30:499–501. doi: 10.1128/aac.30.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan ZK, Katiyar R. Potent antifungal activity of garlic (allium sativum) against experimental murine dissemenated cryptococcosis. Pharm Biol. 2000;38:87–100. doi: 10.1076/1388-0209(200004)3821-1FT087. [DOI] [PubMed] [Google Scholar]
- 35.Davis LE, Shen JK, Cai Y. Antifungal activity in human cerebrospinal fluid and plasma after intravenous administration of Allium sativum. Antimicrob Agents Chemother. 1990;34:651–653. doi: 10.1128/aac.34.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang HX, Ng TB. Purification of allivin, a novel antifungal protein from bulbs of the round-cloved garlic. Life Sci. 2001;70:357–365. doi: 10.1016/s0024-3205(01)01399-6. [DOI] [PubMed] [Google Scholar]
- 37.Lima CM, Freitas FI, Morais LC, Cavalcanti MG, Silva LF, Padilha RJ, et al. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Rev Soc Bras Med Trop. 2011;44:327–330. doi: 10.1590/s0037-86822011005000023. [DOI] [PubMed] [Google Scholar]
- 38.Waag T, Gelhaus C, Rath J, Stich A, Leippe M, Schirmeister T. Allicin and derivates are cysteine protease inhibitors with antiparasitic activity. Bioorg Med Chem Lett. 2010;20:5541–5543. doi: 10.1016/j.bmcl.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 39.Lun ZR, Burri C, Menzinger M, Kaminsky R. Antiparasitic activity of diallyl trisulfide (Dasuansu) on human and animal pathogenic protozoa (Trypanosoma sp Entamoeba histolytica and Giardia lamblia) in vitro. Ann Soc Belg Med Trop. 1994;74:51–59. [PubMed] [Google Scholar]
- 40.Gallwitz H, Bonse S, Martinez-Cruz A, Schlichting I, Schumacher K, Krauth-Siegel RL. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: crystallographic, kinetic, and spectroscopic studies. J Med Chem. 1999;42:364–72. doi: 10.1021/jm980471k. [DOI] [PubMed] [Google Scholar]
- 41.Bany J, Zdanowska D, Zdanowski R, Skopińska-Rózewska E. The effect of herbal remedy on the development of Trichinella spiralis infection in mice. Pol J Vet Sci. 2003;6:6–8. [PubMed] [Google Scholar]
- 42.Anthony JP, Fyfe L, Smith H. Plant active components - a resource for antiparasitic agents. Trends Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Soffar SA, Mokhtar GM. Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in hymenolepiasis nana and giardiasis. J Egypt Soc Parasitol. 1991;21:497–502. [PubMed] [Google Scholar]
- 44.Ahmed M, Laing MD, Nsahlai IV. In vitro anthelmintic activity of crude extracts of selected medicinal plants against Haemonchus contortus from sheep. J Helminthol. 2012;26:1–6. doi: 10.1017/S0022149X1200020X. [DOI] [PubMed] [Google Scholar]
- 45.Klimpel S, Abdel-Ghaffar F, Al-Rasheid KA, Aksu G, Fischer K, Strassen B, et al. The effects of different plant extracts on nematodes. Parasitol Res. 2011;108:1047–1054. doi: 10.1007/s00436-010-2168-4. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Ghaffar F, Semmler M, Al-Rasheid KA, Strassen B, Fischer K, Aksu G, et al. The effects of different plant extracts on intestinal cestodes and on trematodes. Parasitol Res. 2011;108:979–984. doi: 10.1007/s00436-010-2167-5. [DOI] [PubMed] [Google Scholar]
- 47.Kalu IG, Ofoegbu U, Eroegbusi J, Nwachukwa CU, Ibeh B. Larvicidal activities of ethanol extract of Allium sativum (garlic bulb) against the filarial vector, Culex quinquefasciatus. J Med Plants Res. 2010;4:496–498. [Google Scholar]
- 48.Singha S, Chandra G. Mosquito larvicidal activity of some common spices and vegetable waste on Culex quinquefasciatus and Anopheles stephensi. Asian Pac J Trop Med . 2011;4:288–293. doi: 10.1016/S1995-7645(11)60088-6. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Velazquez M, Rosario-Cruz R, Castillo-Herrera G, Flores-Fernandez JM, Alvarez AH, Lugo-Cervantes E. Acaricidal effect of essential oils from Lippia graveolens (Lamiales: Verbenaceae), Rosmarinus officinalis (Lamiales: Lamiaceae), and Allium sativum (Liliales: Liliaceae) against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) J Med Entomol. 2011;48:822–827. doi: 10.1603/me10140. [DOI] [PubMed] [Google Scholar]
- 50.Tedeschi P, Leis M, Pezzi M, Civolani S, Maietti A, Brandolini V. Insecticidal activity and fungitoxicity of plant extracts and components of horseradish (Armoracia rusticana) and garlic (Allium sativum) J Environ Sci Health. 2011;46:486–490. doi: 10.1080/03601234.2011.583868. [DOI] [PubMed] [Google Scholar]
- 51.Park IK, Choi KS, Kim DH, Choi IH, Kim LS, Bak WC, et al. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae) Pest Manag Sci . 2006;62:723–728. doi: 10.1002/ps.1228. [DOI] [PubMed] [Google Scholar]
- 52.Weber ND, Andersen DO, North JA, Murray BK, Lawson LD, Hughes BG. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58:417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- 53.Josling P. Preventing the common cold with a garlic supplement: a double-blind placebo-controlled survey. Adv Ther. 2001;18:189–193. doi: 10.1007/BF02850113. [DOI] [PubMed] [Google Scholar]
- 54.Tatarintsev AV, Vrzhets PV, Ershov DE, Shchegolev AA, Turgiev AS, Karamov EV, etal The ajoene blockade of integrin-dependent processes in an HIV-infected cell system. Vestn Ross Akad Med Nauk . 1992:6–10. [PubMed] [Google Scholar]
- 55.Zhen H, Fang F, Ye DY, Shu SN, Zhou YF, Dong YS, et al. Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate-early genes. Antiviral Res. 2006;72:68–74. doi: 10.1016/j.antiviral.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Qidwai W, Qureshi R, Hasan SN, Azam SI. Effect of dietary garlic (Allium Sativum) on the blood pressure in humans--a pilot study. J Pak Med Assoc. 2000;50:204–207. [PubMed] [Google Scholar]
- 57.Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. 2006;136:736S–740S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 58.Kianoush S, Balali-Mood M, Mousavi SR, Moradi V, Sadeghi M, Dadpour B, et al. Comparison of therapeutic effects of garlic and d-Penicillamine in patients with chronic occupational lead poisoning. Basic Clin Pharmacol Toxicol. 2012;110:476–481. doi: 10.1111/j.1742-7843.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 59.Nwokocha CR, Ozolua RI, Owu DU, Nwokocha MI, Ugwu AC. Antihypertensive properties of Allium sativum (garlic) on normotensive and two kidney one clip hypertensive rats. Niger J Physiol Sci . 2011;26:213–218. [PubMed] [Google Scholar]
- 60.Vazquez-Prieto MA, González RE, Renna NF, Galmarini CR, Miatello RM. Aqueous garlic extracts prevent oxidative stress and vascular remodeling in an experimental model of metabolic syndrome. J Agric Food Chem. 2010;58:6630–6635. doi: 10.1021/jf1006819. [DOI] [PubMed] [Google Scholar]
- 61.Duda G, Suliburska J, Pupek-Musialik D. Effects of short-term garlic supplementation on lipid metabolism and antioxidant status in hypertensive adults. Pharmacol Rep. 2008;60:163–170. [PubMed] [Google Scholar]
- 62.Sener G, Sakarcan A, Yegen BC. Role of garlic in the prevention of ischemia-reperfusion injury. Mol Nutr Food Res. 2007;51:1345–1352. doi: 10.1002/mnfr.200700078. [DOI] [PubMed] [Google Scholar]
- 63.Al-Qattan KK, Thomson M, Al-Mutawa'a S, Al-Hajeri D, Drobiova H, Ali M. Nitric oxide mediates the blood-pressure lowering effect of garlic in the rat two-kidney, one-clip model of hypertension. J Nutr. 2006;136:774S–776S. doi: 10.1093/jn/136.3.774S. [DOI] [PubMed] [Google Scholar]
- 64.Harauma A, Moriguchi T. Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J Nutr. 2006;136:769S–773S. doi: 10.1093/jn/136.3.769S. [DOI] [PubMed] [Google Scholar]
- 65.Zahid Ashraf M, Hussain ME, Fahim M. Antiatherosclerotic effects of dietary supplementations of garlic and turmeric Restoration of endothelial function in rats. Life Sci. 2005;77:837–857. doi: 10.1016/j.lfs.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 66.Budoff MJ, Takasu J, Flores FR, Niihara Y, Lu B, Lau BH, et al. Inhibiting progression of coronary calcification using Aged Garlic Extract in patients receiving statin therapy: a preliminary study. Prev Med. 2004;39:985–991. doi: 10.1016/j.ypmed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Verma SK, Rajeevan v, Jain P, Bordia A. Effect of garlic (Allium sativum) oil on exercise tolerance in patients with coronary artery disease. J Physiol Pharmacol. 2005;49:115–118. [PubMed] [Google Scholar]
- 68.Yang Y, Chan SW, Hu M, Walden R, Tomlinson B. Effects of some common food constituents on cardiovascular disease. ISRN Cardiol. 2011:397136. doi: 10.5402/2011/397136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ali M, Al-Qattan KK, Al-Enezi F, Khanafer RM, Mustafa T. Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins Leukot Essent Fatty Acids. 2000;62:253–259. doi: 10.1054/plef.2000.0152. [DOI] [PubMed] [Google Scholar]
- 70.Chu TC, Ogidigben M, Han JC, Potter DE. Allicin-induced hypotension in rabbit eyes. J Ocul Pharmacol. 1993;9:201–209. doi: 10.1089/jop.1993.9.201. [DOI] [PubMed] [Google Scholar]
- 71.Drobiova H, Thomson M, Al-Qattan K, Peltonen-Shalaby R, Al-Amin Z, Ali M. Garlic increases antioxidant levels in diabetic and hypertensive rats determined by a modified peroxidase method. Evid Based Complement Alternat Med. 2011:703049. doi: 10.1093/ecam/nep011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fallon MB, Abrams GA, Abdel-Razek TT, Dai J, Chen SJ, Chen YF, et al. Garlic prevents hypoxic pulmonary hypertension in rats. Am J Physiol. 1998;275:L283–287. doi: 10.1152/ajplung.1998.275.2.L283. [DOI] [PubMed] [Google Scholar]
- 73.Brankovic S, Radenkovic M, Kitic D, Veljkovic S, Ivetic V, Pavlovic D, et al. Comparison of the hypotensive and bradycardic activity of ginkgo, garlic, and onion extracts. Clin Exp Hypertens. 2011;33:95–99. doi: 10.3109/10641963.2010.531833. [DOI] [PubMed] [Google Scholar]
- 74.Gu X, Zhu YZ. Therapeutic applications of organosulfur compounds as novel hydrogen sulfide. Expert Rev Clin Pharmacol. 2011;4:123–133. doi: 10.1586/ecp.10.129. [DOI] [PubMed] [Google Scholar]
- 75.Lavu M, Bhushan S, Lefer DJ. Hydrogen sulfide-mediated cardioprotection: mechanisms and therapeutic potential. Clin Sci . 2011;120:219–229. doi: 10.1042/CS20100462. [DOI] [PubMed] [Google Scholar]
- 76.Ginter E, Simko V. Garlic (Allium sativum L) and cardiovascular diseases. Bratisl Lek Listy. 2010;111:452–456. [PubMed] [Google Scholar]
- 77.Cruz C, Correa-Rotter R, Sánchez-González DJ, Hernández-Pando R, Maldonado PD, Martínez- Martínez CM, et al. Renoprotective and antihypertensive effects of S-allylcysteine in 5/6 nephrectomized rats. Am J Physiol Renal Physiol. 2007;293:F1691–1698. doi: 10.1152/ajprenal.00235.2007. [DOI] [PubMed] [Google Scholar]
- 78.Sobenin IA, Andrianova IV, Fomchenkov IV, Gorchakova TV, Orekhov AN. Time-released garlic powder tablets lower systolic and diastolic blood pressure in men with mild and moderate arterial hypertension. Hypertens Res. 2009;32:433–437. doi: 10.1038/hr.2009.36. [DOI] [PubMed] [Google Scholar]
- 79.Durak I, Kavutcu M, Aytaç B, Avci A, Devrim E, Ozbek H, Oztürk HS. Effects of garlic extract consumption on blood lipid and oxidant/antioxidant parameters in humans with high blood cholesterol. J Nutr Biochem. 2004;15:373–377. doi: 10.1016/j.jnutbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Castro C, Lorenzo AG, González A, Cruzado M. Garlic components inhibit angiotensin II-induced cell-cycle progression and migration: Involvement of cell-cycle inhibitor p27 (Kip1) and mitogen-activated protein kinase. Mol Nutr Food Res. 2010;54:781–787. doi: 10.1002/mnfr.200900108. [DOI] [PubMed] [Google Scholar]
- 81.Thevenot T, Pastor CM, Cervoni JP, Jacquelinet C, Nguyen-Khac E, Richou C, et al. Hepatopulmonary syndrome. Gastroenterol Clin Biol. 2009;33:565–579. doi: 10.1016/j.gcb.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Asdaq SM, Inamdar MN. Pharmacodynamic and Pharmacokinetic Interactions of Propranolol with Garlic (Allium sativum) in Rats. Evid Based Complement Alternat Med . 2011:824042. doi: 10.1093/ecam/neq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asdaq SM, Inamdar MN. The potential benefits of a garlic and hydrochlorothiazide combination as antihypertensive and cardioprotective in rats. J Nat Med. 2011;65:81–88. doi: 10.1007/s11418-010-0467-9. [DOI] [PubMed] [Google Scholar]
- 84.Asdaq SM, Inamdar MN. The potential for interaction of hydrochlorothiazide with garlic in rats. Chem Biol Interact. 2009;181:472–479. doi: 10.1016/j.cbi.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 85.Asdaq SM, Inamdar MN. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. 2010;17:1016–1026. doi: 10.1016/j.phymed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 86.Younis F, Mirelman D, Rabinkov A, Rosenthal T. S-allyl-mercapto-captopril: a novel compound in the treatment of Cohen-Rosenthal diabetic hypertensive rats. J Clin Hypertens (Greenwich) . 2010;12:451–455. doi: 10.1111/j.1751-7176.2010.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elkayam A, Mirelman D, Peleg E, Wilchek M, Miron T, Rabinkov A, et al. The effects of allicin and enalapril in fructose-induced hyperinsulinemic hyperlipidemic hypertensive rats. Am J Hypertens. 2001;14:377–381. doi: 10.1016/s0895-7061(00)01298-x. [DOI] [PubMed] [Google Scholar]
- 88.Wang HX, Ng TB. Natural products with hypoglycemic, hypotensive, hypocholesterolemic antiatherosclerotic and antithrombotic activities. Life Sci. 1999;65:2663–2677. doi: 10.1016/s0024-3205(99)00253-2. [DOI] [PubMed] [Google Scholar]
- 89.Espirito Santo SM, van Vlijmen BJ, van Duyvenvoorde W, Offerman EH, Havekes LM, Arnault I, et al. Absence of an atheroprotective effect of the garlic powder printanor in APOE*3-Leiden transgenic mice. Atherosclerosis. 2004;177:291–297. doi: 10.1016/j.atherosclerosis.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 90.Calvo-Gómez O, Morales-López J, López MG. Solid-phase microextraction-gas chromatographic-mass spectrometric analysis of garlic oil obtained by hydrodistillation. J Chromatogr A. 2004;1036:91–93. doi: 10.1016/j.chroma.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 91.Jain RC, Konar DB. Effect of garlic oil in experimental cholesterol atherosclerosis. Atherosclerosis . 1978;29:125–129. doi: 10.1016/0021-9150(78)90002-3. [DOI] [PubMed] [Google Scholar]
- 92.Gonen A, Harats D, Rabinkov A, Miron T, Mirelman D, Wilchek M, et al. The antiatherogenic effect of allicin: possible mode of action. Pathobiology . 2005;72:325–334. doi: 10.1159/000091330. [DOI] [PubMed] [Google Scholar]
- 93.Gromnatskiĭ NI, Sereditskaia ZhE, Lazareva NV, Sereditskiĭ AV, Annenkova GV. Effects of garlic allicor tablets on lipid metabolism and risk of fatal cardiovascular complications in patients with atherogenic dyslipoproteinemia. Vopr Pitan . 2007;76:60–64. [PubMed] [Google Scholar]
- 94.Orekhov AN, Tertov VV, Sobenin IA, Pivovarova EM. Direct anti-atherosclerosis-related effects of garlic. Ann Med. 1995;27:63–65. doi: 10.3109/07853899509031938. [DOI] [PubMed] [Google Scholar]
- 95.Orekhov AN, Grünwald J. Effects of garlic on atherosclerosis. Nutrition. 1997;13:656–663. doi: 10.1016/s0899-9007(97)83010-9. [DOI] [PubMed] [Google Scholar]
- 96.Lau BH. Suppression of LDL oxidation by garlic. J Nutr. 2001;131:985S–988S. doi: 10.1093/jn/131.3.985S. [DOI] [PubMed] [Google Scholar]
- 97.Phelps S, Harris WS. Garlic supplementation and lipoprotein oxidation susceptibility. Lipids. 1993;28:475–477. doi: 10.1007/BF02535949. [DOI] [PubMed] [Google Scholar]
- 98.Koscielny J, Klüssendorf D, Latza R, Schmitt R, Radtke H, Siegel G, et al. The antiatherosclerotic effect of Allium sativum. Atherosclerosis. 1999;144:237–249. doi: 10.1016/s0021-9150(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 99.Morcos NC. Modulation of lipid profile by fish oil and garlic combination. J Natl Med Assoc. 1997;89:673–678. [PMC free article] [PubMed] [Google Scholar]
- 100.Mathew B, Biju R. Neuroprotective effects of garlic: a review. Libyan J Med. 2008;3:23–33. doi: 10.4176/071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Asdaq SM, Inamdar MN, Asad M. Effect of conventional antihypertensive drugs on hypolipidemic action of garlic in rats. Indian J Exp Biol. 2009;47:176–181. [PubMed] [Google Scholar]
- 102.Yamaji K, Sarker KP, Abeyama K, Maruyama I. Anti-atherogenic effects of an egg yolk-enriched garlic supplement. Int J Food Sci Nutr. 2004;55:61–66. doi: 10.1080/09637480310001642493. [DOI] [PubMed] [Google Scholar]
- 103.Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. 1996;16:111–124. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 104.Fukao H, Yoshida H, Tazawa Y, Hada T. Antithrombotic effects of odorless garlic powder both in vitro and in vivo. Biosci Biotechnol Biochem. 2007;71:84–90. doi: 10.1271/bbb.60380. [DOI] [PubMed] [Google Scholar]
- 105.Srivastava KC. Aqueous extracts of onion, garlic and ginger inhibit platelet aggregation and alter arachidonic acid metabolism. Biomed Biochim Acta. 1984;43:S335–346. [PubMed] [Google Scholar]
- 106.Hiyasat B, Sabha D, Grotzinger K, Kempfert J, Rauwald JW, Mohr FW, et al. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology. 2009;83:197–204. doi: 10.1159/000196811. [DOI] [PubMed] [Google Scholar]
- 107.MacDonald JA, Marchand ME, Langler RF. Improving upon the in vitro biological activity of antithrombotic disulfides. Blood Coagul Fibrinolysis. 2004;15:447–450. doi: 10.1097/00001721-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 108.Choi YH, Park HS. Apoptosis induction of U937 human leukemia cells by diallyl trisulfide induces through generation of reactive oxygen species. J Biomed Sci. 2012;19 doi: 10.1186/1423-0127-19-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan KC, Yin MC, Chao WJ. Effect of diallyl trisulfide-rich garlic oil on blood coagulation and plasma activity of anticoagulation factors in rats. Food Chem Toxicol. 2007;45:502–507. doi: 10.1016/j.fct.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 110.Bordia A, Verma SK, Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1998;58:257–263. doi: 10.1016/s0952-3278(98)90034-5. [DOI] [PubMed] [Google Scholar]
- 111.Ariga T, Tsuj K, Seki T, Moritomo T, Yamamoto JI. Antithrombotic and antineoplastic effects of phyto-organosulfur compounds. Biofactors . 2000;13:251–255. doi: 10.1002/biof.5520130138. [DOI] [PubMed] [Google Scholar]
- 112.Cavagnaro PF, Camargo A, Galmarini CR, Simon PW. Effect of cooking on garlic (Allium sativum L) antiplatelet activity and thiosulfinates content. J Agric Food Chem. 2007;55:1280–1288. doi: 10.1021/jf062587s. [DOI] [PubMed] [Google Scholar]
- 113.Nishimura H, Takahashi T, WijayaCH , Satoh A, Ariga T. Thermochemical transformation of sulfur compounds in Japanese domestic Allium, Allium victorialis L. Biofactors . 2000;13:257–263. doi: 10.1002/biof.5520130139. [DOI] [PubMed] [Google Scholar]
- 114.Briggs WH, Xiao H, Parkin KL, Shen C, Goldman IL. Differential inhibition of human platelet aggregation by selected Allium thiosulfinates. J Agric Food Chem. 2000;48:5731–5735. doi: 10.1021/jf0004412. [DOI] [PubMed] [Google Scholar]
- 115.Apitz-Castro R, Badimon JJ, Badimon L. A garlic derivative, ajoene, inhibits platelet deposition on severely damaged vessel wall in an in vivo porcine experimental model. Thromb Res. 1994;75:243–249. doi: 10.1016/0049-3848(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 116.Mousa SA. Antithrombotic effects of naturally derived products on coagulation and platelet Function. Methods Mol Biol. 2010;663:229–240. doi: 10.1007/978-1-60761-803-4_9. [DOI] [PubMed] [Google Scholar]
- 117.Apitz-Castro R, Escalante J, Vargas R, Jain MK. Ajoene, the antiplatelet principle of garlic, synergistically potentiates the antiaggregatory action of prostacyclin, forskolin, indomethacin and dypiridamole on human platelets. Thromb Res. 1986;42:303–311. doi: 10.1016/0049-3848(86)90259-8. [DOI] [PubMed] [Google Scholar]
- 118.Apitz-Castro R, Ledezma E, Escalante J, Jain MK. The molecular basis of the antiplatelet action of ajoene: direct interaction with the fibrinogen receptor. Biochem Biophys Res Commun. 1986;141:145–150. doi: 10.1016/s0006-291x(86)80346-1. [DOI] [PubMed] [Google Scholar]
- 119.Srivastava KC, Tyagi OD. Effects of a garlic-derived principle (ajoene) on aggregation and arachidonic acid metabolism in human blood platelets. Prostaglandins Leukot Essent Fatty Acids. 1993;49:587–595. doi: 10.1016/0952-3278(93)90165-s. [DOI] [PubMed] [Google Scholar]
- 120.Stanger MJ, Thompson LA, Young AJ, Lieberman HR. Anticoagulant activity of select dietary supplements. Nutr Rev. 2012;70:107–117. doi: 10.1111/j.1753-4887.2011.00444.x. [DOI] [PubMed] [Google Scholar]
- 121.Mateen AA, Rani PU, Naidu MU, Chandrashekar E. Pharmacodynamic interaction study of Allium sativum (garlic) with cilostazol in patients with type II diabetes mellitus. Indian J Pharmacol. 2011;43:270–274. doi: 10.4103/0253-7613.81514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macan H, Uykimpang R, Alconcel M, Takasu J, Razon R, Amagase H, et al. Aged garlic extract may be safe for patients on warfarin therapy. J Nutr. 2006;136:793S–795S. doi: 10.1093/jn/136.3.793S. [DOI] [PubMed] [Google Scholar]
- 123.Spolarich AE, Andrews L. An examination of the bleeding complications associated with herbal supplements antiplatelet and anticoagulant medications. J Dent Hyg. 2007;81 [PubMed] [Google Scholar]
- 124.Kuda T, Iwai A, Yano T. Effect of red pepper Capsicum annuum var conoides and garlic Allium sativum on plasma lipid levels and cecal microflora in mice fed beef tallow. Food Chem Toxicol. 2004;42:1695–1700. doi: 10.1016/j.fct.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 125.Zacharias NT, Sebastian KL, Philip B, Augusti KT. Hypoglycemic and hypolipidaemic effects of garlic in sucrose fed rabbits. Indian J Physiol Pharmacol. 1980;24:151–154. [PubMed] [Google Scholar]
- 126.Gorinstein S, Leontowicz H, Leontowicz M, Drzewiecki J, Najman K, Katrich E, et al. Raw and boiled garlic enhances plasma antioxidant activity and improves plasma lipid metabolism in cholesterol-fed rats. Life Sci. 2006;78:655–663. doi: 10.1016/j.lfs.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 127.Kuo CF, Jao YC, Yang P. Downregulation of hepatic lipoprotein assembly in rats by fermented products of Monascus pilosus. Nutrition. 2008;24:477–483. doi: 10.1016/j.nut.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 128.Sengupta A, Ghosh S, Bhattacharjee S. Allium vegetables in cancer prevention: an overview. Asian Pac J Cancer Prev. 2004;5:237–245. [PubMed] [Google Scholar]
- 129.Lin MC, Wang EJ, Lee C, Chin KT, Liu D, Chiu JF, et al. Garlic inhibits microsomal triglyceride transfer protein gene expression in human liver and intestinal cell lines and in rat intestine. J Nutr. 2002;132:1165–1168. doi: 10.1093/jn/132.6.1165. [DOI] [PubMed] [Google Scholar]
- 130.Peleg A, Hershcovici T, Lipa R, Anbar R, Redler M, Beigel Y. Effect of garlic on lipid profile and psychopathologic parameters in people with mild to moderate hypercholesterolemia. Isr Med Assoc J. 2003;5:637–640. [PubMed] [Google Scholar]
- 131.Rajasree CR, Rajamohan T, Augusti KT. Biochemical effects of garlic protein on lipid metabolism in alcohol fed rats. Indian J Exp Biol. 1999;37:243–247. [PubMed] [Google Scholar]
- 132.Bobboi A, Augusti KT, Joseph PK. Hypolipidemic effects of onion oil and garlic oil in ethanol-fed rats. Indian J Biochem Biophys. 1984;21:211–213. [PubMed] [Google Scholar]
- 133.Mathew BC, Daniel RS, Augusti KT. Hypolipidemic effect of garlic protein substituted for casein in diet of rats compared to those of garlic oil. Indian J Exp Biol. 1996;34:337–340. [PubMed] [Google Scholar]
- 134.Orellana A, Kawada ME, Morales MN, Vargas L, Bronfman M. Induction of peroxisomal fatty acyl-coenzyme A oxidase and total carnitine acetyl-coenzyme A transferase in primary cultures of rat hepatocytes by garlic extracts. Toxicol Lett. 1992;60:11–17. doi: 10.1016/0378-4274(92)90042-i. [DOI] [PubMed] [Google Scholar]
- 135.Sovová M, Sova P. Pharmaceutical importance of Allium sativum L. 5. Hypolipemic effects in vitro and in vivo. Ceska Slov Farm. 2004;53:117–123. [PubMed] [Google Scholar]
- 136.Kempaiah RK, Srinivasan K. Antioxidant status of red blood cells and liver in hypercholesterolemic rats fed hypolipidemic spices. Int J Vitam Nutr Res. 2004;74:199–208. doi: 10.1024/0300-9831.74.3.199. [DOI] [PubMed] [Google Scholar]
- 137.Pedraza-Chaverrí J, Medina-Campos ON, Granados-Silvestre MA, Maldonado PD, Olivares-Corichi IM, Hernández-Pando R. Garlic ameliorates hyperlipidemia in chronic aminonucleoside nephrosis. Mol Cell Biochem. 2000;211:69–77. doi: 10.1023/a:1007106632313. [DOI] [PubMed] [Google Scholar]
- 138.Kempaiah RK, Srinivasan K. Beneficial influence of dietary curcumin, capsaicin and garlic on erythrocyte integrity in high-fat fed rats. J Nutr Biochem. 2006;17:471–478. doi: 10.1016/j.jnutbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 139.Lii CK, Huang CY, Chen HW, Chow MY, Lin YR, Huang CS, et al. Diallyl trisulfide suppresses the adipogenesis of 3T3-L1 preadipocytes through ERK activation. Food Chem Toxicol. 2012;50:478–484. doi: 10.1016/j.fct.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 140.Rai SK, Sharma M, Tiwari M. Inhibitory effect of novel diallyldisulfide analogs on HMG-CoA reductase expression in hypercholesterolemic rats: CREB as a potential upstream target. Life Sci . 2009;85:211–219. doi: 10.1016/j.lfs.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 141.Liu L, Yeh YY. Water-soluble organosulfur compounds of garlic inhibit fatty acid and triglyceride syntheses in cultured rat hepatocytes. Lipids. 2001;36:395–400. doi: 10.1007/s11745-001-0734-4. [DOI] [PubMed] [Google Scholar]
- 142.Sela U, Ganor S, Hecht I, Brill A, Miron T, Rabinkov A, et al. Allicin inhibits SDF-1alpha-induced T cell interactions with fibronectin and endothelial cells by down-regulating cytoskeleton rearrangement, Pyk-2 phosphorylation and VLA-4 expression. Immunology. 2004;111:391–399. doi: 10.1111/j.0019-2805.2004.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mathew PT, Augusti KT. Studies on the effect of allicin (diallyl disulphide-oxide) on alloxan diabetes I Hypoglycaemic action and enhancement of serum insulin effect and glycogen Synthesis. Indian J Biochem Biophys. 1973;10:209–212. [PubMed] [Google Scholar]
- 144.Vidyashankar S, Sambaiah K, Srinivasan K. Dietary garlic and onion reduce the incidence of atherogenic diet-induced cholesterol gallstones in experimental mice. Br J Nutr. 2009;101:1621–1629. doi: 10.1017/S0007114508118748. [DOI] [PubMed] [Google Scholar]
- 145.Andrianova IV, Demidova OM, Medvedeva LA, Latyshev OA. Correction of hyperlipidemia with Allicor. Klin Med (Mosk) 2004;82:56–58. [PubMed] [Google Scholar]
- 146.Augusti KT, Arathy SL, Asha R, Ramakrishanan J, Zaira J, Lekha V, et al. A comparative study on the beneficial effects of garlic (Allium sativum Linn) amla (Emblica officinalis Gaertn)and onion (Allium cepa Linn) on the hyperlipidemia induced by butter fat and beef fat in rats. Indian J Exp Biol. 2001;39:760–766. [PubMed] [Google Scholar]
- 147.Romagnolo DF, Davis CD, Milner JA. Phytoalexins in cancer prevention. Front Biosci. 2012;17:2035–2058. doi: 10.2741/4036. [DOI] [PubMed] [Google Scholar]
- 148.Lai KC, Kuo CL, Ho HC, Yang JS, Ma CY, Lu HF, et al. Diallyl sulfide, diallyl disulfide and diallyl trisulfide affect drug resistant gene expression in colo 205 human colon cancer cells in vitro and in vivo. Phytomedicine . 2012;19:625–630. doi: 10.1016/j.phymed.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 149.Yu CS, Huang AC, Lai KC, Huang YP, Lin MW, Yang JS, et al. Diallyl trisulfide induces apoptosis in human primary colorectal cancer cells. Oncol Rep. 2012;28:949–954. doi: 10.3892/or.2012.1882. [DOI] [PubMed] [Google Scholar]
- 150.Park HS, Kim GY, Choi IW, Kim ND, Hwang HJ, Choi YW, et al. Inhibition of matrix metalloproteinase activities and tightening of tight junctions by diallyl disulfide in AGS human gastric carcinoma cells. J Food Sci . 2011;76:T105–111. doi: 10.1111/j.1750-3841.2011.02114.x. [DOI] [PubMed] [Google Scholar]
- 151.Liu Z, Li M, Chen K, Yang J, Chen R, Wang T, et al. S-allylcysteine induces cell cycle arrest and apoptosis in androgen-independent human prostate cancer cells. Mol Med Report. 2012;5:439–443. doi: 10.3892/mmr.2011.658. [DOI] [PubMed] [Google Scholar]
- 152.Bar-Chen W, Golan T, Peri I, Ludmer Z, Schwartz B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr Cancer. 2010;62:947–957. doi: 10.1080/01635581.2010.509837. [DOI] [PubMed] [Google Scholar]
- 153.Wang X, Jiao F, Wang QW, Wang J, Yang K, Hu RR, et al. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol Med Report. 2012;5:66–72. doi: 10.3892/mmr.2011.588. [DOI] [PubMed] [Google Scholar]
- 154.Ban JO, Lee DH, Kim EJ, Kang JW, Kim MS, Cho MC, et al. Antiobesity effects of a sulfur compound thiacremonone mediated via down-regulation of serum triglyceride and glucose levels and lipid accumulation in the liver of db/db mice. Phytother Res . 2012;10:3729. doi: 10.1002/ptr.3729. [DOI] [PubMed] [Google Scholar]
- 155.Dkhil MA, Abdel-Baki AS, Wunderlich F, Sies H, Al-Quraishy S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol. 2011;175:66–72. doi: 10.1016/j.vetpar.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 156.Shih PC, Kuo CH, Juang JY, Liu CH, Hsu L, Liu CT. Effects of garlic oil on the migration of neutrophil-like cell studied by using a chemotactic gradient Labchip. J Biomed Biotechnol. 2010;31:9059. doi: 10.1155/2010/319059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiang YH, Jen LN, Su HY, Lii CK, Sheen LY, Liu CT. Effects of garlic oil and two of its major organosulfur compounds, diallyl disulfide and diallyl trisulfide, on intestinal damage in rats injected with endotoxin. Toxicol Appl Pharmacol. 2006;213:46–54. doi: 10.1016/j.taap.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 158.Krishna A, Yadav A. Lead compound design for TPR/COX dual inhibition. J Mol Model. 2012;18:4397–4408. doi: 10.1007/s00894-012-1435-y. [DOI] [PubMed] [Google Scholar]
- 159.Lin GH, Lee YJ, Choi DY, Han SB, Jung JK, Hwang BY, et al. Anti-amyloidogenic effect of thiacremonone through anti-inflamation in vitro and in vivo models. J Alzheimers Dis. 2012;29:659–676. doi: 10.3233/JAD-2012-111709. [DOI] [PubMed] [Google Scholar]
- 160.Chandrashekar PM, Venkatesh YP. Fructans from aged garlic extract produce a delayed immunoadjuvant response to ovalbumin antigen in BALB/c mice. Immunopharmacol Immunotoxicol. 2012;34:174–180. doi: 10.3109/08923973.2011.584066. [DOI] [PubMed] [Google Scholar]
- 161.Chandrashekar PM, Prashanth KV, Venkatesh YP. Isolation, structural elucidation and immunomodulatory activity of fructans from aged garlic extract. Phytochemistry. 2011;72:255–264. doi: 10.1016/j.phytochem.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 162.Hanieh H, Narabara K, Tanaka Y, Gu Z, Abe A, Kondo Y. Immunomodulatory effects of Alliums and Ipomoea batata extracts on lymphocytes and macrophages functions in White Leghorn chickens: in vitro study. Anim Sci J . 2012;83:68–76. doi: 10.1111/j.1740-0929.2011.00918.x. [DOI] [PubMed] [Google Scholar]
- 163.Gamboa-León MR, Aranda-González I, Mut-Martín M, García-Miss MR, Dumonteil E. In vivo and in vitro control of Leishmania mexicana due to garlic-induced NO production. Scand J Immunol. 2007;66:508–514. doi: 10.1111/j.1365-3083.2007.02000.x. [DOI] [PubMed] [Google Scholar]
- 164.Bruck R, Aeed H, Brazovsky E, Noor T, Hershkoviz R. Allicin, the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int. 2005;25:613–621. doi: 10.1111/j.1478-3231.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 165.Lang A, Lahav M, Sakhnini E, Barshack I, Fidder HH, Avidan B, et al. Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin Nutr. 2004;23:1199–1208. doi: 10.1016/j.clnu.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 166.Salman H, Bergman M, Bessler H, Punsky I, Djaldetti M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int J Immunopharmacol. 1999;21:589–597. doi: 10.1016/s0192-0561(99)00038-7. [DOI] [PubMed] [Google Scholar]
- 167.Tattelman E. Health effects of garlic. Am Fam Physician. 2005;72:103–106. [PubMed] [Google Scholar]
- 168.Friedman T, Shalom A, Westreich M. Self-inflicted garlic burns: our experience and literature review. Int J Dermatol. 2006;45:1161–1163. doi: 10.1111/j.1365-4632.2006.02860.x. [DOI] [PubMed] [Google Scholar]
- 169.Hamlaoui-Gasmi S, Mokni M, Limam N, N'guessan P, Carrier A, Limam F, et al. Grape seed and skin extract mitigates garlic-induced oxidative stress in rat liver. Can J Physiol Pharmacol. 2012;90:547–556. doi: 10.1139/y2012-025. [DOI] [PubMed] [Google Scholar]
- 170.Rana SV, Pal R, Vaiphei K, Singh K. Garlic hepatotoxicity: safe dose of garlic. Trop Gastroenterol. 2006;27:26–30. [PubMed] [Google Scholar]
- 171.Banerjee SK, Maulik M, Manchanda SC, Dinda AK, Das TK, Maulik SK. Garlic-induced alteration in rat liver and kidney morphology and associated changes in endogenous antioxidant status. Food Chem Toxicol. 2001;39:793–797. doi: 10.1016/s0278-6915(01)00018-7. [DOI] [PubMed] [Google Scholar]
- 172.Alnaqeeb MA, Thomson M, Bordia T, Ali M. Histopathological effects of garlic on liver and lung of rats. Toxicol Lett. 1996;85:157–164. doi: 10.1016/0378-4274(96)03658-2. [DOI] [PubMed] [Google Scholar]
- 173.Liu CT, Wong PL, Lii CK, Hse H, Sheen LY. Antidiabetic effect of garlic oil but not diallyl disulfide in rats with streptozotocin-induced diabetes. Food Chem Toxicol. 2006;44:1377–1384. doi: 10.1016/j.fct.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 174.Müller AC, Kanfer I. Potential pharmacokinetic interactions between antiretrovirals and medicinal plants used as complementary and African traditional medicines. Biopharm Drug Dispos. 2011;32:458–470. doi: 10.1002/bdd.775. [DOI] [PubMed] [Google Scholar]
- 175.Berginc K, Žakelj S, Kristl A. In vitro interactions between aged garlic extract and drugs used for the treatment of cardiovascular and diabetic patients. Eur J Nutr. 2010;49:373–384. doi: 10.1007/s00394-010-0095-x. [DOI] [PubMed] [Google Scholar]
- 176.Reddy GD, Reddy AG, Rao GS, Kumar MV. Pharmacokinetic interaction of garlic and atorvastatin in dyslipidemic rats. Indian J Pharmacol. 2012;44:246–252. doi: 10.4103/0253-7613.93860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol. 2004;22:2489–2503. doi: 10.1200/JCO.2004.08.182. [DOI] [PubMed] [Google Scholar]
- 178.Fateh R, Kashani MJN, Motevallian M, Falahati M, Yazdanparast A. In vitro antifungal activity of Allium hirtifolium in comparison with the miconazole. Med J Islam Repub Iran. 2010;24:1. [Google Scholar]
- 179.Amin M, Jahangirnezhad M, Rasaei N, Pipelzadeh MH, Rafiee M. Evaluation of the effect of Persian shallot (Allium hirtifolium, boiss) aqueous extract on mouth bacterial count compared with chlorhexidine mouth rinse. Afr J Microbiol Res. 2012;6:5809–5813. [Google Scholar]
- 180.Ghodrati Azadi H, Ghaffari SM, Riazi GH, Ahmadian S, Vahedi F. Antiproliferative activity of chloroformic extract of Persian Shallot, Allium hirtifolium, on tumor cell lines. Cytotechnology. 2008;56:179–185. doi: 10.1007/s10616-008-9145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sela U, Ganor S, Hecht I, Brill A, Miron T, Rabinkov A, et al. Allicin inhibits SDF-1alpha-induced T cell interactions with fibronectin and endothelial cells by down-regulating cytoskeleton rearrangement, Pyk-2 phosphorylation and VLA-4 expression. Immunology. 2004;111:391–399. doi: 10.1111/j.0019-2805.2004.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhu Z, Mao S, Zhu W. Effects of ruminal infusion of garlic oil on fermentation dynamics, fatty acid profile and abundance of bacteria involved in biohydrogenation in rumen of goats. Asian-Aust J Anim Sci. 2012;25:962–970. doi: 10.5713/ajas.2011.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Krumm P, Giraldez T, Alvarez de la Rosa D, Clauss WG, Fronius M, Althaus M. Thiol-reactive compounds from garlic inhibit the epithelial sodium channel (ENaC) Bioorg MedChem. 2012;20:3979–3984. doi: 10.1016/j.bmc.2012.05.021. [DOI] [PubMed] [Google Scholar]