Abstract
Infection of the central nervous system (CNS) is a severe and frequently fatal event during the course of many diseases caused by microbes with predominantly intracellular life cycles. Examples of these include the facultative intracellular bacteria Listeria monocytogenes, Mycobacterium tuberculosis, and Brucella and Salmonella spp. and obligate intracellular microbes of the Rickettsiaceae family and Tropheryma whipplei. Unfortunately, the mechanisms used by intracellular bacterial pathogens to enter the CNS are less well known than those used by bacterial pathogens with an extracellular life cycle. The goal of this review is to elaborate on the means by which intracellular bacterial pathogens establish infection within the CNS. This review encompasses the clinical and pathological findings that pertain to the CNS infection in humans and includes experimental data from animal models that illuminate how these microbes enter the CNS. Recent experimental data showing that L. monocytogenes can invade the CNS by more than one mechanism make it a useful model for discussing the various routes for neuroinvasion used by intracellular bacterial pathogens.
INTRODUCTION
Invasion of the central nervous system (CNS) is a severe and frequently fatal event during the course of many infectious diseases, and those who survive are often left with permanent neurological dysfunction. One of the key events in the pathogenesis of CNS infections is how microbes interact with and cross the blood-brain or blood-choroid barriers to gain access to the central compartment. Great strides have been made in understanding the mechanisms by which bacterial pathogens with a predominantly extracellular life cycle, such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae, invade the CNS from the bloodstream (for recent reviews, see references 172, 196, 206, 274, and 419). However the mechanisms used by obligate and facultative intracellular bacterial pathogens, here collectively referred to as intracellular bacteria, to enter the CNS are less well established.
In most scenarios, neuroinvasion occurs in the context of a systemic disease and typically follows bacterial dissemination via the bloodstream. In this respect, intracellular and extracellular bacteria have developed different strategies to solve the biological problem of how to escape elimination by host defenses such as phagocytes and serum proteins. Extracellular bacteria typically produce a polysaccharide capsule that allows them to resist complement-mediated lysis and phagocytosis by leukocytes. As a result, these pathogens typically achieve a high-density bacteremia that is important for CNS invasion (217, 372). Successful entry into the CNS then results in extremely high densities of extracellular bacteria in the cerebrospinal fluid (CSF) (39, 217).
By comparison, the means used by facultative and obligate intracellular bacteria to survive and disseminate within the host are fundamentally different. These differences are reflected in the lower densities of these organisms in the bloodstream and the CSF than of extracellular pathogens. Although this point is intuitively obvious for obligate intracellular bacteria, recent data cited below indicate that even facultative intracellular pathogens, i.e., those that can replicate inside or outside of a eukaryotic cell, cause disease only if they can survive intracellularly. Put another way, if intracellular replication is prevented, the microbes are not pathogenic, suggesting that the intracellular and extracellular pathways are not equal in terms of pathogenicity. This concept is important for understanding how facultative intracellular bacteria enter the CNS, considering that in some infections both extracellular and intracellular organisms are present in the bloodstream to a greater or lesser extent and it is unclear whether one is more neuroinvasive than the other. For example, recent studies of facultative intracellular bacteria including Listeria monocytogenes, Mycobacterium tuberculosis, and Salmonella enterica serovar Typhimurium show that they take up an intracellular location almost from the initial point of invasion of the host (302, 316, 317, 378, 391). Conversely, even obligate intracellular bacteria like Ehrlichia chaffeensis have an extracellular portion of their life cycle that could, in theory, contribute to CNS invasion (222). Thus, intracellular bacteria could have more options available to them than do extracellular bacteria for entering the CNS.
Mechanisms used by microbial pathogens to enter the CNS are usually divided according to the cellular route involved and whether the organisms breach endothelial cells of blood-brain barrier or specialized epithelial cells of blood-choroid barriers. These routes of entry are commonly referred to as (i) intercellular, i.e., passing between cells, (ii) transcellular, i.e., passing through cells, (iii) leukocyte facilitated, i.e., a Trojan horse-like mechanism, or (iv) nonhematogenous (172, 182, 241, 419). The most common routes of entry for extracellular bacteria are the intercellular and transcellular routes. The transcellular approaches are interesting in that they result in these bacteria temporarily assuming an intracellular location (111, 303, 324). Transcytosis of Streptococcus pneumoniae across endothelial cells is an excellent example of this event. Pneumococci bind to the platelet-activating factor receptor on endothelial cells that are activated by proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1α (IL-1α), in a process dependent on choline binding protein A, a bacterial adhesin (73). Bound bacteria are internalized into vesicles which transport transparent bacterial variants through the endothelial cell or recycle them to the apical surface, whereas most opaque bacterial variants are degraded within the cell (324). Intracellular organisms, on the other hand, typically do not use an intercellular approach, and the transcellular approach is modified by the fact that bacterial replication often takes places within endothelial cells. In addition, at least one bacterium, L. monocytogenes, is able to enter the brain via a nonhematogenous route by retrograde transport within cranial nerves.
The purpose of this overview is to elaborate on the mechanisms by which different facultative and obligate intracellular bacteria achieve CNS invasion in the context of human disease. Predominant clinical features reflective of CNS infection are noted, and the salient aspects of the pathological findings at postmortem examination, particularly as they shed light on entry mechanisms, are reviewed. Similarly, we discuss some studies in experimental or natural infection in animals as far as they provide insight into CNS invasion (Table 1). We refer as much as possible to the primary literature describing the original findings so that this paper can be a useful resource to the reader who wants to go into more detail in given aspects. In keeping with the focus of this review, experiments detailing the innate and adaptive host immune responses that follow bacterial entry the CNS are generally not discussed, nor are treatment modalities and prevention strategies discussed. Finally, readers are directed to several recent reviews for a detailed discussion of the blood-brain and blood-choroid barriers in steady state and infection (173, 259, 289, 330).
TABLE 1.
Possible mechanisms used by intracellular bacteria for CNS invasion
| Microbe | Possible mechanisms for CNS invasiona
|
||
|---|---|---|---|
| Phagocyte-facilitated invasion | Direct invasion of endothelial cells | Retrograde transport within neurons | |
| L. monocytogenes | + | + | + |
| M. tuberculosis | + | ? | − |
| Brucella spp. | + | ? | − |
| Salmonella spp. | + | ? | − |
| R. rickettsii | − | + | − |
| R. prowazekii | − | + | − |
| O. tsutsugamushi | + | + | − |
| E. chaffeensis | + | − | − |
| C. burnetii | + | − | − |
| T. whipplei | + | − | − |
The qualitative scale is as follows: +, likely; ?, questionable; −, unlikely.
This review is arranged according to the different microorganisms rather than by the presumed mechanism(s) of entry into the CNS. For most of the organisms considered here, the cellular and molecular mechanisms that enable neuroinvasion are largely unknown or are just now being discovered. Of these, the neuroinvasive mechanisms of L. monocytogenes and Rickettsia rickettsii are the best studied. This review begins by discussing L. monocytogenes because it is a good model organism for illustrating the various modes of CNS entry available to intracellular pathogens. For other bacteria, however, general aspects of neuroinvasion can be inferred from careful histopathological studies of CNS infections and/or from what is known about the cell biology of the organism. Examples of these organisms are M. tuberculosis, Brucella spp., Salmonella spp., Orientia tsutsugamushi, E. chaffeensis, and Coxiella burnetii. Finally, one organism, Tropheryma whipplei, which causes a rare, poorly understood disease that includes CNS symptoms but for which little is known about its pathophysiology, is discussed.
FACULTATIVE INTRACELLULAR PATHOGENS
Listeria monocytogenes
L. monocytogenes is a gram-positive, nonsporulating bacillus that is facultatively anaerobic and produces weak beta-hemolysis on blood agar. It is divided into 13 serotypes based on antibody reactivity to somatic and flagellar antigens, but serotypes 1/2a, 1/2b, and 4b account for the majority of human disease (189). It is a facultative intracellular parasite of professional and nonprofessional phagocytes, with a similar intracellular life cycle in both cases (299, 390). Phagocytic cells internalize bacteria through a variety of opsonin-dependent and opsonin-independent mechanisms. In addition, L. monocytogenes can induce its own entry into nonprofessional phagocytes by using invasion proteins such as internalin A, internalin B, and P60. Live bacteria delay phagosomal maturation and targeting to the degradative pathway and rapidly lyse the membrane of the acidified phagosome through the action of listeriolysin O (LLO) working in concert with the phospholipases PlcA and PlcB (10, 122, 240, 300, 313, 381). The bacteria then reside freely in the cytoplasm, where they replicate and acquire F-actin-based intracellular motility based on expression of the ActA protein (205). Subsequently, there is invasion of adjacent cells by cell-to-cell spread (326, 381).
Clinical infections with gram-positive rods suggestive of L. monocytogenes were first described in the 1890s in humans (cited by Seeliger [349]) and 1911 in rabbits (by Hülphers [174]). Cases of human meningitis caused by gram-positive diphtheroid bacilli later thought to be L. monocytogenes were identified in 1915 by Atkinson (19), in 1918 by Dumont and Cotoni (99), and in 1919 by Dick (85). A clear association between L. monocytogenes and CNS infection in humans was established in 1945 by Kaplan, who found that meningitis was present in 22 of the first 36 cases reported in the medical literature (184). Clinical reviews in the 1960s by Gray and by Louria et al. supported this association by identifying meningitis and/or meningoencephalitis in 77 and 78% of patients, respectively (145, 229).
Most human infection by L. monocytogenes is acquired by consumption of contaminated food. A recent study estimated that 2,500 invasive L. monocytogenes infections occur in the United States annually (254). Interestingly, this study concluded that L. monocytogenes was responsible for less than 0.1% of all food-borne illnesses but caused 27.6% of deaths attributed to food-borne diseases. L. monocytogenes attacks hosts who are immunocompromised by factors such as pregnancy, the extremes of age, solid and hematological malignancies, and liver disease, as well as chronic illnesses not typically thought of as immunosuppressive such as heart disease and diabetes (140, 344). Pregnancy is the single most common predisposing factor and is associated with approximately 35% of cases worldwide (358). Nonperinatal cases of invasive listeriosis in developed nations have an incidence that ranges from 0.1 to 1.1 cases/105 population, 74% of which are in immunocompromised persons, with an overall mortality rate of 36% (358). Regarding CNS infection, active surveillance in the United States showed that L. monocytogenes is the second leading cause of bacterial meningitis in patients younger than 1 month or older than 60 years (345). In addition, it is frequently isolated throughout the developed world in community-based studies of bacterial meningitis in adults (101, 114, 175, 360).
L. monocytogenes has a striking predilection for invading the CNS in humans. Reviews published in the past 40 years show that CNS infection is present in 28 to 79% of cases of invasive listeriosis in nonpregnant adults (229, 277, 358) and in 13 to 44% of neonates (119, 251, 278, 348, 377). In contrast to extracellular bacteria that commonly cause CNS infection, i.e., S. pneumoniae, N. meningitidis, and H. influenzae, L. monocytogenes infection results in a variety of CNS manifestations including meningitis, meningoencephalitis, rhombencephalitis, cerebritis, and brain abscess. Meningitis and meningoencephalitis are the two most common manifestations in cases not associated with pregnancy (49, 140, 252, 271, 296). The majority of brain abscesses also occur in individuals who are compromised by underlying medical conditions or who are receiving immunosuppressive therapy (77, 102). In contrast, rhombencephalitis, a primary infection of the brain stem, is clearly different since it is found predominantly in noncompromised adults (15, 386). The most common symptoms referable to the CNS are headache and altered sensorium including confusion, lethargy, and coma (44, 140, 215, 271, 296). Seizures and focal neurological findings including cranial nerve palsies are also observed. CSF abnormalities are not diagnostic for L. monocytogenes infection and can manifest either a polymorphonuclear or a mononuclear predominance but are reported to show lower total leukocyte and protein concentrations compared with meningitis caused by extracellular bacterial pathogens (44, 215, 271, 296).
Postmortem pathological findings in the brains of humans include focal hemorrhages and areas of necrosis on a gross level, with purulent meningitis, supperative inflammation with necrosis, microabscesses, and vasculitis with perivascular lymphocytic cuffing and mononuclear cell infiltration of the vessel walls on histological analysis (15, 146). Bacilli are present in the necrotic parenchymal lesions but are not typically found in the perivascular cuffs. Histological findings in sheep and other ruminants are similar to those in humans, with the exception that humans more typically manifest meningitis or meningoencephalitis whereas brain stem encephalitis is more common in ruminants (51, 72). Histological analysis for sheep shows that focal lesions in the parenchyma usually occur around small vessels. The lesions vary in size from 50 to 500 μm, with decreasing numbers of macrophages but increasing numbers of bacteria and neutrophils as they enlarge that ultimately form a microabscess (72). Such microabscesses are most common in the reticular formation of the midbrain, pons, and medulla but also are found elsewhere. In addition to parenchymal lesions, perivascular meningeal infiltrates are present and consist mostly of mononuclear cells with some neutrophils. The infiltrates are often generalized but can be particularly severe basally and in the cerebral and cerebellar sulci. Immunohistochemical analysis of these lesions shows mixed cellular infiltrates consisting of mononuclear phagocytes and neutrophils with relatively few T and B lymphocytes (211). In parenchymal microabscesses and glial nodules, there is evidence of macrophage activation, as shown by the expression of inducible nitric oxide synthase that colocalizes with bacteria (183). In addition, inducible nitric oxide synthase-negative cells that also express major histocompatibility complex (MHC) class II are present in perivascular lesions and could represent infiltrating dendritic cells or transmigrating monocytes that are not yet fully activated. Ultrastructural analysis by electron microscopy of a specimen from a patient with fatal human meningoencephalitis clearly identified intracellular L. monocytogenes free within the cytoplasm of infected macrophages, smooth muscle cells, and endothelial cells (200).
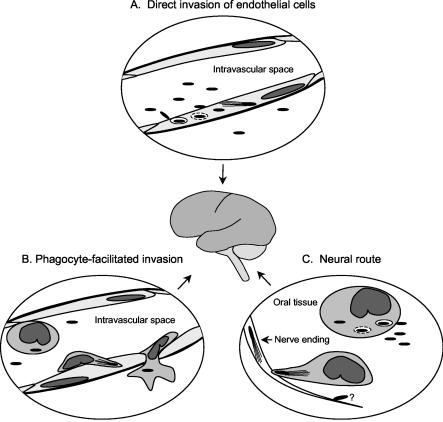
Experimental data indicate that L. monocytogenes can use several different mechanisms to invade the CNS (Fig. 1). Infection of the CNS from the bloodstream could occur by (i) direct invasion of endothelial cells of the blood-brain or blood-choroid barriers by blood-borne bacteria or (ii) transportation of bacteria to the CNS within circulating leukocytes in a phagocyte-facilitated (Trojan horse) mechanism. In addition, a neural route exists whereby bacteria reach the CNS from peripheral tissues by intra-axonal transport.
FIG. 1.
Routes used by L. monocytogenes for neuroinvasion. This cartoon shows a schematic representation of the three different routes used by L. monocytogenes to invade the CNS. It should be noted that the intracellular life cycle of L. monocytogenes, specifically phagosomal escape and F-actin-based motility, is crucial to each case. (A) Direct invasion of endothelial cells. In this scenario, blood-borne bacteria directly invade endothelial cells of the CNS vasculature, possibly via internalin B or other not yet described mechanisms. Bacteria are internalized into phagosomes, which are lysed largely through the action of LLO, and then escape into the cytoplasm and acquire F-actin-dependent intracellular motility requiring the expression of the ActA protein. The bacteria transcytose the endothelium and then invade surrounding cells including neurons and microglia or escape into the subarachnoid space (not shown). (B) Phagocyte-facilitated invasion. Monocytes that are parasitized in the periphery are recruited to the CNS through as yet unidentified signals. Infected leukocytes adhere to endothelial cells of the CNS vasculature, allowing intercellular spread of bacteria from the phagocyte to the endothelial cell. Alternatively, the leukocyte can transmigrate and deliver bacteria to the CNS parenchyma. (C) Neural route. Bacteria are inoculated into the oral tissues when abrasive food is chewed. Tissue macrophages, or blood phagocytes recruited into the infected tissue, phagocytose the bacteria and become parasitized by intracellular bacteria that facilitate the invasion of cranial nerve neurites by cell-to-cell spread. Bacteria move in a retrograde direction through the nerve axons, eventually reaching the CNS, where they continue to spread intercellularly in the parenchyma. Direct invasion of some nerves in vitro has been described, but its role in vivo is unclear.
Invasion by blood-borne L. monocytogenes.
Different lines of evidence support the concept that CNS invasion by blood-borne L. monocytogenes is the predominant route of infection in humans; this evidence includes the high concurrence of bacteremia and CNS invasion in cases of invasive disease and the distribution of histological lesions in the brain. The mouse model of experimental L. monocytogenes infection has provided key insights into how bacteria enter the CNS in vivo. CNS infection as a manifestation of systemic disease develops in mice infected by a variety of routes including intravenous, intraperitoneal, and subcutaneous injections or by introduction of the bacteria into their drinking water (31, 90, 93, 225, 301). In each of these models, the majority of bacteria can be recovered from the liver and spleen, but the bone marrow also is infected (76). Presumably, overwhelming replication of L. monocytogenes in these organs leads to a secondary bacteremia that is composed of cell-free and cell-associated bacteria in an approximately 70:30 ratio (90). CNS invasion follows or coincides with the appearance in the bloodstream of bacteria and parasitized cells and results in histological changes generally similar to those found in humans (31, 226, 301). Data from a gerbil model show that after bacterial infection has been established in the CNS, the majority of bacteria actually reside in the brain parenchyma rather than the CSF (42).
An interesting point in the mouse model is that CNS invasion occurs relatively late in systemic disease, so that animals that succumb early to overwhelming infection die of peripheral organ failure but manifest little in the way of pathological changes in the CNS (18, 72). These experiments suggest that intravenously injected L. monocytogenes organisms are not particularly tropic for the brain endothelium in the same way that they are for other cells, in particular hepatocytes (148). Rather, migration of bacteria into the brain from the bloodstream requires a combination of duration and density of bacteremia, neither of which is fully quantified (31). As a complement to these factors, Autret et al. sought to identify bacterial genes required for brain infection by using in vivo signature-tagged transposon mutagenesis (23). These investigators identified 18 hemolytic L. monocytogenes mutants attenuated for brain invasion. The transposon was mapped to 10 distinct loci, 5 of which corresponded to putative cell wall components including GtcA (five mutants) and YtgP (four mutants), whereas the others represented a variety of cellular processes. This method offers great promise for identifying molecular mechanisms for brain invasion in vivo.
The location where bacteria enter the CNS in experimental infection, as well as the subsequent lesions produced in response to them, is heavily influenced by how the animals are infected and the ensuing systemic illness. For example, the main inflammatory lesions in the CNS following intravenous and subcutaneous inoculation of mice are in the choroid plexus and the ventricles, suggesting that bacteria enter these sites first (31, 225, 301). However, additional studies by López et al. concluded that the meningeal vasculature is the most likely site of bacterial invasion, rather than the choroid plexus, based on the sequence of upregulation of intercellular cell adhesion molecule 1 (ICAM-1) and P-selectin on the cerebral endothelium following subcutaneous injection of L. monocytogenes (226). By comparison, rhombencephalitis is a prominent feature in mice infected by repeated gastrointestinal inoculation or in gerbils that develop chronic, low-density bacteremia secondary to otitis media (7, 8, 42). In contrast, injection of bacteria into the carotid artery of goats produced lesions in the meninges, the ependyma of the cerebral ventricles, and the aqueduct (17). These manifestations are similar to those caused by extracellular bacteria such as S. pneumoniae that produce a high-density bacteremia during clinical infection (419).
Direct invasion of endothelial cells.
Pathological analyses demonstrate that L. monocytogenes can infect human endothelial cells in vivo. In a fatal case of meningoencephalitis, L. monocytogenes organisms were found within vascular endothelial cells, and one bacterium was attached to the luminal surface of a vessel (200). In addition, vasculitis and neutrophilic infiltration of the umbilical vein were identified in a 28-week fetus with congenital listeriosis, suggestive of infection of the endothelium (95). This latter report shows that human umbilical vein endothelial cells (HUVEC), cells that are commonly used in the laboratory but not regarded as in vivo targets, may be infected in naturally occurring disease and elicit neutrophil recruitment as also shown in vitro (91, 212). Nevertheless, widespread vasculitis with readily demonstrable organisms in endothelial cells in the periphery or in the CNS, such as is common in rickettsial diseases, is not common in L. monocytogenes infection. In addition, careful histological analysis of CNS lesions in ruminants showed that perivascular lesions typically do not contain bacteria (72, 183). However, it is possible that endothelial cell infection is an early event, the signs of which become less evident as the disease progresses, and as such is not found on postmortem examination.
In vitro data showing that L. monocytogenes can invade and replicate within endothelial cells were first obtained by using cultured HUVEC as the target cells (94, 151). Later, bacterial infection was also found in primary human brain microvascular endothelial cells (164, 410) and in simian virus 40 transformed human brain microvascular endothelial cells (150). Endothelial invasion requires intact microfilament and microtubule function, as shown by near complete inhibition of invasion by cytochalasin D and nocodazole, respectively (92, 150, 410). Wild-type L. monocytogenes organisms as well as nonpathogenic Listeria species bind and enter endothelial cells in vitro, as shown by gentamicin protection assays (91, 150). L. monocytogenes possesses many adhesins that may play roles in mediating these interactions (50, 135), but most experimental work to date has focused on the role of proteins of the L. monocytogenes inlAB operon in binding to and invasion of endothelial cells. Initial findings using an L. monocytogenes strain with a transposon-induced mutation in inlA suggested a role for internalin A (InlA) in binding to and invading HUVEC under serum-free conditions (94). In apparent contrast, studies using in-frame deletion mutants of L. monocytogenes that lack inlA, inlB or both (ΔinlAB) showed that inlB played a key role in both binding and invasion of HUVEC and transformed human brain microvascular cells (150, 286), whereas other studies showed that inlB promoted invasion but not adhesion (34, 149). The mechanism for triggering invasion is probably due to the interaction of internalin B (InlB) with the hepatocyte growth factor receptor, Met, triggering phosphorylation of this receptor tyrosine kinase (354). Along these same lines, recent data indicate that gene products of the bacterial inlGHE gene cluster suppress InlB-mediated invasion, perhaps by competing for the same receptor (34).
The discrepancies in the experimental evidence showing the importance of InlA versus InlB for endothelial invasion may be caused by several factors. The initial findings supporting a role for InlA could have been compromised by a polar effect of the transposon inhibiting the expression of InlB. Furthermore, different of mutants of L. monocytogenes were used, as well as different endothelial cells and differential experimental conditions. In particular, the presence or absence of serum has a profound influence on the mechanisms of binding of bacteria to these cells (94). This was demonstrated by experiments conducted in the presence of serum that showed InlA- and InlB-independent invasion of HUVEC and primary brain microvascular endothelial cells (BMEC) (151, 410). It is known that antibodies to surface proteins of L. monocytogenes are found in sera from humans following infection with L. monocytogenes and also in normal human sera (128, 208). Recent experiments by Hertzig et al. showed that normal human sera, and particularly the immunoglobulin G (IgG) fraction, strongly inhibited L. monocytogenes invasion of human brain microvascular endothelial cells, a process dependent on InlB in the absence of serum (164). Given that endothelial cells and extracellular bacteria in the bloodstream are continuously bathed by plasma proteins in vivo, these data question whether InlB-mediated mechanisms play a significant role in the establishment of CNS infection in adult humans. Moreover, although newborn serum also demonstrated inhibitory activity, it was approximately 50-fold less potent than the same concentration of adult serum. Concentrations of >1% were not tested, but this finding is consistent with the extreme susceptibility of the neonatal CNS to invasion by L. monocytogenes. The cause of the discrepancy between adult and neonatal serum in the inhibition of binding is not evident because maternal IgG is actively transported across the placenta and into the fetal circulation. At least two possibilities may be considered. First, purified IgG in amounts comparable to a given concentration of serum was not as potent as the serum itself, suggesting that other proteins, e.g., IgA, could also be involved. Second, different IgG subclasses are not transported across the placenta with equal efficiency, and it is possible that the anti-InlB antibodies are of a subtype that does not achieve a sufficiently high concentration in the neonatal circulation (107, 110).
L. monocytogenes invasion of endothelial cells triggers a robust cellular and has recently been reviewed (390). In brief, the NF-κB signal transduction pathway is activated, as are phosphatidylinositol-induced lipid mediator generation and diacylglycerol generation (356, 357). In conjunction, there is increased surface expression of the adhesion molecules P- and E-selectin, ICAM-1, and VCAM-1 (91, 193, 212, 410). Infected endothelial cells also produce IL-6 (151) and the chemoattractants IL-8 and monocyte chemotactic protein 1 (MCP-1) (151, 193). Increased adhesion molecule and chemokine expression leads to increased neutrophil and monocyte adhesion to infected HUVEC and BMEC monolayers (91, 212, 410). A critical role in endothelial cell activation is played by LLO. This is shown by experiments in which purified LLO stimulates endothelial cells while LLO-deficient L. monocytogenes mutants and nonhemolytic Listeria species are significantly less able to trigger adhesion molecule upregulation and mediator release compared to wild-type L. monocytogenes (91, 193). Moreover, transformation of L. innocua with the LLO-encoding hly gene from L. monocytogenes allows these normally nonhemolytic bacteria to produce LLO and also enables them to stimulate endothelial cells in a fashion comparable to that of wild-type L. monocytogenes (193). Recent studies on the mechanism by which LLO activates NF-κB in transfected human embryonic kidney cells (HEK-293) show that LLO activates the IκB kinase complex to phosphorylate and degrade cytoplasmic IκBα (192).
Invasion via infected phagocytes.
The second mechanism for CNS invasion by L. monocytogenes is via infected phagocytes. This is an extension of the Trojan horse model established in studies of neuroinvasion by visna virus in sheep (287) and more recently has been applied to the entry of human immunodeficiency virus type 1 (HIV-1)-infected cells into the CNS of humans (279, 421). The basic concept is that leukocytes are infected in the periphery and then transport intracellular microbes to the CNS and across the blood-brain or blood-choroid barriers. This idea was first applied to a bacterial pathogen when proposed as a means for Streptococcus suis to cross the choroid plexus of swine (407). The likelihood that this mechanism applies to L. monocytogenes-induced pathogenesis derives in part from the facts that these bacteria are so well adapted to the intracellular environment that intracellular parasitism is absolutely required for pathogenesis. The latter point is demonstrated by the finding that L. monocytogenes mutants that lack hly, and thus do not escape the phagosome and replicate intracellularly, are also more than 1,000-fold less virulent in vivo (122). Moreover, intracellular transport of L. monocytogenes from the gut to the lymph nodes by dendritic cells or other phagocytes appears to be involved in the early in vivo dissemination of bacteria following gastrointestinal infection (239, 302). This demonstrates that L. monocytogenes also utilizes host phagocytes for trafficking at another crucial step its pathogenesis.
The notion that parasitized phagocytes are present in the CNS in human disease is supported by the presence of F-actin-coated bacteria within macrophages from a case of human rhombencephalitis (200). Similarly, L. monocytogenes-infected phagocytes were identified in the peripheral blood (90) and the ventricular system of the brain (301) in experimentally infected mice. Differential quantification of cell-free and cell-associated bacteria in whole blood revealed that approximately 30% of bacterial CFU present were isolated with leukocytes (90). In these experiments, more than 90% of cells associated with bacteria were mononuclear cells, suggesting that neutrophils did not participate in dissemination of intracellular bacteria. Further characterization of these cells showed that nearly all of them were CD11b+, supporting their identification as monocytes (D. A. Drevets, P. J. M. Leenen, T. Nikolic, M. J. Dillon, J. E. Schawang, and C. Sunderkötter, Program Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1048, 2003). Moreover, the viability and virulence of cell-associated bacteria was established by showing that infected peripheral blood leukocytes initiated disseminated infection, including CNS infection, when transferred into recipient mice. In addition, bacteria were capable of spreading directly from parasitized peripheral blood leukocytes to endothelial cells in vitro (90). Taken together, these data show that L. monocytogenes-infected cells are present in the peripheral circulation and in the CNS of experimentally infected mice and that cell-associated bacteria retain virulence. Nevertheless, it was not clear whether these cells played a key role in the initiation of CNS infection or were merely present but irrelevant in terms of pathogenesis.
A recent study tested whether leukocyte-associated bacteria could establish CNS infection in the absence of extracellular bacteria. This was accomplished by treating L. monocytogenes-infected mice with a continuous infusion of gentamicin delivered by surgically implanted osmotic pumps (93). Gentamicin is an antibiotic that is rapidly bactericidal against extracellular L. monocytogenes but crosses cell membranes poorly and is not concentrated in the cytoplasm of cells (166). This antibiotic is commonly and effectively used in vitro to kill extracellular L. monocytogenes (see, for example, references 94, 148, and 300), but because intracellular replication and cell-to-cell spread continue unabated, its role in the treatment of human infections is limited to use in combination with other antimicrobials (166). Mice with gentamicin-secreting pumps had serum and plasma gentamicin concentrations that were 16- to 256-fold greater than the MIC for the bacterial strains used for infection (93). Gentamicin-treated animals had 17-fold fewer CFU bacteria in the bloodstream at the time of sacrifice, but essentially all bacteria were cell associated, compared with only 26% in untreated mice. Nevertheless, the quantity of bacteria in the brain was similar in gentamicin-treated and untreated animals. Additionally, brain infection developed in gentamicin-treated mice infected by oral inoculation. Single-cell analysis of blood leukocytes from mice infected with an actA-gfpuv-plcB-transfected L. monocytogenes in which there is a 200 to 500-fold increase of gfpuv expression when bacteria escape from phagosomes to the cytosol (120) confirmed that intracellular bacteria were in fact parasitizing circulating phagocytes. Taken together, these data demonstrate that L. monocytogenes parasitizes circulating phagocytes in vivo and indicate that CNS infection can be established by infected leukocytes in the absence of viable cell-free bacteria.
It is not known whether L. monocytogenes-infected phagocytes are recruited to the CNS by specific or nonspecific mechanisms or whether infected phagocytes have altered migratory characteristics. For the endothelial counterpart in the CNS, it has been shown that it is activated for leukocyte adhesion prior to bacterial invasion as evidenced by upregulation of ICAM-1 on large veins, arterioles, subarachnoid venules, and capillaries and de novo P-selectin expression on subarachnoid vessels and to a lesser extent on parenchymal vessels and in the choroid plexus (226). The mechanism for this endothelial activation involves LLO-dependent triggering of NF-κB nuclear translocation in cerebral vessels (193). In vitro studies show that once infected phagocytes adhere to endothelium, bacteria can invade endothelial cells by cell-to-cell spread in an hly- and actA-dependent process (90, 94, 150). After invading endothelial cells, bacteria presumably continue to spread deeper into the CNS parenchyma, thus causing the characteristic meningoencephalitis of L. monocytogenes. The finding that ΔplcB2 mutants of L. monocytogenes which are deficient in the phosphatidylcholine-specific phospholipase C, but not ΔinlAB mutants have reduced virulence following intracranial injection suggests that cell-to-cell spread rather than direct invasion occurs (341). As an alternative to adhering to and infecting the endothelium, infected phagocytes could transmigrate and enter the brain tissue. In this case, bacteria contained within phagocytes could spread to cells such as neurons and microglia (89).
Invasion by the neural route.
The third route of CNS invasion, infection via neural transport, was explored several decades ago by Asahi et al. These investigators injected L. monocytogenes into the oral membranes of mice and goats or fed the animals with abrasive food that had been soaked in bacterial cultures (18). Using these techniques, 47 (72%) of 65 injected mice and 13 (65%) of 20 mice fed dried boiled fish developed signs of brain stem encephalitis 6 days after infection. The remaining animals either died of systemic disease prior to day 6 (some of these also had CNS lesions at autopsy) or remained asymptomatic. Examination of the brain and cranial nerves revealed mononuclear infiltrations along the length of the trigeminal nerve from the distal end in the lips to the proximal terminus in the medulla, in addition to neutrophilic perivascular cuffing throughout the brain stem. Similarly, injection of bacteria into the lips of goats produced neurological disease in 4 of 11 animals and feeding them with bean shells soaked in bacteria produced symptoms in 2 of 8 animals. Histological analysis also showed lesions similar to those in the mice, in particular a mononuclear infiltrate in the trigeminal nerve from the maxillary and mandibular branches to the medulla, usually with focal meningitis. These pathological finding were very similar to those for naturally occurring cases of L. monocytogenes encephalitis in sheep and goats. These authors concluded that transport of L. monocytogenes from the oral cavity to the brain stem via the trigeminal nerve was the likely mechanism by which CNS disease was established in ruminants.
Some of these conclusions were reconfirmed in a subsequent study by Cordy and Osebold, who found encephalitis in 33% of mice infected by oral scarification (72). They also found that 80% of mice that died early, with a median time to death of 4.9 days, did die of systemic disease without histological encephalitis. In contrast, mice developing encephalitis had a mean time to death of 9.3 days, but fewer than 10% of these mice showed evidence of neuronal involvement. Although the authors did find infected trigeminal nuclei and roots in 4 of 15 field cases of sheep, they suggested that mononuclear infiltrates in the nerve sheaths, including the trigeminal nerve, could have resulted from centrifugal spread of inflammation from the surrounding meninges. Subsequent studies by others using electron microscopy identified intra-axonal bacteria in cases of spontaneous L. monocytogenes encephalitis in sheep (58, 59, 283). This finding was reproduced by experimental inoculation of the L. monocytogenes into the dental pulp of sheep, in which case the animals developed brain stem encephalitis on the same side as the dental infection (28). In addition, recent studies of mice confirmed a direct relationship between the site of neuronal infection and the resulting CNS disease. In these experiments, rhombencephalitis developed ipsilateral to the site of L. monocytogenes injection into the facial muscle and the cut end of the facial nerve, whereas transverse myelitis followed injection into the triceps surae muscle and the cut sciatic nerve (12). Myelitis could be prevented by surgical disruption of the sciatic nerve proximal to the site of bacterial injection. Furthermore, bacteria could be identified in axons and within mononuclear cells in the peripheral nerve fascicles. Taken together, these data clearly indicate that bacterial conveyance along a neural route can ultimately result in brain stem encephalitis or transverse myelitis.
In vitro studies showed that invasion of neurons by cell-free L. monocytogenes is a rare event (290, 291), and until recently it was unclear how L. monocytogenes entered neurons from infected tissue. In vitro studies by Dons et al. compared the infectivity of different types of neurons and showed that this characteristic differs significantly among them (88). They found that cultured rat dorsal root ganglion neurons could be invaded efficiently by internalin-dependent mechanisms whereas hippocampal neurons were resistant to this process. In addition, bacteria were found to move in antegrade as well as retrograde directions. Dramsi et al. also found that rat spinal chord neurons were resistant to direct bacterial invasion (89). However, they showed that these neurons were efficiently invaded via cell-to-cell spread by bacteria from infected phagocytes. Jin et al. subsequently tested the mechanism of cell-to-cell spread of bacteria from phagocytes to neurons in vivo. These investigators compared the bacterial loads in the trigeminal ganglion and brain of RAG-1-deficient mice injected in the snout with wild-type L. monocytogenes or with ΔplcB2 mutants, bacteria which are impaired in phagosomal escape and cell-to-cell spread (182). The results showed that mice infected with ΔplcB2 mutants had a significantly longer survival time and lower bacterial counts in the trigeminal ganglion and brain than did mice infected with wild-type bacteria, even though equivalent bacterial loads had been injected into the snouts of the two groups. In addition, bacteria were identified within MHC class II-expressing cells, presumably tissue histiocytes, in the snout by immunofluorescence microscopy. The authors interpreted these data to indicate that phagocytes, not neurons, are the first cells infected in the snout injection model and that intercellular spread between the infected phagocytes and neurons was important for initiating CNS infection. This interpretation was supported by a second study in which macrophage colony-stimulating factor-deficient op/op mice were infected in the snout and then the bacterial loads in the trigeminal ganglion and in peripheral organs were compared with those in op/+ controls (181). The results showed that op/op mice had fewer bacteria in the brain despite being more susceptible to systemic infection. The finding that op/op mice developed CNS infection at all could be explained by systemic spread of bacteria or by transfer via macrophage colony-stimulating factor-independent macrophages present in the tissues (273).
Taken together, these data provide a clear picture of certain events in the neuronal route of CNS invasion. Bacteria inoculated into the oral tissues are initially taken up by tissue macrophages and/or by mononuclear phagocytes that are rapidly recruited from the circulation. Some phagocytes are parasitized, and bacteria from them invade, presumably from cell-to-cell spreading, the distal ends of nerves that form branches of the trigeminal ganglion. Bacteria move in a centripetal fashion through the trigeminal ganglion into the brain stem. It is not clear if this mobility requires the L. monocytogenes actin-polymerizing machinery, since inert beads also can be transported within axons (403). Once bacteria arrive in the brain stem, encephalitis is established by continued cell-to-cell spread. This mechanism of infection is clearly important in ruminants, which may chew material coarse enough to abrade the oral mucosa and in which L. monocytogenes-contaminated silage has been linked to epidemics of encephalitis (230, 389). However, the relevance of the neuronal route in humans is less clear because most of the foodstuffs linked to human diseases, e.g., salads (22, 334), meat products (57a, 347), jellied pork tongue (49, 141), milk (119), and cheeses (179, 224), do not have the abrasive qualities required to inoculate submucosal tissues with sufficient numbers of bacteria to cause disease. Whether gingivitis or dental caries could be a contributing factor that enables this mechanism to operate in humans is not known.
Brucella Species
Brucellae are small, nonmotile, and non-spore-forming gram-negative coccobacilli. They are generally considered to be facultative intracellular parasites of professional and nonprofessional phagocytes. However, recent data indicate that Brucella spp. are better adapted for intracellular survival than in the extracellular environment and, as such, should be thought of as facultative extracellular pathogens rather than the converse (266). The intracellular niche of virulent brucellae is within membrane-bound endosomes that differ somewhat between professional and nonprofessional phagocytes. In phagocytic cells, the Brucella-containing phagosomes acidify but do not fuse with lysosomes, so that the bacteria reside in and replicate in specialized endosomes recently called “brucellosomes” (14, 207, 325). By comparison, in nonprofessional phagocytes, early endosomes that contain Brucella enter the autophagocytic pathway and bacteria replicate within autophagosome-like compartments in the endoplasmic reticulum (294, 295). Recent data show that the VirB operon, a type IV secretion pathway that is induced on phagosomal acidification, plays a key role in intracellular parasitism and is essential for pathogenicity (280, 359). It is hypothesized that this system secretes effector molecules that alter the host cell endosomal pathways to promote the formation of an endoplasmic reticulum-derived compartment that is conducive to the replication of Brucellae rather than to fusion with lysosomes in a degradative pathway (57, 258).
There are six nomen species in the genus Brucella; however, human disease is caused predominantly by B. abortus, B. melitensis, and B. suis (70). In addition, two cases of human neurobrucellosis were reported recently that were caused by a Brucella sp. closely related to B. pinnipediae, a bacterium found in marine mammals (365). The history of brucellosis is interesting in that bone lesions consistent with Brucella infection have been identified in 17.4% of adult skeletal remains from Herculaneum, a Roman town covered by the Mount Vesuvius eruption in 79 AD (52). In more modern times, systemic infection caused by Brucella spp. was referred to as Malta fever or undulant fever, and its etiologic agent was isolated from humans and identified in 1886 as Micrococcus melitensis (46). A wide variety of acute and chronic CNS infections caused by Brucella spp. were recognized soon thereafter (78).
Pathogenic brucellae are found worldwide, and the incidence of clinical disease in humans varies greatly. It has been nearly eradicated from some countries, but in areas of endemic infection such as Peru, Kuwait, and Saudi Arabia, there are more than 200 cases/100,000 population (69). Human infection typically is acquired by ingestion of unpasteurized milk or cheese or through occupational exposure to infected animals, in particular sheep, goats, swine, camels, and cattle (16, 47, 69, 103, 233, 269). There is no obvious predilection for individuals with underlying diseases (45). The frequency with which Brucella attacks the CNS is relatively low, from 1.3 to 7% of total cases in recent series, and neurobrucellosis is found much less commonly in children than in adults (45, 157, 233, 253). The mortality rate of neurobrucellosis in the postantibiotic era is 0 to 5.5%, but permanent neurological deficits, particularly deafness, are common (27, 253, 270).
Signs and symptoms referable to CNS involvement typically include headache with or without meningeal irritation and can occur early in the course of brucellosis or more than 1 year after the onset of systemic symptoms (27, 253, 270, 276, 353). Neurobrucellosis can result in a multitude of nervous system manifestations that can only be summarized here. The most common presentation is as a typical meningitis or meningoencephalitis that has an acute onset and can occur either as the only site of infection or in the context of systemic disease (45, 253, 353). Patients with acute infection can have cranial nerve palsies that usually resolve completely with antibiotics, whereas those with chronic CNS infection often have permanent neurological deficits. The various chronic manifestations are perhaps best divided into those presenting with peripheral neuropathy or radiculopathy and those presenting with more diffuse CNS involvement that includes myelitis with cranial nerve involvement and a syndrome of parenchymatous dysfunction (253, 353). Symptoms of the peripheral neuropathy and radiculopathy include back pain, areflexia, and paraparesis with involvement of the proximal nerve radicals. In patients with diffuse CNS involvement, myelitis is evidenced by back pain, spastic paraparesis, and demyelination and can also occur with cerebellar dysfunction. The syndrome of parenchymatous dysfunction can occur at any location in the CNS but most commonly affects the cerebellum, spinal cord, and cerebral white matter. Meningovascular complications, in particular mycotic aneurysms, ischemic strokes, and subarachnoid hemorrhage, are relatively common (45, 118, 159, 253).
Examination of the CSF typically reveals an elevated protein concentration, a depressed glucose concentration, and a moderate leukocytosis composed mainly of lymphocytes (27, 253, 270, 353). The exception is the cerebellar syndrome, in which the protein concentration is elevated but there is no leukocytosis. Cultures of CNS tissue and fluid are frequently sterile; however, bacteria are occasionally recovered from the CSF and from the brain granuloma. Pathological changes of the meninges are almost always found. They are typified by diffuse involvement manifested by thickening due to acute and chronic inflammatory cell infiltration as well as connective cell proliferation that is sometimes more prominent in the basal areas (118, 276). Granulomatous inflammation of the meninges and of the brain parenchyma is also found and can be accompanied by central necrosis (276, 365). In the spinal cord, there is chronic inflammatory cell infiltration into the parenchyma and the overlying arachnoid membrane as well as areas of demyelination. Vascular inflammation is present and ranges in character from minimal and mononuclear to massive and neutrophilic; it can lead to significant adventitial thickening, necrosis, and the formation of aneurysms. Furthermore, there is inflammation of the perineurium of nerve roots in the peripheral nervous system that probably accounts for the radiculopathies (253, 276).
The precise mechanisms by which brucellae enter the CNS are not known. The extreme degree to which brucellae are adapted to the intracellular environment suggests that phagocyte-facilitated infection is a likely mechanism. Consistent with this, it has been shown that B. suis prevents apoptosis of infected and uninfected human monocytes in vitro and that monocytes and lymphocytes taken from Brucella-infected patients are resistant to spontaneous apoptosis and to apoptosis triggered by anti-Fas monoclonal antibody (152, 382). This would prolong the life span of infected cells and in theory would give more opportunity for them to enter the CNS. In addition, brucellae can invade nonprofessional phagocytes, and so it is likely that endothelial cell invasion could be another possible route for CNS infection; however, this has not been studied. In terms of potential mechanisms for entry into nonprofessional phagocytes, a two-component regulatory system, BvrR-BvrS, that is important for invasion and intracellular parasitism has been identified in B. abortus (366). Transposon-induced inactivation of bvrR-bvrS inhibits the invasion of mouse peritoneal macrophages and HeLa cells, and mutants that are internalized do not inhibit phagolysosome fusion. Moreover, BvrR-BvrS mutants are avirulent in mice. Further studies showed that this system regulates the expression of B. abortus outer membrane proteins, in particular Omp3a (formerly known as Omp25) and Omp3b (155). Omp3a/Omp25 probably plays an important role since mutants of B. abortus, B. melitensis, and B. ovis that lack this protein are less pathogenic in mice, cattle, and goats (104-106). Internalization of Brucella spp. by cells occurs by a zipper-like mechanism involving as yet unidentified cellular receptors, in which sialic acid-mediated recognition by a bacterial lectin could play an important role (79). Internalization of bound bacteria requires recruitment of F-actin and, in nonprofessional phagocytes, activation of GTPases of the Rho subfamily (154, 325).
Mycobacterium tuberculosis
M. tuberculosis is a member of the M. tuberculosis complex along with M. africanum, M. bovis, and M. microti (121). The characteristic staining quality of these organisms is their ability to resist decolorization of carbol fuchsin by acid-alcohol in the Ziehl-Neelsen process due to the high lipid content of the cell wall. M. tuberculosis is an aerobic, nonmotile, non-spore-forming bacillus that also stains weakly gram positive. The main cellular reservoirs of these organisms in the host are tissue macrophages, although the organisms also exist extracellularly. There are numerous macrophage receptors capable of binding M. tuberculosis that can participate in its internalization, including CD11b, CD14, the macrophage mannose receptor, scavenger receptor A, Toll-like receptor 4, and CD44 (reviewed in references 36, 108, 219, 292, and 392). Intracellular parasitism is accomplished by a variety of mechanisms that include altered trafficking of bacteria during endocytosis, interference with host Ca2+ signaling pathways, and induction of maturational arrest of the phagosome (reviewed in references 82, 293, and 332). The result is that bacteria reside in a membrane-bound compartment which has an elevated pH and does not contain mature lysosomal enzymes.
Tuberculosis is a disease whose history and impact on humanity are impossible to underestimate (331). Morphological and molecular evidence of M. tuberculosis infection has been found in the fossilized remains of Pleistocene era bison that lived approximately 18,000 years ago in North America (329) and in human mummies from ancient Egypt and pre-Columbian Peru (209, 336, 420). Green published the first description of tuberculous meningitis as a specific entity in 1836 (147), approximately half a century prior to Koch's discovery in 1882 that M. tuberculosis was the causative agent of human tuberculosis (204). In the vast majority of human cases, tuberculosis is acquired through inhalation of bacillus-containing droplets into the lungs (reviewed in reference 26). Bacilli are then transported to draining lymph nodes, and the ensuing inflammatory response eventually leads to the formation of the characteristic granuloma and to activation of a type 1 T-helper cell-mediated immune response. During the early stage of infection prior to the development of a containing response, there is a low-titer bacteremia in which bacteria disseminate to distant sites. Active tuberculosis develops when containment fails so that bacillary replication and caseous necrosis occur in preexisting granulomas (332). Pulmonary involvement is the predominant form of clinical tuberculosis and usually accounts for 80 to 90% of cases, depending on the era in which the data were collected. Extrapulmonary tuberculosis, i.e., disease outside the lungs, can be found in nearly every organ system. In the pre-AIDS era, extrapulmonary tuberculosis was found in 10 to 20% of cases of active tuberculosis. Meningitis was the predominant feature in 5 to 8% of extrapulmonary cases, so that the overall estimation of CNS infection was approximately 1% of active cases (9, 115, 195a). The presence of HIV-1-induced immunodeficiency, however, is a powerful cofactor for the development of disseminated tuberculosis, and therefore the proportion of extrapulmonary cases has increased (for reviews, see references 156 and 352). Nevertheless, whether HIV-1 coinfection also increases the occurrence of meningitis is not completely clear. In a study of 2,205 patients diagnosed with tuberculosis, Berenguer et al. identified meningitis in 10% of HIV-1-coinfected patients compared with 2% of non-HIV-1-coinfected individuals (32). Supporting this finding of an increased prevalence of meningitis, an autopsy study from Kenya found meningeal involvement in tuberculosis in 26% of HIV-infected adults (305). However, other studies have reported that HIV-1-infected and uninfected patients have similar rates of CNS tuberculosis (352, 374). Current mathematical models suggest that tuberculosis caused 8.3 million new cases worldwide in 2000 (71). Hence, although the exact incidence of tuberculous meningitis is unknown, these data imply that M. tuberculosis is the most common cause of CNS infection by an intracellular bacterium.
CNS infection by M. tuberculosis occurs in individuals of any age. Large autopsy series of patients dying of tuberculosis identified CNS involvement in 19.3 to 42.2% of pediatric cases compared with only 2.9 to 5.9% of adult cases (21, 234, 321). Most cases in children occurred between the ages of 6 months and 4 years, whereas adult cases clustered in patients aged 20 to 50 years. In addition to HIV-1 coinfection, underlying conditions associated with tuberculous meningitis include recent measles infection and malnutrition in children (416) and alcoholism, malignancies, and immunosuppressive medications in adults (202, 281, 393). In terms of prevention of CNS infection, M. tuberculosis is exceptional in that it is the only intracellular bacterium for which a vaccine, i.e., BCG, is available that has a protective effect against meningitis (327). Nevertheless, the salutary effect of BCG vaccination against tuberculous meningitis is found only in children, not in adults, and some authors suggest that it merely delays the age of onset of meningitis.
M. tuberculosis infection of the CNS has several manifestations, including involvement of the meninges, space-occupying lesions in the brain parenchyma, and focal disease of the spinal chord and its osseous structures (for reviews, see references 123, 265, 369, and 380). Meningitis is the most common form of CNS disease and is discussed in depth here. The typical presenting signs and symptoms include fever, headache, and altered mental status, ranging from lethargy to coma (136, 188, 281, 298, 393). Meningismus, seizures, and focal neurological signs, in particular cranial nerve palsies, are also common. Spinal fluid analysis usually reveals an elevated protein concentration, hypoglycorrachia, and a moderately (typically <500 cells/mm3) increased leukocyte count with lymphocyte predominance. Nevertheless, patients with normal CSF or CSF that shows a neutrophil predominance are occasionally found; most of these individuals have impaired host defenses (188, 214). Interestingly, most series comparing tuberculous meningitis in patients with and without HIV-1 infection do not find striking differences between the two groups of patients in the clinical features of the disease or in the CSF analysis (32, 188, 190, 346, 383). In contrast, HIV-1-coinfected individuals do show more frequent abnormalities such as space-occupying lesions or hydrocephalus on brain-imaging studies. Analysis of cytokines and chemokines in the CSF of patients with tuberculous meningitis shows elevated levels of IL-1β, TNF-α, IL-8, MCP-1, and macrophage inflammatory protein-1α (MIP-1α), in amounts that are usually below those found in patients with bacterial meningitis but remain elevated for a much longer period after treatment (4, 245, 246, 417). Acid-fast bacteria have been recovered from the CSF in as many as 91% of centrifuged specimens (370), but recovery is usually much lower than that due to low concentrations of bacteria in the CSF. The mortality rate of tuberculous meningitis is substantial, and survivors often have permanent neurological deficits. Untreated patients usually die within 3 weeks of the onset, but effective antituberculosis treatment had lowered the mortality rate to 10 to 20% in the pre-AIDS era (265). By comparison, more recent studies report mortality rates ranging from 40 to 70% (137, 188, 298). A correlation of higher mortality with HIV-1 coinfection is sometimes present (190, 383).
The pathological features of tuberculous meningitis have been extensively reviewed and offer the best understanding to date of how the CNS becomes infected (20, 33, 74). A thick, gelatinous exudate covers the surface of the brain and is most abundant on the basilar surface in the optic chiasm and within the ventricles. Dilatation of the ventricles is common, due in part to obstruction of the normal CSF flow by the protein-rich exudate. The fibrinous exudate contains a variety of cells including erythrocytes, neutrophils, and macrophages in early lesions, whereas lymphocytes are more common in older lesions. In the gelatinous exudate, there are focal areas of caseous necrosis with surrounding epithelioid cells and giant cells. The inflammatory process in the meninges typically extends through the perivascular spaces into the cortex, resulting in foci of encephalitis properly described as a diffuse meningoencephalitis. The critical lesions in terms of pathogenesis are called “Rich foci” and were first described in the early 1930s (318). These are small nodules, about 1 mm in diameter, which follow a vascular distribution. They are found most frequently in the meninges but also are present in the ependyma and the brain parenchyma. Interestingly, Rich foci are not found more frequently in the basilar areas of the brain than in other locations, and the localization of the exudate to this area is thought to be due to the normal pattern of CSF flow (234, 318). Microscopically, there is an accumulation of inflammatory cells in the subendothelial region of the intima of the blood vessel, with formation of a nodule in the adventitia. A detailed study of blood vessels in tuberculous meningitis by Winkelman and Moore suggested that the earliest lesions begin in the subendothelium with an infiltration of lymphocytes, monocytes, and plasma cells (411). As the lesion progresses, there is cellular proliferation with displacement of the intact endothelium with or without tuberculoma formation. In later stages, the vessel is completely involved with a contiguous inflammatory process that began within it or was the result of direct extension of inflammation from the subarachnoid space into the vessel. This process can lead to complete occlusion of the vessel.
Early investigators confirmed that there was blood-borne dissemination of M. tuberculosis. Data first reported by Villemin in 1868, and subsequently confirmed by others, showed that injection of blood from experimentally infected animals, or from human patients, transmitted tuberculous infection to recipient animals (66). However, until the pioneering work of Rich and McCordock in 1933, it was thought that tuberculous meningitis was the “direct and immediate result” of hematogenous spread of bacilli during acute miliary tuberculosis (234, 318). These investigators showed that intravenous and intra-arterial injection of large amounts of the virulent H37RV strain of M. tuberculosis into rabbits and guinea pigs did not produce meningitis or meningeal lesions despite the presence of widespread visceral miliary disease (reviewed in reference 21). In addition, autopsy studies of adult humans showed that meningitis also occurred in the absence of miliary involvement elsewhere and that miliary tuberculosis did not always lead to meningitis or even to frequent tuberculous lesions in the CNS vasculature (318). These observations led to the hypothesis that hematogenous dissemination of bacilli from primary foci caused vascular lesions in the meninges and elsewhere but this event was temporally separated from bacillary invasion of the CSF and meningitis. The latter events happened when a vascular lesion ruptured and subsequently introduced bacteria into the CSF. This hypothesis was supported by the identification of CNS vascular foci (Rich foci) in 87 to 94% of patients with tuberculous meningitis (21, 234, 318), as well as in individuals dying of tuberculosis who did not have histological evidence of meningitis (234). Subsequent experiments showed that direct inoculation of acid-fast bacteria into the CSF could replicate many of the pathological findings of natural cases, and that TNF-α is a key proinflammatory cytokine once bacteria enter the CSF (318, 385). Recently, this sequence of events has been demonstrated in experiments by Bolin et al., using swine infected intravenously with M. bovis (43). In these experiments, three of six pigs developed meningitis 17 to 38 days after infection. Postmortem examination of the brains identified granulomatous lesions, some of which also contained acid-fast bacteria, in the walls of meningeal blood vessels. These data substantiate the central hypothesis that Rich foci develop soon after hematogenous dissemination and offer a new animal model for in vivo studies of the pathogenesis of tuberculous meningitis.
The mechanisms used by M. tuberculosis to disseminate and enter blood vessels to establish Rich foci is not completely understood. In theory, hematogenous dissemination of cell-free bacteria and dissemination of bacteria within phagocytes are both possible. With regard to the CNS invasion by cell-free bacteria, data from human autopsies and animal experiments cited above suggest that the vast majority of cell-free bacteria that enter the bloodstream, either from erosion of caseous foci into the vascular or lymphatic systems or by experimental injection, are removed by visceral organs. Direct interactions between mycobacteria and endothelial cells are not well characterized. By comparison, recent data indicate that trafficking of intracellular bacteria by phagocytes could play a key role. For example, experimental data show that alveolar macrophages and newly recruited monocytes are important in transporting mycobacteria from the alveolar spaces to the draining lymph nodes (37, 41, 378) and that dendritic cells also may participate in this process (126, 180, 375). In addition, data from intratracheally infected mice show that mycobacteria enter microfold cells (M-cells), specialized antigen-sampling cells that overlie the mucosa-associated lymphoid tissues that line the bronchial epithelium. The bacteria are then transported to draining lymph nodes by macrophage colony-stimulating factor-dependent cells, i.e., monocytes and most macrophages (378). Once mononuclear phagocytes are infected, a number of physiological and phenotypic changes occur that could alter their cellular trafficking. For instance, infected monocytes and macrophages show increased expression of surface ICAM-1 (227), increased migratory activity across epithelial and endothelial bilayers (37), and activation of intracellular signaling pathways and secretion of proinflammatory cytokines (29).
Salmonella Species
Salmonella spp. are gram-negative, facultative anaerobic, motile, non-lactose-fermenting, non-spore-forming bacilli measuring 2 to 3 by 0.4 to 0.6 μm in size. All Salmonella isolates are currently classified as S. enterica, which is subdivided into more than 2,300 serovars according to somatic O, surface Vi, and flagellar H antigens. For historical reasons and convenience, serovar names are often used as species names. Salmonellae are facultative intracellular bacteria that can invade and replicate within many different cells in vitro. Nevertheless, in vivo data show that intracellular survival of bacteria within macrophages is essential for virulence in mice (117) and that the preferred in vivo niche for Salmonella is within CD11b+ macrophages in the liver and the spleen rather than in the extracellular milieu (247, 319, 335). Intracellular Salmonella organisms replicate within a spacious phagosome, a modified, acidified endosome (6). This is due largely to the actions of virulence genes of the Salmonella pathogenicity island 2 type III secretion systems and the PhoP-PhoQ two-component regulatory system (reviewed in reference 168).
The genus is named after the U.S. veterinary surgeon Daniel E. Salmon, who first isolated S. enterica serovar Choleraesuis from porcine intestine in 1885. Ghon (134) and Cole (68) were the first to recognize human Salmonella meningitis in the first decade of the 20th century. S. enterica serovars Typhi and Paratyphi cause typhoid and paratyphoid fever, respectively, and colonize only humans. Therefore, they are acquired either by close contact with a person who is colonized or infected with these organisms or, more commonly, by ingestion of food or water contaminated by the excreta of such a person (262). It is estimated that there are at least 16 million new cases of typhoid fever globally each year, with 600,000 deaths (177). While it was a scourge of European and U.S. urban areas in the 19th century, most of the burden of typhoid fever is now in the developing world. The annual incidence of typhoid fever in an urban area of Kalkaji, New Delhi, India, has recently been reported as 980 per 100,000 (364). Incidence peaks in 5- to 12-year-old children, but children aged 1 to 5 years are also commonly infected; although less frequently infected, children younger than 1 year experience more severe disease (364). In the developed world, typhoid fever is largely a disease of travelers returning from areas of endemic infection, with occasional point source epidemics (3).
In contrast to S. enterica serovars Typhi and Paratyphi, nontyphoidal Salmonella (NTS) is a prime source of common gastroenteritis. It is widely dispersed in nature, including the gastrointestinal tracts of domesticated and wild mammals, reptiles, birds, and insects. In humans, NTS infections are most often associated with consumption of inadequately prepared food products of animal origin, including meat, poultry, eggs, and dairy products, although a variety of other foods have been implicated. In the United States, the annual incidence has doubled in the last 20 years. There are an estimated 1.4 million cases yearly, resulting in an estimated 16,430 hospitalizations and 582 deaths (254). Globally, by far the most common group with NTS illnesses are children younger 5 years (142). Host conditions associated with an increased risk of salmonellosis include gastric hypoacidity, extremes of age, alteration of the endogenous bowel flora, diabetes mellitus, malignancy, rheumatological disorders, reticuloendothelial blockade (sickle cell disease, malaria, or bartonellosis), and therapeutic immunosuppression (167). In addition, patients with HIV infection have an estimated 20- to 100-fold increased risk of salmonellosis and are more likely to have severe invasive disease (262).
Enteric fever (typhoid or paratyphoid fever) is a severe systemic illness characterized by abdominal symptoms and fever. After an incubation period of 5 to 21 days, diarrhea of several days' duration may develop and typically resolves before the onset of fever. Nonspecific symptoms such as chills, diaphoresis, headache, anorexia, cough, weakness, sore throat, dizziness, and muscle pains are frequently present before the onset of fever. In addition to headache, CNS manifestations of enteric fever in the form of neuropsychiatric manifestations from encephalitis, including confusion and psychosis, occur in 5 to 10% of patients. S. enterica serovar Typhi meningitis has been reported but is very rare (67, 220). Mortality was 15% in the preantibiotic era but is now less than 1% in the United States (262). Gastroenteritis caused by NTS is not clinically distinctive. After an incubation period of 6 to 72 h (depending on the inoculum and host status), patients typically present with acute onset of fever, diarrhea, and abdominal cramping (167). There is a wide spectrum of severity of illness. Approximately 5% of patients with gastroenteritis develop bacteremia, and <1% have focal infections such as osteomyelitis, soft tissue infection, urinary tract infection or, endocarditis (262).
CNS infection is present in approximately 0.1 to 0.9% of cases (167). CNS infection by NTS is primarily a disease of children, particularly neonates and infants (218, 418). Only about 10 cases of Salmonella meningitis have been reported in adults (186, 220). In a recent review of 144 cases of NTS bacteremia in children, the median age was 10.5 months, and only 3 (2.1%) developed meningitis (418). In general, presenting symptoms and signs of Salmonella meningitis are fever (73%), meningismus (51%), vomiting or anorexia (43%), seizures (43%), lethargy or coma (34%), and irritability (12%) (the percentages are frequencies) (67). CSF findings include a neutrophilic pleocytosis, elevated protein concentration, hypoglycorrhachia, and, in 75%, a positive Gram stain (67). In a review of 13 infants with Salmonella meningitis, serious complications were common. In this series, 69% of infants had seizures, 38% developed hydrocephalus, whereas subdural effusions and empyema were present in 31 and 23%, respectively. Overall mortality in the last 40 years has ranged from 0 to 59% (13, 171, 191, 218, 231, 256). Neurological complications, including mental retardation, cerebral palsy, and visual and hearing impairment, have been found in 13 to 62% of survivors. In addition to typical meningitis, focal intracranial infections with NTS can occur with or without meningitis. Principal among these are subdural empyema and brain abscess (178, 213, 232, 236, 328, 337, 404).
It is unknown whether salmonellae invade the CNS primarily as cell-free or intracellular bacteria. With regard to direct interactions of bacteria with endothelial cells, in vitro data showed that smooth, wild-type S. enterica serovar Minnesota was poorly adherent to bovine pulmonary endothelial cells compared with a rough mutant and that phagocytosis of the latter bacterium by the endothelial cells was induced by opsonization with C1q (333). However, interactions between NTS and endothelial cells remain a poorly studied phenomenon. Trafficking of Salmonella-infected phagocytes is a likely but as yet unproven mechanism for CNS infection. In terms of trafficking of infected phagocytes from the blood into other anatomic spaces, monocytes bearing Salmonella antigens show increased binding to synovial endothelium and transendothelial migration, and Salmonella antigens have been identified within synovial cells in patients with reactive arthritis (143, 201). However, this does not necessarily indicate that infected cells may go elsewhere, in particular into the CNS. Nevertheless, recent descriptions of the mechanisms by which leukocytes transport Salmonella across the gastrointestinal epithelium do provide a useful example for how phagocytes can transport bacteria across an epithelial barrier. Vazquez-Torres showed that CD18+ phagocytes transported S. enterica serovar Typhimurium mutants that lacked invasion genes across the gut epithelium in a process that bypassed both epithelial cells and M-cells (391). More recent studies showed that CD11b+ CD8α− dendritic cells are rapidly recruited to the gut epithelium in the presence of intraluminal Salmonella (316, 317). These dendritic cells emerge between the epithelial cells by interacting with the tight-junction proteins claudin-1 and zonula occludins-1 and then extend dendrites into the lumen without disrupting the integrity of the epithelial lining (317). Thus, dendritic cells that sample luminal contents directly can internalize bacteria and then transport them to lymphoid tissues (169, 316, 317).
OBLIGATE INTRACELLULAR PATHOGENS
Rickettsiaceae
Human pathogens of the family Rickettsiaceae are obligate intracellular parasites of professional and nonprofessional phagocytes that invade the CNS as part of a systemic infection. They are for the most part arthropod borne and have a limited capacity to survive outside a host. The notable exception is Coxiella burnetii, an organism that does not have an arthropod intermediate host. It is efficiently transmitted via aerosol and survives well in the environment outside the host. This family of pathogens also varies with regard to their cellular targets and intracellular niches. The Rickettsiaceae can be divided into three categories according to their targets during natural infection: (i) organisms that parasitize vascular endothelial cells, (ii) organisms that parasitize phagocytes, and (iii) organisms that parasitize both endothelial cells and phagocytes (Table 2). The first category includes members of the R. rickettsii group, R. rickettsii and R. conorii, and the typhus group, R. prowazekii and R. typhi (350). The organisms that principally target phagocytes are E. chaffeensis, Anaplasma (Ehrlichia) phagocytophila, and C. burnetii. By comparison, only Orientia tsutsugamushi is routinely found in endothelial cells as well as in circulating phagocytes. In terms of their intracellular niches, O. tsutsugamushi and the rickettsiae lyse the phagosome and replicate predominantly in the cytoplasm of the host cell, whereas E. chaffeensis, A. phagocytophila, and C. burnetii remain in specialized vacuoles.
TABLE 2.
Key parameters of intracellular parasitism and CNS invasion by members of the Rickettsiaceae
| Microbe | Intracellular niche | Cellular target in vivo
|
Predominant CNS manifestation | |
|---|---|---|---|---|
| Endothelium | Leukocyte | |||
| R. rickettsii | Cytoplasm | × | Encephalitis | |
| R. prowazekii | Cytoplasm | × | Encephalitis | |
| O. tsutsugamushi | Cytoplasm | × | × | Meningoencephalitis |
| C. burnetii | Phagosome | × | Meningitis and meningoencephalitis | |
| E. chaffeensis | Phagosome | × | Meningitis | |
Riskettsia spp.
Rickettsiae are small (0.3 by 1 μm) gram-positive coccobacilli that are divided into the R. rickettsii and typhus groups, with R. rickettsii and R. prowazekii, respectively, being the prototypes of these groups. Rocky Mountain spotted fever (RMSF) is the clinical entity caused by R. rickettsii. The first identified case of RMSF was thought to have occurred in 1873 in the Bitter Root Valley in Montana, and by the end of the 19th century, Wood (1896) and Maxey (1899) described this disease as an entity occurring predominantly in Idaho and Montana (cited in references 161 and 409). More recently, however, RMSF has been identified in 42 states, with North Carolina and Oklahoma reporting the greatest number of cases during the period from 1993 to 1996 (384). The etiologic agent of the disease was identified in 1909 by Howard Ricketts, who found R. rickettsii in the eggs of ticks and in blood from infected humans and demonstrated its transmissibility from ticks to guinea pigs (320).
RMSF is a systemic disease that typically manifests as fever, headache, myalgias, gastrointestinal disturbances, and severe dysfunction of multiple organ systems (379). Headache is the most common CNS symptom and is one of the most consistent clinical findings in RMSF. This was noted in an early report by Wilson and Chowning (409), and headache is present in 38 to 93% of cases reviewed by others (260, 379). The presence of headache is not pathognomonic of actual CNS invasion by the organism. However, less frequent symptoms such as lethargy, confusion, stupor, and coma and physical signs including meningismus, focal neurological deficits, and behavioral changes consistent with encephalitis are also present and indicate actual brain involvement. CSF abnormalities are often present but usually are not as impressive as those found in bacterial meningitis (160, 261). In one series, 27 of 33 patients with RMSF had a modest (<50 white blood cells) mononuclear pleocytosis, 28 of 33 showed small but abnormal numbers of erythrocytes, and 7 of 18 had an elevated CSF protein concentration (160). By comparison, boutonneuse fever, also known as Mediterranean spotted fever or tick typhus, which is caused by R. conorii, tends to result in fewer CNS findings and also has a lower case fatality rate than RMSF. For example, headache was reported as present in only 15% of 645 pediatric patients from Sicily, and only 2 children had lymphocytic meningitis (55).
R. prowazekii, the etiological agent of louse-borne epidemic typhus, is the prototype of the typhus group of the genus Rickettsia and was identified in the early 20th century by Rocha-Lima (cited in reference 127). This disease has a long history and is typically associated with war, mass movements of people, especially refugees, and squalid living conditions (422). Humans are the main reservoir for the disease, and the role of the body louse in its transmission was discovered by Nicolle in 1909 (153, 309). Like RMSF, typhus is a systemic disease that can be quite severe and is frequently fatal. A severe headache is nearly invariably present in patients with typhus and has been used as a key clinical criterion for identifying suspected cases during epidemics (127, 288, 308). More severe CNS features such as neck stiffness, neurological weakness, seizures, delirium, and coma are reported in 3 to 78% of cases (127, 288, 308, 310). Delirium and coma were reported in 35 and 39% of fatal cases, respectively (187). Another member of the typhus group, R. typhi, is the etiologic agent of murine typhus. This is a less severe disease than epidemic typhus, and symptoms of severe CNS disease such as seizures, stupor, and ataxia are infrequent, being found in <5% of patients in one study (98); meningitis was reported in only 3 of 137 patients in a different study (361). Despite the low prevalence of CNS symptoms, R. typhi has been demonstrated by immunofluorescence in the brain of an elderly woman who died of murine typhus, indicating that this organism has the potential for CNS invasion (398).
Pathological involvement of the brain is common in fatal cases of rickettsial diseases, with lesions being found in 14 of 15 brains from patients with RMSF (160, 261) and in 63 of 66 brains from patients dying from epidemic typhus (223, 415). Moreover, histological studies have confirmed rickettsial invasion of vascular endothelial cells in the brains of humans (396, 398) and experimentally infected mice (399, 400). Characteristic CNS lesions in rickettsial infections are termed typhus nodules; they are proliferative vascular lesions that are similar to those found in the periphery (223). In the brain, they are associated with blood vessels and usually are 120 to 180 μm in diameter. They are composed of vascular endothelium and neuroglia and undergo a characteristic evolution throughout the course of the disease (223). In addition to nodules, other frequent findings include perivascular accumulations of mononuclear cells and microinfarcts, the latter being the most common lesions in RMSF, but they are also found with epidemic typhus (5, 223). Postmortem examination of patients dying of RMSF shows widely scattered microinfarcts throughout the brain and spinal cord that are usually associated with small to medium-sized blood vessels and necrotic arterioles (5, 261). In some series, these inflammatory lesions were found only in the white matter (5), but others have also reported that they were present in gray matter as well (160, 261). In epidemic typhus, however, the lesions spare the white matter and show a striking predilection for the inferior olivary nuclei; they are also common in the gray matter of the cerebral cortex, pons, medulla, basal ganglia, and cord, presumably reflecting the vascularity and cellularity of these tissues (5, 223, 415). Meningeal lesions in the pia arachnoid are mild in patients with RMSF and consist of lymphocytic infiltrate without frank purulent meningitis or vascular occlusion, whereas acute inflammatory cell infiltrates are frequently present in patients with epidemic typhus (5, 223, 261). R. prowazekii also has been identified by PCR in the CSF of a human patient (244).
The central pathophysiological event of Rickettsia infections, including CNS infection, has long been identified as parasitism of vascular endothelial cells by blood-borne bacteria (415). Hematogenous dissemination of bacteria was demonstrated by inoculating animals and tissue culture cells with blood or plasma from patients infected with R. rickettsii (185, 320) and R. prowazekii (40). In addition, R. rickettsii has been isolated from bone marrow monocytes and from peripheral blood monocytes in experimentally infected animals (48, 84, 195). Nevertheless, whether infected phagocytes also contribute to systemic dissemination is not known. Rickettsial invasion of endothelial cells (362, 397) and other nonprofessional phagocytes, such as chicken embryo cells, Vero cells, and fibroblasts (414), occurs by parasite-directed endocytosis, which requires bacterial metabolic activity and involves bacterial phospholipase A2 (363). Candidate invasion proteins for this are rOmpA (221) and the protein encoded by the recently described R. prowazekii invasion gene homologue invA (125). This protein is a member of the Nidux hydrolase subfamily and is conserved among pathogenic rickettsiae. The invA gene was identified by sequence analysis of the R. prowazekii genome as a gene that encodes a putative protein with significant homology to invasion proteins of other bacterial pathogens (125). Transcriptional analysis indicates that invA is upregulated at several different times during the pathogenic life cycle, including the early entry phase, and may improve bacterial survival in the cytoplasm of the host cell (124). Internalized rickettsiae lyse the phagosomal membrane through an incompletely understood process in which bacterial phospholipase A2 could play a role and then gain access to the cytoplasm. Subsequently, the pathogens proliferate in the cytoplasm and, in the case of R. rickettsii, also in the nucleus (363, 401, 412). R. rickettsii group bacteria exhibit actin-based motility in the cytoplasm similar to that used by L. monocytogenes but with notable differences in the ultrastructure and protein components of the actin tail (139, 163, 388). By comparison, R. prowazekii does not associate with F-actin, whereas R. typhi does form a very short F-actin tail (388). Rickettsial parasitism of endothelial cells as described above induces numerous intracellular events including inhibition of apoptosis, expression of proinflammatory cytokines and chemokines, upregulation of surface adhesion molecule expression, and oxidative stress. Together, these changes lead to altered hemostatic properties that then contribute to the formation of pathological lesions (109).
Orientia tsutsugamushi.
O. tsutsugamushi, the etiologic agent of scrub typhus, was formerly a member of the spotted fever group of Rickettsia species but was placed in its own genus based on molecular phylogenetic studies (376). In vitro experiments show that O. tsutsugamushi infects neutrophils (322) and macrophages (272) as well as nonprofessional phagocytes including fibroblasts and endothelial cells (275, 387). After internalization, it escapes from phagosomes by an unknown mechanism and then proliferates in the cytoplasm (112, 323, 387). In contrast to other rickettsiae, intracellular movement of O. tsutsugamushi depends on microtubular and dynein function rather than actin polymerization (199). The mechanism for cell-to-cell spread is through budding of membrane-coated bacteria from infected cells (112).
These organisms are spread to humans through the bite of the larval stage of trombiculid mites and cause a systemic disease with features like those of other Rickettsia infections (351). The neurological manifestations are similar in many respects to other rickettsial diseases in that headache is nearly always present (35, 284). Signs of more severe CNS disease such as meningismus or meningitis have been found in 5.7 to 13.5% of patients (35, 340, 361). However, in a recent series of 25 patients who underwent lumbar puncture in the absence of overt CNS signs, 48% had a reactive spinal fluid showing a mild mononuclear pleocytosis, and O. tsutsugamushi was identified by PCR in 24% (284). These data suggest that CNS invasion is much more common in this disease than is suggested by symptoms alone. Necropsy studies show brain parenchymal lesions with a similar appearance and distribution to those in epidemic typhus, but the number of lesions is generally smaller (5). In contrast, the meninges are more commonly involved by O. tsutsugamushi than by other rickettsial infections, and the overall histological picture in the CNS is best described as a meningoencephalitis (5).
Dissemination of bacteria from the periphery to the CNS is hematogenous. Compared with other rickettsiae, O. tsutsugamushi is more frequently found in circulating mononuclear cells during naturally acquired infection of humans (402) and during experimental infection of dogs and nonhuman primates (355). Moreover, infection can be transmitted by blood transfusion due to prolonged microbial survival in leukocytes (56, 257). These findings suggest that phagocyte-facilitated infection could play a role in CNS invasion.
O. tsutsugamushi parasitizes endothelial cells in the periphery as well as in the brain, but it also can be found in macrophages of the liver and spleen (267). The mechanism of cellular invasion is not known, but binding to nonprofessional phagocytes may involve interaction between cell surface heparan sulfate proteoglycans and bacterial lectins (176). Infection of host cells triggers a response including activation of the transcription factors NF-κB (in macrophages) and NF-κB and AP-1 (in endothelial cells), leading to subsequent expression of chemokine genes for MIP-1α/β, MIP-2, and MCP-1 in macrophages and MCP-1, IL-8, and RANTES in endothelial cells (61-63). In an interesting contrast to R. rickettsii, which inhibits endothelial apoptosis, O. tsutsugamushi was shown to induce apoptosis in an endothelial cell line. However, it inhibited the apoptotic process in monocyte-like THP-1 cells (194, 198) and might also actively suppress cytokine production by infected macrophages (197).
Ehrlichia chaffeensis.
E. chaffeensis is an obligate intracellular parasite of mononuclear phagocytes and is the etiologic agent of human monocytotropic ehrlichosis (282). Individual organisms are termed elementary bodies and are small (approximately 0.5 μm in diameter) gram-negative bacteria that are found within infected cells (250). Monocytes are the primary cellular targets of E. chaffeensis in vivo; however, bacteria are also found in liver endothelial cells in experimentally infected mice (367, 413) and can infect nonprofessional phagocytes in vitro (60). Ehrlichiae have a characteristic intracellular life cycle that progresses through three developmental stages: elementary bodies, initial bodies, and morulae (reviewed in reference 250). Elementary bodies enter monocytes by phagocytosis. The receptors and ligands for E. chaffeensis invasion of mononuclear phagocytes are not well established. A bacterial 120-kDa outer membrane protein is probably important in this respect, since expression of it by Escherichia coli allows the E. coli cells to bind and invade HeLa cells (297). Once internalized, E. chaffeensis localizes to early endosomal compartments and causes upregulated expression of mRNA for the transferrin receptor with subsequent accumulation of transferrin receptors in phagosomes (30, 268). Bacteria replicate in the phagosomal compartment by binary fission, so that over a period of 3 to 5 days the individual organisms become tightly packed and form structures that are 1.0 to 2.5 μm in diameter, referred to as initial bodies. Over the ensuing 7 to 10 days, initial bodies coalesce, forming a more mature inclusion body known as a morula. When the infected cell ruptures, morulae break up into elementary bodies and the infective cycle repeats itself.
The first monocytotropic Ehrlichia species discovered was E. canis, which was found in tick-infected dogs in 1935 in Algeria (87). Human monocytotropic ehrlichosis was first identified in 1987, and the infecting agent was subsequently named E. chaffeensis (235). Ehrlichial infections are zoonoses transmitted to humans and other mammals through the bite of infected ticks, principally the lone star tick, Amblyomma americanum. Animal reservoirs of E. chaffeensis include white-tailed deer and coyotes (75, 113, 203). Human infection with E. chaffeensis is clinically similar to RMSF, with the major exception that most patients with ehrlichiosis do not develop a rash. The manifestations of infection are nonspecific and include fever, myalgia, and malaise, with headache being the main symptom referable to the CNS and which is found in approximately 80% of patients (97).
A review of CNS manifestations of ehrlichiosis by Ratnasamy et al. identified 22 cases of which 21 were attributed to E. chaffeensis and 1 was attributed to the closely related Anaplasma phagocytophila, formerly known as the agent of human granulocytic ehrlichiosis (312). Patients with severe CNS involvement had headache, nuchal rigidity, confusion, ataxia, hyperreflexia, seizures, photophobia, and cranial nerve palsies. Findings in the CSF were nonspecific and consisted of a lymphocytic pleocytosis with a mildly elevated protein level in almost 75% of cases. The presence of organisms in the CSF has been established by PCR and by microscopic identification of parasitized cells (312). In addition, E. chaffeensis has been isolated from CSF of two patients by primary culture (368). Pathological findings in the CNS from patients with fatal cases either were unremarkable (312) or consisted only of a lymphocytic infiltrate in the leptomeninges with involvement of blood vessel walls and extension into the Virchow-Robin space (144). Thus, the brain parenchyma appears to be spared from direct infection.
As noted above, E. chaffeensis is more frequently found in the CNS in humans than is A. phagocytophila. This relative difference is also noted in animals infected with related organisms. For example, dogs that are experimentally infected with E. canis, a monocytotropic organism closely related to E. chaffeensis, commonly develop meningitis with a mild vascular cuffing and gliosis in the parenchyma (285). In contrast, brain lesions are not found in horses and sheep experimentally infected with the organism formerly known as E. equi, now termed A. phagocytophila comb. nov. (96). The mechanism used by E. chaffeensis to invade the CNS is unknown. Given that its in vivo niche is within monocytes, phagocyte-facilitated invasion is the most likely route by which these bacteria enter the CNS. Nevertheless, recent data by Li and Winslow show that approximately 10% of blood-borne bacteria in E. chaffeensis-infected mice are actually extracellular and that these cell-free organisms are virulent if they are immediately transferred into other mice or are cocultured with the DH82 macrophage-like cell line (222). These authors suggest that cell-free E. chaffeensis organisms are liberated from infected cells by necrotic cell death and are an important target for antibody-mediated clearance mechanisms. Hence, it is possible that these extracellular bacteria could invade endothelial cells of the CNS directly, but the lack of widespread endothelial cell involvement in vivo suggests that this is unlikely. In addition, the greater incidence of CNS infection observed with monocytotropic ehrlichiae compared with granulocytotropic Anaplasma is consistent with the greater ability of monocytes to migrate across the blood-brain barrier under steady-state conditions (reviewed in reference 165).
Coxiella burnetii.
C. burnetii is an obligate intracellular bacterium that is the etiologic agent of Q fever (249). Q fever is a zoonosis that was first described as an outbreak of a febrile disease in an abattoir in Queensland, Australia (83). The pathogenesis of C. burnetii infection in humans is dependent on this microbe parasitizing macrophages and monocytes, although it also can enter and replicate within nonprofessional phagocytes such as HeLa cells and fibroblasts (162, 248). Once internalized by nonprofessional phagocytes, C. burnetii resides in an acidified endosomal compartment characterized as a phagolysosome (162). However, it has been proposed recently that intracellular bacteria delay (170) or completely inhibit (133) phagolysosome fusion in macrophage-like and monocytic cell lines, respectively. Intracellular C. burnetii organisms undergo a complex intracellular life cycle involving a sporulation-like process that leads to the formation of intracellular forms termed “large-cell variants” and “small-cell variants” (reviewed in reference 158).
A recent review of 1,383 cases of Q fever by Raoult et al. found that isolated fever, hepatitis, and pneumonia with or without associated hepatitis accounted for 91% of cases (311). The most common neurological sign is headache, which has been found in 65 to 90% of cases (339). A review of neurological involvement in Q fever identified headache severe enough to warrant lumbar puncture to rule out meningitis in 3.5% of hospitalized patients. After exclusion of patients with a normal CSF examination, the authors concluded that the prevalence of serious neurological symptoms was 2.2% (38). By comparison, a different series reported an incidence of neurological symptoms of 22% (314). A variety of manifestations of CNS disease in Q fever have been reported, including meningitis and meningoencephalitis in 0.5 and 1% of patients, respectively (311), neck stiffness in 5%, and confusion and apathy in 7% (64). Behavioral and psychiatric disturbances, seizures, and peripheral neuritis have also been reported (38). Approximately two-thirds of CSF analyses show increased numbers of leukocytes, typically mononuclear, and one-third of specimens had increased protein levels (38). C. burnetii is difficult to culture from the CSF but has been identified by PCR in one patient (338). One necropsy of a patient who died of Q fever pneumonia showed small perivascular hemorrhages in the brain, with capillary endothelial swelling without perivascular accumulation of leukocytes (406). Rickettsial forms were identified extracellularly and inside neuroglia and endothelial cells by Giemsa staining.
The method by which this organism enters the CNS is not known, but its tropism for monocytes and the ability to survive for extended periods within them suggests that trafficking of infected cells into the CNS is the most likely mechanism. Chronic persistence of C. burnetii within human monocytes is well described and is associated with increased cellular secretion of TNF-α (80), IL-10, and transforming growth factor β1 (53). Overproduction of IL-10 may play a role in inhibiting the bactericidal activity of the host cell by altering cellular responses to TNF-α (130, 132). Interestingly, C. burnetii is one of the few organisms studied from the perspective of the way in which intracellular infection of monocytes alters their interactions with endothelial cells. Dellacasagrande et al. reported that infection of THP-1 cells with a virulent strain of C. burnetii increased cellular adhesion to a TNF-α-stimulated human microvascular cell line but transmigration across the cell monolayers was significantly inhibited (81). The authors suggested that decreased transmigration might interfere with localization of the bacterium to target tissues and/or may contribute to the formation of poorly formed granulomas that are found in chronic Q fever. From the standpoint of CNS invasion, the inability of infected monocytes to migrate across endothelial barriers could contribute to the relatively low incidence of CNS infection found in Q fever in spite of their long-term presence in the circulation.
Tropheryma whipplei
T. whipplei, the causative agent of Whipple's disease, is a gram-positive, periodic acid-Schiff (PAS)-positive, diastase-resistant bacterium that is a member of the high-G+C phylum of gram-positive bacteria (315). In vitro study of this obligate intracellular organism and understanding of how it lives intracellularly have been complicated by the fact that sustainable ex vivo culture of the organism was achieved only in 2000 (306). Studies using infection of HeLa cells for a model show that T. whipplei survives by altering the phagosomal environment. Phagosomes containing bacteria are acidic and colocalize with lysosome-associated protein 1 but not with cathepsin D, indicating that phagolysosome fusion and maturation are incomplete (131). Interestingly, acidification of the vacuole is critical to the survival of the organism, as shown by the finding that agents that increase the intravacuolar pH decrease bacterial viability.
Whipple's disease is rare, with less than 1,000 cases reported to date (116), although this is certainly an underestimate of the number of cases that have occurred (86). It is named after George Whipple, who described an autopsy in 1907 of a 36-year-old physician with gradual loss of weight and strength, diarrhea, ill-defined abdominal signs, and arthritis in multiple joints. The disease was called “intestinal lipodystrophy” based on findings of polyserositis, aortic valve lesions, and prominent deposition of fat within intestinal mucosa and mesenteric lymph nodes. In addition, there was marked infiltration of the intestinal lamina propria by foamy macrophages that contained rod-shaped bacilli (405). In 1991, Wilson et al. reported on the 16S rRNA gene and partial sequence of bacterial DNA extracted from duodenal and lymph node biopsy specimens from patients with Whipple's disease (408). These findings were confirmed and extended the following year, and the proposed name of the new organism as Tropheryma whipplei (Greek trophe for nourishment and eryma for barrier) was advanced (315). In 2001, the causative organism of Whipple's disease was officially named Tropheryma whipplei (216).
Whipple's disease occurs worldwide, but 55% of cases were reported from Europe and 38% were reported from North America (86). The habitat of T. whipplei in the environment is unknown, as is the mode of acquisition of the bacteria. However, data suggest that T. whipplei is an environmental human commensal, which is acquired by drinking water but rarely causes human infection. This notion is based on identification of the organism by PCR in 25 of 38 samples of sewage water from five different sewage plants in Germany (237) and also in a variety of specimens from patients with no evidence of Whipple's disease (371). The reason why this assumed commensal organism rarely causes disease might be found in underlying host conditions. Immune abnormalities have been demonstrated in patients with Whipple's disease, as reviewed in references 243 and 263. If there is an underlying immune defect, it appears to be quite specific, because patients with Whipple's disease do not acquire other opportunistic infections. These abnormalities might be primary, as is suggested by the finding of decreased intracellular killing of Candida tropicalis by monocytes from patients with Whipple's disease in remission compared to normal controls (24). Furthermore, peripheral blood mononuclear cells from patients with Whipple's disease had increased production of IL-4 in response to in vitro stimulation with phytohemagglutinin and reduced secretion of gamma interferon and IL-2 compared to controls (242). Thus, macrophages might be more susceptible to T. whipplei infection in part because of a decreased influence of classically activating cytokines and the increased alternatively activating IL-4 signal (138). The report of a patient with refractory Whipple's disease who had an apparent response to gamma interferon therapy lends credence to this hypothesis (342).
Whipple's disease is a chronic relapsing multisystem disease, and its clinical manifestations have been recently reviewed (243). Almost all reported cases (97%) were in whites (86), with a male-to-female ratio of approximately 8:1 (243). The average age at the time of diagnosis was 50 to 55 years (100, 243), although patients with neurological abnormalities as the initial manifestation may be younger (average age, 42 years) and more commonly female (male-to-female ratio, 1:1) (129). Intermittent arthropathy, which is the initial symptom in 63% of cases, precedes the diagnosis by an average of 8 years and is present at the time of diagnosis in 85% of patients (304). The cardinal symptoms include significant weight loss, intermittent diarrhea or steatorrhea, abdominal pain, fever, and lymphadenopathy. Involvement of the CNS is found in 20 to 40% of patients with Whipple's disease (11, 54, 65, 129, 210, 228, 255, 343, 373, 394). CNS involvement typically occurs as part of a systemic infection and usually develops in the later stage of disease, although cases confined to the CNS have been reported (373). Patients with CNS disease nearly always exhibit diffuse neurological signs. In a review of 122 cases, the neurological signs were classified as follows, in order of decreasing frequency: supranuclear ophthalmoplegia, confusion and dementia, psychiatric signs, myoclonic signs, seizures, hypothalamic involvement, cerebellar forms, myorhythmic forms, cranial nerve involvement, peripheral neuropathies, and spinal cord involvement (129). Meningeal involvement is often associated with all these neurological signs, so that the disease is actually a meningoencephalitis or, in cases of ophthalmologic involvement, meningouveitis (129). The CSF was abnormal in half of all patients with Whipple's disease studied (n = 73) and in two-thirds of those experiencing neurological relapse (n = 29).
The histopathology of Whipple's disease in the small intestine is a readily recognizable characteristic group of findings including villi with many histiocytes containing diastase-resistant PAS-positive granular material in the lamina propria, club-shaped vili, prominent dilated lymphatic spaces, and large-droplet lipid deposits (25). Histopathology of the involved cerebral cortex shows scattered aggregates of foamy macrophages with prominent perivascular cuffing and large reactive astrocytes; the large foamy macrophages are filled with diastase-resistant PAS-positive granules. In patients with florid disease, T. whipplei can be seen apparently in the extracellular compartment, and it has recently been cultivated from the CSF of two patients (238). The mechanism(s) by which T. whipplei invades the CNS has not been studied. However, T. whipplei has been demonstrated by immunocytochemical staining of peripheral blood monocytes of a patient with Whipple's disease (307). This suggests that circulating mononuclear phagocytes could transport the organism into the CNS.
CONCLUDING REMARKS
The intracellular microbes causing CNS disease reviewed in this paper are a diverse group of human pathogens. One way in which this is demonstrated is the wide variety of mechanisms used to enter their human hosts: through contaminated food (e.g., L. monocytogenes, Salmonella, and Brucella spp.), through the bite of infected arthropods (R. rickettsii, R. prowazekii, and E. chaffeensis), and through inhalation (M. tuberculosis and C. burnetii). This is an interesting contrast to extracellular neuroinvasive bacteria such as S. pneumoniae, N. meningitidis, and H. influenzae, which infect humans via the respiratory tract. The diversity of the intracellular pathogens inflicting CNS disease also is reflected in their place in human history. For example, there is evidence of human infection with M. tuberculosis and Brucella spp. dating back several thousand years, whereas human infection with E. chaffeensis has been recognized only in the past two or three decades and T. whipplei was identified only about one decade ago. Typhus and tuberculosis are clearly associated with poverty, calamity, and social upheaval. The former emerged historically in times of war, whereas the latter now is united with a truly modern epidemic, HIV-1, to reestablish itself as the “Captain of all these men of death” (J. Bunyan in The Life and Death of Mr. Badman, 1680). On the other hand, none of these bacteria are particularly associated with prosperity, except that only in a prosperous society do people live long enough that a bacterium like L. monocytogenes can prey on the elderly. Given the limited insight into the etiopathogenesis of many of the CNS infections caused by intracellular bacteria, it is unlikely that new therapies aimed specifically at preventing CNS infection by these microorganisms will be available soon. Thus, continued research and clinical effort in the development of CNS-specific therapies are needed. In spite of profound biological differences, intracellular pathogens are united in their adaptation to the intracellular environment and in the exploitation of host cells for their maintenance and dissemination. It should be underscored that for most of these organisms, the basic cellular and molecular mechanisms used to invade the CNS are largely unknown or are just now being explored. Of the typical routes used by blood-borne bacteria to enter the CNS, the two routes most likely to be used by intracellular organisms are direct invasion of endothelial cells and trafficking of organisms within infected mononuclear phagocytes. The main point in favor of the former mechanism is that most, if not all, of these pathogens can invade and replicate within nonprofessional phagocytes. The clearest examples of this mode of invasion are provided by R. rickettsii and R. prowazekii. In diseases caused by these organisms, endothelial cell invasion is the key pathophysiological event.
By comparison, CNS invasion by infected phagocytes is less well studied and does not leave easily identifiable footprints in vivo. Nevertheless, parasitism of mononuclear phagocytes is an essential feature of several of the organisms considered here. For example, the recent characterization of Brucella spp. as facultative extracellular pathogens emphasizes that these bacteria have an extreme preference for the intracellular environment despite their ability to live outside host cells. For such organisms, CNS invasion via trafficking within infected phagocytes is a logical route. By comparison, some microbes, such as L. monocytogenes and O. tsutsugamushi, may be capable of CNS invasion by more than one mechanism. In this instance, data from experimental infection with L. monocytogenes are helpful in defining the molecular and cellular mechanisms that are operative in vivo. Additionally, data that show an important role for transportation of M. tuberculosis and S. enterica serovar Typhimurium within mononuclear phagocytes across epithelial barriers suggest that trafficking of infected phagocytes could be a central motif for several intracellular bacterial pathogens in the same way that subversion of key intracellular processes, e.g., maturation of phagosomes, is a common feature of intracellular pathogenesis.
ADDENDUM IN PROOF
Readers are directed to new data concerning the role of infected monocytes and transportation of intracellular L. monocytogenes into the brain: D. A. Drevets et al., J. Immunol., in press.
REFERENCES
- 1.Reference deleted.
- 2.Reference deleted.
- 3.Ackers, M. L., N. D. Puhr, R. V. Tauxe, and E. D. Mintz. 2000. Laboratory-based surveillance of Salmonella serotype typhi infections in the United States: antimicrobial resistance on the rise. JAMA 283:2668-2673. [DOI] [PubMed] [Google Scholar]
- 4.Akalin, H., A. C. Akdis, R. Mistik, S. Helvaci, and K. Kilicturgay. 1994. Cerebrospinal fluid interleukin-1 beta/interleukin-1 receptor antagonist balance and tumor necrosis factor-alpha concentrations in tuberculous, viral and acute bacterial meningitis. Scand. J. Infect. Dis. 26:667-674. [DOI] [PubMed] [Google Scholar]
- 5.Allen, A. C., and S. Spitz. 1945. A comparative study of the pathology of scrub typhus (Tsutsugamushi disease) and other rickettsial diseases. Am. J. Pathol. 21:603-681. [PMC free article] [PubMed] [Google Scholar]
- 6.Alpuche-Aranda, C. M., E. L. Racoosin, J. A. Swanson, and S. I. Miller. 1994. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 179:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altimira, J., N. Prats, S. Lopez, M. Domingo, V. Briones, L. Dominguez, and A. Macro. 2000. Effect of selenium deficiency on the development of central nervous system lesions in murine listeriosis. J. Comp. Pathol. 123:104-109. [DOI] [PubMed] [Google Scholar]
- 8.Altimira, J., N. Prats, S. Lopez, M. Domingo, V. Briones, L. Dominguez, and A. Marco. 1999. Repeated oral dosing with Listeria monocytogenes in mice as a model of central nervous system listeriosis in man. J. Comp. Pathol. 121:117-125. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez, S., and W. R. McCabe. 1984. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine (Baltimore) 63:25-55. [PubMed] [Google Scholar]
- 10.Alvarez-Dominguez, C., R. Roberts, and P. D. Stahl. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731-743. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, M. 2000. Neurology of Whipple's disease. J. Neurol. Neurosurg. Psychiatry 68:2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antal, E. A., E. M. Loberg, P. Bracht, K. K. Melby, and J. Maehlen. 2001. Evidence for intraaxonal spread of Listeria monocytogenes from the periphery to the central nervous system. Brain Pathol. 11:432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Applebaum, P. C., and J. Scragg. 1977. Salmonella meningitis in infants. Lancet i:1052-1053. [DOI] [PubMed] [Google Scholar]
- 14.Arenas, G. N., A. S. Staskevich, A. Aballay, and L. S. Mayorga. 2000. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect. Immun. 68:4255-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong, R. W., and P. C. Fung. 1993. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin. Infect. Dis. 16:689-702. [DOI] [PubMed] [Google Scholar]
- 16.Arnow, P. M., M. Smaron, and V. Ormiste. 1984. Brucellosis in a group of travelers to Spain. JAMA 251:505-507. [PubMed] [Google Scholar]
- 17.Asahi, O., and T. Hosoda. 1952. Studies on listeriosis in domestic animals. II. Studies on experimental listeriosis in goats. Med. Biol. (Japan) 24:100-103. [Google Scholar]
- 18.Asahi, O., T. Hosoda, and Y. Akiyama. 1957. Studies on the mechanism of infection of the brain with Listeria monocytogenes. Am. J. Vet. Res. 18:147-157. [PubMed] [Google Scholar]
- 19.Atkinson, E. 1917. Meningitis associated with gram-positive bacilli of the diphteroid type. Med. J. Aust. 1:115-118. [Google Scholar]
- 20.Auerbach, O. 1951. Tuberculous meningitis: correlation of therapeutic results with the pathogenesis and pathologic changes. II. Pathologic changes in untreated and treated cases. Am. Rev. Tuberc. 64:419-429. [DOI] [PubMed] [Google Scholar]
- 21.Auerbach, O. 1951. Tuberculous meningitis: correlation of therapeutic results with the pathogenesis and pathologic changes. I. General considerations and pathogenesis. Am. Rev. Tuberc. 64:408-418. [DOI] [PubMed] [Google Scholar]
- 22.Aureli, P., G. C. Fiorucci, D. Caroli, G. Marchiaro, O. Novara, L. Leone, and S. Salmaso. 2000. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N. Engl. J. Med. 342:1236-1241. [DOI] [PubMed] [Google Scholar]
- 23.Autret, N., I. Dubail, P. Trieu-Cuot, P. Berche, and A. Charbit. 2001. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 69:2054-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai, J. C., L. Sen, R. Diez, S. Niveloni, E. C. Maurino, M. Esteves, and L. A. Boerr. 1996. Impaired monocyte function in patients successfully treated for Whipple's disease. Acta Gatroenterol. Latinoam. 26:85-89. [PubMed] [Google Scholar]
- 25.Baisden, B. L., H. Lepidi, D. Raoult, P. Argani, J. H. Yardley, and J. S. Dumler. 2002. Diagnosis of Whipple disease by immunohistochemical staining. Am. J. Clin. Pathol. 118:743-748. [DOI] [PubMed] [Google Scholar]
- 26.Balasubramanian, V., E. H. Wiegeshaus, B. T. Taylor, and D. W. Smith. 1994. Pathogenesis of tuberculosis: pathway to apical localization. Tubercle Lung Dis 75:168-178. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin, C. L., and A. J. Winter. 1994. Macrophages and Brucella. Immunol. Ser. 60:363-380. [PubMed] [Google Scholar]
- 28.Barlow, R. M., and B. McGorum. 1985. Ovine listerial encephalitis: analysis, hypothesis and synthesis. Vet. Rec. 116:233-236. [DOI] [PubMed] [Google Scholar]
- 29.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnewall, R. E., Y. Rikihisa, and E. H. Lee. 1997. Listeria monocytogenes inclusions are early endosomes which selectively accumulate transferrin receptor. Infect. Immun. 65:1455-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berche, P. 1995. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb. Pathog. 18:323-336. [DOI] [PubMed] [Google Scholar]
- 32.Berenguer, J., S. Moreno, F. Laguna, T. Vicente, M. Adrados, A. Ortega, J. Gonzalez-LaHoz, and E. Bouza. 1992. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 326:668-672. [DOI] [PubMed] [Google Scholar]
- 33.Beres, D., and T. Meltzer. 1938. Tuberculous meningitis and its relation to tuberculous foci in the brain. Am. J. Pathol. 14:59-75. [PMC free article] [PubMed] [Google Scholar]
- 34.Bergmann, B., D. Raffelsbauer, M. Kuhn, M. Goetz, S. Hom, and W. Goebel. 2002. In1A- but not In1B-mediated internalization of Listeria monocytogenes by non-phagocytic mammalian cells needs the support of other internalins. Mol. Microbiol. 43:557-570. [DOI] [PubMed] [Google Scholar]
- 35.Berman, S. J., and W. D. Kundin. 1973. Scrub typhus in South Vietnam. A study of 87 cases. Ann. Intern. Med. 79:26-30. [DOI] [PubMed] [Google Scholar]
- 36.Bermudez, L. E., and F. J. Sangari. 2001. Cellular and molecular mechanisms of internalization of mycobacteria by host cells. Microbes Infect. 3:37-42. [DOI] [PubMed] [Google Scholar]
- 37.Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernit, E., J. Pouget, F. Janbon, H. Dutronc, P. Martinez, P. Brouqui, and D. Raoult. 2002. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch. Intern. Med. 162:693-700. [DOI] [PubMed] [Google Scholar]
- 39.Bingen, E., N. Lambert-Zechovsky, P. Mariani-Kurkdjian, C. Doit, Y. Aujard, F. Fournerie, and H. Mathieu. 1990. Bacterial counts in cerebrospinal fluid of children with meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 9:278-281. [DOI] [PubMed] [Google Scholar]
- 40.Birg, M. L., B. La Scola, V. Roux, P. Brouqui, and D. Raoult. 1999. Isolation of Rickettsia prowazekii from blood by shell vial cell culture. J. Clin. Microbiol. 37:3722-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birkness, K. A., M. Deslauriers, J. H. Bartlett, E. H. White, C. H. King, and F. D. Quinn. 1999. An in vitro tissue culture bilayer model to examine early events in Mycobacterium tuberculosis infection. Infect. Immun. 67:653-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanot, S., M. M. Joly, F. Vilde, F. Jaubert, O. Clement, G. Frija, and P. Berche. 1997. A gerbil model for rhombencephalitis due to Listeria monocytogenes. Microb. Pathog 23:39-48. [DOI] [PubMed] [Google Scholar]
- 43.Bolin, C. A., D. L. Whipple, K. V. Khanna, J. M. Risdahl, P. K. Peterson, and T. W. Molitor. 1997. Infection of swine with Mycobacterium bovis as a model of human tuberculosis. J. Infect. Dis. 176:1559-1566. [DOI] [PubMed] [Google Scholar]
- 44.Bouvet, E., F. Suter, C. Gibert, J. L. Witchitz, C. Bazin, and F. Vachon. 1982. Severe meningitis due to Listeria monocytogenes. A review of 40 cases in adults. Scand. J. Infect. Dis. 14:267-270. [DOI] [PubMed] [Google Scholar]
- 45.Bouza, E., M. Garcia de la Torre, F. Parras, A. Guerrero, M. Rodriguez-Creixems, and J. Gobernado. 1987. Brucellar meningitis. Rev. Infect. Dis. 9:810-822. [DOI] [PubMed] [Google Scholar]
- 46.Bruce, D. 1887. Note on the discovery of a microorganism in Malta fever. Practitioner 39:161. [Google Scholar]
- 47.Buchanan, T. M., S. L. Hendricks, C. M. Patton, and R. A. Feldman. 1974. Brucellosis in the United States, 1960-1972: an abattoir-associated disease. III. Epidemiology and evidence for acquired immunity. Medicine (Baltimore) 53:427-439. [PubMed] [Google Scholar]
- 48.Buhles, W. C., D. L. Huxsoll, G. Ruch, R. H. Kenyon, and B. L. Elisberg. 1975. Evaluation of primary blood monocyte and bone marrow cell culture for the isolation of Rickettsia rickettsii. Infect. Immun. 12:1457-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bula, C. J., J. Bille, and M. P. Glauser. 1995. An epidemic of food-borne listeriosis in western Switzerland: description of 57 cases involving adults. Clin. Infect. Dis. 20:66-72. [DOI] [PubMed] [Google Scholar]
- 50.Cabanes, D., P. Dehoux, O. Dussurget, L. Frangeul, and P. Cossart. 2002. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 10:238-245. [DOI] [PubMed] [Google Scholar]
- 51.Campero, C. M., A. C. Odeon, A. L. Cipolla, D. P. Moore, M. A. Poso, and E. Odriozola. 2002. Demonstration of Listeria monocytogenes by immunohistochemistry in formalin-fixed brain tissues from natural cases of ovine and bovine encephalitis. J. Vet. Med. Ser. B 49:379-383. [DOI] [PubMed] [Google Scholar]
- 52.Capasso, L. 1999. Brucellosis at Herculaneum. Int. J. Osteoarcheol. 9:277-288. [Google Scholar]
- 53.Capo, C., Y. Zaffran, F. Zugun, P. Houpikian, D. Raoult, and J. L. Mege. 1996. Production of interleukin-10 and transforming growth factor beta by peripheral blood mononuclear cells in Q fever endocarditis. Infect. Immun. 64:4143-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carella, F., P. Valla, G. Bernardi, F. Parente, A. Costa, and S. Lodrini. 1998. Cerebral Whipple's disease: clinical and cerebrospinal fluid findings. Ital. J. Neurol. Sci. 19:101-105. [DOI] [PubMed] [Google Scholar]
- 55.Cascio, A., P. Dones, A. Romano, and L. Titone. 1998. Clinical and laboratory findings of boutonneuse fever in Sicilian children. Eur. J. Pediatr. 157:482-486. [DOI] [PubMed] [Google Scholar]
- 56.Casleton, B. G., K. Salata, G. A. Dasch, D. Strickman, and D. J. Kelly. 1998. Recovery and viability of Orientia tsutsugamushi from packed red cells and the danger of acquiring scrub typhus from blood transfusion. Transfusion 38:680-689. [DOI] [PubMed] [Google Scholar]
- 57.Celli, J., C. de Chastellier, D.-M. Franchini, J. Pizarro-Cerda, E. Moreno, and J.-P. Gorvel. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Centers for Disease Control and Prevention. 1999. Update: multistate outbreak of listeriosis—United States, 1998-1999. JAMA 281:317-318. [PubMed] [Google Scholar]
- 58.Charlton, K. M. 1977. Spontaneous listeric encephalitis in sheep. Electron microscopic studies. Vet. Pathol. 14:429-434. [DOI] [PubMed] [Google Scholar]
- 59.Charlton, K. M., and M. M. Garcia. 1977. Spontaneous listeric encephalitis and neuritis in sheep. Light microscopic studies. Vet. Pathol. 14:297-313. [DOI] [PubMed] [Google Scholar]
- 60.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho, N. H., S. Y. Seong, M. S. Choi, and I. S. Kim. 2001. Expression of chemokine genes in human dermal microvascular endothelial cell lines infected with Orientia tsutsugamushi. Infect. Immun. 69:1265-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho, N. H., S. Y. Seong, M. S. Huh, T. H. Han, Y. S. Koh, M. S. Choi, and I. S. Kim. 2000. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect. Immun. 68:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho, N. H., S. Y. Seong, M. S. Huh, N. H. Kim, M. S. Choi, and I. S. Kim. 2002. Induction of the gene encoding macrophage chemoattractant protein 1 by Orientia tsutsugamushi in human endothelial cells involves activation of transcription factor activator protein 1. Infect. Immun. 70:4841-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark, W. H., E. H. Lennette, O. C. Railsback, and M. S. Romer. 1951. Q fever in California. VII. Clinical features in one hundred eighty cases. Arch. Intern. Med. 88:155-161. [DOI] [PubMed] [Google Scholar]
- 65.Clarke, C. E., Z. F. Falope, H. A. Abdelhadi, and A. J. Franks. 1998. Cervical myelopathy caused by Whipple's disease. Neurology 50:1505-1506. [DOI] [PubMed] [Google Scholar]
- 66.Clough, M. C. 1917. The cultivation of tubercule bacilli from the circulating blood in miliary tuberculosis. Am. Rev. Tuberc. 1:598-621. [Google Scholar]
- 67.Cohen, J. I., J. A. Bartlett, and G. R. Corey. 1987. Extra-intestinal manifestations of Salmonella infections. Medicine 66:349-388. [DOI] [PubMed] [Google Scholar]
- 68.Cole, R. I. 1905. Typhoid meningitis. Johns Hopkins Hosp. Rep. 12:379. [Google Scholar]
- 69.Corbel, M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corbel, M. J. 1997. Recent advances in brucellosis. J. Med. Microbiol. 46:101-103. [DOI] [PubMed] [Google Scholar]
- 71.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 72.Cordy, D. R., and J. W. Osebold. 1959. The neuropathogenesis of Listeria encephalomyelitis in sheep and mice. J. Infect. Dis. 104:164-173. [DOI] [PubMed] [Google Scholar]
- 73.Cundell, D. R., C. Gerard, I. Idanpaan-Heikkila, E. I. Tuomanen, and N. P. Gerard. 1996. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv. Exp. Med. Biol. 416:89-94. [DOI] [PubMed] [Google Scholar]
- 74.Dastur, D. K., D. K. Manghani, and P. M. Udani. 1995. Pathology and pathogenetic mechanisms in neurotuberculosis. Radiol. Clin. North Am. 33:733-52. [PubMed] [Google Scholar]
- 75.Dawson, J. E., J. E. Childs, K. L. Biggie, C. Moore, D. Stallknecht, J. Shaddock, J. Bouseman, E. Hofmeister, and J. G. Olson. 1994. White-tailed deer as a potential reservoir of Ehrlichia spp. J. Wildl. Dis. 30:162-168. [DOI] [PubMed] [Google Scholar]
- 76.de Bruijn, M. F., W. van Vianen, R. E. Ploemacher, I. A. Bakker-Woudenberg, P. A. Campbell, W. van Ewijk, and P. J. Leenen. 1998. Bone marrow cellular composition in Listeria monocytogenes infected mice detected using ER-MP12 and ER-MP20 antibodies: a flow cytometric alternative to differential counting. J. Immunol. Methods 217:27-39. [DOI] [PubMed] [Google Scholar]
- 77.Dee, R. R., and B. Lorber. 1986. Brain abscess due to Listeria monocytogenes: case report and literature review. Rev. Infect. Dis. 8:968-977. [DOI] [PubMed] [Google Scholar]
- 78.DeJong, R. N. 1936. Central nervous system involvement in undulant fever, with the report of a case and a survey of the literature. J Nerv. Ment. Dis. 83:430-442. [Google Scholar]
- 79.del Carmen Rocha-Gracia, R., E. I. Castaneda-Roldan, S. Giono-Cerezo, and J. A. Giron. 2002. Brucella sp. bind to sialic acid residues on human and animal red blood cells. FEMS Microbiol. Lett. 213:219-224. [DOI] [PubMed] [Google Scholar]
- 80.Dellacasagrande, J., E. Ghigo, C. Capo, D. Raoult, and J. L. Mege. 2000. Coxiella burnetii survives in monocytes from patients with Q fever endocarditis: involvement of tumor necrosis factor. Infect. Immun. 68:160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dellacasagrande, J., P. A. Moulin, C. Guilianelli, C. Capo, D. Raoult, G. E. Grau, and J. L. Mege. 2000. Reduced transendothelial migration of monocytes infected by Coxiella burnetii. Infect. Immun. 68:3784-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deretic, V., and R. A. Fratti. 1999. Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31:1603-1609. [DOI] [PubMed] [Google Scholar]
- 83.Derrick, E. H. 1983. “Q” fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Rev. Infect. Dis. 5:790-800. [DOI] [PubMed] [Google Scholar]
- 84.DeShazo, R. D., J. R. Boyce, J. V. Osterman, and E. H. Stephenson. 1976. Early diagnosis of Rocky Mountain spotted fever. Use of primary monocyte culture technique. JAMA 235:1353-1355. [PubMed] [Google Scholar]
- 85.Dick, G. F. 1920. Case of cerebrospinal meningitis due to diphteroid bacillus. JAMA 74:84. [Google Scholar]
- 86.Dobbins, W. O. I. 1987. Whipple's disease. Charles C Thomas, Springfield, I11.
- 87.Donatien, A., and F. Lestoquard. 1935. Existence en Algerie d'une Rickettsia du chien. Bull. Soc. Pathol. Exot. 28:418-419. [Google Scholar]
- 88.Dons, L., K. Weclewicz, Y. Jin, E. Bindseil, J. Olsen, and K. Kristenson. 1999. Rat dorsal root ganglia neurons as a model for Listeria monocytogenes infections in culture. Med. Microbiol. Immunol. 188:15-21. [DOI] [PubMed] [Google Scholar]
- 89.Dramsi, S., S. Levi, A. Triller, and P. Cossart. 1998. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect. Immun. 66:4461-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drevets, D. A. 1999. Dissemination of Listeria monocytogenes by infected phagocytes. Infect. Immun. 67:3512-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drevets, D. A. 1997. Listeria monocytogenes infection of cultured endothelial cells stimulates neutrophil adhesion and adhesion molecule expression. J. Immunol. 158:5305-5813. [PubMed] [Google Scholar]
- 92.Drevets, D. A. 1998. Listeria monocytogenes virulence factors that stimulate endothelial cells. Infect. Immun. 66:232-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drevets, D. A., T. A. Jelinek, and N. E. Freitag. 2001. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect. Immun. 69:1344-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Drevets, D. A., R. T. Sawyer, T. A. Potter, and P. A. Campbell. 1995. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect. Immun. 63:4268-4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Driscoll, S. G., G. A., and D. Feldman. 1962. Congenital listeriosis: diagnosis from placental studies. Obstet. Gynecol. 20:216-220. [PubMed] [Google Scholar]
- 96.Dumler, J., A. Barbet, C. Bekker, G. Dasch, G. Palmer, S. Ray, Y. Rikihisa, and F. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 97.Dumler, J. S., and J. S. Bakken. 1998. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu. Rev. Med. 49:201-213. [DOI] [PubMed] [Google Scholar]
- 98.Dumler, J. S., J. P. Taylor, and D. H. Walker. 1991. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA 266:1365-1370. [PubMed] [Google Scholar]
- 99.Dumont, J., and L. Cotoni. 1921. Bacille semblable a celui du rouget du porc rencontre dans le L.C.R. d'un meningitique. Ann. Inst. Pasteur 35:625-633. [Google Scholar]
- 100.Durand, D. V., C. Lecomte, P. Cathebras, H. Rousset, and P. Godeau. 1997. Whipple disease: clinical review of 52 cases. Medicine 76:170-184. [DOI] [PubMed] [Google Scholar]
- 101.Durand, M. L., S. B. Calderwood, D. J. Weber, S. I. Miller, F. S. Southwick, V. S. Caviness, Jr., and M. N. Swartz. 1993. Acute bacterial meningitis in adults. A review of 493 episodes. N. Engl. J. Med. 328:21-28. [DOI] [PubMed] [Google Scholar]
- 102.Eckburg, P. B., J. G. Montoya, and K. L. Vosti. 2001. Brain abscess due to Listeria monocytogenes: five cases and a review of the literature. Medicine (Baltimore) 80:223-35. [DOI] [PubMed] [Google Scholar]
- 103.Eckman, M. R. 1975. Brucellosis linked to Mexican cheese. JAMA 232:636-637. [PubMed] [Google Scholar]
- 104.Edmonds, M. D., A. Cloeckaert, N. J. Booth, W. T. Fulton, S. D. Hagius, J. V. Walker, and P. H. Elzer. 2001. Attenuation of a Brucella abortus mutant lacking a major 25 kDa outer membrane protein in cattle. Am. J. Vet. Res. 62:1461-1466. [DOI] [PubMed] [Google Scholar]
- 105.Edmonds, M. D., A. Cloeckaert, and P. H. Elzer. 2002. Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet. Microbiol. 88:205-221. [DOI] [PubMed] [Google Scholar]
- 106.Edmonds, M. D., A. Cloeckaert, S. D. Hagius, L. E. Samartino, W. T. Fulton, J. V. Walker, F. M. Enright, N. J. Booth, and P. H. Elzer. 2002. Pathogenicity and protective activity in pregnant goats of a Brucella melitensis Deltaomp25 deletion mutant. Res. Vet. Sci. 72:235-239. [DOI] [PubMed] [Google Scholar]
- 107.Einhorn, M. S., D. M. Granoff, M. H. Nahm, A. Quinn, and P. G. Shackelford. 1987. Concentrations of antibodies in paired maternal and infant sera: relationship to IgG subclass. J. Pediatr. 111:783-788. [DOI] [PubMed] [Google Scholar]
- 108.El-Etr, S. H., and J. D. Cirillo. 2001. Entry mechanisms of mycobacteria. Front. Biosci. 6:D737-D747. [DOI] [PubMed] [Google Scholar]
- 109.Elghetany, M. T., and D. H. Walker. 1999. Hemostatic changes in Rocky Mountain spotted fever and Mediterranean spotted fever. Am. J. Clin. Pathol. 112:159-168. [DOI] [PubMed] [Google Scholar]
- 110.Englund, J. A., W. P. Glezen, C. Turner, J. Harvey, C. Thompson, and G. R. Siber. 1995. Transplacental antibody transfer following maternal immunization with polysaccharide and conjugate Haemophilus influenzae type b vaccines. J. Infect. Dis. 171:99-105. [DOI] [PubMed] [Google Scholar]
- 111.Eugene, E., I. Hoffmann, C. Pujol, P. O. Couraud, S. Bourdoulous, and X. Nassif. 2002. Microvilli-like structures are associated with the internalization of virulent capsulated Neisseria meningitidis into vascular endothelial cells. J. Cell Sci. 115:1231-1241. [DOI] [PubMed] [Google Scholar]
- 112.Ewing, E. P., Jr., A. Takeuchi, A. Shirai, and J. V. Osterman. 1978. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect. Immun. 19:1068-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ewing, S. A., J. E. Dawson, A. A. Kocan, R. W. Barker, C. K. Warner, R. J. Panciera, J. C. Fox, K. M. Kocan, and E. F. Blouin. 1995. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32:368-374. [DOI] [PubMed] [Google Scholar]
- 114.Fang, C. T., S. C. Chang, P. R. Hsueh, Y. C. Chen, W. Y. Sau, and K. T. Luh. 2000. Microbiologic features of adult community-acquired bacterial meningitis in Taiwan. J. Formos. Med. Assoc. 99:300-304. [PubMed] [Google Scholar]
- 115.Farer, L. S., A. M. Lowell, and M. P. Meador. 1979. Extrapulmonary tuberculosis in the United States. Am. J. Epidemiol. 109:205-217. [DOI] [PubMed] [Google Scholar]
- 116.Fenollar, F., and D. Raoult. 2001. Whipple's Disease. Clin. Diagn. Lab. Immunol. 8:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fincham, R. W., A. L. Sahs, and R. J. Joynt. 1963. Protean manifestations of nervous system brucellosis. JAMA 184:269-275. [DOI] [PubMed] [Google Scholar]
- 119.Fleming, D. W., S. L. Cochi, K. L. MacDonald, J. Brondum, P. S. Hayes, B. D. Plikaytis, M. B. Holmes, A. Audurier, C. V. Broome, and A. L. Reingold. 1985. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 312:404-407. [DOI] [PubMed] [Google Scholar]
- 120.Freitag, N. E., and K. E. Jacobs. 1999. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect. Immun. 67:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frothingham, R., H. G. Hills, and K. H. Wilson. 1994. Extensive DNA sequence conservation throughout the Mycobacterium tuberculosis complex. J. Clin. Microbiol. 32:1639-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gaillard, J. L., P. Berche, and P. Sansonetti. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect. Immun. 52:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garg, R. K. 1999. Tuberculosis of the central nervous system. Postgrad. Med. J. 75:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gaywee, J., S. Radulovic, J. A. Higgins, and A. F. Azad. 2002. Transcriptional Analysis of Rickettsia prowazekii invasion gene homolog (invA) during host cell infection. Infect. Immun. 70:6346-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gaywee, J., W. Xu, S. Radulovic, M. J. Bessman, and A. F. Azad. 2002. The Rickettsia prowazekii invasion gene homolog (invA) encodes a nudix hydrolase active on adenosine (5′)-pentaphospho-(5′)-adenosine. Mol. Cell. Proteomics 1:179-183. [DOI] [PubMed] [Google Scholar]
- 126.Geijtenbeek, T. B. H., S. J. van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. J. E. Vandenbroucke-Grauls, B. Appelmelk, and Y. van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gelston, A. L., and T. C. Jones. 1977. Typhus fever: report of an epidemic in New York City in 1847. J. Infect. Dis. 136:813-821. [DOI] [PubMed] [Google Scholar]
- 128.Gentschev, I., Z. Sokolovic, S. Kohler, G. F. Krohne, H. Hof, J. Wagner, and W. Goebel. 1992. Identification of p60 antibodies in human sera and presentation of this listerial antigen on the surface of attenuated salmonellae by the HlyB-HlyD secretion system. Infect. Immun. 60:5091-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gerard, A., F. Sarrot-Reynauld, E. Liozon, P. Cathebras, G. Besson, C. Robin, A. Vighetto, J. F. Mosnier, I. Durieu, D. Vital Durand, and H. Rousset. 2002. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore) 81:443-457. [DOI] [PubMed] [Google Scholar]
- 130.Ghigo, E., C. Capo, N. Amirayan, D. Raoult, and J. Mege. 2000. The 75-kD tumour necrosis factor (TNF) receptor is specifically up-regulated in monocytes during Q fever endocarditis. Clin. Exp. Immunol. 121:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ghigo, E., C. Capo, M. Aurouze, C.-H. Tung, J.-P. Gorvel, D. Raoult, and J.-L. Mege. 2002. Survival of Tropheryma whipplei, the agent of Whipple's disease, requires phagosome acidification. Infect. Immun. 70:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghigo, E., C. Capo, D. Raoult, and J. L. Mege. 2001. Interleukin-10 stimulates Coxiella burnetii replication in human monocytes through tumor necrosis factor down-modulation: role in microbicidal defect of Q fever. Infect. Immun. 69:2345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ghigo, E., C. Capo, C. H. Tung, D. Raoult, J. P. Gorvel, and J. L. Mege. 2002. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J. Immunol. 169:4488-4495. [DOI] [PubMed] [Google Scholar]
- 134.Ghon, J. 1907. Bericht uber den XIV Internationelen Kongress fur Hygiene. Berlin, Germany.
- 135.Gilot, P., and J. Content. 2002. Specific identification of Listeria welshimeri and Listeria monocytogenes by PCR assays targeting a gene encoding a fibronectin-binding protein. J. Clin. Microbiol. 40:698-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Girgis, N. I., J. E. Sippel, M. E. Kilpatrick, W. R. Sanborn, I. A. Mikhail, E. Cross, M. W. Erian, Y. Sultan, and Z. Farid. 1993. Meningitis and encephalitis at the Abbassia Fever Hospital, Cairo, Egypt, from 1966 to 1989. Am. J. Trop. Med. Hyg. 48:97-107. [DOI] [PubMed] [Google Scholar]
- 137.Girgis, N. I., Y. Sultan, Z. Farid, M. M. Mansour, M. W. Erian, L. S. Hanna, and A. J. Mateczun. 1998. Tuberculosis meningitis, Abbassia Fever Hospital-Naval Medical Research Unit No. 3-Cairo, Egypt, from 1976 to 1996. Am. J. Trop. Med. Hyg. 58:28-34. [DOI] [PubMed] [Google Scholar]
- 138.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 139.Gouin, E., H. Gantelet, C. Egile, I. Lasa, H. Ohayon, V. Villiers, P. Gounon, P. J. Sansonetti, and P. Cossart. 1999. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J. Cell Sci. 112:1697-1708. [DOI] [PubMed] [Google Scholar]
- 140.Goulet, V., and P. Marchetti. 1996. Listeriosis in 225 non-pregnant patients in 1992: clinical aspects and outcome in relation to predisposing conditions. Scand. J. Infect. Dis. 28:367-374. [DOI] [PubMed] [Google Scholar]
- 141.Goulet, V., J. Rocourt, I. Rebiere, C. Jacquet, C. Moyse, P. Dehaumont, G. Salvat, and P. Veit. 1998. Listeriosis outbreak associated with the consumption of rillettes in France in 1993. J. Infect. Dis. 177:155-160. [DOI] [PubMed] [Google Scholar]
- 142.Graham, S. M. 2002. Salmonellosis in children in developing and developed countries and populations. Curr. Opin. Infect. Dis. 15:507-512. [DOI] [PubMed] [Google Scholar]
- 143.Granfors, K., S. Jalkanen, A. A. Lindberg, O. Maki-Ikola, R. von Essen, R. Lahesmaa-Rantala, H. Isomaki, R. Saario, W. J. Arnold, and A. Toivanen. 1990. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet 335:685-688. [DOI] [PubMed] [Google Scholar]
- 144.Grant, A. C., S. Hunter, and W. C. Partin. 1997. A case of acute monocytic ehrlichiosis with prominent neurologic signs. Neurology 48:1619-1623. [DOI] [PubMed] [Google Scholar]
- 145.Gray, M. L. 1962. Listeric infection in man in the United States, p. 290. In M. L. Gray (ed.), Second Symposium on Listeric Infection. Artcraft Printers, Bozeman, Mont.
- 146.Gray, M. L., and A. H. Killinger. 1966. Listeria monocytogenes and listeric infections. Bacteriol Rev. 30:309-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Green, P. H. 1836. Tubercular meningitis. Lancet ii:232-235. [Google Scholar]
- 148.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157:2514-2520. [PubMed] [Google Scholar]
- 149.Greiffenberg, L., W. Goebel, K. S. Kim, J. Daniels, and M. Kuhn. 2000. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: an electron microscopic study. Infect. Immun. 68:3275-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Greiffenberg, L., W. Goebel, K. S. Kim, I. Weiglein, A. Bubert, F. Engelbrecht, M. Stins, and M. Kuhn. 1998. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: inlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 66:5260-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Greiffenberg, L., Z. Sokolovic, H. J. Schnittler, A. Spory, R. Bockmann, W. Goebel, and M. Kuhn. 1997. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol. Lett. 157:163-170. [DOI] [PubMed] [Google Scholar]
- 152.Gross, A., A. Terraza, S. Ouahrani-Bettache, J. P. Liautard, and J. Dornand. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gross, L. 1996. How Charles Nicolle of the Pasteur Institute discovered that epidemic typhus is transmitted by lice: reminiscences from my years at the Pasteur Institute in Paris. Proc. Natl. Acad. Sci. USA 93:10539-10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guzman-Verri, C., E. Chaves-Olarte, C. von Eichel-Streiber, I. Lopez-Goni, M. Thelestam, S. Arvidson, J. P. Gorvel, and E. Moreno. 2001. GTPases of the Rho subfamily are required for Brucella abortus internalization in nonprofessional phagocytes: direct activation of Cdc42. J. Biol. Chem. 276:44435-44443. [DOI] [PubMed] [Google Scholar]
- 155.Guzman-Verri, C., L. Manterola, A. Sola-Landa, A. Parra, A. Cloeckaert, J. Garin, J. P. Gorvel, I. Moriyon, E. Moreno, and I. Lopez-Goni. 2002. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc. Natl. Acad. Sci. USA 99:12375-12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Haas, D. W., and R. M. Des Prez. 1994. Tuberculosis and acquired immunodeficiency syndrome: a historical perspective on recent developments. Am. J. Med. 96:439-450. [DOI] [PubMed] [Google Scholar]
- 157.Habeeb, Y. K., A. K. Al-Najdi, S. A. Sadek, and E. Al-Onaizi. 1998. Paediatric neurobrucellosis: case report and literature review. J. Infect. 37:59-62. [DOI] [PubMed] [Google Scholar]
- 158.Hackstadt, T. 1998. The diverse habitats of obligate intracellular parasites. Curr. Opin. Microbiol. 1:82-87. [DOI] [PubMed] [Google Scholar]
- 159.Hansmann, G. H., and J. R. Schenken. 1932. Melitensis meningo-encephalitis. Mycotic aneurysm due to Brucella melitensis var. porcine. Am. J. Pathol. 8:435-443. [PMC free article] [PubMed] [Google Scholar]
- 160.Harrell, G. T. 1953. Rickettsial involvement of the nervous system. Med. Clin. North Am. 37:395-422. [DOI] [PubMed] [Google Scholar]
- 161.Harrell, G. T. 1949. Rocky Mountain spotted fever. Medicine (Baltimore) 28:333-370. [DOI] [PubMed] [Google Scholar]
- 162.Heinzen, R., M. Scidmore, D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hertzig, T., M. Weber, L. Greiffenberg, B. S. Holthausen, W. Goebel, K. S. Kim, and M. Kuhn. 2003. Antibodies present in normal human serum inhibit invasion of human brain microvascular endothelial cells by Listeria monocytogenes. Infect. Immun. 71:95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hickey, W. F. 2001. Basic principles of immunological surveillance of the normal central nervous system. Glia 36:118-124. [DOI] [PubMed] [Google Scholar]
- 166.Hof, H., T. Nichterlein, and M. Kretschmar. 1997. Management of listeriosis. Clin. Microbiol. Rev. 10:345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hohmann, E. L. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263-269. [DOI] [PubMed] [Google Scholar]
- 168.Holden, D. W. 2002. Trafficking of the Salmonella vacuole in macrophages. Traffic 3:161-169. [DOI] [PubMed] [Google Scholar]
- 169.Hopkins, S. A., F. Niedergang, I. E. Corthesy-Theulaz, and J. P. Kraehenbuhl. 2000. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell Microbiol. 2:59-68. [DOI] [PubMed] [Google Scholar]
- 170.Howe, D., and L. P. Mallavia. 2000. Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells. Infect. Immun. 68:3815-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang, L. T., S. F. Ko, and C. C. Lui. 1997. Salmonella meningitis: clinical experience with third generation cephalosporins. Acta Paediatr. 86:1056-1058. [DOI] [PubMed] [Google Scholar]
- 172.Huang, S. H., and A. Y. Jong. 2001. Cellular mechanisms of microbial proteins contributing to invasion of the blood-brain barrier. Cell Microbiol. 3:277-287. [DOI] [PubMed] [Google Scholar]
- 173.Huber, J. D., R. D. Egleton, and T. P. Davis. 2001. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 24:719-725. [DOI] [PubMed] [Google Scholar]
- 174.Hülphers, G. 1911. Lefvernekros hos kanin orsakad af en ej förut beskrifven bakterie. Sven. Vet. Tidskr. 2:265-273. [Google Scholar]
- 175.Hussein, A. S., and S. D. Shafran. 2000. Acute bacterial meningitis in adults. A 12-year review. Medicine (Baltimore) 79:360-368. [DOI] [PubMed] [Google Scholar]
- 176.Ihn, K. S., S. H. Han, H. R. Kim, M. S. Huh, S. Y. Seong, J. S. Kang, T. H. Han, I. S. Kim, and M. S. Choi. 2000. Cellular invasion of Orientia tsutsugamushi requires initial interaction with cell surface heparan sulfate. Microb. Pathog. 28:227-233. [DOI] [PubMed] [Google Scholar]
- 177.Ivanoff, B. 1995. Typhoid fever: global situation and WHO recommendations. Southeast Asian J. Trop. Med. Public Health 26:1-6. [Google Scholar]
- 178.Jain, K. C., and A. K. Mahapatra. 1998. Subdural empyema due to Salmonella infection. Pediatr. Neurosurg. 28:89-90. [DOI] [PubMed] [Google Scholar]
- 179.Jensen, A., W. Frederiksen, and P. Gerner-Smidt. 1994. Risk factors for listeriosis in Denmark, 1989-1990. Scand. J. Infect. Dis. 26:171-178. [DOI] [PubMed] [Google Scholar]
- 180.Jiao, X., R. Lo-Man, P. Guermonprez, L. Fiette, E. Deriaud, S. Burgaud, B. Gicquel, N. Winter, and C. Leclerc. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168:1294-1301. [DOI] [PubMed] [Google Scholar]
- 181.Jin, Y., L. Dons, K. Kristensson, and M. E. Rottenberg. 2002. Colony-stimulating factor 1-dependent cells protect against systemic infection with Listeria monocytogenes but facilitate neuroinvasion. Infect. Immun. 70:4682-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jin, Y., L. Dons, K. Kristensson, and M. E. Rottenberg. 2001. Neural route of cerebral Listeria monocytogenes murine infection: role of immune response mechanisms in controlling bacterial neuroinvasion. Infect. Immun. 69:1093-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jungi, T. W., H. Pfister, H. Sager, R. Fatzer, M. Vandevelde, and A. Zurbriggen. 1997. Comparison of inducible nitric oxide synthase expression in the brains of Listeria monocytogenes-infected cattle, sheep, and goats and in macrophages stimulated in vitro. Infect. Immun. 65:5279-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kaplan, M. M. 1945. Listerellosis. N. Engl. J. Med. 232:755-759. [Google Scholar]
- 185.Kaplowitz, L. G., J. V. Lange, J. J. Fischer, and D. H. Walker. 1983. Correlation of rickettsial titers, circulating endotoxin, and clinical features in Rocky Mountain spotted fever. Arch. Intern. Med. 143:1149-1151. [PubMed] [Google Scholar]
- 186.Karim, M., and N. Islam. 2002. Salmonella meningitis: report of three cases in adults and literature review. Infection 30:104-108. [DOI] [PubMed] [Google Scholar]
- 187.Karsner, H. T. 1953. Pathology of epidemic typhus. Arch. Pathol. 56:397-435. [Google Scholar]
- 188.Karstaedt, A. S., S. Valtchanova, R. Barriere, and H. H. Crewe-Brown. 1998. Tuberculosis meningitis in South African urban adults. Q. J. Med. 91:743-747. [DOI] [PubMed] [Google Scholar]
- 189.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 190.Katrak, S. M., P. K. Shembalkar, S. R. Bijwe, and L. D. Bhandarkar. 2000. The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J. Neurol. Sci. 181:118-126. [DOI] [PubMed] [Google Scholar]
- 191.Kavaliotis, J., A. Tsiaousi, D. Papavasiliou, and A. Kansouzidou. 1994. Non-typhoid Salmonella meningitis. Scand. J. Infect. Dis. 26:403-405. [DOI] [PubMed] [Google Scholar]
- 192.Kayal, S., A. Lilienbaum, O. Join-Lambert, X. Li, A. Israel, and P. Berche. 2002. Listeriolysin O secreted by Listeria monocytogenes induces NF-kappaB signalling by activating the IkappaB kinase complex. Mol. Microbiol. 44:1407-1419. [DOI] [PubMed] [Google Scholar]
- 193.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31:1709-1722. [DOI] [PubMed] [Google Scholar]
- 194.Kee, S. H., K. A. Cho, M. K. Kim, B. U. Lim, W. H. Chang, and J. S. Kang. 1999. Disassembly of focal adhesions during apoptosis of endothelial cell line ECV304 infected with Orientia tsutsugamushi. Microb. Pathog. 27:265-271. [DOI] [PubMed] [Google Scholar]
- 195.Keenan, K. P., W. C. Buhles, Jr., D. L. Huxsoll, R. G. Williams, P. K. Hildebrandt, J. M. Campbell, and E. H. Stephenson. 1977. Pathogenesis of infection with Rickettsia rickettsii in the dog: a disease model for Rocky Mountain spotted fever. J. Infect. Dis. 135:911-917. [DOI] [PubMed] [Google Scholar]
- 195a.Kenyan/British Medical Research Council. 1989. Tuberculosis in Kenya 1984: a third national survey and a comparison with earlier surveys in 1964 and 1974. A Kenyan/British Medical Research Council Co-operative Investigation. Tubercle 70:5-20. [DOI] [PubMed] [Google Scholar]
- 196.Kim, K. S. 2002. Strategy of Escherichia coli for crossing the blood-brain barrier. J. Infect. Dis. 186(Suppl. 2):S220-S224. [DOI] [PubMed] [Google Scholar]
- 197.Kim, M. K., and J. S. Kang. 2001. Orientia tsutsugamushi suppresses the production of inflammatory cytokines induced by its own heat-stable component in murine macrophages. Microb. Pathog. 31:145-150. [DOI] [PubMed] [Google Scholar]
- 198.Kim, M. K., S. H. Kee, K. A. Cho, M. H. Chung, B. U. Lim, W. H. Chang, and J. S. Kang. 1999. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol. Immunol. 43:751-757. [DOI] [PubMed] [Google Scholar]
- 199.Kim, S. W., K. S. Ihn, S. H. Han, S. Y. Seong, I. S. Kim, and M. S. Choi. 2001. Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect. Immun. 69:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kirk, J. 1993. Diagnostic ultrastructure of Listeria monocytogenes in human central nervous tissue. Ultrastruct. Pathol. 17:583-592. [DOI] [PubMed] [Google Scholar]
- 201.Kirveskari, J., S. Jalkanen, O. Maki-Ikola, and K. Granfors. 1998. Increased synovial endothelium binding and transendothelial migration of mononuclear cells during Salmonella infection. Arthritis Rheum. 41:1054-1063. [DOI] [PubMed] [Google Scholar]
- 202.Klein, N. C., B. Damsker, and S. Z. Hirschman. 1985. Mycobacterial meningitis. Retrospective analysis from 1970 to 1983. Am. J. Med. 79:29-34. [DOI] [PubMed] [Google Scholar]
- 203.Kocan, A. A., G. C. Levesque, L. C. Whitworth, G. L. Murphy, S. A. Ewing, and R. W. Barker. 2000. Naturally occurring Ehrlichia chaffeensis infection in coyotes from Oklahoma. Emerg. Infect. Dis. 6:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Koch, R. 1882. Die aetologie der tuberculosis. Berl. Klin. Wochenschr. 19:2211. [Google Scholar]
- 205.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 206.Koedel, U., W. M. Scheld, and H. W. Pfister. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2:721-736. [DOI] [PubMed] [Google Scholar]
- 207.Kohler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J. P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kolb-Maurer, A., S. Pilgrim, E. Kampgen, A. D. McLellan, E. B. Brocker, W. Goebel, and I. Gentschev. 2001. Antibodies against listerial protein 60 act as an opsonin for phagocytosis of Listeria monocytogenes by human dendritic cells. Infect. Immun. 69:3100-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Konomi, N., E. Lebwohl, K. Mowbray, I. Tattersall, and D. Zhang. 2002. Detection of mycobacterial DNA in Andean mummies. J. Clin. Microbiol. 40:4738-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kremer, S., G. Besson, B. Bonaz, B. Pasquier, J.-F. Le Bas, and S. Grand. 2001. Diffuse lesions in the CNS revealed by MR imaging in a case of Whipple diseases. Am. J. Neuroradiol. 22:493-495. [PMC free article] [PubMed] [Google Scholar]
- 211.Krueger, N., C. Low, and W. Donachie. 1995. Phenotypic characterization of the cells of the inflammatory response in ovine encephalitic listeriosis. J. Comp. Pathol. 113:263-275. [DOI] [PubMed] [Google Scholar]
- 212.Krull, M., R. Nost, S. Hippenstiel, E. Domann, T. Chakraborty, and N. Suttorp. 1997. Listeria monocytogenes potently induces up-regulation of endothelial adhesion molecules and neutrophil adhesion to cultured human endothelial cells. J. Immunol. 159:1970-1976. [PubMed] [Google Scholar]
- 213.Kumari, P., and V. L. Kan. 2000. Salmonella typhimurium brain abscess: postoperative complication. Clin. Infect. Dis. 30:621-622. [DOI] [PubMed] [Google Scholar]
- 214.Laguna, F., M. Adrados, A. Ortega, and J. M. Gonzalez-Lahoz. 1992. Tuberculous meningitis with acellular cerebrospinal fluid in AIDS patients. AIDS 6:1165-1167. [DOI] [PubMed] [Google Scholar]
- 215.Larsson, S., S. Cronberg, and S. Winblad. 1978. Clinical aspects on 64 cases of juvenile and adult listeriosis in Sweden. Acta Med. Scand. 204:503-508. [DOI] [PubMed] [Google Scholar]
- 216.La Scola, B., F. Fenollar, P. E. Fournier, M. Altwegg, M. N. Mallet, and D. Raoult. 2001. Description of Tropheryma whipplei gen. nov. sp. nov., the Whipple's disease bacillus. Int. J. Syst. Evol. Microbiol. 51:1471-1479. [DOI] [PubMed] [Google Scholar]
- 217.La Scolea, L. J., Jr., and D. Dryja. 1984. Quantitation of bacteria in cerebrospinal fluid and blood of children with meningitis and its diagnostic significance. J. Clin. Microbiol. 19:187-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee, W. S., S. D. Puthucheary, and A. Omar. 1999. Salmonella meningitis and its complications in infants. J. Paediatr. Child Health 35:379-382. [DOI] [PubMed] [Google Scholar]
- 219.Leemans, J. C., S. Florquin, M. Heikens, S. T. Pals, R. van der Neut, and T. Van Der Poll. 2003. CD44 is a macrophage binding site for Mycobacterium tuberculosis that mediates macrophage recruitment and protective immunity against tuberculosis. J. Clin. Investig. 111:681-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Leonard, M. K., J. R. Murrow, R. Jurado, and R. Gaynes. 2002. Salmonella meningitis in adults infected with HIV: case report and review of the literature. Am. J. Med. Sci. 323:266-268. [DOI] [PubMed] [Google Scholar]
- 221.Li, H., and D. H. Walker. 1998. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 24:289-298. [DOI] [PubMed] [Google Scholar]
- 222.Li, J. S., and G. M. Winslow. 2003. Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect. Immun. 71:4229-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lillie, R. D., D. E. Smith, and B. K. Black. 1953. Pathology of epidemic typhus. Arch. Pathol. 56:512-553. [PubMed] [Google Scholar]
- 224.Linnan, M. J., L. Mascola, X. D. Lou, V. Goulet, S. May, C. Salminen, D. W. Hird, M. L. Yonekura, P. Hayes, R. Weaver, and et al. 1988. Epidemic listeriosis associated with Mexican-style cheese. N. Engl. J. Med. 319:823-828. [DOI] [PubMed] [Google Scholar]
- 225.López, S., D. Borras, C. Juan-Salles, N. Prats, M. Domingo, and A. J. Marco. 1997. Immunohistochemical detection of adhesion molecules intercellular adhesion molecule-1 and E-selectin in formalin-fixed, paraffin-embedded mouse tissues. Lab. Investig. 77:543-544. [PubMed] [Google Scholar]
- 226.López, S., N. Prats, and A. J. Marco. 1999. Expression of E-selectin, P-selectin, and intercellular adhesion molecule-1 during experimental murine listeriosis. Am. J. Pathol. 155:1391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lopez Ramirez, G. M., W. N. Rom, C. Ciotoli, A. Talbot, F. Martiniuk, B. Cronstein, and J. Reibman. 1994. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect. Immun. 62:2515-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Louis, E. D., T. Lynch, P. Kaufmann, S. Fahn, and J. Odel. 1996. Diagnostic guidelines in central nervous system Whipple's disease. Ann. Neurol. 40:561-568. [DOI] [PubMed] [Google Scholar]
- 229.Louria, D. B., T. Hensle, D. Armstrong, H. S. Collins, A. Blevins, D. Krugman, and M. Buse. 1967. Listeriosis complicating malignant disease. A new association. Ann. Intern. Med. 67:260-281. [DOI] [PubMed] [Google Scholar]
- 230.Low, J. C., and W. Donachie. 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153:9-29. [DOI] [PubMed] [Google Scholar]
- 231.Low, L. C. K., B. C. C. Lam, W. T. Wong, W. Y. Chan-Lui, and C. Y. Yeung. 1984. Salmonella meningitis in infancy. Aust. Paediatr. J. 20:225-228. [DOI] [PubMed] [Google Scholar]
- 232.Lu, C. S., C. H. Chiu, T. Y. Lin, and S. L. Lin. 1997. Salmonella typhimurium brain abscess in a six-month-old infant: a case report and review of the literature. Changgeng Yi Xue Za Zhi 20:219-225. [PubMed] [Google Scholar]
- 233.Lulu, A. R., G. F. Araj, M. I. Khateeb, M. Y. Mustafa, A. R. Yusuf, and F. F. Fenech. 1988. Human brucellosis in Kuwait: a prospective study of 400 cases. Q. J. Med. 66:39-54. [PubMed] [Google Scholar]
- 234.MacGregor, A. R., and C. A. Green. 1937. Tuberculosis of the central nervous system, with special reference to tuberculous meningitis. J. Pathol. Bacteriol. 45:613-645. [Google Scholar]
- 235.Maeda, K., N. Markowitz, R. C. Hawley, M. Ristic, D. Cox, and J. E. McDade. 1987. Human infection with Ehrlichia canis, a leukocytic rickettsia. N. Engl. J. Med. 316:853-856. [DOI] [PubMed] [Google Scholar]
- 236.Mahapatra, A. K., S. J. Pawar, and R. R. Sharma. 2002. Intracranial Salmonella infections: meningitis, subdural collections and brain abscess. A series of six surgically managed cases with follow-up results. Pediatr. Neurosurg. 36:8-13. [DOI] [PubMed] [Google Scholar]
- 237.Maiwald, M., F. Schumacher, H. J. Ditton, and A. von Herbay. 1999. Environmental occurrence of the Whipple's disease bacterium (Tropheryma whipplei). Appl. Environ. Microbiol. 64:760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Maiwald, M., A. Von Herbay, D. N. Fredricks, C. C. Ouverney, J. C. Kosek, and D. A. Relman. 2003. Cultivation of Tropheryma whipplei from cerebrospinal fluid. J. Infect. Dis. 188:801-808. [DOI] [PubMed] [Google Scholar]
- 239.Marco, A. J., M. Domingo, M. Prats, V. Briones, M. Pumarola, and L. Dominguez. 1991. Pathogenesis of lymphoid lesions in murine experimental listeriosis. J. Comp. Pathol. 105:1-15. [DOI] [PubMed] [Google Scholar]
- 240.Marquis, H., H. Goldfine, and D. A. Portnoy. 1997. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol. 137:1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Marra, A., and D. Brigham. 2001. Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect. Immun. 69:7318-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Marth, T., N. Kleen, A. Stallmach, S. Ring, S. Aziz, C. Schmidt, W. Strober, M. Zeitz, and T. Schneider. 2002. Dysregulated peripheral and mucosal Th1/Th2 response in Whipple's disease. Gastroenterology 123:1468-1477. [DOI] [PubMed] [Google Scholar]
- 243.Marth, T., and D. Raoult. 2003. Whipple's disease. Lancet 361:239-246. [DOI] [PubMed] [Google Scholar]
- 244.Massung, R. F., L. E. Davis, K. Slater, D. B. McKechnie, and M. Puerzer. 2001. Epidemic typhus meningitis in the southwestern United States. Clin. Infect. Dis. 32:979-982. [DOI] [PubMed] [Google Scholar]
- 245.Mastroianni, C. M., L. Lancella, F. Mengoni, M. Lichtner, P. Santopadre, D. A. C, F. Ticca, and V. Vullo. 1998. Chemokine profiles in the cerebrospinal fluid (CSF) during the course of pyogenic and tuberculous meningitis. Clin. Exp. Immunol. 114:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mastroianni, C. M., F. Paoletti, M. Lichtner, D. A. C, V. Vullo, and S. Delia. 1997. Cerebrospinal fluid cytokines in patients with tuberculous meningitis. Clin. Immunol. Immunopathol. 84:171-176. [DOI] [PubMed] [Google Scholar]
- 247.Matsui, H., M. Eguchi, and Y. Kikuchi. 2000. Use of confocal microscopy to detect Salmonella typhimurium within host cells associated with Spv-mediated intracellular proliferation. Microb. Pathog. 29:53-59. [DOI] [PubMed] [Google Scholar]
- 248.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 60:5013-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McDade, J. E. 1990. Ehrlichiosis—a disease of animals and humans. J. Infect. Dis. 161:609-617. [DOI] [PubMed] [Google Scholar]
- 251.McLauchlin, J. 1990. Human listeriosis in Britain, 1967-85, a summary of 722 cases. 1. Listeriosis during pregnancy and in the newborn. Epidemiol. Infect. 104:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.McLauchlin, J. 1990. Human listeriosis in Britain, 1967-85, a summary of 722 cases. 2. Listeriosis in non-pregnant individuals, a changing pattern of infection and seasonal incidence. Epidemiol. Infect. 104:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McLean, D. R., N. Russell, and M. Y. Khan. 1992. Neurobrucellosis: clinical and therapeutic features. Clin. Infect. Dis. 15:582-590. [DOI] [PubMed] [Google Scholar]
- 254.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mendel, E., L. T. Khoo, J. L. Go, D. Hinton, C.-S. Zee, and M. L. J. Apuzzo. 1999. Intracerebral Whipple's disease diagnosed by stereotactic biopsy: a case report and review of the literature. Neurosurgery 44:203-209. [DOI] [PubMed] [Google Scholar]
- 256.Messer, R. D., T. H. Warnock, R. J. Heazlewood, and J. N. Hanna. 1997. Salmonella meningitis in children in Far North Queensland. J. Paediatr. Child Health 33:535-538. [DOI] [PubMed] [Google Scholar]
- 257.Mettille, F. C., K. F. Salata, K. J. Belanger, B. G. Casleton, and D. J. Kelly. 2000. Reducing the risk of transfusion-transmitted rickettsial disease by WBC filtration, using Orientia tsutsugamushi in a model system. Transfusion 40:290-296. [DOI] [PubMed] [Google Scholar]
- 258.Michaux-Charachon, S., E. Jumas-Bilak, A. Allardet-Servent, G. Bourg, M. L. Boschiroli, M. Ramuz, and D. O'Callaghan. 2002. The Brucella genome at the beginning of the post-genomic era. Vet. Microbiol. 90:581-585. [DOI] [PubMed] [Google Scholar]
- 259.Miller, D. W. 1999. Immunobiology of the blood-brain barrier. J. Neurovirol. 5:570-578. [DOI] [PubMed] [Google Scholar]
- 260.Miller, J. Q., and T. R. Price. 1972. Involvement of the brain in Rocky Mountain spotted fever. South. Med. J. 65:437-439. [DOI] [PubMed] [Google Scholar]
- 261.Miller, J. Q., and T. R. Price. 1972. The nervous system in Rocky Mountain spotted fever. Neurology 22:561-566. [DOI] [PubMed] [Google Scholar]
- 262.Miller, S. I., and D. A. Pegues. 2000. Salmonella species, including Salmonella typhi, p. 2344-2363. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 263.Misbah, S. A., and N. P. Mapstone. 2000. Whipple's disease revisited. J. Clin. Pathol. 53:750-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mittal, S. K., V. Aggarwal, A. Rastogi, and N. Saini. 1996. Does B. C. G. vaccination prevent or postpone the occurrence of tuberculous meningitis? Indian J. Pediatr. 63:659-664. [DOI] [PubMed] [Google Scholar]
- 265.Molavi, A., and J. L. LeFrock. 1985. Tuberculous meningitis. Med. Clin. North Am. 69:315-331. [DOI] [PubMed] [Google Scholar]
- 266.Moreno, E., and I. Moriyon. 2002. Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc. Natl. Acad. Sci. USA 99:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Moron, C. G., V. L. Popov, H. M. Feng, D. Wear, and D. H. Walker. 2001. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod. Pathol. 14:752-759. [DOI] [PubMed] [Google Scholar]
- 268.Mott, J., R. E. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67:1368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mousa, A. R., K. M. Elhag, M. Khogali, and A. A. Marafie. 1988. The nature of human brucellosis in Kuwait: study of 379 cases. Rev. Infect. Dis. 10:211-217. [DOI] [PubMed] [Google Scholar]
- 270.Mousa, A. R., T. S. Koshy, G. F. Araj, A. A. Marafie, S. A. Muhtaseb, D. S. Al-Mudallal, and M. S. Busharetulla. 1986. Brucella meningitis: presentation, diagnosis and treatment—a prospective study of ten cases. Q. J. Med. 60:873-885. [PubMed] [Google Scholar]
- 271.Mylonakis, E., E. L. Hohmann, and S. B. Calderwood. 1998. Central nervous system infection with Listeria monocytogenes. 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 77:313-336. [DOI] [PubMed] [Google Scholar]
- 272.Nacy, C. A., and M. S. Meltzer. 1979. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J. Immunol. 123:2544-2549. [PubMed] [Google Scholar]
- 273.Naito, M., S. Umeda, K. Takahashi, and L. D. Shultz. 1997. Macrophage differentiation and granulomatous inflammation in osteopetrotic mice (op/op) defective in the production of CSF-1. Mol. Reprod. Dev. 46:85-91. [DOI] [PubMed] [Google Scholar]
- 274.Nassif, X., S. Bourdoulous, E. Eugene, and P. O. Couraud. 2002. How do extracellular pathogens cross the blood-brain barrier? Trends Microbiol. 10:227-232. [DOI] [PubMed] [Google Scholar]
- 275.Ng, F. K., S. C. Oaks, Jr., M. Lee, M. G. Groves, and G. E. Lewis, Jr. 1985. A scanning and transmission electron microscopic examination of Rickettsia tsutsugamushi-infected human endothelial, MRC-5, and L-929 cells. Jpn. J. Med. Sci. Biol. 38:125-139. [DOI] [PubMed] [Google Scholar]
- 276.Nichols, E. 1951. Meningo-encephalitis due to brucellosis with the report of a case in which B. abortus was recovered from the cerebrospinal fluid, and a review of the literature. Ann. Intern. Med. 35:673-693. [DOI] [PubMed] [Google Scholar]
- 277.Nieman, R. E., and B. Lorber. 1980. Listeriosis in adults: a changing pattern. Report of eight cases and review of the literature, 1968-1978. Rev. Infect. Dis. 2:207-227. [DOI] [PubMed] [Google Scholar]
- 278.Nolla-Salas, J., J. Bosch, I. Gasser, L. Vinas, M. de Simon, M. Almela, C. Latorre, P. Coll, and M. D. Ferrer. 1998. Perinatal listeriosis: a population-based multicenter study in Barcelona, Spain (1990-1996). Am. J. Perinatol. 15:461-467. [DOI] [PubMed] [Google Scholar]
- 279.Nottet, H. S. 1999. Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood-brain barrier function. J. Neurovirol. 5:659-669. [DOI] [PubMed] [Google Scholar]
- 280.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 281.Ogawa, S. K., M. A. Smith, D. J. Brennessel, and F. D. Lowy. 1987. Tuberculous meningitis in an urban medical center. Medicine (Baltimore) 66:317-326. [DOI] [PubMed] [Google Scholar]
- 282.Olano, J. P., and D. H. Walker. 2002. Human ehrlichioses. Med. Clin. North Am. 86:375-392. [DOI] [PubMed] [Google Scholar]
- 283.Otter, A., and W. F. Blakemore. 1989. Observation on the presence of Listeria monocytogenes in axons. Acta Microbiol. Hung. 36:125-131. [PubMed] [Google Scholar]
- 284.Pai, H., S. Sohn, Y. Seong, S. Kee, W. H. Chang, and K. W. Choe. 1997. Central nervous system involvement in patients with scrub typhus. Clin. Infect. Dis. 24:436-440. [DOI] [PubMed] [Google Scholar]
- 285.Panciera, R. J., S. A. Ewing, and A. W. Confer. 2001. Ocular histopathology of ehrlichial infections in the dog. Vet. Pathol. 38:43-46. [DOI] [PubMed] [Google Scholar]
- 286.Parida, S. K., E. Domann, M. Rohde, S. Muller, A. Darji, T. Hain, J. Wehland, and T. Chakraborty. 1998. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol. Microbiol. 28:81-93. [DOI] [PubMed] [Google Scholar]
- 287.Peluso, R., A. Haase, L. Stowring, M. Edwards, and P. Ventura. 1985. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology 147:231-236. [DOI] [PubMed] [Google Scholar]
- 288.Perine, P. L., B. P. Chandler, D. K. Krause, P. McCardle, S. Awoke, E. Habte-Gabr, C. L. Wisseman, Jr., and J. E. McDade. 1992. A clinico-epidemiological study of epidemic typhus in Africa. Clin. Infect. Dis. 14:1149-1158. [DOI] [PubMed] [Google Scholar]
- 289.Perry, V. H., D. C. Anthony, S. J. Bolton, and H. C. Brown. 1997. The blood-brain barrier and the inflammatory response. Mol. Med. Today 3:335-341. [DOI] [PubMed] [Google Scholar]
- 290.Peters, M., and M. Hewicker-Trautwein. 1994. Infection of murine fetal brain cell cultures with Listeria monocytogenes. Vet. Microbiol. 41:19-28. [DOI] [PubMed] [Google Scholar]
- 291.Peters, M., and M. Hewicker-Trautwein. 1996. Studies on the cell tropism of Listeria monocytogenes in ovine fetal brain cell cultures. Vet. Microbiol. 49:169-179. [DOI] [PubMed] [Google Scholar]
- 292.Pieters, J. 2001. Entry and survival of pathogenic mycobacteria in macrophages. Microbes Infect. 3:249-255. [DOI] [PubMed] [Google Scholar]
- 293.Pieters, J., and J. Gatfield. 2002. Hijacking the host: survival of pathogenic mycobacteria inside macrophages. Trends Microbiol. 10:142-146. [DOI] [PubMed] [Google Scholar]
- 294.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pizarro-Cerda, J., E. Moreno, V. Sanguedolce, J. L. Mege, and J. P. Gorvel. 1998. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 66:2387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pollock, S. S., T. M. Pollock, and M. J. Harrison. 1984. Infection of the central nervous system by Listeria monocytogenes: a review of 54 adult and juvenile cases. Q. J. Med. 53:331-340. [PubMed] [Google Scholar]
- 297.Popov, V. L., X. Yu, and D. H. Walker. 2000. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb. Pathog. 28:71-80. [DOI] [PubMed] [Google Scholar]
- 298.Porkert, M. T., M. Sotir, P. Parrott-Moore, and H. M. Blumberg. 1997. Tuberculous meningitis at a large inner-city medical center. Am. J. Med. Sci. 313:325-331. [DOI] [PubMed] [Google Scholar]
- 299.Portnoy, D. A., V. Auerbuch, and I. J. Glomski. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Prats, N., V. Briones, M. M. Blanco, J. Altimira, J. A. Ramos, L. Dominguez, and A. Marco. 1992. Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. Eur. J. Clin. Microbiol. Infect. Dis. 11:744-747. [DOI] [PubMed] [Google Scholar]
- 302.Pron, B., C. Boumaila, F. Jaubert, P. Berche, G. Milon, F. Geissmann, and J. L. Gaillard. 2001. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell Microbiol. 3:331-340. [DOI] [PubMed] [Google Scholar]
- 303.Pron, B., M. K. Taha, C. Rambaud, J. C. Fournet, N. Pattey, J. P. Monnet, M. Musilek, J. L. Beretti, and X. Nassif. 1997. Interaction of Neisseria meningitidis with the components of the blood-brain barrier correlates with an increased expression of PilC. J. Infect. Dis. 176:1285-1292. [DOI] [PubMed] [Google Scholar]
- 304.Puechal, X. 2002. Whipple's disease. Joint Bone Spine 69:133-140. [DOI] [PubMed] [Google Scholar]
- 305.Rana, F. S., M. P. Hawken, C. Mwachari, S. M. Bhatt, F. Abdullah, L. W. Ng'ang'a, C. Power, W. A. Githui, J. D. Porter, and S. B. Lucas. 2000. Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya. J. Acquir. Immune Defic. Syndr. 24:23-29. [DOI] [PubMed] [Google Scholar]
- 306.Raoult, D., M. L. Birg, B. La Scola, P. E. Fournier, M. Enea, H. Lepidi, V. Roux, J. C. Piette, F. Vandenesch, D. Vital-Durand, and T. J. Marrie. 2000. Cultivation of the bacillus of Whipple's disease. N. Engl. J. Med. 342:620-625. [DOI] [PubMed] [Google Scholar]
- 307.Raoult, D., H. Lepidi, and J. Robert. 2001. Tropheryma whipplei circulating in blood monocytes. N. Engl. J. Med. 345:548. [DOI] [PubMed] [Google Scholar]
- 308.Raoult, D., J. B. Ndihokubwayo, H. Tissot-Dupont, V. Roux, B. Faugere, R. Abegbinni, and R. J. Birtles. 1998. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 352:353-358. [DOI] [PubMed] [Google Scholar]
- 309.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Raoult, D., V. Roux, J. B. Ndihokubwayo, G. Bise, D. Baudon, G. Marte, and R. Birtles. 1997. Jail fever (epidemic typhus) outbreak in Burundi. Emerg. Infect. Dis. 3:357-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Raoult, D., H. Tissot-Dupont, C. Foucault, J. Gouvernet, P. E. Fournier, E. Bernit, A. Stein, M. Nesri, J. R. Harle, and P. J. Weiller. 2000. Q fever 1985-1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 79:109-123. [DOI] [PubMed] [Google Scholar]
- 312.Ratnasamy, N., E. D. Everett, W. E. Roland, G. McDonald, and C. W. Caldwell. 1996. Central nervous system manifestations of human ehrlichiosis. Clin. Infect. Dis. 23:314-319. [DOI] [PubMed] [Google Scholar]
- 313.Raveneau, J., C. Geoffroy, J. L. Beretti, J. L. Gaillard, J. E. Alouf, and P. Berche. 1992. Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect. Immun. 60:916-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Reilly, S., J. L. Northwood, and E. O. Caul. 1990. Q fever in Plymouth, 1972-88. A review with particular reference to neurological manifestations. Epidemiol. Infect. 105:391-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Relman, D. A., T. M. Schmidt, R. P. MacDermott, and S. Falkow. 1992. Identification of the uncultured bacillus of Whipple's disease. N. Engl. J. Med. 327:293-301. [DOI] [PubMed] [Google Scholar]
- 316.Rescigno, M., G. Rotta, B. Valzasina, and P. Ricciardi-Castagnoli. 2001. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology 204:572-581. [DOI] [PubMed] [Google Scholar]
- 317.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 318.Rich, A. R., and H. A. McCordock. 1033. The pathogenesis of tuberculous meningitis. Bull. Johns Hopkins Hosp. 52:5-37. [Google Scholar]
- 319.Richter-Dahlfors, A., A. M. J. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ricketts, H. T. 1909. Some aspects of Rocky Mountain spotted fever as shown by recent investigations. Med. Rec. 16:843-855. [DOI] [PubMed] [Google Scholar]
- 321.Riggs, H. E., C. Rupp, and H. Ray. 1956. Clinicopathologic study of tuberculosis meningitis in adults. Am. Rev. Tuberc. 74:830-834. [DOI] [PubMed] [Google Scholar]
- 322.Rikihisa, Y., and S. Ito. 1982. Entry of Rickettsia tsutsugamushi into polymorphonuclear leukocytes. Infect. Immun. 38:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rikihisa, Y., and S. Ito. 1979. Intracellular localization of Rickettsia tsutsugamushi in polymorphonuclear leukocytes. J. Exp. Med. 150:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ring, A., J. N. Weiser, and E. I. Tuomanen. 1998. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Investig. 102:347-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rittig, M. G., M. T. Alvarez-Martinez, F. Porte, J. P. Liautard, and B. Rouot. 2001. Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes. Infect. Immun. 69:3995-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Robbins, J. R., A. I. Barth, H. Marquis, E. L. de Hostos, W. J. Nelson, and J. A. Theriot. 1999. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J. Cell Biol. 146:1333-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rodrigues, L. C., V. K. Diwan, and J. G. Wheeler. 1993. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int. J. Epidemiol. 22:1154-1158. [DOI] [PubMed] [Google Scholar]
- 328.Rodriguez, R. E., V. Valero, and C. Watanakunakorn. 1986. Salmonella focal intracranial infections: review of the world literature (1884-1984) and report of an unusual case. Rev. Infect. Dis. 8:31-41. [DOI] [PubMed] [Google Scholar]
- 329.Rothschild, B. M., L. D. Martin, G. Lev, H. Bercovier, G. K. Bar-Gal, C. Greenblatt, H. Donoghue, M. Spigelman, and D. Brittain. 2001. Mycobacterium tuberculosis complex DNA from an extinct bison dated 17,000 years before the present. Clin. Infect. Dis. 33:305-311. [DOI] [PubMed] [Google Scholar]
- 330.Rubin, L. L., and J. M. Staddon. 1999. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 22:11-28. [DOI] [PubMed] [Google Scholar]
- 331.Rubin, S. A. 1995. Tuberculosis. Captain of all these men of death. Radiol. Clin. North Am. 33:619-639. [PubMed] [Google Scholar]
- 332.Russell, D. G. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell. Biol. 2:569-577. [DOI] [PubMed] [Google Scholar]
- 333.Ryan, U. S., D. R. Schultz, J. D. Goodwin, J. M. Vann, M. P. Selvaraj, and M. A. Hart. 1989. Role of C1q in phagocytosis of Salmonella minnesota by pulmonary endothelial cells. Infect. Immun 57:1356-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Salamina, G., E. Dalle Donne, A. Niccolini, G. Poda, D. Cesaroni, M. Bucci, R. Fini, M. Maldini, A. Schuchat, B. Swaminathan, W. Bibb, J. Rocourt, N. Binkin, and S. Salmaso. 1996. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol. Infect. 117:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Salcedo, S. P., M. Noursadeghi, J. Cohen, and D. W. Holden. 2001. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 3:587-597. [DOI] [PubMed] [Google Scholar]
- 336.Salo, W. L., A. C. Aufderheide, J. Buikstra, and T. A. Holcomb. 1994. Identification of Mycobacterium tuberculosis DNA in a pre-Columbian Peruvian mummy. Proc. Natl. Acad. Sci. USA 91:2091-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sarria, J. C., A. M. Vidal, and R. C. Kimbrough, 3rd. 2000. Salmonella enteritidis brain abscess: case report and review. Clin. Neurol. Neurosurg. 102:236-239. [DOI] [PubMed] [Google Scholar]
- 338.Sawaishi, Y., I. Takahashi, Y. Hirayama, T. Abe, M. Mizutani, K. Hirai, and G. Takada. 1999. Acute cerebellitis caused by Coxiella burnetii. Ann. Neurol. 45:124-127. [DOI] [PubMed] [Google Scholar]
- 339.Sawyer, L. A., D. B. Fishbein, and J. E. McDade. 1987. Q fever: current concepts. Rev. Infect. Dis. 9:935-946. [DOI] [PubMed] [Google Scholar]
- 340.Sayen, J. J., H. S. Pond, J. S. Forrester, and F. C. Wood. 1946. Scrub typhus in Assam and Burma. Medicine (Baltimore) 25:155-214. [DOI] [PubMed] [Google Scholar]
- 341.Schluter, D., E. Domann, C. Buck, T. Hain, H. Hof, T. Chakraborty, and M. Deckert-Schluter. 1998. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun. 66:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Schneider, T., A. Stallmach, A. von Herbay, T. Marth, W. Strober, and M. Zeitz. 1998. Treatment of refractory Whipple diseases with interferon-gamma. Ann. Intern. Med. 129:875-877. [DOI] [PubMed] [Google Scholar]
- 343.Schnider, P. J., E. C. Reisinger, W. Gerschlager, C. Muller, T. Berger, G. J. Krejs, and E. Auff. 1996. Long-term follow-up in cerebral Whipple's disease. Gastroenterol. Hepatol. 8:899-903. [PubMed] [Google Scholar]
- 344.Schuchat, A., K. A. Deaver, J. D. Wenger, B. D. Plikaytis, L. Mascola, R. W. Pinner, A. L. Reingold, C. V. Broome, and The Listeria Study Group. 1992. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. JAMA 267:2041-2045. [PubMed] [Google Scholar]
- 345.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, B. A. Perkins, and the Active Surveillance Team. 1997. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 346.Schutte, C. M. 2001. Clinical, cerebrospinal fluid and pathological findings and outcomes in HIV-positive and HIV-negative patients with tuberculous meningitis. Infection 29:213-217. [DOI] [PubMed] [Google Scholar]
- 347.Schwartz, B., C. A. Ciesielski, C. V. Broome, S. Gaventa, G. R. Brown, B. G. Gellin, A. W. Hightower, and L. Mascola. 1988. Association of sporadic listeriosis with consumption of uncooked hot dogs and undercooked chicken. Lancet ii:779-782. [DOI] [PubMed] [Google Scholar]
- 348.Schwarze, R., C. D. Bauermeister, S. Ortel, and G. Wichmann. 1989. Perinatal listeriosis in Dresden 1981-1986: clinical and microbiological findings in 18 cases. Infection 17:131-138. [DOI] [PubMed] [Google Scholar]
- 349.Seeliger, H. P. 1988. Listeriosis—history and actual developments. Infection 16(Suppl. 2):S80-S84. [DOI] [PubMed] [Google Scholar]
- 350.Sekeyova, Z., V. Roux, and D. Raoult. 2001. Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D’, which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 51:1353-1360. [DOI] [PubMed] [Google Scholar]
- 351.Seong, S. Y., M. S. Choi, and I. S. Kim. 2001. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 3:11-21. [DOI] [PubMed] [Google Scholar]
- 352.Shafer, R. W., and B. R. Edlin. 1996. Tuberculosis in patients infected with human immunodeficiency virus: perspective on the past decade. Clin. Infect. Dis. 22:683-704. [DOI] [PubMed] [Google Scholar]
- 353.Shakir, R. A., A. S. Al-Din, G. F. Araj, A. R. Lulu, A. R. Mousa, and M. A. Saadah. 1987. Clinical categories of neurobrucellosis. A report on 19 cases. Brain 110:213-223. [DOI] [PubMed] [Google Scholar]
- 354.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. inlB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501-510. [DOI] [PubMed] [Google Scholar]
- 355.Shirai, A., V. Sankaran, E. Gan, and D. L. Huxsoll. 1978. Early detection of Rickettsia tsutsugamushi in peripheral monocyte cultures derived from experimentally infected monkeys and dogs. Southeast Asian J. Trop. Med. Public Health 9:11-14. [PubMed] [Google Scholar]
- 356.Sibelius, U., T. Chakraborty, B. Krogel, J. Wolf, F. Rose, R. Schmidt, J. Wehland, W. Seeger, and F. Grimminger. 1996. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J. Immunol. 157:4055-4060. [PubMed] [Google Scholar]
- 357.Sibelius, U., F. Rose, T. Chakraborty, A. Darji, J. Wehland, S. Weiss, W. Seeger, and F. Grimminger. 1996. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect. Immun. 64:674-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Siegman-Igra, Y., R. Levin, M. Weinberger, Y. Golan, D. Schwartz, Z. Samra, H. Konigsberger, A. Yinnon, G. Rahav, N. Keller, N. Bisharat, J. Karpuch, R. Finkelstein, M. Alkan, Z. Landau, J. Novikov, D. Hassin, C. Rudnicki, R. Kitzes, S. Ovadia, Z. Shimoni, R. Lang, and T. Shohat. 2002. Listeria monocytogenes infection in Israel and review of cases worldwide. Emerg. Infect. Dis. 8:305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sigurdardottir, B., O. M. Bjornsson, K. E. Jonsdottir, H. Erlendsdottir, and S. Gudmundsson. 1997. Acute bacterial meningitis in adults. A 20-year overview. Arch. Intern. Med. 157:425-430. [DOI] [PubMed] [Google Scholar]
- 361.Silpapojakul, K., C. Ukkachoke, and S. Krisanapan. 1991. Rickettsial meningitis and encephalitis. Arch. Intern. Med. 151:1753-1757. [PubMed] [Google Scholar]
- 362.Silverman, D. J., and S. B. Bond. 1984. Infection of human vascular endothelial cells by Rickettsia rickettsii. J. Infect. Dis. 149:201-206. [DOI] [PubMed] [Google Scholar]
- 363.Silverman, D. J., L. A. Santucci, N. Meyers, and Z. Sekeyova. 1992. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect. Immun. 60:2733-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sinha, A., S. Sazawal, R. Kumar, S. Sood, V. P. Reddaiah, B. Singh, M. Rao, A. Naficy, J. D. Clemens, and M. K. Bhan. 1999. Typhoid fever in children aged less than 5 years. Lancet 354:734-737. [DOI] [PubMed] [Google Scholar]
- 365.Sohn, A. H., W. S. Probert, C. A. Glaser, N. Gupta, A. W. Bollen, J. D. Wong, E. M. Grace, and W. C. McDonald. 2003. Human neurobrucellosis with intracerebral granuloma caused by a marine mammal Brucella spp. Emerg. Infect. Dis. 9:485-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sola-Landa, A., J. Pizarro-Cerda, M. J. Grillo, E. Moreno, I. Moriyon, J. M. Blasco, J. P. Gorvel, and I. Lopez-Goni. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29:125-138. [DOI] [PubMed] [Google Scholar]
- 367.Sotomayor, E. A., V. L. Popov, H.-M. Feng, D. H. Walker, and J. P. Olano. 2001. Animal model of fatal human monocytotropic ehrlichiosis. Am. J. Pathol. 158:757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Standaert, S. M., T. Yu, M. A. Scott, J. E. Childs, C. D. Paddock, W. L. Nicholson, J. Singleton, Jr., and M. J. Blaser. 2000. Primary isolation of Ehrlichia chaffeensis from patients with febrile illnesses: clinical and molecular characteristics. J. Infect. Dis. 181:1082-1088. [DOI] [PubMed] [Google Scholar]
- 369.Starke, J. R. 1999. Tuberculosis of the central nervous system in children. Semin. Pediatr. Neurol. 6:318-331. [DOI] [PubMed] [Google Scholar]
- 370.Stewart, S. M. 1953. The bacteriological diagnosis of tuberculous meningitis. J. Clin. Pathol. 6:241-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Street, S., H. D. Donoghue, and G. H. Neild. 1999. Tropheryma whipplei DNA in saliva of healthy people. Lancet 354:1178-1179. [DOI] [PubMed] [Google Scholar]
- 372.Sullivan, T. D., L. J. LaScolea, Jr., and E. Neter. 1982. Relationship between the magnitude of bacteremia in children and the clinical disease. Pediatrics 69:699-702. [PubMed] [Google Scholar]
- 373.Suzer, T., N. Demirkan, K. Tahta, E. Coskun, and B. Cetin. 1999. Whipple's disease confined to the central nervous system: case report and review of the literature. Scand. J. Infect. Dis. 31:411-414. [DOI] [PubMed] [Google Scholar]
- 374.Taelman, H., J. Batungwanayo, J. Clerinx, A. Kagame, J. Bogaerts, S. Allen, and P. Van de Perre. 1992. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 327:1171; author reply, 327:1171-1172. [PubMed] [Google Scholar]
- 375.Tailleux, L., O. Schwartz, J.-L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Tamura, A., N. Ohashi, H. Urakami, and S. Miyamura. 1995. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int. J. Syst. Bacteriol. 45:589-591. [DOI] [PubMed] [Google Scholar]
- 377.Teberg, A. J., M. L. Yonekura, C. Salminen, and Z. Pavlova. 1987. Clinical manifestations of epidemic neonatal listeriosis. Pediatr. Infect. Dis. J. 6:817-820. [DOI] [PubMed] [Google Scholar]
- 378.Teitelbaum, R., W. Schubert, L. Gunther, Y. Kress, F. Macaluso, J. W. Pollard, D. N. McMurray, and B. R. Bloom. 1999. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity 10:641-650. [DOI] [PubMed] [Google Scholar]
- 379.Thorner, A. R., D. H. Walker, and W. A. Petri, Jr. 1998. Rocky mountain spotted fever. Clin. Infect. Dis. 27:1353-1359. [DOI] [PubMed] [Google Scholar]
- 380.Thwaites, G., T. T. Chau, N. T. Mai, F. Drobniewski, K. McAdam, and J. Farrar. 2000. Tuberculous meningitis. J. Neurol. Neurosurg. Psychiatry 68:289-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Tolomeo, M., P. Di Carlo, V. Abbadessa, L. Titone, S. Miceli, E. Barbusca, G. Cannizzo, S. Mancuso, S. Arista, and F. Scarlata. 2003. Monocyte and lymphocyte apoptosis resistance in acute and chronic brucellosis and its possible implications in clinical management. Clin. Infect. Dis. 36:1533-1538. [DOI] [PubMed] [Google Scholar]
- 383.Topley, J. M., S. Bamber, H. M. Coovadia, and P. D. Corr. 1998. Tuberculous meningitis and co-infection with HIV. Ann. Trop. Paediatr. 18:261-266. [DOI] [PubMed] [Google Scholar]
- 384.Treadwell, T. A., R. C. Holman, M. J. Clarke, J. W. Krebs, C. D. Paddock, and J. E. Childs. 2000. Rocky Mountain spotted fever in the United States, 1993-1996. Am. J. Trop. Med. Hyg. 63:21-26. [DOI] [PubMed] [Google Scholar]
- 385.Tsenova, L., K. Sokol, V. H. Freedman, and G. Kaplan. 1998. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J. Infect. Dis. 177:1563-1572. [DOI] [PubMed] [Google Scholar]
- 386.Uldry, P. A., T. Kuntzer, J. Bogousslavsky, F. Regli, J. Miklossy, J. Bille, P. Francioli, and R. Janzer. 1993. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 adult cases. J. Neurol. 240:235-242. [DOI] [PubMed] [Google Scholar]
- 387.Urakami, H., T. Tsuruhara, and A. Tamura. 1983. Penetration of Rickettsia tsutsugamushi into cultured mouse fibroblasts (L cells): an electron microscopic observation. Microbiol. Immunol. 27:251-263. [DOI] [PubMed] [Google Scholar]
- 388.Van Kirk, L. S., S. F. Hayes, and R. A. Heinzen. 2000. Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins. Infect. Immun. 68:4706-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Vazquez-Boland, J. A., L. Dominguez, M. Blanco, J. Rocourt, J. F. Fernandez-Garayzabal, C. B. Gutierrez, R. I. Tascon, and E. F. Rodriguez-Ferri. 1992. Epidemiologic investigation of a silage-associated epizootic of ovine listeric encephalitis, using a new Listeria-selective enumeration medium and phage typing. Am. J. Vet. Res. 53:368-371. [PubMed] [Google Scholar]
- 390.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 392.Velasco-Velazquez, M. A., D. Barrera, A. Gonzalez-Arenas, C. Rosales, and J. Agramonte-Hevia. 2003. Macrophage—Mycobacterium tuberculosis interactions: role of complement receptor 3. Microb. Pathog. 35:125-131. [DOI] [PubMed] [Google Scholar]
- 393.Verdon, R., S. Chevret, J. P. Laissy, and M. Wolff. 1996. Tuberculous meningitis in adults: review of 48 cases. Clin. Infect. Dis. 22:982-988. [DOI] [PubMed] [Google Scholar]
- 394.Verhagen, W. I., P. L. Huygen, J. E. Dalman, and M. M. Schuurmans. 1996. Whipple's disease and the central nervous system. A case report and a review of the literature. Clin. Neurol. Neurosurg. 98:299-304. [DOI] [PubMed] [Google Scholar]
- 395.Reference deleted.
- 396.Walker, D. H., H. M. Feng, S. Ladner, A. N. Billings, S. R. Zaki, D. J. Wear, and B. Hightower. 1997. Immunohistochemical diagnosis of typhus rickettsioses using an anti-lipopolysaccharide monoclonal antibody. Mod. Pathol. 10:1038-1042. [PubMed] [Google Scholar]
- 397.Walker, D. H., W. T. Firth, and C. J. Edgell. 1982. Human endothelial cell culture plaques induced by Rickettsia rickettsii. Infect. Immun. 37:301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Walker, D. H., F. M. Parks, T. G. Betz, J. P. Taylor, and J. W. Muehlberger. 1989. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am. J. Clin. Pathol. 91:720-724. [DOI] [PubMed] [Google Scholar]
- 399.Walker, D. H., V. L. Popov, and H. M. Feng. 2000. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab. Investig. 80:1361-1372. [DOI] [PubMed] [Google Scholar]
- 400.Walker, D. H., V. L. Popov, J. Wen, and H. M. Feng. 1994. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab. Investig. 70:358-368. [PubMed] [Google Scholar]
- 401.Walker, T. S., and H. H. Winkler. 1978. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazekii. Infect. Immun. 22:200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Walsh, D. S., K. S. Myint, P. Kantipong, K. Jongsakul, and G. Watt. 2001. Orientia tsutsugamushi in peripheral white blood cells of patients with acute scrub typhus. Am. J. Trop. Med. Hyg. 65:899-901. [DOI] [PubMed] [Google Scholar]
- 403.Wang, C., D. J. Asai, and K. R. Robinson. 1995. Retrograde but not anterograde bead movement in intact axons requires dynein. J. Neurobiol. 27:216-226. [DOI] [PubMed] [Google Scholar]
- 404.Wessalowski, R., L. Thomas, J. Kivit, and T. Voit. 1993. Multiple brain abscesses caused by Salmonella enteritidis in a neonate: successful treatment with ciprofloxacin. Pediatr. Infect. Dis. J. 12:683-688. [DOI] [PubMed] [Google Scholar]
- 405.Whipple, G. H. 1907. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull. Johns Hopkins Hosp. 198:383. [Google Scholar]
- 406.Whittick, J. W. 1950. Necropsy findings in a case of Q fever in Britain. Br. Med. J. 1:979-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Williams, A. E., and W. F. Blakemore. 1990. Pathogenesis of meningitis caused by Streptococcus suis type 2. J. Infect. Dis. 162:474-481. [DOI] [PubMed] [Google Scholar]
- 408.Wilson, K. H., R. Blitchington, R. Frothingham, and J. A. Wilson. 1991. Phylogeny of the Whipple's-disease-associated bacterium. Lancet 338:474-475. [DOI] [PubMed] [Google Scholar]
- 409.Wilson, L. B., and W. M. Chowning. 1904. Studies in Pyroplasmosis hominis (“spotted fever” or “tick fever” of the Rocky Mountains). J. Infect. Dis. 1:31-57. [DOI] [PubMed] [Google Scholar]
- 410.Wilson, S. L., and D. A. Drevets. 1998. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J. Infect. Dis. 178:1658-1666. [DOI] [PubMed] [Google Scholar]
- 411.Winkelman, N. W., and M. T. Moore. 1940. Meningeal blood vessels in tuberculous meningitis. Am. Rev. Tuberc. 42:315-333. [Google Scholar]
- 412.Winkler, H. H., and E. T. Miller. 1982. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells). Infect. Immun. 38:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Winslow, G. M., E. Yager, K. Shilo, D. N. Collins, and F. K. Chu. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 66:3892-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wisseman, C. L., Jr., E. A. Edlinger, A. D. Waddell, and M. R. Jones. 1976. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect. Immun. 14:1052-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wolbach, S. B., J. L. Todd, and F. W. Palfrey. 1922. The etiology and pathology of typhus. Published for the League of Red Cross Societies. Harvard University Press, Cambridge, Mass.
- 416.Yaramis, A., F. Gurkan, M. Elevli, M. Soker, K. Haspolat, G. Kirbas, and M. A. Tas. 1998. Central nervous system tuberculosis in children: a review of 214 cases. Pediatrics 102:E49. [DOI] [PubMed] [Google Scholar]
- 417.Yilmaz, E., M. K. Gurgoze, N. Ilhan, Y. Dogan, and H. Aydinoglu. 2002. Interleukin-8 levels in children with bacterial, tuberculous and aseptic meningitis. Indian J. Pediatr. 69:219-221. [DOI] [PubMed] [Google Scholar]
- 418.Zaidi, E., R. Bachur, and M. Harper. 1999. Non-typhi Salmonella bacteremia in children. Pediatr. Infect. Dis. J. 18:1073-1077. [DOI] [PubMed] [Google Scholar]
- 419.Zhang, J. R., and E. Tuomanen. 1999. Molecular and cellular mechanisms for microbial entry into the CNS. J. Neurovirol 5:591-603. [DOI] [PubMed] [Google Scholar]
- 420.Zink, A., C. J. Haas, U. Reischl, U. Szeimies, and A. G. Nerlich. 2001. Molecular analysis of skeletal tuberculosis in an ancient Egyptian population. J. Med. Microbiol. 50:355-366. [DOI] [PubMed] [Google Scholar]
- 421.Zink, W. E., J. Zheng, Y. Persidsky, L. Poluektova, and H. E. Gendelman. 1999. The neuropathogenesis of HIV-1 infection. FEMS Immunol. Med. Microbiol. 26:233-241. [DOI] [PubMed] [Google Scholar]
- 422.Zinsser, H. 1934. Rats, lice, and history. Atlantic-Little, Brown, and Co., Boston, Mass.