Abstract
The tectorial membrane has long been postulated as playing a role in the exquisite sensitivity of the cochlea. In particular, it has been proposed that the tectorial membrane provides a second resonant system, in addition to that of the basilar membrane, which contributes to the amplification of the motion of the cochlear partition. Until now, technical difficulties had prevented vibration measurements of the tectorial membrane and, therefore, precluded direct evidence of a mechanical resonance. In the study reported here, the vibration of the tectorial membrane was measured in two orthogonal directions by using a novel method of combining laser interferometry with a photodiode technique. It is shown experimentally that the motion of the tectorial membrane is resonant at a frequency of 0.5 octave (oct) below the resonant frequency of the basilar membrane and polarized parallel to the reticular lamina. It is concluded that the resonant motion of the tectorial membrane is due to a parallel resonance between the mass of the tectorial membrane and the compliance of the stereocilia of the outer hair cells. Moreover, in combination with the contractile force of outer hair cells, it is proposed that inertial motion of the tectorial membrane provides the necessary conditions to allow positive feedback of mechanical energy into the cochlear partition, thereby amplifying and tuning the cochlear response.
Full text
PDF
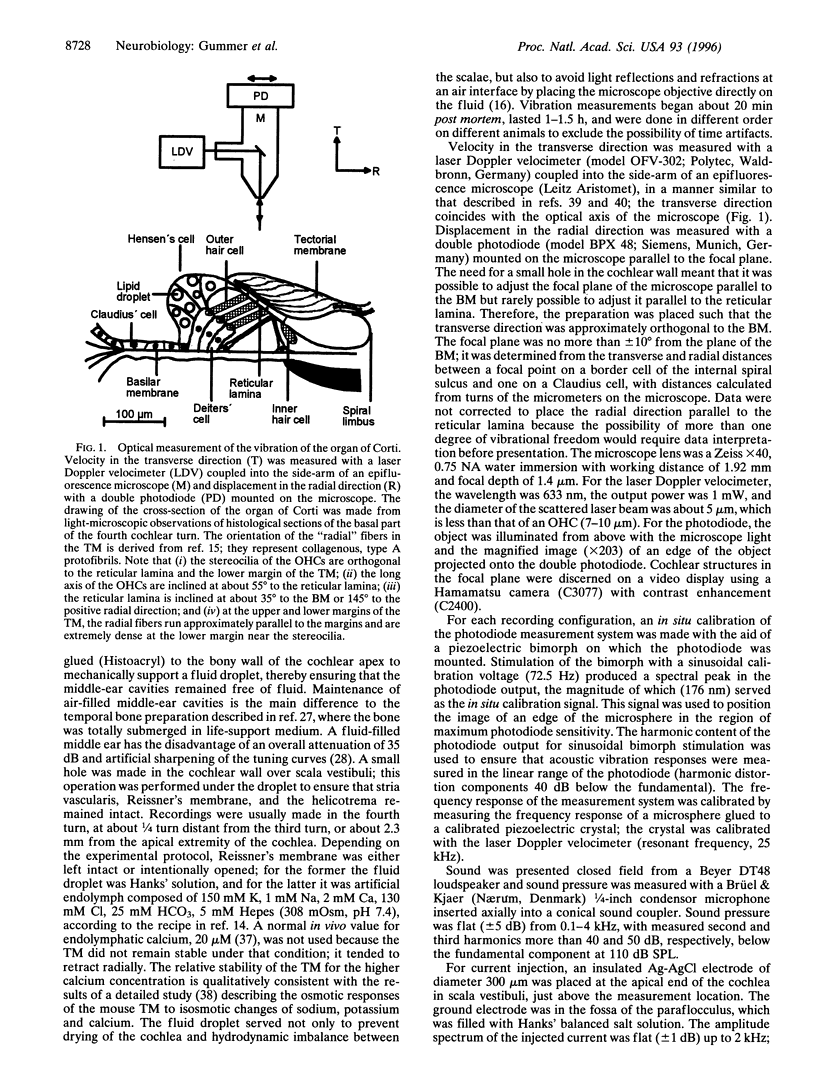

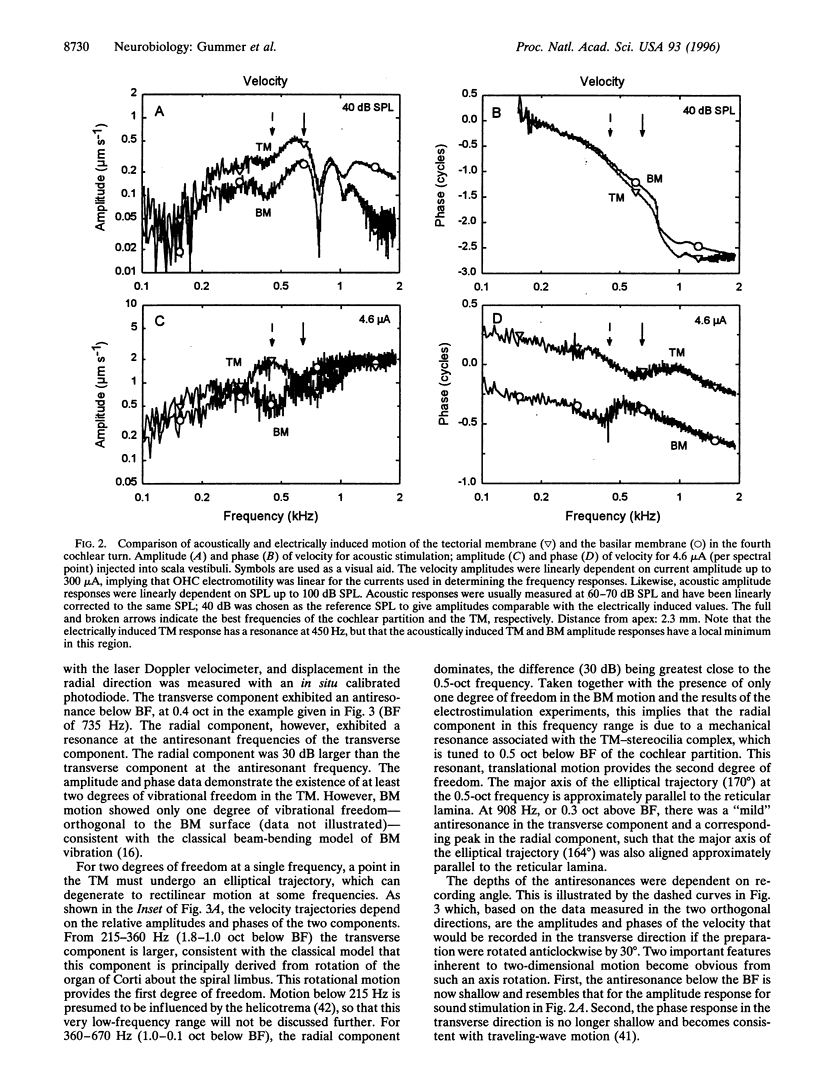
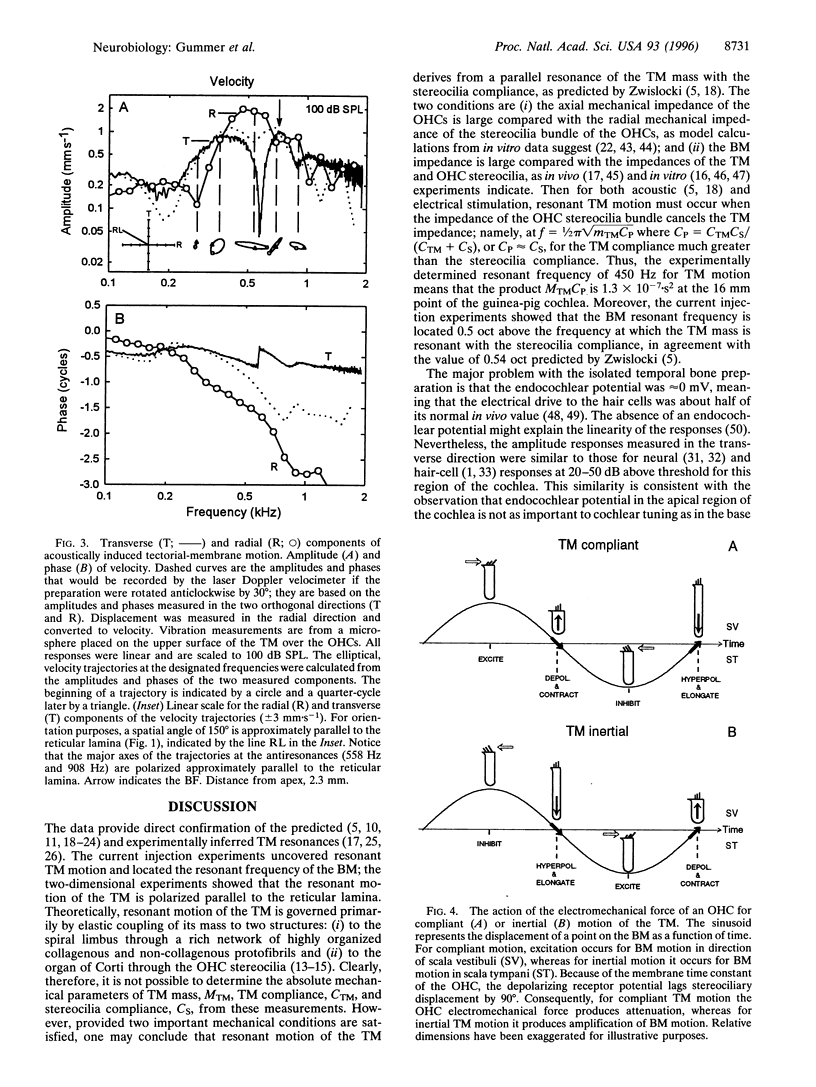

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B. Cochlear micromechanics--a physical model of transduction. J Acoust Soc Am. 1980 Dec;68(6):1660–1670. doi: 10.1121/1.385198. [DOI] [PubMed] [Google Scholar]
- Allen J. B., Fahey P. F. A second cochlear-frequency map that correlates distortion product and neural tuning measurements. J Acoust Soc Am. 1993 Aug;94(2 Pt 1):809–816. doi: 10.1121/1.408182. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Meech R. W. Ionic basis of membrane potential in outer hair cells of guinea pig cochlea. Nature. 1986 Jul 24;322(6077):368–371. doi: 10.1038/322368a0. [DOI] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature. 1978 Jun 1;273(5661):377–378. doi: 10.1038/273377a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Gaskill S. A., Williams D. M. Mechanical filtering of sound in the inner ear. Proc Biol Sci. 1992 Oct 22;250(1327):29–34. doi: 10.1098/rspb.1992.0126. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Dallos P., Evans B. N. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995 Mar 31;267(5206):2006–2009. doi: 10.1126/science.7701325. [DOI] [PubMed] [Google Scholar]
- Dallos P. Low-frequency auditory characteristics: Species dependence. J Acoust Soc Am. 1970 Aug;48(2):489–499. doi: 10.1121/1.1912163. [DOI] [PubMed] [Google Scholar]
- Dallos P. Neurobiology of cochlear inner and outer hair cells: intracellular recordings. Hear Res. 1986;22:185–198. doi: 10.1016/0378-5955(86)90095-x. [DOI] [PubMed] [Google Scholar]
- Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci. 1985 Jun;5(6):1591–1608. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. F. The frequency response and other properties of single fibres in the guinea-pig cochlear nerve. J Physiol. 1972 Oct;226(1):263–287. doi: 10.1113/jphysiol.1972.sp009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko J. A., Richardson G. P. The ultrastructural organization and properties of the mouse tectorial membrane matrix. Hear Res. 1988 Sep 1;35(1):21–38. doi: 10.1016/0378-5955(88)90037-8. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H. A membrane motor model for the fast motility of the outer hair cell. J Acoust Soc Am. 1994 Oct;96(4):2216–2224. doi: 10.1121/1.410094. [DOI] [PubMed] [Google Scholar]
- Johnstone B. M., Sellick P. M. The peripheral auditory apparatus. Q Rev Biophys. 1972 Feb;5(1):1–57. doi: 10.1017/s0033583500000032. [DOI] [PubMed] [Google Scholar]
- Kronester-Frei A. The effect of changes in endolymphatic ion concentrations on the tectorial membrane. Hear Res. 1979 Mar;1(2):81–94. doi: 10.1016/0378-5955(79)90019-4. [DOI] [PubMed] [Google Scholar]
- Lim D. J. Cochlear anatomy related to cochlear micromechanics. A review. J Acoust Soc Am. 1980 May;67(5):1686–1695. doi: 10.1121/1.384295. [DOI] [PubMed] [Google Scholar]
- Lim D. J. Fine morphology of the tectorial membrane. Its relationship to the organ of Corti. Arch Otolaryngol. 1972 Sep;96(3):199–215. doi: 10.1001/archotol.1972.00770090321001. [DOI] [PubMed] [Google Scholar]
- Mammano F., Ashmore J. F. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature. 1993 Oct 28;365(6449):838–841. doi: 10.1038/365838a0. [DOI] [PubMed] [Google Scholar]
- Mammano F., Nobili R. Biophysics of the cochlea: linear approximation. J Acoust Soc Am. 1993 Jun;93(6):3320–3332. doi: 10.1121/1.405716. [DOI] [PubMed] [Google Scholar]
- Miller C. E. Structural implications of basilar membrane compliance measurements. J Acoust Soc Am. 1985 Apr;77(4):1465–1474. doi: 10.1121/1.392041. [DOI] [PubMed] [Google Scholar]
- Mountain D. C., Hubbard A. E. A piezoelectric model of outer hair cell function. J Acoust Soc Am. 1994 Jan;95(1):350–354. doi: 10.1121/1.408273. [DOI] [PubMed] [Google Scholar]
- Neely S. T., Kim D. O. A model for active elements in cochlear biomechanics. J Acoust Soc Am. 1986 May;79(5):1472–1480. doi: 10.1121/1.393674. [DOI] [PubMed] [Google Scholar]
- Nuttall A. L., Dolan D. F., Avinash G. Laser Doppler velocimetry of basilar membrane vibration. Hear Res. 1991 Feb;51(2):203–213. doi: 10.1016/0378-5955(91)90037-a. [DOI] [PubMed] [Google Scholar]
- Olson E. S., Mountain D. C. In vivo measurement of basilar membrane stiffness. J Acoust Soc Am. 1991 Mar;89(3):1262–1275. doi: 10.1121/1.400535. [DOI] [PubMed] [Google Scholar]
- Orman S. S., Geisler C. D. Guinea pig tectorial membrane profile in an in vitro cochlear preparation. Am J Otolaryngol. 1986 Mar-Apr;7(2):140–146. doi: 10.1016/s0196-0709(86)80043-6. [DOI] [PubMed] [Google Scholar]
- Rhode W. S., Geisler C. D. Model of the displacement between opposing points on the tectorial membrane and reticular lamina. J Acoust Soc Am. 1967 Jul;42(1):185–190. doi: 10.1121/1.1910547. [DOI] [PubMed] [Google Scholar]
- Rose J. E., Hind J. E., Anderson D. J., Brugge J. F. Some effects of stimulus intensity on response of auditory nerve fibers in the squirrel monkey. J Neurophysiol. 1971 Jul;34(4):685–699. doi: 10.1152/jn.1971.34.4.685. [DOI] [PubMed] [Google Scholar]
- Ruggero M. A., Rich N. C. Application of a commercially-manufactured Doppler-shift laser velocimeter to the measurement of basilar-membrane vibration. Hear Res. 1991 Feb;51(2):215–230. doi: 10.1016/0378-5955(91)90038-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero M. A., Rich N. C. Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991 Apr;11(4):1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Kössl M., Richardson G. P. Nonlinear mechanical responses of mouse cochlear hair bundles. Proc Biol Sci. 1992 Dec 22;250(1329):217–227. doi: 10.1098/rspb.1992.0152. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983 May;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Asymmetry in voltage-dependent movements of isolated outer hair cells from the organ of Corti. J Neurosci. 1989 Aug;9(8):2954–2962. doi: 10.1523/JNEUROSCI.09-08-02954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W. F. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear Res. 1984 Jun;14(3):305–314. doi: 10.1016/0378-5955(84)90057-1. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Freeman D. M., Weiss T. F. The osmotic response of the isolated, unfixed mouse tectorial membrane to isosmotic solutions: effect of Na+, K+, and Ca2+ concentration. Hear Res. 1995 Jul;87(1-2):187–207. doi: 10.1016/0378-5955(95)00089-m. [DOI] [PubMed] [Google Scholar]
- Strelioff D., Flock A., Minser K. E. Role of inner and outer hair cells in mechanical frequency selectivity of the cochlea. Hear Res. 1985 May;18(2):169–175. doi: 10.1016/0378-5955(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Teich M. C., Khanna S. M., Keilson S. E. Nonlinear dynamics of cellular vibrations in the organ of Corti. Acta Otolaryngol Suppl. 1989;467:265–279. doi: 10.3109/00016488909138347. [DOI] [PubMed] [Google Scholar]
- Ulfendahl M., Khanna S. M., Flock A. Effects of opening and resealing the cochlea on the mechanical response in the isolated temporal bone preparation. Hear Res. 1991 Dec;57(1):31–37. doi: 10.1016/0378-5955(91)90071-g. [DOI] [PubMed] [Google Scholar]
- Wilson J. P., Johnstone J. R. Basilar membrane and middle-ear vibration in guinea pig measured by capacitive probe. J Acoust Soc Am. 1975 Mar;57(3):705–723. doi: 10.1121/1.380472. [DOI] [PubMed] [Google Scholar]
- Xue S., Mountain D. C., Hubbard A. E. Acoustic enhancement of electrically-evoked otoacoustic emissions reflects basilar membrane tuning: experiment results. Hear Res. 1993 Oct;70(1):121–126. doi: 10.1016/0378-5955(93)90056-7. [DOI] [PubMed] [Google Scholar]
- Zenner H. P., Zimmermann U., Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985 May;18(2):127–133. doi: 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Zwislocki J. J. Analysis of cochlear mechanics. Hear Res. 1986;22:155–169. doi: 10.1016/0378-5955(86)90091-2. [DOI] [PubMed] [Google Scholar]
- Zwislocki J. J., Cefaratti L. K. Tectorial membrane. II: Stiffness measurements in vivo. Hear Res. 1989 Nov;42(2-3):211–227. doi: 10.1016/0378-5955(89)90146-9. [DOI] [PubMed] [Google Scholar]
- Zwislocki J. J. Five decades of research on cochlear mechanics. J Acoust Soc Am. 1980 May;67(5):1679–1685. doi: 10.1121/1.384294. [DOI] [PubMed] [Google Scholar]
- Zwislocki J. J., Kletsky E. J. Tectorial membrane: a possible effect on frequency analysis in the cochlea. Science. 1979 May 11;204(4393):639–641. doi: 10.1126/science.432671. [DOI] [PubMed] [Google Scholar]



