Abstract
Recent theories have suggested that cortico-striatal interactions may play an important part in mediating working memory demands and may impact clinical symptomology of schizophrenia. These effects are thought to occur through changes in dopamine signaling from the midbrain and via feedback from the frontal cortex. The COMT Val158Met polymorphism may prove useful for studying these effects in vivo. In this study, patients with schizophrenia, their well siblings, and healthy controls were genotyped and scanned using functional magnetic resonance imaging (fMRI) while they performed a working memory task. We found that patients and their siblings, but not controls, who were Val homozygotes displayed greater activity of the DLPFC, striatum, and the cerebellum during the task than respective Met carriers. Our findings support and extend previous studies of COMT effects on cognition and neural activity, and suggest that changes in dopamine availability may impact cortico-striatal functioning of individuals at risk for schizophrenia differentially.
Keywords: schizophrenia, COMT, dopamine, working memory, striatum
Introduction
Working memory (WM) is a critical part of complex goal directed cognitive control and is a hallmark deficit of serious mental illnesses like schizophrenia, however the neural architecture that supports working memory processing is not fully understood. Previous imaging studies have identified involvement of the prefrontal cortex (PFC) and parietal cortex during WM processing (Braver, Cohen, Nystrom, Jonides, Smith et al., 1997; Cohen, Perlstein, Braver, Nystrom, Noll et al., 1997; Collette, Van der Linden, Laureys, Arigoni, Delfiore et al., 2007; Jonides, Schumacher, Smith, Lauber, Awh et al., 1997; Smith & Jonides, 1997; Smith, Jonides, & Koeppe, 1996). Anatomical connections between the basal ganglia (BG) and the cortex, including the PFC, suggest that subcortical structures may also play a role in mediating cognitive functions such as WM (Middleton & Strick, 1994). Consistent with this idea, a growing body of literature examining subcortical involvement during WM processing has demonstrated BG engagement during various WM tasks (Chang, Crottaz-Herbette, & Menon, 2007; Heyder, Suchan, & Daum, 2004; S. J. Lewis, Dove, Robbins, Barker, & Owen, 2004; McNab & Klingberg, 2008; Ravizza & Ciranni, 2002; Voytek & Knight, 2010). Further, there is some support for the idea that the nigrostriatal dopamine system and its targets in the striatum (the caudate nucleus and putamen) also play a role in schizophrenia etiology (Graybiel, 1997; Heyder, Suchan, & Daum, 2004; Kegeles, Abi-Dargham, Frankle, Gil, Cooper et al., 2010).
Previous cellular and computational studies have indicated that dopamine (DA) availability likely plays a critical role in WM processing (Braver & Cohen, 2000; Cohen, Braver, & Brown, 2002; Cohen, Braver, & O'Reilly, 1996; Durstewitz, Seamans, & Sejnowski, 2000; Frank, Loughry, & O'Reilly, 2001; Goldman-Rakic, 1995a, 1995b, 1996). Catechol-O-methyltransferase (COMT) is an important enzyme involved in the extra-neuronal brdown of dopamine (DA) and has been studied as a candidate risk gene for psychosis (see Craddock, Owen, & O'Donovan, 2006 for a review) as well as an entrée into understanding the interaction of genetic factors, prefrontal cognitive functioning, and its dopaminergic circuits (Dickinson & Elvevag, 2009; Savitz, Solms, & Ramesar, 2006). A functional polymorphism within the Catechol-o-methyl transferace (COMT) gene, at the codon 108/158 (Val158Met), results in a substitution of methionine (Met), for valine (Val). Individuals homozygotic for the Val allele have an approximate 4-fold increase of enzymatic activity compared to Met homozygotes (Lotta, Vidgren, Tilgmann, Ulmanen, Melén et al., 1995), which may result in increased metabolism of DA. Given its putative effect on DA availability in the frontal cortex a number of studies have examined the effects of this polymorphism on the cognitive test performance of healthy controls, patients with schizophrenia, and their well siblings across a variety of cognitive domains and have shown some association between that the Val allele and poorer performance on these tasks (Barnett, Jones, Robbins, & Muller, 2007; Barnett, Scoriels, & Munafò, 2008; Goldberg, Egan, Gscheidle, Coppola, Weickert et al., 2003, MacDonald III, Carter, Flory, Ferrell, & Manuck, 2007, Malhotra, Kestler, Mazzanti, Bates, Goldberg, Goldman, 2002; Rosa, Peralta, Cuesta, Zarzuela, Serrano et al., 2004). Interestingly, a recent study examining the effects of various COMT polymorphisms on WM, IQ, and executive functioning performance of patients with bipolar disorder, schizophrenia, and a healthy control group found that only Val158Met significantly affected WM, such that the Val allele was associated with poorer performance, and it did so only within the schizophrenia group (Wirgenes, Djurovic, Sundet, Agartz, Mattingsdal et al., 2010). However, evidence supporting the association between COMT and schizophrenia is modest (Fan, Zhang, Gu, Li, Sun et al., 2005) and the impact that COMT has on cognitive behavioral performance may be small and are inconsistently found (Barnett, Scoriels, & Munafò, 2008).
Despite the challenges of examining COMT effects on cognition and clinical pathology these studies allow for in vivo hypothesis testing of systems level neurobiological and neurochemical changes and their effect on cognition and clinical symptoms. Further, through such experimentation our understanding of these systems and their effect on relevant phenotypes is sharpened. For example, one possible explanation for performance and neural functioning differences between Val/Met homozygotes is provided by Bilder et al. (2004). He suggests that the relationship may be explained by considering the tonic-phasic DA hypothesis (Grace, 1991), where changes in extrasynaptic DA produced by COMT degradation in the frontal cortex ultimately may lead to changes in the responsiveness of DA neurons in the midbrain to task related information. Studies have shown that COMT is minimally expressed in DA neurons and regions that project to the striatum, but it is expressed in striatal and cortical regions that receive DA input, supporting the idea that COMT influences DA signaling via feedback from nondopaminergic neurons (Akil, Kolachana, Rothmond, Hyde, Weinberger et al., 2003; Kastner, Anglade, Bounaix, Damier, Javoy-Agid et al., 1994). Evidence for this downstream effect is provided by Meyer-Lindenberg et al. (Meyer-Lindenberg, Kohn, Kolachana, Kippenhan, McInerney-Leo et al., 2005), who used positron emission tomography (PET) and fMRI to study the effects of COMT on WM performance in a healthy population. While they did not find a direct effect of COMT on midbrain or striatal DA synthesis they did, consistent with Akil et al. (2003), find strong correlations between prefrontal activity and midbrain DA synthesis during an n-back WM task that differed between COMT genotypes. They suggest that their task, given that it was a WM task, was not optimal for detecting COMT effects in the BG. Other studies have demonstrated that COMT impacts activity in regions of the BG during response inhibition (Congdon, Constable, Lesch, & Canli, 2009), reward processing (Krugel, Biele, Mohr, Li, & Heekeren, 2009; Schmack, Schlagenhauf, Sterzer, Wrase, Beck et al., 2008; Yacubian, Sommer, Schroeder, Gläscher, Kalisch et al., 2007), as well as WM (Caldú, Vendrell, Bartrés-Faz, Clemente, Bargalló et al., 2007; de Frias, Marklund, Eriksson, Larsson, Öman et al., 2009). Thus, while imaging studies of COMT and WM in schizophrenia have tended to focus on involvement of the cortex, given evidence suggesting an indirect influence of COMT on midbrain DA synthesis via its expression in cortical and subcortical regions of the brain and known anatomical connections between the midbrain, the BG, and the cortex, we felt it would be informative to further investigate the effect of COMT on BG activation of patients with schizophrenia during WM processing.
In the current study, we examined the influence of the Val158Met polymorphism on activation of the PFC and BG in individuals with schizophrenia, their siblings, and healthy volunteers. Because siblings of schizophrenic patients share, on average, 50% of their genes, are medication naïve, and are free of severe symptoms that may confound performance on cognitive tasks, studying siblings may enhance sensitivity for detecting subtle gene effects. All participants underwent a functional magnetic resonance imaging (fMRI) scan while performing a WM task. We hypothesized that Val homozygotes would display greater activation of the PFC, specifically in the DLPFC, during WM performance than Met carriers. We also conducted masked analyses of the PFC and BG to increase the activity signal during our task. Additionally, we predicted that COMT would impact striatal activity during WM performance via feedback from nondopaminergic regions in the cortex, such that the Val allele would be associated with increased neural activity. However, in terms of behavioral performance, given the findings of Barnett et al. (2008) we did not predict a large effect of COMT on WM performance.
Methods
Participants
Participants were recruited through the Conte Center for the Neuroscience of Mental Disorders (CCNMD) at Washington University in St. Louis. Participants were 20 individuals with DSM-IV schizophrenia, 15 siblings of individuals with schizophrenia, and 66 healthy controls. Exclusion criteria for controls included a lifetime history of any Axis I psychiatric disorder and having a first-degree relative with a psychotic disorder. Siblings were also excluded for a lifetime history of psychotic disorders. We included siblings of healthy control participants as well as healthy controls to address confounds associated with differential recruitment and screening criteria for controls versus the siblings of patients. Our controls were required to have no family history of psychosis and no personal history of any Axis I disorder. However, we could not impose such a criterion on the siblings of individuals with schizophrenia, as many have past depression or anxiety and to exclude such individuals would result in an unrepresentative sample. Thus, we also recruited the siblings of controls, and allowed them to have the same personal history of non-psychotic AXIS I disorders as the siblings of individuals with schizophrenia. Thus, the two sets of siblings were recruited with the same methods and inclusion/exclusion criteria, other than the diagnosis of their sibling. Participants in any group were also excluded if they met criteria for substance abuse or dependence within the past 6 months, had a clinically unstable or severe medical disorder, a medical disorder that would confound the assessment of psychiatric diagnosis or render research participation dangerous, had head trauma with loss of consciousness, or met DSM-IV criteria for mental retardation. Patients were on stable antipsychotic medication doses for at least 2 weeks before participating in the study. Of the 20 participants with schizophrenia, 13 (65%) were taking atypical antipsychotic medication, 5 (25%) were on a combination of both typical and atypical, and 2 (10%) weren’t taking any antipsychotic medication.
Diagnoses for all participants were determined using the Structured Clinical Interview for DSM-IV (SCID-IV; (First, Spitzer, Miriam, & Williams, 2002). These interviews were conducted by a master’s-level research assistant, who had completed SCID-IV training and participated in regular diagnostic training sessions as par of the CCNMD. The SCID-IV interview had access to all data from present and past hospital records and corroborative family sources. In addition, a psychiatrist conducted a semi-structured interview, also using DSM-IV criteria and all available records. A consensus meeting between the SCID-IV interview and the expert clinician determined the participant’s final diagnosis.
Clinical Rating Scales
Clinical symptoms were assessed using the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983a), the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1983b), and the Structured Interview for Prodromal Syndromes (SIPS; McGlashan, Miller, Woods, Rosen, Hoffman et al., 2000). These assessments were conducted by a psychologist, psychiatrist, or a trained master’s-level research assistant. All participants also completed the Chapman Psychosis Proneness Scales (Chapman, Chapman, & Kwapil, 1995), which included the Perceptual Aberration Scale, the Magical Ideation Scale, the Physical Anhedonia Scale, and the Social Anhedonia Scale. Three clinical symptom clusters were used for assessment: Positive, Negative, and Disorganized symptoms. All rating scale scores were z-scored using the mean and standard deviation of the current sample and averaged within symptom clusters.
Working Memory Task
All participants were scanned while performing a verbal and nonverbal 2-back working memory task. In this task, participants saw a sequence of stimuli presented in the center of a computer screen and were told to push one button (target) any time they saw a stimulus that was the same as the stimulus that they saw two trials back and to push a nontarget button otherwise. The stimuli for each task were presented in 4 blocks of trials, with each block containing 16 trials. Within each 16 trials, one third were targets and two thirds were nontargets. Stimuli for the verbal tasks were concrete visually presented words, 3–10 letters in length, presented in 48-point Geneva font. Stimuli for the nonverbal tasks were nonnameable faces. These are the same stimuli used in a number of prior studies (Braver, Barch, Kelley, Buckner, Cohen et al., 2001; Kelley, Miezin, McDermott, Buckner, Raichle et al., 1998). Participants performed the task in a run lasting 255 s (6 runs total). Each run included 4 task blocks and 3 fixation blocks interleaved in alternating order with the task blocks. Task blocks lasted 40 s and fixation blocks lasted 25 s. Each of the 16 items in a task block was presented for 2 s followed by a 500-ms interstimulus interval. During fixation blocks, a cross-hair appeared continuously, and participants were told to fixate. Visual stimuli were generated by an Apple PowerMac (Cupertino, CA) and PsyScope (Pittsburgh, PA; Cohen, MacWhinney, Flatt, & Provost, 1993) and projected to participants with a Sharp LCD projector (Mahwah, NJ) onto a screen positioned at the head end of the bore. Participants viewed the screen through a mirror attached to the top of the magnetic resonance (MR) head coil. A fiber-optic key press interfaced with the PsyScope button box was used to record the participant’s behavioral performance.
Scanning Procedure
Scanning was performed on the 1.5T Siemens VISION system at the Research Imaging Center of the Mallinckrodt Institute of Radiology at the Washington University Medical School. Functional images were collected using an asymmetric spin-echo echo-planar sequence sensitive to blood oxygenation level-dependent (b) contrast (T2) (TR=2500 ms, TE=50 ms, FOV=24 cm, flip=90°). During each functional run, 102 sets of oblique axial images were acquired parallel to the anterior–posterior commissure plane (3.75°—3.75 mm in plane resolution). Nineteen 7 mm thick slices were acquired in each image. Structural images were acquired using a coronal MP-RAGE 3D T1-weighted sequence (TR=9.7 ms, TE=4 ms, flip=10°; voxel size=1°—1°—1.2 mm). These structural images were used for between subject registration and anatomic localization. See supplemental materials for details of imaging preprocessing.
Genotyping
The procedure used to genotype the participants was similar to that outlined in Li et al. (2005). Using a procedure similar to that outlined by Willis-Owen et al. (2005) and the Sequenom™ system, polymerase chain reactions (PCRs) were carried out in 10-ųL reaction volumes using specific primers. Each reaction included 2 ųL DNA template (2 ng/ųL), .5 ųL primers (1 ųm), .04 ųL Titanium Taq (Clontech) (BD Biosciences, San Jose, California), 1 ųL Titanium Taq buffer (BD Biosciences, San Jose, California), 1 ųL deoxyribonucleotide triphosphates (dNTPs) (2 mmol/L), .4 ųL magnesium chloride (MgCl2) (25 mmol/L), and 5.06 ųL Milli-Q H2O. The reaction was carried out as follows: 95°C for 1 minute (1 cycle), 95°C for 30 seconds, 60°C for 30 seconds, 68°C for 1 minute (45 cycles), and 68°C for 3 minutes (1 cycle). These products were then subject to a shrimp alkaline phosphatase (SAP) digest for removal of nonincorporated dNTPs and a final extension reaction via use of another specific primer. Extension products were cleaned and spotted onto 384 SpectroCHIPs , which were read on a mass spectrometer. Genotyping revealed 17 Met/Met participants, 47 Met/Val participants and 37 Val/Val participants among the three groups of participants. For the purposes of all subsequent analyses, we grouped the Met/Val heterozygotes and Met/Met homozygotes as Met carriers.
Data Analysis
fMRI Image Preprocessing
Preprocessing of the fMRI data included: (1) compensation for slice-dependent time shifts; (2) elimination of odd/even slice intensity differences due to interpolated acquisition; (3) realignment of data acquired in each subject within and across runs to compensate for rigid body motion (Ojemann, Akbudak, Snyder, McKinstry, Raichle et al., 1997); (4) intensity normalization to a whole brain mode value of 1000; and (5) spatial smoothing with an 8-mm FWHM Gaussian kernel. Functional data were transformed into stereotaxic atlas space (Talairach & Tournoux, 1988) by computing a sequence of affine transforms and resampled to 3 mm cubic voxels. Methods for movement correction and cross subject registration are analogous to the linear methods used in AIR (Woods, Grafton, Holmes, Cherry, & Mazziotta, 1998).
Functional Magnetic Resonance Imaging Data (fMRI)
For each participant, we estimated the magnitude of 2-back task-related activation as compared to fixation in each voxel with a general linear model (GLM) using a box-car function convolved with a canonical hemodynamic response, with separate estimates for each stimulus type (e.g., WM-words, WM-faces). However, because we found no effects of stimulus type interactions behaviorally and stimulus type effects were not a focus of the current study, stimulus type effects were not considered further. As such, our primary dependent measure was the average of word and face estimates. These estimates were then entered into an ANOVA with diagnosis (patients, siblings, and controls) and genotype (Val homozygotes and Met carriers) as between subject factors. Given the prior work showing that COMT genotype modulates functional brain activity in both DLPFC and the BG (Schmack et al., 2008; Yacubian et al., 2007), our primary analyses were focused on a priori regions of interest. We generated masks of voxels within the DLPFC (as defined by Rajkowska & Goldman-Rakic, 1995) and BG (as defined by Wang, Mamah, Harms, Karnik, Price et al., 2008), and conducted voxel-by-voxel analyses restricted to these a priori regions of interest.. We used significance and clustersize algorithms described in McAvoy, Ollinger and Buckner (2001) and Ollinger, Corbetta and Shulman (2001) to protect against false positive rates. Our statistical threshold for this ROI based analyses took into account the reduced number of voxels (a cluster-size threshold of 10 contiguous voxels and a per voxel z value of .005) compared to a whole brain analysis. A second, more conservative, whole brain analysis was conducted, using a more stringent threshold of 21 contiguous voxels and a per-voxel alpha of .0001, corresponding to a corrected whole brain false positive rate of ~ .05.
Results
Demographic data and genotype frequencies for each group are shown in Table 1. The observed distribution of genotypes was consistent with Hardy-Weinberg equilibrium expectations (χ2 (2) = 0.1, p = 0.75). The proportion of males and females differed significantly between the patient and sibling groups (χ2 (1) = 8.44, p < .01) and between the patient and control groups (χ2 (1) = 16.2, p < .001). No gender differences were observed between the siblings and control groups. However, when we entered gender as a covariate our results were unchanged. The three diagnostic groups differed in years of education (F (2, 98) = 3.87, p = 0.02), with both siblings and controls having more personal education than patients. However, the groups did not differ in parental socioeconomic status (F (2, 98) = 0.26, p > 0.50), or age (F (2, 98) = 0.64, p > 0.50).
Table 1.
Demographic Data and Genotype Sample for All Diagnostic Groups
| Mean | Standard deviation | |||||
|---|---|---|---|---|---|---|
| Patients | Siblings | Controls | Patients | Siblings | Controls | |
| n | 20 | 15 | 66 | |||
| Age (in years) | 21.7 | 21.5 | 20.8 | 3.3 | 2.9 | 3.5 |
| Sex (% male) | 95 | 53 | 44 | |||
| Parent’s edu. (years) | 14.8 | 14.8 | 14.5 | 2.4 | 2.2 | 1.9 |
| Education (years) | 11.5 | 13.3 | 13.1 | 2.0 | 2.9 | 2.6 |
| Handedness (% right) | 80 | 93 | 81 | |||
| Valine Homozygotes | 10 | 6 | 21 | |||
| Methionine Carriers | 10 | 9 | 45 | |||
Note: The proportion of males and females differed significantly between the patient and sibling groups (χ2 (1) = 8.44, p < .01) and between the patient and control groups (χ2 (1) = 16.2, p < .001). No gender differences were observed between the siblings and control groups. No differences in our results were observed when gender was entered as a covariate. The three diagnostic groups differed in years of education (F (2, 98) = 3.87, p = 0.02), with both siblings and controls having more personal education than patients. However, the groups did not differ in parental socioeconomic status (F (2, 98) = 0.26, p > 0.50), or age (F (2, 98) = 0.64, p > 0.50).
Behavioral Data
Group differences in WM performance (see Table 2) were examined with a repeated measures ANOVA with accuracy as the dependent variable, diagnosis (patients, siblings, and controls) and genotype (Val homozygotes and Met carriers) as between group factors, and stimulus type (word or face) as a within participants factor. The ANOVA revealed a main effect of stimulus type (F (1,99) = 15.8, p < 0.01). In general, participants were more accurate during the word condition than they were during the face condition. We did not observe a diagnosis by stimulus type effect (F (2,98) = 1.4, p > 0.05), a genotype by stimulus type interaction (F (1,99) = 1.8, p > 0.05), nor did we find a 3-way stimulus by diagnosis by genotype interaction (F (2,98) = 2.6, p > 0.05). We did find an omnibus effect of diagnosis (F (2,98) = 15.1, p < 0.01). Overall patients were the least accurate, followed by siblings. Controls had the highest accuracy within our sample. We did not observe a main effect of genotype (F (1,99) = 2.6, p > 0.05). A trend towards an effect of diagnosis by genotype (F (2,98) = 2.9, p = 0.06) was observed, such that patient and sibling Vals performed worse than control Vals, patient Met carriers performed worse than control and sibling Met carriers, and sibling Vals performed worse than sibling Met carriers. However, the performance of patient and control genotype groups was nearly identical to one another. We did test for normality and found that our data was skewed. We then performed an arcsine transform on our data and re-ran our analyses but our results were unchanged. We again found significant main effects of stimulus type (F (1,99) = 23.3, p < 0.01) and diagnosis (F (2,98) = 18.9, p < 0.01), but did not observe significant interactions between any of our variables.
Table 2.
In-Scanner Verbal, Non-Verbal, and Combined Working Memory Performance
| Patients |
Siblings |
Controls |
||||
|---|---|---|---|---|---|---|
| In Scanner Data | Val | Met Carrier | Val | Met Carrier | Val | Met Carrier |
| Word Accuracy | 0.88 (0.13) | 0.89 (0.07) | 0.89 (0.13) | 0.93 (0.11) | 0.97 (0.04) | 0.96 (0.07) |
| Face Accuracy | 0.83 (0.13) | 0.83 (0.16) | 0.802 (0.17) | 0.94 (0.05) | 0.95 (0.11) | 0.94 (0.09) |
| Combined Accuracy | 0.85 (0.13) | 0.86 (0.11) | 0.85 (0.09) | 0.94 (0.08) | 0.96 (0.03) | 0.95 (0.06) |
| Positive symptoms | 1.05 (1.21) | 1.67 (0.81) | −0.29 (0.46) | −0.38 (0.22) | −0.39 (0.32) | −0.33 (0.42) |
| Negative symptoms | 1.31 (0.82) | 1.07 (0.69) | 0.37 (0.74) | −0.31 (0.42) | −0.41 (0.45) | −0.44 (0.21) |
| Disorganized symptoms | 0.77 (0.91) | 0.79 (0.86) | 0.15 (0.51) | −0.33 (0.16) | −0.35 (0.21) | −0.4 (0.2) |
Note: Percent correct with standard deviations.
Imaging
A priori Regions of Interest
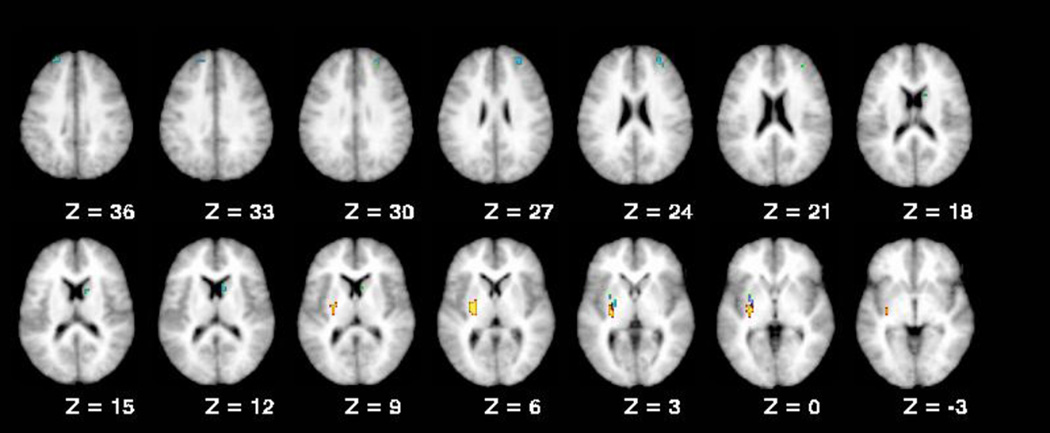
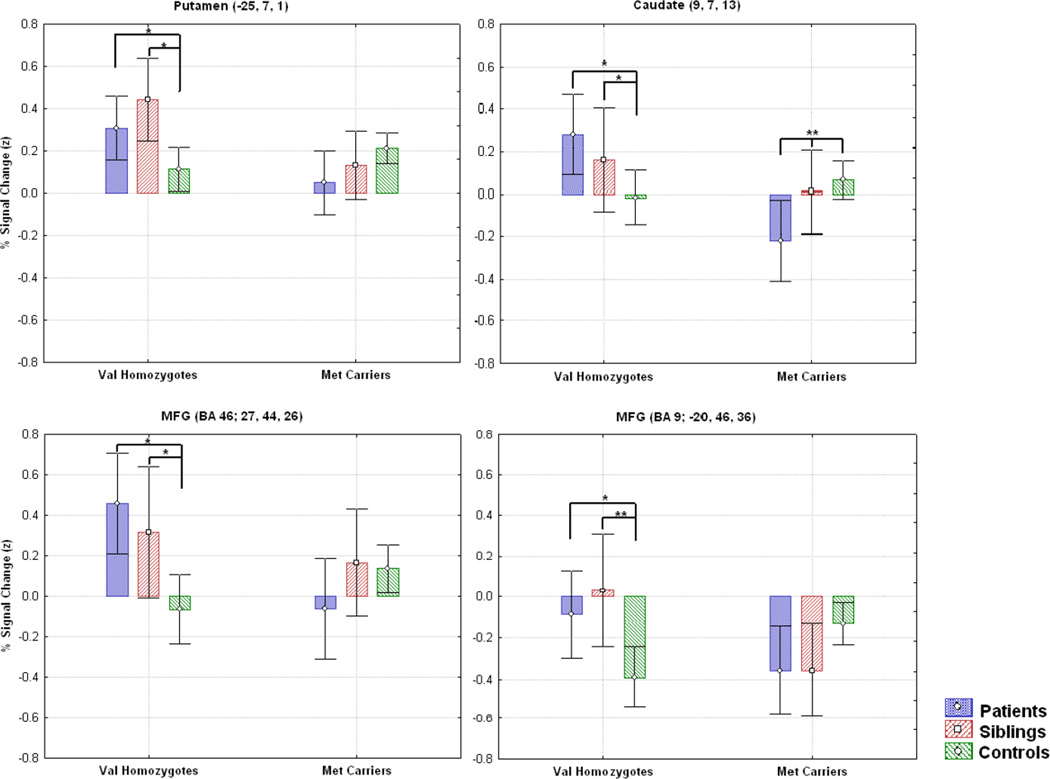
The ROI analysis did not reveal any significant main effects of diagnosis that did not also show higher order interactions (Table 3). A region in the left putamen demonstrated a significant main effect of genotype, with Val homozygotes showing higher activation than Met carriers (F (1,95) = 12.32, p < 0.01). We also observed a significant diagnosis by genotype interactions in the putamen (F (2,95) = 6.6, p < 0.01), caudate (F (2,95) = 6.99, p < 0.01), and two middle frontal gyrus regions (BA 46, F (2,95) = 6.36, p < 0.01 and BA 9, F (2,95) = 7.93, p < 0.01, respectively; Figure 1). Interestingly, in all four of these regions Val homozygotes in the patient and sibling groups showed higher activation than Met carriers (Figure 2). However, we observed no differences between Val and Met carriers in these regions for our control group, although numerically Met carrier controls typically displayed greater activation than control Val homozygotes. Further, for the regions that displayed a diagnosis by genotype interaction, patient and sibling Val homozygotes significantly differed from control Val homozygotes (Figure 2), whereas patient and sibling Met carriers only differed from control Met carriers in one region (caudate region 9, 7, 13; see Figure 2). This pattern is consistent with the diagnosis by genotype effects observed in our behavioral data.
Table 3.
Regions From the A priori Analysis Showing Interaction Effects of Genotype, and Diagnosis
| Region | BAI | X | Y | Z | F value | Pattern | |
|---|---|---|---|---|---|---|---|
| Region of Interest | |||||||
| COMT | |||||||
| Putamen | −26 | −15 | 3 | 12.32 | Val > Met | ||
| DX* COMT | |||||||
| Putamen | −25 | −7 | 1 | 6.60 | Val > Met* | ||
| Caudate | 9 | 7 | 13 | 6.99 | Val > Met* | ||
| Middle Frontal Gyrus | 46 | 27 | 44 | 26 | 6.36 | Val > Met* | |
| Middle Frontal Gyrus | 9 | −20 | 46 | 36 | 7.93 | Val > Met* | |
| Whole Brain Analysis | |||||||
| COMT | |||||||
| Cerebellum | −43 | −65 | −14 | 27.77 | Val > Met | ||
| Putamen | −29 | −17 | 5 | 14.87 | Val > Met | ||
| DX* COMT | |||||||
| Lingual Gyrus | 18 | 0 | −97 | −10 | 15.60 | Val > Met** | |
| Putamen | −29 | 4 | 0 | 9.15 | Val > Met* | ||
Brodmann area.
Pattern observed for patients and siblings only.
Pattern observed for patients only.
Figure 1.
Regions from the ROI analysis that showed COMT effects.
Figure 2.
Regions from the ROI analysis that showed a diagnosis by genotype interaction. In the putamen the caudate and one of the middle frontal gyrus regions (BA 46), the patient and sibling Val homozygotes showed significantly greater activation than control Val homozygotes (ps<.05). In contrast, in the caudate, the patient and sib Met carriers actually showed significantly less activation than control Met carriers (ps<.01), but did not differ from control met carriers in the putamen or the middle frontal gyrus regions.
Whole Brain Analysis
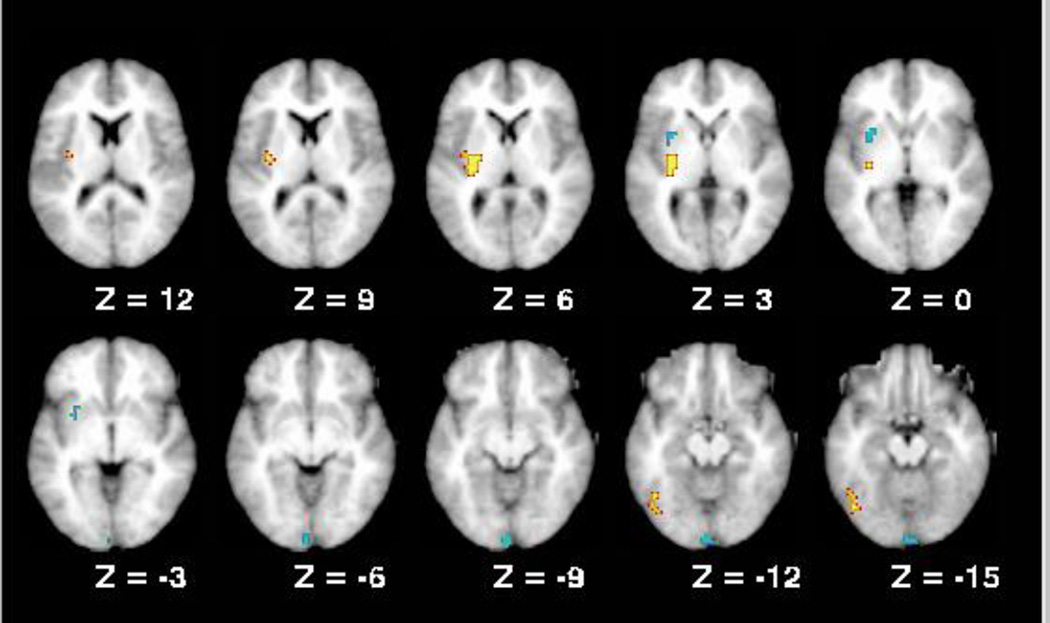
We found no regions that showed a main effect of diagnosis that did not also show higher order interactions. A main effect of genotype was observed in the cerebellum (Figure 3), where Val homozygotes showed higher activation than Met carriers. Regions in the lingual gyrus and putamen showed interactions between diagnosis and genotype. In the putamen, as in the ROI analysis, Val homozygote patients and siblings showed higher activation than Met carriers. This effect was not present in controls. For the lingual gyrus, only patient Vals showed higher activation than Met carriers. In the putamen, there was a trend for patient and sibling Val homozygotes to show greater activation than controls (p=.07), while the patient and sibling Met carriers again showed less activation than controls (p<.05). A similar pattern was observed in the lingual gyrus.
Figure 3.
Regions from the whole brain analysis that showed COMT effects.
The relationship between behavioral performance, symptoms, and brain activation
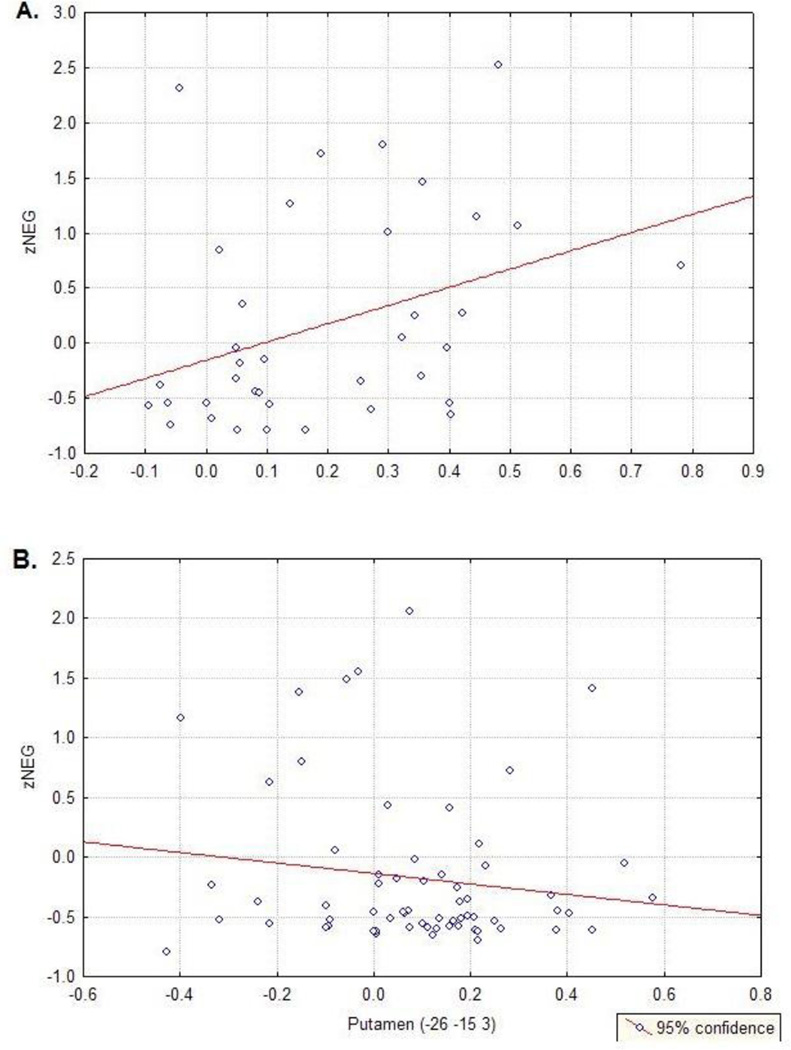
Given the differential performance between our subject groups, we performed ANCOVAs with in-scanner behavioral performance as a covariate for regions that showed significant effects of diagnosis. Previous studies have found a relationship between reaction time duration and brain activation (Honey, Bullmore, & Sharma, 2000, 2002), so we included both accuracy and reaction time as covariates. The results indicated that differences in accuracy and a combination of accuracy and reaction time did not appear to be responsible for differences in activation, as all regions continued to show significant effects of diagnosis (all p < 0.05) when performance was used as a covariate. Further, Graybiel et al. (1997) suggests that, given previous lesion work, disruption of functioning within the striatum could lead to symptoms like apathy and anhedonia, that may be expressed as increased negative symptom expression. To assess whether there was an association between COMT related striatal activity and clinical symptoms we ran correlation analyses between the left putamen region (−26, −15, 3) that showed a main effect of genotype (Table 3) and the 3 symptom categories (positive, negative, and disorganized symptom z-scores). We found that putamen activity of Val participants only significantly correlated with negative symptoms (Figure 4; r = 0.36, p = 0.03), but not positive or disorganized symptoms. Activity within the left putamen of Met carriers did not significantly correlate negative symptoms (Figure 4), nor did it significantly correlate with positive or disorganized symptoms.
Figure 4.
A) Correlation between z-scores of the negative symptom category and the only region that showed a main effect of COMT. The correlation was significant (r = 0.36, p = 0.03), whereas the correlation between this region and z-scores of the positive symptom category (B) and disorganized symptom z-scores were not.
Discussion
Like previous studies, we found that DLPFC activity was increased in Val homozygotes as compared to Met carriers during a task of WM, even when controlling for performance differences. We replicated previous studies that found involvement of the BG, particularly the striatum, during working memory processing (Chang, Crottaz-Herbette, & Menon, 2007; Voytek & Knight, 2010). We also replicated previous studies that found Val homozygotes demonstrated greater DLPFC activity as well as striatal activity during WM processing (de Frias et al., 2009; Tan, Chen, Goldberg, Mattay, Meyer-Lindenberg et al., 2007). Further, we were interested in assaying these results across diagnostic groups by assessing the symptoms, behavioral performance, and neural activity of patients with schizophrenia, their first-degree relatives, and healthy control participants. We found that COMT status influenced the pattern of differences across groups, such that patient and sibling Val homozygotes showed greater activation than controls in both striatal and prefrontal regions. In contrast, patient and sibling Met carriers either showed no difference from controls, or less activation.
COMT Genotype and Behavioral Performance
In-scanner working memory performance of our participants revealed expected differences between the diagnostic groups. However, we did not find a main effect of genotype. Although the pattern of genotype effects suggested that COMT impacted our diagnostic groups differently, we also failed to find a significant genotype by diagnosis interaction. While the pattern of performance was similar to what has been observed previously (Goldberg et al., 2003), such that Val homozygotes performed worse than Met carriers, it may not be surprising that this effect did not reach significance, particularly given the findings of others (Barnett, Scoriels, & Munafò, 2008; Stefanis, van Os, Avramopoulos, Smyrnis, Evdokimidis et al., 2004). While this may suggest that COMT has no impact on working memory performance, it may also be the case that the subtle impact of COMT on cognition is attenuated as we move from its direct impact on dopamine catabolism, to the effect of that catabolism on neural signaling, to the complex series of neural and environmental interactions that are occur during cognition. Neural activation, on the other hand, may be better suited than behavioral metrics to reveal the subtle gene effects of COMT of working memory.
COMT Genotype and Neural Processing
Consistent with our hypothesis, we found that patient and sibling Val homozygotes displayed greater activity in the caudate, putamen, and middle frontal gyrus than Met carriers. This further supports neuroanatomic and functional imaging studies that suggest a role for both the PFC and BG in mediating WM demands (Frank, Loughry, & O'Reilly, 2001; McNab & Klingberg, 2008; Middleton & Strick, 1994). While this finding is intriguing, further work would be need to elucidate the particular role of the striatum during WM processing. Additionally, because this effect was evident in Val homozygotes (with less available DA), our findings are consistent with the hypotheses that adequate levels of DA in both PFC and striatum are necessary for regulated neural processing during WM tasks. Given the likelihood that COMT plays a small role in metabolizing subcortical DA, our findings are also consistent with the proposed relationships between prefrontal DA availability and striatal DA tone (Bilder et al., 2004).
We found that COMT genotype influenced the ways in which patients and siblings differed from controls in terms of functional brain activation. Patients and sibling Val homozygotes not only displayed greater activity than all Met carriers, but they also displayed significantly greater activity than control Val homozygotes. This was true for essentially all regions of our ROI analysis that showed a diagnosis by genotype effect. Given the putative role of COMT as a “risk gene”, one could speculate that influence of genes such as COMT may help explain some of the variability found in patient groups, both within and across studies.
Surprisingly, given previous work, we did not find a similar effect of COMT for our control participants. This may be due to ceiling effects, as the performance of our controls was typically very high. However, the lack of COMT influences in controls could also reflect differences in the influences of other genes on cognition and brain function in controls versus individuals with schizophrenia. Given that the risk of developing schizophrenia may be influenced by multiple risk genes of small effect, or result from an interaction of such genes and environmental stressors, one might expect that patients with schizophrenia and their relatives would possess other genetic alleles that can exert a negative influence on DA function. In this sense, the additive effects of risk genes or epigenetic effects on patient and siblings COMT genotypes may confer cognitive impairment or neural disruption differently than it does for control groups.
Clinical Symptoms
The potential relationship between COMT variation and symptom expression not clear. Recent theories about the role of DA in schizophrenia suggest that decreased available DA in the PFC is associated with negative symptoms, while dysregulated DA subcortically could lead to positive symptoms (Weinberger, Egan, Bertolino, Callicott, Mattay et al., 2001). According to this framework, the Val allele would be associated with negative symptoms and, due to cortico-striatal interactions, also an increase in positive symptoms. Alternatively, Bilder et al (2004) suggested that increased post-synaptic tonic DA associated with the Met allele would lead to decreased phasic DA release within the synapse, and therefore cognitive rigidity and negative symptoms. We found that activity in a region of the putamen that demonstrated an effect of COMT significantly correlated with negative symptoms of Val homozygotes but not Met carriers. Given the proposed role of cortico-striatal loops and dopamine in the expression of apathy (see Levy & Dubois, 2006 for a review) this finding may make some sense. Although examining COMT effects on symptoms expression was not a focus of this study, this finding is intriguing in that it gives some credence to the notion that a putative disruption of DA signaling and associated changes in activity in the BG may impact clinical symptom expression. Further work is needed to explore the influence subcortical structures, like the BG, have on clinical symptom expression.
Limitations
The majority of the individuals with schizophrenia were treated with antipsychotic medication, and most of these individuals were treated with atypical drugs. While medication status could have interacted with the influences of COMT genotype in our study, COMT effects have previously been observed in unmedicated patients (Woodward, Jayathilake, & Meltzer, 2007). Furthermore, many of the same genotype effects on cognition and neural activation of the unmedicated siblings of patients with schizophrenia were observed previously (Egan, Goldberg, Kolachana, Callicott, Mazzanti et al., 2001; Goldberg et al., 2003; Rosa et al., 2004), and were also observed in our unmedicated sibling sample. Another limitation was sample sizes. Given the subtle nature of gene effects from a single allelic variation, larger sample sizes would enhance power to detect differences caused by such variations. However, despite our small samples, we did detect patterns consistent with pervious studies of COMT effects on cognition and neural activation. In addition, our WM contrast compared a 2-back WM condition to fixation, a contrast that may encapsulate processes that immediately relevant to WM, such as encoding, response selection, and motor execution. Of particular concern, given our focus on the BG, is motor execution. While to some degree this may account for the activity we observed in the striatum during WM, we would not expect striatal activity associated with the motor activity of button presses to vary as a function of genotype or diagnostic category. However, additional work will be necessary to address whether our findings are specific to WM as opposed to more general to a range of processing mechanisms, both of which are important to understand.
Conclusions
Our findings support and extend previous studies of COMT genotype variability on neural activity during WM performance. We found further evidence to support an important role for COMT genotype variability in the direct regulation of PFC function, and on the indirect regulation of striatal function. Further, we found that COMT allelic status influenced the pattern of group differences in functional brain activation, with patient and sibling Val homozygotes showing evidence for increased activation in PFC and striatal regions compared to controls. These results add to the growing literature suggest that both cognitive performance and functional brain activation during WM is influenced by genetic variation that controls DA function, and that such effects are important for understanding behavior and brain function among individuals with schizophrenia.
References
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-Methyltransferase Genotype and Dopamine Regulation in the Human Brain. J. Neurosci. 2003;23(6):2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983b. [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12(5):502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biological Psychiatry. 2008;64(2):137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DMD, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. NeuroImage. 2001;14(1 Pt 1):48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen J. On the control of control: The role of dopamine in regulating prefrontal function and working memory. Control of cognitive processes: Attention and performance. 2000;XVIII:713–737. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado MA, et al. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. NeuroImage. 2007;37(4):1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. NeuroImage. 2007;34(3):1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. New York, NY: Cambridge University Press; 1995. [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology. 2002;12(2):223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, O'Reilly RC. A computational approach to prefrontal cortex, cognitive control and schizophrenia: recent developments and current challenges. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1996;351(1346):1515–1527. doi: 10.1098/rstb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. Psy-Scope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, & Computers. 1993;25(2):257–271. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Arigoni F, Delfiore G, Degueldre C, et al. Mapping the updating process: common and specific brain activations across different versions of the running span task. Cortex; a journal devoted to the study of the nervous system and behavior. 2007;43(1):146–158. doi: 10.1016/s0010-9452(08)70452-0. [DOI] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biological psychology. 2009;81(3):144–152. doi: 10.1016/j.biopsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Owen MJ, O'Donovan MC. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Molecular psychiatry. 2006;11(5):446–458. doi: 10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Marklund P, Eriksson E, Larsson A, Öman L, Annerbrink K, et al. Influence of COMT Gene Polymorphism on fMRI-assessed Sustained and Transient Activity during a Working Memory Task. Journal of cognitive neuroscience. 2009 doi: 10.1162/jocn.2009.21318. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Elvevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nature neuroscience, 3 Suppl. 2000:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met Genotype on Frontal Lobe Function and Risk for Schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY, et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biological Psychiatry. 2005;57(2):139–144. doi: 10.1016/j.biopsych.2004.10.018. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clincal Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York, NY: Unpublished manuscript; 2002. [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cognitive, affective & behavioral neuroscience. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive Subprocesses in Working Memory: Relationship to Catechol-O-methyltransferase Val158Met Genotype and Schizophrenia. Arch Gen Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Annals of the New York Academy of Sciences. 1995a;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995b;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1996;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophrenia Bulletin. 1997;23(3):459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychologica. 2004;115(2–3):271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Honey GD, Bullmore ET, Sharma T. Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. NeuroImage. 2000;12(5):495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- Honey GD, Bullmore ET, Sharma T. De-coupling of cognitive performance and cerebral functional response during working memory in schizophrenia. Schizophrenia Research. 2002;53(1–2):45–56. doi: 10.1016/s0920-9964(01)00154-2. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher E, Smith EE, Lauber E, Awh E, Minoshima S, et al. Verbal working memory load affects regional brain activation as measured by PET. Journal of cognitive neuroscience. 1997;9(4):462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kastner A, Anglade P, Bounaix C, Damier P, Javoy-Agid F, Bromet N, et al. Immunohistochemical study of catechol-O-methyltransferase in the human mesostriatal system. Neuroscience. 1994;62(2):449–457. doi: 10.1016/0306-4522(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of General Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20(5):927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Krugel LK, Biele G, Mohr PN, Li SC, Heekeren HR. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(42):17951–17956. doi: 10.1073/pnas.0905191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral cortex (New York, NY : 1991) 2006;16(7):916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: Regional, laminar, and ultrastructural localization. The Journal of Comparative Neurology. 2001;432(1):119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. The European journal of neuroscience. 2004;19(3):755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Di Naro E, Vitucci A, Zimmermann B, Holzgreve W, Hahn S. Detection of paternally inherited fetal point mutations for {beta}-thalassemia using size-fractionated cell-free DNA in maternal plasma. Jama. 2005;293(7):843. doi: 10.1001/jama.293.7.843. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. NeuroImage. 2001;13:6–2. [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L. Structured Interview fro Prodromal Syndromes (SIPS) New Haven, Conn: Yale School of Medicine; 2000. [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana BS, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nature neuroscience. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating Processes within a Trial in Event-Related Functional MRI II. Analysis. NeuroImage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cerebral cortex (New York, NY : 1991) 1995;5(4):323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ciranni MA. Contributions of the prefrontal cortex and basal ganglia to set shifting. Journal of cognitive neuroscience. 2002;14(3):472–483. doi: 10.1162/089892902317361985. [DOI] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martinez-Larrea A, et al. New Evidence of Association Between COMT Gene and Prefrontal Neurocognitive Function in Healthy Individuals From Sibling Pairs Discordant for Psychosis. Am J Psychiatry. 2004;161(6):1110–1112. doi: 10.1176/appi.ajp.161.6.1110. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes, brain, and behavior. 2006;5(4):311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Schmack K, Schlagenhauf F, Sterzer P, Wrase J, Beck A, Dembler T, et al. Catechol-O-methyltransferase val158met genotype influences neural processing of reward anticipation. NeuroImage. 2008;42(4):1631–1638. doi: 10.1016/j.neuroimage.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cognitive Psychology. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cerebral cortex (New York, NY : 1991) 1996;6(1):11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, et al. Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biological Psychiatry. 2004;56(7):510–515. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: Thieme New York. 1988 [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(49):13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, et al. Progressive Deformation of Deep Brain Nuclei and Hippocampal-Amygdala Formation in Schizophrenia. Biological Psychiatry. 2008;64(12):1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biological Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Willis-Owen SAG, Turri MG, MunafÚ MR, Surtees PG, Wainwright NWJ, Brixey RD, et al. The serotonin transporter length polymorphism, neuroticism, and depression: a comprehensive assessment of association. Biological Psychiatry. 2005;58(6):451–456. doi: 10.1016/j.biopsych.2005.04.050. [DOI] [PubMed] [Google Scholar]
- Wirgenes KV, Djurovic S, Sundet K, Agartz I, Mattingsdal M, Athanasiu L, et al. Catechol O-methyltransferase variants and cognitive performance in schizophrenia and bipolar disorder versus controls. Schizophrenia Research. 2010;122(1–3):31–37. doi: 10.1016/j.schres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22(1):139. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Jayathilake K, Meltzer HY. COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophrenia Research. 2007;90(1–3):86–96. doi: 10.1016/j.schres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Gläscher J, Kalisch R, Leuenberger B, et al. Gene-gene interaction associated with neural reward sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(19):8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]