Abstract
Background/Aims
Cardiovascular disease (CVD) is increased in chronic kidney disease (CKD), and contributed to by the CKD-mineral bone disorder (CKD-MBD). The CKD-MBD begins in early CKD and its vascular manifestations begin with vascular stiffness proceeding to increased carotid artery intima-media thickness (cIMT) and vascular calcification (VC). Phosphorus is associated with this progression and is considered a CVD risk factor in CKD. We hypothesized that modifying phosphorus balance with lanthanum carbonate (LaCO3) in early CKD would not produce hypophosphatemia and may affect vascular manifestations of the CKD-MBD.
Methods
We randomized 38 subjects with normophosphatemic stage 3 CKD to a fixed dose of LaCO3 or matching placebo without adjusting dietary phosphorus in a 12-month randomized, double-blind, pilot and feasibility study. The primary outcome was the change in serum phosphorus. Secondary outcomes were changes in measures of phosphate homeostasis and vascular stiffness assessed by carotid-femoral pulse wave velocity (PWV), cIMT and VC over 12 months.
Results
There were no statistically significant differences between LaCO3 and placebo with respect to the change in serum phosphorus, urinary phosphorus, tubular reabsorption of phosphorus, PWV, cIMT, or VC. Biomarkers of the early CKD-MBD such as plasma fibroblast growth factor-23 (FGF23), Dickkopf-related protein 1 (DKK1), and sclerostin were increased 2–3-fold at baseline but were not affected by LaCO3.
Conclusion
12 months of LaCO3 had no effect on serum phosphorus and did not alter phosphate homeostasis, PWV, cIMT, VC, or biomarkers of the CKD-MBD.
Keywords: Cardiovascular disease, chronic kidney disease, phosphate binders, vascular calcification, randomized controlled trials
INTRODUCTION
Chronic kidney disease (CKD) is associated with an increased risk of cardiovascular disease (CVD) compared with the general population [1,2]. CVD risk is inversely related to glomerular filtration rate (GFR), and begins to rise in early stages of CKD [1–3]. The global burden of CKD-associated CVD is immense, as stage 3 CKD (GFR 30–59 ml/min/1.73m2) is prevalent in approximately 10% of the general population [4]. Furthermore, the mortality rate due to kidney disease-associated CVD eclipses progressive CKD [5], thus highlighting its public health importance. Classic CVD risk factors do not fully explain the increased CVD risk in CKD [5]. Vascular calcification (VC) [6,7] and hyperphosphatemia [8–12] may be important CKD-- specific risk factors for CVD, and human and animal studies suggest that hyperphosphatemia stimulates VC [13–17]. Multiple studies have demonstrated an association between serum phosphorus levels and risk factors for CVD, including vascular stiffness [18–20] and VC [19]. In recognition of the roles that disorders of mineral homeostasis and skeletal remodeling play in kidney disease-associated CVD, a syndrome was named in 2006, the chronic kidney disease – mineral bone disorder (CKD-MBD) [22].
In early CKD, the serum phosphorus concentration remains normal until late stage 3 or stage 4, but the CKD-MBD begins in early CKD [23–25] suggesting that vascular perturbations of CKD occur prior to hyperphosphatemia. Furthermore, studies have demonstrated that the pathogenesis of the CKD-MBD and associated VC includes other factors along with changes in phosphate homeostasis. Thus, early intervention in the CKD-MBD may affect vascular stiffness before structural abnormalities such as vascular intima-media thickness or calcification are altered.
We hypothesized that in normophosphatemic patients with stage 3 CKD, intestinal phosphate binding with lanthanum carbonate (LaCO3) would not produce hypophosphatemia but may improve vascular manifestations of the CKD-MBD such as vascular stiffness, carotid intima-media thickness (cIMT) and VC. Secondarily, we sought to produce preliminary data that can be used for powering cardiovascular outcomes in future clinical trials of phosphate binders.
RESULTS
Baseline Demographics
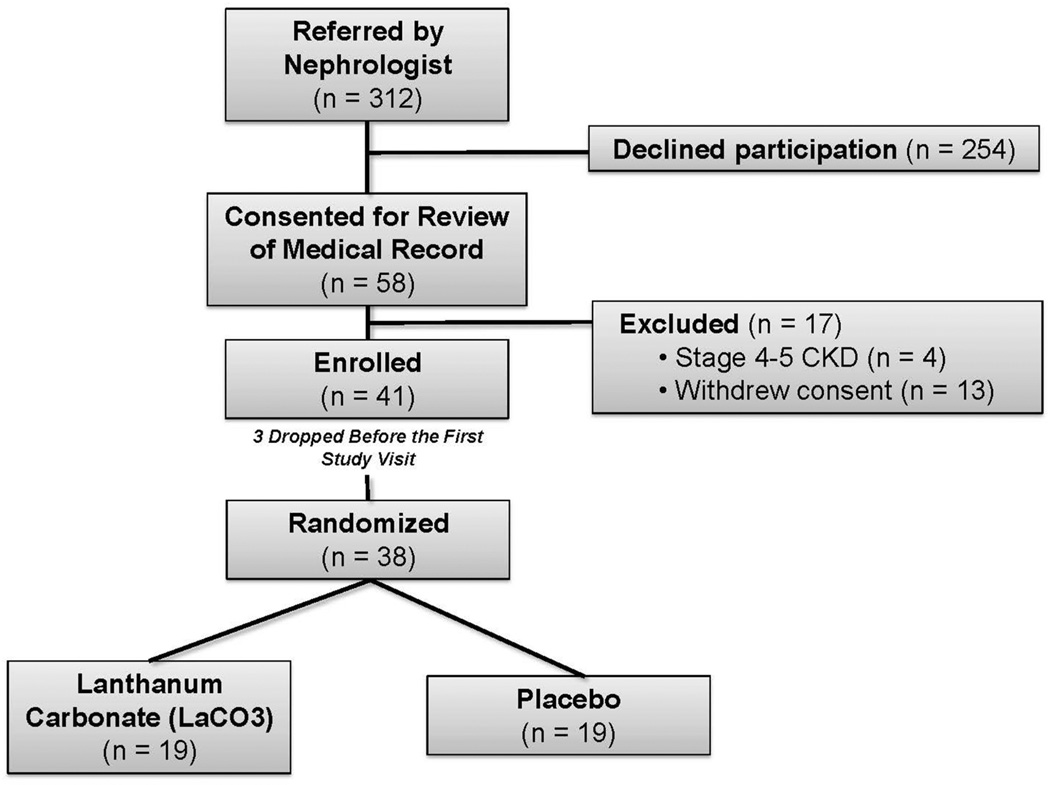
312 subjects that met inclusion criteria were identified by their primary nephrologist between January 1 and December 31, 2010. 58 (19%) met the study entry criteria (including willingness to participate in the study) and were recruited, 41 were enrolled after a formal review of their medical records to confirm eligibility, and three subjects dropped out prior to the first study visit, leaving 38 subjects for the intention-to-treat analysis (Figure 1). Enrollment of a subject required matching for age, sex, race and diabetes status in a subsequent enrollee producing the large gap between screening and enrollment along with consent. The matching is shown in Table 1, and gender matching was less successful as there were more males enrolled in the placebo group, but the difference was not statistically significant. Other baseline clinical, cardiovascular, and biochemical characteristics were similar between the groups (Tables 1 and 2). Compliance was assessed at each study visit using pill counts, and was defined as the percentage of prescribed doses that were taken by each subject. The overall mean compliance was 84% (range 45–100%); compliance was similar in both groups (LaCO3: 85%, Placebo: 84%).
Figure 1. Enrollment Flowchart and Randomization Strategy.
Table 1.
Baseline Demographic Data
| LaCO3 | Placebo | |
|---|---|---|
| (n=19) | (n=19) | |
| Demographics | ||
| Age (yr) | 62 ± 11 | 61 ± 13 |
| Height (cm) | 173 ± 9 | 174 ± 12 |
| Weight (kg) | 97 ± 20 | 94 ± 23 |
| Body mass index (kg/m2) | 33 ± 6 | 31 ± 5 |
| Gender (M/F) | 9/10 | 14/5 |
| Diabetes Mellitus [n, (%)] | 7 (36) | 7 (36) |
| Race | ||
| African-American [n, (%)] | 4 (21) | 5 (26) |
| Caucasian [n, (%)] | 15 (79) | 12 (63) |
| Other [n, (%)] | 0 (0) | 2 (10) |
All values given as mean ± SD or number (percent). There are no significant differences between LaCO3 and Placebo.
Table 2.
Comparison of Changes Phosphorus, Phosphate homeostasis, Biomarkers of CVD, and Biomarkers of CKD-MBD Between Groups.
| LaCO3 (n=19) |
Placebo (n=19) |
||||
|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | P | |
| Phosphate homeostasis | |||||
| Serum phosphorus (mg/dL) | 3.5 ± 0.5 | 3.3 ± 0.5 | 3.3 ± 0.4 | 3.2 ± 0.7 | 0.88 |
| Serum calcium (mg/dL) | 9.2 ± 0.5 | 9.5 ± 0.4 | 9.3 ± 0.3 | 9.5 ± 0.4 | 0.55 |
| Serum creatinine (mg/dL) | 1.5 ± 0.3 | 1.6 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.5 | 0.48 |
| 24 hour creatinine clearance (ml/min/1.73m2) | 47 ± 12 | 47 ± 16 | 45 ± 12 | 46 ± 15 | 0.97 |
| Intact parathyroid hormone (pg/ml) | 76 ± 72 | 77 ± 53 | 57 ± 19 | 65 ± 36 | 0.48 |
| FGF23 (pg/ml) | 69 (24, 117) | 55 (33, 367) | 55 (25, 201) | 55 (33, 299) | 0.87 |
| Tubular reabsorption of phosphorus (%) | 76 ± 10 | 74 ± 8 | 77 ± 11 | 71 ± 13 | 0.28 |
| 24 hour urine phosphorus (mg) | 707 (469, 958) | 605 (530, 803) | 735 (590, 905) | 764 (591, 897) | 0.88 |
| Hemodynamics | |||||
| Systolic BP (mmHg) | 130 ± 17 | 131 ± 14 | 130 ± 12 | 128 ± 10 | 0.65 |
| Diastolic BP (mmHg) | 76 (56, 94) | 70 (54, 88) | 80 (64, 98) | 72 (56, 80) | 0.30 |
| Cardiovascular Structure/Function | |||||
| Carotid artery intima-media thickness (mm) | 0.8 (0.6, 1.0) | 0.9 (0.6, 1.0) | 0.8 (0.5, 0.9) | 0.8 (0.6, 1.0) | 0.98 |
| Left ventricular mass (g/m2.7) | 45 ± 13 | 53 ± 13 | 50 ± 12 | 54 ± 13 | 0.36 |
| Left ventricular ejection fraction (%) | 63 ± 11 | 64 ± 12 | 62 ± 9 | 67 ± 6 | 0.37 |
| Pulse wave velocity (m/s) | 10.6 (7.9, 20.0) | 10.0 (7.2, 13.1) | 10.4 (5.5, 18.4) | 10.0 (6.6, 13.3) | 0.32 |
| Biomarkers of CKD-MBD | |||||
| DKK-1 (pg/ml) | 1968 ± 834 | 1985 ± 814 | 1705 ± 683 | 1772 ± 655 | 0.76 |
| Sclerostin (pg/ml) | 1156 (710, 2282) | 1233 (898, 2361) | 1145 (730, 1756) | 1320 (573, 2002) | 0.40 |
| Bone Mineral Density (SDS Z-score) | 2.4 ± 2.1 | 2.7 ± 2.1 | 1.3 ± 1.1 | 1.1 ± 1.1 | 0.23 |
All values given as mean ± SD or median (range). Calcium volume scores have been square root transformed. Within each group, there were no significant differences from baseline to the 12 month visit. P-values represent the comparison of the change from baseline to the 12 month visit between LaCO3 and Placebo.
LaCO3 was well tolerated during the study period. The most commonly reported adverse effect was nausea, which occurred in 5 subjects (26%) compared to 2 (11%) in the placebo group. Three subjects with nausea left the study before completing all 5 visits. Headache, constipation, and hyperglycemia each occurred in 1 subject. The subject with hyperglycemia was later diagnosed with type 2 diabetes mellitus. There were no deaths or serious adverse events reported during the study period.
Primary Outcome
Mean serum phosphorus levels were 3.3–3.5 mg/dl at baseline in all subjects. There were no instances of hypophosphatemia over the 12 months, and LaCO3 had no significant effect on the primary outcome measure, change in mean fasting serum phosphorus from baseline to month 12 (Table 2).
Secondary Outcomes
Phosphate Homeostasis and Biomarkers of the Early CKD-MBD
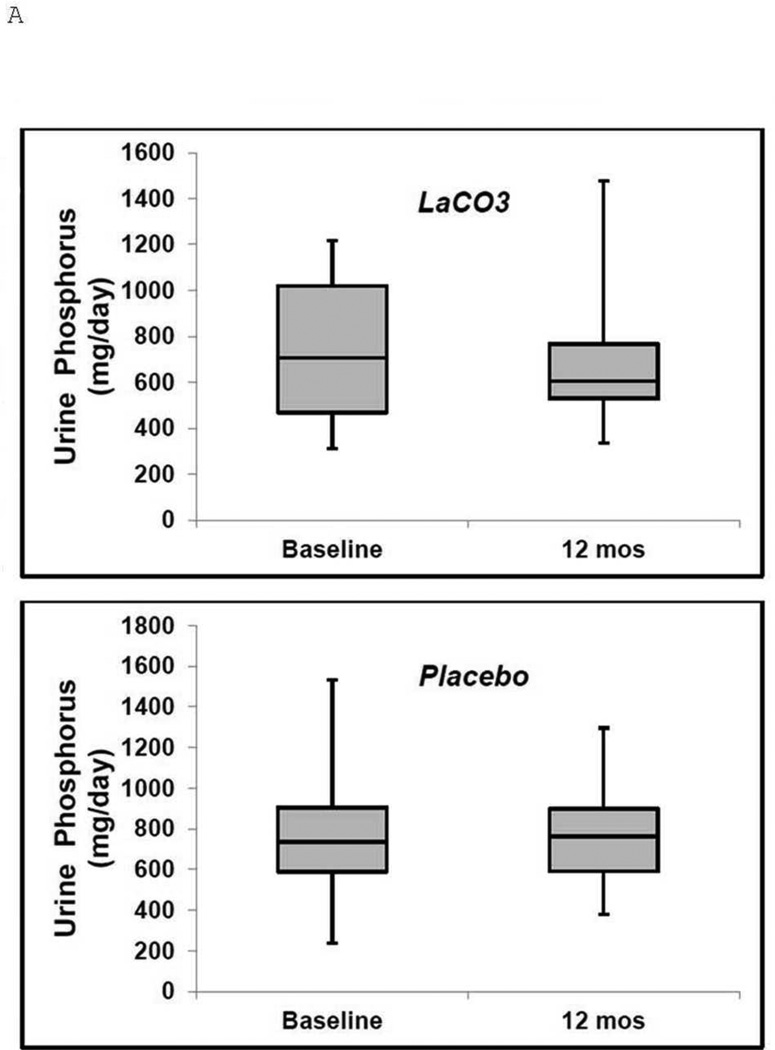
The tubular reabsorption of phosphorus (TRP) was decreased in both groups at baseline (76% and 77% in LaCO3 and placebo, respectively) and was not affected by LaCO3 (Table 2). Baseline median urinary phosphorus excretion was 707 and 735 mg/day in LaCO3 and placebo, respectively (Table 2). Urinary phosphorus excretion decreased to 605 mg/day in LaCO3 and increased to 764 mg/day in placebo by month 12, but these changes were not significant (Table 2, Figure 3A). Baseline mean intact PTH levels were 76 and 57 pg/ml in LaCO3 and placebo, respectively (upper limit of normal in our local assay is 72 pg/ml) (Table 2). PTH levels did not change significantly over the 12 months in either group(Table 2). There were no significant differences in calcium, creatinine, or creatinine clearance between groups at baseline or at month 12 (Table 2).
Figure 3. Urinary phosphate and FGF23 levels in the LaCO3 and placebo groups at baseline and at 12 months.
Boxplot representation of (A), Urinary Phosphate and (B), FGF23 levels at baseline and 12 months. Median values are the horizontal lines within the shaded areas. The shaded areas represent the 75th (top) to 25th (bottom) percentiles, and the vertical lines with whiskers represent the full range.
Median FGF23 levels in the cohort at baseline were 58 pg/ml (range 24–201), which were elevated compared to a reference group of 450 patients in the Diabetes Heart Study with normal glomerular filtration rates that were measured simultaneously with our cohort (37 pg/ml (range 0–539)). LaCO3 did not significantly affect FGF23 levels, which were decreased from 69 pg/ml at baseline to 55 pg/ml at month 12 in the LaCO3 group, compared to no change from 55 pg/ml in placebo (Table 2, Figure 3B).
Baseline levels of DKK1 and sclerostin were 2–3-fold higher in both groups than reference values determined simultaneously in the Diabetes Heart Study cohort that had normal kidney function. After 12 months of treatment there were no differences in plasma DKK1 or sclerostin between or within groups (Table 2). Bone mineral density was normal and remained stable or slightly improved in each group after 12 months (Table 2).
Cardiovascular Endpoints
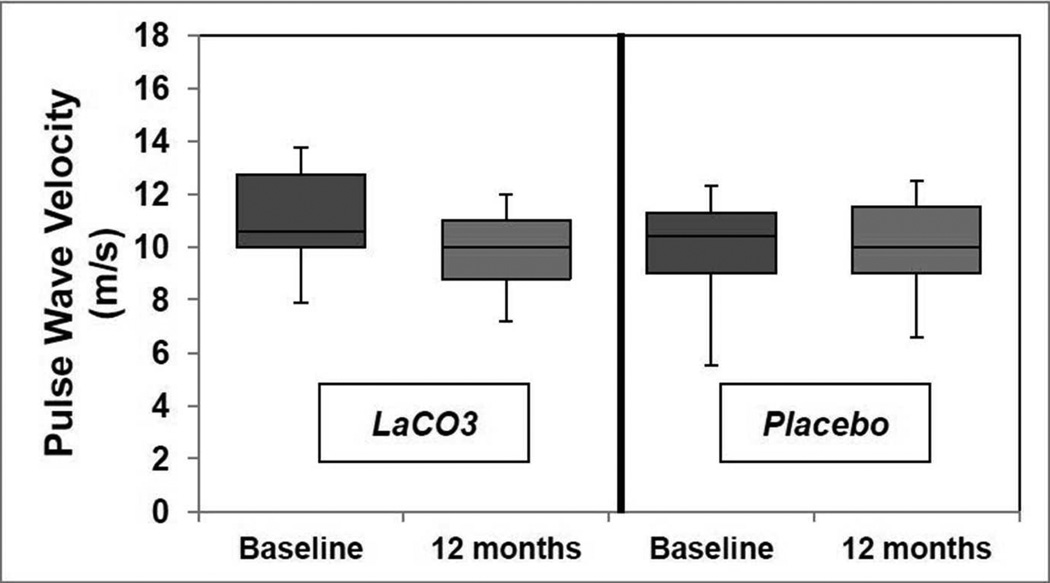
Blood pressure was well controlled in both groups, with 32 of 38 subjects (84%) taking antihypertensive medications or ACEI for proteinuria throughout the study (Table 2). The majority of our cohort demonstrated vascular stiffness at the baseline visit, with PWV greater than the 50th percentile for age using data from “The Reference Values for Arterial Stiffness” Collaboration (Table 2) [26]. The decrease in PWV from baseline to month 12 in the LaCO3 group [10.6 (7.9–20.0) to 10.0 (7.2–13.1) m/s, Figure 2], was not significant when compared to placebo (Table 2). Baseline cIMT was normal in both groups. With our observed effect on pulse wave velocity, a statistical power of 0.8 and a probability level of 0.05, the sample size per group with a two tailed hypothesis would need to be 45 patients per group.
Figure 2. Effect of LaCO3 on Pulse Wave Velocity.
Boxplot representation of carotid-femoral pulse wave velocity data at baseline and the 12-month visit in each group. The line in the shaded area is the median value, the top of the shaded area is the 75th percentile, the bottom of the shaded area is the 25th percentile and the whiskers represent the range of the values.
There was no change in cIMT from baseline to month 12 within LaCO3 or placebo (Table 2). Low grade VC was common in the carotid arteries, coronary arteries, and aorta in both groups at the baseline visit (Table 3) as determined by Agatston score and calcium volume. After 12 months, the progression of the Agatston score or calcium volume was minimal, and there were no differences in VC in the carotid arteries, coronary arteries, or aorta between LaCO3 and placebo (Table 3, supplemental Figure 1). Left ventricular ejection fraction (LVEF) remained stable in both groups. LVM/Ht2.7 increased within the LaCO3 group after 12 months of treatment, but this trend was not statistically significant (Table 2).
Table 3.
Vascular Calcification Between Groups
| LaCO3 (n=19) |
Placebo (n=19) |
||||
|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | P | |
| Agatston Score | |||||
| Right Carotid Artery | 0 (0, 674) | 27 (0, 734) | 0 (0, 513) | 0 (0, 400) | 0.48 |
| Left Carotid Artery | 23 (0, 795) | 111 (0, 902) | 16 (0, 282) | 21 (0, 397) | 0.69 |
| Coronary Arteries | 170 (0, 4357) | 248 (0, 5835) | 246 (0, 2146) | 386 (0, 2443) | 0.86 |
| Aorta | 778 (0, 25269) | 243 (0, 35071) | 1675 (0, 10384) | 2730 (0, 10994) | 0.58 |
| Calcium Volume (mm3) | |||||
| Right Carotid Artery | 7 ± 9 | 7 ± 9 | 4 ± 6 | 5 ± 6 | 0.57 |
| Left Carotid Artery | 8 ± 10 | 9 ± 10 | 5 ± 5 | 5 ± 6 | 0.88 |
| Coronary Arteries | 20 ± 21 | 23 ± 23 | 17 ± 16 | 20 ± 17 | 0.70 |
| Aorta | 42 ± 46 | 46 ± 53 | 39 ± 32 | 43 ± 30 | 0.70 |
All values given as mean ± SD or median (range). Calcium volume scores have been square root transformed. Within each group, there were no significant differences from baseline to the 12 month visit. P-values represent the comparison of the change from baseline to the 12 month visit between LaCO3 and Placebo.
DISCUSSION
This randomized, placebo-controlled, pilot and feasibility study demonstrates that 12 months of treatment with the phosphate binder LaCO3 had no significant effect on the serum phosphorus level in normophosphatemic patients with CKD stage 3a [27]. These results were compatible with our preclinical studies of phosphate binders in models of normophosphatemic early CKD. LaCO3 had no significant effect on urine phosphorus excretion, TRP or plasma levels of the phosphaturic hormone FGF23. Furthermore, LaCO3 did not alter PWV, cIMT or VC compared to placebo.
Other investigators have demonstrated small or no changes in phosphate homeostasis during intervention with phosphate binders in early CKD [28,29]. Hill et al [29] showed that the phosphate binder calcium carbonate produced little change in phosphate homeostasis in normophosphatemic subjects, in agreement with our results using the binder LaCO3. Our cohort size was twice that of Gonzalez-Parra et al [28], who performed an open label study of LaCO3 in hyperphosphatemic patients. They also reported a lack of change in serum phosphorus but significant reductions in urinary phosphate excretion and increased TRP that were not detected in our cohort. Our study was designed as a pilot and feasibility study, and there was insufficient power to detect changes in phosphate homeostasis during the study. Using a two-tailed alpha of 0.05, a post-hoc analysis revealed that our sample size of 38 subjects was adequately powered to detect a 0.5 mg/dl difference in serum phosphorus levels, a 10 percentage point difference in TRP, and 400 mg difference in 24-hour urine phosphorus between groups with 80% power. Alternatively, passive phosphate absorption may be resistant to binders in the presence of normophosphatemia, and therefore the change in phosphate homeostasis in our study was truly insignificant. Several studies of phosphate binders in early CKD have shown lack of change in FGF23 levels but modest changes in phosphate homeostasis [30–32]. These studies were in cohorts containing hyperphosphatemic subjects with more advanced kidney disease than our cohort, which may account for the lack of change in FGF23 levels or phosphate homeostasis that we observed. In contrast, other reports show reductions in FGF23 with phosphate binders [28,33,34]. Closer inspection of these studies reveals higher baseline levels of FGF23 than in our cohort, shorter periods of treatment and changes in levels similar to those reported here (Table 2). Although it is not routine clinical practice, coupling dietary restriction with phosphate binders has recently been shown to decrease FGF23 levels in CKD [35,36]. Sigrist et al suggest that dietary restriction alone may be adequate for reducing FGF23 levels in early CKD, but they demonstrate additivity with aluminum based phosphate binders [35]. Thus, it is possible that phosphate binders plus dietary phosphorus restriction may be necessary to reduce FGF23 levels in early CKD.
Parathyroid hormone levels were normal at baseline in our cohort as a whole (68 pg/ml), and were unchanged by LaCO3 just as in the LaCO3 arm in Block et al [30]. Thus, the changes in phosphate homeostasis in our study were minimal, but consistent across all parameters used to assess phosphate homeostasis.
Recent data demonstrate that the CKD-MBD begins in early CKD. Elevations of FGF23 in the skeleton and the circulation are present in stage 2–3 CKD [24,37,38], along with reductions in the tubular reabsorption of phosphorus (TRP) [38] and the onset of vascular stiffness [18]. Our results are in agreement with these studies, showing that in normophosphatemic subjects with stage 3 CKD, circulating FGF23, DKK1 and sclerostin were increased and TRP was decreased at baseline. All subjects had elevated PWV at baseline, confirming that vascular stiffness was present.
An important contribution of our study is the assessment of vascular stiffness in each treatment arm using carotid-femoral PWV. After 12 months of therapy, LaCO3 did not significantly improve PWV compared to placebo. Recent clinical and translational studies have highlighted the importance of FGF23 signaling in vascular stiffness and overall CVD risk [39,40]. We therefore speculate that the lack of significant improvement in vascular stiffness in our study may be linked to the lack of significant changes in phosphate homeostasis and FGF23 levels. Future studies that combine phosphate binders with dietary phosphorus restriction may produce the necessary changes in phosphate homeostasis and FGF23 to reduce markers of CVD risk such as PWV. For our observed effects on PWV, a statistical power of 0.8 and a probability level of 0.05, the sample size per group with a two tailed hypothesis would need to be 45 patients per group.
Our cohort demonstrated normal ranges of cIMT but significant VC at baseline with no significant change in either parameter after 12 months of LaCO3. The normal cIMT range in our cohort may reflect the early stage of CKD and could explain the observed lack of improvement with LaCO3. Although VC was detectable in the cohort at baseline, the progression over 12 months was minimal in both LaCO3 and Placebo. Our results do not disagree with recent studies from Block et al [30] that reported increased coronary artery calcification in a composite cohort of subjects with stage 3–4 CKD receiving calcium acetate, LaCO3, or sevelamer carbonate. In their study the progression of VC in the LaCO3 arm also did not differ from placebo. The progression of VC in the combined study population appeared to derive from the subgroup treated with calcium acetate. Despite the initial consensus around phosphorus as a CVD risk factor [8–12,41], recent studies have questioned this concept and therefore the impetus to intervene in phosphate homeostasis early in the course of CKD [30,42].
There are several limitations to this study. The first is that the study was under powered for the cardiovascular outcomes, especially for detection of the modest differences we observed for each outcome between groups. Secondly, the period of observation may have been too short to observe progression in the surrogates of cardiovascular disease selected for study. This will influence design of future studies seeking to intervene in the cardiovascular morbidity associated with CKD.
We conclude that LaCO3 therapy is feasible without hypophosphatemia in stage 3a CKD. In this prospective pilot and feasibility study in early CKD, 12 months of LaCO3 was associated with no significant changes in phosphate homeostasis and no improvement in PWV, a measure of vascular stiffness, cIMT or VC. This study does not lend support for further study of phosphate binders in early stage CKD because of the failure to affect phosphate homeostasis. A practical design that incorporates effective change in phosphate homeostasis is worthy of additional study.
STUDY POPULATION AND METHODS
The study protocol was approved by the Human Research Protection Office at Washington University in St. Louis. Subjects were enrolled after giving informed consent in accordance with guidelines from the Declaration of Helsinki. Subjects were eligible if greater than 18 years of age and had stage 3 CKD (estimated GFR 30–59 ml/min/1.73m2) using the Modification of Diet in Renal Disease study equation [46]. Exclusion criteria included pregnancy, bone disease, myocardial infarction, congestive heart failure, diastolic dysfunction or severe hypertension. Each week members of the research team (M.R., S.C., W.R., or D.W.) identified their clinic patients that satisfied the inclusion criteria and were interested in the study. Those who provided informed consent to our research nurse allowed a review of their medical records to determine final eligibility before enrollment. The cohort size was predetermined by the available funding. As subjects were enrolled, they were stratified for age, gender, race and diabetes status and then randomized into 2 groups, allocated 1:1 to receive either 1000 mg of LaCO3 or a matching placebo with meals three times daily for 12 months. The randomization and double-blind strategy was designed and maintained by our research pharmacist and statistician. Because practitioners often do not prescribe dietary phosphate restriction in normophosphatemic CKD, dietary phosphate intake was not regulated. All participants were encouraged to track phosphate sources in the diet but these data were not collected. The purpose of the study was to determine the cohort sizes needed to adequately power primary and secondary outcomes in definitive prospective trials.
Cardiovascular evaluations
Cardiovascular assessments were performed at baseline and 12 months. Pulse wave velocity (PWV) was determined by use of applanation tonometry of the carotid and femoral arteries (SphygmoCor, AtCor Medical, Australia) as previously described and validated [47–51]. Briefly, subjects were placed in the supine position and after 10 minutes of rest, heart rate and three brachial artery blood pressure measurements were obtained in each arm using a noninvasive manual sphygmomanometer. The high-fidelity transducer was then placed on the subject’s right carotid artery and the recorded pressure waveforms were calibrated using an average of the peripheral brachial artery blood pressure. Then the transducer was applied to the right femoral artery and waveforms were again acquired. PWV was determined as the difference in travel time of the pulse-wave between the heart and each the right carotid and right femoral arteries, divided by the travel distance of the pulse-wave form. The onset of the Q-wave from the surface electrocardiogram (ECG) is used to determine the start of the pulse-wave. Using a tape measure, the distance between the right carotid artery and suprasternal notch was subtracted from the distance between the right femoral artery and suprasternal notch. The applanation tonometry measurements were performed by a research technician who was blinded to clinical data, echocardiographic results and treatment group.
Carotid artery intima-media thickness (cIMT) was measured by a single vascular sonographer from B-mode images of both carotid arteries expressed as the average of the far-- walls of the right and left common carotid arteries; each site represents the average of three separate measurements [52]. The intraclass correlation coefficient for repeated measures of the cIMT is 0.91 and for echocardiographic measurements ranges from 0.85–0.90 at our laboratory.
Two-dimensional and M-mode echocardiograms and carotid artery ultrasound were performed by use of commercially-available ultrasound equipment (Sequoia-C256, Acuson-- Siemens, Mountain View, CA). Two-dimensional directed echocardiographic measurements included the LV ejection fraction (LVEF) calculated using the biplane method of discs (modified Simpson’s method). LV mass was measured by the M-mode-derived cubed method and indexed to height2.7 (LVM/Ht2.7) [53]. All measurements were performed in accordance to published guidelines and represent the average of three consecutive cardiac cycles obtained by a single observer blinded to all clinical parameters and treatment group [54].
Multidetector CT (MDCT) Imaging for Assessment of Vascular Calcification
A 64-slice multidetector CT (MDCT) scanner (Somatom Sensation 64, Siemens Medical Systems, Forchheim, Germany) measured calcium scores and volumes by the Agatston method [55]. After initial scout imaging, the scan fields were set for the neck, chest, and abdomen for measurement of arterial calcification in the carotids (from the arch to 1 mm above the carotid bifurcation), coronary arteries, aortic arch and thoracic aorta (to the top endplate of the T12 vertebral body). The scan parameters included 24 × 1.2 mm collimation, 3 mm slice thickness, 0.37 second rotation time, spiral mode, and 120 kVp at 80 mAs with reconstruction at 60% of the R-R interval. Vascular calcium scores and volumes were measured by use of commercially available software (Vitrea; Vital Images, Inc., Minnetonka, Minnesota) as previously described [55]. All images were evaluated by a single radiologist (A.B.) blinded to all clinical characteristics and the treatment group.
Biomarkers of the chronic kidney disease-mineral bone disorder (CKD-MBD)
Blood samples were obtained from each subject at the baseline visit and after 12 months of treatment. There was intersubject variation in the time of day that blood samples were obtained. Aliquots of plasma were generated from each sample and used immediately for biochemical testing or frozen at 80°C. Plasma levels of FGF23, DKK1 and sclerostin were measured in duplicate using commercially available ELISA kits, according to the manufacturer’s instructions (FGF23: Kainos Laboratories, Tokyo, Japan; DKK1: R&D Systems, Minneapolis, MN; sclerostin: Teco Medical Group, Sissach, Switzerland).
For safety monitoring, plasma phosphorus, calcium, creatinine, and intact parathyroid hormone (iPTH) levels were measured using standard laboratory methods. A random urine sample was obtained concomitantly to calculate the tubular reabsorption of phosphorus. A separate 24 hour urine collection was performed at the baseline and 12 month visits to detect a change in creatinine clearance or phosphorus excretion. We used the 24-hour creatinine excretion to determine the adequacy of each urine collection. Bone mineral density was assessed using DXA scan of the lumbar spine at baseline and 12 months.
Outcome Definitions
The primary outcome was the change in serum phosphorus. Secondary outcomes included change in mean carotid-femoral PWV, a surrogate for vascular stiffness, from baseline to month 12 and changes in: serum phosphorus, 24-hour urine phosphorus, tubular reabsorption of phosphorus (TRP), VC, cIMT, left ventricular mass indexed to height2.7 (LVM/Ht2.7), left ventricular ejection fraction (LVEF), and plasma FGF23, DKK1 and sclerostin levels. Given the pilot nature to the design, an empiric number of subjects were enrolled based on available funds.
Statistical Analysis
Statistical analysis was performed by a statistician who remained blinded to the identity of the subjects and their corresponding study groups (J. M.). The data were analyzed using the software package SAS 9.1 (Cary, NC). Calcium volume scores were square-root transformed to limit the likelihood of differences due to variability in MDCT scans, as validated and reported previously [56]. Wilcoxon two-sample test and student’s t test were used to test the differences of continuous variables at baseline and the differences from baseline to the 12 month visit between LaCO3 and Placebo. Signed-rank test (non-normally distributed data) and paired t test (normally distributed data) were used to test differences of continuous variables from baseline to the 12 month visit within LaCO3 or Placebo. Normally distributed data are presented as mean ± standard deviation (SD). Non-normally distributed data are presented as median (range). Categorical variables were compared using Chi-square test. All tests were two-tailed; statistical significance was considered at p < 0.05.
Supplementary Material
ACKNOWLEGMENTS
The authors are grateful to Julie Nobbe, Pharm.D., who maintained the randomization and double-blind strategy. We thank Jingnan Mao for her assistance as a statistician, and Daniel Coyne for reviewing the results and adverse events in lieu of a data and safety monitoring committee. The LaCO3 and matched placebo were provided by Shire U.S. Pharmaceuticals, Inc. The study was funded by Shire U.S. Pharmaceuticals, Inc., and by NIH grants DK 070790, DK 089137 (K.A.H.), KL2RR024994, and UL1 RR024992 (Washington University).
Footnotes
Conflict of Interest:
K.A.H. has been a consultant for or the recipient of research funding from Shire, Genzyme and Fresenius.
REFERENCES
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu Cy. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Culleton BF, Larson MG, Wilson PWF, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 6.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in endstage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 7.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in endstage renal diseases: Impact on all cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 8.Block GA, HulbertShearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 9.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. for the C, Recurrent Events Trial I: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 11.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 12.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GFM. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 13.ElAbbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM. Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor23, and osteopontin. Kidney Int. 2009;75:1297–1307. doi: 10.1038/ki.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 16.Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in endstage renal disease. Kidney Int. 2001;60:472–479. doi: 10.1046/j.1523-1755.2001.060002472.x. [DOI] [PubMed] [Google Scholar]
- 17.Mathew S, Lund R, Strebeck F, Tustison KS, Geurs T, Hruska KA. Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol. 2007;18:122–130. doi: 10.1681/ASN.2006050490. [DOI] [PubMed] [Google Scholar]
- 18.Ix JH, de Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in mesa. Clin J Am Soc Nephrol. 2009;4:609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng J, Wassel CL, Kestenbaum BR, Collins TC, Criqul MH, Lewis CE, Cummings SR, Ix JH. Serum phosphorus levels and the spectrum of ankle brachial index in older men. The osteoporotic fractures in men (MROS) study. Am J Epidemiol. 2010;171:909–916. doi: 10.1093/aje/kwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 22.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 23.Fang Y, Ginsberg C, Sugatani T, Faugere MC, Malluche H, Hruska KA. The early chronic kidney disease-mineral bone disorder (CKD-MBD) stimulates vascular calcification. Kidney Int. 2013 doi: 10.1038/ki.2013.271. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1 and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabbagh Y, Graciolli FG, O'Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani MEF, Carvalho AB, Jorgetti V, Moyses RMA, Schiavi SC. Repression of osteocyte wnt/β catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27:1757–1772. doi: 10.1002/jbmr.1630. [DOI] [PubMed] [Google Scholar]
- 26.Collaboration TRVfAS: Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘ Establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.KDIGO 2013 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Parra E, Gonzalez-Casaus ML, Galán A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A. Lanthanum carbonate reduces FGF23 in chronic kidney disease stage 3 patients. Nephrol Dial Transplant. 2011;26:2567–2571. doi: 10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 29.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M. Oral calcium carbonate affects calcium but not phosphorus balance in stage 34 chronic kidney disease. Kidney Int. 2013;83:959–966. doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, Wolf M. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block GA, Persky MS, Ketteler M, Kestenbaum B, Thadhani R, Kooienga L, Spiegel D, Asplin J, Ehrlich J, Dennis V, Nissenson A, Chertow GM, Wheeler DC. A randomized double-blind pilot study of serum phosphorus normalization in chronic kidney disease: A new paradigm for clinical outcomes studies in nephrology. Hemodialysis Int. 2009;13:360–362. doi: 10.1111/j.1542-4758.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Mallamaci F, Zoccali C. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: A randomized clinical trial. Am J Kidney Dis. 2012;59:177–185. doi: 10.1053/j.ajkd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira RB, Cancela ALE, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RMA. Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigrist M, Tang M, Beaulieu M, Espino-Hernandez G, Er L, Djurdjev O, Levin A. Responsiveness of FGF23 and mineral metabolism to altered dietary phosphate intake in chronic kidney disease (CKD): Results of a randomized trial. Nephrology Dialysis Transplantation. 2013;28:161–169. doi: 10.1093/ndt/gfs405. [DOI] [PubMed] [Google Scholar]
- 36.Isakova T, Barchi-Chung A, Enfield G, Smith K, Vargas G, Houston J, Xie H, Wahl P, Schiavenato E, Dosch A, Gutierrez OM, Diego J, Lenz O, Contreras G, Mendez A, Weiner R, Wolf M. Effects of dietary phosphate restriction and lanthanum carbonate on FGF23 in CKD. J Am Soc Nephrol. 2012;23:P45A. doi: 10.2215/CJN.09250912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor23 and early decrements in kidney function: The heart and soul study. Nephrol Dial Transplant. 2010;25:993–997. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CAM, Lash JP, Hsu Cy, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, AquillonPrada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandi NC, Brenner H, Hahmann H, Wüsten B, März W, Rothenbacher D, Breitling LP. Calcium, phosphate and the risk of cardiovascular events and all-cause mortality in a population with stable coronary heart disease. Heart. 2012;98:926–933. doi: 10.1136/heartjnl-2011-300806. [DOI] [PubMed] [Google Scholar]
- 43.Fang Y, Zhang Y, Mathew S, Futhey J, Lund RJ, Hruska KA. Early chronic kidney disease (CKD) stimulates vascular calcification (VC) and decreased bone formation rates prior to positive phosphate balance. J Am Soc Nephrol. 2009;20:36A. [Google Scholar]
- 44.Graciolli FC, Oliveira RB, Dos Reis LM, Cancela ALE, Cuppari L, Canziani MEF, Carvalho AB, Sabbagh Y, Jorgetti V, Schiavi S, Moyses RM. Wnt pathway inhibition: Another actor in CKD-MBD pathophysiology? J Am Soc Nephrol. 2010;21:774A. [Google Scholar]
- 45.Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, Haas M, Malluche HH. Sclerostin and dickkopf1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6:877–882. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 47.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 48.Van Bortel LM, Balkestein EJ, van der HeijdenSpek JJ, Vanmolkot FH, Staessen JA, Kragten JA, Vredeveld JW, Safar ME, Boudier HAS, Hoeks AP. Noninvasive assessment of local arterial pulse pressure: Comparison of applanation tonometry and echo-tracking. J Hypertens. 2001;19:1037–1044. doi: 10.1097/00004872-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 49.O'Rourke MF, Adji A. An updated clinical primer on large artery mechanics: Implications of pulse waveform analysis and arterial tonometry. Curr Opin Cardiol. 2005;20:275–281. doi: 10.1097/01.hco.0000166595.44711.6f. [DOI] [PubMed] [Google Scholar]
- 50.O'Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51:507–522. doi: 10.1046/j.0306-5251.2001.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 52.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 53.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 54.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiography. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 55.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J AM Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 56.Mathews SJ, de las Fuentes L, Podaralla P, Cabellon A, Zheng S, Bierhals A, Spence K, Slatopolsky E, Davila-Roman VG, Delmez JA. Effects of sodium thiosulfate on vascular calcification in end-stage renal disease: A pilot study of feasibility, safety and efficacy. Am J Nephrol. 2011;33:131–138. doi: 10.1159/000323550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.