Abstract
The insular cortex has been associated with the processing of rewarding stimuli and with the neural bases of drug addiction. Ischemic damage to the insula has been associated with decreased desire to smoke cigarettes. Which component of insular function is involved in the neural basis of cigarette smoking is not clear. Dopamine systems are crucial for the reinforcing value of addictive drugs. The DA projection from the ventral tegmental area to the nucleus accumbens (NAc) has been shown to be a vital pathway for the primary reinforcement caused by taking a variety of abused drugs. In the current set of studies, the roles of D1 and D2 receptors in the insular cortex in the self-administration of nicotine by rats were assessed. Adult female Sprague-Dawley rats were fitted with jugular catheters and given access to self-administer nicotine. Bilateral local infusion cannulae were implanted into the agranular insular cortex to locally administer D1 and D2 antagonists (SCH-23390 and haloperidol). Acute local infusions of the D1 antagonist SCH-23390 into the insula (1–2 μg/side) significantly decreased nicotine self-administration by more than 50%. Repeated infusions of SCH-23390 into the agranular insula caused continuing decreases in nicotine self-administration without signs of tolerance. In contrast, local infusions of the D2 antagonist haloperidol 0.5–2 μg/side did not have any discernable effect on nicotine self-administration. These studies show the importance of DA D1 systems in the insula for nicotine reward.
Keywords: Insular cortex, Dopamine, D1, D2, Nicotine, Self-administration
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are considered to be the primary therapeutic targets in the treatment of tobacco addiction. However, nicotinic receptor systems interact with a variety of other neural systems, which are also important components of reinforcement circuitry and are key to the development and maintenance of addiction. In particular, dopamine receptor systems have been found to play important roles for the rewarding properties of nicotine. For example, Corrigall and Coen [1] examined the effects of subcutaneous injections of the selective D1 dopamine antagonist SCH-23390 and D2 dopamine antagonists spiperone and haloperidol on nicotine self-administration, food-maintained responding, and locomotor activity in rats. Results showed that all three dopaminergic antagonists reduced both nicotine self-administration and locomotor activity. Furthermore, O'Neill et al. [2] found that nicotine induced hyperactivity is reduced with the systemic injections of the SCH-23390, as well as the D2 antagonist raclopride, and D1/D2 antagonist fluphenzine. Specifically, the DA activity in the NAc was found to play an important role in the processing of reward salience (e.g. [3,4]). Dopaminergic innervation of the NAc has also long been known to be key for the reinforcing effects of a variety of addictive drugs including nicotine (see [5] for a review). For instance, several studies [6] showed that both systemic and local injections of nicotine into the NAc increased extracellular DA levels. Furthermore, dopaminergic neurons in the NAc are populated with nAChRs, which cause DA release when nicotine is injected into the NAc. Fu et al. [7] showed that blocking a subtype of nAChRs (α7-nAChRs) also blocks the DA release in response to nicotine administration.
Other brain areas have also been found to play important roles in tobacco addiction. Ischemic damage to the insular cortex, which plays an important role in monitoring and preserving physiological homeostasis, encoding the incentive value of the taste of foods [8] and other rewards [9] has been associated with decreased desire to smoke [10]. Studies have linked the insula with incentive learning [8], conditioned taste-aversion [11] and disgust [12]. According to Naqvi and Bechera [13], along with the ventromedial prefrontal cortex, anterior cingulate cortex, NAc, and amygdala, the insula is a part of the network which is responsible for the conscious pleasure from drugs and urge for drug taking as well as relapse of this behavior. Specifically, the insula processes interoceptive information about the drugs received from a thalamocortical pathway and relays this information to the prefrontal regions and the amygdala which evoke pleasure related effects of the drugs through dopaminergic activation in the ventral tegmental area (VTA). Similarly, the model suggests that the insula contributes to the urge of drug taking by integrating the internal representation of the drug effects received from the ventromedial prefrontal cortex and the information about the environmental cues received from the anterior cingulate cortex. Finally, this information is fed into the NAc and gives rise to the motivational elements of drug taking behavior.
The insula consists of three main subregions: posterior granular insula, intermediate dysgranular, and anterior agranular insula. The granular insula receives projections from a thalamocortical pathway including the thalamus, parietal, occipital, and temporal association cortices and it is responsible for processing of the interoceptive information and of somatosensory, vestibular, and motor integration. This subregion of the insula also plays an important role in the addictive behavior. For example, Forget et al. [14] demonstrated that inactivation of the granular insula by using the GABAergic agonist Muscimol decreased nicotine self-administration in rats. Moreover, the agranular insula was found to mediate cocaine self-administration. The agranular insula, on the other hand, is responsible for the emotional and motivational integration of visceral information and has reciprocal connections with the limbic areas responsible for reward-processing such as the anterior cingulate cortex, the ventromedial prefrontal cortex, the amygdala, and the ventral striatum [13,15,16]. In line with its role in motivation, the agranular insula contains a high density of dopaminergic D1 receptors [17] as well as μ-opioid receptors which play a role in pain modulation and the rewarding effects of drugs [18]. These receptors make the agranular insula a natural target for the studies of reward and addiction. Di Pietro et al. [19], demonstrated that when dopaminergic activity in the prefrontal dorsal agranular insula was blocked with the D1-like receptor antagonist SCH 23390, the cocaine self-administration was reduced. However neural system interactions within the insula that are responsible for this effect are not well understood.
The current studies were conducted to determine the role of dopaminergic D1 and D2 receptors in the agranular insular cortex for the control of IV nicotine self-administration. It was hypothesized that dopaminergic innervation of the agranular insular cortex plays an important role in controlling nicotine self-administration. Furthermore, we expect to find similar effects of D1 and D2 antagonists on nicotine self-administration.
2. Methods
2.1. Subjects
Young adult female Sprague-Dawley rats (Taconic Lab, Germantown, NY, USA) were used in the present study. Animals were individually housed in a temperature controlled vivarium room located adjacent to the nicotine self-administration testing room. Animals were maintained on a 12:12 reverse light-dark cycle so that experimental sessions occurred during the active part of the rats' diurnal cycle. Animals were given ad lib. access to water at all times excluding experimental sessions, and were fed daily 20–30 min after the completion of their experimental session and were maintained at roughly 85% of free-feeding weight. Animals progressively and healthily gained weight throughout the study.
2.2. Behavioral procedures
Before the start of nicotine self-administration sessions, all animals were trained to lever press in a standard dual-lever experimental chamber (Med Associates, St. Albans, VT, USA) for food reinforcement. Each chamber was equipped with two levers (one active, one inactive), two cue lights located directly above each lever, a house light, and a tone generator. After lever pressing was established, animals experienced three 30 min sessions of lever pressing for food under a fixed ratio (FR) 1 schedule of reinforcement. After rats learned to press for 50 food pellets in 30 min, subjects had catheters surgically implanted into the jugular vein to enable them to receive nicotine infusions (see Section 2.3).
Following catheterization surgeries, animals were trained to self-administer nicotine (0.03 mg/kg/infusion, IV) via operant lever response (FR1) with a visual secondary reinforce for 5 sessions. Two levers were available to be pressed and only one caused the delivery of nicotine on an FR1 schedule. Pressing the lever on the active side resulted in the activation of the feedback tone for 0.5 s and the immediate delivery of one 50-μl infusion of nicotine in less than 1 s. Each infusion was immediately followed by a 1-min period in which the cue lights went out, the house light came on and responses were recorded but not reinforced. Each session lasted for 45-min.
After the 5th training session, guide cannulae for drug microinfusions were stereotaxically implanted into the agranular insular area (see Section 2.4). Following a 3-day hiatus, rats were given 8 nicotine self-administration test sessions. These sessions were identical to the training sessions except 5 min prior to the test sessions D1 antagonist SCH-23390 or D2 antagonist Haloperidol was infused through the implanted cannulae (see Section 2.5).
2.3. Catheterization surgery
Following the completion of their final training session with food reinforcement, animals were anesthetized with a mixture of ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA; 60 mg/kg) and dex-dormitor (Pfizer, New York, NY, USA; 0.075 mg/kg) and a catheter (Strategic Application Inc., Libertyville, IL, USA) was implanted into their jugular vein. The jugular catheter was attached to a harness that could be tethered to the infusion pump during experimental sessions. Animals were given a minimum of 24 h to recover from surgery before experiencing nicotine self-administration sessions. Catheters were flushed daily, before the sessions began, with a 0.3 ml solution containing 100 U/ml heparinized saline (Baxter Health Corporation, Deerfield, IL, USA). When sessions were over, the nicotine remaining in the ports was drawn out and replaced by a 0.25 ml sterile lock consisting of heparinized saline 500 U/ml with 8-mg/ml gentamicin (American Pharmaceutical Partners, Schaumburg, IL, USA). At the end of the study catheter patency tests were conducted and animals with an obstructed catheter were dropped from the study.
2.4. Cannulation surgery
After the 5th nicotine self-administration sessions, bilateral local infusion cannulae were implanted into the agranular insula by stereotaxic surgery (David Kopf Instruments, Tujunga, CA, USA) to locally administer D1 and D2 antagonists (SCH-23390 and haloperidol) into the insula. The rats were anesthetized as described above. The skull was positioned in a flat-skull position where the incisor bar was lowered 3.3 ±0.4 mm below horizontal zero. To target the agranular insula we used following coordinates, relative to bregma, anteroposterior +1.00 mm, lateral ±5.2 mm, and dorsoventral −5.80 mm [20]. Figs. 1 and 2 show the injection sites for the animals included in the statistical analysis. The cannulae were secured using a screw and wire structure, and then covered with dental cement.
Fig. 1.
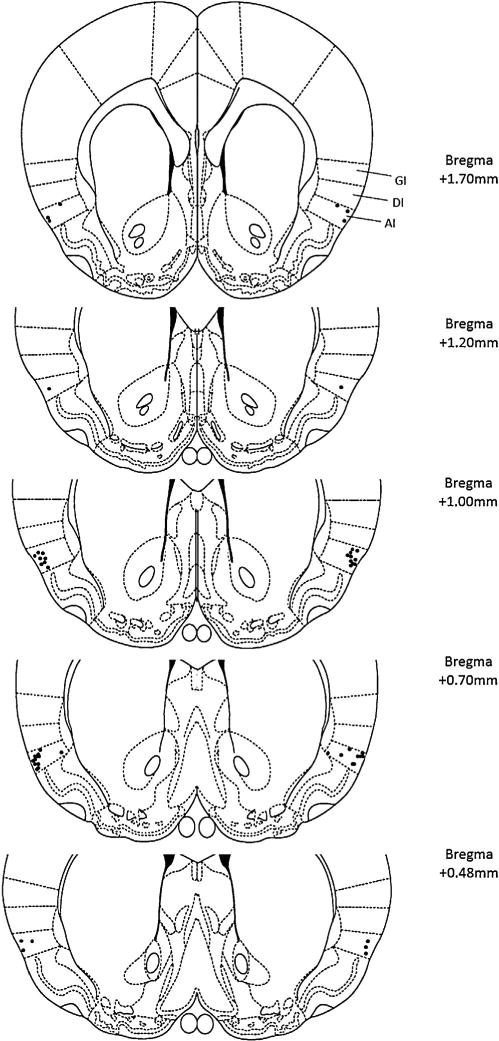
For the acute dose-effect studies, histological reconstruction of the injection sites in the agranular insula (GI: granular insula; DI: dysgranular insula; AI: agranular insula) for Experiments 1–3. Each black dot represents the tip of the cannulae.
Source: Adapted from The Rat Brain in Stereotaxic Coordinates, 4th ed., by Paxinos and Watson, Copyright Elsevier (1997).
Fig. 2.
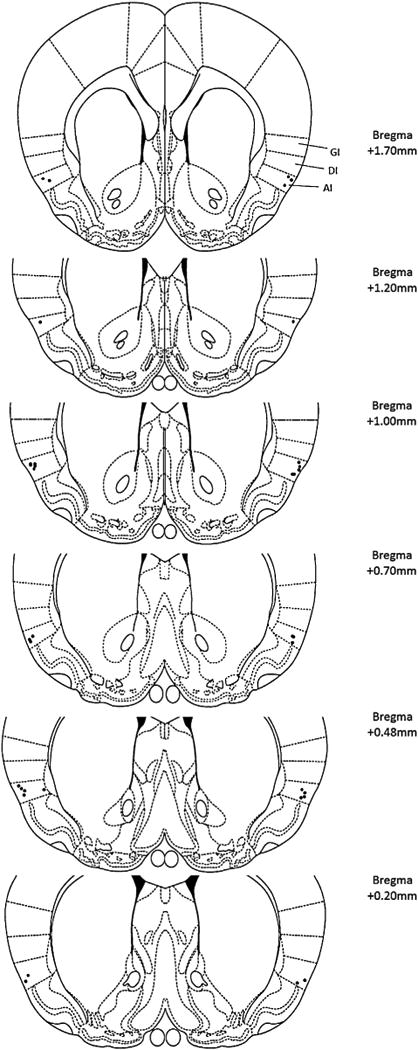
For the repeated infusion study, histological reconstruction of the injection sites in the agranular insula (GI: granular insula, DI: dysgranular insula, AI: agranular insula) for Experiment 4. Each black dot represents the tip of the cannulae.
Source: Adapted from The Rat Brain in Stereotaxic Coordinates, 4th ed., by Paxinos and Watson, Copyright Elsevier (1997).
2.5. Drug preparation and local infusions
Nicotine bitartrate solutions were prepared weekly in sterilized isotonic saline. The dose used for self-administration (0.03 mg/kg/infusion) was calculated as a function of the nicotine free base weight. The pH of the nicotine solution was adjusted to 7.0 using NaOH and the solution was filtered in a Nalgene filter (Nalgene Nunc International, Rochester, NY, USA) for sterilization. Between sessions all nicotine was kept in a dark refrigerator.
Microinfusion drugs, D1 antagonist SCH-23390 and D2 antagonist Haloperidol provided by Sigma (St. Louis, MO, USA), were prepared in 0.5 ml and 1 ml artificial cerebrospinal fluid (aCSF) respectively. Two Hamilton injection syringes were attached to the injection cannulae via plastic tubes. The cannulae were filled with drugs and inserted into the guide cannulae after dummy cannulae were removed. The infusion pump rate was 0.250 μg/min, running for 2 min for SCH-23390 and 0.333 μg/min running for 3 min for the haloperidol. After each drug microinfusion rats were given 5 min for diffusion before the nicotine session started. Finally, the dummy cannulae replaced the injection cannulae. The doses of SCH-23390 and haloperidol were chosen based on the doses used in Di Pietro et al. [19]. D1 and D2 antagonist doses were as follows:
2.5.1. SCH-23390 higher dose-effect function
Using a counterbalanced repeated measures design done twice, rats were given a single self-administration test session on each day preceded by the infusion of the D1 antagonist SCH-23390 into their insular cannulae in doses of 0, 1, 2, and 4 μg/side (N = 13).
2.5.2. SCH-23390 lower dose-effect function
Using a counterbalanced repeated measures design done twice, rats were given a single self-administration test session on each day preceded by the infusion of lower doses of the D1 antagonist SCH-23390:0, 0.125, 0.250, and 0.5 μg/side (N = 7).
2.5.3. Acute haloperidol dose-effect function
Using a counterbalanced repeated measures design done twice, rats were given a single self-administration test session on each day preceded by the infusion of D2 antagonist haloperidol into their insular cannulae in doses of 0.5, 1, and 2 μg/side (N = 8). Joseph et al. [21] injected haloperidol 0.5 μg/side and found that it enhances latent inhibition. The doses of haloperidol used in the current experiment followed that study.
2.5.4. Repeated dosing SCH-23390
We used a between subjects design with each cohort split with half receiving a single self-administration test session on each day after insular infusions of D1 antagonist SCH-23390 (N = 10) at a dose of 2 μg/side and the other half after insular infusions of ACSF vehicle (N = 9). Rats were given total 10 daily infusion sessions of either ACSF or SCH-23390 in a two week duration (5 sessions during the first week and 5 sessions during the following week).
2.6. Data analysis
Analysis of variance was used to assess the statistical significance of the data. The dependent measure was nicotine infusions per session. In the acute dose-effect function studies of SCH-23390 and haloperidol the within subjects factor was drug dose. Planned comparisons were made between, the vehicle control treatment and each dose level. In the repeated dosing study the between subjects factor in the repeated dosing study was 0 vs. 2 mg/kg of SCH-23390. The repeated measure in the repeated dosing study was day of treatment. In all cases the threshold for significance was p < 0.05.
3. Results
3.1. Histological localization
Only those rats with both of the bilateral infusion cannulae within the agranular insular cortex were used for statistical analysis of the effects of local insular infusion of dopamine antagonists on nicotine self-administration.
3.2. SCH-23390 higher dose-effect function
The main effect of the D1 antagonist SCH-23390 was significant (F(3,36) = 3.94, p < 0.025). Paired comparisons of each dose with control showed that doses, 2 (p < 0.025) and 4 μg/side (p < 0.005) caused significant decreases in nicotine self-administration (Fig. 3).
Fig. 3.
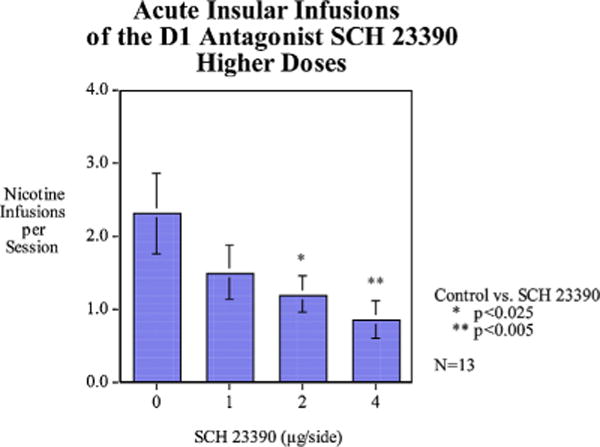
Acute infusions of a higher dose range of SCH-23390 (mean ± sem), N = 13.
3.3. SCH-23390 lower dose-effect function
To determine the threshold for the effect of D1 blockade with SCH-23390 for reducing nicotine self-administration we tested a lower dose-effect function. None of the doses in the lower dose range of 0.125, 0.25 and 0.5 μg/side was effective in lowering nicotine self-administration (Fig. 4).
Fig. 4.
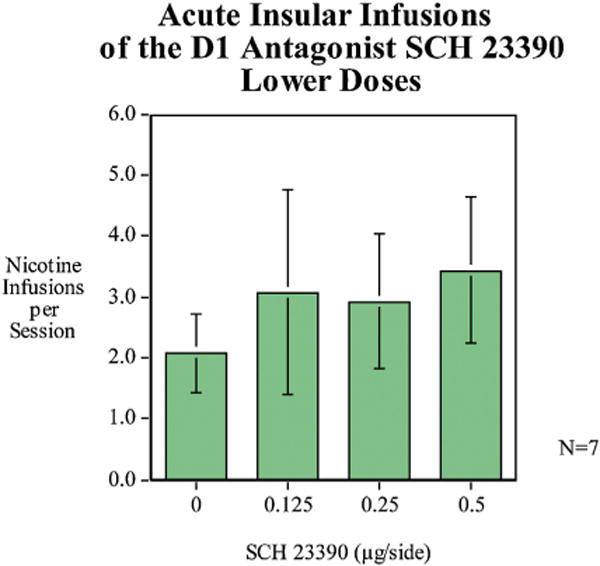
Acute infusions of a lower dose range of SCH-23390 (mean ± sem), N = 7.
3.4. Acute haloperidol dose-effect function
None of the doses of the D2 antagonist haloperidol were effective in significantly changing nicotine self-administration (Fig. 5).
Fig. 5.
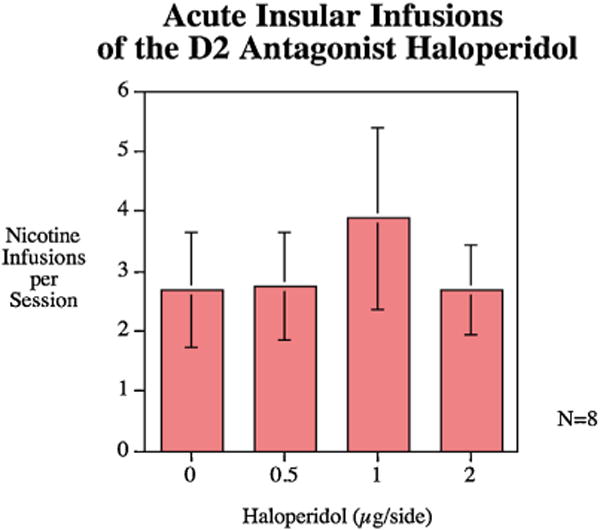
Acute infusions of haloperidol (mean ± sem), N = 8.
3.5. Repeated dosing SCH-23390
The repeated dosing study was conducted for two reasons: to determine if the carryover of SCH-23390 in the repeated measures studies reduced nicotine self-administration in the control condition and to determine whether the efficacy of SCH-23390 decreased or increased with repeated administration. The 2 μg/side SCH-23390 dose or the ACSF vehicle was infused bilaterally into the insular cortex for ten sessions. There was a significant (F(1,17) = 5.78, p <0.05) main effect of SCH-23390. There was no indication of decreased efficacy from the first week to the second (Fig. 6).
Fig. 6.
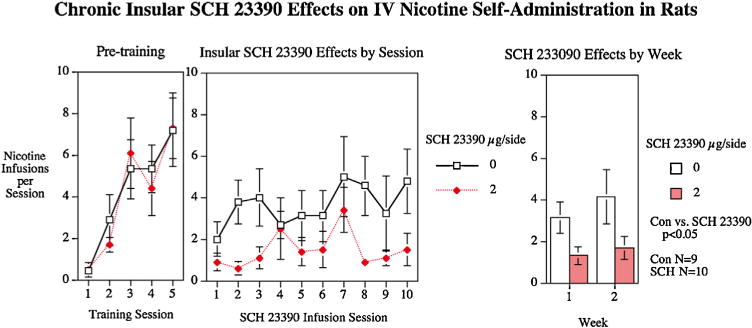
Repeated dosing insular infusions of SCH-23390 during two weeks of nicotine self-administration and one week of resumed access after a week of enforced abstinence. Nicotine infusions (mean ±sem), group sizes control N = 9, SCH-23390 at a dose of 2 μg/side N = 10.
4. Discussion
The insular cortex was found in previous studies to be significantly associated with the loss of desire to smoke in people with stroke damage to this area as compared with damage to other brain regions [10]. Our results showed that while 2 and 4 μg/side doses of the D1 antagonist SCH-23390 decrease nicotine self-administration in rats, neither the 1 μg/side dose nor the lower doses (0.125, 0.250, and 0.5 μg/side) of the drug affected the self-administration behavior. Our results suggest that the threshold for the effect of SCH-23390 infusion into the agranular insular cortex seems to be between 1 and 2 μg/side. Interestingly, microinjections of the D2 antagonist haloperidol also did not affect the level of nicotine infusions per session. Finally, the repeated dosing of SCH-23390 study showed no sign of attenuation of effectiveness over the course of ten sessions. The longer-term efficacy remains to be determined in future studies.
The current studies show that there is a cause-and-effect relationship between decreased activity in the insula and decreased nicotine self-administration. In addition, this research showed that more specifically it was the D1, but not D2 innervation of the agranular insula that was critically involved in promoting nicotine self-administration. D1 and D2 antagonists when given systemically decrease nicotine self-administration. With systemic administration these drugs affect dopamine receptors in all parts of the brain. The current study shows that the insula is a critical site for D1 effects. D2 effects in the insula were not seen, implying that their effects seen after systemic administration has bases elsewhere in the brain. Ikemoto et al. [22] demonstrated that rats self-administer a mixture of the D1 agonist SKF-38393 and the D2 agonist Quinelorane into the NAc shell but not D1 or D2 agonists alone. Furthermore, the self-administration of SKF-38393/Quinelorane mix was eliminated with the coadministration of D1 antagonist SCH-23390 and D2 antagonist sulpiride. Together with our study Ikemoto et al.'s [22] results show that D1 and D2 receptors might have an interactive effect in the NAc but not in the insula.
Overall, our results are in line with other experimental results suggesting that the insula plays an important role in reinforcement learning and reward processing. For example, Kiefer et al. [23] found that rats lacking the gustatory cortex consisting of the agranular and dysgranular regions of the insula had difficulties learning taste aversion to a NaCl–sucrose solution. Interestingly, Balleine and Dickinson [8] found that the gustatory cortex is not necessary for the acquisition of lever press responses to a food reward but the lesions of this region attenuated the effects of outcome devaluation in rats. Moreover, in humans, fMRI studies showed increased insular activity when cigarette smokers are subcutaneously injected nicotine reward [24]; or cocaine-dependent subjects were injected cocaine reward intravenously [25]. Moreover, insular reactivity to smoking cues has been found to predict smoking abstinence [26].
Our results are also in line with Forget et al. [14] results showing decreased nicotine self-administration as a result of GABAergic agonist infusions. It is known that GABA can inhibit dopaminergic activity especially in the mesolimbic areas [27,28]. For example, Reid et al. [28] showed that injections of GABA into the substantia nigra resulted in a decreased levels of DA and simultaneously increased levels of GABA. Therefore, it is not surprising to find that GABA agonists used by Forget et al. [14] and DA antagonist SCH-23390 used in our study produced similar results when they are injected into the insula.
Finally, our results are consistent with Di Pietro et al.'s [19] results demonstrating decreased cocaine self-administration as a result of D1 antagonist SCH-23390 infusions into the insular cortex. They did find that the high dose of 4 μg/side of SCH-23390 in the insula significantly also reduced food motivated responding indicating a less specific effect on motivated behavior. They did not find the lower dose of 2 μg/side of SCH-23390 to impact cocaine self-administration whereas in the current study we did find this dose to significantly lower nicotine self-administration, pointing to greater selectivity of effect of this dose. Interestingly, SCH-23390 has the opposite effect on cocaine self-administration when injected into the NAc. Previously, in a series of studies, Koob et al. demonstrated that SCH-23390 infusions into the NAc increased cocaine self-administration [29]. In contrast, other studies showed that SCH-23390 infusions into the NAc shell attenuate drug-seeking behavior when induced by cocaine injections [30]. However, possible differential effects of the D1 antagonist SCH-23390 injections into the NAc on nicotine self-administration have yet to be studied.
SCH-23390 does have agonist effects at 5HT2c receptors [31]. This action may be relevant to its effects on nicotine self-administration. We have recently found that systemic administration of the selective 5HT2c agonist lorcaserin significantly decreases nicotine self-administration in rats [32].
Recently, insular hypocretin (orexin) activity has been linked to the rewarding properties of nicotine (see [33] for a review). Hypocretin is a neuropeptide produced in the lateral hypothalamus and hypocretin neurons send projections to many brain regions including regions related to reward and addiction such as VTA, Substantia Nigra, and NAc (see [34], 2008 for a review). Hypocretin and dopaminergic activity in these regions are closely related. For example, hypocretin has been show to increase the fire rate of the dopaminergic neurons in the VTA slices [35]. Also, microinfusion of hypocretin into the VTA increased the dopamine levels in the NAc [36]. Interestingly, Hollander et al. [37], found that blockade of the hypocretin receptors in the insula decreases nicotine-self administration in rats. Considering reciprocal connections between the insula, hypothalamic hypocretin neurons, and the VTA, it is possible that insular hypocretin transmission plays a critical role in nicotine addiction. Future studies will shed light on this critical relationship between insular hypocretin and DA activity in the insula and other reward related regions.
In sum, these studies show that D1, but not D2 receptors in the agranular insular cortex play critical roles in promoting nicotine self-administration. D1 antagonist treatment for smoking cessation has not yet been adequately investigated as a novel treatment strategy. In light of the current studies it shows promise and should be further studied.
Highlights.
Acute insular infusion of a D1 antagonist reduced nicotine self-administration.
Chronic insular infusion of a D1 antagonist reduced nicotine self-administration.
Acute insular infusion of a D2 antagonist was not found to reduce nicotine self-administration.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse of the National Institutes of Health (P50 Center grant DA027840).
References
- 1.Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology. 1991;104:171–6. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill MF, Dourish CT, Iversen SD. Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology. 1991;104:343–50. doi: 10.1007/BF02246034. [DOI] [PubMed] [Google Scholar]
- 3.Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature Neuroscience. 2007;10:1020–8. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- 5.Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. European Journal of Pharmacology. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Kleijn J, Folgering JH, van der Hart MC, Rollema H, Cremers TI, Westerink BH. Direct effect of nicotine on mesolimbic dopamine release in rat nucleus accumbens shell. Neuroscience Letters. 2011;493:55–8. doi: 10.1016/j.neulet.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y, Matta SG, Gao W, Sharp BM. Local alpha-bungarotoxin-sensitive nicotinic receptors in the nucleus accumbens modulate nicotine-stimulated dopamine secretion in vivo. Neuroscience. 2000;101:369–75. doi: 10.1016/s0306-4522(00)00371-7. [DOI] [PubMed] [Google Scholar]
- 8.Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. Journal of Neuroscience. 2000;20:8954–64. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. Journal of Neuroscience. 2007;27:4587–97. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez H, Hernandez-Echeagaray E, Ramirez-Amaya V, Bermudez-Rattoni F. Blockade of N-methyl-d-aspartate receptors in the insular cortex disrupts taste aversion and spatial memory formation. Neuroscience. 1999;89:751–8. doi: 10.1016/s0306-4522(98)00360-1. [DOI] [PubMed] [Google Scholar]
- 12.Calder AJ. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- 13.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function. 2010;214:435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biological Psychiatry. 2010;68:265–71. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. Journal of Comparative Neurology. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- 16.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurd YL. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. Chemical Neuroanatomy. 2001;22:127–37. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner U. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30:692–9. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacology. 2008;200:81–91. doi: 10.1007/s00213-008-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sixth. New York: Academic Press; 2007. [Google Scholar]
- 21.Joseph MH, Peters SL, Moran PM, Grigoryan GA, Young AM, Gray JA. Modulation of latent inhibition in the rat by altered dopamine transmission in the nucleus accumbens at the time of conditioning. Neuroscience. 2000;101:921–30. doi: 10.1016/s0306-4522(00)00437-1. [DOI] [PubMed] [Google Scholar]
- 22.Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. Journal of Neuroscience. 1997;17:8580–7. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiefer SW, Orr MR, Missy R. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behavioral Neuroscience. 1992;106:140–6. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- 24.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. American Journal of Psychiatry. 1998;155:1009–15. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 25.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 26.Janes AC, Pizzagalli DA, Richardt S, Frederick B, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010;67:722–9. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuxe K, Hokfelt T, Agnati L, Johansson O, Perez de la Mora M. Evidence for an inhibitory GABAergic control of the mesolimbic dopamine neurons: possibility of improving the treatment of schizophrenia by combined treatment with neuroleptics and GABAergic drugs. Medical Biology. 1975;53:175–83. [PubMed] [Google Scholar]
- 28.Reid MS, O'Connor WT, Herrera-Marschitz M, Ngerstedt U. The effects of intranigral GABA and dynorphin A injections on striatal dopamine and GABA release: evidence that dopamine provides inhibitory regulation of striatal GABA neurons via D2 receptors. Brain Research. 1990;519:255–60. doi: 10.1016/0006-8993(90)90086-q. [DOI] [PubMed] [Google Scholar]
- 29.Koob GF, Le HT, Creese I. The D-1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neuroscience Letters. 1987;79:315–20. doi: 10.1016/0304-3940(87)90451-4. [DOI] [PubMed] [Google Scholar]
- 30.Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology. 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- 31.Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The selective dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology. 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- 32.Levin ED, Johnson J, Slade S, Wells C, Cauley M, Petro A, et al. Lorcaserin decreases nicotine self-administration in female rats. Journal of Pharmacology and Experimental Therapeutics. 2011;338:890–6. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenny PJ. Tobacco dependence, the insular cortex and the hypocretin connection. Pharmacology, Biochemistry and Behavior. 2011;97:700–7. doi: 10.1016/j.pbb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman A. Neurobiology of addiction: an integrative review. Biochemical Pharmacology. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orex-ins/hypocretins. Journal of Neuroscience. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. Journal of Neuroscience. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:480–5. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]


