Abstract
A truly 4-component reaction! In analogy to a galaxy consisting of millions of stars a multicomponent reaction scaffold can result in millions of compound variations. The MCR of α-amino acids, oxocomponents, isocyanides and primary or secondary amines is such a high-number high-diversity reaction providing an enormous potential for drug discovery or catalyst screening.
Keywords: multicomponent reaction, iminodicarboxamide scaffold, 4 component reaction, Ugi, isocyanide
To Ivar Ugi, the father of modern multicomponent reaction chemistry
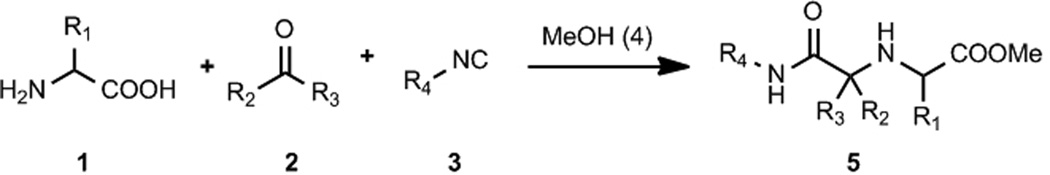
Multicomponent reaction technology (MCR) is now widely recognized for its impact on drug discovery projects and is strongly endorsed by industry in addition to academia.[1] Thus an increasing number of products based on MCRs are marketed or in development. Recent examples include boceprevir,[2] retosiban[3] or mandipropamide,[4] just to name a few. While the number of described MCRs is enormous, only a small number has a wide breath in all classes of educts and allows for the quasi infinite variation of all starting materials.[5] The size of the chemical space of the different MCR scaffolds, however, has major implications on the usefulness of the particular MCR. For example, the classical Ugi four component reactions allows for the simultaneous variation of four very common starting materials (amine, oxo-component, carboxylic acid and isocyanide, which is derived from a primary amine).[6] Thus the number of synthesizable products is very large.[7] On the other hand, the three component reaction of sulfur, carbon monoxide and epoxides yielding oxathiolan-2-ones, although synthetically very useful can yield only a rather limited number of products.[8] Different strategies for the design of molecular complexity using MCR chemistry have been devised.[9] We would like to report here on a novel stereoselective Ugi-type reaction of the four highly variable starting materials α-amino acid, oxo-component, isocyanide and primary or secondary amine, thus comprising a novel true 4-CR.
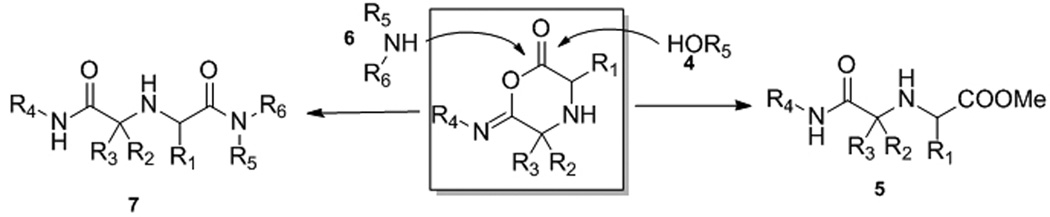
15 Years ago Ugi et al. described the first time the discovery of a novel variant of his reaction in this journal (Scheme 1).[10] The reaction was termed U-5C-4CR (5-center-4-component) because of the use of bireactive α-amino acids, oxo-components, isocyanides and alcohol as a solvent and reactant. The reaction proved to be versatile and several reports document their usefulness.[11] Remarkably, this reaction also lead to the first orally available potent, selective and non peptide oxytocin antagonist, currently undergoing clinical trials for preterm birth.[3, 12]
Scheme 1.
The Ugi-5-C-4CR.
This reaction, however only comprises a MCR where three components show a great variability: the α-amino acid 1, the oxo-component 2 and the isocyanide 3. The variability of the alcohol component 4, which is also the solvent, is rather restricted towards low molecular weight liquids, e.g. MeOH and EtOH. The restriction is a result of the poor solubility of the amino acids in higher alcohols and their reduced nucleophilic reactivity.
The key reaction intermediate is the six-membered α-adduct of the α-amino acid, the oxo-component and the isocyanide which is reacting to the linear product 5 by the nucleophilic attack of the solvent methanol 4 (Scheme 2). We envisioned that an N-nucleophile, a primary or secondary amine 6, could potentially also work as a nucleophile and successfully compete with the alcohol solvent to attack the 6-membered α-adduct leading to the iminodicarboxamide derivative 7. The outcome of the projected reaction however was a priori unclear since the amino acid amine and the external primary or secondary amine could compete for the oxo-component and result into different types of Ugi or Passerini products (e.g. via U-5C-4CR, U-4CR, U-3CR or P-3CR) or mixtures thereof. We hypothesized however, that an intramolecular reaction involving the Schiffbase of the bifunctional α-amino acid as opposed to an intermolecular reaction of the Schiffbase or enamine formed by the additional primary or secondary amine should be more favorable and would preferentially form the cyclic α-adduct leading to the new scaffold vs. the acyclic α-adduct leading to the intermolecular classic Ugi reaction respectively. Additionally a reaction funneling into the projected reaction pathway would be of high value since this would comprise one of the very few four component reactions which are truly variable in all components.
Scheme 2.
Proposed key intermediate and two competing reaction pathways
To test our MCR hypothesis we first reacted leucine (1{7}), 2-fluorobenzaldehyde (2{16}), benzyl isocyanide (3{1}), and morpholine (6{14}) and surprisingly found the expected compound (7{7,16,1,14}) as the major product. Extensive optimization was performed including the parameter solvent, temperature, w/o microwave, reaction time and catalyst and their influence on different combinations of starting materials. In previous Ugi type reactions where intramolecular cyclization of the α-adduct occurs trifluoroethanol (TFE) or similar solvents were advantageously used due to their reduced nucleophilicity.[13] Unexpectedly when TFE was attempted in this reaction it was found to produce a significant amount of trifluoroethylester 5’ as side product. An optimal solvent mixture was found to be MeOH/water 4/1, which is a compromise in solubility for the different classes of starting materials. Due to the poor solubility of most amino acids we attempted to speed up the reaction via microwave conditions, from 60–120 °C for anywhere from 30min to 2 hours. While under these conditions the amino acid completely solubilized in the reaction mixture, however no increase in yield was found. Also due to the formation of unwanted side products, and therefore an increased difficulty in separation the benefit from the time gained by using microwave conditions was outweighed by the amount of extra time spent optimizing separation conditions; instead room temperature for 3 days was used.
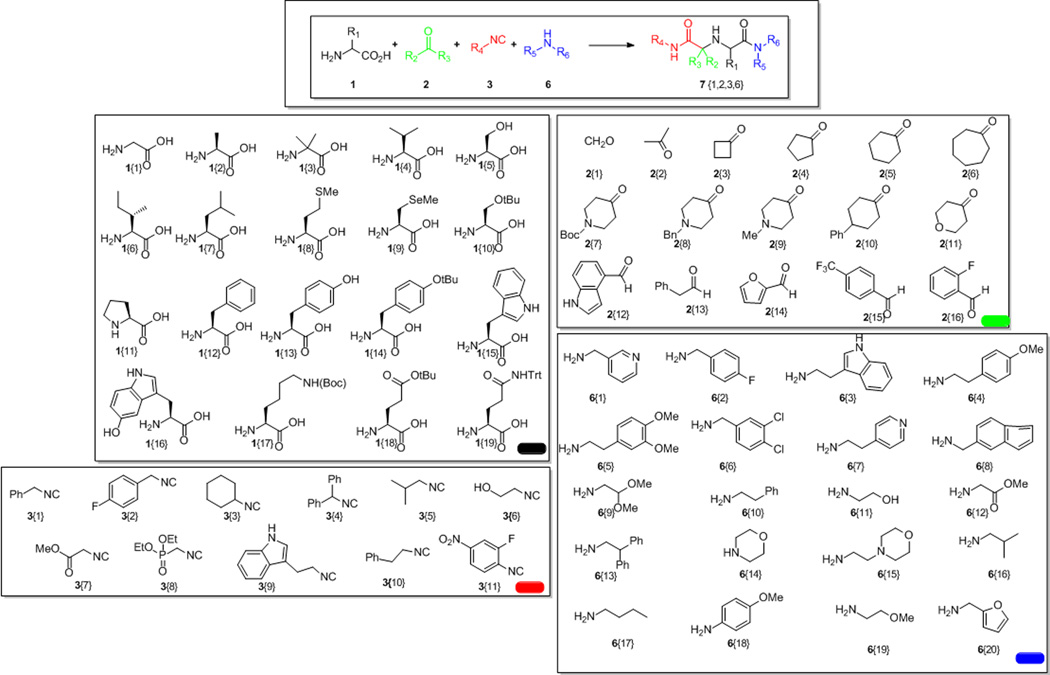
Next we investigated the scope of the reaction by using representative starting materials of each class and synthesizing a library of iminobisamides (Figure 1). Not surprisingly it was found that the identity of the starting materials and their specific combinations played a role for the overall yields and selectivity of the reaction. We successfully used virtually all natural α-amino acids and some non natural ones (1), including hindered 1{3} or oxidation labile 1{9} and 1{16}. It was found that while use of Tyr (1{13}) gave the desired product, (7{13,7,9,17}); using tert-butyl protected Tyr (1{14}) gave product (7{14,7,9,17} in much better yields (25% vs 47% respectively). Similar results were seen between Ser (1{5}) and tert-butyl protected Ser (1{10}) for products 7{5,5,4,3} and 7{10,5,4,3} (23% and 40% respectively). Therefore amino acids with reactive side chains (e.g. Ser, Glu, Asp, Lys) were used in their side chain protected form. As oxo-components (2) we often used symmetrical ketones for the sake of formation of only one stereoisomer, however either aldehydes or ketones worked equally well in this reaction. Cyclic ketones, cyclobutanone (2{3}), cyclopentanone (2{4}), and cyclohexanone (2{5}) worked well in the reaction, while cycloheptanone (2{6}) and ketones of higher ring size worked with sub-optimal yields, e.g. (7{14,6,5,9}, 23%). Different isocyanides were used including aromatic, heteroaromatic, aliphatic and bulky ones (3) and worked generally well. Primary and secondary amines were used as the forth component (6), and generally worked well including functionalized, heterocyclic, aromatic and heteroaromatic ones. As part of the NIH roadmap initiative, a project aiming to address roadblocks to research and to transform the way biomedical research is conducted by providing a large and diverse publicly available compound library for the discovery of molecular probes, we synthesized up to now more than 400 compounds based on this reaction.[14] Yields of representative compounds are summarized in Table 1.
Fig 1.
The new 4CR and the investigated starting material classes
Table 1.
Products of the 4CR {amino acid, ketone or aldehyde, isocyanide, 1° or 2° amine} and their yields.
| Compound | Yield [a] | Compound | Yield [a] |
|---|---|---|---|
| 7{2,11,9,6} | 54 | 7{12,13,5,16} | 40 |
| 7{9,5,9,10} | 50 | 7{7,15,9,19} | 34 |
| 7{14,7,9,17} | 47 | 7{10,5,4,3} | 40 |
| 7{15,6,1,16} | 43 | 7{19,4,2,20} | 41 |
| 7{1,10,4,17} | 42 | 7{12,4,3,13} | 38 |
| 7{6,3,1,3} | 54 | 7{15,4,3,13} | 37 |
| 7{11,2,4,19} | 40 | 7{12,5,9,12} | 63 |
| 7{15,9,5,10} | 51 | 7{17,5,9,18} | 62 |
| 7{15,2,8,6} | 52 | 7{12,5,9,11} | 30 |
| 7{14,5,7,16} | 47 | 7{7,5,3,13} | 48 |
| 7{10,7,3,7} | 41 | 7{7,5,3,3} | 46 |
| 7{11,2,2,6} | 45 | 7{14,14,5,2} | 31 |
| 7{18,5,1,8} | 41 | 7{2,16,2,14} | 32 |
| 7{6,4,6,5} | 33 | 7{2,16,2,15} | 28 |
| 7{4,5,9,2} | 54 | 7{15,2,10,15} | 38 |
| 7{7,8,4,9} | 44 | 7{3,12,5,9} | 30 |
| 7{6,9,2,1} | 51 | 7{1,12,10,4} | 41 |
| 7{4,8,1,4} | 53 | 7{8,8,5,3} | 40 |
| 7{15,5,4,9} | 47 | 7{16,5,4,15} | 34 |
isolated yields after preparative SFC purification (SI)
All reactions were performed on a 0.5 mmol scale and the products were purified to their enantiomers by efficient and fast supercritical fluid carbon dioxide (SFC) technology. The yields in most cases are satisfactory taking into account the complexity of the reaction, between 40 and 60% after chromatographic separation.
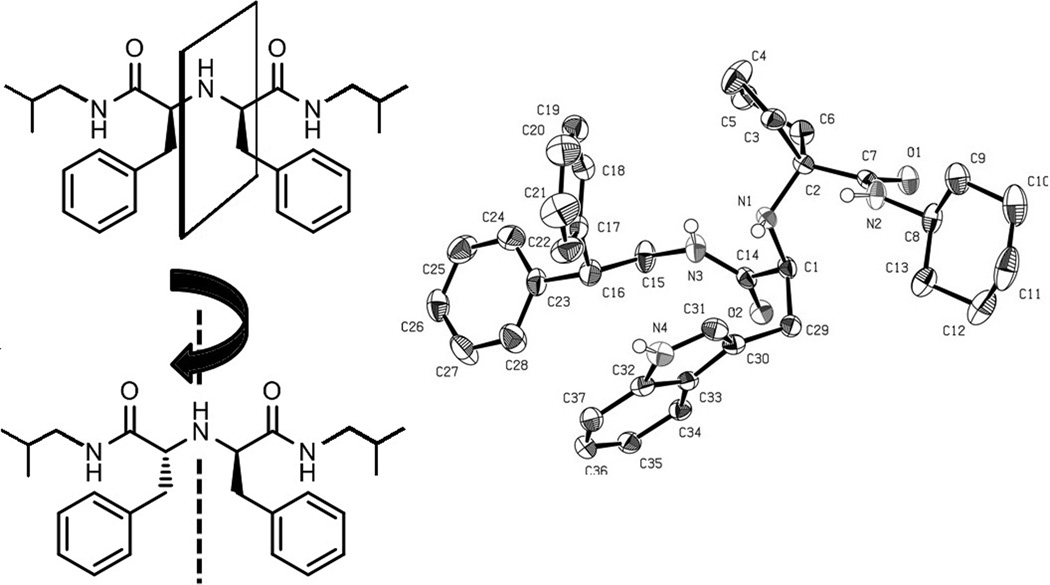
Next we asked the question of the stereochemical integrity of the formed products. Thus all reactions performed were investigated by chiral supercritical fluid chromatography (SFC, SI). We investigated the influence of the amine component on the integrity of the amino acid stereocenter. We found that primary amines lead to retention of the stereochemical integrity of the amino acid stereocenter, whereas secondary amines are leading to partial racemization. This can be rationalized by the typical 1–2 units higher pKB of secondary amines. For example use of aldehyde 2{17} with L-Ala 1{2}, benzyl isocyanide 3{1} and morpholine 6{14}, for examples lead to the formation of 4 stereoisomers which however have been conveniently separated using chiral SFC; when 2-morpholinoethylamine 6{15} was used only 2 stereoisomers were found. Racemic D,L-Ala in these reactions was used as a control (SI Fig 1–4). As a further proof of the structures we solved several compounds in molecular detail using single crystal x-ray analysis; 7{15,4,3,13} is shown in Figure 2. An example of a noteworthy combination is the use of phenylacetaldehyde 2{13}, Phe 1{12}, iso-butyl isocyanide 3{5} and iso-butyl amine 6{16} which is leading to C2 and Cs symmetrical products in a ratio of 6:1 (Fig. 1). Such, otherwise difficult to access, C2 symmetrical compounds could potentially serve as chiral tridental ligands for catalytic organic transformations.
Fig 2.
Left: Example of Cs and C2 symmetrical products conveniently formed by the new 4CR. Right: ORTEP style plot of compound 7{15,4,3,13} in the solid state.
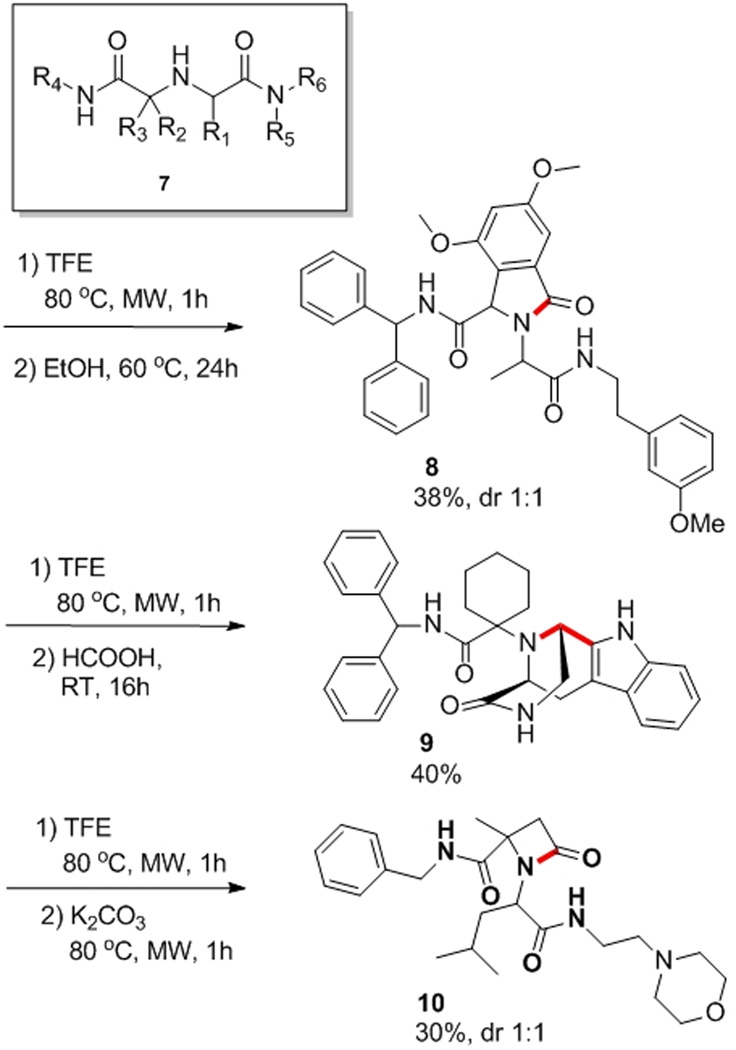
In another attempt to explore the potential of this 4-component reaction we used bifunctional starting materials to cyclize the initially formed Ugi products. Thus we performed cyclization based on the Pictet-Spengler reaction, and deprotective cyclization. Representative examples (8 – 10) are shown in Scheme 3. Remarkably those cyclization can be performed without purification of the initial Ugi product.
Scheme 3.
Example of products of backbone cyclization conveniently formed from suitable intermediates of the new 4CR. The newly formed bonds of the cyclization are indicated in red.
Iminodicarboxamides are a particular useful class of scaffold and have been reported annotated with divers biological activities, including factor Xa,[15] HIV-1 protease,[16] renin[17], thrombin,[18] and most recently p53/mdm2 inhibition.[19] Our herein reported one-pot synthesis towards this scaffold comprises a major advancement and is superior to reported stepwise sequential or MCR approaches, in terms of yield, time, effort, stereochemistry and breath of useful starting materials.[20] The scope of the extended Ugi 4-CR is good in all investigated starting materials and a very large number of products are theoretically accessible. This is significant since recent trends in drug discovery using MCR chemistry more and more leverage the usage of virtual screening tools.[19, 21] Towards this end we introduced a freely accessible virtual compound library of ~5 million compounds based on this backbone in the recently published ANCHOR.QUERY software for the discovery of protein-protein interaction antagonists.[19] In conclusion we described a novel and rare case of a 4CR where all four starting material classes can be widely combined which each other.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants 1R21GM087617-01, 1P41GM094055-01 and 1R01GM097082-01.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Dömling A, Wang W, Wang K. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatraman S, Bogen SL, Arasappan A, Bennett F, Chen K, Jao E, Liu Y-T, Lovey R, Hendrata S, Huang Y, Pan W, Parekh T, Pinto P, Popov V, Pike R, Ruan S, Santhanam B, Vibulbhan B, Wu W, Yang W, Kong J, Liang X, Wong J, Liu R, Butkiewicz N, Chase R, Hart A, Agrawal S, Ingravallo P, Pichardo J, Kong R, Baroudy B, Malcolm B, Guo Z, Prongay A, Madison V, Broske L, Cui X, Cheng K-C, Hsieh Y, Brisson J-M, Prelusky D, Korfmacher W, White R, Bogdanowich-Knipp S, Pavlovsky A, Bradley P, Saksena AK, Ganguly A, Piwinski J, Girijavallabhan V, Njoroge FG. J. Med. Chem. 2006;49:6074–6086. doi: 10.1021/jm060325b. [DOI] [PubMed] [Google Scholar]
- 3.Liddle J, Allen MJ, Borthwick AD, Brooks DP, Davies DE, Edwards RM, Exall AM, Hamlett C, Irving WR, Mason AM, McCafferty GP, Nerozzi F, Peace S, Philp J, Pollard D, Pullen MA, Shabbir SS, Sollis SL, Westfall TD, Woollard PM, Wu C, Hickey DMB. Bioorg. Med. Chem. Lett. 2008;18:90–94. doi: 10.1016/j.bmcl.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Lamberth C, Jeanguenat A, Cederbaum F, De Mesmaeker A, Zeller M, Kempf H-J, Zeun R. Bioorg. Med. Chem. Lett. 2008;16:1531–1545. doi: 10.1016/j.bmc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 5.a) Passerini M, Simone L. Gazz. Chim. Ital. 1921;51 [Google Scholar]; b) Van Leusen AM, Wildeman J, Oldenziel OH. J. Org. Chem. 1977;42:1153–1159. [Google Scholar]; c) Szardenings AK, Burkoth TS, Lu HH, Tien DW, Campbell DA. Tetrahedron. 1997;53:6573–6593. [Google Scholar]; d) Bon RS, van Vliet B, Sprenkels NE, Schmitz RF, de Kanter FJJ, Stevens CV, Swart M, Bickelhaupt FM, Groen MB, Orru RVA. J. Org. Chem. 2005;70:3542–3553. doi: 10.1021/jo050132g. [DOI] [PubMed] [Google Scholar]
- 6.Ugi I. Angew. Chem. 1959;71:386. [Google Scholar]
- 7.Dömling A, Ugi I. Angew. Chem., Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama Y, Katahira C, Sonoda N. Tetrahedron Lett. 2004;45:8539–8540. [Google Scholar]
- 9.a) Weber L, Illgen K, Almstetter M. Synlett. 1999;1999:366–374. [Google Scholar]; b) Dömling A. Curr. Opin. Chem. Biol. 2000;4:318–323. doi: 10.1016/s1367-5931(00)00095-8. [DOI] [PubMed] [Google Scholar]; c) Ruijter E, Scheffelaar R, Orru RVA. Angew. Chem., Int. Ed. 2011;50:6234–6246. doi: 10.1002/anie.201006515. [DOI] [PubMed] [Google Scholar]
- 10.Demharter A, Hörl W, Herdtweck E, Ugi I. Angew. Chem., Int. Ed. 1996;35:173–175. [Google Scholar]
- 11.a) Ugi I, Demharter A, Hörl W, Schmid T. Tetrahedron. 1996;52:11657–11664. [Google Scholar]; b) Park SJ, Keum G, Kang SB, Koh HY, Kim Y, Lee DH. Tetrahedron Lett. 1998;39:7109–7112. [Google Scholar]; c) Kim YB, Choi EH, Keum G, Kang SB, Lee DH, Koh HY, Kim Y. Org. Lett. 2001;3:4149–4152. doi: 10.1021/ol016716w. [DOI] [PubMed] [Google Scholar]; d) Sung F-L, Chen M-J, Chung K. Mol. Diversity. 2003;6:213–221. doi: 10.1023/b:modi.0000006783.21086.0d. [DOI] [PubMed] [Google Scholar]; e) Godet T, Bonvin Y, Vincent G, Merle D, Thozet A, Ciufolini MA. Org. Lett. 2004;6:3281–3284. doi: 10.1021/ol048850x. [DOI] [PubMed] [Google Scholar]; f) Sollis SL. J. Org. Chem. 2005;70:4735–4740. doi: 10.1021/jo0501137. [DOI] [PubMed] [Google Scholar]; g) Ku IW, Cho S, Doddareddy MR, Jang MS, Keum G, Lee J-H, Chung BY, Kim Y, Rhim H, Kang SB. Bioorg. Med. Chem. Lett. 2006;16:5244–5248. doi: 10.1016/j.bmcl.2006.05.031. [DOI] [PubMed] [Google Scholar]; h) Kadzimirsz D, Hildebrandt D, Merz K, Dyker G. Chem. Commun. 2006:661–662. doi: 10.1039/b516017k. [DOI] [PubMed] [Google Scholar]
- 12.Borthwick AD, Liddle J, Davies DE, Exall AM, Hamlett C, Hickey DM, Mason AM, Smith IED, Nerozzi F, Peace S, Pollard D, Sollis SL, Allen MJ, Woollard PM, Pullen MA, Westfall TD, Stanislaus DJ. J. Med. Chem. 2012;55:783–796. doi: 10.1021/jm201287w. [DOI] [PubMed] [Google Scholar]
- 13.a) Beck B, Srivastava S, Khoury K, Herdtweck E, Domling A. Mol. Diversity. 2010;14:479–491. doi: 10.1007/s11030-010-9249-2. [DOI] [PubMed] [Google Scholar]; b) Isaacson J, Kobayashi Y. Angew. Chem., Int. Ed. 2009;48:1845–1848. doi: 10.1002/anie.200805709. [DOI] [PubMed] [Google Scholar]
- 14.NIH. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): National Center for Biotechnology Information (US); 2010. [ http://www.ncbi.nlm.nih.gov/books/NBK47352/] [PubMed] [Google Scholar]
- 15.Zbinden KG, Anselm L, Banner DW, Benz J, Blasco F, Decoret G, Himber J, Kuhn B, Panday N, Ricklin F, Risch P, Schlatter D, Stahl M, Thomi S, Unger R, Haap W. Eur. J. Med. Chem. 2009;44:2787–2795. doi: 10.1016/j.ejmech.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Gehlhaar DK, Verkhivker GM, Rejto PA, Sherman CJ, Fogel DB, Fogel LJ, Freer ST. Chem. Biol. 1995;2:317–324. doi: 10.1016/1074-5521(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 17.Jensen C, Herold P, Brunner HR. Nat. Rev. Drug Discovery. 2008;7:399–410. doi: 10.1038/nrd2550. [DOI] [PubMed] [Google Scholar]
- 18.Matthews JH, Krishnan R, Costanzo MJ, Maryanoff BE, Tulinsky A. Biophys. J. 1996;71:2830–2839. doi: 10.1016/S0006-3495(96)79479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koes D, Khoury K, Huang Y, Wang W, Bista M, Popowicz GM, Wolf S, Holak TA, Dömling A, Camacho CJ. PLoS One. 2012;7:e32839. doi: 10.1371/journal.pone.0032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) Moderhack D. Justus Liebigs Ann. Chem. 1973;1973:359–364. [Google Scholar]; b) Smith HK, Beckett RP, Clements JM, Doel S, East SP, Launchbury SB, Pratt LM, Spavold ZM, Thomas W, Todd RS, Whittaker M. Bioorg. Med. Chem. Lett. 2002;12:3595–3599. doi: 10.1016/s0960-894x(02)00790-4. [DOI] [PubMed] [Google Scholar]; c) Chang J-y, Shin E-k, Kim HJ, Kim Y, Park YS. Bull Korean Chem Soc. 2005;26:989–992. [Google Scholar]
- 21.a) Weber L, Wallbaum S, Broger C, Gubernator K. Angew. Chem., Int. Ed. 1995;34:2280–2282. [Google Scholar]; b) Shoda M, Harada T, Kogami Y, Tsujita R, Akashi H, Kouji H, Stahura FL, Xue L, Bajorath J. J. Med. Chem. 2004;47:4286–4290. doi: 10.1021/jm040103i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.