Abstract
While the literature on prostate cancer health-related quality of life has grown extensively, little is known about symptom management strategies used by men to manage treatment-related side effects and the effectiveness of those strategies. We collected 628 symptom management reports from 98 men treated for localized prostate cancer. Participants were recruited from email lists and a prostate cancer clinic in Northern California. Data were collected using the Critical Incident Technique. Symptom management reports were assigned to categories of urinary, sexual, bowel, mental health, systemic, or “other.” We calculated descriptive statistics by symptom type and management strategy effectiveness. The most common symptoms were urinary (26 %) and sexual (23 %). Participants’ symptom management strategies varied widely, from medical and surgical interventions (20 %) to behavioral strategies (11 %) to diet and lifestyle interventions (12 %). The effectiveness of symptom management strategies varied, with sexual symptoms being managed effectively only 47 % of the time to mental health symptom management strategies considered effective 89 % of the time. Doing nothing was a commonly reported (15 %) response to symptoms and was effective only 14 % of the time. Men report the least effectiveness in symptom management for sexual dysfunction after prostate cancer treatment. Including men’s experience with managing treatment side effects may be an important way to improve survivorship programs and make them more acceptable to men. More work is needed to find out why men frequently do nothing in response to symptoms when effective solutions exist and how providers can successfully engage such men.
Keywords: Prostate cancer, Symptom management, Quality of life, Survivorship
Introduction
Since Litwin and colleagues introduced the University of California-Los Angeles Prostate Cancer Index (UCLA PCI) in 1995 [1], researchers have developed a large and growing literature concerning the impact of the diagnosis and treatment of prostate cancer on patients’ lives. Both small cross-sectional studies and large ongoing observational studies like the Prostate Cancer Outcomes Study [2] and the Cancer of the Prostate Strategic Research Endeavor [3] have enriched our understanding of how treatments can affect disease-specific health-related quality of life (HRQOL). Another work has demonstrated the effect of treatment-related symptoms on general HRQOL in both patients and their spouses [4].
Empirical evidence has demonstrated the equivalence of various treatments for most localized prostate cancers (PCa) [5]. Similar efficacy leaves the patient to choose his preferred therapy based on recommendations from his provider or the side-effect profiles for each treatment and his personal preferences. Symptoms vary by treatment type. In addition, symptom profiles evolve over time for many men [1, 6]. Patients treated with surgery frequently report substantial improvements in their urinary and sexual functioning 12 months after treatment, while those managed with radiotherapy experience generally stable urinary and declining sexual function [7, 8].
Prostate cancer survivors report symptom management to be an area of unmet need [9]. Symptom management was singled out as the area in greatest need of improvement for prostate cancer education materials [10]. Unfortunately, few studies of prostate cancer patients’ symptom management practices exist. Although some intervention studies have included physical symptom management as one component [11], most interventions have been developed to manage psychosocial concerns [12].
Therefore, we undertook a qualitative study to understand how men manage symptoms related to prostate cancer treatment. The qualitative technique used in data collection and analysis, the Critical Incident Technique, has been used in other disease areas to develop patient education interventions where such interventions have not previously existed [13, 14]. Results from this study have been used to develop a new telehealth symptom management educational intervention that is tailored to fit a man’s symptom profile, which is being evaluated in an ongoing randomized controlled trial. This report presents results from our qualitative study.
Methods
The Prostate Cancer Patient Education Project (PCPEP) is an ongoing program of research to develop effective, patient-centered, symptom management education materials for men treated for PCa. Telehealth interventions developed through this program are created to be accessible to low-health-literacy men, rural men, and others with limited access to large medical centers.
Participants
We identified prostate cancer patients through prostate cancer support groups in the San Francisco Bay Area and via email lists maintained by the University of California, San Francisco, prostate cancer patient advocates. Data were collected between May 2004 and August 2005. Potential participants were screened to determine study inclusion criteria: (1) diagnosed with clinically localized PCa within 2 years of the screening date and (2) received some form of active treatment for their cancer, including hormone therapy. Men who reported being on active surveillance for their PCa were excluded. The institutional review board at the University of California, San Francisco approved the data collection protocol and other study methods.
Data Collection
Telephone interviews were conducted by one of the authors (DML) and were guided by the Critical Incident Technique [15], a structured, qualitative data collection and analysis technique used to develop new symptom management education programs in other disease areas [13, 14]. All data collected were obtained from the participating survivors. Each participant was asked about what led to his prostate cancer diagnosis, what treatments he completed, and what symptoms resulted. For each symptom reported, a participant was asked what he did to manage that symptom, what influenced him to take that action, and whether the symptom management actions were helpful or not and why. Each interview lasted 30–60 min, depending on the number of symptoms and symptom management strategies reported by each man.
Information collected during the interview was abstracted onto a standardized coding sheet and entered in a Microsoft Access database. As the incident reports were entered into Access, they were edited using guidelines provided by Anderson and Wilson [16]. Editing ensured that each incident was reported in a standard format, with sufficient level of detail provided for analysis and with corrections to spelling, grammar, and punctuation. Redundant incident reports were grouped together. Men’s exact words were recorded as closely as possible and entered in each incident report. Records in the database were checked for errors against the interviewer notes, and corrections were made as needed.
Data Analysis
Each symptom management report was coded by two trained, independent coders, with discrepancies resolved by one of the investigators (DML). Each report was assigned to one of six a priori symptom types: urinary, sexual, bowel, mental health, systemic, or “other.” Examples for each category include nocturia, leakage, and urgency for urinary symptoms; erectile dysfunction, low desire, and climacturia for sexual symptoms; urgency, leakage, and pain for bowel symptoms; anxiety, depression, and changes in sense of self for mental health symptoms; hot flashes for systemic symptoms; and fatigue, joint pain, and weight change under the “other” category. We computed descriptive statistics for each category overall and then examined relationships between symptom reports and participant demographic and clinical variables.
Results
Participant Characteristics
Demographic data for the 98 participants are shown in Table 1. The participants were primarily white. All participants were originally diagnosed with localized disease. Eighty-nine participants (90.8 %) had localized disease at the time of the interview, while nine (9.2 %) were originally diagnosed with localized disease that had metastasized. Data collection focused on symptoms experienced before the participant developed metastatic disease.
Table 1.
Patient characteristics (N=98)
| Mean | Range | |
|---|---|---|
| Age at diagnosis (years) | 61.5 | 47–79 |
| Gleason Score at diagnosis | 6.6 | 5–9 |
| PSA at diagnosis | 10.2 | 3.5–40 |
| Total no. of treatments received | 1.7 | 1–4 |
| Total no. of incidents reported | 6.4 | 1–22 |
| N | % | |
| Ethnicity | ||
| White | 89 | 90.8 |
| Nonwhite | 9 | 9.2 |
| Disease extent | ||
| Local | 89 | 90.8 |
| Advanced | 9 | 9.2 |
| Time since treatment | ||
| Ongoing | 13 | 13.5 |
| <6 months | 13 | 13.5 |
| 6–12 months | 13 | 13.5 |
| >12 months | 57 | 59.5 |
| Type of treatment | ||
| Multiple treatments | 47 | 48.0 |
| Hormone + external beam RT | 18 | 38.3 |
| Radical prostatectomy + EBRT + hormone | 9 | 19.1 |
| Radical prostatectomy + EBRT | 2 | 4.3 |
| Radical prostatectomy + other therapy | 1 | 2.1 |
| Other multiple treatment | 17 | 36.2 |
| Radical prostatectomy | 25 | 25.5 |
| External beam radiation therapy | 11 | 11.2 |
| Brachytherapy | 7 | 7.1 |
| Hormonal therapy | 5 | 5.1 |
| Other monotherapy | 3 | 3.1 |
Symptom Management Reports
After treatment, participants reported a mean of 6.4 symptom incidents (range 1–22). A total of 628 symptoms were categorized according to the above scheme. The most common symptoms were urinary (26 %) and sexual (23 %). Systemic symptoms made up 19 % of the total, mental health complaints represented 10 %, and bowel problems comprised 9 %. Other symptoms made up the remaining 14 %. Participants reported that not all interventions were equally effective (see Fig. 1).
Fig. 1.
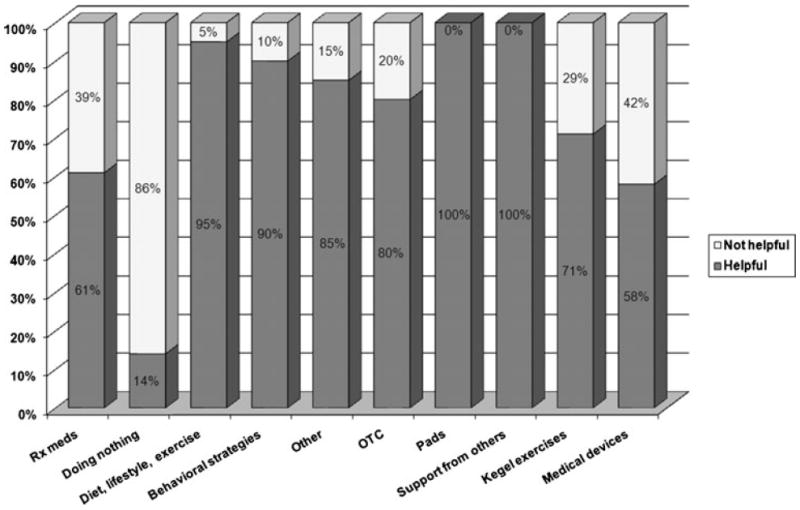
Symptom management strategies by reported effectiveness
The most common method of treating symptoms was the use of prescription medications (20 %). For 15 % of the symptoms, the respondents reported doing nothing. Diet, lifestyle modifications, and exercise were used in 12 % of cases. Behavioral strategies were used in 11 % of instances. Over-the-counter medications were used to intervene in 10 % of cases. Pads were used in 5 % of overall symptom reports, psychosocial support from others was sufficient in 4 % of cases, and Kegel exercises were used in 3 % of cases. Other interventions were used to manage 10 % of reported symptoms.
Urinary Symptoms
The breakdown of frequency of intervention for each system-specific symptom type is presented in Table 2. For urinary symptoms, reported by 73.5 % of the sample, patients most frequently used alpha blockers and other prescription medications and pads. Many respondents reported trying a number of different types of pads before settling on one type that kept them dry and could be worn without discomfort. Kegel exercises and behavioral strategies such as limiting fluid intake in the evenings were the next most frequently used symptom intervention. A few participants reported doing nothing for their symptoms, and a few reported using over-the-counter medications. Other interventions were reported in 17 % of cases. Of the reported interventions for urinary symptoms, 81 % were reported to be successful.
Table 2.
Top ten symptom management strategies employed across different symptom types (N =628)
| Urinarya | Sexuala | Bowel | Systemica | Mental health | Other | |
|---|---|---|---|---|---|---|
| Prescription medications | 17 % | 40 % | 12 % | 13 % | 11 % | 10 % |
| Doing nothing | 8 % | 29 % | 0 % | 21 % | 0 % | 21 % |
| Diet, lifestyle, exercise | 0 % | 0 % | 21 % | 15 % | 35 % | 37 % |
| Behavioral strategies | 10 % | 0 % | 0 % | 27 % | 12 % | 11 % |
| Other | 17 % | 13 % | 10 % | 4 % | 4 % | 6 % |
| OTC medications | 6 % | 0 % | 57 % | 10 % | 0 % | 15 % |
| Pads | 17 % | 0 % | 0 % | 0 % | 0 % | 0 % |
| Support from others | 0 % | 0 % | 0 % | 0 % | 39 % | 1 % |
| Kegel exercises | 10 % | 0 % | 0 % | 0 % | 0 % | 0 % |
| Medical devices | 0 % | 11 % | 0 % | 0 % | 0 % | 0 % |
Values do not sum to 100 % due to other management strategies not listed here
Sexual Symptoms
For sexual symptoms, reported by 77.6 % of the sample, the most common intervention was the use of prescription medications, primarily phosphodiesterase type 5 inhibitors. Doing nothing was the next most frequent response, followed by using another intervention and then medical devices. Some men reported the need for extra time to mentally prepare for sex by relaxing or otherwise getting in the mood. Only 47 % of strategies used for sexual dysfunction were reported by respondents to be successful.
Bowel Symptoms
Bowel symptoms were reported by 32.7 % of the sample. A little over half of participants used over-the-counter medications to solve their bowel-related symptoms, followed by diet, lifestyle modifications, and exercise. Participants reported increasing their fiber intake, either through diet or over-the-counter medications. Others chose to use prescription medications or another intervention, including analgesic pads and soothing baths to ease rectal pain after radiation treatment. For bowel symptoms, 84 % of reported strategies were successful.
Systemic Symptoms
Systemic symptoms were reported by 66.3 % of the sample. The most common intervention for systemic symptoms were behavioral strategies, which included changing attire, drinking cold drinks, or using ice packs during hot flashes. “No intervention” was the next most frequent response, followed by diet, lifestyle modifications, and exercise. Prescription medications were used by some participants reporting systemic symptoms; over-the-counter medications, by others. Other strategies, such as reframing the experience of hot flashes from being a discomfort to being a reminder that the participant had a medication that was working to control his PCa, were used by a few participants. Systemic symptoms were successfully treated in 61 % of instances.
Mental Health Symptoms
For mental health concerns, reported by 46.9 % of the sample, the most frequently relied upon strategy was psychosocial support from others, including a spouse or partner, family member, or other prostate cancer survivors in a support group. Diet, lifestyle modifications, and exercise were the next most frequently used strategy to help alleviate symptoms, followed by behavioral strategies and then antidepressant or anti-anxiety prescription medications. Mental health symptoms were alleviated by 89 % of reported interventions. Of particular note, many men reported being told they had been “lucky” to get a “good” cancer with several effective treatments available. Men reporting this phenomenon indicated some distress, as they felt their experience was being trivialized by others.
Other Symptoms
For symptoms in the “other” category (e.g., fatigue, muscle loss, joint pain, weight gain), reported by 12.2 % of the sample, more participants turned to diet, lifestyle changes, and exercise than to any other strategy. The next most frequently used strategy was doing nothing, followed by over-the-counter medications and then behavioral strategies. Some used prescription medications, a few used other interventions, and even fewer relied on support from others. Seventy percent of the interventions for other symptoms were considered effective.
Discussion
Psychosocial and patient education interventions for men treated for localized PCa have been limited. Using the Critical Incident Technique, we asked men to report the symptoms associated with the treatment(s) they received, strategies used to manage those symptoms, and the effectiveness of those strategies. Ninety-eight men contributed 628 symptom management reports.
Many men responded that they did “nothing” to manage their symptoms. Across their lifespan, men make fewer contacts with physicians than women and are more likely to have gone 2 years since their last contact with a physician [17]. Data for ambulatory care visits show that women have 376.8 office visits while men have 283.1 visits per 100 person years [18]. The number of men reporting doing nothing in response to their prostate cancer symptoms may be related to a number of factors, but health-care providers should be aware of this tendency in men not to seek medical help and be proactive in discussing HRQOL concerns with prostate cancer survivors.
Men in our study reported difficulties in communicating their concerns to providers and partners. Sexual dysfunction may be a problem for which men have particular difficulty seeking treatment. Some men with erectile dysfunction (ED) may feel that poorer sexual functioning is a natural part of aging, or their partner may have sexual dysfunction that makes sexual activity for the couple problematic [19]. In one study, only about a third of men with ED of mixed etiologies had sought treatment [20]. Many men report that embarrassment or lack of information underlies their decision not to seek treatment. Men who do seek treatment may do so to improve self-esteem or because their partner insists [20]. Other work suggests that the discrepancy between expectations and posttreatment outcomes may engender a sense of loss and changes in masculine self-confidence that should be addressed in men where it is sufficient to be clinically meaningful to survivors [21-24].
Few men reported any organized education or information offered to them by their health-care provider to manage treatment-related side effects. Some men reported tips or information provided by a nurse or other provider, but these were generally offhand comments, not a formalized educational intervention. ED treatment guidelines recommend psychosexual counseling and patient education for patients with ED, but patients do not widely use such services, even when they are available [24, 25]. However, even brief psychosocial interventions can improve satisfaction with ED treatment and erectile functioning [26, 27]. Thus, PCa survivors should be offered psychosocial interventions appropriate to their literacy level to help improve their HRQOL.
Men in our study reported more than localized concerns suggested by the type of treatment they received. While validated, disease-specific HRQOL instruments provide invaluable information on the impact of treatment, and use of these measures constrains our view of how prostate cancer treatment may affect men. Our data suggest that providers and researchers should continue using validated HRQOL instruments but be mindful that PCa may affect other aspects of a man’s health not well described by existing questionnaires.
Localized PCa is highly treatable, with disease-free survival rates of nearly 100 % at 5 years and 91 % at 10 years after treatment [28]. Some participants included in this study reported feeling marginalized by others as being “lucky” to have gotten a “good” cancer, in that they are likely to have many years of life ahead of them. However, these negative comments may trivialize the sometimes substantial decrements in HRQOL some prostate cancer survivors experience and discourage them from asking for help managing treatment-related symptoms. This feeling of having their concerns minimized, in comparison with concerns about mortality with other cancers, may be another reason some men report doing nothing to manage symptom concerns.
There are some limitations to our study. Our population was a primarily white convenience sample obtained mainly from email distribution lists of prostate cancer support groups in Northern California. Many of our participants were treated with surgery as monotherapy or as first- or second-line therapy. As such, our participants may not be representative of men who do not attend support groups or do not have email access or were treated primarily with radiotherapy. Our participants were recruited between May 2004 and August 2005 after receiving various treatments. The maximum time that patients had to recall symptom experiences was 2 years, but it is possible that men either overestimated or underestimated the strategies used. The reports are also subjective and susceptible to recall bias. In addition, the treatment of localized PCa has been refined in the interval between participant accrual and the time of this report. Perhaps the intervening increase in experience with robotic-assisted approach to radical prostatectomy, photon- or proton-based radiotherapy techniques, and the emerging acceptance of active surveillance in lieu of immediate treatment may result in fewer or less severe treatment-related complications in patients treated since 2005.
Our data are the first look at symptom management strategies employed by patients treated for localized PCa. In addition to expected reports of medical and surgical interventions, a number of behavioral and lifestyle intervention methods were identified that have been incorporated into a patient education intervention currently being evaluated. Two important results emerged from this study that bear further investigation. First, we identified “doing nothing” as a more common than expected reaction to symptoms. Further research is needed to understand whether many of these untreated symptoms may have resolved without treatment and how many could have been resolved if health-care providers were aware of and addressed these concerns. Second, we identified sexual symptoms as the area where men reported the lowest effectiveness. Work is needed to understand how to improve sexual symptom management, including patient education, and how to improve HRQOL as much as is possible after prostate cancer treatment.
Prostate cancer treatment continues to evolve. As new approaches to treatment are developed, similar studies are needed to understand symptom management strategies of men treated with newer forms of radiation therapy such as intensity-modulated radiation therapy and proton beam therapy [29]. Additionally, men who choose active surveillance may use different strategies to manage the primarily psychosocial symptoms they experience as part of the surveillance process [30-32]. Further work is needed to understand how men adapt or accommodate to these new symptom challenges so that medical and psychosocial clinicians can provide needed support that can improve quality of life after treatment or during surveillance.
Acknowledgments
This work was funded by grant R03 CA101586 from the National Cancer Institute to David M. Latini. This material is partly the result of work supported with resources and the use of facilities at the Health Services Research & Development Center of Excellence (HFP90-020), Michael E. DeBakey Veterans Affairs Medical Center. Dr. Latini is supported by Mentored Research Scholar Grant 06-083-01-CPPB from the American Cancer Society. We also would like acknowledge the assistance of Sarah Howard Joost in recruiting participants, David V. Flores and Thomas D. McNeese II in data management and coding, and Sonora Hudson, MA, in editing the manuscript. Sandra R. Wilson, PhD, provided her expertise with the Critical Incident Technique to the study team. The views expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs/Baylor College of Medicine.
Footnotes
Conflict of Interest The authors have no financial interests to disclose.
Contributor Information
Alok Vij, Baylor College of Medicine, Houston, TX, USA.
Marc A. Kowalkowski, Baylor College of Medicine, Houston, TX, USA VA Health Services Research & Development Center of Excellence, Michael E. DeBakey VA Medical Center, Houston, TX, USA.
Tae Hart, Department of Psychology, Ryerson University, Toronto, ON, Canada.
Heather Honoré Goltz, Baylor College of Medicine, Houston, TX, USA; VA Health Services Research & Development Center of Excellence, Michael E. DeBakey VA Medical Center, Houston, TX, USA.
David J. Hoffman, University of Texas Southwestern Medical School, Dallas, TX, USA
Sara J. Knight, San Francisco VA Medical Center, San Francisco, CA, USA Department of Urology and Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Peter R. Caroll, Department of Urology and Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA
David M. Latini, Email: latini@bcm.edu, Scott Department of Urology and Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, USA; Mental Health Care Line (116), Michael E. DeBakey Veterans Affairs Medical Center, 2002 Holcombe Boulevard, Houston, TX 77030, USA.
References
- 1.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273(2):129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 2.Potosky AL, Harlan LC, Stanford JL, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 1999;91(20):1719–1724. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 3.Lubeck DP, Litwin MS, Henning JM, Carroll PR. Measurement of health-related quality of life in men with prostate cancer: the CaPSURE database. Qual Life Res. 1997;6(5):385–392. doi: 10.1023/a:1018439528024. [DOI] [PubMed] [Google Scholar]
- 4.Eton DT, Lepore SJ, Helgeson VS. Psychological distress in spouses of men treated for early-stage prostate carcinoma. Cancer. 2005;103(11):2412–2418. doi: 10.1002/cncr.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 6.Talcott JA. Quality of life in early prostate cancer: do we know enough to treat? Hematol Oncol Clin N Am. 1996;10:691–701. doi: 10.1016/s0889-8588(05)70361-0. [DOI] [PubMed] [Google Scholar]
- 7.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2004;96(18):1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 8.Janssen E, Vorst H, Finn P, Bancroft J. The Sexual Inhibition (SIS) and Sexual Excitation (SES) Scales: II. Predicting psychophysiological response patterns. J Sex Res. 2002;39(2):127–132. doi: 10.1080/00224490209552131. [DOI] [PubMed] [Google Scholar]
- 9.Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. The supportive care needs of men with prostate cancer. Psychooncology. 2001;10(1):66–75. doi: 10.1002/1099-1611(200101/02)10:1<66::aid-pon493>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Boberg EW, Gustafson DH, Hawkins RP, et al. Assessing the unmet information, support and care delivery needs of men with prostate cancer. Patient Educ Couns. 2003;49(3):233–242. doi: 10.1016/s0738-3991(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 11.Giesler RB, Given B, Given CW, et al. Improving the quality of life of patients with prostate carcinoma: a randomized trial testing the efficacy of a nurse-driven intervention. Cancer. 2005;104(4):752–762. doi: 10.1002/cncr.21231. [DOI] [PubMed] [Google Scholar]
- 12.Latini DM, Hart SL, Coon DW, Knight SJ. Sexual rehabilitation after localized prostate cancer: current interventions and future directions. Cancer J. 2009;15(1):34–40. doi: 10.1097/PPO.0b013e31819765ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson SR, Mitchell JH, Rolnick S, Fish L. Effective and ineffective management behaviors of parents of infants and young children with asthma. J Pediatr Psychol. 1993 Feb;18(1):63–81. doi: 10.1093/jpepsy/18.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Starr NJ, Likosky WH, Hayes BW, Casey D, Wilson SR, Javerbaum J. Improved quality of life (QOL) outcomes in MS patients after participation in the “Coping with MS Effectively” program. Neurology. 1996;46:S63.004. [Google Scholar]
- 15.Flanagan JC. The critical incident technique. Psychol Bull. 1954;51:327–358. doi: 10.1037/h0061470. [DOI] [PubMed] [Google Scholar]
- 16.Anderson LE, Wilson SR. The critical incident technique. In: Whetzel DL, Wheaton GR, editors. Applied measurement methods in industrial psychology. Davies-Black Publishing; Palo Alto: 1997. pp. 89–112. [Google Scholar]
- 17.Department of Health and Human Services. Services DoHaH. Hyattsville, MD: National Center for Health Statistics; 1998. Health, United States, with socioeconomic status and chartbook. [Google Scholar]
- 18.Cherry DK, Woodwell DA, Rechtsteiner EA. Advance data from vital and health statistics. Center for Health Statistics N; Hyattsville, MD: 2007. National ambulatory medical care survey: 2005 summary. [PubMed] [Google Scholar]
- 19.Shabsigh R, Perelman MA, Laumann EO, Lockhart DC. Drivers and barriers to seeking treatment for erectile dysfunction: a comparison of six countries. BJU Int. 2004;94(7):1055–1065. doi: 10.1111/j.1464-410X.2004.05104.x. [DOI] [PubMed] [Google Scholar]
- 20.Ansong KS, Lewis C, Jenkins P, Bell J. Help-seeking decisions among men with impotence. Urology. 1998;52(5):834–837. doi: 10.1016/s0090-4295(98)00397-5. [DOI] [PubMed] [Google Scholar]
- 21.Wittmann D, Foley S, Balon R. A biopsychosocial approach to sexual recovery after prostate cancer surgery: the role of grief and mourning. J Sex Marital Ther. 2011;37(2):130–144. doi: 10.1080/0092623X.2011.560538. [DOI] [PubMed] [Google Scholar]
- 22.Wittmann D, He C, Coelho M, Hollenbeck B, Montie JE, Wood DP., Jr Patient preoperative expectations of urinary, bowel, hormonal and sexual functioning do not match actual outcomes 1 year after radical prostatectomy. J Urol. 2011;186(2):494–499. doi: 10.1016/j.juro.2011.03.118. [DOI] [PubMed] [Google Scholar]
- 23.Bokhour BG, Clark JA, Inui TS, Silliman RA, Talcott JA. Sexuality after treatment for early prostate cancer: exploring the meanings of “erectile dysfunction”. J Gen Intern Med. 2001;16(10):649–655. doi: 10.1111/j.1525-1497.2001.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latini DM, Penson DF, Colwell HH, et al. Psychological impact of erectile dysfunction: validation of a new health related quality of life measure for patients with erectile dysfunction. J Urol. 2002;168(5):2086–2091. doi: 10.1016/S0022-5347(05)64302-9. [DOI] [PubMed] [Google Scholar]
- 25.American Urological Association. The management of erectile dysfunction: an update. American Urological Association; Linthicum: 2006. [Google Scholar]
- 26.McCabe MP, Price E, Piterman L, Lording D. Evaluation of an internet-based psychological intervention for the treatment of erectile dysfunction. Int J Impot Res. 2008;20(3):324–330. doi: 10.1038/ijir.2008.3. [DOI] [PubMed] [Google Scholar]
- 27.Canada AL, Neese LE, Sui D, Schover LR. Pilot intervention to enhance sexual rehabilitation for couples after treatment for localized prostate carcinoma. Cancer. 2005;104(12):2689–2700. doi: 10.1002/cncr.21537. [DOI] [PubMed] [Google Scholar]
- 28.Altekruse SF, Kosary CL, Krapcho M, et al. Services DoHH ed Vol based on November 2009 SEER data submission, posted to the SEER web site, 2010. Bethesda, MD: National Cancer Institute; 2010. SEER cancer statistics review, 1975–2007. [Google Scholar]
- 29.Budaus L, Bolla M, Bossi A, et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61(1):112–127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Goh AC, Kowalkowski MA, Bailey DE, Jr, Kazer MW, Knight SJ, Latini DM. Perception of cancer and inconsistency in medical information are associated with decisional conflict: a pilot study of men with prostate cancer who undergo active surveillance. BJU Int. 2012;110(2 Pt 2):E50–E56. doi: 10.1111/j.1464-410X.2011.10791.x. [DOI] [PubMed] [Google Scholar]
- 31.Kazer MW, Psutka SP, Latini DM, Bailey DE., Jr Psychosocial aspects of active surveillance. Curr Opin Urol. 2013;23(3):273–277. doi: 10.1097/MOU.0b013e32835eff24. [DOI] [PubMed] [Google Scholar]
- 32.Bailey DE, Jr, Wallace M, Latini DM, et al. Measuring illness uncertainty in men undergoing active surveillance for prostate cancer. Appl Nurs Res ANR. Nov;24(4):193–199. doi: 10.1016/j.apnr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


