Abstract
Background/Aim
Cyclin D1 is a mediator of cell-cycle control that is frequently overexpressed in primary ductal breast carcinomas, but its role is controversial. A polymorphism in the CCND1 gene, G870A, results in an aberrantly spliced protein (cyclin D1b) lacking the Thr-286 phosphorylation site necessary for nuclear export. Studies of murine fibroblasts have shown that although overexpression of canonical cyclin D1 (cyclin D1a) alone is not sufficient to drive malignant transformation, expression of nuclear cyclin D1b is oncogenic. Our objectives were to determine whether cyclin D1b is expressed in human breast carcinomas and to characterize the relationship of this protein to both cyclin D1a and clinical outcome in breast cancer patients.
Patients and Methods
We performed a prospective cohort study of women with early-stage breast cancer and analyzed cyclin D1a and D1b expression in primary breast tumor sections. Expression was tested for correlation with other breast cancer prognostic factors and clinical outcome, including recurrence or death.
Results
A total of 118 patients were included in this analysis, with a median follow-up of 44 months. Cyclin D1b was expressed in 26% of tumors and cyclin D1a was overexpressed in 27%; co-expression occurred in 4%. Cyclin D1a and/or D1b expression were not significantly associated with estrogen or progesterone receptor negativity, Her2 overexpression, young age, lymph node positivity, high tumor grade, nor large tumor size. The risk of recurrence was higher in those co-expressing D1a and D1b compared to the expression of either alone (relative risk=5.3, 95% confidence interval 1.27 to 22.1, p=0.02). The hazard ratio for those with co-expression compared with those without was 6.05 (p=0.04).
Conclusion
Expression of cyclin D1b occurs in primary human breast carcinomas and its coexpression with cyclin D1a may be a marker for increased recurrence risk, independently of other factors.
Keywords: Breast cancer, cyclin D1, cyclin D1b, cyclin-dependent kinase inhibitor, recurrence
Over 200,000 American women are diagnosed with breast cancer annually, with 40,000 deaths per year (1). Breast cancer involving axillary lymph nodes is potentially curable, but up to 50% of women with positive nodes will eventually develop metastatic disease despite optimal adjuvant treatment (2). Estrogen and progesterone receptor (ER/PR) and HER-2 status provide some predictive power for recurrence and response to targeted therapies (3). However, despite a plethora of data, few other markers clinically impact prognosis and treatment.
Cyclin D1, a protein derived from the CCND1 gene on chromosome 11q13, is involved in both normal regulation of the cell cycle and neoplasia (4–6). It is one of four cyclins that function during the G1 stage of the cell cycle by binding to cyclin-dependent kinases (CDK) 4 and 6 to promote progression to the S phase (7). The heterodimeric molecule formed by cyclin D1 and CDK 4/6 is an active protein kinase that phosphorylates specific substrates to modulate the G1/S interphase (8). After entering the S phase, phosphorylation of the cyclin D1 protein by glycogen synthase kinase-3β at a specific threonine residue (Thr-286) enables its export from the nucleus and subsequent proteolysis by Skp1-Cul1-F box4 (9, 10), thereby limiting the effect of this protein in promoting cell cycle progression (Figure 1).
Figure 1.

Normal function of cyclin D1a. Binding of cyclin D1a leads to phosphorylation of Rb and progression of the cell cycle into the G1 phase. Cyclin D1a is subsequently phosphorylated at Thr-286 by GSK-3β, leading to its nuclear export and degradation.
A polymorphism at nucleotide 870 of the CCND1 gene, G870A, results in an aberrantly spliced cyclin D1 variant (termed cyclin D1b) (11). This mutant transcript differs from the native cyclin D1 isoform (termed cyclin D1a) in the last 55 amino acids of the carboxy-terminus, thereby lacking the Thr-286 phosphorylation site required for nuclear export and subsequent proteasome-mediated degradation (12) (Figure 2). Preliminary studies in human esophageal and breast carcinoma cell lines indicate that cyclin D1b is expressed exclusively in neoplastic cells and likely represents an oncogenic alternatively spliced isoform of canonical cyclin D1a (13). Although overexpression of cyclin D1a alone is not sufficient to drive malignant transformation in murine fibroblasts, expression of cyclin D1b, which is stabilized in the nucleus, does transform murine fibroblasts without the presence of a collaborating oncogene (13). This suggests that a polymorphism leading to the production of cyclin D1b could be the key event triggering malignancy in some patients.
Figure 2.

Cyclin D1b. An alternatively spliced cyclin D1a produces mRNA encoding an isoform with a different C-terminus which lacks Thr-286 which is necessary for GSK-3β mediated nuclear export.
We analyzed cyclin D1a and D1b expression in a cohort of women with early-stage breast carcinoma to determine the frequency of cyclin D1b expression in human breast carcinoma, determine whether it is coexpressed with cyclin D1a, and to evaluate whether cyclin D1b expression is associated with a more aggressive phenotype and a higher risk of recurrence of breast cancer.
Patients and Methods
We performed a prospective cohort study of patients diagnosed with early-stage breast carcinoma at the University of Pennsylvania. Patients were eligible for study participation if they had a primary tumor measuring at least 2 cm or histologically involved axillary lymph nodes, had a negative metastatic evaluation (consisting of a chest x-ray, bone scan, and serum liver function tests) and were willing to provide tumor specimens for study. At the time of enrollment, patients were required to have completed definitive surgery and lymph node evaluation with no evidence of gross or microscopic tumor at the surgical resection margins, and to have received no prior chemotherapy or radiation for the current malignancy, including pre-operative chemotherapy. Exclusion criteria included prior history of malignancy (except cervical intraepithelial neoplasia of the uterine cervix, or basal cell carcinoma), locally advanced or inflammatory breast cancer. All patients provided written informed consent prior to enrollment on the study. The study protocol was approved by the Institutional Review Board of the University of Pennsylvania and the Abramson Cancer Center Scientific Review and Monitoring Committee.
Demographic information, as well as medical, reproductive, social and family histories was collected through a questionnaire completed by each patient upon entry to the study. Information about tumor size, histological subtype, tumor grade, number of axillary lymph nodes with cancer, type of surgery, margin status, ER/PR and Her2 status were collected from each patient’s primary pathology report. Treatment information was abstracted from patient medical records. Patients were contacted on an annual basis by phone or mail to document treatment and disease status with verification via medical record review.
Immunohistochemical analysis was performed on paraffin-embedded primary tumor sections using isoform-specific antibodies to cyclin D1a and cyclin D1b by a laboratory technician blinded to patient-specific clinical data. The cyclin D1b antibody was previously described (11). Briefly, slides were sectioned at 4 to 6 microns and pretreated by microwaving in 10 mM citric acid buffer, pH 6.0, prior to treatment with 30% hydrogen peroxide to quench endogenous peroxidases. Pairs of slides from each study case were stained for cyclin D1a as well as cyclin D1b. Cyclin D1a staining was undertaken using the primary mouse anti-cyclin D1a(ab3) (Oncogene Research Products, San Diego, CA, USA) and diluted with DAKO Antibody Dilutent (DAKO, Carpenteria, California, USA) at a 1:100 dilution and incubated overnight at 4°C. A matching set of slides was stained with primary rabbit anti-cyclin D1b (R3), produced by the Diehl lab at a 1:2,500 dilution in DAKO Antibody Dilutent and incubated overnight at 4°C. Slides were then washed with 1x phosphate-buffered saline (PBS) and incubated with either Labeled Polymer HRP anti-mouse for cyclin D1a slides (K4006; Dakocytomation, DAKO, Carpinteria, California, USA) or Labeled Polymer-HRP anti-rabbit for cyclin D1b (K4003; Dakocytomation) at room temperature for thirty minutes. Slides were again rinsed with 1xPBS solution and developed with DAB+Chromogen (K3468; Dakocytomation). Slides were counterstained with Gill’s #2 Haematoxylin (Sigma-Aldrich, St. Louis, MO, USA).
Slides stained with cyclin D1a and D1b antibodies were reviewed by an independent study pathologist blinded to patient identity. The overall intensity of protein staining was recorded on a scale of 0 (no staining), 1+ (weak staining), 2+ (moderate staining), or 3+ (strong staining). Protein expression was evaluated in the nucleus and cytoplasm separately, and the percentage of cells with staining in either compartment, along with the percentage of cells with both nuclear and cytoplasmic staining, was recorded. Tumors with 2+ or 3+ staining and with greater than 50% staining for cyclin D1a in either the nucleus or cytoplasm were considered positive for cyclin D1a. Because cyclin D1b is not expected to be found to be in normal cells, tumors were considered positive for cyclin D1b if any cells showed staining in the nucleus.
Patient clinical and tumor characteristics were summarized using means, standard deviations, medians and ranges for continuous variables, and frequencies for categorical variables. Expression of cyclin D1a and D1b expression was summarized, and tested for correlation with clinical factors. Expression levels were compared between groups of patients with t-tests or their nonparametric equivalents (Wilcoxon rank sum tests); for continuous variables, Pearson and Spearman correlation coefficients were estimated. To assess the association of cyclin D1a and D1b expression with time to recurrence of disease and survival, we generated Kaplan-Meier estimates and compared these using log-rank tests. Multivariate Cox models were fit for disease-free survival (DFS) and logistic models were fit for recurrence to determine prognostic factors. Statistical analysis was performed using Stata Version 10 (College Station, TX, USA) and all tests used a two-sided significance level of 0.05.
Results
A total of 118 patients were included in this analysis, with a median follow-up of 44 months. Table I summarizes the clinical characteristics of the study population. The median age of the patients at diagnosis was 50 years, and 46% were postmenopausal. The majority of patients had positive axillary lymph nodes and high-grade tumors (79% and 60%, respectively). As expected in an early-stage cohort, the majority of tumors (80%) were hormone receptor positive, while 22% overexpressed Her2. Of the 94 patients with ER/PR-positive disease, almost all (94%) received adjuvant hormones. Most patients (76%) also received adjuvant chemotherapy with doxorubicin and cyclophosphamide, and 58% also received adjuvant taxane based chemotherapy.
Table I.
Characteristics of study population (n=118).
| Median age (years) | 50 (range:26–84) |
| Postmenopausal | 54 (46%) |
| Median tumor size (cm) | 2.2 (range:1–6) |
| Lymph node-positive | 93 (79%) |
| ER/PR-positive | 94 (80%) |
| Adjuvant hormonal treatment | 88 (94%) |
| No adjuvant hormonal treatment | 2 (2%) |
| Adjuvant hormonal treatment unknown | 4 (4%) |
| Her2-positive | 26 (22%) |
| Recurrence | 17 (14%) |
| Adjuvant chemotherapy | |
| Doxorubicin and cyclophosphamide | 90 (76%) |
| Additional taxane therapy | 69 (58%) |
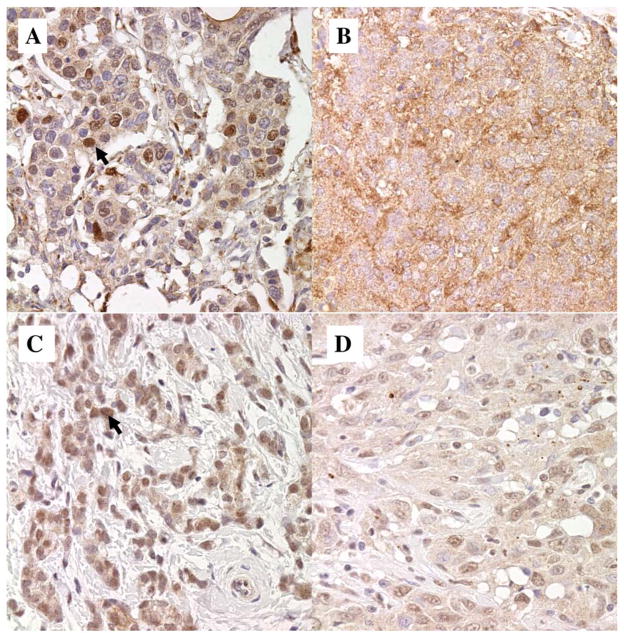
Table II shows the immunohistochemical staining results for cyclin D1a and cyclin D1b. Overall, 26% of the tumors in this cohort expressed cyclin D1b protein. Of those expressing cyclin D1b, 11% had nuclear expression only and 15% had both nuclear and cytoplasmic expression. Cyclin D1a was overexpressed at a frequency of 27%. A total of 4% of patients overexpressed both canonical and variant cyclin D1, while 23% overexpressed only cyclin D1a. A total of 22% of patients were cyclin D1a-negative and cyclin D1b-positive, and 51% were negative for both. Figure 3 shows representative immunohistochemical staining for cyclin D1a and D1b from four different breast tumors. We did not find any significant correlation between cyclin D1a and/or D1b expression and ER/PR status, Her2 status, age, lymph node status, grade of tumor, or tumor size (Table III).
Table II.
Cyclin D1a and D1b expression patterns.
| Marker | Frequency (%) |
|---|---|
| Cyclin D1a(+) | 32 (27%) |
| Cyclin D1b(+) | 31 (26%) |
| Nuclear expression only | 13 (11%) |
| Nuclear and cytoplasmic expression | 18 (15%) |
| Combination frequencies | |
| D1a(+)/D1b(+) | 5 (4%) |
| D1a(+)/D1b(−) | 27 (23%) |
| D1a(−)/D1b(+) | 26 (22%) |
| D1a(−)/D1b(−) | 60 (51%) |
Figure 3.
Sample IHC stains of cyclin D1a and cyclin D1b. A: Cyclin D1a with strong nuclear staining (arrow) and weak cytoplasmic staining. B: Cyclin D1a with 2+ cytoplasmic staining and no nuclear staining. C: Cyclin D1b with 2+ nuclear only staining (arrow) in 100% of the cells. D: Cyclin D1b with 1+ nuclear and 1+ cytoplasmic staining in 80% of the cells.
Table III.
Associations between cyclin D1a, D1b and other tumor prognostic markers.
| Cyclin D1a+ | Cyclin D1b+ | p-Value | |
|---|---|---|---|
| ER-positive | 25 (78%) | 23 (74%) | 0.57 |
| Her2-positive | 8 (27%) | 5 (18%) | 0.68 |
| Median tumor size, cm (range) | 1.75 (0.1–5) | 2.5 (0.5–6) | 0.13 |
| Positive lymph nodes, n | 2 (0–17) | 1 (0–15) | 0.21 |
| Median age, years (range) | 50 (38–84) | 47 (26–81) | 0.86 |
| Premenopausal | 13 (42%) | 12 (39%) | 0.56 |
At a median follow-up of 3.6 years, 17 patients (14%) had recurrence of breast cancer. Figure 4 shows the Kaplan-Meier curve for recurrence based on cyclin D1a and D1b positivity. In general, there were no significant differences in DFS comparing patients who overexpressed cyclin D1a and cyclin D1b to those who did not overexpress either isoform, or only one of the two isoforms (log-rank p=0.12). Among patients who overexpressed either cyclin D1a or cyclin D1b, however, coexpression of cyclin D1a and cyclin D1b appeared to increase the relative risk (RR) of recurrence compared to overexpression of D1a or D1b alone (RR 5.3, 95% confidence interval (CI) 1.27 to 22.1, p=0.02). In addition, there was a significant difference in the length of DFS according to cyclin D1a and cyclin D1b status (hazard ratio, HR=6.05, p=0.04). When multivariate models were fit including other prognostic factors and cyclin D1a and D1b positivity, no significant predictive effects were found. The final models for both DFS and recurrence showed only tumor size as a predictive factor (p=0.04 and p=0.06 for Cox modeling of DFS and logistic modeling of recurrence, respectively).
Figure 4.
Kaplan-Meier curve for risk of recurrence. Patients co-expressing cyclins D1a and D1b (line 3) had a higher risk of recurrence compared to those expressing either cyclin D1a (line 1) or D1b (line 2) alone. Relative risk=5.3, 95% confidence interval 1.27 to 22.1; Kaplan-Meier hazard ratio: 6.05, p=0.04.
Discussion
In this cohort of women with early-stage breast cancer, we found that 26% of tumors expressed the splice variant, cyclin D1b. The vast majority of these tumors expressed cyclin D1b in the absence of canonical cyclin D1a (4% coexpression rate), suggesting that cyclin D1a overexpression is not necessary for cyclin D1b production. Patients expressing cyclin D1b alone did not have an increased risk of relapse in our study. However, the 4% of patients with concurrent expression of cyclin D1a and cyclin D1b did show a trend toward increased risk of recurrence of breast cancer.
Cyclin D1b is expected to be found exclusively in the nucleus. We found cyclin D1b staining restricted to the nucleus in 13 breast cancers in this study; however, 18 tumors showed stainable D1b in both the cytoplasm and nucleus. These findings are consistent with studies of mantle cell lymphoma in which D1b was found in both nuclear and cytosol-protein fractions from cell lines and primary cells (14). This would seem to counter preclinical studies showing cyclin D1b restricted to the nucleus, as would be predicted, and is unexplained.
Overexpression of canonical cyclin D1a is common in breast carcinoma, with up to 50% of primary ductal breast carcinomas overexpressing cyclin D1a in various studies (15, 16). Increased expression has been shown to be evident in all histological types of breast cancer and is present in both ER+ and ER− malignancies (17, 18). Once acquired, cyclin D1a overexpression is maintained through all stages of disease, including metastatic lesions (19). Despite the undisputed presence of cyclin D1a in breast cancer, data thus far have produced conflicting results regarding the biological and clinical significance of cyclin D1a overexpression. Cyclin D1a overexpression alone has not been shown to induce malignant transformation in cell lines and mouse models, but nuclear cyclin D1 alleles have exhibited increased oncogenic potential (20). Our data are consistent with these findings. Overall, 27% of patients overexpressed cyclin D1a and its overexpression alone did not correlate with an increased risk of relapse of breast cancer. Whether cyclin D1 overexpression is an oncogenic mechanism or simply an indication of cell proliferation driven by other upstream or downstream mechanisms remains unclear (21, 22).
Since cyclin D1b lacks the threonine-286 phosphorylation site which is required for its export out of the nucleus, it may continuously bind to CDK 4/6 to phosphorylate positive modulators of the G1/S interphase in the cell cycle. Our finding that 22% of breast tumors in our cohort expressed cyclin D1b without concurrent overexpression of cyclin D1a suggests that cyclin D1b may play a role independently of that previously attributed to canonical cyclin D1. Previous studies examining cyclin D1a expression may have erroneously measured cyclin D1b due to a lack of specificity of the antibody. It is possible that cyclin D1a may merely be a marker of increased proliferation in most patients with breast cancer, but it may represent a key driver of oncogenesis in those who express cyclin D1b as well. Thus, cyclin D1b could be the oncogenic mechanism that allows overexpression of cyclin D1a to function in an oncogenic capacity.
We are aware of only one other study testing the correlation of overexpression of the two cyclin D1 isoforms and clinical outcome in breast cancer (23, 24). These investigators studied 175 women followed for a median of 75 months, using immunohistochemical staining to identify and semi-quantitate cyclin D1a and cyclin D1b expression in formalin-fixed, paraffin-embedded tumor tissue sections, as we did (23, 24). They found a higher prevalence of both isoforms of cyclin D1 – one or both were overexpressed in 61% of tumors compared to 49% of tumors in our study. The difference was mostly in D1a, which was overexpressed in 49% of tumors they studied vs. 27% of ours; D1b was overexpressed in 31% of their tumors vs. 26% in our study. They found the D1a(+)/D1b(+) phenotype in 18% of tumors, D1a(+)/D1b(−) in 30%, and D1a(−)/D1b(+) in 12%, compared to our 4%, 23% and 22%, respectively (23, 24).
One possible reason for different staining results may be the antibodies used for detecting the D1a and D1b isoforms. Both studies used commercially available antibodies for D1a (Ab-3), although from different venders. Wang et al. used the same strategy we did to develop a similar, but not identical, antibody for detecting the D1b isoform (23). They raised antibodies to an 18-amino acid peptide derived from the C-terminal of translated intron 4 sequences that overlaps 9 peptides with the 22-amino acid sequence that we used for this purpose. Differences in the antibodies themselves or in adapting them to stain fixed, paraffin-embedded tumor tissues may account for some differences in detecting cyclin D1 overexpression.
Millar et al. (24) found that D1b overexpression was significantly associated with poor outcome in the form of shorter time to recurrence, distant metastasis and breast cancer-specific death; patients with the D1a(+)/D1b(+) and D1a(−)/D1b(+) had the worst outcomes (24). In contrast, we saw poorer outcome only in patients with the D1a(+)/D1b(+) phenotype. Like us, they did not find any significant associations between D1b expression and tumor size, lymph node status, hormone receptor expression or Her2 status, nor did they find an association between high cyclin D1a expression and outcome (24).
The lack of a greater prognostic effect of cyclin D1b in our cohort may be due to several factors. Clearly, the sample size and small number of relapses at the time of this analysis may have limited our ability to detect a significant difference. Notably, after a median of 44 months of follow-up, only 14% of our patients had relapse of their breast cancer, suggesting that these patients are doing well in the short term with improvements in therapy, especially dose-dense treatment in patient with large tumors or with lymph node-positive disease and the increased use of aromatase inhibitors in postmenopausal women since the start of the study. Addressing the possible effects of treatment, we note in Millar et al., only 41% of their patients received adjuvant chemotherapy vs. 76% of our patients. Similarly, 51% of their patients received adjuvant endocrine therapy vs. 94% in our group (24). Longer follow-up may provide greater evidence of a correlation between cyclin D1a overexpression, cyclin D1b expression, and risk of relapse of breast cancer in our cohort.
In conclusion, our study shows that cyclin D1b is found in human breast carcinoma and that cyclin D1a overexpression or cyclin D1b expression alone does not correlate with any adverse prognostic markers or with clinical outcome. However, coexpression of cyclin D1a and D1b proteins may have an implication for risk of recurrence. The recent development of CDK inhibitors targeting the cyclin D1 pathway lends special import to exploring this pathway and identifying patient populations who would benefit most from these drugs. Identifying patients in whom cyclin D1a or D1b overexpression is a key activator of oncogenesis is crucial in selecting patients rationally for future trials of drugs targeting cyclin D1 modulators. These findings suggest that cyclin D1b warrants further investigation as an oncogenic marker in breast cancer.
Acknowledgments
This study was made possible through generous funding from the NCI (Angela DeMichele: K23-CA81009; Alan Diehl: CA 11360; Vandana Gupta Abramson: 5-K12-CA-076931-10), and through the Leukemia Lymphoma Society (Alan Diehl: Leukemia Lymphoma Society Scholar).
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer Statistics, 2004. CA Cancer J Clin. 2004;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Sunderland MC, McGuire WL. Prognostic indicators in invasive breast cancer. Surg Clin North Am. 1990;70(5):989–1004. doi: 10.1016/s0039-6109(16)45226-6. [DOI] [PubMed] [Google Scholar]
- 4.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 5.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 6.Donnellan R, Chetty R. Cyclin D1 and human neoplasia. Mol Pathol. 1998;51(1):1–7. doi: 10.1136/mp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 8.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 9.Lin DI, Barbash O, Kumar KGS, Weber JD, Harper JW, Klein-Szanto AJP, Rustgi A, Fuchs SY, Diehl JA. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF (FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 11.Betticher DC, Thatcher N, Altermatt HJ, Hoban P, Ryder WD, Heighway J. Alternate splicing produces a novel cyclin D1 transcript. Oncogene. 1995;11:1005–1011. [PubMed] [Google Scholar]
- 12.Solomon DA, Wang Y, Fox SR, Lambeck TC, Giesting S, Lan Z, Senderowicz AM, Knudsen ES. Cyclin D1 splice variants. Differential effects on localization, RB phosphorylation, and cellular transformation. J Biol Chem. 2003;278:30339–30347. doi: 10.1074/jbc.M303969200. [DOI] [PubMed] [Google Scholar]
- 13.Lu F, Gladden AB, Diehl JA. An alternatively spliced cyclin D1 isoform, cyclin D1b, is a nuclear oncogene. Cancer Res. 2003;63:7056–7061. [PubMed] [Google Scholar]
- 14.Krieger S, Gauduchon J, Roussel M, Troussard X, Sola B. Relevance of cyclin D1b expression and CCND1 polymorphism in the pathogenesis of multiple myeloma and mantle cell lymphoma. BMC Cancer. 2006;6:238. doi: 10.1186/1471-2407-6-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntosh GG, Anderson JJ, Milton I, Steward M, Parr AH, Thomas MD, Henry JA, Angus B, Lennard TW, Horne CH. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene. 1995;11:885–891. [PubMed] [Google Scholar]
- 16.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 17.Weinstat-Saslow D, Merino MJ, Manrow RE, Lawrence JA, Bluth RF, Wittenbel KD, Simpson JF, Page DL, Steeg PS. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med. 1995;1:1257–1260. doi: 10.1038/nm1295-1257. [DOI] [PubMed] [Google Scholar]
- 18.Wani G, Noyes I, Milo GE, D’Ambrosio SM. Expression of molecular biomarkers in primary breast tumors implanted into a surrogate host: increased levels of cyclins correlate with tumor progression. Mol Med. 1997;3:273–283. [PMC free article] [PubMed] [Google Scholar]
- 19.Bartkova J, Lukas J, Müller H, Lützhøft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- 20.Lin DI, Lessie MD, Gladden AB, Bassing CH, Wagner KU, Diehl JA. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene. 2008;27:1231–1242. doi: 10.1038/sj.onc.1210738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 22.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Dean JL, Millar EDA, Tran TH, McNeil CM, Burd CJ, Henshall SM, Utama FE, Witkiewicz A, Rui H, Sutherland RL, Knudsen KE, Knudsen ES. Cyclin D1b is aberrantly regulated in response to therapeutic challenge and promotes resistance to estrogen antagonists. Cancer Res. 2008;68:5628–5638. doi: 10.1158/0008-5472.CAN-07-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar EKA, Dean JL, McNeil CM, O’Toole SA, Henshall SM, Tran T, Lin J, Quong A, Comstock CES, Witkiewicz A, Musgrove EA, Rui H, LeMarchand L, Setiawan VW, Haiman CA, Knudsen KE, Sutherland RL, Knudsen ES. Cyclin D1b protein expression in breast cancer is independent of cyclin D1a and associated with poor disease outcome. Oncogene. 2009;28:1813–1820. doi: 10.1038/onc.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]