Abstract
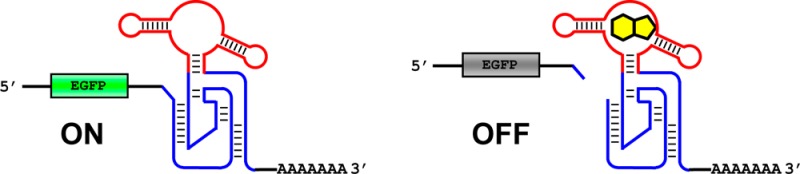
We engineered small molecule responsive allosteric ribozymes based on the genomic hepatitis delta virus (HDV) ribozyme by replacing the P4-L4 stem-loop with an RNA aptamer through a connector stem. When embedded in the 3′ untranslated region of a reporter gene mRNA, these RNA devices enabled regulation of cis-gene expression by theophylline and guanine by up to 29.5-fold in mammalian cell culture. Furthermore, a NOR logic gate device was constructed by placing two engineered ribozymes in tandem, demonstrating the modularity of the RNA devices. The significant improvement in the regulatory dynamic range (ON/OFF ratio) of the RNA devices based on the HDV ribozyme should provide new opportunities for practical applications.
Keywords: ribozyme, HDV ribozyme, riboswitch, aptazyme, logic gate
RNA devices designed to regulate gene expression in response to various chemical stimuli are a promising class of genetic devices for interfacing synthetic circuits with practical chemical information.1−5 A widely adopted strategy to engineer RNA gene regulatory devices exploits a self-cleaving ribozyme fused to an RNA aptamer (i.e., aptazyme) that serves as a chemical sensor. With a suitable connection between the ribozyme and the aptamer, the ribozyme activity can be allosterically regulated by the aptamer ligand. These aptazymes can be integrated into various genetic and biological contexts to facilitate chemical gene regulation in Escherichia coli,6,7 yeast,8,9 and mammalian cells.10−14
However, the majority of the aptazymes that have been used to control gene expression in living cells have focused on a single class of self-cleaving ribozyme, namely, the hammerhead ribozyme. While the hammerhead ribozymes have been successfully exploited in many RNA devices, the regulatory dynamic ranges of these devices are often rather modest with up to 5- to 6-fold maximum change in gene expression in response to the ligand.
Hepatitis delta virus (HDV) ribozymes are an alternative class of self-cleaving ribozymes.15 HDV ribozymes possess some distinct characteristics that may complement the widely used hammerhead ribozymes in engineering RNA devices for synthetic biology applications. For example, the HDV ribozyme structure has been found to be exceptionally stable, with in vitro activity reported in the presence of 5 M urea or 50% formamide or at 80 °C.16−18 Although analogues of the HDV ribozyme have been recently discovered in diverse organisms,15 the mammalian origin of the ribozyme provides confidence that the HDV ribozyme derivatives would be functional in mammalian cells.
Despite its long history of investigation, HDV ribozymes have not been extensively exploited for engineering applications except for trans-acting HDV ribozymes for targeted gene knockdown.19 To our knowledge, only two allosteric HDV ribozymes characterized in vitro have been described in the literature. Kertsburg and Soukup described a theophylline-activated HDV ribozyme,20 and Beaudoin and Perreault incorporated a G-quadruplex structure that regulates HDV ribozyme activity in response to potassium ion.21
Most recently, the Perreault group demonstrated the first chemically regulated HDV ribozymes embedded in the 5′ untranslated region (UTR) of mRNA in mammalian cell culture.22 The chemical inputs used in this system, however, are 13- or 14-mer synthetic chemically modified oligonucleotides designed to hybridize with the complementary sequences strategically placed to modulate the ribozyme activity. Moreover, the dynamic ranges of gene expression (ON/OFF ratios) were modest, topping at about 2-fold.
We investigated the possibility of using HDV ribozymes as a platform for RNA devices for applications in mammalian cells. In this article, we report our engineering efforts of the first small molecule responsive HDV ribozymes that function in living cells. Our RNA devices exhibit excellent gene expression control in response to the two small molecules theophylline and guanine with ON/OFF ratios up to 29.5. Additionally, the modularity of the HDV aptazymes was highlighted by the integration of two aptazymes to construct a NOR logic gate device.
Methods
Library and Plasmid Construction
All plasmids were prepared by standard recombinant DNA techniques. Plasmids encoding the cis-acting (3′ UTR) HDV aptazymes were derived from pEGFP-N1 (Clontech). Appropriate aptazyme sequences were cloned in the 3′ UTR of the EGFP transcript. All plasmids were purified using Zyppy Plasmid Miniprep kit (Zymo Research). Nucleotide sequences of the plasmids are provided in Supporting Information.
Cell Culture and Transfection
HEK293 cells were maintained in a 5% CO2 humidified incubator at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1× antibiotic-antimycotic (Gibco). One day before transfection, HEK293 cells were trypsinized and diluted appropriately with fresh complete medium, and 2.4 × 104 cells/well (∼100 μL) were seeded onto 96-well plates. Fifty nanograms of an EGFP/SEAP-aptazyme plasmid or an appropriate control plasmid and 10 ng of pCMV-mCherry plasmid (constitutively expresses mCherry) were cotransfected using 1 μL of PolyFect reagent (QIAGEN) per well according to the manufacturer’s instruction. After 3.5 h of incubation, the medium was removed and replaced with 100 μL of fresh complete medium containing appropriate concentrations of theophylline or guanine. Guanine (Acros) was first dissolved in 100× concentrations in 0.2 M NaOH and was diluted 100-fold with the complete medium immediately before use. The cells were incubated for additional 18 h before EGFP or SEAP assay.
EGFP Assay
Cellular fluorescence was measured and normalized according to our previous report.12 Briefly, the cell culture medium was replaced with phosphate buffered saline (PBS) (150 μL per well) and incubated at 37 °C until measurement. Fluorescence intensities were measured for EGFP (484 nm excitation/510 emission/5 nm bandwidth) and mCherry (587 nm excitation/610 nm emission/10 nm bandwidth) using Safire2 microplate reader (Tecan). The raw fluorescence values were first subtracted with that of the untransfected cells (background). For each well, EGFP fluorescence was normalized by mCherry ([EGFP fluorescence]/[mCherry fluorescence]) to account for variations in transfection efficiency. The values were further normalized by the cells transfected with pEGFP-wtHDVRz(inactive)/pCMV-mCherry (= 1.0). The reported values are mean ± SD from four replicate samples.
SEAP Assay
Approximately 100 μL of the medium from each well containing the secreted SEAP was sampled and centrifuged in a 1.5 mL microcentrifuge tube. Supernatant (80 μL) from each well was transferred to a fresh tube and stored at −20 °C until SEAP assay. After removing the remaining medium, PBS (150 μL) was added to each well, and the cells were incubated at 37 °C until mCherry fluorescence was measured as described above. SEAP assay of the medium was performed using Great EscAPe SEAP Fluorescence Detection Kit (Clontech) according to the manufacturer’s instructions. The samples were diluted 16-fold before measurement after confirming that the SEAP activities fall within the linear range of the assay. Fluorescence of the SEAP-cleaved substrate was measured (360 nm excitation/449 nm emission/20 nm bandwidth) using a Safire2 microplate reader. The values were normalized by mCherry fluorescence after subtracting the background values obtained using untransfected cells.
Acknowledgments
This work was supported by National Institutes of Health (GM099748).
Supporting Information Available
Sequence information of the plasmids described and a supporting figure. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Isaacs F. J.; Dwyer D. J.; Collins J. J. (2006) RNA synthetic biology. Nat. Biotechnol. 24, 545–554. [DOI] [PubMed] [Google Scholar]
- Liang J. C.; Bloom R. J.; Smolke C. D. (2011) Engineering biological systems with synthetic RNA molecules. Mol. Cell 43, 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H.; Inoue T. (2009) Synthetic biology with RNA motifs. Int. J. Biochem. Cell Biol. 41, 398–404. [DOI] [PubMed] [Google Scholar]
- Weigand J. E.; Suess B. (2009) Aptamers and riboswitches: perspectives in biotechnology. Appl. Microbiol. Biotechnol. 85, 229–236. [DOI] [PubMed] [Google Scholar]
- Wieland M.; Fussenegger M. (2010) Ligand-dependent regulatory RNA parts for Synthetic Biology in eukaryotes. Curr. Opin. Biotechnol. 21, 760–765. [DOI] [PubMed] [Google Scholar]
- Ogawa A.; Maeda M. (2008) An artificial aptazyme-based riboswitch and its cascading system in E. coli. ChemBioChem 9, 206–209. [DOI] [PubMed] [Google Scholar]
- Wieland M.; Hartig J. S. (2008) Improved aptazyme design and in vivo screening enable riboswitching in bacteria. Angew. Chem., Int. Ed. 47, 2604–2607. [DOI] [PubMed] [Google Scholar]
- Wittmann A.; Suess B. (2011) Selection of tetracycline inducible self-cleaving ribozymes as synthetic devices for gene regulation in yeast. Mol. Biosyst. 7, 2419–2427. [DOI] [PubMed] [Google Scholar]
- Win M. N.; Smolke C. D. (2007) A modular and extensible RNA-based gene-regulatory platform for engineering cellular function. Proc. Natl. Acad. Sci. U.S.A. 104, 14283–14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausländer S.; Ketzer P.; Hartig J. S. (2010) A ligand-dependent hammerhead ribozyme switch for controlling mammalian gene expression. Mol. Biosyst. 6, 807–814. [DOI] [PubMed] [Google Scholar]
- Chen Y. Y.; Jensen M. C.; Smolke C. D. (2010) Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc. Natl. Acad. Sci. U.S.A. 107, 8531–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D.; An C. I.; Yokobayashi Y. (2009) Conditional RNA interference mediated by allosteric ribozyme. J. Am. Chem. Soc. 131, 13906–13907. [DOI] [PubMed] [Google Scholar]
- Nomura Y.; Kumar D.; Yokobayashi Y. (2012) Synthetic mammalian riboswitches based on guanine aptazyme. Chem. Commun. (Cambridge) 48, 7215–7217. [DOI] [PubMed] [Google Scholar]
- Wieland M.; Auslander D.; Fussenegger M. (2012) Engineering of ribozyme-based riboswitches for mammalian cells. Methods 56, 351–357. [DOI] [PubMed] [Google Scholar]
- Webb C. H.; Lupták A. (2011) HDV-like self-cleaving ribozymes. RNA Biol. 8, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta A. T.; Shih I.; Been M. D. (1999) Imidazole rescue of a cytosine mutation in a self-cleaving ribozyme. Science 286, 123–126. [DOI] [PubMed] [Google Scholar]
- Rosenstein S. P.; Been M. D. (1990) Self-cleavage of hepatitis delta virus genomic strand RNA is enhanced under partially denaturing conditions. Biochemistry 29, 8011–8016. [DOI] [PubMed] [Google Scholar]
- Wu H. N.; Lai M. M. (1990) RNA conformational requirements of self-cleavage of hepatitis delta virus RNA. Mol. Cell. Biol. 10, 5575–5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif-Ullah M.; Lévesque M.; Robichaud G.; Perreault J. P. (2007) Development of ribozyme-based gene-inactivations; the example of the hepatitis delta virus ribozyme. Curr. Gene Ther. 7, 205–216. [DOI] [PubMed] [Google Scholar]
- Kertsburg A.; Soukup G. A. (2002) A versatile communication module for controlling RNA folding and catalysis. Nucleic Acids Res. 30, 4599–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin J. D.; Perreault J. P. (2008) Potassium ions modulate a G-quadruplex-ribozyme’s activity. RNA 14, 1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau S. G.; Jodoin R.; Bisaillon M.; Perreault J. P. (2012) Programming a highly structured ribozyme into complex allostery using RNA oligonucleotides. ACS Chem. Biol. 7, 1802–1806. [DOI] [PubMed] [Google Scholar]
- Ruminski D. J.; Webb C. H.; Riccitelli N. J.; Lupták A. (2011) Processing and translation initiation of non-long terminal repeat retrotransposons by hepatitis delta virus (HDV)-like self-cleaving ribozymes. J. Biol. Chem. 286, 41286–41295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. K. R.; Suh Y. A.; Miyashiro H.; Nishikawa F.; Kawakami J.; Taira K.; Nishikawa S. (1992) Random mutations to evaluate the role of bases at two important single-stranded regions of genomic HDV ribozyme. Nucleic Acids Res. 20, 3919–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenison R. D.; Gill S. C.; Pardi A.; Polisky B. (1994) High-resolution molecular discrimination by RNA. Science 263, 1425–1429. [DOI] [PubMed] [Google Scholar]
- Mandal M.; Boese B.; Barrick J. E.; Winkler W. C.; Breaker R. R. (2003) Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113, 577–586. [DOI] [PubMed] [Google Scholar]
- Zuker M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win M. N.; Smolke C. D. (2008) Higher-order cellular information processing with synthetic RNA devices. Science 322, 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketzer P.; Haas S. F.; Engelhardt S.; Hartig J. S.; Nettelbeck D. M. (2012) Synthetic riboswitches for external regulation of genes transferred by replication-deficient and oncolytic adenoviruses. Nucleic Acids Res. 40, e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C. H.; Riccitelli N. J.; Ruminski D. J.; Lupták A. (2009) Widespread occurrence of self-cleaving ribozymes. Science 326, 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


