Abstract
Context:
Aging changes the biology, healing capacity, and biomechanical function of tendons and ligaments and results in common clinical pathologies that present to orthopedic surgeons, primary care physicians, physical therapists, and athletic trainers. A better understanding of the age-related changes in these connective tissues will allow better patient care.
Evidence Acquisition:
The PubMed database was searched in December 2012 for English-language articles pertaining to age-related changes in tendons and ligaments.
Level of Evidence:
Level 5.
Results:
The mature athlete faces challenges associated with age-dependent changes in the rotator cuff, Achilles tendon, lateral humeral epicondylar tendons, quadriceps tendon, and patellar tendon. The anterior cruciate ligament and the medial collateral ligament are the most studied intra-articular and extra-articular ligaments, and both are associated with age-dependent changes.
Conclusion:
Tendons and ligaments are highly arranged connective tissue structures that maintain joint motion and joint stability. These structures are subject to vascular and compositional changes with increasing age that alter their mechanotransduction, biology, healing capacity, and biomechanical function. Emerging research into the etiology of age-dependent changes will provide further information to help combat the age-related clinical complications associated with the injuries that occur to tendons and ligaments.
Keywords: tendon, ligament, age-related, biomechanics, rotator cuff, Achilles tendon, ACL
Tendon
Tendon Structure
Tendons are dense, regularly arranged connective tissues that attach muscle to bone and produce joint motion by transferring force from muscle to bone. Tendons are composed primarily of type I collagen arranged in parallel fibrils with the remaining 20% to 30% of dry weight composed of proteoglycans, glycosaminoglycans, other collagens (type III, V, XII, and others), and elastin.61,108,118 These minor constituents, such as type V collagen and decorin, help regulate fibrillogenesis.20,86,114,115 Tendon structure is highly regular with collagen forming triple helices (approximately 300 nm in length and 1.5 nm in diameter), which pack together to form microfibrils,45 which interdigitate to form fibrils (50 to 200 nm in diameter), which coalesce to form fibers (3 to 7 µm in diameter), which combine to form fascicles, which are bundled together to form a tendon (mm or cm in diameter).57 The mechanical properties of tendon come from its highly oriented structure. It is able to resist tensile stress in the direction of its fiber orientation because of the collagen structure and it is able to resist some compressive stress because of its proteoglycan content.
Tendons have different mechanical properties dependent on anatomic location, exercise, immobilization, and age of the tendon. Material and structural properties of the tendon increase from birth through maturity and then decrease from maturity through old age. Tendon injuries correlate positively with patient age, but the cellular changes in the tendon associated with age are somewhat less clear. Some of the more commonly studied tendons are rotator cuff, Achilles, lateral humeral epicondylar, quadriceps, and patellar tendons because as people age, these areas become clinically problematic.
Vascular Supply
Tendons are metabolically active and are provided with a rich vascular supply during development.82 Tendons do not undergo neovascularization under normal circumstances, but during pathologic processes, changes in vascularity may take place. Tendons receive vascular supply through the musculotendinous junction, the osseotendinous junction, and the vessels from the various surrounding tissues including the paratenon, mesotenon, and vincula.2,24,27,102 Tendons in different areas of the body receive different amounts of blood supply. The vascular supply of the specific tendon also relates to whether or not it is a sheathed tendon. If the tendon is sheathed, such as the digital flexor tendons, it receives blood supply from the mesotenon, vincula, and diffusion from vascularized surrounding segments. Tendons that are not sheathed are covered with a paratenon and have the advantage of a local extrinsic vascular supply with branches forming an intratendinous vascular network with multiple anastamoses. Tendon vascularity can be compromised at junctional zones and at sites of friction, torsion, or compression.
The vascular supply to the rotator cuff tendons is a 6-artery supply.27 However, it is not uniform to each tendon, with the supraspinatus having relatively reduced vascularity.85 The region of relative avascularity in the supraspinatus, called “Codman’s critical zone,” was described by Codman and Akerson in 1931.32 This area is actually hypovascular as the vascularity increases when the compression applied by the humeral head is removed.69
In addition to the supraspinatus having reduced tendon vascularity, the biceps,85 Achilles,95,99 patella,31 and posterior tibial tendon37 have areas of reduced vascularity. The Achilles tendon receives blood supply from the musculotendinous junction, the osseotendinous junction, and the paratenon, with the posterior tibial artery supplying the major contribution. However, histological analyses proved that the Achilles tendon has a poor vascular supply through its length, as shown by the low number of blood vessels per cross-sectional area.2 The Achilles tendon has a hypovascular zone approximately 2 to 7 cm proximal to its bony insertion, with this area at the highest risk of rupture and surgical complications.29
Biology of Tendon Aging
Healthy tendon relies on a normal vascular supply and efficient mechanotransduction with cells that are capable of responding to mechanical cues with biochemical signals to maintain tendon development, homeostasis, healing, and degeneration.12,111
Changes in Vascular Supply
Astrom and Rausing14 noted that patients with Achilles tendinopathy demonstrated hypervascularity of the tendon with unevenly distributed thick-walled vessels as compared with healthy controls. A recent ultrasound study evaluating the volume of neovascularity in tendinopathic Achilles tendons revealed that 97.3% of the tendons had evidence of neovascularization and 55.6% of the tendons had neovascularization at the location of the tendon thickening.122
The origin of rotator cuff disease is controversial, with tendon ischemia, extrinsic compression, and chronic repetitive microtrauma having been cited as factors. There are both extrinsic as well as intrinsic reasons for tendon failure and age-related degeneration. A recent in vivo study evaluating the vascularity of rotator cuff tears using ultrasound showed that there was a significant decrease in blood flow in the intratendinous region in elderly subjects compared with younger subjects but no differences in the bursal blood flow suggesting an age-related decrease in intratendinous vascularity.39 Rudzki et al93 corroborated those results by finding a significant decrease in blood flow in the supraspinatus tendon in patients older than age 40 years compared with younger patients after exercise (Figure 1). Several studies have hypothesized that tendon vascularity is compromised at the articular surface of the distal aspect of the supraspinatus tendon.25,70,85,92 Adler et al1 reported an in vivo ultrasound study demonstrating a consistent region of decreased vascularity at the articular medial margin of the rotator cuff with significantly less flow compared with the bursal side. This study also suggested a trend toward decreased blood flow with increasing patient age.1
Figure 1.
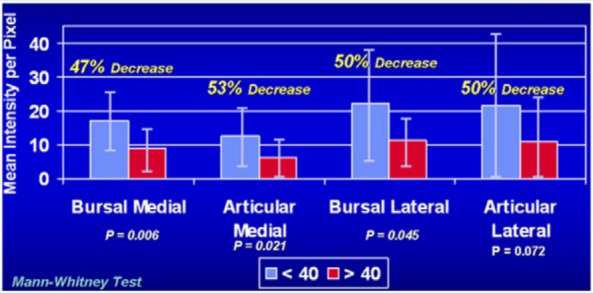
Intratendinous vascularity by age. Comparative analysis of intratendinous supraspinatus vascularity in patients younger than and older than 40 years old. Error bars show the standard deviation. Reprinted with permission from Rudzki et al.93
Vascular changes also play a role in the pathogenesis of patellar tendinitis. In a study of chronic patellar tendinitis, there was capillary proliferation and prominent angiogenesis in the degenerated region of tendon.58,124 The paratenon surrounding the patellar tendon can also be a site of chronic pain with marked neovascularization and degenerative vascular changes,16,63 with hypervascular changes resulting in abnormal blood flow and ischemic pain during exercise.63 An ultrasound study on the neovascularization of the patellar tendon in symptomatic elite athletes with patellar tendinitis noted that 60% had neovascularization.47 A recent study on patellar and Achilles tendons of elite badminton players showed that intratendinous vascularity tended to increase with strenuous activity, but it was only significantly increased in the dominant leg after repetitive loading.21
Bales et al17 performed a microvascular anatomic study on the lateral epicondyle of the humerus and found 2 hypovascular zones. The first was at the proximal lateral epicondyle just distal to the supracondylar ridge and the second was distal to the lateral epicondyle on the deep surface of the common extensor tendon. The presence of these hypovascular zones may preclude the normal inflammatory cascade and healing response to microtearing in this region of tendon.17 Thus the common sites of clinical tendon degeneration with age show significantly altered tendon vascularity occurs with age and activity.
Changes in Mechanobiologic Environment
Tendon tissue homeostasis is based on the ability of the tendon cells to sense and respond to mechanical load through mechanotransduction.19,48,94,113 The exact level of mechanical and biological stimulation required to maintain normal tendon homeostasis is not currently known, but it is widely believed that an abnormal level of stimulation (underload or overload) may play a role in the pathogenesis of tendinopathy.8,10,52 Archambault et al6 proposed an algorithm for the onset of overuse tendinopathy in response to repetitive loading. Repetitive strains below injury threshold resulted in degenerative changes in the tendon-matrix composition and organization, which led to transient weakness of the tissue making it more susceptible to continued load. Over time, the damage continued until tendinopathy developed.6 Cyclic strain is beneficial to tendon health, but repetitive strain may result in overuse tendon injuries.6,98
Based on the theory that excessive loading of tendons during vigorous physical activity is the main stimulation for degeneration of the extracellular tendon matrix, several studies have looked at in vitro analyses of strain patterns and extrinsic factors that induce tendinopathy. Overstimulation in vitro of tendon cells increases inflammatory cytokines and degenerative enzymes.4,5,18,19,105,112 The in situ environmental conditions in these studies are in a monolayer cell culture and may not replicate the 3-dimensional collagenous matrix found in vivo. In addition, the high strain magnitudes and durations may provide an artificially enhanced cellular response to the repetitive loading stimulus, suggesting that these in situ conditions may not be clinically relevant. Further study is necessary under clinical conditions to evaluate the theory of repetitive loading resulting in overuse.
An increase in the degradative enzyme production in aging tendons or tendons unable to maintain homeostasis has been postulated in several biochemical studies. Fu et al38 showed that matrix metalloproteinase-1 (MMP-1) was increased in human patellar tendinosis tissue, and Riley et al88 showed that MMP-1 levels were high in ruptured tendons compared with normal tendons. Tendinosis may result from increased MMP production as the pathology associated with tendinosis results in irregular orientation of collagen, fiber disruption, changes in fiber diameter, decrease in density of collagen, and an upregulation of collagen type III production.50,53,55 The increase in MMP production has been associated with significant reductions in the tensile modulus and tensile strength of tendons.65 In addition, MMP inhibitors have been shown in vitro to prevent the decrease in mechanical properties of stress-deprived tendons.9
In addition to the increase in MMP production, other studies have suggested a role for increased apoptosis in clinical cases of tendinopathy.106,125,126 In degenerative supraspinatus tendons compared with normal controls, there was a significant increase in the number of apoptotic cells.106,125 Egerbacher et al33 reported an increase in the number of apoptotic cells in the stress-deprived rat tail tendon model.
Thus, as the tendon ages, it is subjected to more mechanical load and the sequela of that repetitive use may result in an increase in degradative enzymes, apoptosis, and resulting clinical tendinopathy or tendon rupture. Some authors, however, have proposed an alternative theory to tendon over-stimulation as the etiology of tendon degeneration. Arnoczky et al7 proposed that understimulation may be a cause of tendinopathy as well. In tendons that have undergone an injury from a mechanical load, there are resulting damaged collagen fibers. These tendons are then understimulated because of the release of cellular tension on the remainder of that tendon structure. This understimulation may then induce apoptosis. Understimulation of tendon cells can produce a histological picture consistent with tendinopathy.41 In an in situ rat tail tendon model, Arnoczky and colleagues showed that the alteration in cell-matrix interactions secondary to isolated tendon fibrillar damage could result in mechanobiological understimulation of tendon cells thereby resulting in an upregulation of collagenase mRNA expression and protein synthesis.12,65-67 This results in an initial degeneration of the pericellular matrix, a decrease in the material properties of the tendon, risk of further damage or rupture with subsequent mechanical loading, and clinical and histological signs of tendinopathy eventually.
Alteration in Tenocyte Biochemistry and Failure of Healing Response
Ippolito et al49 showed that with aging, rabbit tendon tissue extracellular matrix volume increases and the relative number of cells per unit of tendon decreases. The tenocytes also become longer and thinner and have decreased protein synthesis, and the collagen fibers become more disoriented with more variations in thickness due to an increase in collagen, a decrease in mucopolysaccharies, and a decrease in water content.49 Riley et al89 showed a significant decrease in total glycomaminoglycan, chondroitin sulphate, and dermatan sulphate with age in the supraspinatus tendon.
Tenocyte biology has been a particularly exciting topic of research for tendon healing and whether age has an effect on the ability of tenocytes to repair the surrounding tissue. Gerber et al40 and Rodeo et al90 demonstrated in animal studies that tendon to bone healing is a complex process that forms biomechanically inferior scar tissue rather than regenerated native tendon to bone attachments. Several studies on rotator cuff healing have noted that patient age is associated with increased healing complications.22,78,97,121 Klatte-Schulz et al60 showed that tenocyte-like cells from aged donors compared with younger donors showed a decreased cell growth and stem cell potential including potential for self-renewal and osteogenic differentiation, but no differences in cell density. This suggests a slower metabolic rate for aged tenocyte-like cells and thus, possibly, a weaker tendon to bone healing response. Both aged and younger donor tenocyte-like cells can be stimulated with BMP-2 and BMP-7.60 There is significantly increased cell activity, cell proliferation, and collagen type I synthesis following BMP-7 treatment in in vivo tendon studies.104,120,123 Several in vivo studies have also shown improved tendon to bone healing and higher biomechanical strength following treatment with BMP-2 and BMP-7.44,72,74,77,91 Importantly, Klatte-Schulz et al60 showed no differences in decorin production based on age, which is an important factor given that decorin reduces scar formation and may improve the biomechanical properties of tendons.51
The histopathology associated with degeneration of rotator cuff tendons and lateral epicondyle tendons includes blood vessel wall changes, tenocyte loss, calcification, glycosaminoglycan infiltration, and fibrocartilaginous transformation.28 These changes were variably and mildly present in younger patients (less than 39 years old) with only 17% of cadaveric tendons having these changes, but the abnormalities occurred in 40% to 50% of patients older than 40 years of age.28
Biomechanics of Tendon Aging
Tendon microarchitecture is disrupted with tendinopathy.13,71 Specimens taken from torn tendons show disorientation of collagen fibers, thinning of the fibers, myxoid degeneration, chondroid metaplasia, calcification, and vascular infiltration.43 Degeneration of tendons significantly reduces the tensile modulus and tensile strength of tendons.65 However, it is unclear whether normal aging is always synonymous with changes in the biomechanical properties of tendons. Plate et al83 demonstrated in rat Achilles tendons that the passive biomechanical properties of the muscle-tendon unit were altered by normal aging with a decreased relaxation response and increased stiffness in the middle-aged tendons as compared with the younger tendons.
Aging is associated with a decrease in muscle mass and muscle fiber cross-sectional area, which in combination with the structural changes in tendon aging such as collagen disorganization and decreased collagen content, can alter the biomechanical response of tendon tissue.87 The current literature is not consistent, however, with Kubo et al62 showing decreased Achilles tendon strain in older compared with younger patients, Onambele et al81 showing increased strain, and Karamanidis and Arampatzis56 showing no strain differences. Mouse tibialis anterior tendon modulus increased with age but was independent of changes in collagen fibril morphology or force- generating capacity of muscle.119 Zhou et al128 further showed that tendon self-renewal and differentiation capacity decreased with age by showing that progenitor stem cells, while present in both the young and old tendons, are reduced by 70% in stem cell number, have a lower cell proliferation, and have delayed cell cycle progression in older tendons.
Clinical Implications
Tendinopathy is a common clinical problem in patients, particularly with increasing age. The most common clinical tendon problems for the aging population are in the rotator cuff, Achilles, lateral elbow epicondyle, quadriceps, and patellar tendon. Yamaguchi et al121 found in his landmark ultrasound study on symptomatic and asymptomatic rotator cuff tears that there was a high correlation between the onset of rotator cuff tears (either partial or full thickness) and increasing age. In a group of patients with shoulder pain evaluated prior to surgical intervention, patients age 65 years and older had a full-thickness rotator cuff tear prevalence of 22%.34 In addition, for each 10-year age increase, the odds of a rotator cuff tear increased 2.69-fold (P = 0.005).34 Patients who are more than 60 years old and are exposed to prolonged quinolone antibiotics are at increased risk of Achilles tendonitis and tendon rupture.116
Ligament
Ligament Structure
Ligaments connect bone to bone and thus stabilize, guide, and restrict joint motions.3,26,36,46,54 Like tendons, ligaments function to resist tensile load.46 Ligaments are composed of collagen type I (70% dry weight), elastin fibers, proteoglycans, and other minor collagens.23 Collagen fibrils within each collagen fiber vary in size from 60 nm to 4000 nm in diameter.35 The collagen fibers transfer the force within the ligaments.64,84 The multiple collagen fiber bundles are interdigitated and function together to maintain normal joint motion.
Ligaments can be classified either as intra-articular or extra-articular. A majority of the research performed on ligaments has been on the anterior cruciate ligament (ACL), which is an intra-articular ligament. Mesenchymal stem cells have been found within the ACL.96,100 The number of stem cells within the ligament decreases with age.101 Stem cells have been found in both the ACL and the medial collateral ligament (MCL) of the knee, which is an extra-articular ligament. Zhang et al127 found that the stem cells found in the ACL are intrinsically different from those found in the MCL, which may help explain why injuries to the MCL are commonly treated conservatively while injuries to the ACL require operative reconstruction to restore function. This concept of conservative management for extra-articular ligaments and operative reconstruction for intra-articular ligaments is related to the healing potential for each of the types of ligaments.
Vascular Supply
The microvascular circulation of the ACL and posterior cruciate ligament (PCL), intra-articular ligaments, is primarily from the infrapatellar fat pad and the synovial membrane, which form a vascular envelope with the vascular supply to the PCL greater than that of the ACL.11 The ACL has a relatively hypovascular segment in the central portion, which is common in intra-articular ligaments.11 The ACL has been shown to contain a population of vascular-derived stem cells that may contribute to ligament regeneration and repair at the site of rupture.76 In contrast to the ACL, the MCL is a relatively well-vascularized ligament, with high magnification histology revealing numerous capillaries in the substance of the MCL while there were none in the ACL.109
Biology of Ligament Aging
The ACL is subject to degeneration based on increasing age. Hasegawa et al42 reported on the pattern of spontaneous age-related changes in the ACL in a histologic cadaveric study; ACL substance scores and ligament sheath inflammation scores increased with age. Collagen fiber disorientation was the most prevalent change that occurred earliest. Cadaveric human knee joints were evaluated histologically with special emphasis on the ACL, PCL, and cartilage.68 The most significant histologic change was fiber disorientation, with only 6% of the intra-articular ligaments classified as normal and 76% showing mild degeneration.68 There was a correlation between age and total histologic PCL scores and an even stronger correlation between age and total histologic ACL scores.68 ACL cell metabolism has been previously studied; cell proliferation and migration are higher in skeletally immature animals75 and an improved biomechanical response to healing was found in skeletally immature animals79 possibly due to a decrease in growth factor receptor number with age.107 In addition, with ACL cell maturity decreases in metabolic activity, collagen production and response to platelet-rich plasma occur along with an increase in apoptosis.30
Wang et al110 studied the age-dependent changes in the matrix and organization of the ligament to bone insertion and found that there were age-dependent structural and compositional changes at the insertion site, with the skeletally immature group resembling articular cartilage while the adult interface resembled fibrocartilaginous tissue. There were marked differences in collagen fiber orientation that became more pronounced with age. The extracellular matrix composition and cellularity were also found to be age-dependent.110 Normal aging results in decreased numbers and altered morphology of mechanoreceptors in the ACL, which correlates positively with the deficits in proprioception associated with aging.15 Interestingly, the sulfur content in the ACL decreases gradually with aging whereas the content of calcium, phosphorus, and magnesium increased with aging.103
Biomechanics of Ligament Aging
Ligament biomechanics are also age-dependent. Murray et al79 evaluated the biomechanical outcomes of ACL healing in skeletally immature and mature minipigs and found that immature animals healed the ligament better than mature animals. In addition, they found that the structural properties of the skeletally immature ligament were significantly better than those of the mature animal.79 Woo et al117 evaluated the structural properties of the femur-ACL-tibia complex in younger (22-35 years), middle aged (40-50 years), and older (60-97 years) knees and found that linear stiffness, ultimate load, and energy absorbed decreased significantly with specimen age. This correlates well with the original data from Noyes and Grood,80 who found a decreased linear stiffness and ultimate load in the ACL with age.
Clinical Implications
ACL tears are a common problem in active patients, including both younger and older cohorts. In a recent study of second-look arthroscopy on double-bundle ACL reconstructions, synovial coverage was significantly decreased in elderly patients (50 years and older) as compared with either of the younger cohorts (29 years and younger; 30 to 49 years).59 This alteration in synovial coverage was not reflected in clinical outcomes, which were not different between the age groups.59 In addition, in a study evaluating the use of hamstring autograft, no difference in clinical outcome was found when comparing patients greater than 40 years old and a younger population.73
Conclusion
Tendons and ligaments are regularly arranged connective tissues with extremely important functions in the maintenance of joint stability and joint motion. With increasing age, these tissues are subject to vascular and compositional changes that alter their mechanotransduction, biology, healing capacity, and biomechanical function. Emerging theories, such as understimulation changing the mechanotransduction properties of the remaining tissue, will provide further information to help combat the age-related clinical complications associated with the injuries that occur to tendons and ligaments.
Footnotes
The authors declared no potential conflicts of interest in the development and publication of this manuscript.
References
- 1. Adler RS, Fealy S, Rudzki JR, et al. Rotator cuff in asymptomatic volunteers: contrast-enhanced US depiction of intratendinous and peritendinous vascularity. Radiology. 2008;248:954-961 [DOI] [PubMed] [Google Scholar]
- 2. Ahmed IM, Lagopoulos M, McConnell P, et al. Blood supply of the Achilles tendon. J Orthop Res. 1998;16:591-596 [DOI] [PubMed] [Google Scholar]
- 3. Ali AF, Taha MM, Thornton GM, et al. Biomechanical study using fuzzy systems to quantify collagen fiber recruitment and predict creep of the rabbit medial collateral ligament. J Biomech Eng. 2005;127:484-493 [DOI] [PubMed] [Google Scholar]
- 4. Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med Sci Sports Exerc. 1993;25:603-607 [PubMed] [Google Scholar]
- 5. Archambault J, Tsuzaki M, Herzog W, et al. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36-39 [DOI] [PubMed] [Google Scholar]
- 6. Archambault JM, Wiley JP, Bray RC. Exercise loading of tendons and the development of overuse injuries. A review of current literature. Sports Med. 1995;20:77-89 [DOI] [PubMed] [Google Scholar]
- 7. Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnoczky SP, Lavagnino M, Egerbacher M. The response of tendon cells to changing loads: implications in the etiopathogenesis of tendinopathy. In: Woo SL, Renstrom P, Arnoczky SP, eds. Tendinopathy in Athletes: Encyclopedia of Sports Medicine. Oxford, UK: Blackwell; 2007:46-59 [Google Scholar]
- 9. Arnoczky SP, Lavagnino M, Egerbacher M, et al. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763-769 [DOI] [PubMed] [Google Scholar]
- 10. Arnoczky SP, Lavagnino M, Whallon JH, et al. In situ cell nucleus deformation in tendons under tensile load: a morphological analysis using confocal laser microscopy. J Orthop Res. 2002;20:29-35 [DOI] [PubMed] [Google Scholar]
- 11. Arnoczky SP, Rubin RM, Marshall JL. Microvasculature of the cruciate ligaments and its response to injury. An experimental study in dogs. J Bone Joint Surg Am. 1979;61:1221-1229 [PubMed] [Google Scholar]
- 12. Arnoczky SP, Tian T, Lavagnino M, et al. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328-333 [DOI] [PubMed] [Google Scholar]
- 13. Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108:670-675 [DOI] [PubMed] [Google Scholar]
- 14. Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;(316):151-164 [PubMed] [Google Scholar]
- 15. Aydog ST, Korkusuz P, Doral MN, et al. Decrease in the numbers of mechanoreceptors in rabbit ACL: the effects of ageing. Knee Surg Sports Traumatol Arthrosc. 2006;14:325-329 [DOI] [PubMed] [Google Scholar]
- 16. Backman C, Friden J, Widmark A. Blood flow in chronic Achilles tendinosis. Radioactive microsphere study in rabbits. Acta Orthop Scand. 1991;62:386-387 [DOI] [PubMed] [Google Scholar]
- 17. Bales CP, Placzek JD, Malone KJ, et al. Microvascular supply of the lateral epicondyle and common extensor origin. J Shoulder Elbow Surg. 2007;16:497-501 [DOI] [PubMed] [Google Scholar]
- 18. Banes AJ, Horesovsky G, Larson C, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis Cartilage. 1999;7:141-153 [DOI] [PubMed] [Google Scholar]
- 19. Banes AJ, Tsuzaki M, Hu P, et al. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J Biomech. 1995;28:1505-1513 [DOI] [PubMed] [Google Scholar]
- 20. Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228-244 [DOI] [PubMed] [Google Scholar]
- 21. Boesen AP, Boesen MI, Koenig MJ, et al. Evidence of accumulated stress in Achilles and anterior knee tendons in elite badminton players. Knee Surg Sports Traumatol Arthrosc. 2011;19:30-37 [DOI] [PubMed] [Google Scholar]
- 22. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240 [DOI] [PubMed] [Google Scholar]
- 23. Bray DF, Frank CB, Bray RC. Cytochemical evidence for a proteoglycan-associated filamentous network in ligament extracellular matrix. J Orthop Res. 1990;8:1-12 [DOI] [PubMed] [Google Scholar]
- 24. Brockis JG. The blood supply of the flexor and extensor tendons of the fingers in man. J Bone Joint Surg Br. 1953;35-B:131-138 [DOI] [PubMed] [Google Scholar]
- 25. Brooks CH, Revell WJ, Heatley FW. A quantitative histological study of the vascularity of the rotator cuff tendon. J Bone Joint Surg Br. 1992;74:151-153 [DOI] [PubMed] [Google Scholar]
- 26. Butler DL, Kay MD, Stouffer DC. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech. 1986;19:425-432 [DOI] [PubMed] [Google Scholar]
- 27. Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10:807-822 [PubMed] [Google Scholar]
- 28. Chard MD, Cawston TE, Riley GP, et al. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994;53:30-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen TM, Rozen WM, Pan WR, et al. The arterial anatomy of the Achilles tendon: anatomical study and clinical implications. Clin Anat. 2009;22:377-385 [DOI] [PubMed] [Google Scholar]
- 30. Cheng M, Johnson VM, Murray MM. Effects of age and platelet-rich plasma on ACL cell viability and collagen gene expression. J Orthop Res. 2012;30:79-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clancy WG, Jr, Narechania RG, Rosenberg TD, et al. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63:1270-1284 [PubMed] [Google Scholar]
- 32. Codman EA, Akerson IB. The pathology associated with rupture of the supraspinatus tendon. Ann Surg. 1931;93:348-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egerbacher M, Arnoczky SP, Caballero O, et al. Stress-deprivation induces apoptosis in tendon cells: a mechanistic explanation for the histologic changes seen in tendinopathy. Trans Orthop Res Soc. 2007;(32):862 [Google Scholar]
- 34. Fehringer EV, Sun J, VanOeveren LS, et al. Full-thickness rotator cuff tear prevalence and correlation with function and co-morbidities in patients sixty-five years and older. J Shoulder Elbow Surg. 2008;17:881-885 [DOI] [PubMed] [Google Scholar]
- 35. Frank C, Bray D, Rademaker A, et al. Electron microscopic quantification of collagen fibril diameters in the rabbit medial collateral ligament: a baseline for comparison. Connect Tissue Res. 1989;19:11-25 [DOI] [PubMed] [Google Scholar]
- 36. Frank CB, Hart DA, Shrive NG. Molecular biology and biomechanics of normal and healing ligaments—a review. Osteoarthritis Cartilage. 1999;7:130-140 [DOI] [PubMed] [Google Scholar]
- 37. Frey C, Shereff M, Greenidge N. Vascularity of the posterior tibial tendon. J Bone Joint Surg Am. 1990;72:884-888 [PubMed] [Google Scholar]
- 38. Fu SC, Chan BP, Wang W, et al. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73:658-662 [DOI] [PubMed] [Google Scholar]
- 39. Funakoshi T, Iwasaki N, Kamishima T, et al. In vivo visualization of vascular patterns of rotator cuff tears using contrast-enhanced ultrasound. Am J Sports Med. 2010;38:2464-2471 [DOI] [PubMed] [Google Scholar]
- 40. Gerber C, Schneeberger AG, Perren SM, et al. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81:1281-1290 [DOI] [PubMed] [Google Scholar]
- 41. Hannafin JA, Arnoczky SP, Hoonjan A, et al. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907-914 [DOI] [PubMed] [Google Scholar]
- 42. Hasegawa A, Otsuki S, Pauli C, et al. Anterior cruciate ligament changes in the human knee joint in aging and osteoarthritis. Arthritis Rheum. 2012;64:696-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;(415):111-120 [DOI] [PubMed] [Google Scholar]
- 44. Higuera CA, Inoue N, Lim JS, et al. Tendon reattachment to a metallic implant using an allogenic bone plate augmented with rhOP-1 vs. autogenous cancellous bone and marrow in a canine model. J Orthop Res. 2005;23:1091-1099 [DOI] [PubMed] [Google Scholar]
- 45. Hodge AJ, Petruska JA, Ramachandran GN, eds. Aspects of protein structure. New York, NY: Academic Press; 1963 [Google Scholar]
- 46. Hoffmann A, Gross G. Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop. 2007;31:791-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoksrud A, Ohberg L, Alfredson H, et al. Color Doppler ultrasound findings in patellar tendinopathy (jumper’s knee). Am J Sports Med. 2008;36:1813-1820 [DOI] [PubMed] [Google Scholar]
- 48. Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575-599 [DOI] [PubMed] [Google Scholar]
- 49. Ippolito E, Natali PG, Postacchini F, et al. Morphological, immunochemical, and biochemical study of rabbit Achilles tendon at various ages. J Bone Joint Surg Am. 1980;62:583-598 [PubMed] [Google Scholar]
- 50. Jarvinen M, Jozsa L, Kannus P, et al. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86-95 [DOI] [PubMed] [Google Scholar]
- 51. Jarvinen TA, Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci USA. 2010;107:21671-21676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jozsa L, Kannus P. Human Tendons: Anatomy, Physiology, and Pathology. Champaign, IL: Human Kinetics; 1997 [Google Scholar]
- 53. Jozsa L, Reffy A, Kannus P, et al. Pathological alterations in human tendons. Arch Orthop Trauma Surg. 1990;110:15-21 [DOI] [PubMed] [Google Scholar]
- 54. Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312-320 [DOI] [PubMed] [Google Scholar]
- 55. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507-1525 [PubMed] [Google Scholar]
- 56. Karamanidis K, Arampatzis A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech. 2006;39:406-417 [DOI] [PubMed] [Google Scholar]
- 57. Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11-23 [DOI] [PubMed] [Google Scholar]
- 58. Khan KM, Bonar F, Desmond PM, et al. Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology. 1996;200:821-827 [DOI] [PubMed] [Google Scholar]
- 59. Kinugasa K, Mae T, Matsumoto N, et al. Effect of patient age on morphology of anterior cruciate ligament grafts at second-look arthroscopy. Arthroscopy. 2011;27:38-45 [DOI] [PubMed] [Google Scholar]
- 60. Klatte-Schulz F, Pauly S, Scheibel M, et al. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur Cell Mater. 2012;24:74-89 [DOI] [PubMed] [Google Scholar]
- 61. Koob TJ, Vogel KG. Site-related variations in glycosaminoglycan content and swelling properties of bovine flexor tendon. J Orthop Res. 1987;5:414-424 [DOI] [PubMed] [Google Scholar]
- 62. Kubo K, Morimoto M, Komuro T, et al. Age-related differences in the properties of the plantar flexor muscles and tendons. Med Sci Sports Exerc. 2007;39:541-547 [DOI] [PubMed] [Google Scholar]
- 63. Kvist M, Jozsa L, Jarvinen MJ, et al. Chronic Achilles paratenonitis in athletes: a histological and histochemical study. Pathology. 1987;19:1-11 [DOI] [PubMed] [Google Scholar]
- 64. Lanir Y. Structure-strength relations in mammalian tendon. Biophys J. 1978;24:541-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lavagnino M, Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res. 2005;23:1211-1218 [DOI] [PubMed] [Google Scholar]
- 66. Lavagnino M, Arnoczky SP, Frank K, et al. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J Biomech. 2005;38:69-75 [DOI] [PubMed] [Google Scholar]
- 67. Lavagnino M, Arnoczky SP, Tian T, et al. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res. 2003;44:181-187 [DOI] [PubMed] [Google Scholar]
- 68. Levy YD, Hasegawa A, Patil S, et al. Histopathological changes in the human posterior cruciate ligament during aging and osteoarthritis: correlations with anterior cruciate ligament and cartilage changes. Ann Rheum Dis. 2013;72:271-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ling SC, Chen CF, Wan RX. A study on the vascular supply of the supraspinatus tendon. Surg Radiol Anat. 1990;12:161-165 [DOI] [PubMed] [Google Scholar]
- 70. Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res. 1990;(254):35-38 [PubMed] [Google Scholar]
- 71. Longo UG, Franceschi F, Ruzzini L, et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36: 533-538 [DOI] [PubMed] [Google Scholar]
- 72. Ma CB, Kawamura S, Deng XH, et al. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med. 2007;35:597-604 [DOI] [PubMed] [Google Scholar]
- 73. Marquass B, Hepp P, Engel T, et al. The use of hamstrings in anterior cruciate ligament reconstruction in patients over 40 years. Arch Orthop Trauma Surg. 2007;127:835-843 [DOI] [PubMed] [Google Scholar]
- 74. Martinek V, Latterman C, Usas A, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002;84-A:1123-1131 [DOI] [PubMed] [Google Scholar]
- 75. Mastrangelo AN, Magarian EM, Palmer MP, et al. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28:644-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Matsumoto T, Ingham SM, Mifune Y, et al. Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cells Dev. 2012;21:859-872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mihelic R, Pecina M, Jelic M, et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619-1625 [DOI] [PubMed] [Google Scholar]
- 78. Milgrom C, Schaffler M, Gilbert S, et al. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77:296-298 [PubMed] [Google Scholar]
- 79. Murray MM, Magarian EM, Harrison SL, et al. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92:2039-2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am. 1976;58:1074-1082 [PubMed] [Google Scholar]
- 81. Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100:2048-2056 [DOI] [PubMed] [Google Scholar]
- 82. Peacock EE., Jr. A study of the circulation in normal tendons and healing grafts. Ann Surg. 1959;149:415-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Plate JF, Wiggins WF, Haubruck P, et al. Normal aging alters in vivo passive biomechanical response of the rat gastrocnemius-Achilles muscle-tendon unit. J Biomech. 2013;46:450-455 [DOI] [PubMed] [Google Scholar]
- 84. Provenzano PP, Vanderby R., Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71-84 [DOI] [PubMed] [Google Scholar]
- 85. Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52:540-553 [PubMed] [Google Scholar]
- 86. Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249-255 [DOI] [PubMed] [Google Scholar]
- 87. Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise in humans. Exp Physiol. 2006;91:483-498 [DOI] [PubMed] [Google Scholar]
- 88. Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185-195 [DOI] [PubMed] [Google Scholar]
- 89. Riley GP, Harrall RL, Constant CR, et al. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1994;53:367-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rodeo SA, Potter HG, Kawamura S, et al. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485-2497 [DOI] [PubMed] [Google Scholar]
- 91. Rodeo SA, Suzuki K, Deng XH, et al. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476-488 [DOI] [PubMed] [Google Scholar]
- 92. Rothman RH, Parke WW. The vascular anatomy of the rotator cuff. Clin Orthop Relat Res. 1965;41:176-186 [PubMed] [Google Scholar]
- 93. Rudzki JR, Adler RS, Warren RF, et al. Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J Shoulder Elbow Surg. 2008;17(1 suppl):96S-100S [DOI] [PubMed] [Google Scholar]
- 94. Ruoslahti E. Stretching is good for a cell. Science. 1997;276:1345-1346 [DOI] [PubMed] [Google Scholar]
- 95. Schmidt-Rohlfing B, Graf J, Schneider U, et al. The blood supply of the Achilles tendon. Int Orthop. 1992;16:29-31 [DOI] [PubMed] [Google Scholar]
- 96. Segawa Y, Muneta T, Makino H, et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435-441 [DOI] [PubMed] [Google Scholar]
- 97. Sorensen AK, Bak K, Krarup AL, et al. Acute rotator cuff tear: do we miss the early diagnosis? A prospective study showing a high incidence of rotator cuff tears after shoulder trauma. J Shoulder Elbow Surg. 2007;16:174-180 [DOI] [PubMed] [Google Scholar]
- 98. Soslowsky LJ, Thomopoulos S, Esmail A, et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057-1063 [DOI] [PubMed] [Google Scholar]
- 99. Stein V, Laprell H, Tinnemeyer S, et al. Quantitative assessment of intravascular volume of the human Achilles tendon. Acta Orthop Scand. 2000;71:60-63 [DOI] [PubMed] [Google Scholar]
- 100. Steinert AF, Kunz M, Prager P, et al. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A. 2011;17:1375-1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163-173 [DOI] [PubMed] [Google Scholar]
- 102. Takasugi H, Akahori O, Nishihara K, et al. Three-dimensional architecture of blood vessels of tendons demonstrated by corrosion casts. Hand. 1978;10:9-15 [DOI] [PubMed] [Google Scholar]
- 103. Tohno Y, Moriwake Y, Takano Y, et al. Age-related changes of elements in human anterior cruciate ligaments and ligamenta capitum femorum. Biol Trace Elem Res. 1999;68:181-192 [DOI] [PubMed] [Google Scholar]
- 104. Tsai AD, Yeh LC, Lee JC. Effects of osteogenic protein-1 (OP-1, BMP-7) on gene expression in cultured medial collateral ligament cells. J Cell Biochem. 2003;90:777-791 [DOI] [PubMed] [Google Scholar]
- 105. Tsuzaki M, Bynum D, Almekinders L, et al. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89:556-562 [DOI] [PubMed] [Google Scholar]
- 106. Tuoheti Y, Itoi E, Pradhan RL, et al. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005;14:535-541 [DOI] [PubMed] [Google Scholar]
- 107. Vavken P, Saad FA, Murray MM. Age dependence of expression of growth factor receptors in porcine ACL fibroblasts. J Orthop Res. 2010;28: 1107-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vogel KG, Sandy JD, Pogany G, et al. Aggrecan in bovine tendon. Matrix Biol. 1994;14:171-179 [DOI] [PubMed] [Google Scholar]
- 109. Wallace CD, Amiel D. Vascular assessment of the periarticular ligaments of the rabbit knee. J Orthop Res. 1991;9:787-791 [DOI] [PubMed] [Google Scholar]
- 110. Wang IE, Mitroo S, Chen FH, et al. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745-1755 [DOI] [PubMed] [Google Scholar]
- 111. Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563-1582 [DOI] [PubMed] [Google Scholar]
- 112. Wang JH, Jia F, Yang G, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128-133 [DOI] [PubMed] [Google Scholar]
- 113. Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181-2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331-53337 [DOI] [PubMed] [Google Scholar]
- 115. Wenstrup RJ, Smith SM, Florer JB, et al. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem. 2011;286: 20455-20465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wise BL, Peloquin C, Choi H, et al. Impact of age, sex, obesity, and steroid use on Quinolone-associated tendon disorders. Am J Med. 2012;125:1228. e23-1228.e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Woo SL, Hollis JM, Adams DJ, et al. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19:217-225 [DOI] [PubMed] [Google Scholar]
- 118. Woo SL, Lee TQ, Abramowitch SD, et al. Structure and function of ligaments and tendons. In: Mow VC, Huiskes R, eds. Basic Orthopaedic Biomechanics and Mechano-biology. Philadelphia, PA: Lippincott Williams and Wilkins; 2005:301-342 [Google Scholar]
- 119. Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol. 2011;111:999-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yamada M, Akeda K, Asanuma K, et al. Effect of osteogenic protein-1 on the matrix metabolism of bovine tendon cells. J Orthop Res. 2008;26:42-48 [DOI] [PubMed] [Google Scholar]
- 121. Yamaguchi K, Ditsios K, Middleton WD, et al. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699-1704 [DOI] [PubMed] [Google Scholar]
- 122. Yang X, Coleman DP, Pugh ND, et al. The volume of the neovascularity and its clinical implications in Achilles tendinopathy. Ultrasound Med Biol. 2012;38:1887-1895 [DOI] [PubMed] [Google Scholar]
- 123. Yeh LC, Tsai AD, Lee JC. Bone morphogenetic protein-7 regulates differentially the mRNA expression of bone morphogenetic proteins and their receptors in rat Achilles and patellar tendon cell cultures. J Cell Biochem. 2008;104:2107-2122 [DOI] [PubMed] [Google Scholar]
- 124. Yu JS, Popp JE, Kaeding CC, et al. Correlation of MR imaging and pathologic findings in athletes undergoing surgery for chronic patellar tendinitis. AJR Am J Roentgenol. 1995;165:115-118 [DOI] [PubMed] [Google Scholar]
- 125. Yuan J, Murrell GA, Wei AQ, et al. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372-1379 [DOI] [PubMed] [Google Scholar]
- 126. Yuan J, Wang MX, Murrell GA. Cell death and tendinopathy. Clin Sports Med. 2003;22:693-701 [DOI] [PubMed] [Google Scholar]
- 127. Zhang J, Pan T, Im HJ, et al. Differential properties of human ACL and MCL stem cells may be responsible for their differential healing capacity. BMC Med. 2011;9:68-7015- 9-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9:911-915 [DOI] [PMC free article] [PubMed] [Google Scholar]


