Abstract
Objectives
The present study of a representative sample of older adults quantified everyday physical activity (EPA) by having participants wear actigraphs. Our objectives were to examine whether poor health may partly explain why older adults become less physically active with advancing age and whether gender might moderate the extent to which health status predicts EPA.
Methods
We performed multiple regression analyses on a sample of older, community-dwelling adults (aged 80–98 years, N = 198; women = 63.1%).
Results
The results imply that age-related declines in EPA may be partially accounted for by health (in men) and by living arrangements (in women).
Discussion
We consider reasons why poorer health might erode EPA for men (but not women) and why living alone might erode EPA for women (but not men).
Keywords: Activity, Actigraph, Gender, Health, Living arrangements
To be alive is to be in motion. Although one does not observe the motion that exists at the molecular, cellular, metabolic, and sensory levels, one can easily observe physical motion at a broader level in the simple actions of daily life. In this regard, activity is studied from various disciplines including sociology, psychology, physiology, nursing, kinesiology, and medical rehabilitation. Having been the focus of research that cuts across the physical and social sciences, the study of physical activity has spawned an enormous body of empirical research covering the life span.
Over the life span, physical activity appears to decline, the decrease having been documented from infancy to young childhood (Chipperfield, 1986; Eaton, 1994) and more so from 11 years of age to Grade 12 (Brodersen, Steptoe, Boniface, & Wardle, 2007; Schoenborn, 1986; Trost, Pate, et al., 2002). Age is inversely related to physical activity among adults (Trost, Owen, Bauman, Sallis, & Brown, 2002) and among 18- to 95-year-old participants of the Baltimore Study of Aging (Talbot, Metter, & Fleg, 2000). Declines in physical activity are evident into very late life (e.g., Sihvonen, Rantanen, & Heikkinen, 1998; Talbot et al., 2000) and even continue into the 80s and 90s (Chipperfield, 2008).
Because physical activity has implications for many health conditions such as osteoarthritis, cardiovascular disease, and diabetes, it is critical to study why very old adults have reduced levels of physical activity. Beyond the implications for health, age-related declines in physical activity are also germane to many issues in gerontology. For example, declining physical activity may be accompanied by negative expectations, perpetuating a self-fulfilling prophecy of continuing decline and self-induced dependency. At the societal level, declining physical activity may reinforce negative stereotypes and discrimination. Given the widespread implications of declining physical activity, it is imperative to develop a better understanding of why this happens.
The logical premise that underpinned the current study is that age per se is not likely the sole causal factor for declining levels of everyday physical activity (EPA). Rather, declining EPA is more likely partly due to something that co-occurs with advancing age, the most obvious candidate being failing health. That poor health undermines physical activity was suggested by a longitudinal study (aged 60+ years) in which inactive participants were more likely than active participants to cite poor health as a barrier to activity (Booth, Bauman, & Owen, 2002). We examined the question of whether poorer health might explain age-related declines in activity was examined by assessing the integrated, physical motions that occur as one navigates through daily life, defined here as EPA.
EPA represents a broad, nonspecific index of physical activity or movement over a period of time. In its focus on simple movement, EPA shares similarities with concepts such as Levine and colleagues’ (2005) nonexercise activity thermogenesis. Because one cannot engage in the simplest of daily activities or social exchanges without physical movement, EPA is an integral component necessary for any interaction in the environment and for the simplest of goal striving. Of course, EPA is also necessary for the more complex, aggregated movements that characterize activities of daily living (ADLs), leisure activities, and the periodic, vigorous forms of exercise. However, it does not distinguish between activities that vary in their fitness-enhancing or functional capacity, thus differing from exercise and the basic ADLs. The study of EPA goes beyond an analysis of exercise or leisure activity to assess the quantity or level of physical movement as it occurs (or does not occur) within one’s daily endeavors. Thus, studying EPA has advantages over assessing formal exercise programs that can be impossible, especially for those who are confined to their homes or have reduced muscle strength and/or slow reaction times (Williams & Lord, 1995).
We assessed EPA in our study by using data from mechanical, computerized motion recorders (actigraphs) that individuals wear as they go about their normal daily activities. The actigraph is relatively unobtrusive and offers an objective alternative to the measurement of physical activity via self-report items (e.g., Menec & Chipperfield, 1997; Schroll, 2003), activity diaries (Washburn, Jette, & Janney, 1990), and questionnaires such as the Zutphen Physical Activity Questionnaire and the Baecke Questionnaire for Older Adults (see Washburn, 2000, for a review). Because actigraphs produce an objective measure, they are less subject to bias and unreliability than self-reports (Dishman, Washburn, & Schoeller, 2001; Hoyt & Kerns, 1999). Although researchers have traditionally used computerized motion recorders in research on infants and children (Chipperfield & Eaton, 1992; Eaton, Chipperfield, & Singbeil, 1989; Rabbia et al., 2002), they are beginning to apply actigraphs in the study of older populations (e.g., Buchheit et al., 2004; Fukukawa et al., 2004).
Despite some limitations (Esliger, Copeland, Barnes, & Tremblay, 2005; Kolbe-Alexander, Lambert, Harkins, & Ekelund, 2006), actigraphs produce scores that have greater variation than self-report ratings of physical activity (e.g., 1 = very inactive, 5 = very active), and reliability and validity are well established (e.g., Focht, Sanders, Brubaker, & Rejeski, 2003; Melanson & Freedson, 1995; Resnick & Galik, 2007; Welk, Schaben, & Morrow, 2004). Finally, by accurately documenting a complete lack of physical movement (scores of 0), actigraphs can capture the inactivity that often defines later life (Esliger et al., 2005; Steele et al., 2003). This allows for an absolute definition of “sedentary” and a precise analysis of just how sedentary older adults are on a daily basis. The importance of studying sedentary life is implied by the physical restraint research showing that muscles can weaken and shorten and abnormal changes can occur in metabolic rates, body chemistry, and blood volume (Mott, Poole, & Kenrick, 2005). Moreover, being sedentary significantly predicts the likelihood of dying within the next 2 years (Chipperfield, 2008).
The present study assessed EPA among a representative sample of older, community-dwelling adults to consider what might explain the previously reported age-related declines in EPA (Chipperfield, 2008). If health (both physical and functional) negatively predicts EPA, this would partly explain the link between age and EPA. Because men experience an earlier decline in late-life EPA than women (Chipperfield, 2008), and women have poorer physical and functional health (Arber & Cooper, 1999; Austad, 2006; Bird & Fremont, 1991; Evert, Lawler, Bogan, & Perls, 2003), we conducted analyses separately for men and women to test the hypothesis that health status should negatively predict EPA.
Finally, because socioeconomic status (or income as a proxy) and social factors predict physical activity or exercise (Clark, 1996; Ford et al., 1991; Terry, Biddle, Chatzisarantis, & Bell, 1997; Wister, 1996), we included these background variables in the analysis. The use of gender-specific analyses was important because men have higher incomes and women are more likely to live alone (Canetto, 2001). Although it is not clear whether income would more strongly predict EPA for men or women, some empirical evidence suggests that social factors such as living arrangements can play a predominant role in women’s exercise (e.g., Terry et al., 1997). If social interactions play a weaker role for men, living alone would be of little relevance to their EPA. Although this literature might suggest that the presence of another acts as a stronger motivator of EPA for women (relative to men), we did not specify hypotheses with regard to the background variables. Rather, we examined separate models for men and women in an exploratory way, allowing for a consideration of whether lower incomes or living with another person differentially predict EPA for men and women.
Methods
Study Procedure
The Study of Everyday Physical Activity is a satellite study of the larger Aging in Manitoba (AIM) Longitudinal Project, which comprises data on nearly 9,000 older adults who were interviewed over a 35-year period. The original wave of AIM participants began in 1971, and new participants were added in 1976 and 1983. For each new wave, rigorous random sampling techniques were used to create samples stratified by age, gender, and region. Follow-up of all surviving individuals was done through reinterviewing participants at six subsequent points in time. Previous analyses have shown that the sampling procedures successfully achieved representativeness and minimized selection bias and selective attrition (Chipperfield, Havens, & Doig, 1997).
In 2003, the sample for the Study of Everyday Physical Activity was drawn from the pool of AIM respondents who participated in the 2001 follow-up interview (see Chipperfield et al., 2006, for full details). The participants were deemed eligible for the EPA if they (a) lived in or very near the major cities in Manitoba, Canada; (b) needed no or only little assistance to complete interviews; and (c) had satisfactory comprehension of English. Interviews were conducted with 232 participants, with actigraph data being obtained from 198 individuals. Excluded from the EPA sample were 34 individuals because their data were unusable (n = 7), they refused to wear the actigraph (n =12), or they participated at a time when an actigraph was unavailable (n = 15). Research has shown no differences between the nonrespondents (n = 34) and the respondents on a variety of variables, including age, income, education, gender, severity of chronic health conditions, positive affect, and survival status (Chipperfield, 2008).
Two visits were made to participants’ homes, typically over a 2- to 3-day time period. On Visit 1, in-depth interviews were conducted to gather such data as participants’ living arrangements and health. Prior to the interview, the actigraph was placed on the participant’s nondominant wrist much like a wristwatch, and the start time was recorded. Interviewers encouraged participants to wear the actigraph continuously over the next day and to go about their daily activities as they normally would. They were also shown how to remove the actigraph and reattach it if this became necessary (e.g., while bathing) and were asked to record the times and reasons for doing so.
Interviewers returned on the next day for Visit 2, at which time the actigraph was removed, the end time was recorded, and participants were asked several questions concerning their activity on the day the actigraph was worn. In particular, participants reported the types of activities they engaged in during the day and the times they went to bed at night and got up in the morning. If a Visit 2 appointment could not be scheduled for the next day, participants were asked to remove the actigraph themselves (approximately 24 hr after the start time) and to note the exact time this was done. After retrieving the actigraph from a participant, researchers downloaded the data and reinitialized it for use by the next participant.
Measures
Background characteristics were obtained during the interviews. On average, participants were 85 years of age (SD = 4.39, range = 80–98 years). The majority of participants were women (63.1%), and most lived alone (55.6%). Participants’ average annual incomes were calculated by asking them to provide a best estimate of their total yearly personal income from all sources before deductions. Outliers were adjusted and, as described by Tabachnick and Fidell (2001), missing values were replaced with the predicted values from a regression equation that included education level, gender, and marital status. On average, participants had a total annual personal income of $21,368.67 CDN (SD = $10,977.15).
A measure of functional status was available (from AIM 2001) that included 22 items selected from existing ADL measures (e.g., Lawton & Brody, 1969). In particular, participants were asked whether they were independently capable (0 = needs help; 1 = yes, can do) of performing 12 instrumental IADLs (e.g., light housework, laundry) and 10 basic ADLs (e.g., eating, bathing). A composite measure was created by summing those activities that participants were capable of undertaking, such that higher scores reflected greater functional capability (M = 18.6, SD = 3.0, range = 7–22).
Three measures of health status were available from the EPA interview to assess both subjective and objective physical health. First, we included a single-item self-rated health measure that has been used extensively in the gerontological literature and has been shown to predict mortality beyond more objective measures (Mossey & Shapiro, 1982). This involved participants’ global subjective ratings of their health compared to that of other people their own age. We derived a 3-point scale (1 =poor, 2=fair, 3 =good) after recoding the small number of responses that reflected extreme scores (“bad” and “excellent”) and reverse-coding responses so that higher scores reflected better health (M = 2.6, SD = 0.6).
Second, to capture subjective health problems of a more immediate nature, we assessed recent health. This involved asking participants to report the truth of the following statement: “In the past month I have often felt physically unwell” (1 = almost always true to 5 =almost never true). Thus, high scores reflected feeling physically well (M = 3.8, SD = 1.2).
Third, as outlined elsewhere (Chipperfield, 2008), we created a severity of chronic conditions score from participants’ responses to being asked whether (0 = no, 1 = yes), within the past year, they had experienced or had had aftereffects from each of 22 chronic conditions (e.g., heart attack, arthritis). We assigned a severity score to each of the conditions in our study by borrowing severity scores from the Seriousness of Illness Rating Scale introduced by Wyler, Masuda, and Holmes (1968) and revised by Rosenberg, Hayes, and Peterson (1987). For each participant in our study, we created a total severity of chronic conditions score by summing the Seriousness of Illness Rating Scale scores assigned to each of his or her reported chronic conditions (M =382.1, SD =198.1). Thus, this measure included objective information to capture the severity of poor health, distinguishing it from the two other self-report measures that were purely subjective. The importance of examining more objective measures along with global subjective self-ratings is underscored by the fact that the two are not always entirely congruent (Chipperfield, 1993; Ruthig & Chipperfield, 2007).
We obtained an objective measure of EPA by using uniaxial accelerometers (Model 7164), also referred to as MTI acti-graphs (MTI Health Services, 2000). Because the actigraph captures 10 acceleration signals per second, these can be summed at the end of every 1-min cycle, thereby combining 600 accelerations (10 × 60 s) before the integrator is reset to zero. Powered by a 2430 coin cell lithium battery, Actigraph® uniaxial accelerometers are small (2.0 × 1.6 × 0.6 in) and weigh only 1.5 ounces. This means they can be worn comfortably while they obtain a continuous recording of activity from acceleration signal magnitudes. These acceleration scores, also called activity counts, are stored in memory and are easily downloaded onto a computer. They can be summarized for each person to represent physical movement over a time period, specified in our study as a 1-min (user-defined) time interval. The overall activity count can then be used as a precise, quantifiable index of simple, physical movement (Resnick & Galik, 2007) or converted into other metrics such as metabolic equivalents (i.e., METs) or energy expenditure. The actigraph data are distinguishable from indicators such as step counts detected by pedometers (see Steele et al., 2003).
As has been done in other studies (Carvalho-Bos, Reimersmavan der Lek, Waterhouse, & Van Someren, 2007; Steele et al., 2003), we placed the actigraph on the wrist rather than the ankle or waist. This simplified the participants’ removal and reattachment of actigraphs if this was necessary during the study. More important, wrist placement allowed us to capture the kind of activity that is common in late life, in particular the fine, upper body movements involved in such everyday activities that occur while both sitting (e.g., sewing, playing cards) or standing (e.g., washing dishes, moving about the house).
For the present purposes, as done by others who have examined daily activity (Jilcott et al., 2006; Rowe, Kemble, Robinson, & Mahar, 2007), we created an EPA score after excluding acceleration data that were associated with times a person had removed the actigraph or was in bed at night. Of note, 31% (61/198) of participants removed the actigraph for a mean period of 1.44 hr (SD = 2.72, range = 0.08–11.00 hr). The majority of those who removed the actigraph (56/61) did so once, with only 5 people removing it twice over the course of the study. Participants’ most common reasons for removing the actigraph included bathing, showering, and washing hair. That these participants did not differ on EPA from those who had worn the actigraph continuously, t(196) =−0.57, p > .05.
Because there were slight variations in the amount of time each participant wore the actigraph, we derived the EPA score for each participant by calculating a mean over the 1-min acceleration scores. Thus, for our purposes, we divided the sum of the 1-min acceleration scores by the total number of acceleration scores obtained for that participant. This score can be broadly interpreted as an index of physical activity per minute. Women had relatively higher EPA levels than men (Ms = 769 vs 756), the overall mean EPA score for the sample being 764 (SD = 321, range = 78–1745). Although we cannot compare our EPA scores to those of other studies that have reported summed acceleration scores (e.g., Resnick & Galik, 2007), the mean level of EPA in our study fell into what others have defined as “light” activity (<1,952 counts per min; Freedson, Melanson, & Sirard, 1998).
Because it is not very informative to describe participants’ average activity levels as being “light,” we further attempted to contextualize the meaning of EPA scores by examining participants’ qualitative reports of the four major activities they had engaged in during the day the actigraph was worn. It is interesting that the activities reported by the individual with the highest EPA level in our study clearly indicated that she played a caregiving role, which we later argue may partly account for EPA. In contrast, among those with the lowest EPA scores, watching TV and reading were reported as major activities of the day.
To obtain additional information on the meaning of EPA scores, two research assistants wore an actigraph for a period of 20 min while undertaking specified activities. The means of the two scores were as low as 44 for reading and as high as 3,106 for walking at a moderate pace. Further activities that provide some context for interpreting the EPA scores included watching TV (52), driving a car (346), walking very slowly (843), and preparing a meal (1,749), although it is important to underscore that these values are based on the activities of young adults.
Reliability
As described more fully elsewhere, we conducted several analyses to provide further supportive evidence for the reliability of the EPA measure (Chipperfield, 2008). First, the very few participants (10.7%) who reported that the study day was atypical had a similar EPA score to those who reported that their day was typical. Thus, atypical days did not tend to bias the EPA scores in such a way that they were either higher or lower than the EPA scores for participants who reported typical days. Second, we conducted test–retest reliability on the EPA score using a subset of the participants in our study who wore the actigraph approximately 1 year later (n = 68). The Time 1 and Time 2 scores did not differ significantly from each other, t(67) =−1.69, p > .05, and they were highly correlated (r =.77, p < .05; Chipperfield et al., 2006). Thus, test–retest reliability showed that the actigraphs were reliable indicators of physical movement over this period of time. Although reliability would likely have been even higher had we measured EPA over many days, we chose a 1-day period for several reasons. Recognizing that there is always a compromise between validity and subject comfort (Westerterp, 1999), we opted for a 1-day period in order to increase compliance and minimize discomfort experienced due to older adults’ sensitive skin. We garnered further confidence in the reliability of the measure from findings showing that there are not large gains from having participants wear the actigraph for 2 or 3 days (Lambert, 2006).
Results
Table 1 provides correlations for all study variables. Of direct relevance to our subsequent analysis was the significant correlation between age and EPA (r = −.28, p < .01) that has previously been demonstrated (Chipperfield, 2008). Age was also associated with functional status, living arrangements, and health (recent). That each of these variables also correlated with age suggested that one or more of these covarying variables may have accounted for the negative association between EPA and advancing age.
Table 1.
Correlations Between All Study Variables for Total Sample (N = 198)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age (years) | — | ||||||||
| 2. Annual income | −.09 | — | |||||||
| 3. Gender | .03 | −.40** | — | ||||||
| 4. Functional status (ADL) | −.34** | .18* | −.18* | — | |||||
| 5. Living arrangements | −.24** | −.01 | −.30** | .11 | — | ||||
| 6. Self-rated health | .04 | .11 | −.03 | .24** | −.04 | — | |||
| 7. SCCS | .04 | −.07 | −.03 | −.34** | −.03 | −.35** | — | ||
| 8. Recent health | .15* | .15* | −.16* | .21** | −.05 | .41** | −.38** | — | |
| 9. Everyday physical activity | −.28** | .11 | .02 | .25** | .09 | .11 | −.14* | .12 | — |
| M | 85.03 | 21,368.67 | 1.63 | 18.56 | 1.43 | 2.57 | 382.08 | 3.79 | 763.88 |
| SD | 4.39 | 10,977.15 | 0.48 | 3.01 | 0.50 | 0.56 | 198.12 | 1.18 | 320.75 |
| Range | 79–98 | 4800.00–60,002.00 | 1–2 | 7–22 | 1–2 | 1–3 | 2–965 | 1–5 | 78.31–1745.17 |
Notes: ADL = activities of daily living; SCCS = severity of chronic conditions score.
p < .05;
p < .01.
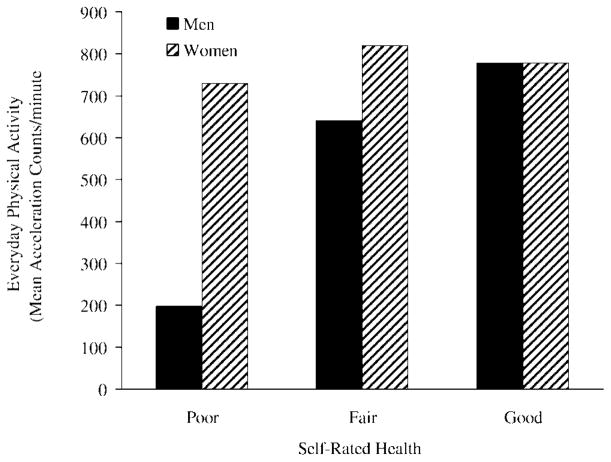
We conducted separate regression models for men and women to examine possible alternative explanations for why EPA would be negatively associated with advancing age. Table 2 shows that the findings for men (but not women) provided support for our hypothesis that poorer self-rated health would be significantly associated with lower EPA (β=.28, t =2.44, p < .05). Figure 1 shows the distinct patterns for men and women: Health negatively predicted EPA for men but not women.
Table 2.
Regression Coefficients for Predictors of Everyday Physical Activity for Women and Men
| Variable | Women (n = 125)
|
Men (n = 73)
|
||||||
|---|---|---|---|---|---|---|---|---|
| β | B | SE | t | β | B | SE | t | |
| Constant | 1240.39 | 650.90 | 1.91 | 2003.90 | 1051.83 | 1.91 | ||
| Age | −.17 | −11.53 | 6.35 | −1.82 | −.25 | −21.73 | 10.59 | −2.05* |
| Annual income | .13 | .005 | .003 | 1.45 | .05 | .001 | .003 | 0.43 |
| Functional statusa | .14 | 15.59 | 10.92 | 1.43 | .14 | 13.76 | 12.40 | 1.11 |
| Living arrangementsb | .19 | 123.42 | 61.37 | 2.01* | −.15 | −105.04 | 76.63 | −1.37 |
| Healthc | −.02 | −8.63 | 47.21 | −0.18 | .28 | 179.94 | 73.81 | 2.44* |
| R2 | .13 | .23 | ||||||
Notes:
Activities of daily living.
1 = alone, 2 = with others.
Self-rated.
p < .05.
Figure 1.
Relationship between self-rated health and everyday physical activity for men and women.
We conducted regression analyses identical to those shown in Table 2 replacing the self-rated health measure first with the alternative subjective measure of recent health and second with the severity of chronic conditions score. The pattern of findings was identical to that shown in Table 2 and Figure 1. For men (but not women), better recent health (feeling well) was associated with higher EPA (β =.24, t =2.13, p < .05), and the severity of chronic conditions score was marginally negatively associated with EPA (β =−.24, t =−1.91, p = .06).
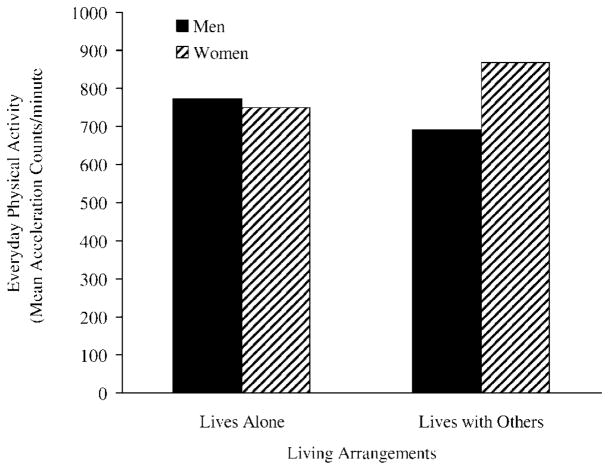
It is interesting to note that Table 2 also shows that, although health did not predict women’s EPA, living arrangements did (β = .19, t =−2.01, p < .05). In contrast, for men, living with another person failed to predict EPA. Figure 2 shows the pattern in which living with another person, typically a spouse, predicted higher EPA for women but not for men.
Figure 2.
Relationship between living arrangements and everyday physical activity for men and women.
Discussion
Our assessment of EPA allowed us to capture a large amount of quantifiable information from each respondent. The findings provide partial support for the notion that failing physical health associated with advancing age predicts lower EPA or, conversely, that good physical health predicts higher EPA. This was true, however, only for men. Whereas better physical health predicted EPA for men, living arrangement predicted EPA for women. If we can infer causality from these findings, they imply that good health facilitates EPA for men (but not women) and that living with another person facilitates EPA for women (but not men). Speculation for why EPA might be determined by different factors for men and women could include the gender-based impact of many forces such as social roles, coping mechanisms, genetic factors, or physiological processes.
Health Status as a Predictor of Men’s EPA
Why health predicted EPA for men but not women could also be the result of men and women having different types of physical health problems. However, the results of a supplemental analysis did not support this logic. In particular, we obtained very little support for this explanation when we considered whether men had more of the types of health problems that should curtail their EPA. Although men were more likely than women to have had a heart attack, χ2(1, N = 198) = 8.6, p < .05; and hearing problems, χ2(1, N = 198) = 6.7, p < .05; women were more likely to suffer from arthritis, χ2(1, N = 198) =17.0, p < .05. More important, gender differences failed to emerge for all other health conditions examined. Thus, it seems unlikely that gender differences in the types of health conditions explain why health status predicted EPA differently for men and women.
The most interesting question to arise with regard to physical health may be the following: Why did poor health not undermine women’s EPA? Perhaps due to their experience with menstruation, menopause, and pregnancy, women learn to persist with activity in the face of physical discomfort. This history might prepare women to respond with higher levels of EPA than men when facing health problems. In a similar way, the daily demands of childrearing may have resulted in women growing accustomed to persisting in daily physical endeavors even when feeling physically unwell. Of course, men of this generation whose work involved manual labor would have also become proficient at physical persistence when their health was compromised. However, this persistence may be more specific to work demands outside the home, perhaps not carrying over to daily life in the same way as women’s. This would be reflected in men’s day-to-day physical activity being suppressed when physical health is compromised, as suggested by our findings.
Although our finding supported the idea that physical health status predicts EPA (at least for men), functional status did not predict EPA. Functional status correlated positively with EPA in the overall sample (see Table 1) but did not significantly predict EPA in the regressions for either men or women. It is possible that the magnitude of a relationship between functional status and EPA was attenuated because we obtained our measure of functional status from 2 years earlier, and this measure may have failed to capture declining function that had occurred during the intervening years. Thus, one should exercise caution with the interpretation of this finding. Further research should also consider gender differences in the underlying norms and gender roles associated with specific activities of daily living because, for example, women of this generation might be less likely than men to report that they need help with yardwork simply because they do less of it. To the extent that reported functional status is influenced by such underlying norms, these need to be taken into account to understand the relationship between functional status and EPA.
Living Arrangements as a Predictor of Women’s EPA
Why would women’s physical activity be higher in the presence of another individual, whereas this was not true for men? As previously mentioned, perhaps the presence of another individual acts as a motivator or energizer for women but not for men. This would be consistent with the finding that social factors facilitate women’s time spent on productive activities and leisure (Klumb & Baltes, 1999). In contrast, consistent with past research showing a lack of difference in the activity of men who lived alone versus with a spouse (Muraki, Nagao, & Ishikawa, 2001), our findings suggest that social context is less important for men’s everyday physical activity. Perhaps men are more motivated by factors other than social context.
Our findings might also be informed by the caregiving literature that shows that women typically provide more physical care for their spouses than do men (Ingersoll-Dayton & Raschick, 2004; Katz, Kabeto, & Langa, 2000). If women who are living with another person are heavily engaged in day-to-day caregiving activities that demand a high level of physical movement (e.g., dressing, bathing, feeding, etc.), their caregiving role offers a plausible explanation for high EPA levels. This was the case for the woman in our study who had the highest level of EPA and who reported that her major activities the day she wore the actigraph involved caregiving. Of course, our results could be explained in this way only if it were true that women cohabited with individuals requiring physical care.
Future Directions and Implications
Empirical data are needed to show if the association between living arrangements and physical activity (for women) is due to the provision of a vehicle for activity (e.g., a walking partner), the demands of caregiving (e.g., providing care to a spouse), or motivational forces (e.g., an energizing effect of another’s presence). One viable approach would be to compare women whose history had or had not included the provision of care to a spouse, hypothesizing that physical activity would be higher for the former compared to the latter. Research could also test our speculation for why women persist with physical activity even when facing poor health by comparing physical activity among unhealthy women who had and had not raised children, predicting that activity should be higher among those who had parented.
Although it is reasonable to interpret our data as suggesting that men’s poor health negatively impacts EPA, there is a large body of research showing that physical activity also determines health. If men’s higher EPA plays a protective role against physical health decline and morbidity (e.g., Laukkanen, Kauppinen, & Heikkinen, 1998), physical inactivity should be viewed as both contributing to and being a consequence of poor health. This would imply a bidirectional or reciprocal relationship that can be expressed as a downward spiral (↓EPA → ↓ Health → ↓EPA), ultimately threatening survival. Future research should examine the bidirectionality of this relationship and consider the determinants of a downward spiral. Extrapolating from our findings, we would hypothesize such a downward spiral to more accurately characterize the relationship for men than women.
Future research is also needed to provide a clearer interpretation of the meaning of the EPA scores. This requires a systematic study of older adults who wear the actigraph while performing meaningful and typical daily activities for specific periods of time. Observations that tap into the type, duration, speed, and/or length of activities should be accompanied by self-reports of physical activity in order to permit a comparison of the actigraph methods to self-report. It would be useful to document, for example, whether the actigraph produces similar activity counts for two activities that differ in functional value (e.g., a helper lifts my arms to help me put on a sweater vs me putting on my own sweater). In addition, because many older people use aids such as walkers and canes, researchers should attend to whether actigraphs might under- or overestimate EPA among those who vary in upper versus lower extremity function (Guralnik et al., 2000). Of note, because only 13% of our participants required the use of these aids, and supplemental analyses showed that these participants did not differ significantly in EPA from their counterparts, this form of under- or over-estimation was not a threat in our study.
Finally, our results have implications for potential future interventions. Gerontologists have long been interested in determining how to encourage physical activity, especially in the face of physical/functional, structural, and social challenges that accompany growing old. Due to late-life impairment in balance, agility, and flexibility, exercise and leisure activities such as golf and gardening can become difficult and even risky. Moreover, unlike formalized exercise programs that are plagued by adherence problems (Martin & Sinden, 2001), EPA interventions would avoid these problems by encouraging people to build increased levels of physical movement into their current repertoire of activities and daily routines. Increased movement over the course of a day, a week, or a month could be encouraged even among those older adults who suffer from arthritis, pain, or functional limitations or who are confined to wheelchairs. The messages contained in an EPA intervention would differ from those that have characterized past mass public health campaigns aimed at improving fitness through vigorous physical activity or exercise. Rather than presenting images of people engaging in sport and exercise, an EPA intervention would feature people simply engaging in daily behaviors with a health promotion message that focuses on how easy it is to “keep moving, even if only slowly.”
Of course, the design of interventions must recognize that motivation to move is essential. The promotion of EPA should therefore build in motivation, perhaps by refocusing people’s thoughts away from internal attributions for inactivity (e.g., I don’t have the strength/energy to move) or encouraging thoughts about the positive outcomes of moving (e.g., If I get up and get moving, I will feel better). To the extent that interventions can be shown to effectively enhance EPA, it would remain important to target those who need them most. Our findings imply that older men in poor health and older women who live alone would be prime candidates for an intervention to enhance EPA. A final step would be to identify the best delivery mechanism to promote EPA. Ideally, the delivery of such messages would be through both informal mechanisms, such as the older adult’s own family, as well formal mechanisms, including the physician or geriatrician (Ross & Teasdale, 2005).
Concluding Comment
The social context that exists in late life may help to explain women’s age-related declines in EPA as suggested by the finding that age no longer predicted their EPA levels after we controlled for the effects of living arrangements. In contrast, age remained a strong determinant for men’s EPA, even after we controlled for various factors, including physical health status, that also predicted EPA. Thus, uncovering a more complete explanation for men’s age-related declines in EPA may require expanding the net of predictors.
Nonetheless, our data convincingly show that, for men, good health is a major predictor of higher EPA levels. Confidence in the robustness of the effect is gained by the effect being replicated across the three health measures, despite differences in their time frames and their subjectivity/objectivity. In conclusion, our findings show the need to examine the predictors of EPA separately for men and women, highlighting the possibility that the strong influence of differential traditional gender roles on levels of EPA becomes even more critical in later life.
Acknowledgments
This research was supported by a Canadian Institutes of Health Research (CIHR) operating grant (200609MOP-165097-SDA-CDAA-49702) and a Mid-Career CIHR Award (Institute on Aging) to Judith G. Chipperfield, CIHR Graduate Scholarship doctoral awards to Nancy E. Newall and Audrey U. Swift, and a Social Sciences and Humanities Council of Canada Graduate Scholarship doctoral award to Tara L. Haynes.
J. G. Chipperfield provided funding for this project, designed the study, and wrote the manuscript. N. E. Newall conducted literature reviews, provided conceptual input, assisted in the analyses and interpretation of data, and helped edit and revise the manuscript. L. P. Chuchmach managed the study data, performed statistical analyses, and assisted in revising the paper. A. U. Swift and T. L. Haynes assisted with the literature review and with editing the manuscript.
References
- Arber S, Cooper H. Gender differences in health in later life: The new paradox? Social Science & Medicine. 1999;48:61–76. doi: 10.1016/s0277-9536(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Austad SN. Why women live longer than men: Sex differences in longevity. Gender Medicine. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- Bird CE, Fremont AM. Gender, time use, and health. Journal of Health and Social Behavior. 1991;32:114–129. [PubMed] [Google Scholar]
- Booth ML, Bauman A, Owen N. Perceived barriers to physical activity among older Australians. Journal of Aging and Physical Activity. 2002;10:271–280. [Google Scholar]
- Brodersen NH, Steptoe A, Boniface DR, Wardle J. Trends in physical activity and sedentary behaviour in adolescence: Ethnic and socioeconomic differences. British Journal of Sports Medicine. 2007;41:140–144. doi: 10.1136/bjsm.2006.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit M, Simon C, Viola AU, Doutreleau S, Piquard F, Brandenberger G. Heart rate variability in sportive elderly: Relationship with daily physical activity. Medicine & Science in Sports & Exercise. 2004;36:601–605. doi: 10.1249/01.mss.0000121956.76237.b5. [DOI] [PubMed] [Google Scholar]
- Canetto SS. Older adult women: Issues, resources and challenges. In: Unger RK, editor. Handbook of the psychology of women and gender. New York: Wiley; 2001. pp. 183–190. [Google Scholar]
- Carvalho-Bos S, Riemersmavan der Lek R, Waterhouse J, Van Someren E. Strong association of the rest-activity rhythm with well-being in demented elderly women. Journal of Sleep Research. 2006;15(Suppl 1):216–223. doi: 10.1097/01.JGP.0000236584.03432.dc. [DOI] [PubMed] [Google Scholar]
- Chipperfield JG. Unpublished master’s thesis. University of Manitoba; Winnipeg, Canada: 1986. Child activity level: A longitudinal analysis of its relationships to prenatal cigarette exposure. [Google Scholar]
- Chipperfield JG. Perceived barriers in coping with health problems: A twelve-year longitudinal study of survival among elders. Journal of Aging and Health. 1993;5:123–139. [Google Scholar]
- Chipperfield JG. Everyday physical activity as a predictor of mortality. The Gerontologist. 2008;48:349–357. doi: 10.1093/geront/48.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield JG, Eaton WO. Reactivity and environmental stimulation as predictors of motor activity level in children. Personality and Individual Differences. 1992;13:591–601. [Google Scholar]
- Chipperfield JG, Havens B, Doig WD. Method and description of the Aging in Manitoba project: A 20-year longitudinal study. Canadian Journal on Aging. 1997;16:606–625. [Google Scholar]
- Chipperfield JG, Newall NE, Chuchmach LP, Haynes TL, Ruthig JC, Perry RP, et al. Study of Everyday Physical Activity (EPA) 2003 in later life: Methods and description. Winnipeg, Canada: University of Manitoba, Health, Leisure, & Human Performance Research Institute; 2006. (Tech. Rep. No. HLHPRI112) [Google Scholar]
- Clark DO. Age, socioeconomic status, and exercise self-efficacy. The Gerontologist. 1996;36:157–164. doi: 10.1093/geront/36.2.157. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Washburn RA, Schoeller DA. Measurement of physical activity. Quest. 2001;53:295–309. [Google Scholar]
- Eaton WO. Temperament, development, and the five-factor model: Lessons from activity level. In: Halvorsen CF, Kohnstamm GF, editors. The developing structure of temperament and personality from infancy to adulthood. Hillsdale, NJ: Erlbaum; 1994. pp. 173–187. [Google Scholar]
- Eaton WO, Chipperfield JG, Singbeil CE. Birth order and activity level in children. Developmental Psychology. 1989;25:668–672. [Google Scholar]
- Esliger DW, Copeland JL, Barnes JD, Tremblay MS. Standardizing and optimizing the use of accelerometer data for free-living physical activity monitoring. Journal of Physical Activity and Health. 2005;3:366–383. [Google Scholar]
- Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: Survivors, delayers, and escapers. Journal of Gerontology: Medical Sciences. 2003;58A:M232–M237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- Focht BC, Sanders WM, Brubaker PH, Rejeski WJ. Initial validation of the CSA activity monitor during rehabilitative exercise among older adults with chronic disease. Journal of Aging and Physical Activity. 2003;11:293–304. [Google Scholar]
- Ford ES, Merritt RK, Heath GW, Powell KE, Washburn RA, Kriska A, et al. Physical activity behaviors in lower and higher socioeconomic status populations. American Journal of Epidemiology. 1991;133:1246–1256. doi: 10.1093/oxfordjournals.aje.a115836. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine & Science in Sports & Exercise. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Fukukawa Y, Nakashima C, Tsuboi S, Kozakai R, Doyo W, Niino N, et al. Age differences in the effect of physical activity on depressive symptoms. Psychology and Aging. 2004;19:346–351. doi: 10.1037/0882-7974.19.2.346. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability. Journal of Gerontology: Medical Sciences. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- Hoyt WT, Kerns MD. Magnitude and moderators of bias in observer ratings: A meta-analysis. Psychological Methods. 1999;4:403–424. [Google Scholar]
- Ingersoll-Dayton DB, Raschick M. Relationship between care-recipient behaviors and spousal caregiving stress. The Gerontologist. 2004;44:318–327. doi: 10.1093/geront/44.3.318. [DOI] [PubMed] [Google Scholar]
- Jilcott SB, Keyserling TC, Samual-Hodge CD, Rosamond W, Garcia B, Will JC, et al. Linking clinical care to community resources for cardiovascular disease prevention: The North Carolina Enhanced WISEWOMAN Project. Journal of Women’s Health. 2006;15:569–583. doi: 10.1089/jwh.2006.15.569. [DOI] [PubMed] [Google Scholar]
- Katz SJ, Kabeto M, Langa KM. Gender disparities in the receipt of home care for elderly people with disability in the United States. Journal of the American Medical Association. 2000;284:3022–3027. doi: 10.1001/jama.284.23.3022. [DOI] [PubMed] [Google Scholar]
- Klumb PL, Baltes MM. Time use of old and very old Berliners: Productive and consumptive activities as functions of resources. Journal of Gerontology: Social Sciences. 1999;54B:S271–S278. doi: 10.1093/geronb/54b.5.s271. [DOI] [PubMed] [Google Scholar]
- Kolbe-Alexander TL, Lambert EV, Harkins JB, Ekelund U. Comparison of two methods of measuring physical activity in South African older adults. Journal of Aging and Physical Activity. 2006;14:98–114. doi: 10.1123/japa.14.1.98. [DOI] [PubMed] [Google Scholar]
- Lambert P. Unpublished master’s thesis. University of Manitoba; Winnipeg, Canada: 2006. Physical activity and the oldest-old: A comparison of self-report and accelerometer readings. [Google Scholar]
- Laukkanen P, Kauppinen M, Heikkinen E. Physical activity as a predictor of health and disability in 75- and 80-year-old men and women: A five-year longitudinal study. Journal of Aging and Physical Activity. 1998;6:141–156. [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: Possible role in human obesity. Science. 2005 Jan 25;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- Martin KA, Sinden AR. Who will stay and who will go? A review of older adults’ adherence to randomized controlled trials of exercise. Journal of Aging and Physical Activity. 2001;9:91–114. [Google Scholar]
- Melanson ER, Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Medicine & Science in Sports & Exercise. 1995;27:934–940. [PubMed] [Google Scholar]
- Menec VH, Chipperfield JG. Remaining active in later life: The role of locus of control in senior’s leisure activity participation, health, and life satisfaction. Journal of Aging and Health. 1997;9:105–125. doi: 10.1177/089826439700900106. [DOI] [PubMed] [Google Scholar]
- Mossey JM, Shapiro E. Self-rated health: A predictor of mortality among the elderly. American Journal of Public Health. 1982;72:800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott S, Poole J, Kenrick M. Physical and chemical restraints in acute care: Their potential impact on the rehabilitation of older people. International Journal of Nursing Practice. 2005;11:95–101. doi: 10.1111/j.1440-172X.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- MTI Health Services. Actigraph Analysis Software (Version 3.0) 2000 Computer software user’s manual. Retrieved November 30 2007, from www.mtiactigraph.com/
- Muraki T, Nagao T, Ishikawa Y. A preliminary investigation to explore the effects of daytime physical activity patterns on health-related QOL in healthy community-dwelling elderly subjects. Physical & Occupational Therapy in Geriatrics. 2001;19(2):51–62. [Google Scholar]
- Rabbia F, Grosso T, Cat Genova G, Conterno A, De Vito B, Mulatero P, et al. Assessing resting heart rate in adolescents: Determinants and correlates. Journal of Human Hypertension. 2002;16:327–332. doi: 10.1038/sj.jhh.1001398. [DOI] [PubMed] [Google Scholar]
- Resnick B, Galik E. The reliability and validity of the physical activity survey in long-term care. Journal of Aging and Physical Activity. 2007;15:439–458. doi: 10.1123/japa.15.4.439. [DOI] [PubMed] [Google Scholar]
- Rosenberg SJ, Hayes JR, Peterson RA. Revising the Seriousness of Illness Rating Scale: Modernization and re-standardization. International Journal of Psychiatry in Medicine. 1987;17:85–92. doi: 10.2190/jwmw-8q1u-71dj-an6e. [DOI] [PubMed] [Google Scholar]
- Ross KM, Teasdale TA. Prescribing physical activity for older adults. Journal of the Oklahoma State Medical Association. 2005;98:443–446. [PubMed] [Google Scholar]
- Rowe DA, Kemble CD, Robinson TS, Mahar MT. Daily walking in older adults: Day-to-day variability and criterion-referenced validity of total daily step counts. Journal of Physical Activity and Health. 2007;4:434–446. [PubMed] [Google Scholar]
- Ruthig JC, Chipperfield JG. Health incongruence in later life: Implications for subsequent well-being and health care. Health Psychology. 2007;26:753–761. doi: 10.1037/0278-6133.26.6.753. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA. Health habits of U.S. adults, 1985: The “Alameda 7” revisited. Public Health Reports. 1986;101:571–580. [PMC free article] [PubMed] [Google Scholar]
- Schroll M. Physical activity in an ageing population. Scandinavian Journal of Medicine & Science in Sports. 2003;13:63–69. doi: 10.1034/j.1600-0838.2003.20226.x. [DOI] [PubMed] [Google Scholar]
- Sihvonen S, Rantanen T, Heikkinen E. Physical activity and survival in elderly people: A five-year follow-up study. Journal of Aging and Physical Activity. 1998;6:133–140. [Google Scholar]
- Steele BG, Belza B, Cain K, Warms C, Coppersmith J, Howard J. Bodies in motion: Monitoring daily activity and exercise with motion sensors in people with chronic pulmonary disease. Journal of Rehabilitation Research and Development. 2003;40(Suppl 2):45–58. doi: 10.1682/jrrd.2003.10.0045. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4. Needham Heights, MA: Allyn & Bacon; 2001. [Google Scholar]
- Talbot LA, Metter EJ, Fleg JL. Leisure-time physical activities and their relationship to cardiorespiratory fitness in healthy men and women 18–95 years old. Medicine & Science in Sports & Exercise. 2000;32:417–425. doi: 10.1097/00005768-200002000-00024. [DOI] [PubMed] [Google Scholar]
- Terry PC, Biddle SJH, Chatzisarantis N, Bell RD. Development of a test to assess the attitudes of older adults toward physical activity and exercise. Journal of Aging and Physical Activity. 1997;5:111–125. [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: Review and update. Medicine & Science in Sports & Exercise. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Trost SG, Pate RR, Sallis JF, Freedson PS, Taylor WC, Dowda M, et al. Age and gender differences in objectively measured physical activity in youth. Medicine & Science in Sports & Exercise. 2002;34:350–355. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Washburn RA. Assessment of physical activity in older adults. Research Quarterly for Exercise and Sport. 2000;71:79–88. doi: 10.1080/02701367.2000.11082790. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Jette AM, Janney CA. Using age-neutral physical activity questionnaires in research with the elderly. Journal of Aging and Health. 1990;2:341–356. [Google Scholar]
- Welk GJ, Schaben JA, Morrow JR., Jr Reliability of accelerometry-based activity monitors: A generalizability study. Medicine & Science in Sports & Exercise. 2004;36:1637–1645. [PubMed] [Google Scholar]
- Westerterp KR. Assessment of physical activity level in relation to obesity: Current evidence and research issues. Medicine & Science in Sports & Exercise. 1999;31(Suppl 1):S522–S525. doi: 10.1097/00005768-199911001-00006. [DOI] [PubMed] [Google Scholar]
- Williams P, Lord SR. Predictors of adherence to a structured exercise program for older women. Psychology and Aging. 1995;10:617–624. doi: 10.1037//0882-7974.10.4.617. [DOI] [PubMed] [Google Scholar]
- Wister AV. The effects of socioeconomic status on exercise and smoking: Age-related differences. Journal of Aging and Health. 1996;8:467–488. doi: 10.1177/089826439600800401. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Masuda M, Holmes TH. Seriousness of Illness Rating Scale. Journal of Psychosomatic Research. 1968;11:363–374. doi: 10.1016/0022-3999(68)90033-0. [DOI] [PubMed] [Google Scholar]