Abstract
Background
Maternal asthma is associated with serious pregnancy complications but newborn morbidity is understudied.
Objective
To determine if infants of asthmatic mothers have more neonatal complications.
Methods
The Consortium on Safe Labor (2002–2008), a retrospective cohort, included 223,512 singleton deliveries at ≥ 23 weeks’ gestation. Newborns of mothers with asthma (n=17,044) were compared to newborns of non-asthmatic women using logistic regression models with generalized estimating equations to calculate adjusted odds ratios (OR) and 95% confidence intervals (CI). Electronic medical record data included gestational week at delivery, birthweight, resuscitation, neonatal intensive care unit (NICU) admission, NICU length of stay, hyperbilirubinemia, respiratory distress syndrome, apnea, sepsis, anemia, transient tachypnea of the newborn, infective pneumonia, asphyxia, intracerebral hemorrhage, seizure, cardiomyopathy, peri- or intraventricular hemorrhage, necrotizing enterocolitis, aspiration, retinopathy of prematurity and perinatal mortality.
Results
Preterm delivery was associated with maternal asthma for each week after 33 completed weeks of gestation and not earlier. Maternal asthma also increased the adjusted odds of small for gestational age (SGA, OR=1.10, CI:1.05–1.16), NICU admission (OR=1.12, CI:1.07–1.17), hyperbilirubinemia (OR=1.09, CI:1.04–1.14), respiratory distress syndrome (OR=1.09, CI:1.01–1.19), transient tachypnea of the newborn (OR=1.10, CI:1.02–1.19), and asphyxia (OR=1.34, CI:1.03–1.75). Findings persisted for term infants (≥ 37 weeks) who had additional increased odds of intracerebral hemorrhage (OR=1.84, CI: 1.11–3.03) and anemia (OR=1.30, CI: 1.04–1.62).
Conclusions
Maternal asthma was associated with prematurity and SGA. Adverse neonatal outcomes including respiratory complications, hyperbilirubinemia, and NICU admission were increased in association with maternal asthma even among term deliveries.
Keywords: neonatal health, maternal asthma, respiratory distress syndrome, transient tachypnea of the newborn, neonatal jaundice, preterm birth
Introduction
Maternal asthma is the most common chronic disease in pregnancy1 and the rate of asthma reported during labor and delivery has nearly doubled between 1993–1997 and 2001–20052. A recent meta-analysis3 concluded that maternal asthma increased the risk of preeclampsia, low-birth weight and small for gestational age (SGA) infants as well as preterm delivery. In addition, we recently reported that women with asthma in the Consortium on Safe Labor also have increased risk for serious obstetric complications including placental abruption and pulmonary embolism as well as a higher likelihood of maternal intensive care unit admission4. Since pregnancy, labor and delivery complications are elevated among asthmatic mothers, their neonates may be expected to also have increased risks for complications. Indeed, a recent systematic review and meta-analysis5 found maternal asthma increased the risk for congenital malformations, perinatal mortality and neonatal hospitalization. However, few detailed analyses have been conducted to compare the neonatal health of the offspring of women with asthma to those without asthma, especially for less frequent adverse perinatal outcomes.
Few population-based studies have examined neonatal risks beyond preterm birth and growth restriction. Two historic population-based cohorts6;7 evaluated a small set of neonatal outcomes associated with maternal asthma; however, both had relatively modest numbers of asthmatic women which limited their statistical power. Transient tachypnea of the newborn has been associated with maternal asthma in prior studies with the association reported to be stronger in males and term infants.7 An increased risk of hyperbilirubinemia was reported among infants of mothers who took corticosteroids to manage their asthma, but no association was observed for other asthmatic mothers making it unclear whether this association was due to more severe maternal disease or a drug effect.6
The objective of this study was to examine whether the infants of asthmatic mothers experience a greater burden of complications during the newborn hospital admission compared to infants of non-asthmatic mothers in a recent, large, nationwide US cohort of singleton newborns.
Methods
The Consortium on Safe Labor (CSL) included 12 clinical centers (with 19 hospitals) across 9 American Congress of Obstetricians and Gynecologists U.S. districts. Details of the study and data collection procedures are described elsewhere8. Briefly, electronic medical records and discharge International Classification of Diseases, 9th revision (ICD-9) codes from both mother and infant hospital records were available for 228,562 deliveries among 208,695 women from 2002–2008. This analysis was restricted to singleton infants (n=223,512) among 204,180 women. Most women (n=185,785; 91.0%) contributed only one pregnancy. Women with more than one pregnancy could contribute to both the ‘no asthma’ and ‘asthma’ groups if they were diagnosed with asthma between pregnancies. Once an asthma diagnosis was recorded, it was assumed to affect all subsequent pregnancies. No information on asthma treatment was available in the delivery admission medical records. Institutional review board approval was obtained by all participating institutions.
Most neonatal complications as well as the diagnosis of maternal asthma were recorded in the medical record and supplemented with ICD-9 codes from discharge summaries (Supplemental Table 1). Neonatal complications studied included perinatal mortality (defined as fetal deaths > 23 weeks’ gestation, intrapartum deaths and neonatal deaths within the first week of life), delivery room resuscitation and the level needed, neonatal intensive care unit (NICU) admission, NICU length of stay, hyperbilirubinemia, respiratory distress syndrome, apnea, sepsis, anemia, transient tachypnea of the newborn, pneumonia, asphyxia, intracerebral hemorrhage, seizure, cardiomyopathy, peri- or intraventricular hemorrhage, necrotizing enterocolitis, aspiration, and retinopathy of prematurity. Gestational age and birth weight were reported both as continuous and categorized variables. We calculated the average gestational age and conducted fetuses-at-risk models9 comparing the risk for delivery at each gestational week from 23 to 37 weeks (e.g., <24 compared to ≥24 weeks, <25 compared to ≥25 weeks, etc.). SGA (lowest 10% of birth weight for age and sex) and large for gestational age (LGA, highest 10% of birth weight for age and sex) were calculated based on the distributions in our data after excluding missing or implausible values (n=2,649; 1.2%) and infants with unknown or ambiguous sex (n=316; 0.1%)10.
Covariates selected for inclusion in risk models were based on our prior study of obstetric risks associated with maternal asthma4. In addition, we evaluated mode of delivery and infant sex as potential confounders. Mode of delivery (vaginal or cesarean section) was included in final models but infant sex was not associated with maternal asthma.
The neonate was the unit of analysis for all statistical testing. Descriptive statistics were calculated for all study variables and significance testing was based on either logistic or Poisson regression using generalized estimating equations to account for correlations between multiple pregnancies contributed by the same woman. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using a first order autoregressive covariance structure comparing infants born to women with and without asthma. All reported odds ratios were adjusted for site and fully adjusted models included site, maternal age, maternal race/ethnicity, marital status, pre-pregnancy body mass index (BMI, weight in kilograms/height in meters squared), insurance status, smoking and alcohol use during pregnancy, presence of chronic disease (pre-existing diabetes, chronic hypertension, thyroid disease, or HIV), parity, and mode of delivery.
Additional analyses were stratified by infant sex and restricted to term infants (≥ 37 weeks). Multiple sensitivity analyses were conducted to test the robustness of our findings given potential bias or error in medical record ascertainment including: restriction to women with ICD-9 coded asthma, removing sites with asthma rates at the tails of the distribution (two sites each at the high and the low end), restriction to sites with complete data, restriction to infants with no missing data and finally, restriction to neonates from nulliparous women. Results from these analyses yielded similar findings, so only the main analysis is presented. All statistical analyses were performed using PROC GENMOD in SAS software (version 9.3, SAS Institute Inc., North Carolina, US).
Results
Newborns of mothers with asthma were more likely to be preterm, weighed significantly less and were more likely to be delivered by cesarean section, particularly after induction or spontaneous labor (Table 1). The proportion of male and female infants was similar in both groups.
Table 1.
Infant characteristics among singleton pregnancies with and without maternal asthma (n=223,512), Consortium on Safe Labor, 2002–2008.
| Infant characteristics | No Asthma n=206,468 | Asthma n=17,044 | p-valuea |
|---|---|---|---|
| Sex | |||
| Female | 100644 (48.9) | 8353 (49.1) | 0.62 |
| Male | 105354 (51.1) | 8646 (50.9) | |
| Unknown | 470 (0.2) | 45 (0.3) | |
| Gestational age, weeks (mean, sd) | 38.6 (2.3) | 38.4 (2.5) | <0.0001 |
| Term (37+ weeks) | 182850 (88.6) | 14518 (85.2) | <0.0001 |
| 34–36 weeks | 15719 (7.6) | 1653 (9.7) | |
| 29–33 weeks | 5194 (2.5) | 631 (3.7) | |
| 23–28 weeks | 2705 (1.3) | 242 (1.4) | |
| Route of delivery | |||
| Vaginal | 149199 (72.3) | 11721 (68.8) | <0.0001 |
| Cesarean section (all) | 57269 (27.7) | 5323 (31.2) | <0.0001 |
| Prelabor | 23688 (41.4) | 2193 (41.2) | |
| After induction | 14746 (25.7) | 1381 (25.9) | |
| After spontaneous labor | 18835 (32.9) | 1749 (32.9) | |
| Birth weight, grams (mean, sd)b | 3249.6 (604.4) | 3173.5 (629.6) | <0.0001 |
| Singleton infants per woman | |||
| 1 | 172355 (91.2) | 14074 (90.7) | 0.06 |
| 2 | 15878 (8.4) | 1355 (8.7) | |
| 3 | 724 (0.4) | 80 (0.5) | |
| 4 | 45 (0.02) | 5 (0.03) | |
| 5 | 1 (<.01) | 0 (0.0) | |
Numbers are frequency (%) unless otherwise specified.
P-values are based on generalized estimating equations that account for multiple pregnancies to the same woman and adjusted for site.
Birth weight was missing or implausible for 2486 infants: 2347 (1.1%) and 139 (0.8%) for neonates of mothers without and with asthma.
In fully adjusted models (Table 2), we observed the expected differences in outcomes related to fetal growth. Term infants of mothers with asthma were more likely to weigh <2500 grams (2.9% versus 2.2%; OR=1.13, CI: 1.02–1.26) and less likely to weigh ≥4000 grams (7.2% versus 8.4%; OR=0.84, CI: 0.79–0.90) compared to infants of mothers without asthma. Weight for gestational age had a similar pattern, with increased odds of SGA (11.9% versus 9.8%; OR=1.10, CI: 1.05–1.16) and lower odds of LGA (9.2% versus 10.0%; OR=0.91, CI: 0.86–0.96).
Table 2.
Infant outcomes for singleton deliveries among US women with and without asthma, Consortium on Safe Labor, 2002–2008.
| Outcomes | No Asthma n=206,468 Frequency (%) |
Asthma n=17,044 Frequency (%) |
Site-Adjusted p-valuea | Site-Adjusted Odds Ratio (95% CI)a | Fully-Adjusted Odds Ratio (95% CI)b |
|---|---|---|---|---|---|
| Perinatal mortalityc | 1333 (0.7) | 129 (0.8) | 0.18 | 1.13 [0.94, 1.36] | 1.10 [0.92, 1.33] |
| Level of resuscitation in delivery room CPAP or higherd | 3431 (1.7) | 354 (2.1) | 0.17 | 1.08 [0.97, 1.21] | 1.02 [0.91, 1.14] |
| Term birth weighte | |||||
| <2500g | 3929 (2.2) | 412 (2.9) | <0.0001 | 1.26 [1.13, 1.39] | 1.13 [1.02, 1.26] |
| 2500–3999g (ref) | 161951(89.5) | 12976 (89.9) | |||
| ≥4000g | 15133 (8.4) | 1041 (7.2) | <0.0001 | 0.84 [0.79, 0.90] | 0.84 [0.79, 0.90] |
| Size for gestational agef | |||||
| SGA | 20256 (9.8) | 2008 (11.8) | <0.0001 | 1.18 [1.13, 1.25] | 1.10 [1.05, 1.16] |
| AGA (ref) | 163094 (79.0) | 13315 (78.1) | |||
| LGA | 20616 (10.0) | 1574 (9.2) | 0.001 | 0.91 [0.86, 0.96] | 0.91 [0.86, 0.96] |
| NICU admission | 24656 (11.9) | 2589 (15.2) | <0.0001 | 1.22 [1.17, 1.28] | 1.12 [1.07, 1.17] |
| NICU length of stay, days (mean, sd) | 17.3 (27.9) | 17.0 (24.7) | 0.65 | 0.99 [0.92, 1.05] | 0.96 [0.90, 1.02] |
| Hyperbilirubinemia | 30252 (14.7) | 2554 (15.0) | 0.001 | 1.06 [1.02, 1.10] | 1.09 [1.04, 1.14] |
| Respiratory distress syndrome | 6641 (3.2) | 694 (4.1) | <0.0001 | 1.21 [1.11, 1.31] | 1.09 [1.01, 1.19] |
| Apneag | 4272 (2.3) | 447 (2.7) | 0.12 | 1.08 [0.98, 1.20] | 1.02 [0.92, 1.13] |
| Sepsis | 5659 (2.7) | 570 (3.3) | 0.007 | 1.13 [1.03, 1.24] | 1.02 [0.93, 1.12] |
| Anemia | 3826 (1.9) | 381 (2.2) | 0.07 | 1.10 [0.99, 1.23] | 1.02 [0.92, 1.14] |
| Transient tachypnea | 7230 (3.5) | 783 (4.6) | <0.0001 | 1.21 [1.12, 1.30] | 1.10 [1.02, 1.19] |
| Infective pneumonia | 1291 (0.6) | 132 (0.8) | 0.41 | 1.08 [0.90, 1.30] | 1.01 [0.84, 1.22] |
| Asphyxia | 527 (0.3) | 63 (0.4) | 0.003 | 1.50 [1.15, 1.95] | 1.34 [1.03, 1.75] |
| Intracerebral hemorrhage | 519 (0.3) | 52 (0.3) | 0.30 | 1.17 [0.87, 1.56] | 1.09 [0.81, 1.46] |
| Seizure | 419 (0.2) | 50 (0.3) | 0.03 | 1.38 [1.03, 1.87] | 1.24 [0.92, 1.69] |
| Cardiomyopathy | 188 (0.1) | 23 (0.1) | 0.85 | 1.04 [0.68, 1.61] | 0.91 [0.58, 1.43] |
| Peri- or intraventricular hemorrhage | 1192 (0.6) | 119 (0.7) | 0.49 | 1.07 [0.88, 1.29] | 0.97 [0.80, 1.18] |
| Necrotizing enterocolitis | 420 (0.2) | 35 (0.2) | 0.87 | 1.03 [0.72, 1.48] | 0.86 [0.60, 1.23] |
| Aspiration | 1046 (0.5) | 98 (0.6) | 0.51 | 1.07 [0.87, 1.32] | 1.00 [0.81, 1.24] |
| Retinopathy of prematurity | 932 (0.5) | 77 (0.5) | 0.93 | 1.01 [0.80, 1.28] | 0.93 [0.73, 1.18] |
Abbreviations: CPAP, continuous positive airway pressure; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; NICU, neonatal intensive care unit.
P-values are based on generalized estimating equations that account for multiple pregnancies to the same woman.
Adjusted for site; maternal age; maternal race/ethnicity; marital status; insurance; pre-pregnancy body-mass index; smoking and alcohol use during pregnancy; history of either pre-existing diabetes, HIV, chronic hypertension, or thyroid disease; parity; and cesarean delivery.
Perinatal mortality was defined as intrauterine death, intrapartum death or death during the first seven days of NICU admission.
Missing for 498 infants: 476 (0.2%) and 22 (0.1%) of mothers without and with asthma.
Birth weight was missing or implausible for 2486 infants: 2347 (1.1%) and 139 (0.8%) for neonates of mothers without and with asthma.
Small for gestational age (SGA) was defined as lowest 10% of birth weight for age and sex; Large for gestational age (LGA) was defined as highest 10% of birth weight for age and sex; Appropriate for gestational age (AGA) was the reference category.
Apnea is missing all observations from one study site (20,596 neonates).
Newborns whose mothers had asthma were more likely to be admitted to the NICU (15.2% versus 11.9%, OR=1.12, CI: 1.07–1.17) but had a similar NICU length of stay as infants of non-asthmatic mothers. Hyperbilirubinemia was increased when mothers had asthma (OR=1.09, CI:1.04–1.14) as were the odds of respiratory distress syndrome (4.1% versus 3.2%; OR=1.09, CI: 1.01–1.19), transient tachypnea of the newborn (4.6% versus 3.5%; OR=1.10, CI: 1.02–1.19) and asphyxia (0.4% versus 0.3%; OR=1.34, CI: 1.03–1.75). No differences in perinatal mortality, delivery room resuscitation, apnea, sepsis, anemia, pneumonia, intracerebral hemorrhage, seizure, cardiomyopathy, peri- or intraventricular hemorrhage, necrotizing enterocolitis, aspiration and retinopathy of prematurity were observed in the adjusted analyses.
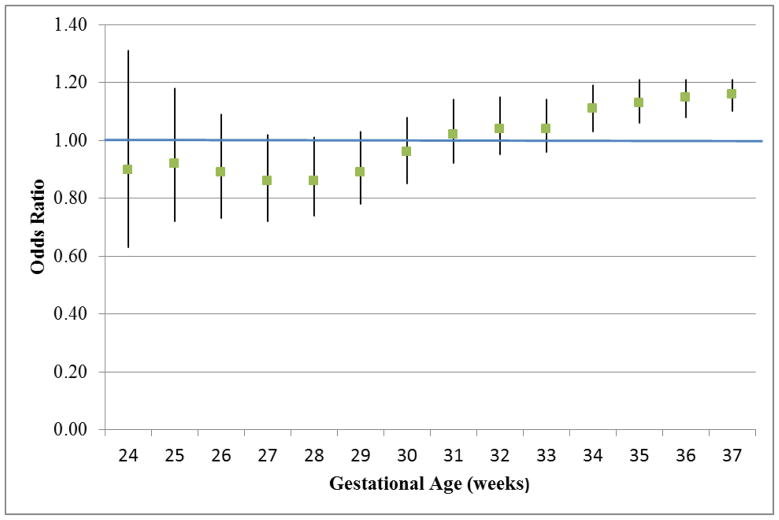
Examining the risk of preterm birth at each gestational week demonstrated that risks were comparable between women with and without asthma before 33 weeks and became significantly increased at 34, 35 and 36 completed weeks for infants born to mothers with asthma (Figure 1). There were no substantive differences when analyses were stratified by infant sex (supplemental tables 2 and 3), although these analyses have less precision due to smaller sample sizes. Restricting the analyses to term infants also yielded similar findings (supplemental table 4) with additional elevated odds of anemia (OR=1.30, CI: 1.04–1.62) and intracerebral hemorrhage (OR=1.84, CI: 1.11–3.03).
Figure 1.
The odds ratios (ORs) with 95% confidence intervals of preterm birth for each gestational week for infants born to mothers with asthma compared to infants of mothers without asthma, with a fetuses-at-risk approach where each gestational age defines the cutpoint of the comparison groups.
Discussion
Asthma is known to increase complications during pregnancy, labor and delivery4, including increased risk for preeclampsia, preterm delivery, low birth weight, and SGA3. In this large U.S. cohort study, we also found increased risk for neonatal complications associated with maternal asthma that persisted after adjustment for mode of delivery and maternal clinical and demographic factors. Newborns of mothers with asthma were more likely to be admitted to the NICU, to experience hyperbilirubinemia, and to have higher risk of respiratory complications including respiratory distress syndrome, transient tachypnea of the newborn and asphyxia. Among term infants born to mothers with asthma, the risk for these neonatal morbidities were still higher with additional increased risk for intracerebral hemorrhage and anemia compared to neonates born to mothers without asthma. Our large sample permitted a novel analysis of preterm birth risk at each gestational week from 23 to 37 and we observed that the increase in risk associated with maternal asthma was only significantly elevated after 33 weeks. We also found the anticipated decrements in fetal growth and birth weight among the infants of asthmatic mothers. Given the increasing prevalence of asthma in reproductive age women, it is reassuring that we did not observe an increased risk of perinatal mortality, apnea, sepsis, pneumonia, seizure, cardiomyopathy, peri- or intraventricular hemorrhage, necrotizing enterocolitis, aspiration or retinopathy of prematurity among the newborns of mothers with asthma.
With respect to respiratory complications, our finding for asphyxia appears to be novel with a greater than 30% increased risk among newborns of asthmatics. The risk for asphyxia was also increased greater than 80% in a sensitivity analysis restricted to term infants. Transient tachypnea of the newborn has been previously reported in association with maternal asthma, but studies were either unadjusted for cesarean delivery11 or had relatively small numbers of mothers with asthma7;11;12. We report an independent increase in risk for transient tachypnea of the newborn, even after controlling for important risk factors such as cesarean delivery as well as other maternal clinical and demographic characteristics. Both of the historic cohorts6;7 evaluated respiratory distress syndrome and reported non-significantly elevated point estimates which is consistent with our finding of a significant increase in risk associated with maternal asthma given that we have greater statistical power.
The mechanisms underlying the impact of maternal asthma and neonatal respiratory complications are not clear. It is possible that some newborn respiratory complications are due to shared genetic factors with their asthmatic mothers. For example, infants of asthmatic mothers may have variations in surfactant protein A haplotypes that could confer a non-specific risk for less optimal respiratory function13. Animal studies also suggest potential genetic pathways associated with beta-adrenergic hyporesponsiveness14 which may interfere with pulmonary fluid resorption and decreased production that normally occurs after birth15.
In our data, the odds of neonatal jaundice were elevated among infants born to women with asthma which was not entirely consistent with findings from one other cohort study with 817 asthmatic women from Halifax county in Nova Scotia. 6 In that study, hyperbilirubinemia was increased but only among the small subgroup of infants of asthmatic mothers (17% of the sample) who took steroids, resulting in 18 cases among 139 infants. Infants whose mothers took no medication (46%) or beta-agonists only (37%) had no jaundice risk with OR=1.0, CI:0.6–1.6 and OR=1.0, CI:0.6–1.7, respectively. The authors hypothesized that this could have been due to a drug effect, but whether the risk was due to more severe maternal disease was unable to be determined. It is important to note that prior studies have identified neonatal jaundice16 or treatment for jaundice17 as a predictor of childhood asthma, but these reports have not taken maternal asthma into account. Perhaps maternal asthma, which increases risk for asthma among offspring,18 is a common cause for both neonatal jaundice and childhood asthma. This intriguing possibility merits further study.
We conducted several sensitivity analyses to assess the robustness of our findings. Results restricted to term births are reported in order to lessen any potential bias due to unmeasured confounding given that preterm birth could operate as a potential mediator of the neonatal effects under study.19;20 Although the association between maternal asthma and preterm birth was strong, it is important to note that the infant complications we observed were not primarily a function of prematurity. In general, the odds of adverse outcomes were higher for term infants.
Infant sex has been posited as an influential effect modifier of both maternal and fetal/infant outcomes associated with maternal asthma21, perhaps due to sexually dimorphic placental characteristics22 or a difference in adaptation to pregnancy determined by infant sex.23 We observed very similar effects among males and females, although with less precision than in the main analysis. Prior studies that report effect modification by preterm/term status and infant sex generally have elevated point estimates with substantially overlapping confidence intervals, suggesting low statistical power and lack of precision as a likely explanation for the subgroup differences. For example, in a New Jersey cohort7 with less than 2300 asthma cases, maternal asthma increased the risk for transient tachypnea of the newborn for males (OR=1.91, CI: 1.35–2.71) but not significantly for females (OR=1.51, CI: 0.94–2.47) where precision was a bit less.
The major limitation of our study was the lack of information on asthma control, exacerbations, and treatment as these characteristics were not recorded in the delivery admission medical records. Several studies have shown that adverse outcomes are more common (or only occur) in pregnancies with poor asthma control 24–26. In a study conducted in the Maternal Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, no difference in perinatal risks were associated with asthma medication with the exception of inhaled corticosteroids which increased risks for preterm and low birth weight, likely a consequence of more severe asthma. Presumably, the asthma cases in our study represent a mix of severity and treatment methods so we cannot evaluate how well our findings apply to the subgroup of women with well controlled asthma. If treatment and good control does improve neonatal outcomes, then our findings are a conservative estimate of the impact of maternal asthma on infant health for women with more severe disease. Given that our data come from a nationwide study intended to capture current obstetric practice8 and were not selected in any way for asthma or other maternal morbidity, we feel confident that a proportion of the women studied had mild asthma or were well controlled and yet the population level risks we observed for the infants of asthmatic mothers remained high for several serious neonatal complications. We considered the possibility that the electronic medical records could have been biased and more likely to include the diagnosis of maternal asthma when complications arose, but the overall prevalence of asthma we observed (7.6%) was similar to the general population estimates of 10% for women of reproductive age and of asthma in pregnancy reported in the early 2000’s (4%–8%)1. The lack of reporting bias is further supported by the fact that our findings were similar when analyses were restricted to maternal asthma coded in the discharge summary, in case asthma only noted in the medical record was a childhood history or not active during pregnancy. Our results were also similar when we excluded sites that reported the highest and lowest prevalence of maternal asthma, suggesting that site-specific variations in reporting or true differences in asthma prevalence in the underlying populations did not drive the observed associations.
Our study is the largest to date with detailed clinical information on the impact of maternal asthma and the potential to investigate uncommon neonatal morbidities among more than 17,000 singleton infants of asthmatic mothers. The guidelines for treatment of asthma in pregnancy27 and clinical strategies to manage asthma28 are well described. Recent reports suggests that medication use is lower during early pregnancy than before pregnancy29;30 which appears to be consistent with active step therapy, although it may also indicate a reluctance of patients to continue medications. To that end, patients should be reassured that continuing asthma medication during pregnancy at the level needed to control the disease is important to improve both maternal and fetal outcomes.31 An ongoing clinical trial32 is investigating enhanced monitoring of lung function during pregnancy as a strategy for improving maternal asthma control which may, in turn, improve obstetric and neonatal outcomes. Our findings suggest that more work needs to be done in this area to further reduce neonatal morbidity.
In conclusion, maternal asthma is the most common chronic disease in pregnancy and is known to increase risk for both preterm delivery and infant growth restriction. Our large contemporary US cohort allowed us to examine preterm risk for each gestational week from 23 to 37 and to evaluate serious but less common adverse neonatal outcomes. It is reassuring that earlier preterm birth was not increased among infants of women with asthma, with comparable risks from 23 to 33 weeks gestation and significant increases appearing at 34, 35, and 36 weeks. Many serious complications were not elevated in newborns of asthmatic mothers despite their mothers increased risk of pregnancy and obstetric complications4. However, we did find a higher risk of NICU admission, hyperbilirubinemia and respiratory complications in neonates of mothers with asthma compared to newborns of women without asthma and these risks were independent of prematurity or the increase in cesarean delivery. In particular, respiratory distress syndrome, asphyxia and transient tachypnea of the newborn occurred more frequently in infants whose mothers have asthma. Future work should consider the impact of maternal asthma on neonatal health including whether optimizing maternal asthma control can mitigate newborn risks.
Supplementary Material
Clinical Implications.
Both term and preterm infants of asthmatic mothers experience more newborn complications, including serious morbidity. Additional research is needed on asthma management during pregnancy to attempt to mitigate newborn morbidity.
Acknowledgments
Funding Source: This research was supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. The Consortium on Safe Labor was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through Contract No. HHSN267200603425C.
Institutions involved in the Consortium on Safe Labor include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas.
Abbreviations
- AGA
appropriate for gestational age
- BMI
body mass index
- CSL
Consortium on Safe Labor
- CPAP
continuous positive airway pressure
- ICD-9
International Classification of Diseases, version 9
- LGA
large for gestational age
- NICU
neonatal intensive care unit
- OR
odds ratio
- SGA
small for gestational age
- CI
95% confidence interval (CI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kwon HL, Triche EW, Belanger K, Bracken MB. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin North Am. 2006;26:29–62. doi: 10.1016/j.iac.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet Gynecol. 2009;113:1075–81. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 3.Murphy VE, Namazy JA, Powell H, Schatz M, Chambers C, Attia J, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118:1314–23. doi: 10.1111/j.1471-0528.2011.03055.x. [DOI] [PubMed] [Google Scholar]
- 4.Mendola P, Laughon SK, Mannisto TI, Leishear K, Reddy UM, Chen Z, et al. Obstetric complications among US women with asthma. Am J Obstet Gynecol. 2013;208:127–28. doi: 10.1016/j.ajog.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy V, Wang G, Namazy J, Powell H, Gibson P, Chambers C, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG. 2013;120:812–22. doi: 10.1111/1471-0528.12224. [DOI] [PubMed] [Google Scholar]
- 6.Alexander S, Dodds L, Armson BA. Perinatal outcomes in women with asthma during pregnancy. Obstet Gynecol. 1998;92:435–40. doi: 10.1016/s0029-7844(98)00191-4. [DOI] [PubMed] [Google Scholar]
- 7.Demissie K, Marcella SW, Breckenridge MB, Rhoads GG. Maternal asthma and transient tachypnea of the newborn. Pediatrics. 1998;102:84–90. doi: 10.1542/peds.102.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yudkin PL, Wood L, Redman CW. Risk of unexplained stillbirth at different gestational ages. Lancet. 1987;1:1192–94. doi: 10.1016/s0140-6736(87)92154-4. [DOI] [PubMed] [Google Scholar]
- 10.Mannisto T, Mendola P, Reddy U, Laughon SK. Neonatal Outcomes and Birth Weight in Pregnancies Complicated by Maternal Thyroid Disease. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark JM, Hulme E, Devendrakumar V, Turner MA, Baker PN, Sibley CP, et al. Effect of maternal asthma on birthweight and neonatal outcome in a British inner-city population. Paediatr Perinat Epidemiol. 2007;21:154–62. doi: 10.1111/j.1365-3016.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 12.Schatz M, Zeiger RS, Hoffman CP, Saunders BS, Harden KM, Forsythe AB. Increased transient tachypnea of the newborn in infants of asthmatic mothers. Am J Dis Child. 1991;145:156–58. doi: 10.1001/archpedi.1991.02160020046013. [DOI] [PubMed] [Google Scholar]
- 13.Pettigrew MM, Gent JF, Zhu Y, Triche EW, Belanger KD, Holford TR, et al. Respiratory symptoms among infants at risk for asthma: association with surfactant protein A haplotypes. BMC Med Genet. 2007;8:15. doi: 10.1186/1471-2350-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes PJ. Endogenous catecholamines and asthma. J Allergy Clin Immunol. 1986;77:791–95. doi: 10.1016/0091-6749(86)90375-1. [DOI] [PubMed] [Google Scholar]
- 15.Wyszogrodski I, Taeusch HW, Jr, Avery ME. Isoxsuprine-induced alterations of pulmonary pressure-volume relationships in premature rabbits. Am J Obstet Gynecol. 1974;119:1107–11. doi: 10.1016/0002-9378(74)90267-1. [DOI] [PubMed] [Google Scholar]
- 16.Ku MS, Sun HL, Sheu JN, Lee HS, Yang SF, Lue KH. Neonatal jaundice is a risk factor for childhood asthma: a retrospective cohort study. Pediatr Allergy Immunol. 2012;23:623–28. doi: 10.1111/j.1399-3038.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- 17.Aspberg S, Dahlquist G, Kahan T, Kallen B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21:e733–e739. doi: 10.1111/j.1399-3038.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 18.Martinez FD. Maternal risk factors in asthma. Ciba Found Symp. 1997;206:233–39. [PubMed] [Google Scholar]
- 19.VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23:1–9. doi: 10.1097/EDE.0b013e31823aca5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–68. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clifton VL, Davies M, Moore V, Wright IM, Ali Z, Hodyl NA. Developmental Perturbation Induced by Maternal Asthma during Pregnancy: The Short- and Long-Term Impacts on Offspring. J Pregnancy. 2012;2012:741613. doi: 10.1155/2012/741613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clifton VL. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell Tissue Res. 2005;322:63–71. doi: 10.1007/s00441-005-1117-5. [DOI] [PubMed] [Google Scholar]
- 23.Dodds L, Armson BA, Alexander S. Use of asthma drugs is less among women pregnant with boys rather than girls. BMJ. 1999;318:1011. doi: 10.1136/bmj.318.7189.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enriquez R, Griffin MR, Carroll KN, Wu P, Cooper WO, Gebretsadik T, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120:625–30. doi: 10.1016/j.jaci.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Bakhireva LN, Schatz M, Jones KL, Chambers CD. Asthma control during pregnancy and the risk of preterm delivery or impaired fetal growth. Ann Allergy Asthma Immunol. 2008;101:137–43. doi: 10.1016/S1081-1206(10)60201-3. [DOI] [PubMed] [Google Scholar]
- 26.Schatz M, Dombrowski MP, Wise R, Momirova V, Landon M, Mabie W, et al. Spirometry is related to perinatal outcomes in pregnant women with asthma. Am J Obstet Gynecol. 2006;194:120–26. doi: 10.1016/j.ajog.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 27.National Asthma Education and Prevention Program. Working Group Report on Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment, Update 2004. Department of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute; 2005. [Google Scholar]
- 28.Dombrowski MP, Schatz M. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 90, February 2008: asthma in pregnancy. Obstet Gynecol. 2008;111:457–64. doi: 10.1097/AOG.0b013e3181665ff4. [DOI] [PubMed] [Google Scholar]
- 29.Zetstra-van der Woude PA, Vroegop JS, Bos HJ, de Jong-van den Berg LT. A population analysis of prescriptions for asthma medications during pregnancy. J Allergy Clin Immunol. 2013;131:711–17. doi: 10.1016/j.jaci.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Enriquez R, Wu P, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol. 2006;195:149–53. doi: 10.1016/j.ajog.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 31.Dombrowski MP, Schatz M. Asthma in pregnancy. Clin Obstet Gynecol. 2010;53:301–10. doi: 10.1097/GRF.0b013e3181de8906. [DOI] [PubMed] [Google Scholar]
- 32.Lim A, Stewart K, Abramson MJ, Walker SP, George J. Multidisciplinary approach to management of maternal asthma (MAMMA [copyright]): the PROTOCOL for a randomized controlled trial. BMC Public Health. 2012;12:1094. doi: 10.1186/1471-2458-12-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.