Abstract
Background
The Asthma Control Questionnaire (ACQ) is a patient-centered tool for evaluating asthma control. It has been validated in adults but not well-validated among children.
Objective
We evaluated the reliability, validity, and responsiveness to change of the ACQ for assessing asthma control in children ages 6 to 17. A threshold value for poor disease control and a minimally important difference (MID) were also determined.
Methods
Data from 305 asthmatic children enrolled in a clinical trial were examined. The ACQ was administered at 8 visits. We analyzed results for the combined age group and for age groups 6 to 11 and 12 to 17 separately.
Results
Overall, the Cronbach’s alpha (internal consistency) for the ACQ was 0.74 at baseline, and the intraclass correlation coefficient (test-retest reliability) for repeated questionnaires among stable patients was 0.53. The Pearson correlations between the ACQ and other asthma questionnaires were moderate to strong (−0.64 to −0.73). Mean ACQ scores were higher (worse) in patients whose peak flow decreased, who used more rescue medications, or who sought medical care for asthma than in patients who were stable (P<0.0001 for all measures). Change in ACQ scores were significantly different among patients with deteriorating, improving, or stable asthma symptoms (P ≤0.01). The optimal threshold indicating poor asthma control was ≥1.25. The MID was established to be 0.40. Results for the separate age groups were similar.
Conclusion
The ACQ is a moderately reliable, valid, and responsive tool with adequate psychometric properties for assessing recent asthma control among children.
Keywords: asthma, asthma control, Asthma Control Questionnaire, validity, minimally important difference
INTRODUCTION
Establishing asthma control is a cornerstone of asthma therapy. Asthma control is defined by several factors including pulmonary function, symptoms, and activity limitation. Several measurement tools have been validated for use in assessing asthma control among adults including the Asthma Control Test (ACT) and the Asthma Control Questionnaire (ACQ)1, 2. At present, the childhood ACT (cACT) is the only questionnaire recommended for assessing asthma control in children under the age of 123.
The ACQ is an attractive alternative to the cACT because it would be useful to have a single instrument that could be used in both adults and children. The ACQ scale is the same in adults and children and scoring is the same, whereas the cACT and ACT have different scales for adults and children making it difficult to combine the scores in studies that include adults and children. Validation of the ACQ among children is limited. A study by Juniper et al. with 35 pediatric patients from a single center demonstrated the ACQ to be valid and reliable in assessing asthma control among children4. However, this study used a U.K. version of the questionnaire that offered alternative wording to younger children administered by trained interviewers.
In this study, we evaluated the reliability, validity, and responsiveness of the ACQ among pediatric-age patients with poorly-controlled asthma enrolled in a multicenter clinical trial. We examined threshold values to distinguish controlled versus uncontrolled asthma. Moreover, we estimated the minimally important difference, or the smallest difference in the mean score that is associated with a clinically significant change in asthma control level as assessed by daily diary records of beta-agonist use, urgent asthma care, prednisone use, nocturnal awakenings and peak expiratory flow rate (PEFR).
METHODS
ACQ Questionnaire
The ACQ consists of 6 questions about key symptoms (dyspnea and wheezing), daytime and nighttime symptoms, activity limitation, and rescue bronchodilator use in the past 7 days. One response is determined by the FEV1% predicted2. Each item is scored on a 7-point scale ranging from 0 to 6. The final score is generated by averaging the total scores for the 7 items. Higher scores indicate worse asthma control. In this study, children ages 11 to17 self-completed the questionnaire. Children ages 6 to 10 completed the questionnaire with the assistance of an accompanying adult, either a parent or guardian5.
Data collection
Patients
Data were collected from patients enrolled in a clinical trial, the Study of Acid Reflux in Children with Asthma (SARCA)6. Three hundred and five patients with inadequately controlled asthma despite treatment with inhaled corticosteroids age 6–17 years were enrolled. Poor asthma control was defined as one or more of the following: use of short-acting bronchodilators 2 or more times weekly; nocturnal asthma awakenings more than once weekly during the prior month; 2 or more asthma exacerbations requiring urgent medical care in the prior year; or a score of 1.25 or higher on the Asthma Control Questionnaire (ACQ) at the screening visit. One patient who turned 18 during the study was excluded from the analysis. The study was approved by institutional review boards of all participating centers and registered at clinicaltrials.gov (NCT00442013). Parents of participants provided written informed consent for enrollment in the clinical trial. We received permission from all questionnaire copyright holders to administer their respective questionnaires to participants enrolled in the trial. Elizabeth Juniper approved the use and format of the ACQ for this trial, and provided instructions for its administration.
Procedures
Participants were evaluated at 8 visits separated by approximately 4 weeks. Patients, or parents for patients ages 6 to 10, kept daily asthma diaries that included morning PEFR, asthma symptom scores, beta-agonist use, nocturnal asthma awakenings, asthma treatments, and health care use. At each visit, questionnaires were completed by the patient (ages 11 and above) or with the assistance of a parent/guardian (ages 6 to 10), including: the ACQ, cACT (ages 6 to11), ACT (ages 12 to 17), the Asthma Symptom Utility Index (ASUI), and the Pediatric Asthma Quality of Life Questionnaire (pAQLQ). The cACT is a 7-question instrument for use among children ages 4 to 11 with 4 questions answered by the child and 3 questions answered by the caretaker7. The ACT is a 5-question, well-validated tool for assessing asthma control among individuals ages 12 and above1. The ASUI is a10-item instrument designed to assess the frequency and severity of four asthma symptoms (cough, wheeze, dyspnea, and awakening at night) and side effects, weighted by patient preferences8. The pAQLQ is a 23-item survey in three domains (symptoms, activity limitation, and emotional function) that measures functional impairments in children ages 7 to 179.
During the course of the study, patients were monitored for episodes of poor asthma control (EPACs), which were defined as the occurrence of one or more of the following clinical events: decrease of ≥30% AM PEFR (from personal best during 2 week run-in period) for 2 consecutive days; addition of oral corticosteroids to treat asthma symptoms; urgent care for asthma; or increased use of rescue medication(s) from baseline (i.e., either 4 or more additional puffs of bronchodilator or 2 or more additional nebulizer treatments in one day)6. The EPAC was determined from the daily asthma diaries over a four week period between visits.
Assessments
Statistical Analysis
All analyses were stratified by age, 6 to 11 versus 12 to 17, and then for the entire sample. We analyzed these two groups separately because they correspond to the age ranges used for the cACT and ACT respectively. We used diary card data to evaluate a patient’s asthma control for the period between visits with either the ACQ score at the end of the period or the change in ACQ scores between visits as the dependent variable. Analyses that included multiple measurements from patients used generalized estimating equations (GEE) to account for the correlation between repeated measures from the same patient10.
Reliability
Internal consistency and test-retest statistical methods were used to evaluate the reliability of the ACQ. For internal consistency, the Cronbach’s alpha value was calculated based on ACQ scores in patients at baseline visit. Test-retest reliability was assessed with the intraclass correlation coefficient based on ACQ scores in stable patients who maintained good asthma control between two consecutive follow-up visits without an EPAC.
Construct validity
Construct validity was evaluated by determining the Pearson correlation coefficient between the ACQ scores at baseline visit with cACT or ACT, ASUI and pAQLQ scores.
The ability of the ACQ to discriminate patient groups with different levels of asthma control was assessed by comparing mean ACQ scores across groups who had the presence or absence of clinical events that constitute an EPAC during the prior period. Mean ACQ scores were compared between the patient groups who did or did not have the specific clinical event with ANOVA adjusted for repeated measures.
Responsiveness
Four levels of asthma control were defined based on the patient’s record of EPACs during follow-up: ‘good control’ -- no events between visits; ‘worsening control’ -- good control followed by an event before the next visit; ‘improved control’ -- an event in the prior period but no event in the subsequent period; and ‘continuing poor control’ -- an event in the prior period and in the subsequent period. Responsiveness of the ACQ was assessed by evaluating the change in the ACQ from the beginning to the end of a period. The mean change in ACQ scores were compared across the four control levels using ANOVA with adjustment for repeated measures.
Diagnostic Assessment
Mean ACQ scores were compared by ANOVA between patient groups who did or did not have an EPAC. ACQ threshold values for determining uncontrolled asthma were evaluated with receiver operating characteristic (ROC) analyses. Occurrence of an EPAC in the prior period was used as the gold standard for determining uncontrolled asthma. We analyzed multiple threshold values of ACQ from 0.25 to 2.00 to calculate sensitivity, specificity, positive and negative predictive values, percent correctly classified, and area under the ROC curve.
Minimally Important Difference (MID)
Distribution- and anchor-based approaches were used to determine the MID, the smallest mean change in the ACQ score considered clinically-meaningful. The distribution-based approach analyzes the properties of the scale using the statistical properties of the scale, whereas the anchor-based approach employs clinical reference points to determine the MID11–15. These methods have been used for estimation of the MID for quality of life, health status, and other patient reported outcomes13. For the distribution-based analysis, baseline data were used to determine the standard deviation (SD) and standard error of measurement (SEM) of the ACQ. The SEM was calculated according to the following formula: SEM=SDACQ*[square root(1- ReliabilityACQ)], where reliability corresponded to Cronbach’s alpha. The MID was defined by both 0.5*SD16 and 1*SEM17. For the anchor-based approach, the mean ACQ scores between patient groups who differed on the presence or absence of four clinical components that constitute an EPAC during the prior period were used to estimate the magnitude and significance of differences in mean ACQ scores between these groups, which corresponded to the MID.
RESULTS
Study Population
Three hundred and five children participated in the study. Completed diaries and corresponding ACQ scores were obtained on 96% of participants, and were available for 1555 out of 1836 possible visits (85%). Participant characteristics are shown in Table I.
Table I.
Sample characteristics.
| Age (years), No. (%) | |
| All | 305 (100) |
| 6 to 11 | 164 (54) |
| 12 to 17 | 141 (46) |
| Age at randomization, mean (SD), years | 11(3) |
| Sex, No. (%) | |
| Female | 118 (39) |
| Male | 187 (61) |
| Race, No. (%) | |
| White | 102 (34) |
| Black | 154 (50) |
| Hispanic | 34 (11) |
| Other | 15 (5) |
| Asthma Characteristics | |
| Age at asthma onset, mean (SD), years | 3 (3) |
| Urgent care for asthma in past year, No. (%) | 224 (73) |
| Oral corticosteroids for asthma in past year, No. (%) | 206 (67) |
| Use of rescue inhaler 2 or more times/week*, No. (%) | 227 (74) |
| Daily use of ICS/LABA in past 6 months, No. (%) | 177 (58) |
| Daily use of leukotriene modifying agent, No. (%) | 167 (55) |
| Self-Reported Atopic Conditions, No. (%) | |
| Rhinitis | 181 (59) |
| Eczema | 133 (43) |
| Food allergies | 81 (26) |
| Allergies worsen asthma | 245 (80) |
| Asthma Questionnaires, mean (SD) | |
| ACQ at randomization+ | 1.2 (0.8) |
| cACT (ages 6-11) ++ | 19 (4) |
| ACT (ages 12-17)# | 20 (4) |
| ASUI## | 0.82 (0.15) |
| Lung Function, mean (SD) | |
| Pre-bronchodilator FEV1, % predicted | 91.9 (15.9) |
| Post-bronchodilator FEV1, % predicted | 99.7 (14.8) |
| Pre-bronchodilator FVC, % predicted | 100.8 (14.2) |
| Post-bronchodilator FVC, % predicted | 103.0 (14.2) |
| Change in FEV1 after bronchodilator | 9.7 (11.1) |
| Change in FVC after bronchodilator | 2.8 (6.2) |
Use of rescue inhaler is based on self-report of average use in month prior to screening visit.
Range of ACQ score is 0 to 6 with lower scores indicating better asthma control.
Range of cACT score is 0 to 27 with higher scores indicating better asthma control.
Range of ACT score is 5 to 25 with higher scores indicating better asthma control.
Range of ASUI score is 0 to 1 with higher scores indicating better asthma control.
Reliability
The Cronbach’s alpha of the ACQ was 0.74 for the entire sample (n=305), 0.75 for ages 6 to 11 group (n=164), and 0.72 for ages 12 to 17 group (n=141) at the baseline visit. A value greater than 0.70 is considered sufficiently internally consistent to compare different study groups whereas a value greater than 0.90 is considered necessary to apply the metric to an individual. The intraclass correlation coefficient (ICC) for ACQ scores between two consecutive visits among stable subjects who reported no EPAC for that period ranged between 0.42 to 0.82 for the overall group (Table II), 0.34 to 0.95 for the 6 to 11 age group (see Table E1in the Online Repository) and 0.22 to 0.74 for the 12 to 17 age group (see Table E1in the Online Repository). Taking into account all stable visit pairs, the ICC from a random effects model is 0.53 including 911 of 1566 visits from 258 participants with one or more stable interval. For children age 6–11 ICC is 0.62 and 0.45 for children 12 and older. The reliability of the instrument is therefore moderate for the overall group but only fair in older children.
Table II.
Intraclass correlation coefficient for ACQ between consecutive visits for stable patients.
| Overall | N | ICC |
|---|---|---|
| Visit | ||
| V2-V4 | 60 | 0.60 |
| V4-V5 | 60 | 0.50 |
| V5-V6 | 54 | 0.70 |
| V6-V7 | 53 | 0.42 |
| V7-V8 | 49 | 0.55 |
| V8-V9 | 49 | 0.82 |
Construct validity
Statistically significant correlations were present between the ACQ versus cACT/ACT for the entire sample, for ages 6 to 11(Figure I, Panel A), and for ages 12 to 17(Figure I, Panel B) at the baseline visit (p<0.0001 for all groups). Similar findings were seen for the ACQ versus pAQLQ and ACQ versus ASUI (Table III, see Table E2 in the Online Repository).
Figure I.
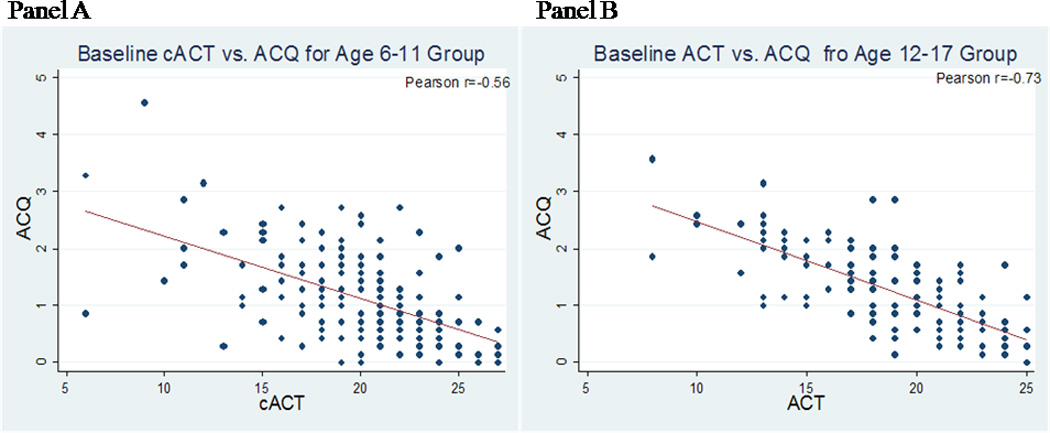
A and B. Scatter plots, regression line, and Pearson correlation coefficient for the ACQ and cACT (age 6 to 11), Panel A, with equation of y(ACQ) = 3.31−0.11x(cACT), or ACT (age 12 to 17), Panel B, with equation of y(ACQ) = 3.82−0.14x(ACT).
Table III.
Correlation of ACQ to other asthma questionnaires for the entire sample.
| Questionnaire | Pearson Correlation Coefficient (r) | 95% CI |
|---|---|---|
| cACT/ACT | −0.64 | −0.57,−0.70 |
| pAQLQ | −0.68 | −0.62,−0.74 |
| ASUI | −0.73 | −0.68,−0.78 |
All groups were statistically significant with P<0.0001.
Mean ACQ scores were different between patients who had experienced an EPAC in the prior period versus those who did not (Table IV; see Table E3 in the Online Repository). The mean ACQ scores were significantly higher, indicating worse control, among patients who had experienced an EPAC compared to those who did not for the overall group and each age subgroup. Depending on the type of event indicating poor control, the group difference ranged from 0.34 to 0.59.
Table IV.
Mean ACQ Scores and mean differences in ACQ scores between patients with and without an EPAC in the prior period for the entire sample.
| Overall | ACQ Mean (SD) | Difference in ACQ | |||
|---|---|---|---|---|---|
| Clinical Event | #EPAC/%visits | EPAC | No EPAC | Mean (SD) | P-value |
| EPAC | 655/42% | 1.31 (0.04) | 0.93 (0.04) | 0.38 (0.01) | <0.0001 |
| EPAC components | |||||
| Decrease in PEFR | 458/29% | 1.33 (0.05) | 0.99 (0.04) | 0.34 (0.02) | <0.0001 |
| Increase Rescue Medication Use | 309/20% | 1.50 (0.06) | 0.98 (0.03) | 0.52 (0.02) | <0.0001 |
| Urgent Care* | 87/6% | 1.64 (0.10) | 1.05 (0.04) | 0.59 (0.07) | <0.0001 |
| Systemic Corticosteroid | 153/10% | 1.51 (0.09) | 1.04 (0.04) | 0.47 (0.05) | <0.0001 |
Urgent care is defined as “urgent unscheduled healthcare contact for asthma” and includes emergency department, hospital, clinic or doctor visits
A subgroup of children had measures of airway inflammation measured. We found no significant baseline correlation of exhaled NO (n=146) or expired breath condensate pH (n=239) with ACQ scores.
Responsiveness
Responsiveness of the ACQ to changes in asthma control was demonstrated by evaluating the change in ACQ scores between visits. Mean change in scores in patients who did not experience a clinical event during the period or continued to have poor asthma control were close to zero (Table V). In contrast, the change in scores for patients with either improved or deteriorating control during the period either decreased (better control) or increased (worsening control), respectively (Table V; see Table E4 in the Online Repository). Mean change in ACQ score differed significantly across groups of patients based on their state for the overall group (p<0.0001), 6 to 11 age group (p<0.001), and 12 to 17 age group (p=0.01).
Table V.
Mean change in ACQ scores between consecutive visits by asthma control status for the entire sample.
| Status* | N | Visit Periods | Mean Change in ACQ | 95% CI |
|---|---|---|---|---|
| Overall | ||||
| Good Control | 196 | 547 | −0.02 | −0.05, 0.01 |
| Worsening Control | 155 | 177 | 0.26 | 0.11, 0.41 |
| Improved Control | 165 | 200 | −0.33 | −0.44, −0.22 |
| Continuing Poor Control | 148 | 337 | 0.06 | 0.00, 0.12 |
Status: Good Control= no EPAC for two consecutive visits, where EPAC is defined as ≥30% decrease in PEFR on 2 consecutive days or requirement for rescue bronchodilator usage, urgent care visit, or systemic cortico therapy; Worsened Control= no EPAC prior to first visit, EPAC between first and second visit; Improved Control= EPAC prior to first visit, no EPAC between first and second visit; Continued Poor Control= EPAC before and after first visit in sequence. Differences between categories of control was significant by ANOVA (P<0.0001).
Diagnostic Assessment for detecting poorly-controlled asthma
The mean ACQ scores presented in Table IV were used to generate the range of ACQ scores applied as threshold values for distinguishing controlled from uncontrolled asthma. Table VI lists the diagnostic test accuracy of various ACQ score thresholds for detecting poorly-controlled asthma with their respective sensitivity, specificity, positive and negative predictive values, percent correctly classified, and area under the ROC curve for the overall group using events from the asthma diary as the reference standard. ACQ scores<0.4 had good sensitivity that was >90% (but poor specificity) while ACQ scores >2.0 had good specificity that was >90% (but poor sensitivity). The ACQ score associated with the highest area under the ROC curve was 1.25, with a sensitivity of 53%, specificity 67%, and positive and negative predictive values of 58 and 62%, respectively. The threshold value corresponding to an episode of poor asthma control was less (0.25) in the 6–to−11 year olds than the 12−to−17 year olds (0.4) (see Table E5 in the Online Repository).
Table VI.
Testing parameters for ACQ threshold values for determining poorly-controlled asthma for the entire sample.
| Overall ACQ Threshold Score |
Sensitivity (%) |
Specificity (%) |
Positive Predictive Value (%) |
Negative Predictive Value (%) |
Correctly Classified (%) |
Area under ROC curve |
|---|---|---|---|---|---|---|
| ≥0.40 | 90.2 | 15.9 | 48.4 | 64.9 | 50.5 | 0.530 |
| ≥0.55 | 86.4 | 23.2 | 49.6 | 66.0 | 52.7 | 0.548 |
| ≥0.70 | 81.1 | 34.4 | 51.9 | 67.5 | 56.2 | 0.577 |
| ≥0.85 | 72.7 | 40.4 | 51.6 | 62.9 | 55.5 | 0.566 |
| ≥1.00 | 65.9 | 51.0 | 54.0 | 63.1 | 58.0 | 0.585 |
| ≥1.10 | 59.8 | 57.6 | 55.2 | 62.1 | 58.7 | 0.587 |
| ≥1.25 | 53.0 | 66.9 | 58.3 | 62.0 | 60.4 | 0.600 |
| ≥1.40 | 47.0 | 71.5 | 59.0 | 60.7 | 60.1 | 0.592 |
| ≥1.50 | 38.6 | 76.8 | 59.3 | 58.9 | 59.0 | 0.577 |
| ≥1.70 | 33.3 | 81.5 | 61.1 | 58.3 | 59.0 | 0.574 |
| ≥1.85 | 25.0 | 87.4 | 63.5 | 57.1 | 58.3 | 0.562 |
| ≥2.00 | 23.5 | 91.4 | 70.5 | 57.7 | 59.7 | 0.574 |
Minimally Important Difference
The MID or smallest difference in the score that represents a clinically meaningful change was determined through two approaches. The distribution-based method gave an MID of 0.375 using 0.5*SD and 0.382 using SEM for the overall group; 0.40 using both 0.5*SD and SEM for the 6–to−11 age group; and 0.35 using 0.5*SD and 0.37 using SEM for the 12−to−17 age group. For the anchor-based approach, the mean ACQ scores among patients experiencing a specified clinical event related to an EPAC between visits were compared to those who did not. The difference between those groups was statistically significant for all of the EPAC components (Table IV; see Table E3 in the Online Repository). For example, patients reported use of systemic corticosteroids for asthma 153 times during the study; the difference between ACQ scores for those patient-visits versus patient-visits not reporting corticosteroid use in the prior interval was 0.47 (SD 0.05). Overall, the mean difference in ACQ scores was 0.4–0.5 (range from 0.34 to 0.59 points) for the combined age group, 0.5–0.6 (range from 0.39 to 0.65 points) for the 6-to-11 age group, and 0.3–0.4 (range from 0.27 to 0.54 points) for the 12-to-17 age group. Anchoring the ACQ against the ACT using 3-points as the MID of the ACT gives an estimated MIDACQ of 0.42 for the 12-to-17 age group as based on the equation generated from the regression line (Figure I, Panel B). Although no MID has been established for the cACT, a 3-point change in the cACT corresponds to an estimated MIDACQ of 0.33 for children ages 6 to 11 based on the regression equation (Figure I, Panel A). Thus, using the triangulation method18, the estimated MID is 0.40 for the overall group.
DISCUSSION
The ACQ is a moderately reliable and responsive tool to assess asthma control in a sample of 6-to-17 year old children with poor asthma control at baseline enrolled in a clinical trial. We estimated the MID for the ACQ to be 0.40 and the optimal threshold value for distinguishing between well-controlled and poorly-controlled pediatric asthma groups to be 1.25, similar to values established for adults19, 20. The results of this study support findings from the previous study of the ACQ in children using a slightly different questionnaire format4. Our study extends these findings in a larger population studied over multiple visits at multiple sites. We did not find any substantial difference between younger and older children for most of the metrics evaluated.
Asthma control questionnaires are commonly used to evaluate individual patient’s asthma control and also as outcome assessment tools for comparing treatments. Although several well-validated questionnaires, including the ACQ and ACT, are available for assessing asthma control among adults, there are few well-validated tools for use in children. The recent NIH workshop recommended the cACT in ages 5 to 11 and ACT in ages 12 to 17 for use in children3. The cACT assesses asthma control in the past four weeks, and thus may be prone to imperfect recall. The ACQ assesses control over the past week, and thus, may be more appropriate than the cACT when a short-term estimate of asthma control is needed.
In this study, the ACQ demonstrated fair to good internal consistency for the overall group and separate age groups based on Cronbach’s alpha. Test-retest reliability as determined by the intraclass correlations was moderate for the overall group but only fair in children 12 and older. The construct validity, as well as the responsiveness of the ACQ, were also examined in this study. Given that there is no gold standard for assessing asthma control, other asthma questionnaires and the occurrence of clinical events related to asthma control (EPACs) were employed to evaluate these properties. Measures of lung function were not used as a reference standard because FEV1 is a component of the ACQ. The results of this study showed the ACQ to have good construct validity as it correlated well with the ACT and cACT, pediatric AQLQ, and ASUI for the overall and separate age groups. Moreover, the ACQ could distinguish between groups of patients who differed on the presence or absence of clinical events related to asthma control. Similarly, the ACQ demonstrated responsiveness by showing changes in the ACQ score that tracked with changes in asthma control as assessed by diaries for the overall and separate age groups.
The diagnostic test characteristics of the ACQ for determining uncontrolled asthma were also examined in this study. The optimal threshold for distinguishing good asthma control from poor control was 1.25 for the overall group. However, the diagnostic accuracy for the ACQ was lower than the ACT and cACT. Perhaps this was due to the different criteria used in defining asthma control for these questionnaires, namely, physician’s global assessment1,7, rather than asthma diaries used in our study. In the previous validation study of ACQ in children, there was a modest correlation between change in ACQ scores and physician global change scores; the difference in ACQ between those who were stable and those who changed status on the physician global change score was not significant (p=0.07)4.
The minimally important difference that would indicate a clinically-meaningful change for the ACQ among children was also assessed. There was consistency among the values obtained by distributive and anchor-based approaches of 0.40, although the 6 to 11 age group showed slightly higher MID values compared to the 12 to 17 age group. This is similar, but not identical to the previous MID estimates of 0.50 for adults and children4, 21. Moreover, results for the overall and separate age groups were similar in all measures evaluated, though the minimally important difference showed slightly higher values for the 6 to 11 age group and slightly lower values for the 12 to 17 age group.
There were several strengths in this study. It involved a large sample from multiple sites and a diverse population with a substantial number of minorities. Moreover, this study used objective measures of asthma control, including symptoms and physiology, which were collected during the look-back period at each visit. Additionally, patients were selected for poor asthma control so there was considerable variability from visit to visit, which allowed responsiveness and the MID to be assessed.
This study also had several limitations. There were a small number of stable patients, which limited the ability to assess test-retest reliability. Also, the treatment given in the trial ultimately was determined to be ineffective, therefore responsiveness to known beneficial treatment could not be assessed and, instead, variation of asthma control over time was used. These are similar but not identical phenomena. Moreover, concurrent physician assessment of asthma control was not available in this study, which precluded direct comparison with prior studies. Our subgroups of children in each of the age strata who had serious asthma attacks (Table E3) were too small to allow us to draw conclusions about the utility of the instrument to predict risk. In the prior validation study in children, the questionnaire that was used incorporated the U.K. English version and permitted alternative wording for younger children. In this study, we used the North American English version and permitted an adult to assist the younger children in completing the questions. This may have accounted for the higher reliability of the test in the younger children than the adolescents.
The results of this study demonstrated the ACQ to be a valid and moderately responsive tool in assessing asthma control among pediatric patients ages 6 to 17 in a clinical trial. It provides support for an additional tool that can be used in assessing asthma control among children. The ACQ has certain advantages and disadvantages compared to the cACT/ACT; comparison of the psychometric properties of the ACQ based on the results of our study to that of the cACT based on a previous study7 is listed (see Table E6 in the Online Repository). The cACT/ACT have an advantage in that they are in the public domain, therefore less costly to use. Moreover, the cACT/ACT do not require measurement of FEV1, which may not be available in all settings. However, shorter versions of the ACQ, namely, the ACQ5 and ACQ6, which do not use FEV1, have been used in adults but require further evaluation in children21.
The main advantage of the ACQ is that it has similar psychometric characteristics in adults and children, permitting the use of a single instrument in clinical trials that include both children and adults. In contrast, the cACT/ACT have different ranges, which make it difficult to analyze combined child and adult data. Overall, the ACQ represents a useful, concise tool with symptom and physiologic measures to assess asthma control among adults and children in clinical trials. The ACQ may also be used in a clinical setting but the moderate reliability and modest diagnostic sensitivity and specificity limit its utility for diagnosing and following individual children.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge the contribution of the following American Lung Association Asthma Clinical Research Centres towards these studies.
Baylor College of Medicine, Houston: N. A. Hanania (principal investigator), M. Sockrider (co-principal investigator), L. Bertrand (principal clinic coordinator), M. Atik (coordinator), L. Giraldo (coordinator), B. Lopez (coordinator);
Columbia University–New York University Consortium, New York: J. Reibman (Principal investigator), E. DiMango (co-principal investigator), L. Rogers (co-principal investigator), E. Fiorino (co-investigator at New York University), C. Cammarata and K.Carapetyan (clinic coordinators at New York University), J. Sormillon and E. Simpson (clinic coordinators at Columbia University);
Duke University Medical Center, Durham, N.C.:L. Williams (principal investigator), J. Sundy (co-principal investigator), P. Michelson, B. Vickery, G. Dudek (principal clinic coordinator), R. Newton, A Dugdale, C. Foss, and D. Jaggers (coordinators);
Emory University School of Medicine, Atlanta: W.G. Teague (principal investigator), A. Fitzpatrick, S. Khatri (co-principal investigators), R. Patel (principal clinic coordinator), J. Peabody, E. Hunter, D. Whitlock (coordinators);
Illinois Consortium, Chicago: L. Smith (principal investigator), J. Moy, A. Prestridge (co-principal investigators), J. Hixon (principal clinic coordinator), A. Brees, J. Judge (coordinators);
Indiana University, Asthma Clinical Research Center, Indianapolis: M. Busk (principal investigator), P. Puntenney (principal clinic coordinator), N. Busk, J. Hutchins (coordinators);
University of Pennsylvania, Philadelphia: F. Leone (principal investigator), M. Hayes-Hampton (principal clinic coordinator);
National Jewish Medical and Research Center, Denver: R. Katial (principal investigator), M. Krawiecz (co-principal investigator), H. Currier (principal clinic coordinator);
Nemours Children’s Clinic–University of Florida Consortium, Jacksonville: J. Lima (principal investigator), K. Blake (co-principal investigator), J. Lang (co-principal investigator), D. Schaeffer (investigator), D. George (investigator), M. Warder (principal coordinator), N. Archer, M. McRae, A. Santos (coordinators);
Hofstra University School of Medicine (formerly North Shore–Long Island Jewish Health System), New Hyde Park, N.Y.: R. Cohen (Principal investigator), M. Santiago (co-principal investigator), R. Ramdeo (principal clinic coordinator);
Northern New England Consortium (formerly Vermont Lung Center at theUniversity of Vermont), Colchester, Vt.: C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky, T. Lahiri, P. Shapiro (co-principal investigators), R. Colletti (gastrointestinal consultant), S.M. Burns, L.V. Griffes, R. Pratt, M. Doucette, and P. Oertel(coordinators);
Ohio State University Medical Center/Columbus Children’s Hospital, Columbus: J. Mastronarde (principal investigator), K. McCoy (co-principal investigator), J. Parsons (co-investigator), J. Drake (principal clinic coordinator), R. Compton, L. Raterman, D. Cosmar (coordinators);
Children’s Hospital at Westchester Medical Center and New York Medical College, New York: A.J. Dozer (principal investigator), S. Krishnan, J. Boyer, N. Traeger (coinvestigators), I. Gherson (principal clinic coordinator), L. Monchil (research coordinator);
University of Alabama at Birmingham, Birmingham: L.B. Gerald (principal investigator), W.C. Bailey, R. Grad (co-principal investigators), S. Erwin (principal clinic coordinator), A. Kelley, D. Laken (coordinators);
University of Miami, Miami–University of South Florida, Tampa: A. Wanner (principal investigator, Miami), R. Lockey (principal investigator, Tampa), E. Mendes (principal clinic coordinator for University of Miami), S. McCullough (principal clinic coordinator for University of South Florida) M.Grandstaff-Singleton, D. Miller (coordinators);
University of Minnesota, Minneapolis: M.N. Blumenthal (principal investigator), G. Brottman, J. Hagen (co-principal investigators), A. Decker, D. Lascewski, S. Kelleher (principal clinic coordinators), K. Bachman, C. Quintard, C. Sherry (coordinators);
University of Missouri, Kansas City School of Medicine, Kansas City: G. Salzman (principal investigator), C. Dinakar, D. Pyszczynski (co-investigators), P. Haney (principal clinic coordinator);
St. Louis Asthma Clinical Research Center, Washington University, St. Louis: M. Castro (principal investigator), L. Bacharier, K. Sumino (co-investigators), J. Tarsi (Principal coordinator), B. Patterson (coordinator);
University of California San Diego: S. Wasserman (principal investigator), J. Ramsdell (co-principal investigator), P. Ferguson, K. Kinninger, T. Greene (clinic coordinators);
Chairman’s Office, University of Alabama, Birmingham (formerly Respiratory Hospital, Winnipeg, Manitoba, Canada): W.C. Bailey, N. Anthonisen(research group chair);
Co-Principal Investigators’ Office, University of Virginia School of Medicine, Department of Pediatrics (formerly at Emory University): W.G. Teague (study co-principal investigator);
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), D. Amend-Libercci, K. Barry, M. Daniel, A. Lears, G. Leatherman, C. Levine, G. Leatherman, D. Nowakowski, N. Prusakowski, S. Rayapudi, S. Roettger, A. Thurman, D. Shade, E. Sugar, J. Ukken, C. Wei;
Esophageal pH Probe Quality Control Center, Children’s Center for Digestive Healthcare Pediatric Gastroenterology, Hepatology, and Nutrition (formerly at Emory University School of Medicine):B. Gold (center director);
Data and Safety Monitoring Board: S. Lazarus(chair), W. Calhoun, M. Cloutier, P. Kahrilas, B. McWilliams, A. Rogatko, C. Sorkness;
Project Office, American Lung Association New York: E. Lancet(project officer), N. Edelman (scientific consultant), S. Rappaport;
Project Office, National Heart Lung and Blood Institute: V. Taggart, R. Smith (project officers), G. Weinmann (DSMB executive secretary; deputy directory, Division of Lung Diseases)
ALA Scientific Advisory Committee: E. Schacter(chair), W. Bailey, M. Castro, B. Christman, A. Chuang, C. Holloway, J. Neubauer, J. Samet, E. Swenson, D. Upson, D. Weiss, R.A. Wise.
Sources of Funding
The SARCA trial was supported by: NIH/NHLBI U01 HL080433 and U01 HL080450, and the American Lung Association and is registered at ClinicalTrials.Gov:NCT00442013.
Abbreviations used
- ANOVA
Analysis of Variance
- ACRC
Asthma Clinical Research Center
- ACQ
Asthma Control Questionnaire
- ACT
Asthma Control Test
- ASUI
Asthma Symptom Utility Index
- ED
Emergency Room
- EPAC
Episode of Poor Asthma Control
- FEV1
Forced Expiratory Volume in 1 Second
- GEE
Generalized Estimating Equations
- ICS
Inhaled Corticosteroids
- LABA
Long-Acting Beta-Agonist
- MID
Minimally-Important Difference
- NIH
National Institutes of Health
- PEFR
Peak Expiratory Flow Rate
- pAQLQ
Pediatric Asthma Quality of Life Questionnaire
- ROC
Receiver Operating Characteristic
- SEM
Standard Error of Measurement
- SARCA
Study of Acid Reflux in Children with Asthma
- UC
Urgent Care
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications: In a clinical trial, the ACQ is a valid instrument that can identify patient groups under age 18 with controlled versus uncontrolled asthma and their response to treatment.
REFERENCES
- 1.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, Pendergraft TB, Jhingran P. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Juniper E, O'Byrne P, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. European Respiratory Journal. 1999 Oct 01;14(4):902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 3.Cloutier M, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, Sheller J, Sorkness C, Stoloff S, Gergen P. Asthma outcomes: Composite scores of asthma control. J Allergy Clin Immunol. 2012;129:S24–S33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juniper E, Gruffyd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36:1410–1416. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 5.Guyatt GH, Juniper EF, Griffith LE, Feeny DH, Ferrie PJ. Children and Adult Perceptions of Childhood Asthma. Pediatrics. 1997;99:165–168. doi: 10.1542/peds.99.2.165. [DOI] [PubMed] [Google Scholar]
- 6.American Lung Association Asthma Clinical Research Centers. Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, Dozer AJ, Lima JJ, Mastronarde JG, Sockrider MM, Teague WG. Lansoprazole for Children with Poorly Controlled Asthma: A Randomized Controlled Trial. JAMA. 2012;307(4):373–381. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu A, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig J, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 8.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: The multi-attribute asthma symptom utility index. Chest. 1998 Oct 4;114:998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 9.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 10.Hardin J, Hilbe J. Generalized Estimating Equations. London: Chapman and Hall/CRC; 2003. [Google Scholar]
- 11.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Osoba D, Wu A, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 13.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD. 2005;2:63–67. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 15.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences. Eval Health Prof. 2005;28:172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 16.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 17.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting a SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life instruments. J Clin Epidemiol. 1999;52:861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 18.Leidy NK, Wyrwich KW. Bridging the gap: using triangulation methodology to estimate minimal clinically important differences (MCIDs) COPD. 2005;(1):157–165. doi: 10.1081/copd-200050508. [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Bousquet J, Abetz L, Bateman ED. The GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Sastre J, Olaguibel J, Vega JM, Del Pozo V, Picado C, Vina AL. Cut-off points for defining asthma control in three versions of the Asthma Control Questionnaire. J Asthma. 2010;47:865–870. doi: 10.3109/02770903.2010.491149. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respiratory Medicine. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


