Abstract
Background
Identifying feasible therapeutic interventions is crucial for ameliorating the intellectual disability and other afflictions of Fragile X Syndrome (FXS), the most common inherited cause of intellectual disability and autism. Hippocampal glycogen synthase kinase-3 (GSK3) is hyperactive in the mouse model of FXS (FX mice), and hyperactive GSK3 promotes locomotor hyperactivity and audiogenic seizure susceptibility in FX mice, raising the possibility that specific GSK3 inhibitors may improve cognitive processes.
Methods
We tested if specific GSK3 inhibitors improve deficits in N-methyl-D-aspartate receptor (NMDAR)-dependent long term potentiation (LTP) at medial perforant path synapses onto dentate granule cells (MPP-DGC) and dentate gyrus-dependent cognitive behavioral tasks.
Results
GSK3 inhibitors completely rescued deficits in LTP at MPP-DGC synapses in FX mice. Furthermore, synaptosomes from the dentate gyrus of FX mice displayed decreased inhibitory serine-phosphorylation of GSK3β compared with wild-type littermates. The potential therapeutic utility of GSK3 inhibitors was further tested on dentate gyrus-dependent congnitive behaviors. In vivo administration of GSK3 inhibitors completely reversed impairments in several cognitive tasks in FX mice, including novel object detection, coordinate and categorical spatial processing, and temporal ordering for visual objects.
Conclusions
These findings establish that synaptic plasticity and cognitive deficits in FX mice can be improved by intervention with inhibitors of GSK3, which may prove therapeutically beneficial in FXS.
Keywords: cognition, Fragile X syndrome, glycogen synthase kinase-3, learning, LTP, synaptic plasticity
Introduction
Currently there are no adequate therapies for the treatment of Fragile X Syndrome (FXS), the most prevalent form of inherited mental retardation and the most common cause of autism (1, 2). FXS results from suppressed FMR1 gene expression and deficiency in fragile X mental retardation protein (FMRP). The predominant characteristic of FXS is intellectual disability, which can be accompanied by hyperactivity, attention deficit, anxiety, seizures, and behaviors characteristic of autism spectrum disorders (3–7). Although some symptoms can be alleviated by anticonvulsants, antidepressants, stimulants and antipsychotics (8), or newly developed drugs affecting glutamatergic (9) and GABAergic (10) neurotransmission, no approved agent improves the central feature of FXS, impaired cognition. Insight into this fundamental issue has been attained using mice with genetic deletion of the Fmr1 gene to model FMRP deficits in FXS (5, 11, 12). Initial studies using Fmr1 knockout (FX) mice surprisingly reported normal N-methyl-D-aspartate receptor (NMDAR)-dependent long-term potentiation (LTP) and depression (LTD) at hippocampal CA1 synapses (13, 14), forms of synaptic plasticity that underlie learning and memory, whereas metabotropic glutamate receptor dependent LTD (mGluR-LTD) was pathologically enhanced (14). This and additional evidence led to the mGluR theory of FXS (15), which proposes that enhanced mGluR signaling is a major cause of the pathological deficits in FXS. Decreasing mGluR5 function corrects several behavioral and morphological phenotypes in developing and adult FX mice (16). Additionally, chronic, but not acute, inhibition of mGluR5 reverses cognitive deficits in young adult FX mice (17). However, recent studies investigating the dentate gyrus, a subregion of the hippocampal formation important for pattern separation, discovered NMDAR hypofunction and deficits in NMDAR-dependent LTP at medial perforant path synapses onto dentate granule cells (MPP-DGC) (18, 19). Interestingly, these deficits were accompanied by deficits in context discrimination, a behavior requiring normal function of the dentate gyrus (18, 20). These findings represent a clear link between synaptic dysfunction and learning deficits in FX mice and provide a synaptic and behavioral paradigm to investigate potential treatments.
The clinically used mood stabilizer lithium is a promising therapeutic agent for FXS because it reverses several behavioral phenotypes, attenuates enhanced mGluR-LTD in FX mice (21–23), and improves a cognitive task in FXS patients (24). Because lithium has a low therapeutic index and can cause side effects at serum concentrations modestly above the therapeutic level, identifying its target in FX mice could lead to the design of more specific and efficacious treatments for FXS. Lithium was first identified as a potential treatment for FXS by the seminal finding that lithium treatment was effective in a Drosophila model of FXS, which may have been due to inhibition of inositol monophosphatase (25). Other evidence shows that glycogen synthase kinase-3 (GSK3) is a target inhibited by lithium (26). The two isoforms of the Ser/Thr kinase GSK3 are primarily regulated by inhibitory phosphorylation at Ser21 in GSK3α and Ser9 in GSK3β which is often mediated by Akt (27). GSK3 has been implicated in the pathology of FXS because it is hyperactive in hippocampus, striatum, and cerebral cortex in FX mice and GSK3 inhibition decreases locomotor hyperactivity and audiogenic seizure susceptibility in FX mice (21, 28). Futhermore, LTP induction requires inhibition of GSK3β through increased Ser-9-phosphorylation, while GSK3β activity is required for LTD induction (29). Therefore, GSK3 is a bidirectional regulator of NMDAR-dependent synaptic plasticity. Additionally, artificially increasing GSK3 activity in adult wild-type (WT) mice impairs LTP (30, 31), a finding consistent with the concept that pathologically increased GSK3 activity in FX mice could be causally related to deficits in LTP at MPP-DGC synapses. Thus, inhibition of GSK3 is likely an important component of the benefits of lithium in FX mice, raising the possibility that GSK3 may be a target for the development of new treatments for FXS. Here we tested the hypothesis that pharmacological inhibition of GSK3 rescues deficits in LTP at MPP-DGC synapses and dentate gyrus-dependent learning tasks. We report that lithium and selective GSK3 inhibitors, but not mGluR5 inhibition, reverse both the LTP deficit and cognitive impairments in FX mice, establishing GSK3 as an independent target for therapeutic development to treat FXS.
Methods and Materials
Antibodies were obtained from Cell Signaling Technology (Beverly, MA), LiCl and picrotoxin from Sigma (St. Louis, MO), Chir99021 (CT99021) from Biovision (Milpitas, CA) and 2-methyl-6-(phenylethynyl)pyridine (MPEP) from Tocris Bioscience (Ellisville, MO). Thiadiazolidindione-8 (TDZD-8) and N′-dodecanoyl-1-ethyl-4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carbohydrazide (VP0.7) were prepared in the Martinez laboratory (35,36) and administered in 5% Tween80, 5% DMSO in saline.
Mice were housed in light and temperature controlled rooms and treated in accordance with National Institutes of Health, the University of Miami, and the University of Alabama at Birmingham Institutional Animal Care and Use Committee regulations. Crude synaptosomes were isolated from hippocampal subfields through a series of centrifugation steps and protein levels were determined using standard procedures. (32). Assessments of visual object novelty detection, temporal ordering for visual objects, coordinate and categorical spatial processing were carried out by published methods (33). Supplementary Information contains further details.
Coronal slices (400uM) were prepared from dorsal hippocampus of WT and FX mice. Extracellular dendritic field potential recordings (fEPSPs) at either Schaffer collateral (CA3-CA1) or MPP-DGC synapses were generated by baseline stimulation (0.1 Hz, 200μs duration). LTP was induced using high-frequency stimulation (HFS, 100 Hz, 1 sec duration × 4, 60 sec interval). To isolate excitatory inputs, all recordings were carried out in the presence of 100 μM picrotoxin. All n values represent animal number. See Supplementary Information for further details.
Data are expressed as mean ± SEM. Student’s t test, one-way ANOVA followed by Bonferroni’s post-hoc multiple comparison, and Kruskall-Wallis with Dunn’s multiple comparison test were used as noted. Significance was taken as p<0.05.
Results
Selective LTP deficit at medial perforant path-dentate granule cell synapses is accompanied by decreased serine-phosphorylated GSK3β
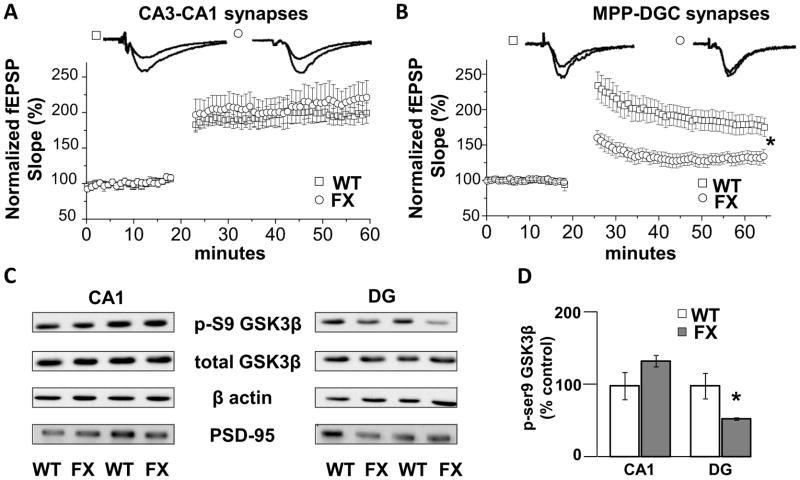
Using acute brain slices and high frequency stimulation (HFS), the LTP magnitude at CA3-CA1 synapses in slices from FX mice is not different from WT mice (WT: 197±12% of baseline fEPSP slope vs FX: 215±23% of baseline fEPSP slope, p>0.05) (Fig. 1A). In contrast, there is a significant LTP deficit in FX mice at MPP-DGC synapses (WT: 183±14% vs FX: 130±8%, p<0.05) (Fig. 1B). These results confirm previous reports (14, 18), and importantly, demonstrate hippocampal region-specific deficits in NMDAR-dependent synaptic plasticity in FX mice.
Figure 1.
Deficits in LTP at MPP-DGC synapses in FX mice are accompanied by decreased inhibitory serine-phosphorylation of GSK3β. (A) Summary plots of the magnitude of LTP induced by HFS at CA3-CA1 Schaffer collateral synapses in slices from WT (n=7) and FX (n=6) mice. (B) Summary plots of the magnitude of LTP induced by HFS at MPP-DGC synapses in slices from WT (n=6) and FX (n=9) mice. (C) Representative Western blots showing a reduction in phospho-serine9-GSK3β (p-S9 GSK3) protein in dentate gyrus (DG) but not in CA1 from FX versus WT mice. Total GSK3β protein levels are not different between groups. β-Actin and PSD-95 were used as cytosolic and synaptic protein loading controls. (D) Quantitation of the ratio of p-S9 GSK3β to total GSK3β normalized to values of WT mice (CA1, n=4, and DG, n=6, for each genotype). *p<0.05 (Student’s t test).
In hippocampal homogenates from FX mice, inhibitory serine-phosphorylation of GSK3β (p-GSK3β) is decreased, indicating increased activity (21, 28). However, it is not known whether the decrease in p-GSK3β is hippocampal region specific. Because GSK3 negatively regulates NMDAR-dependent LTP (29–31), the decrease in p-GSK3 should occur in DG but not CA1, if pathologically increased GSK3 activity is causing the specific deficit in LTP at MPP-DGC synapses. Western blot analysis of synaptosomal fractions revealed that p-GSK3β levels are unchanged in CA1 (135±8% of control, p>0.05) but decreased in DG (53±2% of control, p<0.05) (Fig. 1C and D) from FX mice compared to WT mice, whereas total GSK3β levels were equivalent. These results raise the possibility that hyperactive GSK3 in DG contributes to the deficit in LTP at MPP-DGC synapses.
Pharmacological blockade of GSK3 rescues the deficit in LTP at MPP-DGC synapses
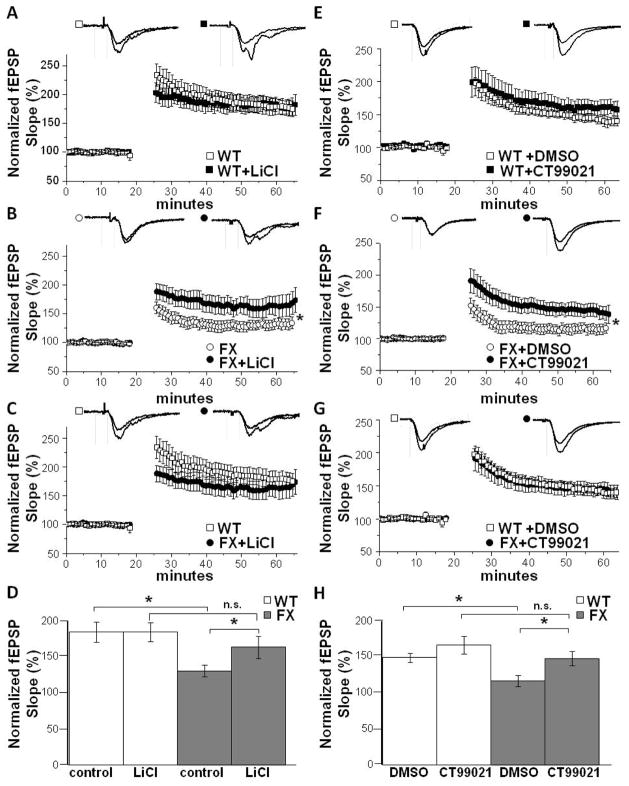
Reduced p-GSK3β levels coupled with the LTP deficit specifically in DG suggest that pharmacological GSK3β inhibition might reverse the synaptic dysfunction. Because lithium rescues some behavioral phenotypes in FX mice and it inhibits GSK3 (21, 22), we first investigated whether bath application of LiCl (20 mM) rescues the LTP deficit. Indeed, LiCl, applied 30 min prior to HFS, significantly increases the LTP magnitude at MPP-DGC synapses in slices from FX mice (FX: 130±8% vs FX+Li: 162±15%, p<0.05) (Fig. 2B) without affecting the LTP magnitude at WT synapses (WT: 183±14% vs WT+Li: 183±12%, p>0.05) (Fig. 2A). In fact, the LTP magnitude is equivalent in WT and FX+Li treated slices (WT: 183±14% vs FX+Li: 162±15%, p>0.05) (Fig. 2C and D), indicating a complete rescue of the LTP deficit.
Figure 2.
Pharmacological blockade of GSK3 reverses the deficit in LTP at MPP-DGC synapses. Summary plots of the magnitude of LTP induced by HFS at MPP-DGC synapses in slices from (A) WT mice with (n=6) and without (n=6) bath application of LiCl (20 mM), and (B) FX mice with (n=6) and without (n=9) bath application of LiCl (20 mM). (C) Data from WT mice (from A) and FX mice (bath application of LiCl from B) replotted to compare the magnitudes of LTP.(D) Summary of data A–C. (E) WT mice with (n=8) and without (n=12) bath application of CT99021 (2 uM). (F) FX mice with (n=7) and without (n=7) bath application of CT99021. (G) WT (from E) and FX (bath application of CT99021 from F) replotted to compare the magnitudes of LTP. *p<0.05 (Student’s t test). (H) Summary of data E–F
In order to confirm that lithium is rescuing LTP specifically through inhibition of GSK3 rather than off-target effects, we utilized CT99021, a highly selective GSK3 inhibitor (34). Slices were perfused with either vehicle or CT99021 (2 μM) for at least 30 min prior to HFS. Similar to LiCl treatment, CT99021 completely rescued the deficit in LTP in FX slices but had no significant effect on WT slices (WT: 146±5% vs WT+CT99021: 164±12%, p>0.05) (FX: 115±7% vs FX+CT99021: 146±10%, p<0.05) (WT: 146±5% vs FX+CT99021: 146±10%, p>0.05) (Fig. 2E-H). Collectively, these results conclusively demonstrate that inhibition of GSK3 reverses the deficit in LTP at MPP-DGC synapses.
Pharmacological blockade of GSK3 reverses the deficit in steady-state depolarization in FX slices during HFS
Because LTP induction requires sufficient depolarization to relieve the voltage-dependent Mg2+ block from NMDARs, we next investigated whether the deficit in LTP and subsequent rescue by GSK3 inhibition was due to alterations in the depolarization during HFS. We measured steady-state depolarization during the fourth round of HFS in WT and FX slices and found a significant decrease in FX slices compared to WT slices, which was reversed by LiCl (Fig. 3A and B) and CT99021 (Fig. 3C and D). These findings raise the possibility that GSK3 inhibition rescues the LTP deficit by increasing neurotransmitter release. However, we found no significant differences in the paired pulse ratio, an indirect measure of presynaptic neurotransmitter release probability (35), between WT and FX slices under any condition or interstimulus interval (Fig. S1 A and B), indicating that enhanced neurotransmitter release may not be the mechanism underlying rescued LTP.
Figure 3.
Pharmacological blockade of GSK3 does not alter steady-state depolarization. (A) Averaged traces during the 4th tetanus from experiments in Fig 2A–C show reduced steady-state depolarization during tetanus in FX slices. (B) Pooled data from all LTP experiments in Fig 2 show LiCl treatment reverses the deficit in steady state depolarization in FX slices. (WT: 110±12 vs FX: 72±8) (FX: 71±8 vs FX+Li: 123±23) (WT: 110±12 vs FX+Li: 123±23) *p<0.05 (Student’s t test). (C) Averaged traces during the 4th tetanus from experiments in Fig 2D–E show reduced steady-state depolarization during tetanus in FX slices. (D) Pooled data from experiments in Fig 2 show CT99021 treatment reverses the deficit in steady state depolarization in FX slices. (WT: 116±8 vs FX: 71±8) (FX: 88±13 vs FX+CT99021: 117±20)(WT: 116±8 vs FX+CT99021: 117±20) *p<0.05 (Student’s t test)
Cognitive deficits in FX mice are rescued by in vivo GSK3 inhibition
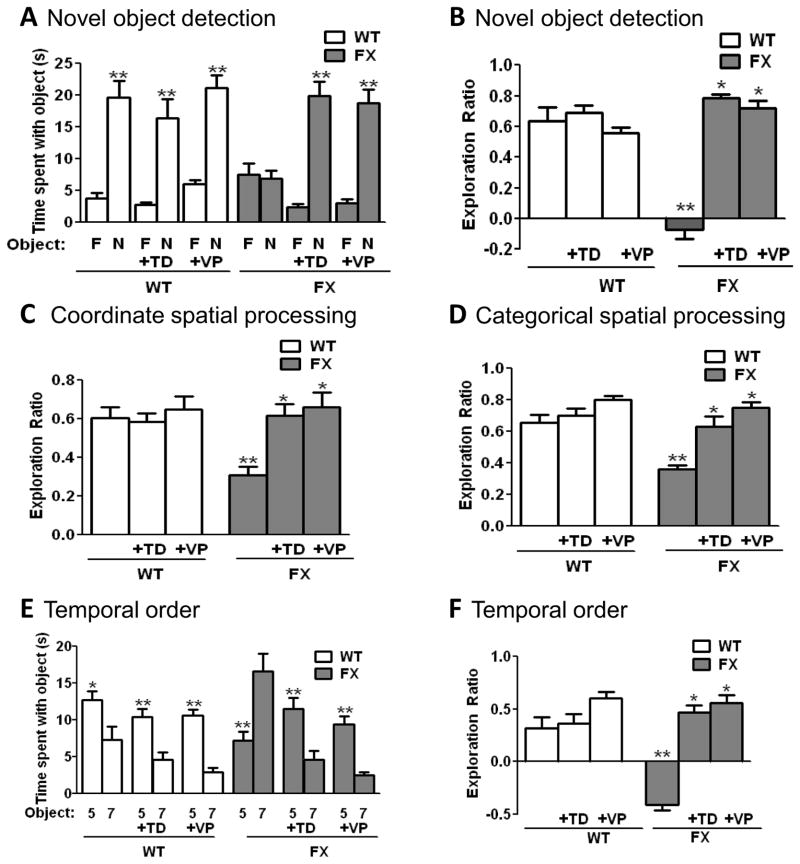
The rescue of the LTP deficit at MPP-DGC synapses suggests that GSK3 inhibition should also rescue deficits in dentate gyrus-dependent learning in FX mice previously reported (18, 36–38). Thus a visual object novelty detection task, which requires the dentate gyrus (39–41) and assesses the ability to discriminate between a familiar and novel object, was used to evaluate the potential benefits of GSK3 inhibition on learning deficits in FX mice. WT mice spent significantly more time exploring the novel versus familiar object (20±3 sec vs 4±1 sec, p<0.01), demonstrating learning (Fig. 4A). In contrast, FX mice spent equivalent amounts of time exploring the novel versus familiar object (8±2 sec vs 7±1 sec), indicating that FX mice are unable to learn the task. The exploration ratio (calculated by dividing the difference between the time spent with the novel object versus the familiar object divided by total time exploring) was significantly different between WT and FX mice (exploration ratio WT: 0.64±0.09; FX: −0.07±0.06, p<0.05) (Fig. 4B).
Figure 4. Inhibition of GSK3 ameliorates cognitive impairments in FX mice.
FX and WT mice were treated with 5 mg/kg of TDZD-8 (TD) or VP0.7 (VP) 1 hr prior to cognitive assessments. (A,B) Performance in the novelty detection for visual objects task. (A) Times spent exploring the novel (N) and familiar (F) object. **p<0.01 compared to time spent with familiar object (Student’s t test). (B) Exploration ratio. (C) Exploration ratio in the coordinate spatial processing task. (D) Exploration ratio in the categorical spatial processing task. (E,F) Performance in the temporal order for visual objects task. (E) Times spent exploring Object 5 and Object 7 (most recently explored). **p<0.01, *p<0.05 compared to time spent with Object 7 (Student’s t test). (F) Exploration ratio. B, C, D and F: **p<0.05 compared to untreated wild-type mice; *p<0.05 compared to same genotype without treatment (Kruskall-Wallis [genotype x treatment] with Dunn’s multiple comparison test). n=10–20 mice per group.
Due to the non-selective effects of LiCl and the limited solubility and CNS penetration of CT99021, GSK3 inhibition in vivo was achieved using two other selective GSK3 inhibitors with CNS bioavailability, TDZD-8 (5 mg/kg; ip), a highly selective ATP non-competitive inhibitor (42), and VP0.7 (5 mg/kg; ip), an allosteric (not competitive with ATP or substrate) selective GSK3 inhibitor (43). The GSK3 inhibitors did not alter the performance of WT mice, which spent more time investigating the novel versus familiar object (TDZD-8: 16±3 sec vs 3±1 sec, p<0.01; VPO.7: 21±2 sec vs 6±1 sec, p<0.01) (Fig. 4A). However, FX mice treated with TDZD-8 or VPO.7 spent significantly more time exploring the novel versus familiar object (TDZD-8: 20±2 sec vs 2±1 sec, p<0.01; VPO.7: 19±2 sec vs 3±1 sec, p<0.01), indicating that under conditions of GSK3 inhibition FX mice are capable of learning the task. Furthermore, the exploration ratio was significantly increased in FX mice treated with TDZD-8 or VP0.7, but had no effect in WT mice (FX exploration ratio: TDZD-8: 0.79±0.03; p<0.05; VP0.7: 0.72±0.04; p<0.05) (WT exploration ratio: TDZD-8: 0.69±0.05; VP0.7: 0.56±0.03) (Fig. 4B), indicating that GSK3 inhibition completely reverses the learning deficit in FX mice.
Next, we assessed whether FX mice displayed deficits in pattern separation using coordinate and categorical tasks, which require the dentate gyrus (33, 40, 44). In the coordinate spatial learning task, the distance between two identical objects is altered between the habituation and testing periods. Pattern separation is indicated when significantly more time is spent exploring objects during the 5 min testing period after repositioning the objects compared to the last 5 min of the habituation phase. WT mice displayed increased object exploration time during testing compared to the last 5 min of the habituation phase (WT exploration ratio: 0.61±0.05) (Fig. 4C), indicating successful pattern separation. In contrast, FX mice spent significantly less time than WT exploring the objects during the test period, indicating impaired behavior in this task (FX exploration ratio: 0.31±0.05, p<0.05). While neither TDZD-8 nor VP0.7 altered behavior of WT mice (WT exploration ratio TDZD-8: 0.60±0.05; VP0.7: 0.65±0.07), both drugs reversed the deficit in FX mice, as they spent significantly more time exploring the objects during testing compared to habituation (FX exploration ratio TDZD-8: 0.62±0.06, p<0.05; VP0.7: 0.66±0.08, p<0.05). The categorical spatial learning task involves interchanging the positions of two identical objects following the habituation phase, while maintaining the same distance between them. FX mice spent significantly less time than WT mice exploring the objects after they had been transposed (FX exploration ratio: 0.36±0.03; WT exploration ratio: 0.66±0.05, p<0.05) (Fig 4D), again revealing impaired spatial pattern separation in FX mice. Administration of GSK3 inhibitors did not alter the amount of time WT mice spent exploring the objects after they were transposed (WT exploration ratio: TDZD-8: 0.70±0.05; VP0.7: 0.80±0.03), but significantly increased the exploration times of FX mice (FX exploration ratio: TDZD-8: 0.63±0.06, p<0.05; VP0.7: 0.75 ± 0.04, p<0.05), demonstrating a reversal of the deficit. Thus, the results of the coordinate and categorical spatial learning tests demonstrate impaired function of the dentate gyrus in FX mice that is normalized by the administration of GSK3 inhibitors.
Finally, we assessed whether FX mice have deficits in temporal ordering of visual objects, a dorsal and ventral hippocampal CA1-dependent task in which rodents spend less time exploring the object most recently presented during a previous habituation period (33, 45–49). In this task, we exposed mice to a series of 3 pairs of objects and then measured the time spent with the initial object when it was reintroduced along with the most recent object. Successful temporal ordering is evident when more time is spent exploring the initial object. WT mice displayed successful temporal ordering because more time was spent exploring the initial object (13±1 sec vs 7±2 sec, p<0.05), whereas FX mice spent significantly less time exploring the initial object presented (7±1 sec vs 16±2 sec, p<0.01) (Fig. 4E). Thus, the object exploration ratio (calculated by dividing the difference between the time spent with the initial object (object 5) versus the more recent object (object 7) by total time exploring), differed significantly between FX and WT mice, revealing a temporal order deficit (WT exploration ratio: 0.32±0.10; FX exploration ratio: −0.41±0.05, p<0.05) (Fig. 4F). FX mice treated with either GSK3 inhibitor, TDZD-8 or VP0.7, spent significantly more time exploring the first object compared to the most recent object presented (TDZD-8: 12±2 sec vs 4±2 sec, p<0.01; VP0.7: 9±3 sec vs 3±1 sec, p<0.01). Similarly, WT mice treated with TDZD-8 or VP0.7 spent significantly more time exploring the first versus the recent object (TDZD-8: 10±1 sec vs 5±1 sec, p<0.01; VP0.7: 11±1 sec vs 3±1 sec, p<0.01). Thus, administration of TDZD-8 or VP0.7 significantly increased the exploration ratio in FX mice (FX exploration ratio: TDZD-8: 0.47±0.07, p<0.05; VP0.7: 0.56±0.07; p<0.05), (Figure 4F), eliminating the impairment in temporal ordering. TDZD-8 or VP0.7 treatment also tended to improve the behavior of wild-type mice in this task (WT exploration ratio: TDZD-8: 0.37±0.08; VP0.7: 0.60±0.06) (Figure 4F). These results demonstrate that temporal ordering of visual objects is impaired in FX mice and that this deficit is corrected by inhibition of GSK3.
Synaptic and cognitive deficits in FX mice are not rescued by mGluR5 inhibition
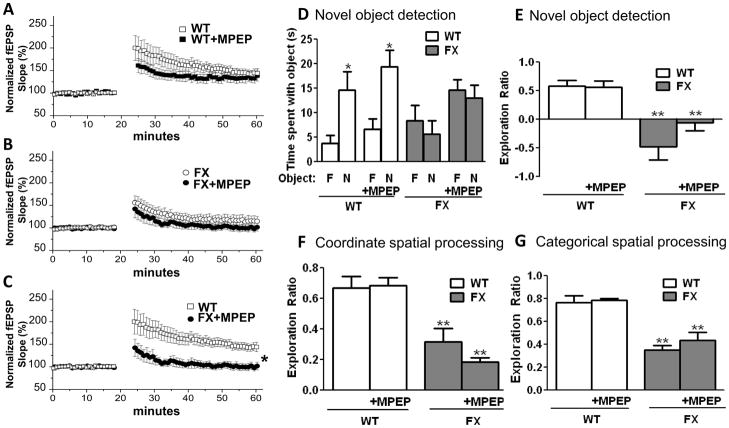
Because inhibition of mGluR reverses many of the synaptic and behavioral phenotypes in FX mice (17, 50), we investigated whether MPEP, an mGluR5 antagonist, would also reverse the dentate gyrus associated deficits in synaptic plasticity and learning and memory. We show using bath application of MPEP (100 uM) that mGluR5 inhibition fails to rescue LTP magnitude in FX slices (WT: 143±8%; WT+MPEP 127±9%, p>0.05) (FX: 116±11%; FX+MPEP: 101±7%, p>0.05) (WT:143±8%; FX+MPEP: 101±7%, p<0.05) (Fig. 5A–C). Treatment of FX mice with MPEP did not affect the impairments in novel object detection (FX: 6±3 sec vs 8±3 sec vs FX+MPEP: 13±3 sec vs 15±2 sec) (Fig. 5D) (FX exploration ratio: −0.48±0.23; MPEP: −0.06±0.13) (Fig. 5E), coordinate spatial processing (FX exploration ratio: 0.31±0.09; MPEP: 0.18±0.03) (Fig. 5F), and categorical spatial processing (FX exploration ratio: 0.35±0.04; MPEP: 0.43±0.07) (Fig. 5G). Administration of MPEP did not alter the performance of WT mice in any of these tasks. Thus, impaired LTP in the dentate gyrus and cognitive deficits in FX mice are not rescued by acute inhibition of mGluR5.
Figure 5.
Synaptic and cognitive deficits in FX mice are not altered by mGluR inhibition. Summary plots of the magnitude of LTP induced by HFS at MPP-DGC synapses in slices from (A) WT mice with (n=5) and without (n=5) bath application of MPEP (100 uM), and (B) FX mice with (n=7) and without (n=6) bath application of MPEP. (C) Data
Discussion
Here we report a significant decrease in inhibitory serine p-GSK3 levels specifically in synaptosomes from the dentate gyrus of FX mice that is associated with a deficit in NMDAR-dependent LTP at MPP-DGC synapses and severe impairments in behavioral tasks dependent upon normal function of the dentate gyrus. Pharmacological inhibition of GSK3, but not an inhibitor of mGluR5, completely reverses both synaptic and behavioral deficits, strongly suggesting that GSK3 should be considered as a therapeutic target for the treatment of cognitive dysfunction in FXS.
Intellectual disability is the predominant trait of FXS, but it is not improved by currently available medications. Advances in treatment strategies for cognitive impairment that is characteristic of FXS were initially slowed by the difficulty in identifying deficits in FX mice, as FX mice behave normally or display only mild impairments in several cortical- and hippocampal-dependent cognitive tasks, such as the Morris water maze, radial arm maze, operant conditioning paradigms and contextual and conditioned fear memory (11, 51–59), rather than the severe impairments that would be expected in a valid FXS model. However, deficits in passive avoidance behavior have been observed, and more recently, robust deficits were identified in tasks that are heavily dependent upon normal function of the dentate gyrus, specifically, novel object recognition and context discrimination, which assesses pattern separation (18, 36–38). Here we extend these findings by demonstrating significant deficits, not only in novel object detection, but also in coordinate and categorical spatial processing tasks. Deficits in pattern separation are associated with NMDAR hypofunction and deficits in LTP at MPP-DGC synapses in FX mice previously reported (18) and because LTP is a cellular correlate of learning and memory (60), this deficit in LTP likely contributes to the impairment in dentate gyrus-dependent cognitive tasks in FX mice evaluated here.
GSK3 inhibitors have been proposed as potential therapeutic candidates for treating FXS, based to a large extent on the multiple beneficial effects resulting from administration of the GSK3 inhibitor lithium to FX mice (61). Lithium treatment of FX mice reverses locomotor hyperactivity, audiogenic seizure hypersensitivity, increased spine density, enhanced mGluR-mediated LTD, reactive astrocytes, macroorchidism, excess protein synthesis, and social behavior deficits (21–23, 28, 62–64). Furthermore, lithium improved deficient passive avoidance behavior, the only reported test of lithium’s effect on cognitive impairments in FX mice. These actions of lithium are likely due to inhibition of GSK3 because GSK3 inhibitors other than lithium also have been reported to control locomotor hyperactivity, susceptibility to audiogenic seizures, trace conditioning, delayed non-matching-to-place radial arm maze and neurogenesis in FX mice (28, 65). Thus it appears that GSK3 inhibitors, including lithium, are able to normalize many abnormal characteristics of FX mice and thus are potential therapeutic interventions for FXS. Importantly, lithium treatment also proved beneficial in a cognitive task in FXS patients (24), suggesting that cognitive deficits in FX mice may be ameliorated by administration of specific inhibitors of GSK3, leading us to directly test this hypothesis. We found that both the impaired MPP-DGC LTP and impaired novel object detection and coordinate and categorical spatial processing in FX mice were repaired by administration of GSK3 inhibitors, suggesting that GSK3 inhibition reversed these cognitive deficits by normalization of LTP. These findings are in accordance with reports that hyperactive GSK3 impairs LTP (30, 31), and that GSK3 is hyperactive in the hippocampus of FX mice (21, 28), specifically in synapstosomes from the dentate gyrus, reported here.
Despite finding no difference in inhibitory serine p-GSK3 levels in CA1 synaptosomes or LTP magnitude at CA3-CA1 synapses between WT and FX mice, we observed a deficit in temporal ordering of objects, a visual task dependent upon area CA1 (48), which is surprisingly reversed by GSK3 inhibition (13, 14). However, because normal function of the hippocampal trisynaptic circuit is required during behavioral tasks, deficits at MPP-DGC synapses are likely propagated to downstream CA3-CA1 synapses, impacting CA1 dependent behavior, even though LTP at CA3-CA1 synapses is normal in slices from FX mice. In support of this concept, GSK3 inhibition, which reverses LTP deficits at MPP-DGC synapses, also normalizes temporal ordering deficits indicating that systemic GSK3 inhibition improves the overall function of the hippocampal tri-synaptic circuit. Another possibility is that there are deficits in plasticity present at the temporoammonic pathway, the monosynaptic projection from entorhinal cortex directly onto distal dendrites of CA1, that contribute to deficits in temporal ordering of objects. Our results indicate that if such deficits exist and underlie termporal order learning, inhibition of GSK3 will also rescue deficits at temporoammonic synapses although this possibility has not been explored.
Because aberrant mGluR5 function is known to regulate several of the phenotypes of FX mice and acute administration of the mGluR5 antagonist MPEP to FX mice reverses deficits in several behaviors and mEPSC frequency in the amygdala (28, 58, 66–69), we tested if administration of MPEP also reversed deficits in LTP and cognition. Acute administration of MPEP was completely ineffective in reversing the impaired MPP-DGC LTP and cognitive impairments in FX mice, in accordance with a report that MPEP fails to rescue LTP deficits in amygdala (67). Collectively, these results indicate that although mGluR5 is a promising therapeutic target (17, 50), GSK3 inhibitors must also be considered for treatment of FXS. Additonally, because acute GSK3, but not acute mGluR5, inhibition reverses these deficits, mGluR5 is not directly driving the pathologically hyperactive GSK3 in FX mice. However, a recent study showed that chronic, but not acute, mGluR5 inhibition reversed novel object recognition deficits (70), so aberrant mGluR function in FX could indirectly modulate GSK3 activity.
To fully understand how hyperactive GSK3 leads to synaptic and cognitive impairments in FX, identification of downstream targets of GSK3 is needed. Both LTP and pattern separation require proper NMDAR function. Hyperactive GSK3 may diminish NMDAR transmission which can be reversed by GSK3 inhibition, an idea consistent with GSK3-dependent NMDAR internalization in cortical neurons (71). Alternatively, inhibition of GSK3 may rescue LTP and cognition through effector targets downstream of NMDAR activation. GSK3 regulates AMPAR trafficking (72), however this mechanism is unlikely since there are no deficits in baseline AMPAR transmissison in FX mice. Other mechanisms upstream and downstream of GSK3 suspected to be involved in modulation of plasticity have been eloquently discussed (73). It will be of great interest in future studies to elucidate the precise mechanisms by which hyperactive GSK3 decreases plasticity and learning and memory, as these mechanisms represent novel targets for therapeutic development for the treatment of intellectual disability in FXS.
The cognitive assessments we studied in FX mice may model the nonverbal measures of intelligence used in FXS patients. Patients with FXS display impaired recognition memory, spatial memory, working memory and short-term memory (3, 74–76). FXS patients also have difficulty with inhibition and attentional control that is consistent with the memory deficits (77). The visual object novelty detection task and the temporal order memory task were used to assess recognition memory, working memory and short-term memory in FX mice. The coordinate metrical and categorical topological spatial learning tasks were used to assess spatial memory in FX mice. All of these cognitive abilities were impaired in FX mice and were corrected by administration of GSK3 inhibitors. Thus, abnormally active GSK3 in the hippocampus of FX mice (21, 22, 28, 78) appears to play an important role in these cognitive deficits, further supporting the possibility that GSK3 inhibitors may be beneficial for multiple aspects of FXS, including intellectual disability.
Supplementary Material
Acknowledgments
This research was supported by grants from the NIMH (MH097362; MH038752; F31MH097362-01A), T32 NS061788, Civitan Emerging Scholars Fellowship, HHMI and from the FRAXA Foundation.
Footnotes
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Curr Opin Genet Dev. 2006;16:270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Bhakar AL, Dolen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses) Annu Rev Neurosci. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ornstein PA, Schaaf JM, Hooper SR, Hatton DD, Mirrett P, Bailey DB., Jr Memory skills of boys with fragile X syndrome. Am J Ment Retard. 2008;113:453–465. doi: 10.1352/2008.113:453-465. [DOI] [PubMed] [Google Scholar]
- 4.Dissanayake C, Bui Q, Bulhak-Paterson D, Huggins R, Loesch DZ. Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. J Child Psychol Psychiatry. 2009;50:290–299. doi: 10.1111/j.1469-7610.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 5.Errijgers V, Kooy RF. Genetic modifiers in mice: the example of the fragile X mouse model. Cytogenet Genome Res. 2004;105:448–454. doi: 10.1159/000078218. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: a longitudinal evaluation. Am J Med Genet A. 2009;149A:1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012;122:4314–4322. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3:64ra61. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 10.Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med. 2012;4:152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- 11.Bakker DB. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 12.Gross C, Berry-Kravis EM, Bassell GJ. Therapeutic strategies in fragile X syndrome: dysregulated mGluR signaling and beyond. Neuropsychopharmacology. 2012;37:178–195. doi: 10.1038/npp.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godfraind JM, Reyniers E, De Boulle K, D’Hooge R, De Deyn PP, Bakker CE, et al. Long-term potentiation in the hippocampus of fragile X knockout mice. Am J Med Genet. 1996;64:246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2012;22:241–254. doi: 10.1002/hipo.20890. [DOI] [PubMed] [Google Scholar]
- 19.Yun SH, Trommer BL. Fragile X mice: reduced long-term potentiation and N-Methyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus. J Neurosci Res. 2011;89:176–182. doi: 10.1002/jnr.22546. [DOI] [PubMed] [Google Scholar]
- 20.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 21.Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol. 2010;79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol. 2011;14:618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, Gertner MJ, et al. Pharmacological reversal of synaptic plasticity deficits in the mouse model of Fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- 25.McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, et al. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56:463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27:12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallett PJ, Collins TL, Standaert DG, Dunah AW. Biochemical fractionation of brain tissue for studies of receptor distribution and trafficking. Curr Protoc Neurosci. 2008;Chapter 1(Unit 1):16. doi: 10.1002/0471142301.ns0116s42. [DOI] [PubMed] [Google Scholar]
- 33.Hunsaker MR, Kim K, Willemsen R, Berman RF. CGG trinucleotide repeat length modulates neural plasticity and spatiotemporal processing in a mouse model of the fragile X premutation. Hippocampus. 2012;22:2260–2275. doi: 10.1002/hipo.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 36.Ventura R, Pascucci T, Catania MV, Musumeci SA, Puglisi-Allegra S. Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: involvement of dopamine in the medial prefrontal cortex. Behav Pharmacol. 2004;15:433–442. doi: 10.1097/00008877-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Pacey LK, Doss L, Cifelli C, van der Kooy D, Heximer SP, Hampson DR. Genetic deletion of regulator of G-protein signaling 4 (RGS4) rescues a subset of fragile X related phenotypes in the FMR1 knockout mouse. Mol Cell Neurosci. 2011;46:563–572. doi: 10.1016/j.mcn.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya A, Klann E. Fragile X syndrome therapeutics S(C)TEP through the developmental window. Neuron. 2012;74:1–3. doi: 10.1016/j.neuron.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18:955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- 41.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 42.Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 43.Palomo V, Soteras I, Perez DI, Perez C, Gil C, Campillo NE, et al. Exploring the binding sites of glycogen synthase kinase 3. Identification and characterization of allosteric modulation cavities. J Med Chem. 2011;54:8461–8470. doi: 10.1021/jm200996g. [DOI] [PubMed] [Google Scholar]
- 44.Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behav Neurosci. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- 45.Honey RC, Watt A, Good M. Hippocampal lesions disrupt an associative mismatch process. J Neurosci. 1998;18:2226–2230. doi: 10.1523/JNEUROSCI.18-06-02226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 47.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kooy RF, D’Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, et al. Transgenic mouse model for the fragile X syndrome. Am J Med Genet. 1996;64:241–245. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 52.D’Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, et al. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997;76:367–376. doi: 10.1016/s0306-4522(96)00224-2. [DOI] [PubMed] [Google Scholar]
- 53.Fisch GS, Hao HK, Bakker C, Oostra BA. Learning and memory in the FMR1 knockout mouse. Am J Med Genet. 1999;84:277–282. doi: 10.1002/(sici)1096-8628(19990528)84:3<277::aid-ajmg22>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 54.Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 55.Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet. 2000;9:1145–1159. doi: 10.1093/hmg/9.8.1145. [DOI] [PubMed] [Google Scholar]
- 56.Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100:423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- 57.Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- 58.Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004;3:337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 59.Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010;9:562–574. doi: 10.1111/j.1601-183X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 60.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 61.Mines MA, Jope RS. Glycogen synthase kinase-3: a promising therapeutic target for fragile x syndrome. Front Mol Neurosci. 2011;4:35. doi: 10.3389/fnmol.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mines MA, Yuskaitis CJ, King MK, Beurel E, Jope RS. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS One. 2010;5:e9706. doi: 10.1371/journal.pone.0009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuskaitis CJ, Beurel E, Jope RS. Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of Fragile X syndrome. Biochim Biophys Acta. 2010;1802:1006–1012. doi: 10.1016/j.bbadis.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu ZH, Huang T, Smith CB. Lithium reverses increased rates of cerebral protein synthesis in a mouse model of fragile X syndrome. Neurobiol Dis. 2012;45:1145–1152. doi: 10.1016/j.nbd.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo W, Murthy AC, Zhang L, Johnson EB, Schaller EG, Allan AM, et al. Inhibition of GSK3beta improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum Mol Genet. 2012;21:681–691. doi: 10.1093/hmg/ddr501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gross C, Berry-Kravis EM, Bassell GJ. Therapeutic strategies in fragile X syndrome: dysregulated mGluR signaling and beyond. Neuropsychopharmacology. 2011;37:178–195. doi: 10.1038/npp.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl) 2012;219:47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- 70.Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013 doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 71.Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol Pharmacol. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- 72.Wei J, Liu W, Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. J Biol Chem. 2010;285:26369–26376. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bradley CA, Peineau S, Taghibiglou C, Nicolas CS, Whitcomb DJ, Bortolotto ZA, et al. A pivotal role of GSK-3 in synaptic plasticity. Front Mol Neurosci. 2012;5:13. doi: 10.3389/fnmol.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gatto CL, Broadie K. The fragile X mental retardation protein in circadian rhythmicity and memory consolidation. Mol Neurobiol. 2009;39:107–129. doi: 10.1007/s12035-009-8057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kemper MB, Hagerman RJ, Altshul-Stark D. Cognitive profiles of boys with the fragile X syndrome. Am J Med Genet. 1988;30:191–200. doi: 10.1002/ajmg.1320300118. [DOI] [PubMed] [Google Scholar]
- 76.Cornish KM, Munir F, Cross G. Spatial cognition in males with Fragile-X syndrome: evidence for a neuropsychological phenotype. Cortex. 1999;35:263–271. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- 77.Cornish K, Munir F, Wilding J. A neuropsychological and behavioural profile of attention deficits in fragile X syndrome. Rev Neurol. 2001;33(Suppl 1):S24–29. [PubMed] [Google Scholar]
- 78.Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat Med. 2011;17:559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.