Abstract
Background
Splenectomy may be an effective therapeutic option for treating massive splenomegaly in patients with myeloproliferative neoplasms (MPNs). There is still limited data on its short- and long-term benefits and risks.
Methods
Efficacy and short-term complications were analyzed in 94 patients with different MPNs who underwent splenectomy at MD Anderson. The long-term impact of splenectomy on overall survival (OS) and transformation free survival (TFS) was evaluated in 461 patients with myelofibrosis (MF) seen at MD Anderson including 50 who underwent splenectomy during disease evolution.
Results
Splenectomy improved anemia and thrombocytopenia in 47% and 66% of patients, respectively. Most common complications were leukocytosis (76%), thrombocytosis (43%), and venous thromboembolism (16%). Post-operative mortality was 5%. Among patients with MF, splenectomy during disease evolution was associated with decreased OS (Hazard Ratio [HR] =2.17, p<0.0001) and TFS (HR=2.17, p<0.0001). This effect was independent of the Dynamic International Prognostic Scoring System.
Conclusions
Splenectomy is a possible therapeutic option for patients with MF and other MPNs, and its greatest benefits are related to improvement in spleen pain and discomfort, anemia and thrombocytopenia. However, in patients with MF it appears to be associated with increased mortality.
Keywords: Myelofibrosis, Myeloproliferative Neoplasms, Splenectomy, Survival, Acute Myeloid Leukemia
Introduction
Splenomegaly is a frequent finding in patients with myeloproliferative neoplasms (MPNs), particularly in patients with advanced myelofibrosis (MF).1,2 Increased spleen size is due to extramedullary hematopoiesis. It can range from few centimeters below the left costal border to massive splenomegaly1, and is associated with debilitating symptoms and complications including abdominal pain, difficulty bending and walking, increased satiety, weight loss, cytopenias, portal hypertension and splenic infarction.3,4
Splenectomy may be an effective therapeutic option for treating massive splenomegaly in patients with MPNs.5,6 Past reports have described the efficacy of splenectomy for this purpose, resulting in the elimination of splenomegaly-related symptoms, and improvements in anemia, thrombocytopenia and portal hypertension.7,8 However, splenectomy in these patients can also be associated with several complications, including development of thrombocytosis in the post-operative period, thrombohemorrhagic phenomena, and increased in-hospital mortality from complications.7–10
More recently, JAK inhibitors have come to the forefront of therapy for patients with MF.11,12 These drugs can decrease spleen size in the great majority of patients, and are associated with significant improvement in both constitutional symptoms and symptoms due to splenomegaly.11,12 In light of these recent advances, a reappraisal of the role of splenectomy in the therapy of MPNs, particularly in MF, is needed. The objectives of the present study are to describe the short-term efficacy and complications of splenectomy in a cohort of patients with MPNs who underwent splenectomy at our center and, in patients with MF, to evaluate long-term impact of splenectomy on survival and risk of transformation to acute myeloid leukemia (AML).
Patients and Methods
Patients
The medical records of 94 patients with MPN (chronic myelogenous leukemia [CML], myelofibrosis [MF] and polycythemia vera [PV]) or chronic myelomonocytic leukemia (CMML) who had underwent splenectomy at M.D. Anderson Cancer Center (MDACC) from 1981 until 2009 were reviewed. This first cohort of patients was analyzed for short-term efficacy and complications post-splenectomy. Next, the medical records of 461 patients with MF who were referred to MDACC within one year of diagnosis (to decrease time bias in assessing influence of splenectomy on survival) between 1984 and 2009 were reviewed to evaluate long-term impact of splenectomy on overall survival [OS] and transformation-free survival [TFS]. In this second cohort there were 50 patients who underwent splenectomy at some point during disease course and 411 patients (control group) who did not undergo the surgery.
Diagnosis of MF and other neoplasms was according to WHO criteria.13 Blast phase transformation was considered in patients with ≥20% blasts in the bone marrow as per the IWG-MRT criteria.14 Cytogenetic results were reported according to the International System for Human Cytogenetic Nomenclature.15 Similar to other published reports, patients without cytogenetic abnormalities and fewer than 20 metaphases were considered diploid as long as ≥10 diploid metaphases had been examined.16 Cytogenetic result was stratified as good-risk (diploid, sole del(13q), sole del(20q), +9 sole or with one abnormality), poor-risk (-5/del(5q), -7/del(7q), chromosome 17 abnormalities or complex karyotype (>3 abnormalities)) and intermediate-risk (all others), based on a previous report from our group.17 Risk stratification according to the Dynamic International Prognostic Scoring System (DIPSS) in patients with MF was as previously published.18 Transfusion dependency was assessed at the time of referral to MDACC and did not consider the actual number of transfusions in order to define a patient as being transfusion dependent. Constitutional symptoms were defined as weight loss ≥10%, night sweats and fever. The post-operative time period was considered as the first two months post-splenectomy, and complications at this time were attributed to the surgical procedure unless a clear secondary cause could be discerned. Post-operative leukocytosis was defined as a white blood cell (WBC) count >10×109/L (for patients with a WBC ≤10×109/L prior to splenectomy) or a 50% increase in the WBC count (for patients with WBC >10×109/L prior to splenectomy) in the post-operative period. Post-operative thrombocytosis was defined as a platelet count >450×109/L (for patients with a platelet ≤450×109/L prior to splenectomy) or a 50% increase in the platelet count (for patients with platelet count >450×109/L prior to splenectomy) in the post-operative period.
Response and Survival Definitions
Responses in anemia and thrombocytopenia were assessed using the IWG-MRT criteria both for patients with MF and other MPNs.14 Response was assessed in all evaluable patients at the time of splenectomy (i.e. for anemia response: all patients with hemoglobin (Hb) <10g/dL or transfusion dependency; for platelets response: all patients with platelet counts <100×109/L). OS was defined as the time from diagnosis until death from any cause. TFS was defined as the time from diagnosis until transformation to blast phase or death from any cause. Patients who were alive at last follow-up were censored. Freedom from blast transformation was defined as the time from diagnosis until transformation to blast phase, censoring patients who were alive or died without transforming. Since several patients underwent splenectomy prior to allogeneic hematopoietic stem cell transplantation (HSCT), survival outcomes were censored at the time of HSCT to prevent any possible confounding of survival effect.
Statistical Analysis
Categorical and continuous variables were compared by the Chi-Square/Fischer exact test and Mann-Whitney U Test, respectively.19 Median follow-up was calculated with the inverted Kaplan-Meier method.20 OS, TFS and freedom from blast transformation were estimated using Kaplan Meier plots. Univariable and multivariable proportional hazards Cox models were fit for survival outcomes to estimate hazard ratios (HRs) and assess the predictive effects of splenectomy and other prognostic factors, considering splenectomy as a dichotomous time-dependent covariate.21,22 Variables entered into multivariable models were those with a p-value less than 0.05 in the univariable analysis. Statistical analyses were conducted in STATA 11.0.
Results
Short-term Efficacy and Safety of Splenectomy in MPN
Clinical features of the first cohort of 94 patients at the time of splenectomy are summarized in Table 1. There was a change in the MPN diagnosis profile according to the year of splenectomy. CML was the most common diagnosis in the pre-1992 and 1992–1996 time periods (56% and 42% of surgeries, respectively), while MF was the most prevalent diagnosis from 1997 onwards (52% of cases during 1997–2001 and 77% during 2002–2009). In the 2002–2009 time period no patient with CML underwent splenectomy at MDACC.
Table 1.
Patients’ characteristics at time of splenectomy (1st cohort, n=94)
| Characteristics | Median [range] or No. (%) |
|---|---|
| Age, years | 58 [16–78] |
| Male Sex | 54 (57) |
| Performance Status ≥ 2 | 15 (16) |
| Disease Type: | |
| • MF | 50 (53) |
| • CML-CP | 18 (19) |
| • Other MPN* | 15 (16) |
| • MPN-Blast Phase** | 11 (12) |
| Time from diagnosis to splenectomy, months | 8.2 [0–251] |
| Number of prior therapies | 2 [0–7] |
| Most frequent previous Therapies | |
| • Hydroxyurea/Interferon | 60 (64) |
| • Alkylating agents | 10 (11) |
| • Chemotherapy | 16 (17) |
| • Splenic Radiation | 4 (4) |
| • Tyrosine Kinase Inhibitors | 11 (12) |
| Hb, g/dL | 8.9 [5.3–17.3] |
| WBC, × 109/L | 16.3 [1–260.5] |
| Platelets, × 109/L | 115 [4–818] |
| PB Blasts, % | 1 [0–81] |
| BM Blasts, % | 2 [0–88] |
| Constitutional Symptoms | 65 (69) |
| Transfusion dependency | 48 (51) |
| Previous VTE | 4 (4) |
| Spleen size, cm from left costal margin | 16 [0–46] |
| JAK2 V617F Positive (N=14) | 6 (43) |
| Cytogenetics (N=88) | |
| • Diploid | 32 (36) |
| • Philadelphia Chromosome | 26 (29) |
| • Other abnormal | 30 (35) |
| Indications for Splenectomy† | |
| • Pain | 45 (48) |
| • Anemia | 36 (38) |
| • Thrombocytopenia | 31 (33) |
| • Preparation for HSCT | 13 (14) |
| • Other‡ | 12 (13) |
includes CMML (N=9), MPN-unclassified (N=4) and hypereosinophilic syndrome (N=2);
includes BP of CML (N=8), MF (N=2) and MPN-unclassified (N=1);
patients could have more than one indication for splenectomy;
includes: for diagnostic evaluation (N=3), non-response/intolerance to previous therapy for splenomegaly (N=3), portal hypertension (N=1), hydronephrosis (N=1) and unclear reasons (N=4)
Among 45 patients who underwent splenectomy for anemia, 47% had an improvement in hemoglobin by IWG-MRT criteria, with a median duration of 24 months (95% CI 8–40 months). Response rate for thrombocytopenia was 66%, and median duration was 24 months (95% CI 13–35 months). Among patients with MF, anemia and thrombocytopenia response rates were 44% and 75%, respectively. Thirteen patients underwent splenectomy as preparation for HSCT (autologous=1, matched related donor=10, matched unrelated donor=2). Eighty-five percent of patients needed therapy for MPN after splenectomy, most commonly hydroxyurea (N=59) and/or interferon-α (N=30). Median time to next therapy was 14 days (range 1–731 days).
Median duration of hospital stay was 8 days (range 3–74 days). Post-operative mortality was 5% (5 cases). Causes of death were post-operative pneumonia (N=2), congestive heart failure (N=2) and sepsis/multi-organ failure (N=1). Three deaths occurred in the pre-1992 period, one in the 1996–2001 period, and one death in the 2002–2009 time period. Complications in the post-operative period are summarized in Table 2. The most common hematological complications were development of post-splenectomy leukocytosis and/or thrombocytosis. Early intervention (<7 days of therapy) for controlling WBC and/or platelet count was needed in thirty-five patients (37%) (usually hydroxyurea [N=30; 32%]) and 7 patients required apheresis in the early post-operative period. Non-hematological clinical complications occurred in 44 patients (47%), and the most common was venous thromboembolism (VTE; N=15, Table 2). Among patients who developed VTE, 7 (47%) had post-operative thrombocytosis (median platelet count 1444×109/L; range 475–4870×109/L) and 13 (87%) had post-operative leukocytosis (median WBC count 64.8×109/L; range 20.8–191×109/L). Post-operative VTE was seen in patients with CML (N=6), MF (N=8) and CMML (N=1). Due to the small number of patients, no pre-splenectomy characteristic was found to be associated with post-operatory development of VTE (data not shown). Treatment for VTE included anticoagulation in 12 (80%) patients. Nine patients (60%) who developed VTE required therapy with hydroxyurea post-splenectomy, of which 5 received hydroxyurea within 7 days of surgery to control post-operative thrombocytosis and/or leukocytosis.
Table 2.
Complications observed post-splenectomy in the 1st cohort
| Hematologic Complication | N [%] or Median (range) |
|---|---|
| Post-operative Leukocytosis | 71 [76] |
| Median Peak White Blood Cell Count | 51.8 × 109/L (12.7–247 × 109/L) |
| Time to Leukocytosis | 1 day (0–42 days) |
| Post-operative Thrombocytosis | 40 [43] |
| Median Peak Platelet Count | 1108 × 109/L (450–4870 × 109/L) |
| Time to Thrombocytosis | 5 days (0–53 days) |
| Non-Hematologic Complication | N [%] |
| Venous Thromboembolism* | 15 [16] |
| Pneumonia | 11 [12] |
| Abdominal Abscess | 7 [8] |
| Rebound Hepatomegaly | 5 [5.5] |
| Sepsis and Multi-Organ Failure | 3 [3.2] |
| Ascites | 2 [2.1] |
| Atrial Fibrillation | 2 [2.1] |
| Congestive Heart Failure | 2 [2.1] |
| Liver failure and Coagulopathy | 2 [2.1] |
| Clostridium difficile diarrhea | 1 [1] |
| Dehiscence of surgical wound | 1 [1] |
| Hemothorax and empyema | 1 [1] |
| Pancreatitis | 1 [1] |
| Pneumothorax | 1 [1] |
| Small Bowel Obstruction | 1 [1] |
sites included portal vein (N=11), supra-hepatic veins (N=3), superior vena cava, pulmonary embolism and splenic vein (N=1 each; two patients had VTE at two different sites).
Median follow-up was 5.5 years. Of 94 patients, 78 (83%) have died. Median survival post-splenectomy was 584 days (95% CI 425–804) or 19.2 months, and 5-years survival was 16.1% (95% CI 9–25%) (Figure 1A). There was no statistically significant difference in OS by MPN subtype (data not shown). Patients who underwent splenectomy in the time period of 2002–2009 had a better OS compared to patients who underwent surgery before that time period (HR=0.41; 95% CI 0.25–0.73; p=0.002) (Figure 1B).
Figure 1.
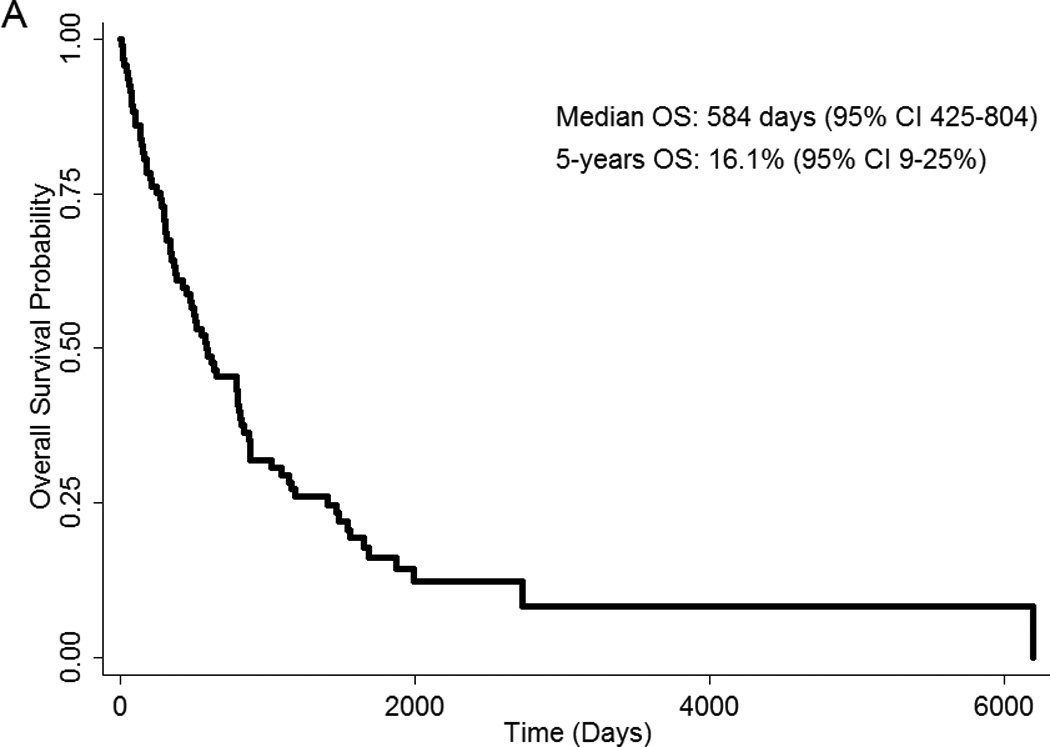
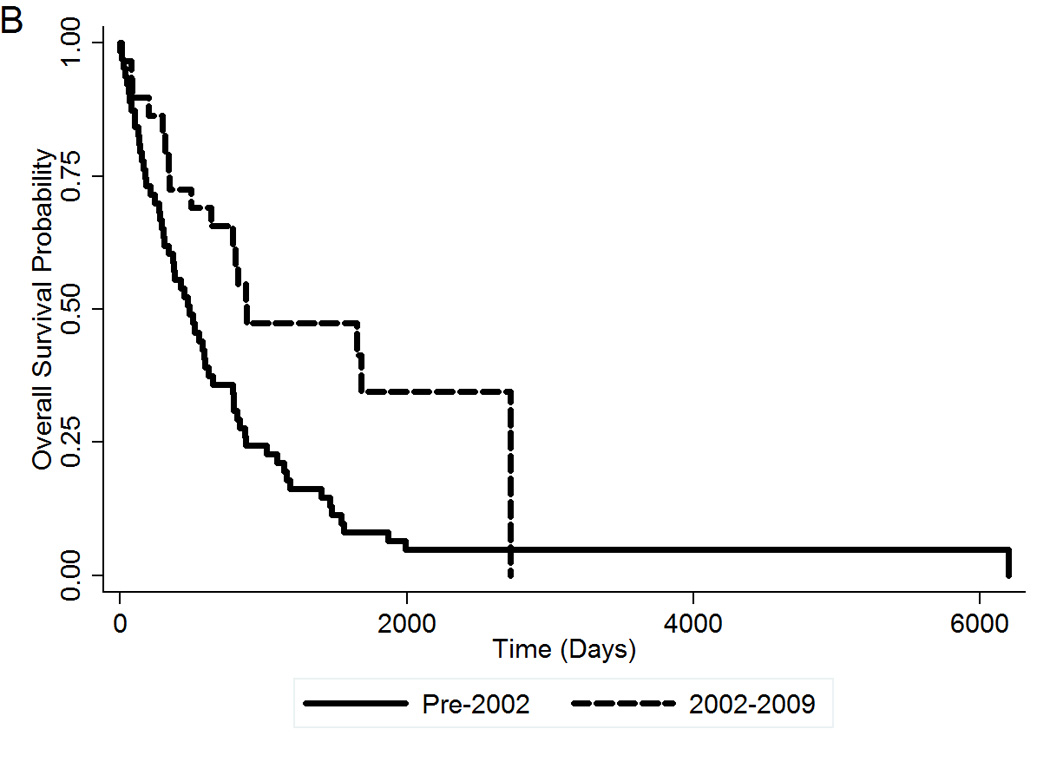
(A) OS post-splenectomy of MPN patients who underwent splenectomy at MDACC. (B) OS post-splenectomy by time period of surgery. OS, overall survival.
Long-term Impact of Splenectomy on Overall Survival and Incidence of Blast Transformation in MF
Clinical features of the second cohort of 461 patients with MF are summarized in Table 3. Median follow-up of the whole cohort was 4 years. At the time of this analysis, 164 patients (36%) have died, 35 patients (8%) have transformed to AML, and 144 deaths (31%) were unrelated to AML transformation. Among the 50 patients who were submitted to splenectomy, 30 (60%) have died and 9 (18%) have transformed to AML. The presence of splenectomy during disease evolution was associated with inferior OS (Figure 2A). At 5 years, OS for patients who underwent splenectomy was 20% (95% CI 9–35%) compared to 47% (95% CI 39–64%) for patients who did not underwent splenectomy. The HR for splenectomy was 2.17 (95% CI 1.43–3.29; p<0.0001). Survival in the splenectomy group was inferior regardless of the moment of the surgery during disease course (data not shown). Patients who were submitted to splenectomy also had an increased risk of transforming to blast phase. At 5 years, TFS was 43% (95% CI 36–50%) in the no-splenectomy group, compared to 17% (95% CI 7–32%) in the splenectomy group (Figure 2B). The HR for splenectomy was 2.17 (95% CI 1.44–3.26; p<0.0001). The freedom from blast transformation at 5 years was 55% (95% CI 27–76%) in the splenectomy group, versus 85% (95% CI 76–90%) in the no-splenectomy group (Figure 2C). The HR was 3.88 (95% CI 1.80=8.35; p=0.001).
Table 3.
Clinical features of patients with Myelofibrosis (2nd cohort)
| Clinical Features | Median [range] or No. (%) |
P | |
|---|---|---|---|
| No Splenectomy (n = 411) |
Splenectomy (N=50) |
||
| Age, years | 64 [20–86] | 59 [26–85] | 0.0011 |
| Age > 65 years | 179 (44) | 12 (24) | 0.008 |
| Male sex | 260 (63) | 29 (58) | 0.46 |
| Diagnosis | 0.34* | ||
| • PMF | 303 (74) | 32 (64) | |
| • Post-PV MF | 61 (15) | 10 (20) | |
| • Post-ET MF | 47 (11) | 8 (16) | |
| Performance Status > 1 | 46 (11) | 4 (8) | 0.30 |
| Constitutional Symptoms | 180 (44) | 25 (50) | 0.40 |
| Hb, g/dL | 10.0 [6.2–18] | 10.0 [7–16] | 0.89 |
| Hb< 10g/dL | 157 (38) | 17 (34) | 0.56 |
| WBC, × 109/L | 9.4 [0.4–191] | 11.8 [1.3–70.6] | 0.02 |
| WBC > 25 × 109/L | 59 (14) | 14 (28) | 0.01 |
| Platelets, × 109/L | 214 [3–1299] | 239 [16–1958] | 0.24 |
| Platelet < 50 × 109/L | 54 (13) | 7 (14) | 0.83 |
| PB Blasts, % | 0 [0–16] | 0 [0–6] | 0.70 |
| PB Blasts > 1% | 199 (48) | 23 (46) | 0.74 |
| BM Blasts, % | 2 [0–17] | 1 [0–8] | 0.99 |
| BM Blasts ≥10% | 22 (5) | 0 (0) | 0.15 |
| LDH, IU/L | 1215 [189–10353] | 1445 [378–5059] | 0.22 |
| Transfusion Dependency | 100 (24) | 12 (24) | 0.95 |
| Exposure to Alkylating Agents | 4 (1) | 4 (8) | 0.006 |
| Cytogenetic Risk Group | 0.36* | ||
| • Low Risk | 290 (70) | 31 (62) | |
| • Intermediate Risk | 59 (14) | 9 (18) | |
| • Poor Risk | 21 (5) | 5 (10) | |
| • No metaphases or not done | 41 (10) | 5 (10) | |
| DIPSS Risk Group | 0.99* | ||
| • Low | 59 (14) | 7 (14) | |
| • Intermediate-1 | 180 (44) | 22 (44) | |
| • Intermediate-2 | 134 (33) | 17 (34) | |
| • High | 38 (9) | 4 (8) | |
p-value for trend
Figure 2.
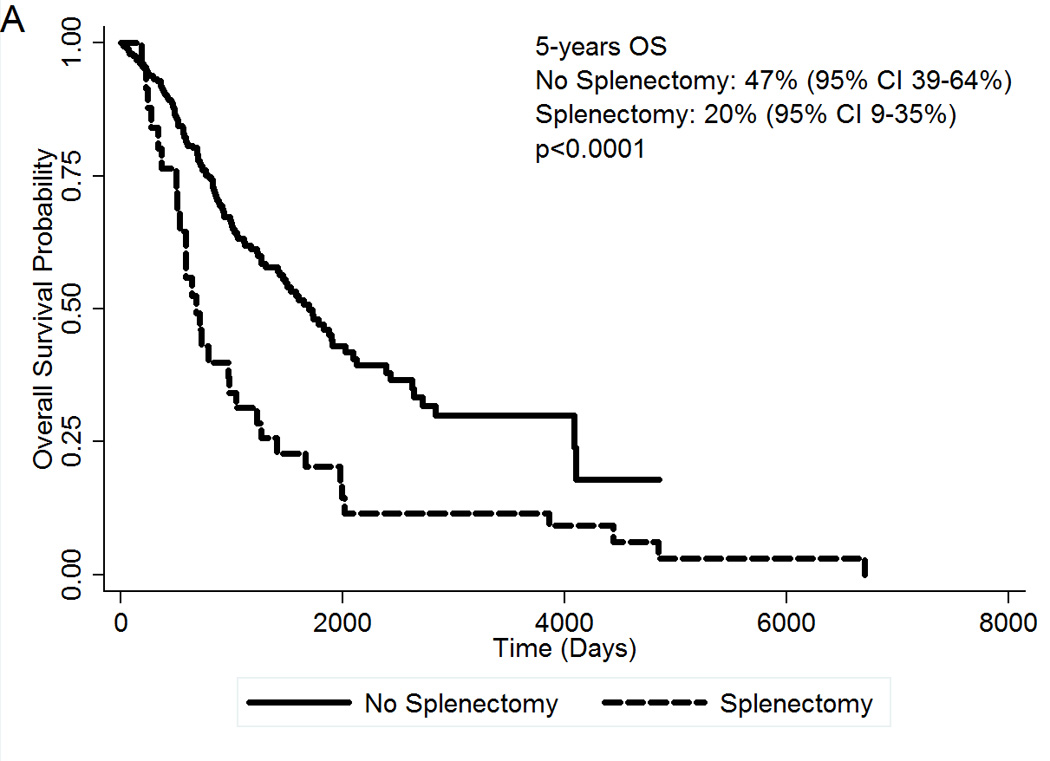
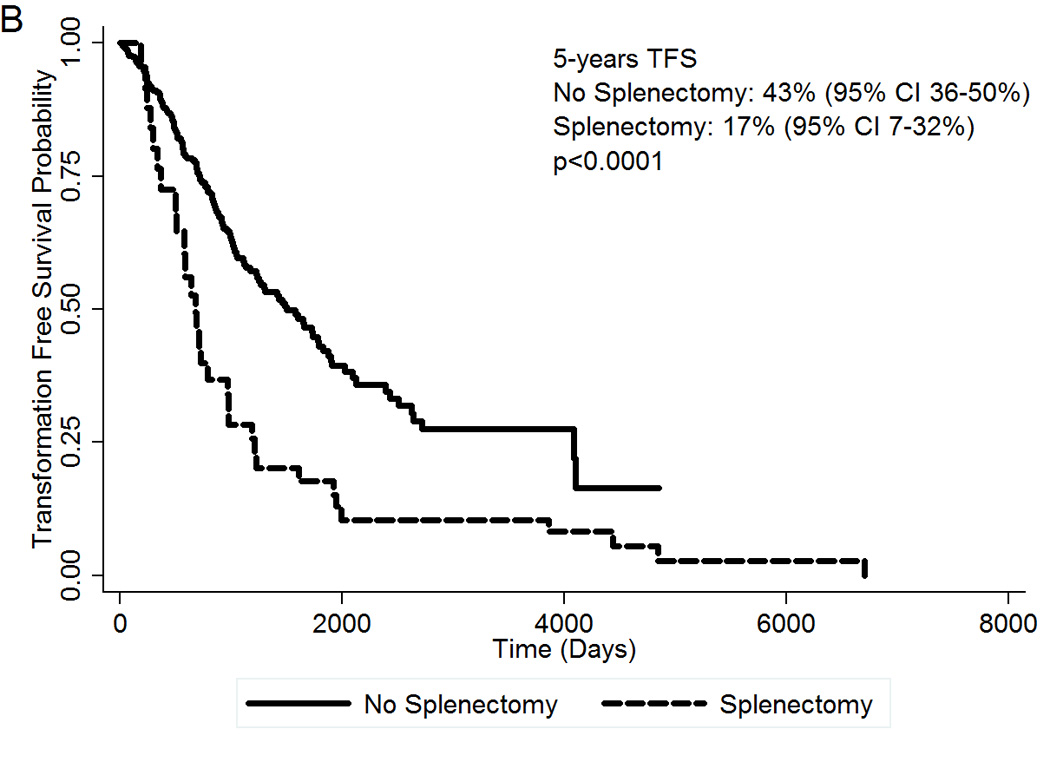
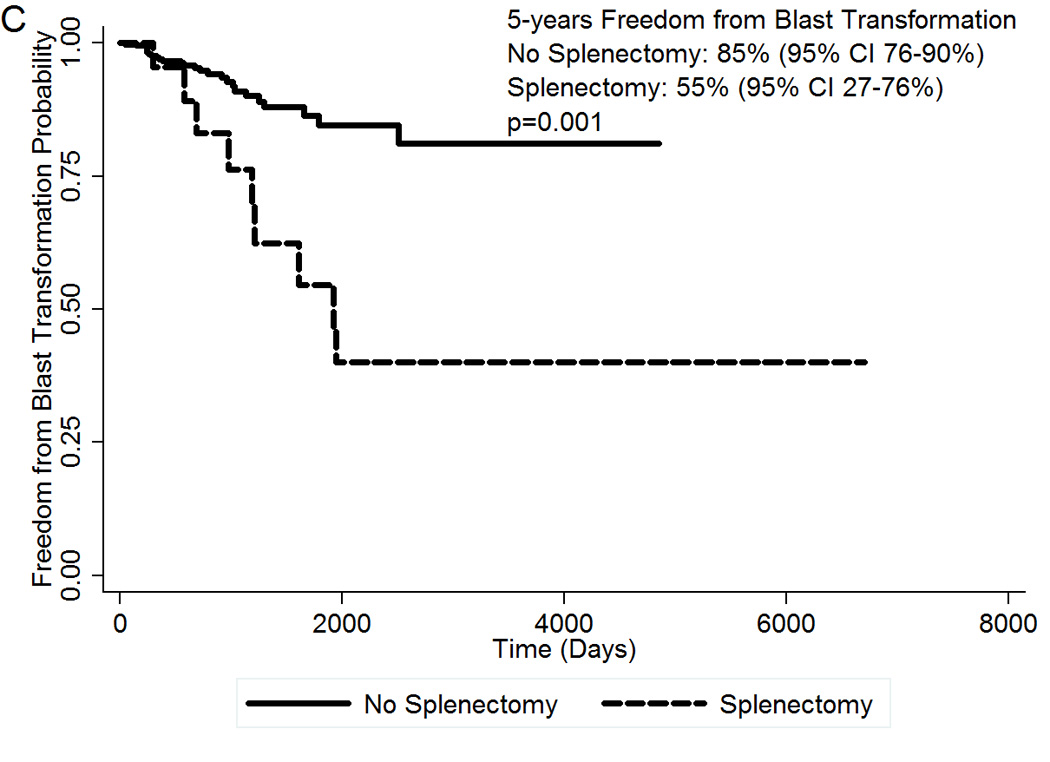
(A) OS of 50 MF patients who underwent splenectomy during disease evolution compared to 411 control MF patients; (B) TFS by splenectomy status; (C) Freedom from blast transformation by presence of splenectomy during disease evolution. CI, confidence interval; HR, Hazard Ratio; OS, Overall Survival; TFS, transformation-free survival.
We then analyzed different variables (all listed in Table 3) for their association with OS and TFS in Cox proportional hazard models (age, Hb, WBC, constitutional symptoms and PB blasts were entered as part of the DIPSS risk score). By univariable analysis, the following covariates were associated with decreased TFS: poor risk chromosomal abnormalities (p<0.0001), male sex (p=0.008), performance status >1 (p<0.0001), splenectomy (p<0.0001), transfusion dependency (p<0.0001), platelet count <50×109/L (p<0.0001), BM blast count ≥10% (p<0.0001) increasing DIPSS risk score (p<0.0001). In the multivariable model (table 4), splenectomy, transfusion dependency, DIPSS risk score, thrombocytopenia, increased BM blasts and poor risk cytogenetic abnormalities were associated with inferior TFS.
Table 4.
Multivariate model for TFS and OS in patients with MF (2nd cohort)
| Covariate | HR (95% CI) | P |
|---|---|---|
|
TFS | ||
| Splenectomy | 1.69 (1.09–2.64) | 0.018 |
| BM Blasts ≥10% (yes vs. no) | 2.92 (1.51–5.65) | 0.001 |
| Transfusion dependency (yes vs. no) | 2.26 (1.60–3.18) | <0.0001 |
| Platelet count <50 × 109/L (yes vs. no) | 2.99 (2.00–4.47) | <0.0001 |
| Cytogenetic Risk Group | ||
| • Intermediate Risk (vs. Low) | 1.19 (0.76–1.84) | 0.43 |
| • Poor Risk (vs. Low) | 2.47 (1.41–4.33) | 0.002 |
| DIPSS risk category | ||
| • Intermediate-1 (vs. Low) | 2.55 (1.28–5.04) | 0.007 |
| • Intermediate-2 (vs. Low) | 4.30 (2.15–8.61) | <0.0001 |
| • High (vs. Low) | 8.59 (4.01–18.37) | <0.0001 |
| OS | ||
| Splenectomy | 1.61 (1.02–2.53) | 0.03 |
| Male Sex (yes vs. no) | 1.54 (1.05–2.25) | 0.02 |
| Performance Status >1 (yes vs. no) | 2.00 (1.03–3.87) | 0.03 |
| BM Blasts ≥10% (yes vs. no) | 2.27 (1.12–4.57) | 0.02 |
| Transfusion dependency (yes vs. no) | 2.58 (1.78–3.75) | <0.0001 |
| Platelet count <50 × 109/L (yes vs. no) | 3.29 (2.13–5.07) | <0.0001 |
| Cytogenetic Risk Group | ||
| • Intermediate Risk (vs. Low) | 1.10 (0.69–1.77) | 0.67 |
| • Poor Risk (vs. Low) | 2.68 (1.46–4.93) | 0.001 |
| DIPSS risk category | ||
| • Intermediate-1 (vs. Low) | 2.42 (1.17–5.00) | 0.01 |
| • Intermediate-2 (vs. Low) | 4.06 (1.94–8.49) | <0.0001 |
| • High (vs. Low) | 7.80 (3.41–17.80) | <0.0001 |
The following covariates were associated with OS in a univariable model: splenectomy (p<0.0001), performance status >1 (p<0.0001), male sex (p=0.006), increasing LDH (p=0.02), transfusion dependency (p<0.0001), platelet count <50×109/L (p<0.0001), BM blasts ≥10% (p=0.001), poor risk chromosomal abnormalities (p<0.0001) and DIPSS risk category (p<0.0001). In the multivariable model (table 4), the splenectomy covariate remained an independent risk factor for inferior OS in patients with MF.
Discussion
We analyzed two cohorts of patients to better ascertain the short- and long-term implications of splenectomy in patients with MPNs. In the first cohort of 94 patients with different MPNs that had undergone splenectomy at our institution, the most common diagnosis overall was MF. However, in the past splenectomy was most often performed in patients with CML. This change is very likely due to the development of effective therapy with BCR/ABL inhibitors for CML, thus virtually abolishing splenectomy as a therapeutic procedure for CML patients. Splenectomy was highly effective for alleviating abdominal pain and discomfort, and resulted in high rates of erythroid and platelet response by the IWG-MRT criteria. Among patients with MF, the rate of erythroid response (44%) compares favorably to what has been reported with drug therapy for treating anemia in MF (e.g. lenalidomide 19–21%: pomalidomide 16–36%).23–25 Improvement in thrombocytopenia is also a very important benefit, since an increase in platelets is rarely seen with conventional therapy.23,24 Available thrombomimetic agents (i.e. romiplostin, eltrombopag) have been associated with an increase in bone marrow fibrosis, and possibly an increased risk of leukemic transformation, and thus their activity has not been explored in patients with MPNs.26
The post-operative mortality was relatively low at our center (5%), which compares favorably with published data (≥9%).7,10 The benefits of splenectomy must be weighted against the substantial risk of post-operative morbidity (47% had non-hematologic complications). The most common complications post-splenectomy were development or worsening of leukocytosis and thrombocytosis. They occurred rapidly (within days of the procedure) and were associated with thromboembolic phenomena in 16% of patients (most frequent in splanchnic veins). Thus, clinicians should aggressively manage patients who display marked elevation of WBC and platelet counts in the immediate post-operative state, and consider extended prophylactic anticoagulation if there are no contra-indications. Benefits of splenomegaly were also offset by short survival after the procedure, with a median OS less than two years. We thus proceeded to analyze the impact of splenectomy in a larger cohort of patients, focusing this time solely on MF, and compared their outcomes with a control group who was not submitted to splenectomy. In this analysis, splenectomy during disease evolution was associated with an increased risk of mortality and of transformation to AML. In a multivariate analysis, the risk attributed to splenectomy was independent of the previously validated risk score DIPSS. Other variables that were found to be associated with decreased survival and increased risk of secondary AML are a reflection of more advanced disease and compromised hematopoiesis.16,18,27
Two previous studies have reported an increased risk of death and transformation to AML post-splenectomy in MF,28,29 but others have not found such an association.8,30 Increased risk of leukemic transformation and secondary AML was reported in patients with aplastic anemia and Hodgkin’s lymphoma post-splenectomy.31–33 At least two possible mechanisms could explain association of splenectomy with poor survival in MF. Accelerated progression of MF, as evidenced by thromboembolic episodes, liver failure and pulmonary hypertension has been reported following splenectomy.34,35 In addition, Porcu et al. reported, in a small case series of patients with MF who underwent splenectomy for various reasons, an increased number of clusters of immature mononuclear cells in the spleens from patients with more advanced MF compared to patients with early MF.36 This suggests that the need for splenectomy, and not the procedure itself, identifies patients with more aggressive disease, with occult leukemic transformation in the spleen extramedullary hematopoiesis. Patients who were submitted to splenectomy were also more likely to be those who have inadequate responses to conventional cytoreductive therapy (e.g. persistent splenic pain and debility despite best available medical treatment), and this might reflect a more aggressive underlying disease with increased risk of transformation. Lastly, 6 to 12 months post-splenectomy there is an increase in circulating CD34+ cells in the blood of patients with MF following an initial, temporary decrease.37 Increased number of CD34+ cells in the peripheral blood of MF patients has been associated with poor outcome in one study,38 but its independent prognostic value has been challenged in the face of more recent prognostic models.2 Despite our results, we cannot definitively conclude that there is a direct association between splenectomy and increased risk of transformation to AML in patients with MF due to several possible confounding risk factors, including the state of disease at the time of surgery.
Splenectomy is an effective treatment for MPN-related splenic pain and/or cytopenias, but is associated with substantial operative morbidity and mortality. It is also associated with reduced survival and a potential increased risk of blast phase transformation in MF. The recent development of JAK2 inhibitors (e.g. ruxolitinib) as an effective and safe therapy for patients with MF diminishes the role of splenectomy in everyday management of MF patients. The pivotal phase III studies of ruxolitinib have confirmed its efficacy in controlling splenectomy and MF-related constitutonal symptoms, and there is early data to suggest that it may also prolong survival.11,12 Therefore, splenectomy should be used cautiously and considered in highly-selected cases, mainly for patients with severe, refractory cytopenias and extremely large spleens not responding to optimal medical management.
Summary.
The authors analyzed the efficacy and long term complications of splenectomy in patients with myelofibrosis and other myeloproliferative neoplasms. Splenectomy was found to be an effective therapy for improving cytopenias, but was associated with increased mortality and risk of transformation to blast-phase.
Acknowledgments
This research is supported in part by the MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Authorship Contributions: FPSS collected data, analyzed data and wrote the manuscript; CST collected data, analyzed data and reviewed the manuscript; HK, JC, DT and RP treated patients and reviewed the manuscript; SV designed project, analyzed data and wrote the manuscript.
Conflict of Interest Disclosure: No conflicts of interest exist for any author
References
- 1.Visani G, Finelli C, Castelli U, et al. Myelofibrosis with myeloid metaplasia: clinical and haematological parameters predicting survival in a series of 133 patients. Br J Haematol. 1990;75:4–9. doi: 10.1111/j.1365-2141.1990.tb02609.x. [DOI] [PubMed] [Google Scholar]
- 2.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 3.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33:1199–1203. doi: 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Hilal M, Tawaker J. Portal hypertension secondary to myelofibrosis with myeloid metaplasia: a study of 13 cases. World J Gastroenterol. 2009;15:3128–3133. doi: 10.3748/wjg.15.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesa RA, Elliott MA, Tefferi A. Splenectomy in chronic myeloid leukemia and myelofibrosis with myeloid metaplasia. Blood Rev. 2000;14:121–129. doi: 10.1054/blre.2000.0132. [DOI] [PubMed] [Google Scholar]
- 6.Mesa RA, Tefferi A. Palliative splenectomy in myelofibrosis with myeloid metaplasia. Leuk Lymphoma. 2001;42:901–911. doi: 10.3109/10428190109097709. [DOI] [PubMed] [Google Scholar]
- 7.Mesa RA, Nagorney DS, Schwager S, et al. Palliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo Clinic. Cancer. 2006;107:361–370. doi: 10.1002/cncr.22021. [DOI] [PubMed] [Google Scholar]
- 8.Tefferi A, Mesa RA, Nagorney DM, et al. Splenectomy in myelofibrosis with myeloid metaplasia: a single-institution experience with 223 patients. Blood. 2000;95:2226–2233. [PubMed] [Google Scholar]
- 9.Akpek G, McAneny D, Weintraub L. Risks and benefits of splenectomy in myelofibrosis with myeloid metaplasia: a retrospective analysis of 26 cases. J Surg Oncol. 2001;77:42–48. doi: 10.1002/jso.1064. [DOI] [PubMed] [Google Scholar]
- 10.Benbassat J, Gilon D, Penchas S. The choice between splenectomy and medical treatment in patients with advanced agnogenic myeloid metaplasia. Am J Hematol. 1990;33:128–135. doi: 10.1002/ajh.2830330210. [DOI] [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 13.Thiele J, Kvasnicka HM, Tefferi A, et al. Primary Myelofibrosis. In: Swerdlow SH, Campo E, Harris NL, et al., editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 44–47. [Google Scholar]
- 14.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT) Blood. 2006;108:1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 15.Shaffer LG, Slovak ML, Campbell LJ. ISCN 2009: An International System for Human Cytogenetic Nomenclature: Recommendations of the International Standing Committee on Human Cytogenetic Nomenclature. Basel, Switzerland: Karger; 2009. [Google Scholar]
- 16.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 17.Tam CS, Abruzzo LV, Lin KI, et al. The role of cytogenetic abnormalities as a prognostic marker in primary myelofibrosis: applicability at the time of diagnosis and later during disease course. Blood. 2009;113:4171–4178. doi: 10.1182/blood-2008-09-178541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 19.Snedecor G, Cochran W. Statistical Methods. ed 7th. Armes, IA: Iowa State University Press; 1980. [Google Scholar]
- 20.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 21.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 22.Mantel N, Byar D. Evaluation of response-time data involving transient states: An illustration using heart-transplant data. J Am Stat Assoc. 1974;69:81–86. [Google Scholar]
- 23.Jabbour E, Thomas D, Kantarjian H, et al. Comparison of thalidomide and lenalidomide as therapy for myelofibrosis. Blood. 2011;118:899–902. doi: 10.1182/blood-2010-12-325589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thapaliya P, Tefferi A, Pardanani A, et al. International working group for myelofibrosis research and treatment response assessment and long-term follow-up of 50 myelofibrosis patients treated with thalidomide-prednisone based regimens. Am J Hematol. 2011;86:96–98. doi: 10.1002/ajh.21892. [DOI] [PubMed] [Google Scholar]
- 25.Tefferi A, Verstovsek S, Barosi G, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27:4563–4569. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghanima W, Junker P, Hasselbalch HC, et al. Fibroproliferative activity in patients with immune thrombocytopenia (ITP) treated with thrombopoietic agents. Br J Haematol. 2011;155:248–255. doi: 10.1111/j.1365-2141.2011.08845.x. [DOI] [PubMed] [Google Scholar]
- 27.Tam CS, Kantarjian H, Cortes J, et al. Dynamic model for predicting death within 12 months in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. J Clin Oncol. 2009;27:5587–5593. doi: 10.1200/JCO.2009.22.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morel P, Duhamel A, Hivert B, et al. Identification during the follow-up of time-dependent prognostic factors for the competing risks of death and blast phase in primary myelofibrosis: a study of 172 patients. Blood. 2010;115:4350–4355. doi: 10.1182/blood-2009-12-255943. [DOI] [PubMed] [Google Scholar]
- 29.Barosi G, Ambrosetti A, Centra A, et al. Splenectomy and risk of blast transformation in myelofibrosis with myeloid metaplasia. Italian Cooperative Study Group on Myeloid with Myeloid Metaplasia. Blood. 1998;91:3630–3636. [PubMed] [Google Scholar]
- 30.Huang J, Li CY, Mesa RA, et al. Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008;112:2726–2732. doi: 10.1002/cncr.23505. [DOI] [PubMed] [Google Scholar]
- 31.Socie G, Henry-Amar M, Bacigalupo A, et al. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. N Engl J Med. 1993;329:1152–1157. doi: 10.1056/NEJM199310143291603. [DOI] [PubMed] [Google Scholar]
- 32.Tura S, Fiacchini M, Zinzani PL, et al. Splenectomy and the increasing risk of secondary acute leukemia in Hodgkin's disease. J Clin Oncol. 1993;11:925–930. doi: 10.1200/JCO.1993.11.5.925. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich PY, Henry-Amar M, Cosset JM, et al. Second primary cancers in patients continuously disease-free from Hodgkin's disease: a protective role for the spleen? Blood. 1994;84:1209–1215. [PubMed] [Google Scholar]
- 34.Lopez-Guillermo A, Cervantes F, Bruguera M, et al. Liver dysfunction following splenectomy in idiopathic myelofibrosis: a study of 10 patients. Acta Haematol. 1991;85:184–188. doi: 10.1159/000204888. [DOI] [PubMed] [Google Scholar]
- 35.Jais X, Ioos V, Jardim C, et al. Splenectomy and chronic thromboembolic pulmonary hypertension. Thorax. 2005;60:1031–1034. doi: 10.1136/thx.2004.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porcu P, Neiman RS, Orazi A. Splenectomy in agnogenic myeloid metaplasia. Blood. 1999;93:2132–2134. [PubMed] [Google Scholar]
- 37.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 38.Barosi G, Viarengo G, Pecci A, et al. Diagnostic and clinical relevance of the number of circulating CD34(+) cells in myelofibrosis with myeloid metaplasia. Blood. 2001;98:3249–3255. doi: 10.1182/blood.v98.12.3249. [DOI] [PubMed] [Google Scholar]


