Abstract
Osteoporotic fractures result in significant morbidity and mortality. Anabolic agents reverse the negative skeletal balance that characterizes osteoporosis by stimulating osteoblast-dependent bone formation to a greater degree than osteoclast-dependent bone resorption. Parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP) are peptide hormones which have anabolic actions when administered intermittently. The only FDA-approved anabolic bone treatment for treatment of osteoporosis in the United States is PTH 1-34, or teriparatide, administered by daily subcutaneous injections. However, PTH 1-84 is also available in Europe. Synthetic human PTHrP 1-36 and a PTHrP 1-34 analog, BA058, have also been shown to increase lumbar spine bone density. These agents and several other PTH and PTHrP analogs, including some which are not administered as injections, continue to be investigated as potential anabolic therapies for osteoporosis.
Keywords: Parathyroid hormone, Parathyroid hormone-related protein, Osteoporosis, Anabolic
1. Introduction
Osteoporosis is a disorder characterized by compromised bone strength which increases risk of fragility fractures. A patient with osteoporosis may have a lifetime fracture risk as high as 40%. Osteoporotic fractures impair mobility, independence and quality of life; fractures of the hip also increase mortality by up to 20% [1]. Bone mineral density (BMD) as assessed by dual x-ray absorptiometry (DXA) is the most reliable test available to estimate bone strength [2]. The World Health Organization defines osteoporosis as BMD 2.5 standard deviations below the mean for young healthy women (a T-score of <-2.5 SD) [3].
Osteoporosis typically occurs in a state of negative skeletal balance in which bone resorption exceeds bone formation. The most commonly used medications for treatment of osteoporosis are antiresorptive agents, including the bisphosphonates, estrogen, selective estrogen receptor modulators and denosumab, which address this imbalance by inhibiting osteoclast-mediated bone resorption [4]. The antiresorptive agents increase bone density by decreasing the number of bone remodeling units and allowing for mineralization of osteoid. In doing so they, particularly the bisphosphonates, significantly reduce the risk of vertebral fractures in osteoporotic patients by about 40–50% and hip or non-spine fractures by 20–30% [5–9]. Although the antiresorptives increase BMD and reduce fracture, they do not stimulate new bone formation and are therefore limited in their capacity to restore bone architecture.
In contrast to the antiresorptive agents, anabolic agents can directly stimulate osteoblastic formation of new bone. The only anabolic agent currently FDA-approved in the United States is parathyroid hormone (1-34) (PTH 1-34), or teriparatide . However, full-length recombinant human parathyroid hormone (PTH 1-84), is also available for treatment of postmenopausal osteoporosis in many European countries [10, 11]. While both PTH 1-34 and PTH 1-84 stimulate osteoblast-mediated bone formation, they also stimulate osteoclast-dependent bone breakdown, albeit to a lesser degree. The ideal agent for treatment of osteoporosis would be purely anabolic, stimulating bone formation without stimulating resorption. Thus there has been much recent interest in studying other PTH and parathyroid hormone related protein (PTHrP) analogs as potential pure anabolic agents.
2. Physiology
Parathyroid hormone is an 84-amino acid polypeptide which is secreted by the parathyroid glands in response to decreases in calcium concentration. Its main actions are to increase renal tubular calcium reabsorption, to stimulate renal calcitriol, or 1,25 dihydroxyvitamin D, production thereby indirectly increasing intestinal calcium absorption, and to regulate bone remodeling [12]. Its ligand is the PTH - 1 receptor, a G protein-coupled receptor expressed primarily in kidney and bone [11, 13]. PTH results in an increase in the number of bone-forming cells by promoting osteoblast growth and decreasing osteoblast cell death or apoptosis [13]. Interestingly, PTH also stimulates osteoclastogenesis. Mice that do not have osteoclasts do not respond to PTH, suggesting that osteoclast activity is required for PTH to have its full anabolic action [11]. Additionally, PTH regulates certain skeletal growth factors (such as IGF-1) and growth factor antagonists (such as sclerostin) to further promote the building of bone [13].
Parathyroid hormone stimulates both osteoclast-mediated bone resorption and osteoblast-mediated bone formation. This increased bone turnover is evidenced by marked increases in biochemical markers of both bone formation and resorption beginning soon after administration [12]. The predominant skeletal action of PTH depends on the pattern of administration [14]. Short-term or intermittent exposure to PTH, as occurs after a single subcutaneous injection, leads to a predominance of osteoblast-mediated bone formation. This bone formation occurs in cortical bone and to a lesser extent trabecular bone [15, 16]. In contrast, continuous administration of PTH or persistently high PTH levels, as occurs in primary hyperparathyroidism, results in predominance of osteoclast-mediated bone resorption and consequent net bone loss [17, 18]. The anabolic response to daily transient peak levels of PTH is the greatest during the first 6–12 months of therapy, the anabolic window, and subsequently tends to wane as bone resorption increases to match the increased bone formation [19]. The mechanisms determining whether PTH has anabolic or catabolic actions are not known as the skeletal effects of PTH are complex and not yet completely understood.
Parathyroid hormone related protein (PTHrP) is widely expressed in normal tissues throughout development, though it does not normally appear in the circulation except during lactation. PTH and PTHrP have significant homology in the N-terminal region, allowing them to bind to the same PTH - 1 receptor, though each protein favors a different conformational state, resulting in somewhat different downstream effects on calcium metabolism [20, 21]. PTHrP acts as a paracrine and autocrine factor to regulate cellular growth, differentiation, development and cell death as well as epithelial calcium transport in cartilage, bone, mammary glands, and a variety of other tissues [21, 22]. Studies in mice have demonstrated that PTHrP is required for normal bone development [23]. PTHrP also plays a role in mobilizing calcium during lactation [22].
Similar to PTH, continuous secretion of PTHrP causes a pathological state, humoral hypercalcemia of malignancy, which is characterized by hypercalcemia and increased bone resorption. Continuous infusion of both PTHrP and PTH result in elevated serum calcium levels, decreased renal calcium excretion, an increase in markers of bone resorption and a decrease in markers of bone formation [24]. In contrast, intermittent administration of PTHrP (1-36) has been found to increase bone mass in rodents [25, 26] and in humans [27, 28], and some data suggest that intermittent PTHrP may be more purely anabolic than PTH [27, 29].
3. PTH analogs as therapeutic agents
Teriparatide (PTH 1-34) is approved in the United States for treatment of osteoporosis in those at high risk of fracture including postmenopausal women, men with primary or hypogonadal osteoporosis and men and women with glucocorticoid-associated osteoporosis [30]. In postmenopausal women with osteoporosis, the risk of vertebral fractures was decreased by 65% and risk of nonvertebral fractures was decreased by 53% with teriparatide 20 µg/day treatment compared to placebo. Increases in vertebral and femoral bone mineral density were also observed [31].
Adverse events with teriparatide include hypercalcemia in approximately 10% of patients. The hypercalcemia generally occurs within the first six months of treatment and is usually easily corrected with reduction of calcium and vitamin D supplementation. Calcium levels greater than 11 mg/dl have been reported in less than 1% of patients treated [31, 32]. Other adverse events associated with teriparatide use include orthostasis, dizziness, muscle cramps, and injection site reactions. Due to concern for osteosarcoma, a black box warning advises against teriparatide use in patients with risk factors for osteosarcoma such as Paget’s disease of the bone, unexplained elevations of alkaline phosphatase, pediatric and young adult patients with open epiphyses, or prior external beam or implant radiation therapy involving the skeleton. Other contraindications include skeletal malignancies or bone metastases, hypercalcemic disorders such as primary hyperparathyroidism, and renal failure [30]. Teriparatide can increase urinary calcium and serum uric acid levels, so its use should be avoided in patients with history of nephrolithiasis or gout unless careful monitoring of the appropriate laboratory values is done [12].
Another form of parathyroid hormone available for therapeutic use in Europe but not in the United States is PTH 1-84. Daily subcutaneous injection of PTH 1-84 100µg for 18 months has been shown to reduce the risk of new or worsened vertebral fractures by 58% in postmenopausal women with osteoporosis. Despite increases in BMD at the hip as well as spine, incidence of nonvertebral fractures in the subjects receiving PTH 1-84 was similar to placebo [33]. Since PTH 1-84 does not have superior effects on fracture risk as compared to teriparatide and since there is higher frequency of hypercalcemia and hypercalciuria [10], approval as a treatment for osteoporosis in the United States is no longer being sought. Interestingly, in a recent study using high-resolution peripheral quantitative computed tomography (HR-pQCT) to characterize bone microarchitecture and estimated strength in postmenopausal osteoporotic women treated with PTH 1-34 or PTH 1-84 for 18 months, cortical thickness and trabecular number increased in the tibia with both agents. Estimated bone strength was preserved with PTH 1-34 but decreased with PTH 1-84 at the radius and tibia [34].
Although PTH 1-84 is not available in the United States for the treatment of osteoporosis, there are ongoing studies exploring the role of PTH 1-84 as a treatment for patients with hypoparathyroidism. PTH 1-84 has a slightly longer half-life than PTH 1-34 which is a disadvantage for treating osteoporosis and may account for the increase in hypercalcemia but may be advantageous in treating hypoparathyroidism [35].
Treatment with both teriparatide and recombinant human intact hormone 1-84 is limited to 18-24 months since safety and efficacy were not evaluated beyond two years in clinical trials. Initial preclinical studies demonstrated risk of osteosarcoma in rats with prolonged, high-dose use [36], leading to the recommendation to limit exposure to a maximum of 2 years [37]. Though three cases of osteosarcoma in patients using teriparatide have been reported [19], the incidence of osteosarcoma in patients using teriparatide appears similar to that in the general population. Furthermore, after the first year of intermittent PTH administration, bone turnover markers begin to plateau and then decline, suggesting that after a certain duration of therapy, gains in bone mass decrease [38]. In contrast to bisphosphonates, which continue to act on bone after administration is stopped, the protective effects of PTH begin to decline soon after the medication is discontinued if alternative therapy is not started [39–41].
Ideally, teriparatide should be administered prior to use of bisphosphonates, particularly in patients with preexisting osteoporotic fractures or very low bone density, as bisphosphonates seem to delay the anabolic response to teriparatide [42–44]. However, it is often difficult to obtain insurance coverage for teriparatide until a treatment course of a bisphosphonate has been tried and failed. In the European Forsteo Observational Study, postmenopausal women with osteoporosis and prior bisphosphonate use were followed for up to 18 months of teriparatide treatment and up to 18 months afterwards. The odds of vertebral and non-vertebral fragility fractures progressively decreased during this period; compared to the first six months of teriparatide treatment, there was a 37% decrease in the adjusted odds of fracture during the final six months of teriparatide treatment. Even after teriparatide treatment was discontinued, odds of fracture were 76% lower than during the first 6 months of treatment, although many of the patients resumed use of an antiresorptive drug after completing the course of teriparatide [45]. Thus, use of teriparatide can reduce fracture risk despite prior bisphosphonate treatment, though the response to teriparatide may be delayed.
In terms of starting bisphosphonate and teriparatide simultaneously in treatment-naïve patients, clinical studies suggest that a single infusion of zoledronic acid plus daily teriparatide increases BMD at the hip and spine more than either therapy alone [46]. This finding does not appear to extend to concomitant use of teriparatide and other bisphosphonates [47, 48]. Due to cost and lack of fracture data, it is most common to use the agents sequentially rather than simultaneously except in the most severe cases of osteoporosis.
Data is conflicting regarding the effects of readministering teriparatide after completing one course of therapy. One recent study showed significant increases in spine BMD with teriparatide retreatment after a 12 month hiatus during which they were on alendronate alone. In that study, by Cosman et al, mean spine BMD increased 6.2% after the first 15 months of daily teriparatide treatment and 4.7% after retreatment for an additional 15 months [49]. Another study showed an attenuated BMD response to readministration of teriparatide after a 12 month hiatus, though the initial increases in BMD in this study were greater [39].
4. PTHrP analogs
PTHrP was initially identified as the cause of humoral hypercalcemia of malignancy, a condition characterized by unopposed bone resorption [22]. Since PTHrP binds the PTH-1 receptor and activates signal transduction with equal potency to PTH, we sought to determine whether PTHrP might also act as a skeletal anabolic agent if administered intermittently. In an initial clinical trial in 16 healthy postmenopausal women with osteoporosis, women were randomized to receive daily subcutaneous injections of synthetic human PTHrP 1-36 (6.56 µg/kg) or placebo for three months. All subjects were on hormone replacement therapy which was continued and received supplementation with vitamin D and calcium beginning at least 2 weeks before starting the study drug or placebo injections. Lumbar spine BMD increased 4.7% from baseline in patients on PTHrP 1-36. Markers of bone formation were increased whereas markers of bone resorption were unchanged, suggesting that PTHrP 1-36 might have purely anabolic effects on bone and might lack the concomitant increase in bone resorption seen with PTH in this select population [27]. A subsequent dose escalation study demonstrated that at doses as high as 750µg/day, bone formation was selectively activated [29].
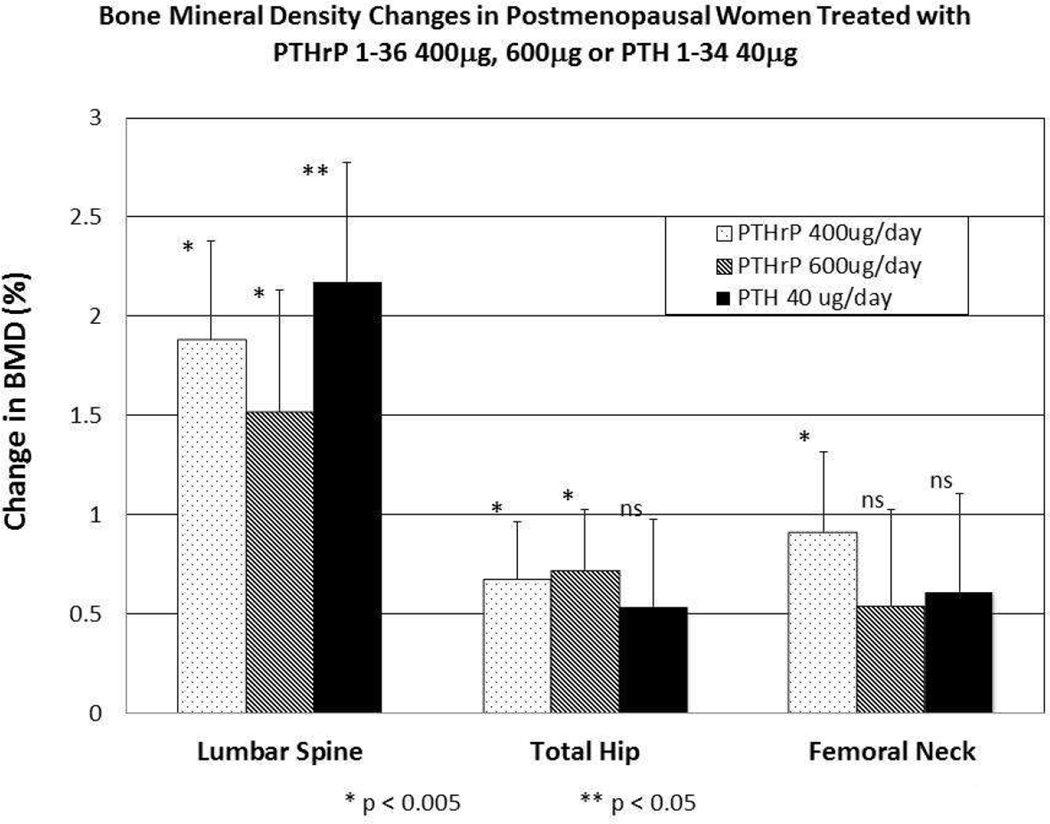
A recent study by our group compared daily subcutaneous injections of synthetic human PTHrP 1-36 to PTH 1-34 for three months in postmenopausal women with osteoporosis or low BMD who had not recently received other osteoporosis treatment [28]. While PTH induced a greater increase in markers of both bone formation and resorption, PTHrP (at 400 and 600 µg doses) and PTH resulted in equivalent significant increases in lumbar spine BMD. Although there was no significant difference between groups in hip BMD, only the PTHrP groups had a significant increase in hip BMD (figure 1). The incidence of mild hypercalcemia was somewhat greater with PTHrP 1-36 than for PTH 1-34 indicating that further studies are needed to determine the ideal dose and role of PTHrP 1-36 for the treatment of postmenopausal osteoporosis [28].
Figure 1.
Lumbar Spine and Hip Bone Mineral Density Changes in Postmenopausal Women Treated with PTHrP 1-36 400µg, 600µg or PTH 1-34 40µg.
The three groups are indicated by the patterns shown in the legend within the figure. There was no significant difference in BMD change between groups at any site. Bars indicate SEM, and the * and ** symbols refer to statistical significance compared to baseline values. Lumbar spine BMD increased equivalently and significantly by day 90 in all three groups. Total hip and femoral neck BMD increased equivalently in all three groups, but was significant only for the two PTHrP(1-36) groups (p–0.05 vs baseline) at the total hip, and for the PTHrP(1-36) 400μg group at the femoral neck (p–0.05 vs baseline). Results are presented as percent change from baseline.
Modified with permission from the American Society for Bone and Mineral Research [28].
A novel PTHrP 1-34 analog, BA058 (Radius Health Inc.), is currently undergoing phase 3 clinical trials as a therapy for postmenopausal women with severe osteoporosis [50]. Phase 2 trials showed improvements of spine BMD at 24 weeks of 5.2% and 6.7% with BA058 40 and 80 µg respectively, compared to 1.6% improvement with placebo and 5.5% improvement with teriparatide. After a 24-week extension phase in 20–30% of subjects, further increases in spine BMD and an increase in total hip BMD were also noted. The most common adverse events were influenza and headaches; serum calcium levels were higher with teriparatide compared to BA058 [51].
5. Future therapeutics
Animal studies and numerous case reports suggest that teriparatide and PTH 1-84 may accelerate fracture healing. The case reports have used recombinant PTH for durations between 2 months and 2 years for a variety of acute fractures and particularly cases of delayed unions [52]. Aspenberg et al. conducted the only randomized, placebo-controlled trial published to date examining use of teriparatide 20µg or 40µg daily for an 8 week course beginning one week after fracture. 102 postmenopausal women were followed for 44 weeks after the teriparatide or placebo course was completed. The only significant difference in median time to cortical bridging of 3 of 4 cortices was between the 20µg group and placebo (7.4 and 9.1 weeks respectively). Interestingly, there was no significant difference in time to cortical bridging between teriparatide 40µg/day and placebo [53]. A trial by Peichl et al. using PTH 1-84 100µg vs placebo in postmenopausal women with pelvic fracture showed mean time to fracture healing was 7.8 weeks in the treatment group, significantly shorter than 12.6 weeks in the control group [54]. These studies suggest that some reduction in fracture healing time can be achieved with daily PTH administration. Larger randomized controlled trials are needed to determine the optimal duration of therapy, timing of initiation, and expected outcomes. Furthermore, it remains to be determined whether the relatively minor improvements in healing time provide a sufficient cost benefit to warrant expensive treatment with daily PTH. These authors feel that it is likely that, in the near future, the anabolic agents may have a greater role in enhancing healing in patients with non-union rather than in acute fracture care.
Both of the currently available PTH analogs are administered as daily subcutaneous injections. In the TOWER phase 3 clinical trial an alternate dosing regimen of once-weekly injections of teriparatide 56.5µg for 72 weeks reduced fracture risk by 80% compared to placebo. BMD was increased by 6.4% at the lumbar spine, 3.0% at the total hip and 2.3% at the femoral neck after 72 weeks of treatment compared to placebo [55]. Reduced fracture risk was maintained after treatment, and BMD gains were maintained in patients started on bisphosphonate treatment [56]. This study suggests that once-weekly teriparatide injections may be an alternative regimen for osteoporosis treatment.
Although oral administration would be more convenient for patients, as a peptide, PTH is susceptible to degradation by proteases and does not diffuse easily though the intestinal wall. Any form of PTH or PTHrP used as therapy for osteoporosis must have a pharmacokinetic profile that results in a rapidly reached peak level with a brief half-life to take advantage of the anabolic window as discussed above. Nevertheless, an oral tablet formulation of recombinant human PTH(1-31) has been shown to result in 2.2% increase in lumbar spine BMD over 24 weeks of administration compared to 5.1% increase in the PTH 1-34 arm [57]. An oral version of PTH 1-34 is also being developed [58].
A number of agents with alternate delivery routes are currently being investigated as well. A transdermal patch which is worn for 30 minutes daily has been tested as a delivery system for teriparatide. The 40 µg patch increased total hip BMD compared to both placebo and 20µg subcutaneous injection after six months of treatment without prolonged hypercalcemia [59]. A short wear time transdermal patch for BA058 is also being developed. In addition, a nasal spray and an implantable microchip have also been tested as alternative means of administering daily doses of PTH 1-34 [60, 61].
6. Conclusion
Anabolic agents which stimulate bone formation to a greater degree than bone resorption are the ideal therapies for patients with osteoporosis. As the only anabolic agent currently FDA-approved, teriparatide has been an effective treatment for osteoporosis, offering a significant reduction in fracture risk with an excellent safety profile in the decade since it came on the market. However, teriparatide and PTH 1-84 stimulate bone resorption as well as bone formation, both have reduced efficacy after a certain duration of therapy, and they can be inconvenient to administer as daily subcutaneous injections. Further investigation of other PTH and PTHrP analogs is warranted, as they have the potential to act as alternate anabolic skeletal agents which might overcome some of the issues of current therapies.
Footnotes
Conflict of Interest
M Augustine works for an institution that has received an NIH grant.
MJ Horwitz works for an institution that has received an NIH grant, is an NPS consultant, and has received royalties as a chapter author for Up To Date.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Reference list
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed May 2013];WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Available at http://www.who.int/chp/topics/Osteoporosis.pdf.
- 4.Dempster DW, Zhou H, Recker RR, et al. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:2799–2808. doi: 10.1210/jc.2012-1262. [DOI] [PubMed] [Google Scholar]
- 5.Watts NB, Harris ST, Genant HK, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323:73–79. doi: 10.1056/NEJM199007123230201. [DOI] [PubMed] [Google Scholar]
- 6.Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 8.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 9.McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res. 2012;27:1480–1486. doi: 10.1002/jbmr.1606. [DOI] [PubMed] [Google Scholar]
- 10.Migliore A, Broccoli S, Massafra U, et al. Mixed-treatment comparison of anabolic (teriparatide and PTH 1-84) therapies in women with severe osteoporosis. Curr Med Res Opin. 2012;28:467–473. doi: 10.1185/03007995.2012.659724. [DOI] [PubMed] [Google Scholar]
- 11.Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol. 2013;85:1417–1423. doi: 10.1016/j.bcp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 13.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 14.Frolik CA, Black EC, Cain RL, et al. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33:372–379. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 15.Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 16.Frost ML, Siddique M, Blake GM, et al. Differential effects of teriparatide on regional bone formation using (18)F-fluoride positron emission tomography. J Bone Miner Res. 2011;26:1002–1011. doi: 10.1002/jbmr.305. [DOI] [PubMed] [Google Scholar]
- 17.Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1-34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612. doi: 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- 18.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res. 2012;27:2419–2428. doi: 10.1002/jbmr.1800. This review article summarizes available data about the safety of teriparatide and PTH 1-84 including risk of osteosarcoma.
- 20.Dean T, Vilardaga JP, Potts JT, Jr, et al. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab. 2012;97:2947–2956. doi: 10.1210/jc.2012-2142. This review article summarizes PTHrP functions throughout the body with particular focus on research from the past decade.
- 22.Philbrick WM, Wysolmerski JJ, Galbraith S, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- 23.Miao D, Li J, Xue Y, et al. Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology. 2004;145:3554–3562. doi: 10.1210/en.2003-1695. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz MJ, Tedesco MB, Sereika SM, et al. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab. 2003;88:1603–1609. doi: 10.1210/jc.2002-020773. [DOI] [PubMed] [Google Scholar]
- 25.Stewart AF, Cain RL, Burr DB, et al. Six-month daily administration of parathyroid hormone and parathyroid hormone-related protein peptides to adult ovariectomized rats markedly enhances bone mass and biomechanical properties: a comparison of human parathyroid hormone 1-34, parathyroid hormone-related protein 1-36, and SDZ-parathyroid hormone 893. J Bone Miner Res. 2000;15:1517–1525. doi: 10.1359/jbmr.2000.15.8.1517. [DOI] [PubMed] [Google Scholar]
- 26.de Castro LF, Lozano D, Portal-Nunez S, et al. Comparison of the skeletal effects induced by daily administration of PTHrP (1-36) and PTHrP (107-139) to ovariectomized mice. J Cell Physiol. 2012;227:1752–1760. doi: 10.1002/jcp.22902. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz MJ, Tedesco MB, Gundberg C, et al. Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88:569–575. doi: 10.1210/jc.2002-021122. [DOI] [PubMed] [Google Scholar]
- 28. Horwitz MJ, Augustine M, Kahn L, et al. A comparison of parathyroid hormone-related protein (1-36) and parathyroid hormone (1-34) on markers of bone turnover and bone density in postmenopausal women: The PrOP study. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1978. Epub 2013/05/11. doi: 10.1002/jbmr.1978. PubMed PMID: 23661240. This paper describes the results of the first randomized, controlled trial comparing the effects daily subcutaneous PTHrP 1-36 or teriparatide administration on bone turnover markers and bone density in women with postmenopausal osteoporosis.
- 29.Horwitz MJ, Tedesco MB, Garcia-Ocana A, et al. Parathyroid hormone-related protein for the treatment of postmenopausal osteoporosis: defining the maximal tolerable dose. J Clin Endocrinol Metab. 2010;95:1279–1287. doi: 10.1210/jc.2009-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [Accessed June 2013];Eli Lilly and Company: Forteo Prescribing information. Available at http://pi.lilly.com/us/forteo-pi.pdf.
- 31.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 32.Gold DT, Pantos BS, Masica DN, et al. Initial experience with teriparatide in the United States. Curr Med Res Opin. 2006;22:703–708. doi: 10.1185/030079906X100159. [DOI] [PubMed] [Google Scholar]
- 33.Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- 34. Hansen S, Hauge EM, Beck Jensen JE, et al. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28:736–745. doi: 10.1002/jbmr.1784. This study demonstrates the benefits of anabolic therapy as opposed to bisphosphonate use on bone microarchitecture and strength.
- 35. Cusano NE, Rubin MR, McMahon DJ, et al. Therapy of hypoparathyroidism with PTH(1-84): a prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2013;98:137–144. doi: 10.1210/jc.2012-2984. This study describes the effects of extended use of PTH 1-84 for treatment of hypoparathyroidism.
- 36.Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 37. Andrews EB, Gilsenan AW, Midkiff K, et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res. 2012;27:2429–2437. doi: 10.1002/jbmr.1768. These are findings from US postmarketing data for teriparatide, indicating that teriparatide is a safe treatment.
- 38.Rubin MR, Bilezikian JP. The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med. 2003;19:415–432. doi: 10.1016/s0749-0690(02)00074-5. [DOI] [PubMed] [Google Scholar]
- 39.Finkelstein JS, Wyland JJ, Leder BZ, et al. Effects of teriparatide retreatment in osteoporotic men and women. J Clin Endocrinol Metab. 2009;94:2495–2501. doi: 10.1210/jc.2009-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leder BZ, Neer RM, Wyland JJ, et al. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009;94:2915–2921. doi: 10.1210/jc.2008-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 42.Finkelstein JS, Hayes A, Hunzelman JL, et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 43.Ettinger B, San Martin J, Crans G, et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745–751. doi: 10.1359/JBMR.040117. [DOI] [PubMed] [Google Scholar]
- 44.Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23:1591–1600. doi: 10.1359/jbmr.080506. [DOI] [PubMed] [Google Scholar]
- 45.Jakob F, Oertel H, Langdahl B, et al. Effects of teriparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-month results from the European Forsteo Observational Study. Eur J Endocrinol. 2012;166:87–97. doi: 10.1530/EJE-11-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:503–511. doi: 10.1002/jbmr.238. This study demonstrates effect of IV bisphosphonate in combination with teriparatide compared to either therapy alone, suggesting that combination therapy may provide the most benefit when hip and spine are both considered.
- 47.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 48.Finkelstein JS, Wyland JJ, Lee H, et al. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95:1838–1845. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cosman F, Nieves JW, Zion M, et al. Retreatment with teriparatide one year after the first teriparatide course in patients on continued long-term alendronate. J Bone Miner Res. 2009;24:1110–1115. doi: 10.1359/JBMR.081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. [Accessed May 2013];Study to Evaluate the Safety and Efficacy of BA058 for Prevention of Fracture in Postmenopausal Women. Available at http://www.clinicaltrials.gov/ct2/show/study/NCT01343004?term=ba058&rank=3.
- 51.Hattersley G, Bilezikian J, Guerriero J, et al., editors. The Endocrine Society's 94th Annual Meeting. Houston: 2012. Bone Anabolic Efficacy and Safety of BA058, a Novel Analog of hPTHrP: Results from a Phase 2 Clinical Trial in Postmenopausal Women with Osteoporosis. [Google Scholar]
- 52. Zhang D, Potty A, Vyas P, et al. The Role of Recombinant Pth in Human Fracture Healing: A Systematic Review. J Orthop Trauma. 2013 doi: 10.1097/BOT.0b013e31828e13fe. This paper reviews the case reports and RCTs to date exploring use of PTH analogs in fracture healing.
- 53.Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 54.Peichl P, Holzer LA, Maier R, et al. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura T, Sugimoto T, Nakano T, et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97:3097–3106. doi: 10.1210/jc.2011-3479. [DOI] [PubMed] [Google Scholar]
- 56.Sugimoto T, Shiraki M, Nakano T, et al. Vertebral fracture risk after once-weekly teriparatide injections: follow-up study of Teriparatide Once-Weekly Efficacy Research (TOWER) trial. Curr Med Res Opin. 2013;29:195–203. doi: 10.1185/03007995.2012.761956. [DOI] [PubMed] [Google Scholar]
- 57.Henriksen K, Andersen JR, Riis BJ, et al. Evaluation of the efficacy, safety and pharmacokinetic profile of oral recombinant human parathyroid hormone [rhPTH(1-31)NH(2)] in postmenopausal women with osteoporosis. Bone. 2013;53:160–166. doi: 10.1016/j.bone.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 58.Hammerle SP, Mindeholm L, Launonen A, et al. The single dose pharmacokinetic profile of a novel oral human parathyroid hormone formulation in healthy postmenopausal women. Bone. 2012;50:965–973. doi: 10.1016/j.bone.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Cosman F, Lane NE, Bolognese MA, et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2010;95:151–158. doi: 10.1210/jc.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsumoto T, Shiraki M, Hagino H, et al. Daily nasal spray of hPTH(1-34) for 3 months increases bone mass in osteoporotic subjects: a pilot study. Osteoporos Int. 2006;17:1532–1538. doi: 10.1007/s00198-006-0159-1. [DOI] [PubMed] [Google Scholar]
- 61.Farra R, Sheppard NF, Jr, McCabe L, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4:122ra121. doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]