Abstract
We have previously determined that there is a significant benefit of vaccination against influenza in patients hospitalized due to an acute coronary event. The purpose of this study is to determine whether the observed benefits of vaccination were maintained over a 2-year follow-up among those who were re-vaccinated during the subsequent winter season.
During the winter season of 2001, a total of 301 acute coronary patients were prospectively enrolled within 72 hours of the onset of symptoms. Follow-up was conducted at 6 and 12 months.
Patients who survived participated in a registry 1 year after the 2nd influenza vaccination period (winter 2002), as a cohort of chronic and stable coronary patients.
The incidence of the primary endpoint cardiovascular death at 1 year was significantly lower in patients receiving vaccination than in controls (6% vs 17%, respectively) by intention-to-treat analysis. The relative risk with vaccination in comparison with controls was 0.34; 95% confidence interval, 0.17–0.71; P = 0.002. In the winter of 2002, 116 patients were vaccinated according to their physicians' instructions, and 114 subjects remained unvaccinated. The combined endpoints of total death plus myocardial infarction 1 year later were 4 (3.4 %) in the vaccinated group vs 11 (9.7%) among those who were not vaccinated (P = 0.05).
Influenza vaccination may reduce the risk of death and ischemic events in patients admitted with acute coronary syndromes. There is also a beneficial trend in the quiescent phase of ischemia.
Key words: Acute disease, adult, atherosclerosis, clinical trials, comparative study, coronary disease/prevention & control, coronary disease/therapy, follow-up studies, influenza vaccine, myocardial infarction/prevention & control, seasons, treatment outcome
Despite the early benefits shown from the original studies on the effect of antithrombotic therapy on acute coronary syndromes, 1–4 the acute coronary event rates in patients who present with myocardial necrosis remain high both in the days following discharge and at 1 year thereafter. Indeed, death or new infarction occurs in approximately 6 of 100 patients within 1 month. An additional 6 deaths or myocardial infarctions per 100 patients can be expected within the year, and the revascularization rate is close to 9% from 1 month to 1 year. 5
In an effort to lower the incidence of ischemic events over time, a more aggressive medical approach (early cardiac revascularization when appropriate) was used in combination with a random 3-month assignment to dalteparin for moderate-to high-risk patients with myocardial infarction. 6 Despite policies of aggressive revascularization, the incidence of subsequent adverse events remains high. 7 The conditions that cause new ischemic events are poorly understood. Previous research has implicated inflammation as a critical component in the development of atherosclerotic disease and in the delay of healing; and several possible causes—including a prothrombotic state, autoimmune reactions, and infection—are potentially linked to the severity of inflammation. 8
We have recently published our findings of a significant benefit of a single intramuscular dose of an influenza vaccination in reducing rates of cardiovascular death and combined traditional end points at 6 months of follow-up. 9
The aim of this present study is to report the 1-year follow-up results of the Flu Vaccination Acute Coronary Syndromes (FLUVACS) study, 9 and to relate the impact of this therapy when administered prophylactically during the subsequent winter season in the southern hemisphere, among the same patients during the quiescent phase of their ischemia.
Methods
Study Design and Population. This was a randomized, prospective, controlled pilot study that used parallel groups from multiple centers. The enrollment criteria, the study design, the treatment protocol, and the endpoint definitions have been published. 9 From 6 care units in Argentina, we enrolled 301 patients from 2 different cohorts: a myocardial infarction group of 200 patients with a diagnosis of recent (within the last 72 hours) ST- or non-ST-segment elevation myocardial infarction, and an interventional group of 101 patients who were to undergo angioplasty and stenting. Our Institutional Review Board approved the study, and informed consent was obtained from all patients.
At admission, the 200 patients who were experiencing ST-segment elevation myocardial infarction or non-ST-segment myocardial infarction were so classified in accordance with new diagnostic criteria arrived at by recent consensus. 10 They were divided into 2 groups. Group A (100 patients) received a single intramuscular vaccination containing 0.5 mL of A/Moscow/10/99-like virus, A/New Caledonia/20/99 (H1N1)-like virus, and AB/Sichuan/379/99-like virus. Follow-up telephone visits were scheduled at 6 and 12 months after treatment. Group B (100 patients) served as a control group and received a saline infusion as placebo.
The PCI (Percutaneous Intervention)-Stenting Group comprised 101 subjects who were to undergo stenting angioplasty at 2 sites in the city of Buenos Aires. Before the interventional procedure, 51 of these patients received vaccination, and the other 50, as members of a control group, received the placebo.
The study began in May 2001. Because the timing of influenza activity varies by region, the vaccine for the present trial was administered after May (June marks the onset of winter in the southern hemisphere). Vaccination is usually recommended during the winter, because that is influenza season. 11 This strategy was adopted on the hypothesis that a nonspecific stimulation of the immune system could reduce the incidence and severity of subsequent ischemic events, in addition to serving as prophylaxis against influenza infection.
Endpoints. One-year follow-up was obtained in a prospective manner, in which each clinical site was responsible for telephone contact with all randomized patients. If a patient was lost to follow-up by telephone, a study investigator conducted a home visit to evaluate the patient. For the 1-year follow-up, the same endpoint definitions were used, and the primary endpoint was cardiovascular death.
Cardiovascular deaths were defined as those due to myocardial infarction, sudden death, death in the hospital after possible myocardial infarction, or death due to heart failure or another coronary cause. Cardiovascular deaths were confirmed by the patients' own physicians or by a death registry. Deaths for other reasons were not considered as primary endpoints.
The FLUVACS Registry. All the cases studied were those of patients who participated in the FLUVACS Study, 9 whether or not they had been vaccinated during the winter of 2001.
During the summer of 2003 (January–March), we recorded the deaths and myocardial infarctions of those patients who participated in the initial FLUVACS Study. 9
Information on demographic characteristics was collected with the use of a structured questionnaire.
The patients or their survivors were asked whether the patients had been vaccinated during the 2nd influenza vaccination campaign (April 2002 in Argentina), whether they had been re-hospitalized for a new cardiovascular infarction, and whether death (if it occurred) was cardiovascular or noncardiovascular. In accordance with international recommendations made in 2002, those patients who were vaccinated received the same intramuscular vaccination (0.5 mL of A/Moscow/10/99-like virus, A/New Caledonia/20/99 (H1N1)-like virus, and AB/Sichuan/379/99-like virus) that they had received during the winter of 2001.
Endpoint Definitions for the Registry. The aim was to assess the composite endpoints of cardiovascular or noncardiovascular death (total deaths) and myocardial infarction defined as any value of creatine kinase-MB (CK-MB) above normal (5% or more of total CK, or a total CK value at least twice the upper limit of the normal reference range). A Q wave myocardial infarction (MI) was defined as chest pain lasting 20 minutes or longer, followed by the appearance of new significant Q waves (≥0.03 seconds) in at least 2 leads in the electrocardiogram (ECG).
Statistical Analysis. The analyses for the 1-year follow-up were done according to the intention-to-treat principle.
Statistical comparisons between groups were made using the time-to-event with the Kaplan-Meier survival technique (2-sided log-rank test, alpha = 0.05).
A χ2 statistic was calculated to test differences between proportions. The Mantel-Cox test was applied to evaluate differences between functions. In measuring the time to an event in cases in which a patient had multiple endpoints, the worst event was considered the endpoint. All patients who experienced no events or were lost to follow-up were recorded at the time of study termination or last contact. When it was possible only to establish whether a patient was alive or dead, the confirmation date determined the length of follow-up for death. However, if full information was available on an earlier date, that date determined the length of follow-up in the analysis of all events.
Results
A total 301 eligible patients were assigned to vaccine therapy (n = 151) or control (n = 150) through the randomization process.
Demographic information has been published elsewhere. 9 There were no significant differences between groups.
One-Year Outcome. Of the 301 patients enrolled, 15 (5%) died within the initial 6 months of follow-up. Nine patients (3%) had no follow-up information beyond 6 months. Complete 1-year follow-up event information was available in the remaining 292 patients (96% in the active vaccination group and 98% in the control group).
There were no differences in terms of medical therapy between groups. All of the surviving patients were on aspirin, 64% on β-blockers, and 34% on statins.
Two patients in the control group were given influenza vaccinations during the follow-up period on the instructions of their own physicians and were withdrawn from the study. Two patients died of noncardiovascular causes.
The incidence of cardiovascular death at 1 year was significantly lower in the vaccination group than in the control group (6% vs 17%; P = 0.002; hazard ratio, 0.34; 95% confidence interval [CI], 0.17–0.71). Figure 1 shows the Kaplan-Meier estimates of the time to cardiovascular death. Noncardiovascular death was not recorded.
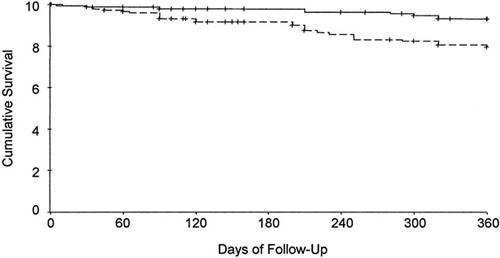
Fig. 1 Kaplan-Meier estimates of the time to cardiovascular death at 1-year follow-up. Noncardiovascular deaths are not taken into account.
Solid line = vaccine group; broken line = control group
Twelve months after randomization, the need for coronary revascularization was significantly less frequent among patients assigned to vaccine (5%) than among those assigned to control (9%).
Follow-Up Registry. Data on the subjects' vaccination status were collected from both vaccination and control patients during the summer of 2003. Of the original 301 patients included in 2001, 257 survived the 1st year of follow-up of the FLUVACS Study, 9 and 256 of these accepted involvement in the registry. By the conclusion of the study, final information was available on 230 patients.
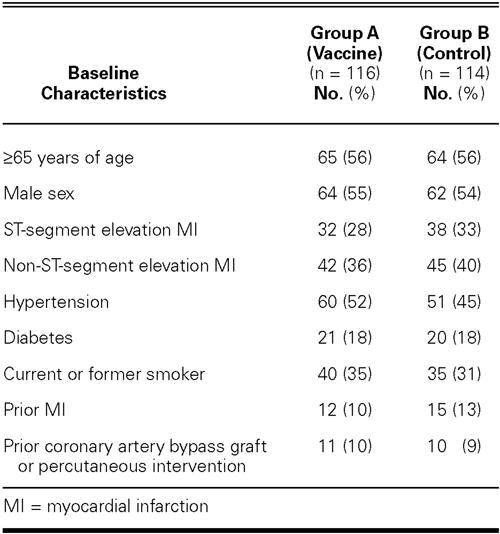
We divided these 230 patients into 2 groups according to the prophylactic influenza treatment given during 2002: Group A comprised 64 patients who were originally in the control arm of the study but were vaccinated during the winter of 2002, together with 52 patients who were re-vaccinated in the same season. Group B comprised 43 patients from the original control group who remained as controls during the winter of 2002, together with 71 patients who refused to be vaccinated during the same period. The baseline characteristics were appropriately balanced between groups (Table I).
TABLE I. Demographic Characteristics for the Registry Population, and the Endpoint at 2 Years of Follow-Up
The composite double endpoint judged in the registry was significantly lower in the vaccination group, in comparison with the control group (3.5% vs 9.7%; P = 0.05; hazard ratio, 0.36; 95% CI, 0.12–1.09) (Table II).
TABLE II. Primary Endpoint Rate during the FLUVACS Registry
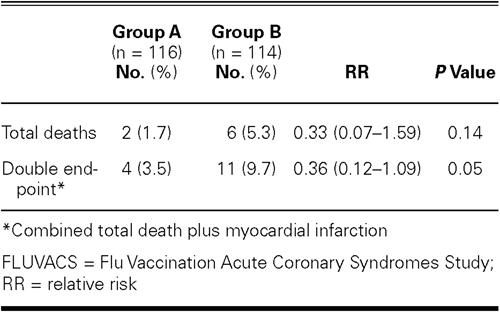
At 2 years, the incidence of death showed a downward trend in the vaccination group, in comparison with controls (1.7% vs 5.3%; P = 0.14; hazard ratio, 0.33; 95% CI, 0.07–1.59) (Table II).
Discussion
The trial was originally intended to vaccinate against influenza a cohort of acute coronary patients and patients scheduled for percutaneous intervention. The Data Review Board Committee of the Investigation performed a second analysis after 12 months' follow-up. In this analysis, an unexpected significant reduction of cardiovascular death was observed in the active group.
In addition, influenza vaccination was more effective, in the majority of subgroups, in reducing the incidence of death and of myocardial infarction and recurrent angina that necessitated urgent revascularization. 12 After stabilization, patients were reclassified into 2 groups: those who received influenza vaccination and those who did not. The intervention proved to be safe and was associated with a significant decrease in the rate of major coronary events, which findings are consistent with some observational trials. 13
Epidemiologic data suggested a possible association between infectious processes and coronary artery disease. For example, the rates of myocardial infarction and cardiac death increase in the winter and during influenza epidemics. 14,15 In case-control studies, influenza vaccination has been reported to protect against myocardial infarction, primary cardiac death, and stroke. 16–18 We hypothesized that the immune system does not provide a sufficiently broad response to combat infection unless vaccination reinforces it. Other hypotheses have also been set forth: micro-organisms might induce autoimmune disease, which involves bystander activation, a nonspecific mechanism. Alternatively, microbial infection causes the release of previously sequestered self-antigens, resulting in activation of self-antigen-expressing antigen-presenting cells. 19
The humoral response after vaccination stimulus may reflect migration of committed B-lymphocytes. Some elegant animal models of atherosclerosis have been developed for this purpose. Dimayuga and colleagues 20 investigated the effect of B-cell reconstitution in immune-deficient Rag-1 knockout mice subjected to arterial injury. They found that the B cells modulated the response to arterial injury. Caligiuri and coworkers 21 found that splenectomy dramatically aggravated atherosclerosis in hypercholesterolemic apoE (apolipoprotein E) knockout mice. Those authors transferred spleen cells from atherosclerotic apoE mice and found a significant reduction in disease development in young apoE-deficient mice, which suggests that B-cell–associated protective immunity develops during atherosclerosis and reduces disease progression.
It is noteworthy that in our study there was a relatively high rate of death at 1 year of follow-up. In light of inter-regional differences detected in clinical trials, it is plausible that general management could differ between sites and physicians participating in the study. 22 This controversial issue is still under debate. 23 However, the fact that we observed consistent results after 2 consecutive influenza seasons still points in the direction of an association between influenza vaccination and major cardiovascular events.
Limitation of the Present Study. In PCI patients, we found no statistically significant differences between groups during the 1-year follow-up period. Of note, the study was underpowered for the elective PCI group, who would be expected to have a low morbidity rate because of their lower rate of restenosis. On the other hand, it is possible to think that influenza vaccination worsens the clinical outcomes of patients allocated to this group. Our findings support the hypothesis that hospitalizations and deaths can be reduced by vaccinating acute coronary patients against influenza.
Footnotes
Address for reprints: Enrique P. Gurfinkel, MD, PhD, Foundation Favaloro, Av. Belgrano 1746 (1093), Capital Federal, Buenos Aires, Argentina
E-mail: epgurfinkel@ffavaloro.org
This paper has its basis in a presentation made at the First Symposium on Influenza and Cardiovascular Disease: Science, Practice, and Policy, held on 26 April 2003, at the Texas Heart Institute, Houston, Texas.
Supported by Foundation Favaloro, Argentina
References
- 1.Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med 1983;309:396–403. [DOI] [PubMed]
- 2.Gurfinkel EP, Manos EJ, Mejail RI, Cerda MA, Duronto EA, Garcia CN, et al. Low molecular weight heparin versus regular heparin or aspirin in the treatment of unstable angina and silent ischemia. J Am Coll Cardiol 1995;26:313–8. [DOI] [PubMed]
- 3.Chazov EI, Matueeva LS, Mazaev AV, Sargin KE, Sadovskaia GV, Ruda MI. Intracoronary administration of fibrinolysis in acute myocardial infarction [in Russian]. Ter Arkh 1976;48(4):8–19. [PubMed]
- 4.Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med 1994;330:956–61. [DOI] [PubMed]
- 5.Goodman SG, Cohen M, Bigonzi F, Gurfinkel EP, Radley DR, Le Iouer V, et al. Randomized trial of low molecular weight heparin (enoxaparin) versus unfractionated heparin for unstable coronary artery disease: one-year results of the ESSENCE Study. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q Wave Coronary Events. J Am Coll Cardiol 2000;36:693–8. [DOI] [PubMed]
- 6.Long-term low-molecular-mass heparin in unstable coronary-artery disease: FRISC II prospective randomised multicentre study FRagmin and Fast Revascularisation during InStability in Coronary artery disease. Investigators [published erratum appears in Lancet 1999;354:1478]. Lancet 1999;354:701–7. [PubMed]
- 7.Yusuf S, Flather M, Pogue J, Hunt D, Varigos J, Piegas L, et al. Variations between countries in invasive cardiac procedures and outcomes in patients with suspected unstable angina or myocardial infarction without initial ST elevation. OASIS (Organisation to Assess Strategies for Ischaemic Syndromes) Registry Investigators. Lancet 1998;352:507–14. [DOI] [PubMed]
- 8.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation 1997;96:4095–103. [DOI] [PubMed]
- 9.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the Flu Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation 2002;105:2143–7. [DOI] [PubMed]
- 10.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) [published erratum appears in J Am Coll Cardiol 2000;38:294–5]. J Am Coll Cardiol 2000;36:970–1062. [DOI] [PubMed]
- 11.Bridges CB, Fukuda K, Uyeki TM, Cox NJ, Singleton JA; Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2002;51(RR-3):1–31. [PubMed]
- 12.Leon De La Fuente R, Gurfinkel EP, Toledo D, Mautner B. Flu vacination in patients with acute coronary syndromes: treatment benefit in prespecified subgroups [in English, Spanish]. Rev Esp Cardiol 2003;56(10):949–54. [DOI] [PubMed]
- 13.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med 2003;348:1322–32. [DOI] [PubMed]
- 14.Woodhouse PR, Khaw KT, Plummer M, Foley A, Meade TW. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: winter infections and death from cardiovascular disease. Lancet 1994;343(8895):435–9. [DOI] [PubMed]
- 15.Tillet HE, Smith JW, Gooch CD. Excess death attributable to influenza in England and Wales: age at death and certified cause. Int J Epidemiol 1983;12(3):344–52. [DOI] [PubMed]
- 16.Siscovick DS, Raghunathan TE, Lin D, Weinmann S, Arbogast P, Lemaitre RN, et al. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol 2000;152:674–7. [DOI] [PubMed]
- 17.Naghavi M, Barlas Z, Siadaty S, Naguib S, Madjid M, Casscells W. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation 2000;102:3039–45. [DOI] [PubMed]
- 18.Lavallee P, Perchaud V, Gautier-Bertrand M, Grabli D, Amarenco P. Association between influenza vaccination and reduced risk of brain infarction [published erratum appears in Stroke 2002;33:1171]. Stroke 2002;33:513–8. [DOI] [PubMed]
- 19.Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet 2003; 362(9396):1659–66. [DOI] [PubMed]
- 20.Dimayuga P, Cercek B, Oguchi S, Fredrikson GN, Yano J, Shah PK, et al. Inhibitory effect on arterial injury-induced neointimal formation by adoptive B-cell transfer in Rag-1 knockout mice. Arterioscler Thromb Vasc Biol 2002;22:644–9. [DOI] [PubMed]
- 21.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 2002;109(6):745–53. [DOI] [PMC free article] [PubMed]
- 22.Van de Werf F, Topol EJ, Lee KL, Woodlief LH, Granger CB, Armstrong PW, et al. Variations in patient management and outcomes for acute myocardial infarction in the United States and other countries. Results from the GUSTO trial. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. JAMA 1995;273:1586–91. [DOI] [PubMed]
- 23.Gurfinkel E, Bozovich G, Mautner B. International comparison of mortality rates in patients with non-ST elevation acute coronary events. Heart 2003;89:1083–4. [DOI] [PMC free article] [PubMed]


