SUMMARY
Nervous systems process information by integrating the electrical activity of neurons in complex networks. This motivates the long-standing interest in using optical methods to simultaneously monitor the membrane potential of multiple genetically targeted neurons via expression of genetically encoded fluorescent voltage indicators (GEVIs) in intact neural circuits. No currently available GEVIs have demonstrated robust signals in intact brain tissue that enable reliable recording of individual electrical events simultaneously in multiple neurons. Here, we show that the recently developed “ArcLight” GEVI robustly reports both subthreshold events and action potentials in genetically targeted neurons in the intact Drosophila fruit fly brain and reveals electrical signals in neurite branches. In the same way that genetically encoded fluorescent sensors have revolutionized the study of intracellular Ca2+ signals, ArcLight now enables optical measurement in intact neural circuits of membrane potential, the key cellular parameter that underlies neuronal information processing.
INTRODUCTION
Nervous systems process information by integrating the electrical activity of neurons in complex networks. This motivates the long-standing interest in using optical methods to simultaneously monitor the membrane potential of multiple genetically targeted neurons in intact functional circuits (Akemann et al., 2012; Baker et al., 2008). Optical measurement of the membrane potential of a cell was first achieved over 40 years ago (Cohen et al., 1968). The currently most popular approach employs hydrophobic, small-molecule, fluorescent dyes that integrate into the plasma membrane and exhibit altered fluorescence with changes in membrane potential (Salzberg et al., 1973). This allows direct interrogation of membrane potential at any location on the neuronal cell membrane and enables optical detection of the spatiotemporal propagation of electrical events in single neurons and neuronal circuits, an experimental achievement that is difficult, at best, with electrode-based methods and frequently impossible. Chemical voltage-sensitive dyes have several disadvantages, including being difficult or impossible to deliver deep into neural tissue and not being targetable to genetically specified subsets of neurons in intact neural circuits.
Genetically encoded fluorescent voltage indicator proteins (GEVIs) whose expression can be driven by cell-type-specific promoters would allow targeted, sustained expression in intact preparations. The first generation of GEVIs was based on fusion of fluorescent proteins with voltage-gated ion channels (Ataka and Pieribone, 2002; Sakai et al., 2001; Siegel and Isacoff, 1997). The second generation has relied on fusion of the S1–S4 voltage-sensor domain of the Ciona intestinalis voltage-sensitive phosphatase (VSP) (Murata et al., 2005) with a single or pair of fluorescent proteins (Baker et al., 2008; Barnett et al., 2012; Dimitrov et al., 2007; Lundby et al., 2008). Some of these first- and second-generation GEVIs function in cultured cells and in a few cases have allowed detection of membrane activity in neurons in intact nervous system (Akemann et al., 2012, 2010). Unfortunately, none have demonstrated robust signals in intact organisms that enable reliable recording of individual (not averaged) electrical events in multiple neurons. An alternative GEVI approach relies not on S1–S4 voltage-sensor domains, but on the intrinsic voltage-dependent fluorescence of microbial rhodopsin proteins (Kralj et al., 2012). However, the extremely weak fluorescence of these molecules—with quantum yields orders of magnitude lower than GFP (Kralj et al., 2012)—will impede use in intact neural circuits in vivo or in brain tissue explants.
To overcome these problems with existing GEVIs, we recently engineered a next-generation VSP-based fluorescent voltage indicator termed ArcLight with dramatically improved signal size and signal-to-noise ratio (Jin et al., 2012). These improved characteristics are largely the consequences of a point mutation (A227D) in the superecliptic pHlourin GFP variant employed, which introduces a negative charge on the outward-facing surface of the GFP structure (Jin et al., 2012). Here, we show that ArcLight robustly reports both subthreshold events and action potentials in genetically targeted neurons in the intact Drosophila fruit fly brain. Using ArcLight, we visualized synchronous barrages of synaptic inputs to circadian clock neurons that have previously been recorded only with whole-cell patch-clamp electrophysiology. We also optically detected a daily rhythm of electrical activity in the electrophysiologically inaccessible distal secretory terminals of circadian clock neurons. As confirmation that ArcLight is effective in other neural circuits, we visualized presynaptic and postsynaptic odor-induced membrane activity in vivo in the first synaptic relay of the fly olfactory system. Our results demonstrate that ArcLight enables robust single-trial optical electrophysiology of multiple individual genetically targeted neurons in neural circuits of intact brain.
RESULTS AND DISCUSSION
ArcLight Imaging of Spontaneous Membrane Activity in Intact Brain
We expressed ArcLight specifically in the lateral ventral circadian clock neurons (LNVs) of Drosophila using the GAL4/UAS binary expression system. These neurons are key pacemakers of the circadian control circuit and are essential for proper timekeeping (Nitabach and Taghert, 2008). Although some aspects of the electrophysiology of these neurons have been extensively studied using whole-cell patch-clamp electrophysiology methods (Cao and Nitabach, 2008; McCarthy et al., 2011), much about their physiology has remained refractory to electrode-based approaches. ArcLight is expressed at high levels in the somata, neurites, and distal peptidergic secretory terminals of both the large and small LNV neurons (lLNVs and sLNVs; Figure 1A). We employ an in situ whole-brain explant preparation for combined whole-cell patch-clamp and optical imaging experiments because it permits simultaneous electrophysiological and optical access to the fly brain. Simultaneous whole-cell current-clamp recording and high-speed (500 Hz) fluorescence imaging demonstrate that ArcLight robustly reports spontaneous sub-threshold events and action potentials in lLNVs, with membrane depolarization causing a decrease in fluorescence intensity and hyperpolarization causing an increase (Figure 1B), as previously reported for cultured mammalian neurons (Jin et al., 2012). Importantly, the external and internal recording solutions have been previously optimized to ensure that spontaneous action potential firing patterns are identical in cell-attached mode (when the cytoplasm is intact) and after breaking in to whole-cell mode (when the cytoplasm has been mixed with internal electrode solution) (Cao and Nitabach, 2008). Subthreshold events and action potentials recorded electrically correspond with slow and rapid changes in ArcLight fluorescence, respectively, in each of these three representative simultaneous optical-electrical measurements. Action potentials in the somata of the lLNVs are readily detectable optically despite the fact that they are small in amplitude (<20 mV) and nonovershooting, as is the case for most Drosophila neurons that have been recorded electrically with whole-cell patch-clamp. Optical recordings of action potentials are of sufficient signal-to-noise ratio (mean ± SD: 8.5 ± 3.1) to permit reliable automated spike picking, as validated in simultaneous optical and patch-clamp recordings (Figure S1 available online). ArcLight robustly reports both depolarization and hyperpolarization of the neuronal membrane (Figure 1C and S2), and thus will also allow optical detection of inhibitory synaptic potentials.
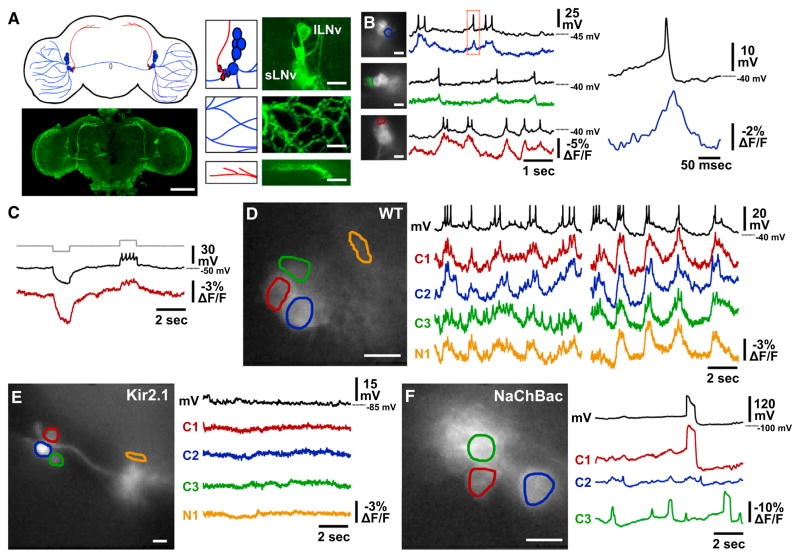
Figure 1. Single-Trial Optical Recordings of Subthreshold and Action Potentials in Intact Neural Circuits using ArcLight Genetically Encoded Voltage Sensor.
(A) ArcLight expression in Drosophila melanogaster lateral ventral clock neurons (LNVs) is schematized at the top left. Large LNVs (lLNVs) and their neurites are illustrated in blue, and small LNVs (sLNVs) and their dorsomedial peptidergic terminal projections in red. Confocal images of anti-GFP immunofluorescence of a whole-brain explant (bottom, left), LNV somata (top, right), lLNV neurites in the optic lobe (middle, right), and sLNV dorsomedial peptidergic terminal projections (bottom, right) are shown. Scale bar, 100 μm on the left, 10 μm on the right.
(B) Three examples of simultaneous, single-trial, whole-cell patch-clamp and optical recordings of lLNvs in situ in whole-brain explants. Sample frames of 80 × 80 depixelated images of each recording are shown on the left, with ROI for optical analysis outlined to indicate the neuron recorded from electrically; black traces corresponding to each image are whole-cell patch-clamp recordings, whereas colored traces are optical recordings. Expanded view of boxed region is shown on the right. Scale bars in all images, 10 μm. Three representative examples are shown from eight total dual patch-optical recordings.
(C) Simultaneous patch-clamp and optical recordings of lLNV in a whole-brain explant injected with 10 pA steps of hyperpolarizing and depolarizing current. The current is shown in gray, patch-clamp membrane voltage in black, and ArcLight optical signal in red. This example is representative of seven experiments.
(D) Optical recording of somata of multiple neurons (C1–3) and one neurite (N1) reveal synchronous membrane activity of wild-type lLNVs. ROIs are outlined in colors corresponding to the optical traces, with the simultaneous whole-cell patch-clamp recording of the cell in the red ROI shown in black. This example is representative of eight experiments.
(E) Kir2.1 expression silences lLNV membrane activity. This example is representative of seven experiments.
(F) NaChBac expression induces large long-duration action potentials. This example is representative of eight experiments.
See also Figures S1, S2, S3, S4, S5, and S6.
To determine in detail the relationship between membrane voltage and ArcLight fluorescence in the intact fly brain, we delivered a series of voltage steps in voltage-clamp mode to Arc-Light-expressing lLNVs and measured changes in fluorescence (Figure S2). Interestingly, this ΔV/ΔF curve is somewhat shifted in the hyperpolarizing direction compared to that for ArcLight-expressing HEK293 cells (Jin et al., 2012), underlying the larger change in ArcLight fluorescence induced by hyperpolarization than by depolarization (Figure 1C). This difference in ArcLight voltage dependence could be due to differences in lipid composition of the plasma membrane between these two cell types (Villalba-Galea et al., 2009). In light of the possibility that ArcLight expression could increase the electrical capacitance of the cell membrane and thereby alter cellular physiological properties (Sjulson and Miesenbock, 2008), we performed a battery of detailed biophysical tests on ArcLight-expressing lLNVs (Figure S3). While there is a statistically significant increase in membrane capacitance in ArcLight-expressing lLNVs (compared to control lLNVs expressing a cytoplasmic fluorescent protein) and an alteration in action potential kinetics, these changes do not dramatically alter the functional information processing properties of the LNVs. For example, the input-output relationship of the neurons as assayed by measuring the number of action potentials induced by current injections of varying magnitude is not different between ArcLight-expressing and control lLNVs (Figure S3). ArcLight-expressing flies also exhibit normal circadian locomotor activity (Figure S4), a behavioral output that is exquisitely sensitive to the membrane biophysical properties of the sLNVs and lLNVs (Choi et al., 2012; Nitabach et al., 2002; Nitabach et al., 2006; Wu et al., 2008). These results indicate that the optical signal strength of ArcLight is sufficient to provide robust cellular signals at an expression level that does not substantially alter neuronal physiological properties. If expression in other cell types with different GAL4 drivers results in too-low or too-high levels of ArcLight, then ArcLight cDNA can be cloned into variant UAS expression vectors that increase or decrease expression level relative to the version employed here (Pfeiffer et al., 2010).
One of the key advantages of optical electrophysiology over whole-cell patch clamp is the ability to easily monitor the membrane potential of multiple cells simultaneously. In Figure 1D, we show two 10 s imaging epochs revealing spontaneous activity in three separate lLNV somata and one dendritic region, with simultaneous patch-clamp recording of one of the cells. These optical recordings reveal rhythmic depolarizations induced by excitatory cholinergic synaptic inputs that are synchronous between lLNVs, with asynchronous action potential trains riding on their depolarized phases, as has been previously observed in dual-electrode whole-cell patch-clamp experiments (McCarthy et al., 2011). It is noteworthy that the amplitudes and time courses of the slow and fast optical changes that correspond to subthreshold synaptic inputs and action potentials, respectively, are not substantially different between the soma that is subject to whole-cell patch clamp and the other somata. The high synchrony of the subthreshold events and action potentials between one of these somata (green) and a neurite (orange) is consistent with the conclusion that this neurite arises from the green soma. In some preparations, due to the geometric relationship between the multiple cells expressing ArcLight, it is not possible to define regions-of-interest for optical analysis that effectively separate the optical signals in different cells. In many preparations, however, such as the one shown in Figure 1D, we do obtain good optical separation. This is demonstrated by the fact that, although the putative somatic optical signals exhibit synchronous slow changes (which correspond to the expected synchronous cholinergic synaptic inputs McCarthy et al., 2011), the rapid changes corresponding to action potentials are asynchronous.
Expression of the inward rectifier K+ channel, Kir2.1, electrically silences lLNVs (Nitabach et al., 2002; Wu et al., 2008), whereas expression of the bacterial voltage-gated Na+ channel, NaChBac, induces large amplitude action potentials (70–80 mV) that last for hundreds of milliseconds followed by long-duration after-hyperpolarizations (Nitabach et al., 2006; Sheeba et al., 2008). Simultaneous electrical and optical measurements reveal that ArcLight reports with high fidelity both the silence of Kir2.1-expressing lLNVs (Figure 1E) and the large action potentials of NaChBac-expressing lLNVs, including their after-hyperpolarizations (Figure 1F). Interestingly, ArcLight optical electrophysiology reveals that the NaChBac-mediated action potentials are frequently asynchronous between lLNVs (Movie S1).
ArcLight Imaging of Odor-Induced Presynaptic and Postsynaptic Membrane Activity In Vivo
We have also tested the ability of ArcLight to report odor-induced membrane activity in the presynaptic and postsynaptic compartments of the first olfactory relay in the glomeruli of the Drosophila antennal lobe using an in vivo preparation that permits simultaneous computer-controlled odorant delivery and fluorescence imaging (Figure 2A). ArcLight expressed in all olfactory sensory neurons (OSNs) robustly reports depolarization or hyperpolarization of their central presynaptic terminals in distinct subsets of glomeruli, depending on whether the odorant delivered is 1-butanol or propionic acid (Figure 2B). The specific patterns of glomerular excitation or inhibition for these two odorants are mostly consistent with what has been directly measured with extracellular recordings of odor-induced action potential firing rate at the somata of the receptor neurons in the periphery (Hallem and Carlson, 2006; Wilson et al., 2004) or inferred using a Ca2+ indicator that reports indirect downstream second-messenger signaling induced by membrane activity changes (Silbering et al., 2008). ArcLight expressed in all of the projection neurons that receive inputs from the OSNs reveals odor-induced depolarization of their postsynaptic terminals (Figure 2C). These postsynaptic activation patterns largely overlap with the presynaptic, but—consistent with whole-cell patch-clamp (Wilson et al., 2004) and Ca2+ imaging studies (Silbering et al., 2008)—are less restricted, in part due to the activity of excitatory local interneurons (Shang et al., 2007).
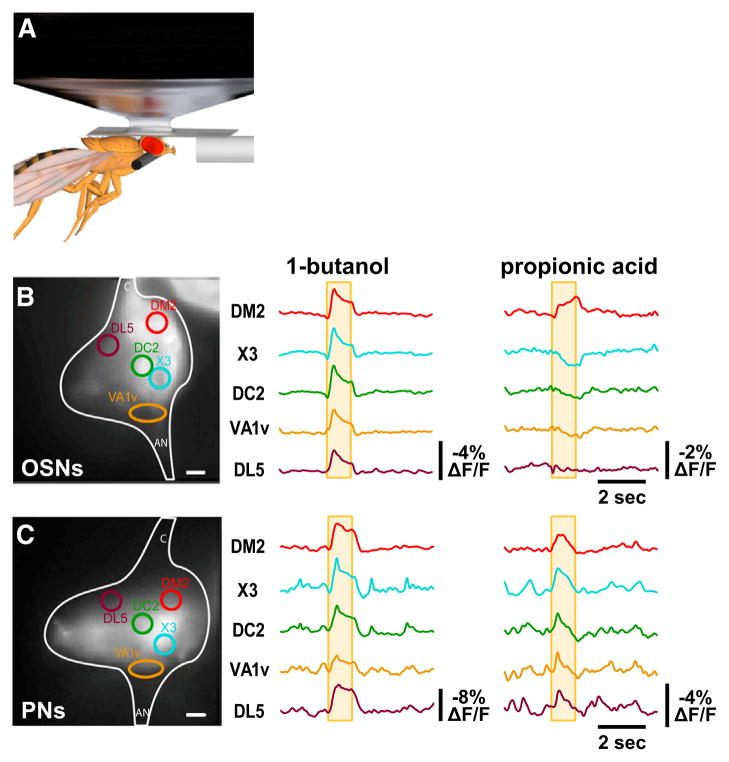
Figure 2. Single-Trial In Vivo Optical Recordings of Odor-Induced Membrane Activity in all OSNs or Projection Neurons.
(A) Schematic diagram of in vivo preparation for optical recording of odor-induced membrane activity in the antennal lobe.
(B) Optical recordings of odor-induced membrane activity in the presynaptic terminals of olfactory sensory neurons in response to either butanol or propionic acid. The yellow box indicates the timing of the odor application, and the antennal lobe glomeruli are identified by standard names as in Hallem and Carlson (2006) and Silbering et al. (2008). This example is representative of three experiments. Scale bar, 10 μm.
(C) Optical recordings of odor-induced membrane activity in the postsynaptic terminals of projection neurons. This example is representative of three experiments. Scale bar, 10 μm.
Importantly, however, there are differences for some glomeruli between the observed presynaptic ArcLight responses and expected responses predicted by peripheral sensillum recordings. For example, extracellular recordings in the periphery reveal substantially bigger excitatory responses of Or22a-expressing OSNs to 1-butanol than those expressing Or13a (Hallem and Carlson, 2006; Kreher et al., 2008), whereas we observe similar ArcLight responses to 1-butanol in the corresponding DM2 and DC2 glomeruli (Figure 2B). In addition, the sensory neurons projecting to the VA1v glomerulus are predicted by peripheral recordings to be inhibited by 1-butanol (Hallem and Carlson, 2006), whereas ArcLight imaging reveals apparent excitation (Figure 2B). One likely source of such discrepancies is contamination of the ArcLight signals arising from these regions-of-interest intended to encompass the indicated glomeruli lying on the surface of the antennal lobe with light emitted by other glomeruli lying underneath. This points out the importance of carefully considering the geometry of ArcLight-labeled neural structures when imaging with wide-field microscopy. Future work is required to determine if ArcLight-mediated optical electrophysiology can be performed with confocal or two-photon scanning microscopy, which would reduce or eliminate this potential confound.
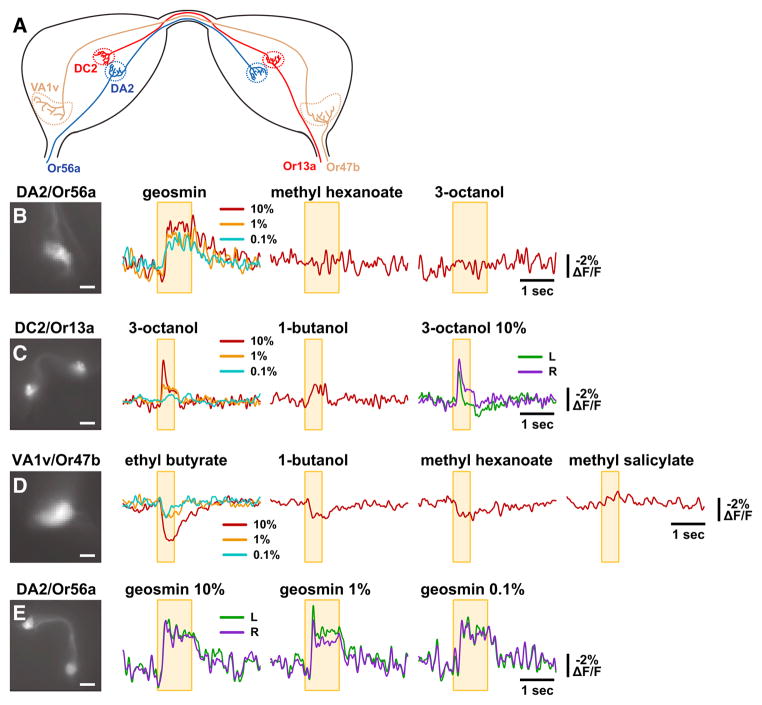
In order to confirm that ArcLight-mediated optical electrophysiology of the central presynaptic terminals faithfully reports odor-specific responses of unambiguously identified OSN populations, we expressed ArcLight in single glomeruli using GAL4 driver transgenes derived from the promoters of three different olfactory receptor genes (Vosshall et al., 2000) (Figure 3A). The Or56a receptor has recently been shown to be exquisitely tuned to respond to the aversive odorant geosmin, a compound emitted by pathogenic microorganisms, and not to any other tested odorants (Stensmyr et al., 2012). We imaged presynaptic odorant responses of the OSNs that express Or56a, which project to the DA2 glomerulus, by expressing ArcLight using an Or56a-GAL4 driver (Vosshall et al., 2000). ArcLight imaging reveals robust excitatory responses to geosmin, but not to methyl hexanoate or 3-octanol, in the central presynaptic terminals of the Or56a-expressing OSNs in the DA2 glomerulus (Figure 3B). This is consistent with the tuning of the Or56a receptor revealed by peripheral sensillum recordings, which show responses to geosmin, but not to methyl hexanoate or 3-octanol (Stensmyr et al., 2012). We also imaged odorant-induced Arc-Light signals in the presynaptic terminals of the Or13a-expressing OSNs in the DC2 glomerulus using an Or13a-GAL4 driver (Vosshall et al., 2000). We observe robust concentration-dependent excitatory responses in DC2 to 3-octanol and a weaker response to 1-butanol (Figure 3C). This is as predicted by peripheral sensillum recordings, which indicate excitatory Or13a responses to both 3-octanol and 1-butanol, but with the latter weaker than the former (Table S1 of Kreher et al., 2008).
Figure 3. Single-Trial In Vivo Optical Recordings of Odor-Induced Membrane Activity in Specific OSNs.
(A) Schematic diagram of the bilateral antennal lobes depicting the bilateral projections to their respective glomeruli of olfactory sensory neurons expressing either Or56a, Or13a, or Or47b.
(B–E) Optical recordings of odor-induced membrane activity in the presynaptic terminals of olfactory sensory neurons expressing the indicated Or in response to the indicated odorants. The yellow box indicates the timing of the odor application, and the antennal lobe glomeruli are identified by standard names. Odorant concentrations are indicated by the color of the traces for the unilateral recordings, or directly for the bilateral recordings, where the left and right glomeruli are indicated by the color of the traces. Scale bars, 10 μm in C and E and 20 μm in D and F. These examples are representative of three recordings of each glomerulus/Or, with each odorant shown tested in at least two of the three recordings.
ArcLight is also effective at detecting inhibitory responses to odorants. We imaged presynaptic odorant responses of the Or47b-expressing OSNs innervating the VA1v glomerulus (also known as VA1lm) using an Or47b-GAL4 driver (Vosshall et al., 2000). ArcLight imaging reveals inhibitory responses to ethyl butyrate, 1-butanol, and methyl hexanoate, but not to methyl salicylate (Figure 3D). This is as predicted by peripheral sensillum recordings, which indicate inhibitory Or47b responses to ethyl butyrate and methyl hexanoate, but not to methyl salicylate (Hallem and Carlson, 2006). In the peripheral sensillum recordings of Hallem and Carlson (2006), the inhibitory Or47b response to 1% ethyl butyrate is smaller than to 1% 1-butanol or 1% methyl hexanoate, whereas we observe using ArcLight a larger inhibitory response to 10% ethyl butyrate than to 10% 1-butanol or 10% methyl hexanoate (Figure 3D). One possible explanation is that this outcome would be expected if the Hallem and Carlson (2006) presentation of 1% 1-butanol and 1% methyl hexanoate saturated the OSN response, but their presentation of 1% ethyl butyrate did not. In some experiments, we were able to image odorant-induced ArcLight responses bilaterally. These measurements reveal similar presynaptic responses in both bilateral glomeruli, with substantial fine-scale synchrony (Figures 3C and 3E). This synchrony of presynaptic electrical activity is as predicted by the bilateral projections of individual OSNs, and consequent bilateral invasion of spike trains from each OSN (Stocker et al., 1990; Stocker et al., 1983; Vosshall et al., 2000). Another noteworthy aspect of our recordings is the apparent smoothness of the ArcLight signals measured with all glomeruli labeled as compared to the signals measured with a single glomerulus labeled (compare, e.g., the DC2/Or13a glomerulus response to 1-butanol in Figure 2B to that in Figure 3C). This difference is most likely attributed to the contamination of signals by neighboring glomeruli when all are labeled, with the higher frequency components seen with single glomeruli labeled (prominently visible in Figure 3E for the DA2/Or56a glomerulus) possibly representing uncontaminated correlated variation in presynaptic electrical activity among the OSN terminals innervating that glomerulus.
These measurements from three different identified glomeruli demonstrate that ArcLight faithfully reports odor-specific pre-synaptic electrical activity, with the response profiles for each glomerulus as predicted by previous peripheral measurements of OSN spiking. This direct detection of odor-specific glomerular presynaptic membrane activity has previously only been inferred indirectly through extracellular recordings from the somata of OSNs in the periphery (e.g., Hallem and Carlson, 2006; Wilson et al., 2004) or through changes in presynaptic fluorescent Ca2+ signals (e.g., Silbering et al., 2008; Wang et al., 2003). In this regard, it is also noteworthy that ArcLight permits optical imaging of membrane activity in contexts where Ca2+ indicators are silent (Figure S5). ArcLight-based optical detection of membrane electrical activity in the OSN terminals in the antennal lobe will now permit the direct detection of presynaptic inhibition and peptidergic presynaptic facilitation, which have previously only been inferred (Ignell et al., 2009; Root et al., 2011; Root et al., 2008).
ArcLight Imaging of Signal Propagation in Neurite Arbors
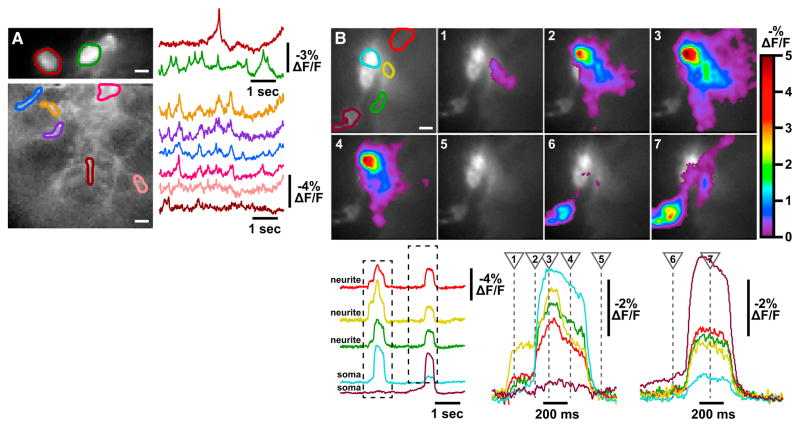
Another major advantage of optical electrophysiology is the ability to monitor membrane potential in the somata and neurites of multiple neurons simultaneously. To test the utility of ArcLight for this purpose, we observed asynchronous tonic firing of two lLNV somata in a whole-brain explant preparation (Figure 4A, top), and then shifted the field of view so that we could optically monitor membrane activity of neurites emanating from these somata (Figure 4A, bottom). Brief action-potential-like events were captured in all neurite branches, and although there is substantial synchrony among the branches, there are also noticeable differences between them. This likely reflects the fact that these neurites are fasciculated with those of some of the other lLNVs (a total number of eight to ten), and so at each branch location we are optically capturing a mixture of signals from multiple lLNVs. To more closely study electrical signal propagation along the neurite branches of lLNVs, we exploited NaChBac-induced action potentials. Simultaneous ArcLight imaging of both somata and neurites revealed two action-potential events occurring during a 5 s epoch (Figure 4B and Movie S1). As expected from the fasciculation of lLNV neurites, all three neurite regions exhibit an action potential when either the blue or brown soma fires. However, a higher time-resolution view reveals that the first action potential initiates in the yellow neurite region and then triggers an action potential in the blue soma, with the brown soma remaining silent. In contrast, the second event initiates by graded depolarization of the brown soma triggering an action potential simultaneously in soma and neurites. This experiment establishes the utility of ArcLight for single-trial optical analysis of spatiotemporal propagation electrical events in genetically targeted neurons in intact circuits.
Figure 4. Optical Detection of Signal Propagation in Neuronal Networks.
(A) Top: optical recording of asynchronous spontaneous activity of two lLNV somata in whole-brain explant. Bottom: optical recording of spontaneous activity in neurite branches in the same brain. This example is representative of ten experiments.
(B) Optical recording of spontaneous activity in NaChBac-expressing lLNVs in whole-brain explant. ROIs in the top left image indicate two lLNV somata (blue and brown) and three neurite regions (red, yellow, green). ArcLight fluorescence changes at seven time points during a 5 s trial are shown in pseudocolor on the image frames depicting those time points. Optical signals corresponding to each ROI are shown below, with expanded views of the boxed regions on the right, and with the times of the image frames indicated with dashed lines. This example is representative of eight experiments.
Scale bars, horizontal, 10 μm in all ROI images. See also Movie S1.
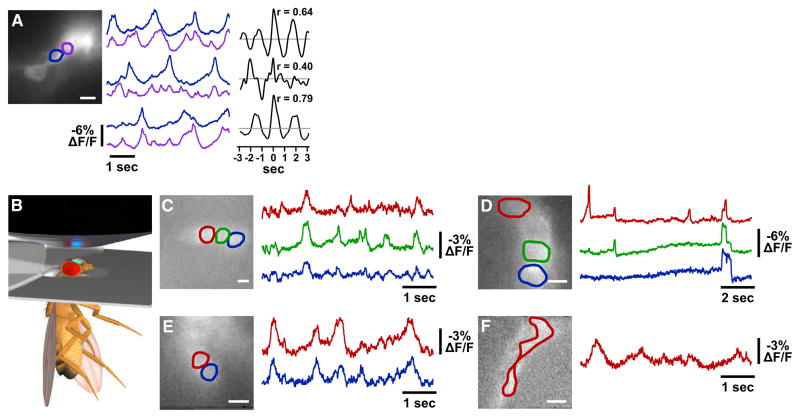
ArcLight Imaging of Multiple Individual Neurons In Vivo
Although the lLNV subset of peptidergic circadian clock neurons have been well-studied electrophysiologically (Cao and Nitabach, 2008; McCarthy et al., 2011; Sheeba et al., 2008), the sLNVs have been mostly refractory, with those whole-cell recordings that have been obtained rarely revealing action potential-like events (Cao and Nitabach, 2008; Choi et al., 2012). This is most likely due to the extremely small size of the sLNV somata (3–4 μm) and their electrotonic isolation from active regions of the neuronal membrane (i.e., the neurites). Using Arc-Light, we optically detected large-amplitude electrical activity in two sLNV somata simultaneously in a whole-brain explant (Figure 5A). Interestingly, the correlation between cells’ membrane activity varied over time, in this case being greater in the first and third recording trials and less in the second. We also developed an in vivo recording preparation that allows optical access to the LNVs through a window in the head of an intact fly head (Figure 5B). Large-amplitude electrical activity is readily detectable in vivo in lLNV somata, both normal spontaneous activity and NaChBac-induced action potentials, as well as sLNV spontaneous activity both in somata and in the distal peptidergic secretory terminals (Figures 5C–5F). These experiments demonstrate the utility of optical electrophysiology in contexts where electrode-based approaches are infeasible.
Figure 5. Optical Recordings of Multiple Individual Neurons in Brain Explants and In Vivo.
(A) Simultaneous optical recording of spontaneous activity in two sLNVs (blue and purple) in a whole-brain explant for three consecutive epochs. Sliding-window linear cross-correlations of the two sLNVs for each trial are shown, also indicating the peak Pearson’s correlation coefficient, r. The gray lines indicate r = 0. This example is representative of eight experiments. Scale bar, 10 μm.
(B) Schematic diagram of in vivo preparation for optical recording of membrane activity in clock neurons.
(C–F) In vivo optical recording of spontaneous activity in three wild-type lLNV somata, three NaChBac-expressing lLNV somata, two wild-type sLNV somata, and wild-type slLNV distal terminal projections, respectively. These examples are representative of ten, six, four, and four experiments, respectively. Scale bars, 10 μm.
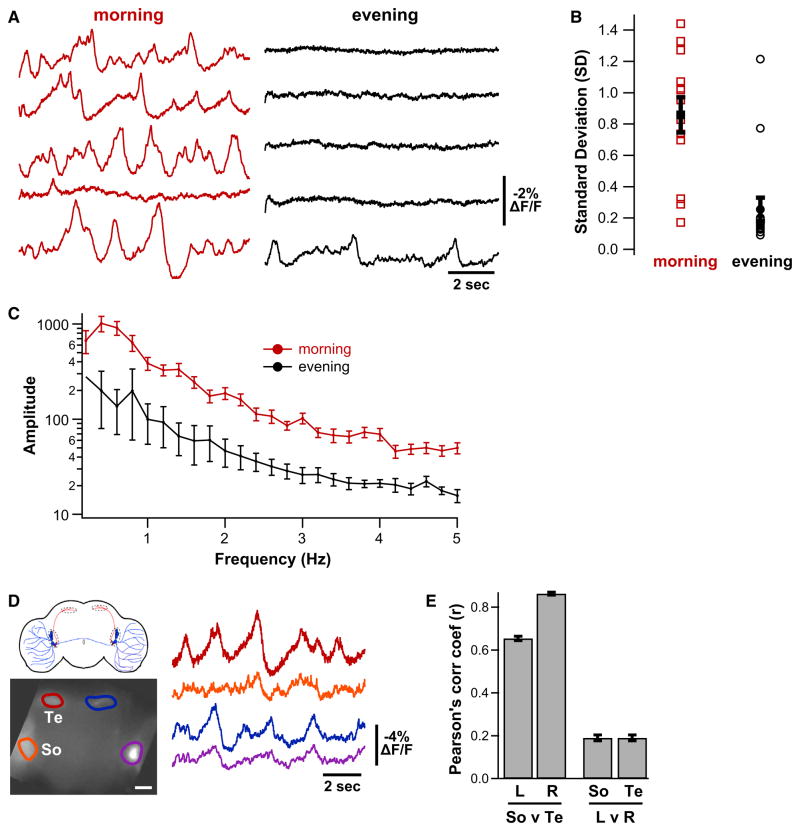
ArcLight Reveals Physiological State Dependence of Peptidergic Terminal Activity
A key question in neuropeptide biology about which little is known is the physiological state or event that triggers membrane activity-dependent neuropeptide secretion from dense-core vesicle-containing terminals (Taghert and Nitabach, 2012). Several lines of indirect evidence suggest that secretion of the neuropeptide PDF by the distal sLNV terminals is greatest in the morning and least in the evening (Nitabach and Taghert, 2008), but no direct evidence of the temporal pattern of electrical activity in the PDF-secreting terminals exists. We used ArcLight to directly demonstrate that membrane electrical activity of the distal sLNV PDF-secreting terminals is highly dependent on the time of day: much greater electrical activity was observed in whole-brains explanted in the morning than in the evening (Figures 6A–6C). This provides evidence for greater activity-dependent PDF secretion in the morning than in the evening and raises the question whether this distal terminal membrane activity is synchronous with that in the sLNV soma. To address this question, we imaged ArcLight at low magnification to allow noncellular resolution of membrane activity simultaneously in the regions of the sLNV and lLNV somata and the sLNV distal terminals of both hemispheres of the brain. We found that there is substantial synchrony of membrane electrical activity between the ipsilateral somata and distal sLNV terminals on each side of the brain, but very little contralateral synchrony (Figures 6D–6E). This indicates coupling of electrical activity of the somata and distal terminals of the sLNVs (which only project ipsilaterally), and suggests that—unlike the lLNVs (McCarthy et al., 2011)—contralateral sLNVs do not receive substantial synchronous synaptic inputs.
Figure 6. Optical Recording of Daily Rhythm of Peptidergic Terminal Membrane Activity in Intact Brain.
(A) Representative 10 s optical recordings of spontaneous membrane activity in sLNV distal peptidergic terminals in independent hemispheres of whole-brains explanted either in the morning (just after lights-on) or in the evening (just after lights-off) from flies maintained in 12 hr:12 hr light:dark conditions. Total Ns > 8 brains and 12 terminal fields for each time point.
(B) Standard deviations (SDs) over the recording trial were computed for each terminal field (unfilled symbols), and the mean ± SEM is plotted in filled symbols. Morning SD is significantly greater than evening (unpaired t test, p < 0.0001).
(C) Power spectrum was computed for each terminal field using fast Fourier transform with 0.2 Hz bin width. Powers at each frequency were averaged (±SEM) across terminal fields within morning and evening groups. Morning power is significantly greater than evening power (two-way ANOVA with repeated-measures, p < 0.0001).
(D) Simultaneous optical recording of spontaneous activity in the LNV soma region (So) and the distal sLNV terminals (Te) in both hemispheres of a whole-brain explant. Scale bar, 20 μm.
(E) Linear no-offset cross-correlation analysis between the indicated brain regions in (D) (bars depict r ± SEM).
CONCLUSIONS
Taken together, these results establish that the ArcLight GEVI enables robust genetically targeted optical electrophysiology in intact neural circuits, reporting membrane potential in somata, neurites, presynaptic terminals, and postsynaptic terminals, both in whole-brain explants and in vivo. Our results also demonstrate simultaneous optical recording of somatic and neuritic—both local dendrites and distal synaptic terminals—membrane electrical activity in genetically targeted neurons. It is thus now possible to fully characterize the electrophysiological properties of each neuronal membrane compartment—soma, neurites, and synaptic terminals—using solely optical methods. The large signal-to-noise ratio provided by ArcLight in intact neural tissue allows the recording of discrete subthreshold events and action potentials in multiple genetically targeted neurons simultaneously, which is almost always impossible with traditional electrode-based electrophysiology.
Exploiting this unique tool for optical electrophysiology, we show that the distal peptidergic terminals of circadian clock neurons exhibit a diurnal pattern of membrane activity. We also directly measure membrane electrical activity in the central presynaptic terminals of OSNs and postsynaptic terminals of projection neurons. In the same way that genetically encoded fluorescent sensors have revolutionized the study of intracellular Ca2+ signals (Grienberger and Konnerth, 2012; Tian et al., 2012), ArcLight now permits optical measurement in intact neural circuits of membrane potential, the key cellular parameter that underlies neuronal information processing. The ArcLight GEVI provides substantial advantages over the use of either GECIs or chemical voltage-sensitive dyes. These advantages include the ability to directly measure action potentials and subthreshold depolarizations and hyperpolarizations, the absence of apparent cellular toxicity, genetic targetability, and the accessibility of deep brain regions that cannot be reached with chemical dyes. The current disadvantages of ArcLight are that its absolute signal strength is smaller (and decreases with depolarization) than that of latest-generation GECIs and that its temporal response properties are not quite as fast as the voltage-gated ion channels that underlie action potential generation. We are currently engaged in further engineering and refinement of S1–S4 voltage-sensor-based GEVIs and expect substantial future progress on improving these parameters (as has been the historical case for GECIs).
EXPERIMENTAL PROCEDURES
Transgenic ArcLight Flies
Codon-optimized cDNA for expression in Drosophila melanogaster was synthesized based on ArcLight A227D amino acid sequence with deletion of two amino acids in the unconserved intracellular N terminal domain (Pro38 and Ala109) (Jin et al., 2012), cloned into the pJFRC7-20xUAS vector (Pfeiffer et al., 2010) to generate the UAS-ArcLight transgene, and inserted in the attP2 and attP40 phiC landing sites by injection of fertilized embryos (Groth et al., 2004). Experiments presented here were all performed using the attP2 insertion of UAS-Arclight. ArcLight was specifically expressed in PDF-positive clock neurons using pdf-GAL4 driver (Renn et al., 1999), in olfactory projection neurons using GH146-GAL4 (Stocker et al., 1997), in all OSNs using Or83b-GAL4 (Larsson et al., 2004), and in specific OSNs using Or56a-GAL4, Or13a-GAL4, and Or47b-GAL4 (Vosshall et al., 2000). Kir2.1 and NaChBac were expressed in LNVs using pdf-GAL4 driver and either UAS-Kir2.1 or UAS-NaChBac, as previously described (Baines et al., 2001; Nitabach et al., 2002; Nitabach et al., 2006). GCaMP5 was expressed in LNVs using pdf-GAL4 driver and UAS-GCaMP5 flies (Akerboom et al., 2012).
ArcLight Imaging of Intact Brain
Imaging was performed on an Olympus BX61WI upright microscope using either a LUMFL 60× N.A. 1.10, a LUMPlan FL 40× N.A. 0.80, or a XLUMPlan FI 20× N.A. 0.95 water immersion objective (Olympus, Japan). ArcLight was excited with a 488 nm 50 mW laser (DL488-050, CrystaLaser, Reno, NV), a 495 nm dichroic mirror, 520/35 nm emission filter (Semrock, Rochester, NY). Laser power measured at the preparation ranged from 1–12 W/cm2, and was adjusted for each recording using a continuous circular neutral density filter to the minimum required to record robust optical signals. The objective C-mount image was projected onto the 80 × 80 pixel chip of a NeuroCCD-SM camera controlled by NeuroPlex software (RedShirtImaging, Decatur, GA). For image demagnification, we used either an Optem zoom system A45731 0.1× or Optem C-to-C mount 25-70-54 0.38× (Qioptiq LINOS, Fairport, NY). Images of LNVs were recorded at a frame rate of 500 Hz and of glomeruli at 125 Hz and depicted optical traces were spatial averages of intensity of all pixels within the region of interest (ROI), with signals processed as previously reported (Jin et al., 2012; Popovic et al., 2011) with double-exponential fitting to compensate for rapid and slow photobleaching (see Figure S6) followed by eight rounds of box-car smoothing. GCaMP5 imaging was performed identically to ArcLight, at a frame-rate of 125 Hz. Whole-brain explant and whole-cell patch-clamp recording was as previously reported (Cao and Nitabach, 2008). External solution consists of (in mM) 101 NaCl, 3 KCl, 1 CaCl2, 4 MgCl2, 1.25 NaH2PO4, 5 glucose, and 20.7 NaHCO3 (pH 7.2), with an osmolarity of 250 mmol/kg. Recording pipettes are filled with internal solution consisting of (in mM) 102 potassium gluconate, 17 NaCl, 0.085 CaCl2, 4 Mg-ATP, 0.5 Na-GTP, 0.94 EGTA, and 8.5 HEPES (pH 7.2), with an osmolarity of 235 mmol/kg. The pH of these solutions has been chosen to best suit the normal physiology of fly brain neurons (Cao and Nitabach, 2008), and is a pH at which the ArcLight superecliptic GFP-based fluorophore is strongly fluorescent (Jin et al., 2012).
In Vivo Preparation and Odor Delivery
Olfactory responses were imaged in 3–14 day old female flies using the in vivo preparation previously reported (Fiala and Spall, 2003). For in vivo imaging of circadian clock neurons, the entire head was pushed through a hole in sticky tape and fixed using epoxy glue. For odor delivery, a constant air stream was directed through two pathways over filter papers with pure mineral oil in one and the mineral oil + odorant in the other. Nearly pure (>99%) stocks of odorants (Sigma-Aldrich) were diluted in mineral oil, and a total of 50 μl was placed on the filter paper. Odorants were applied by switching from the mineral oil pathway to the mineral oil + odorant pathway using an automated valve controller (Warner Instruments).
Supplementary Material
Figure S1. ArcLight Allows Accurate Optical Identification of lLNV Action Potentials Seen in Simultaneous Patch-Clamp Electrophysiology, Related to Figure 1
Signal-to-noise ratio for all action potentials analyzed in this figure (mean ± SD: 8.5 ± 3.1) was computed as SNR = abs(s-n)/σ, where s = peak optical signal during the action potential, n = average of preaction potential noise, σ = standard deviation of the noise.
(A) The slope of a linear fit to the fluorescence signal is computed for a 50 ms sliding window at each point in the optical recording. A threshold is set for the slope (0.005% ΔF/F / ms) above which the event was considered a spike. When this threshold is exceeded, a local search (within 50 ms) is performed for the maximum rate of change followed by a local search (within 50 ms) for peak fluorescence change. The time of occurence of this peak change in fluorescence intensity is defined as the time of occurrence of a spike. The search is then restarted 50 ms beyond this peak to find the next spike. After-hyperpolarizations in these neurons limit the maximum spike frequency, and constrain the duration of the local search window.
(B) This method was applied blindly to optical recordings from three different preparations, and then compared to the corresponding electrical recordings. This reveals accurate automated identification of action potentials from optical data, resulting in only a 10% error rate (false negatives plus false positives).
(C) A selection of optical signals from the cells in (B). Optical signals derived from ROIs covering the soma of the patched cell are in red. Voltage recordings produced from the patch electrode are in black. The blue triangles represent the location of spikes identified by the above algorithm. Circled triangles indicate false-positive errors made by the algorithm.
(D) Image showing regions of interest corresponding to the optical signals depicted in (E). Bar = 10 μm.
(E) Using this algorithm, the spikes identified in the optical signal from the patched cell (dark red) are revealed to occur at different times from spikes identified in optical signals from the other two cells. Blue vertical lines indicate the occurence of spikes identified automatically in the optical signals.
Figure S2. Boltzmann Curve Relating Change in ArcLight Fluorescence to Membrane Potential Was Determined in Voltage-Clamped lLNvs in Whole-Brain Explant Preparation in the Presence of 2 μM TTX to Silence Action Potentials and Network-Dependent Synaptic Potentials, Related to Figure 1
(A) ArcLight optical signals from a representative cell and the corresponding voltage-clamp protocol, which was a family of hyperpolarizing and depolarizing steps in 10 mV increments from the holding potential of −50 mV.
Figure S3. Membrane Biophysical Properties of ArcLight-Expressing lLNVs Were Compared with Control lLNVs, Related to Figure 1
Membrane biophysical properties of ArcLight-expressing lLNVs were compared with control lLNVs expressing a cytoplasmic fluorescent protein in 3–8 days old flies with somatic whole-cell patch-clamp recordings in whole-brain explants in the presence of the AChR antagonist (+)-tubocurarine (200 μM), to block all network activity and silence synaptic inputs. All data are presented as mean ± SEM.
(A) Resting membrane potential (RMP) of lLNVs was measured in whole-cell current-clamp recording mode. Average RMP was −48.4 ± 2.1 mV (n = 17) in ArcLight-expressing lLNVs and −44.0 ± 1.2 mV (n = 10) in control lLNVs, which was not significantly different (p = 0.15, unpaired t test).
(B) The membrane capacitance of lLNVs was measured by imposing a hyperpolarizing voltage step in whole-cell voltage-clamp mode and measuring the time constant of the resulting time-varying membrane current by fitting to a single exponential curve. Membrane capacitance in ArcLight-expressing lLNVs was 9.15 ± 0.66 pF (n = 25), which is significantly higher than in control lLNVs (7.0 ± 0.63 pF, n = 12) (p < 0.05, unpaired t test).
(C) Representative traces of spontaneous action potentials (APs) recorded from ArcLight-expressing (red) and control (black) lLNVs.
(D) AP maximum rise slope was 7.52 ± 0.93 mV/ms (n = 10) in ArcLight-expressing lLNvs and 4.58 ± 0.51 mV/ms (n = 10) in control, which is significantly different (p < 0.05, unpaired t test).
(E) AP maximum decay slope was −3.21 ± 0.23 mV/ms in ArcLight-expressing lLNVs (n = 11) and 2.92 ± 0.32 mV/ms in WT (n = 10), which is not significantly different (p = 0.48, unpaired t test).
(F) Active responses were evoked by 500 ms current steps from −10 to +25 pA in 5pA increments, and the numbers of evoked APs were quantified. There was no significant difference in evoked AP number between ArcLight-expressing (n = 11) and control lLNvs (n = 10) (p = 0.132, two-way repeated-measures ANOVA).
(G) AP threshold was defined as the membrane voltage at the beginning of the rapid upward AP slope induced by current injection. AP threshold was −33.3 ± 0.84 mV in ArcLight-expressing lLNVs (n = 11) and −35.0 ± 0.80 mV in controls (n = 10), which was not significantly different (p = 0.185, unpaired t test).
(H) Average amplitudes of the first current-evoked APs were 22.43 ± 2.13 mV in ArcLight-expressing lLNVs (n = 11) and 18.57 ± 1.40 mV in controls (n = 10), which was not significantly different (p = 0.19, unpaired t test).
Figure S4. Diurnal and Circadian Locomotor Activity of Male Flies Expressing ArcLight or Membrane-Associated CD4-GFP as a Negative Control in the LNVs using pdf-GAL4 Driver, Related to Figure 1
Locomotor activity was measured using the standard Drosophila Activity Monitor system (TriKinetics, Waltham, MA), in which individual flies are housed in glass tubes with food at one end, a gas permeable plug at the other, and an infrared beam crossing the center of the tube to detect longitudinal movement of the fly in the tube. All values are shown as mean ± SEM.
(A) Flies expressing ArcLight (n=29) in the LNVs exhibit normal diurnal patterns of locomotor activity in 12 hr:12 hr light:dark conditions (white bars = light; black bars = dark). This includes morning and evening anticipatory increases in activity prior to the lights-on and lights-off environmental transitions, as well as morning startle increase in activity in response to lights-on and evening startle decrease in activity in response to lights-off. The proper phase and magnitude of morning and evening anticipatory activity depends on normal functioning of the LNVs and their ability to communicate with other downstream clock neurons in the circadian control network.
(B) Flies expressing ArcLight in the LNVs exhibit normal circadian patterns of locomotor activity on the first day in constant dark conditions after transition from entraining 12 hr:12 hr light:dark (gray bars = subjective day; black bars = subjective night). This includes morning and evening peaks of activity driven by the circadian control network and reliant on normal functioning of the LNVs and their ability to communicate with other downstream clock neurons in the circadian control network.
(C) Double-plotted actograms of averaged locomotor activity indicates normal free-running circadian locomotor activity of flies expressing ArcLight in the LNVs in constant darkness, including the more prominent evening peak of activity compared to the morning. Gray bars above the actogram indicate subjective day, whereas black bars indicate subjective night.
(D) Lomb-Scargle periodogram analysis of individual flies reveals no significant difference in average free-running period between ArcLight-expressing and control GFP-expressing flies (n = 30).
Figure S5. Cytoplasmic Ca2+ Levels in lLNVs Measured Optically with GCaMP5 Genetically Encoded Ca2+ Sensor Expressed using pdf-GAL4 LNV-Specific Driver and UAS-GCaMP5, Related to Figure 1
Optical imaging was performed identically to that of ArcLight, albeit with a lower frame rate of 125 Hz.
(A) GCaMP5 reports no Ca2+ increases in the lLNV soma as a consequence of spontaneous APs or by a train of APs induced by current injection (representative of ten experiments).
(B) GCaMP5 reports large Ca2+ increases in the lLNV soma as a consequence of giant NaChBac-induced APs (representative of six experiments).
Figure S6. Analysis of Photobleaching Characteristics of ArcLight in a lLNV Imaged in a Whole-Brain Explant, Related to Figure 1
(A) During each cycle, there is a rapid (~1 s in duration) reversible decrease in fluorescence intensity followed by a slower, smaller nonreversible decrease.
(B) Plotting the fluorescence intensity at the end of the illumination phase of each cycle for nine consecutive cycles reveals a slight decline in the fuorescence intensity, reflecting the accumulation of the nonreversible slower component.
(C) Over the entire course of the 54 cycles of illumination (~260 s of total cumulative excitation), there is very little detectable accumulation of ArcLight photobleaching. The larger, more rapid increases and decrease in fluorescence intensity compared to the slight changes seen in (B) reflect movements of the preparation in the recording chamber.
The preparation was exposed to 54 cycles of intermittent laser excitation illumination (duty cycle of 5 s on/55 s off), resulting in 270 s total laser excitation illumination. (AU = arbitrary units).
Acknowledgments
The authors thank the Bloomington Stock Center (Bloomington, IN), Loren Looger, and Vivek Jayaraman (both at Janelia Farm Research Campus, Howard Hughes Medical Institute) for Drosophila stocks, Lawrence B. Cohen (Yale University School of Medicine) and the members of the Nitabach and Pieribone labs for discussion, and Glenn Turner (Cold Spring Harbor Laboratory) for advice concerning in vivo imaging of fly olfactory responses. Work in the laboratory of M.N.N. is supported in part by the National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of General Medical Sciences, National Institutes of Health (NIH) (R01NS055035, R01NS056443, R01GM098931, and R01NS083875). Work in the laboratory of V.A.P. is supported in part by NINDS, NIH (U24NS057631 and R01NS083875) and The John B. Pierce Laboratory.
Footnotes
Supplemental Information includes Extended Experimental Procedures, six figures, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2013.07.027.
References
- Akemann W, Mutoh H, Perron A, Rossier J, Knöpfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat Methods. 2010;7:643–649. doi: 10.1038/nmeth.1479. [DOI] [PubMed] [Google Scholar]
- Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knöpfel T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophysiol. 2012;108:2323–2337. doi: 10.1152/jn.00452.2012. [DOI] [PubMed] [Google Scholar]
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataka K, Pieribone VA. A genetically targetable fluorescent probe of channel gating with rapid kinetics. Biophys J. 2002;82:509–516. doi: 10.1016/S0006-3495(02)75415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BJ, Mutoh H, Dimitrov D, Akemann W, Perron A, Iwamoto Y, Jin L, Cohen LB, Isacoff EY, Pieribone VA, et al. Genetically encoded fluorescent sensors of membrane potential. Brain Cell Biol. 2008;36:53–67. doi: 10.1007/s11068-008-9026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett L, Platisa J, Popovic M, Pieribone VA, Hughes T. A fluorescent, genetically-encoded voltage probe capable of resolving action potentials. PLoS ONE. 2012;7:e43454. doi: 10.1371/journal.pone.0043454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Cao G, Tanenhaus AK, McCarthy EV, Jung M, Schleyer W, Shang Y, Rosbash M, Yin JC, Nitabach MN. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LB, Keynes RD, Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968;218:438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Dimitrov D, He Y, Mutoh H, Baker BJ, Cohen L, Akemann W, Knöpfel T. Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS ONE. 2007;2:e440. doi: 10.1371/journal.pone.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala A, Spall T. In vivo calcium imaging of brain activity in Drosophila by transgenic cameleon expression. Sci STKE. 2003;2003:PL6. doi: 10.1126/stke.2003.174.pl6. [DOI] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Ignell R, Root CM, Birse RT, Wang JW, Nässel DR, Winther AM. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci USA. 2009;106:13070–13075. doi: 10.1073/pnas.0813004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012;75:779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods. 2012;9:90–95. doi: 10.1038/nmeth.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lundby A, Mutoh H, Dimitrov D, Akemann W, Knöpfel T. Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS ONE. 2008;3:e2514. doi: 10.1371/journal.pone.0002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy EV, Wu Y, Decarvalho T, Brandt C, Cao G, Nitabach MN. Synchronized bilateral synaptic inputs to Drosophila melanogaster neuropeptidergic rest/arousal neurons. J Neurosci. 2011;31:8181–8193. doi: 10.1523/JNEUROSCI.2017-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic MA, Foust AJ, McCormick DA, Zecevic D. The spatio-temporal characteristics of action potential initiation in layer 5 pyramidal neurons: a voltage imaging study. J Physiol. 2011;589:4167–4187. doi: 10.1113/jphysiol.2011.209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nässel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, Repunte-Canonigo V, Raj CD, Knöpfel T. Design and characterization of a DNA-encoded, voltage-sensitive fluorescent protein. Eur J Neurosci. 2001;13:2314–2318. doi: 10.1046/j.0953-816x.2001.01617.x. [DOI] [PubMed] [Google Scholar]
- Salzberg BM, Davila HV, Cohen LB. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973;246:508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel MS, Isacoff EY. A genetically encoded optical probe of membrane voltage. Neuron. 1997;19:735–741. doi: 10.1016/s0896-6273(00)80955-1. [DOI] [PubMed] [Google Scholar]
- Silbering AF, Okada R, Ito K, Galizia CG. Olfactory information processing in the Drosophila antennal lobe: anything goes? J Neurosci. 2008;28:13075–13087. doi: 10.1523/JNEUROSCI.2973-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjulson L, Miesenbock G. Rational optimization and imaging in vivo of a genetically encoded optical voltage reporter. J Neurosci. 2008;28:5582–5593. doi: 10.1523/JNEUROSCI.0055-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Singh RN, Schorderet M, Siddiqi O. Projection patterns of different types of antennal sensilla in the antennal glomeruli of Drosophila melanogaster. Cell Tissue Res. 1983;232:237–248. doi: 10.1007/BF00213783. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Akerboom J, Schreiter ER, Looger LL. Neural activity imaging with genetically encoded calcium indicators. Prog Brain Res. 2012;196:79–94. doi: 10.1016/B978-0-444-59426-6.00005-7. [DOI] [PubMed] [Google Scholar]
- Villalba-Galea CA, Miceli F, Taglialatela M, Bezanilla F. Coupling between the voltage-sensing and phosphatase domains of Ci-VSP. J Gen Physiol. 2009;134:5–14. doi: 10.1085/jgp.200910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cao G, Nitabach MN. Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila. J Biol Rhythms. 2008;23:117–128. doi: 10.1177/0748730407312984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ArcLight Allows Accurate Optical Identification of lLNV Action Potentials Seen in Simultaneous Patch-Clamp Electrophysiology, Related to Figure 1
Signal-to-noise ratio for all action potentials analyzed in this figure (mean ± SD: 8.5 ± 3.1) was computed as SNR = abs(s-n)/σ, where s = peak optical signal during the action potential, n = average of preaction potential noise, σ = standard deviation of the noise.
(A) The slope of a linear fit to the fluorescence signal is computed for a 50 ms sliding window at each point in the optical recording. A threshold is set for the slope (0.005% ΔF/F / ms) above which the event was considered a spike. When this threshold is exceeded, a local search (within 50 ms) is performed for the maximum rate of change followed by a local search (within 50 ms) for peak fluorescence change. The time of occurence of this peak change in fluorescence intensity is defined as the time of occurrence of a spike. The search is then restarted 50 ms beyond this peak to find the next spike. After-hyperpolarizations in these neurons limit the maximum spike frequency, and constrain the duration of the local search window.
(B) This method was applied blindly to optical recordings from three different preparations, and then compared to the corresponding electrical recordings. This reveals accurate automated identification of action potentials from optical data, resulting in only a 10% error rate (false negatives plus false positives).
(C) A selection of optical signals from the cells in (B). Optical signals derived from ROIs covering the soma of the patched cell are in red. Voltage recordings produced from the patch electrode are in black. The blue triangles represent the location of spikes identified by the above algorithm. Circled triangles indicate false-positive errors made by the algorithm.
(D) Image showing regions of interest corresponding to the optical signals depicted in (E). Bar = 10 μm.
(E) Using this algorithm, the spikes identified in the optical signal from the patched cell (dark red) are revealed to occur at different times from spikes identified in optical signals from the other two cells. Blue vertical lines indicate the occurence of spikes identified automatically in the optical signals.
Figure S2. Boltzmann Curve Relating Change in ArcLight Fluorescence to Membrane Potential Was Determined in Voltage-Clamped lLNvs in Whole-Brain Explant Preparation in the Presence of 2 μM TTX to Silence Action Potentials and Network-Dependent Synaptic Potentials, Related to Figure 1
(A) ArcLight optical signals from a representative cell and the corresponding voltage-clamp protocol, which was a family of hyperpolarizing and depolarizing steps in 10 mV increments from the holding potential of −50 mV.
Figure S3. Membrane Biophysical Properties of ArcLight-Expressing lLNVs Were Compared with Control lLNVs, Related to Figure 1
Membrane biophysical properties of ArcLight-expressing lLNVs were compared with control lLNVs expressing a cytoplasmic fluorescent protein in 3–8 days old flies with somatic whole-cell patch-clamp recordings in whole-brain explants in the presence of the AChR antagonist (+)-tubocurarine (200 μM), to block all network activity and silence synaptic inputs. All data are presented as mean ± SEM.
(A) Resting membrane potential (RMP) of lLNVs was measured in whole-cell current-clamp recording mode. Average RMP was −48.4 ± 2.1 mV (n = 17) in ArcLight-expressing lLNVs and −44.0 ± 1.2 mV (n = 10) in control lLNVs, which was not significantly different (p = 0.15, unpaired t test).
(B) The membrane capacitance of lLNVs was measured by imposing a hyperpolarizing voltage step in whole-cell voltage-clamp mode and measuring the time constant of the resulting time-varying membrane current by fitting to a single exponential curve. Membrane capacitance in ArcLight-expressing lLNVs was 9.15 ± 0.66 pF (n = 25), which is significantly higher than in control lLNVs (7.0 ± 0.63 pF, n = 12) (p < 0.05, unpaired t test).
(C) Representative traces of spontaneous action potentials (APs) recorded from ArcLight-expressing (red) and control (black) lLNVs.
(D) AP maximum rise slope was 7.52 ± 0.93 mV/ms (n = 10) in ArcLight-expressing lLNvs and 4.58 ± 0.51 mV/ms (n = 10) in control, which is significantly different (p < 0.05, unpaired t test).
(E) AP maximum decay slope was −3.21 ± 0.23 mV/ms in ArcLight-expressing lLNVs (n = 11) and 2.92 ± 0.32 mV/ms in WT (n = 10), which is not significantly different (p = 0.48, unpaired t test).
(F) Active responses were evoked by 500 ms current steps from −10 to +25 pA in 5pA increments, and the numbers of evoked APs were quantified. There was no significant difference in evoked AP number between ArcLight-expressing (n = 11) and control lLNvs (n = 10) (p = 0.132, two-way repeated-measures ANOVA).
(G) AP threshold was defined as the membrane voltage at the beginning of the rapid upward AP slope induced by current injection. AP threshold was −33.3 ± 0.84 mV in ArcLight-expressing lLNVs (n = 11) and −35.0 ± 0.80 mV in controls (n = 10), which was not significantly different (p = 0.185, unpaired t test).
(H) Average amplitudes of the first current-evoked APs were 22.43 ± 2.13 mV in ArcLight-expressing lLNVs (n = 11) and 18.57 ± 1.40 mV in controls (n = 10), which was not significantly different (p = 0.19, unpaired t test).
Figure S4. Diurnal and Circadian Locomotor Activity of Male Flies Expressing ArcLight or Membrane-Associated CD4-GFP as a Negative Control in the LNVs using pdf-GAL4 Driver, Related to Figure 1
Locomotor activity was measured using the standard Drosophila Activity Monitor system (TriKinetics, Waltham, MA), in which individual flies are housed in glass tubes with food at one end, a gas permeable plug at the other, and an infrared beam crossing the center of the tube to detect longitudinal movement of the fly in the tube. All values are shown as mean ± SEM.
(A) Flies expressing ArcLight (n=29) in the LNVs exhibit normal diurnal patterns of locomotor activity in 12 hr:12 hr light:dark conditions (white bars = light; black bars = dark). This includes morning and evening anticipatory increases in activity prior to the lights-on and lights-off environmental transitions, as well as morning startle increase in activity in response to lights-on and evening startle decrease in activity in response to lights-off. The proper phase and magnitude of morning and evening anticipatory activity depends on normal functioning of the LNVs and their ability to communicate with other downstream clock neurons in the circadian control network.
(B) Flies expressing ArcLight in the LNVs exhibit normal circadian patterns of locomotor activity on the first day in constant dark conditions after transition from entraining 12 hr:12 hr light:dark (gray bars = subjective day; black bars = subjective night). This includes morning and evening peaks of activity driven by the circadian control network and reliant on normal functioning of the LNVs and their ability to communicate with other downstream clock neurons in the circadian control network.
(C) Double-plotted actograms of averaged locomotor activity indicates normal free-running circadian locomotor activity of flies expressing ArcLight in the LNVs in constant darkness, including the more prominent evening peak of activity compared to the morning. Gray bars above the actogram indicate subjective day, whereas black bars indicate subjective night.
(D) Lomb-Scargle periodogram analysis of individual flies reveals no significant difference in average free-running period between ArcLight-expressing and control GFP-expressing flies (n = 30).
Figure S5. Cytoplasmic Ca2+ Levels in lLNVs Measured Optically with GCaMP5 Genetically Encoded Ca2+ Sensor Expressed using pdf-GAL4 LNV-Specific Driver and UAS-GCaMP5, Related to Figure 1
Optical imaging was performed identically to that of ArcLight, albeit with a lower frame rate of 125 Hz.
(A) GCaMP5 reports no Ca2+ increases in the lLNV soma as a consequence of spontaneous APs or by a train of APs induced by current injection (representative of ten experiments).
(B) GCaMP5 reports large Ca2+ increases in the lLNV soma as a consequence of giant NaChBac-induced APs (representative of six experiments).
Figure S6. Analysis of Photobleaching Characteristics of ArcLight in a lLNV Imaged in a Whole-Brain Explant, Related to Figure 1
(A) During each cycle, there is a rapid (~1 s in duration) reversible decrease in fluorescence intensity followed by a slower, smaller nonreversible decrease.
(B) Plotting the fluorescence intensity at the end of the illumination phase of each cycle for nine consecutive cycles reveals a slight decline in the fuorescence intensity, reflecting the accumulation of the nonreversible slower component.
(C) Over the entire course of the 54 cycles of illumination (~260 s of total cumulative excitation), there is very little detectable accumulation of ArcLight photobleaching. The larger, more rapid increases and decrease in fluorescence intensity compared to the slight changes seen in (B) reflect movements of the preparation in the recording chamber.
The preparation was exposed to 54 cycles of intermittent laser excitation illumination (duty cycle of 5 s on/55 s off), resulting in 270 s total laser excitation illumination. (AU = arbitrary units).