Abstract
Background
Ulcerative colitis (UC) is generally described as a superficial diffuse inflammation restricted to the colon and rectum. However, several case reports have described a distinct and rare type of UC-related pan-enteritis, typically occurring after colectomy. Corticosteroids are effective for induction of remission of this condition, but it is not clear how these patients should be managed long-term.
Goals
To further describe and define the entity of UC-related pan-enteritis, and to investigate the efficacy of azathioprine for maintenance of remission.
Results
We describe five patients with superficial diffuse ulcerative inflammation of the stomach, small bowel, and pouch if present. Four of the five patients developed enteritis after colectomy for ulcerative pancolitis. Pathology showed severe mucosal inflammation with infiltration of neutrophils and plasma cells from the stomach to the ileum. Video capsule endoscopy in one patient confirmed the presence of mucosal inflammation throughout the small bowel. All patients were started on a standardized treatment with IV corticosteroids for induction of remission and azathioprine for maintenance therapy. They all rapidly improved and subsequently, four patients were in full remission on azathioprine monotherapy, despite failure of this UC therapy prior to surgery, while one patient continues to have steroid-dependent disease.
Conclusions
The outcomes of five cases of UC-related pan-enteritis as described in this report support a role for azathioprine in remission maintenance. Future research is needed to improve our understanding of this rare but distinct intestinal inflammatory disorder.
Keywords: azathioprine, colectomy, duodenitis, enteritis, gastritis, video capsule endoscopy, ulcerative colitis
Introduction
Ulcerative colitis (UC) is characterized by superficial diffuse inflammation limited to the colon. Occasionally, the distal small bowel can be affected, usually represented by a backwash ileitis or post-colectomy pouchitis.1 However, several case reports have documented another entity, ‘UC-related pan-enteritis’, characterized by diffuse small bowel mucosal inflammation following colectomy for UC.2–3 Induction of remission is typically achieved with corticosteroids, but various maintenance therapies have been utilized in case reports, including mesalamines, azathioprine, cyclosporine, corticosteroids and anti-TNF therapy.2 The small number of reported cases, usually one or two per report, have limited the efforts to establish a standardized treatment strategy for UC-related pan-enteritis. In this report, we describe five patients with a previous diagnosis of pan-UC, who developed mucosal inflammation of the stomach, duodenum, ileum and pouch, resembling diffuse small bowel enteritis as previously described.2–3 Furthermore, we report the results of standardized therapy with corticosteroids and azathioprine for these five patients with UC-related pan-enteritis.
Cases
Case 1
A 23-year-old male who was initially diagnosed at age 17 with ulcerative pancolitis and backwash ileitis. Serology at that time was positive for perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) and negative for anti-Saccharomyces cereviciae antibodies (ASCA). In the following years, he failed to respond to mesalamine, corticosteroids, azathioprine and adalimumab. After six years of disease, he underwent a total abdominal colectomy and end-ileostomy. Pathology of the colectomy specimen confirmed severe ulcerative colitis and revealed a normal terminal ileum. Three months later, he experienced a partial, distal small bowel obstruction just proximal to the stoma. Ileoscopy showed a patchy, serpiginous ulcer with some cobblestoning along with areas of normal mucosa, but a small bowel follow-through was unremarkable. Pathology of ileal biopsies showed focal acute ileitis. Two months later, a follow-up endoscopy confirmed spontaneous resolution. Four months later, he underwent construction of a restorative ileal pouch-anal anastomosis (IPAA) and a loop ileostomy. Three months after surgery, he was hospitalized for vomiting, abdominal pain, diarrhea and weight loss. An upper and lower endoscopy showed diffuse edematous inflamed mucosa with superficial ulcers in the stomach, duodenum and pouch (Figure 1). The distal ileum appeared normal. Pathology showed severe active inflammation with significant neutrophil infiltration in the epithelium and lamina propria of the stomach, duodenum, ileum and pouch. Meanwhile, stool cultures, celiac serology and antibodies to enterocytes were negative. UC-related pan-enteritis was suspected and he started IV methylprednisolone 40 mg. Total parenteral nutrition (TPN) was started as well in order to improve his nutritional status. His symptoms improved within 24 hours and he was discharged on an oral prednisone taper and azathioprine. Six months later, he developed a small bowel obstruction and underwent extensive adhesiolysis, however, repeat endoscopy demonstrated normalization of the ileal mucosa with residual mild inflammation within the pouch. Currently, he is in steroid-free remission on azathioprine 3 mg/kg/d based on azathioprine metabolites. Takedown of his loop ileostomy is scheduled.
Figure 1.
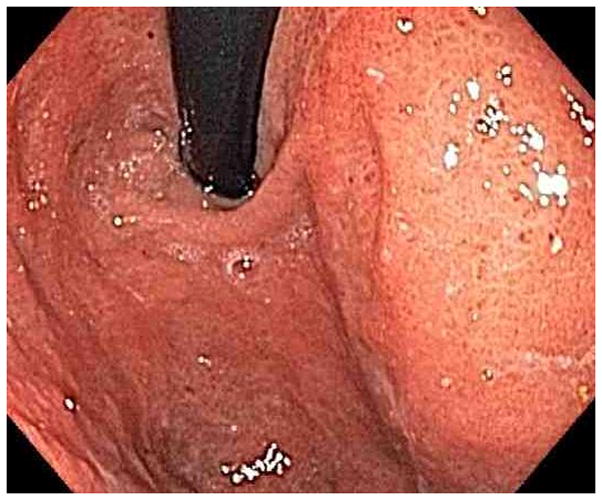
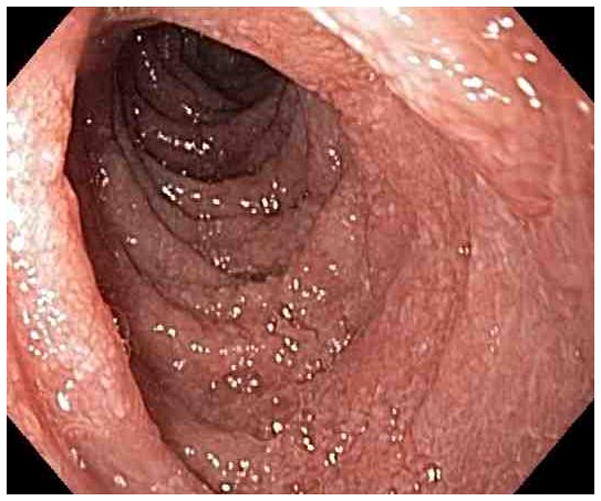
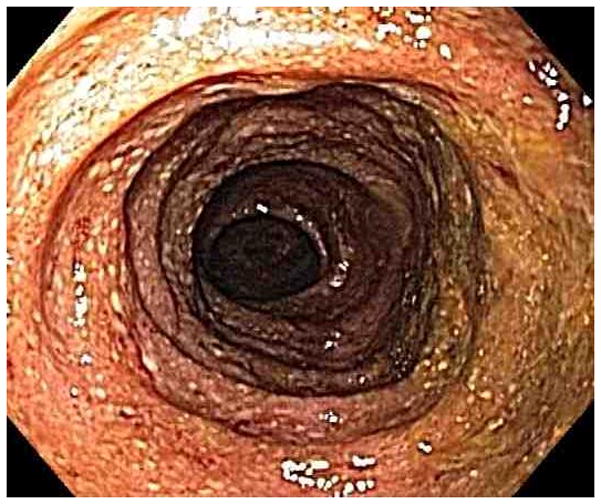
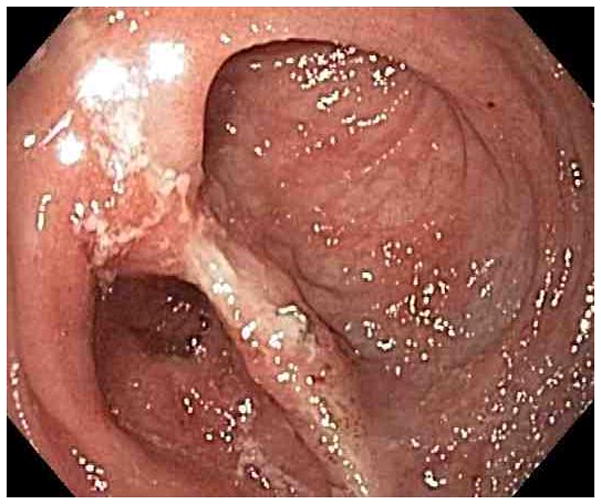
Representative endoscopy images. 1A: stomach in retroflexion, showing granular, erythematous and friable mucosa (case 4). 1B: 2nd portion duodenum, showing erythematous, edematous mucosa with superficial ulceration (case 1). 1C: distal ileum, demonstrating continuous extensive superficial ulcers and spontaneous bleeding (case 4). 1D: pouch with erythematous mucosa and decreased vascular pattern (case 1).
Case 2
A 29-year-old female was diagnosed with UC of the entire colon at age 21. Active disease during the years following diagnosis required multiple courses of corticosteroids, as well as tacrolimus, azathioprine, and later, 6-mercaptopurine. Corticosteroid-dependent disease despite immunosuppressive therapy led to the initiation of infliximab, but this was discontinued after anaphylactic infusion reactions. At the age of 24 she underwent a total abdominal colectomy, followed by construction of an IPAA and ileostomy takedown. Review of the colectomy specimen by multiple pathologists confirmed active inflammation of the colon, compatible with ulcerative colitis. The terminal ileum appeared normal. Three months later, she developed symptoms of vomiting, diarrhea, weight loss and anemia. Six emergency room visits and extensive evaluation in other hospitals revealed mild duodenitis and pouchitis, and an infectious cause was suspected. Eventually, she was transferred to our hospital with persistent symptoms. An upper and lower endoscopy revealed contiguous diffuse ulcerative inflammation in duodenum, ileum and pouch while the stomach appeared normal (Figure 1). Pathology showed dense mixed cell infiltrates in the lamina propria, including a prominent component of plasma cells and clusters of neutrophils in all biopsies (Figure 3). The biopsies were negative for intraepithelial lymphocytosis, viral inclusions, crypt cell apoptosis and decreased goblet cells. Viral pathogens (herpes simplex virus, cytomegalovirus and HIV) were excluded and celiac serology and antibodies to enterocytes were negative. UC-related pan-enteritis was suspected and she started IV methylprednisolone 40 mg and TPN. Her symptoms quickly resolved and she was discharged with an oral steroid taper and azathioprine. In the subsequent year the dosage of azathioprine was adjusted, and allopurinol was added to optimize levels of 6-thioguanine. However, she remained steroid-dependent and she was switched to certolizumab pegol. Despite dose adjustment and shortening of injection interval as well as the addition of methotrexate, she has remained steroid-dependent and her symptoms were reasonably controlled with 10 to 20 mg of prednisone. Recently, she delivered her first child after a pregnancy that was complicated by steroid-induced gestational diabetes. Following delivery, her symptoms of diarrhea worsened and she is currently treated with 40 mg of prednisone.
Figure 3.
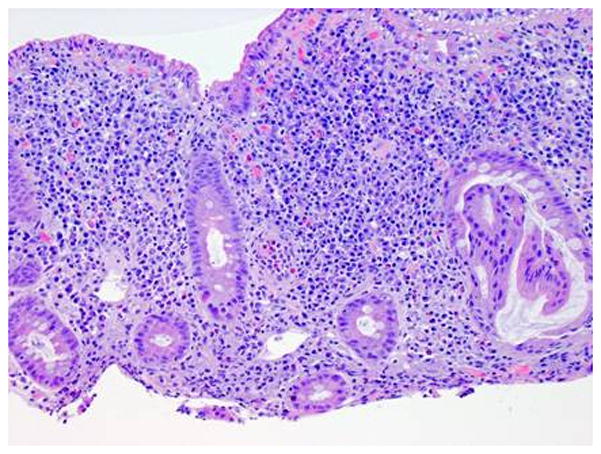
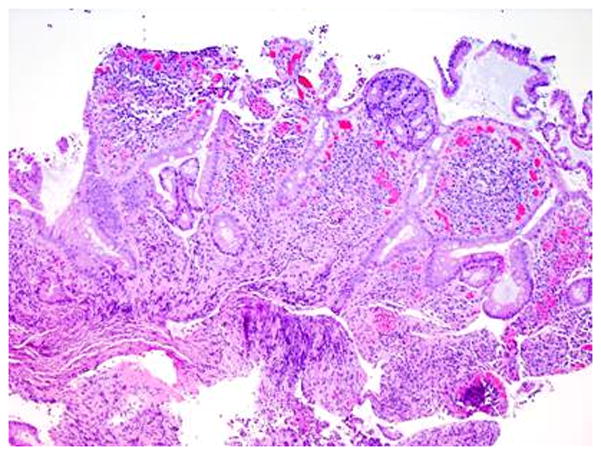
Representative histology sections of the duodenum (A) and pouch (B) from case 2. The findings in both the duodenal and pouch biopsies are nearly identical and show very dense lamina propria inflammatory cell infiltrates, including a very prominent component of plasma cells as well as large clusters of neutrophils. The duodenal biopsies exhibit moderate villous blunting as well.
Case 3
A 30-year-old male underwent a total abdominal colectomy and IPAA for refractory ulcerative colitis at the age of two. He did well until the age of 27 when he developed severe diarrhea and lost 18 pounds. He was hospitalized for further workup and rehydration. 24 hour stool collection yielded eight liters. An infectious work-up was negative and serology was negative for ASCA but positive for pANCA. An upper and lower endoscopy revealed diffuse erythema plus granular and friable mucosa throughout in the stomach, duodenum, pouch, and visualized ileum. Pathology confirmed active inflammation in the stomach and the entire duodenum with prominent eosinophils in the lamina propria. The ileal and pouch biopsies were remarkable for architectural distortion. He started methylprednisolone 40 mg IV daily and improved within 24 hours. At discharge, he was switched to an oral prednisone taper and started azathioprine maintenance therapy 2 mg/kg/d. Currently, he is doing well and has not experienced a recurrence of symptoms.
Case 4
A 57-year-old physician was diagnosed with left-sided ulcerative colitis at the age of 48. Six years later, the colitis progressed to a pancolitis and he eventually became steroid-dependent and refractory to azathioprine and adalimumab. He, subsequently, underwent a subtotal colectomy and end-ileostomy as obesity prevented the construction of a J-pouch. Pathology of the colectomy specimen demonstrated severely active ulcerative pancolitis. The terminal ileum was normal. Following colectomy, his did well for two years, but then he developed fulminant diarrhea and peristomal ulcerations. He was admitted to an outside hospital and was treated with multiple courses of antibiotics for suspected infectious diarrhea, despite the fact that his stool cultures remained negative. His diarrhea did not improve and he was transferred to our hospital. On admission, an upper endoscopy and ileoscopy showed diffuse erythematous and edematous mucosa of the stomach, duodenum and ileum (Figure 1). Pathology confirmed diffuse and active Helicobacter pylori-negative gastritis, duodenitis and ileitis. A video capsule endoscopy showed scattered erythematous lesions and ulcerations throughout the visualized duodenum, jejunum and ileum (Figure 2). His diarrhea and peristomal pyoderma significantly improved within 12 hours after starting IV methylprednisolone 40 mg. He was discharged on a steroid taper and started maintenance therapy with azathioprine. Currently, he is on azathioprine 2.2 mg/kg/d monotherapy and is doing well.
Figure 2.
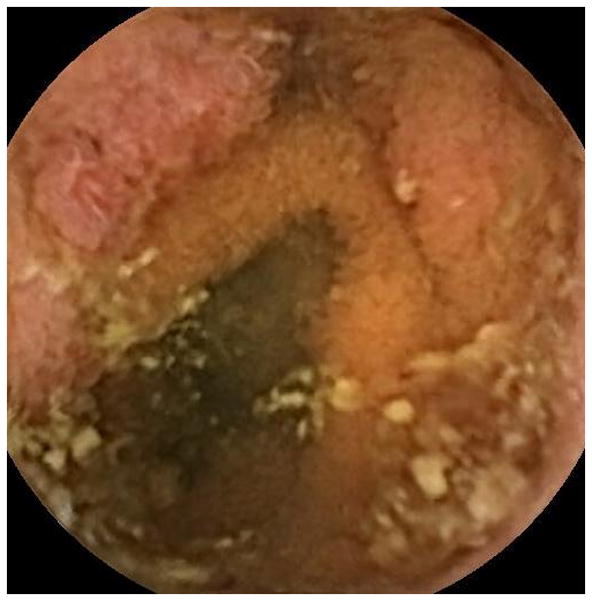
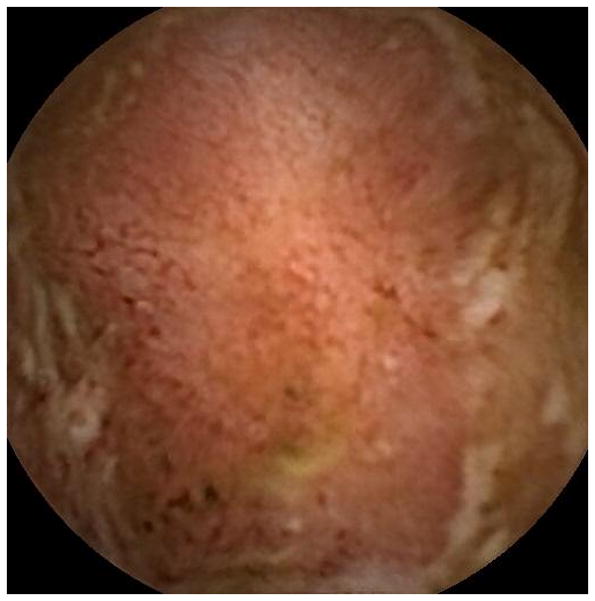
Representative images of the jejunum (A) and ileum (B) during videocapsule endoscopy in case 4. The images show erythematous and edematous mucosa with superficial ulceration.
Case 5
A 36-year-old gentleman was diagnosed with left-sided ulcerative colitis which was managed with mesalamine and oral prednisone. However, bloody diarrhea persisted and he also developed vomiting and weight loss. He was first admitted to an outside hospital and then transferred to our hospital. A colonoscopy revealed severe, pan-ulcerative colitis with histologic confirmation. An upper endoscopy showed moderate gastritis and diffuse, severe ulcerative duodenitis. Histology showed severe, active inflammation with prominent neutrophilic infiltrates in both the gastric and duodenal mucosa. Stool cultures remained negative and he started IV methylprednisolone 40 mg and azathioprine. He slowly improved over the course of one week and was discharged on oral steroids. The steroids were tapered and he is currently doing well on monotherapy with azathioprine 2 mg/kg/d.
Discussion
Ulcerative colitis is generally restricted to the rectum and colon. However, there are exceptions to this rule and multiple variants of small bowel disease in ulcerative colitis are well-described. These conditions include backwash ileitis, post-colectomy pouchitis and pre-stomal ileitis.1 In addition, pediatric UC is often associated with gastric inflammation.4–5 In the last decade, multiple authors have reported another variant of small bowel inflammation, also referred to as UC-related pan-enteritis.3–4, 6–14 Multiple case reports in Japanese journals confirmed and further defined this inflammatory condition.15–18 This enteritis is characterized by diffuse, superficial, ulcerative mucosal inflammation of the entire small bowel, with a typical onset after colectomy for UC.2–3, 10–11 The five patients in our report confirm these characteristics of UC-related pan-enteritis (Table 1). They were all previously diagnosed with ulcerative colitis and underwent a colectomy. Subsequently, all patients developed severe diffuse gastro-enteritis.
Table 1.
Characteristics of five patients with ulcerative colitis (UC)-related pan-enteritis.
| Case | M/F | Age | Extent UC | Onset enteritis | Extent enteritis | Maintenance therapies | FU | Serology | Remission |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23 | pancolitis | 3 mo | Stomach, duodenum, Ileum, pouch | AZA | 10 mo | pANCA(+) ASCA(−) |
Since diagnosis |
| 2 | F | 23 | pancolitis | 4 mo | Stomach, duodenum, Ileum, pouch | AZA, certolizumab, methotrexate, prednisone | 57 mo | ND | Steroid-dependent |
| 3 | M | 27 | pancolitis | 25y | Stomach, duodenum, Ileum, pouch | AZA | 43 mo | pANCA(+) ASCA(−) |
Since diagnosis |
| 4 | M | 57 | pancolitis | 27 mo | Stomach, small bowel | AZA | 6 mo | pANCA(+) ASCA(−) |
Since diagnosis |
| 5 | M | 36 | pancolitis | N/A | Stomach, duodenum | AZA | 17 mo | ND | Since diagnosis |
Age: at presentation of UC-related pan-enteritis symptoms. Timing: interval between colectomy and onset of UC-related pan-enteritis symptoms. ASCA = Anti-Saccharomyces Cereviciae Antibodies, AZA = azathioprine, FU = follow-up, mo = months, ND = not determined, pANCA = perinuclear Anti-Neutrophil Cytoplasmic Antibody.
Several alternative diagnoses were considered in our patients during the course of their hospitalizations. Most importantly, Crohn’s disease was considered, but results from the diagnostic workup supported a diagnosis of UC-related pan-enteritis rather than Crohn’s disease. First, abdominal imaging did not show signs of Crohn’s disease, such as a thickened bowel wall, infiltrated mesenteric fat or strictures. Second, the endoscopic images did not reveal typical features of Crohn’s disease (ie. aphthous ulcers, deep ulcerations, strictures or skip lesions). Third, the continuous and superficial character of the described inflammation in our patients in the absence of granulomas is more “UC-like” and different from the patchy and transmural inflammation as seen in Crohn’s disease. Fourth, the colectomy specimens from all five patients were reviewed retrospectively and the diagnosis of ulcerative colitis was confirmed. Finally, all patients were followed on an outpatient basis with a mean follow-up duration of 27 months and no distinct features of Crohn’s disease developed during this time.
Other causes were also considered during the diagnostic workup including: infections, immune-mediated conditions, malignancies, ischemia, and toxic effects. Evaluation for intestinal infections, including parasitic, viral and bacterial causes, as well as HIV, was negative in all patients. Celiac disease was ruled out by negative serology and the absence of intraepithelial lymphocytosis in duodenal biopsies. Auto-immune enteropathy was ruled out by serology, and CT imaging excluded malignancies and ischemia. NSAIDs can cause intestinal ulceration, but none of our patients reported excessive use of NSAIDs. The eosinophilia in case 3 was an unexpected finding and other infectious causes were considered. However, the negative cultures, rapid response to corticosteroids and history of colectomy for UC supported a diagnosis of UC-related pan-enteritis. Thus, extensive workup revealed enteritis compatible with UC-related pan-enteritis and ruled out alternative causes of small bowel disease in the five reported patients.
The diagnosis of UC-related pan-enteritis is usually delayed due to the very low incidence of this condition (42 reported cases worldwide) and the fact that most physicians are unfamiliar with this condition. The diagnostic evaluation is typically focused on infectious causes, and avoids treatment with corticosteroids. Delayed diagnosis led to malnutrition and the need for TPN in two of the patients presented in this report. One fatal case of UC-related pan-enteritis was previously reported and treatment in that case focused on infectious causes as well.7 In our case series, we included all patients with UC-related pan-enteritis who were reported by our gastroenterologist, GI surgeons and/or GI pathologist in the previous five years. Three patients were initially referred by other hospitals for further evaluation while two patients were already treated for UC by our IBD team. Due to the very low incidence of UC-related pan-enteritis, it remains difficult to clearly define this disease. Common features in our series that affected most if not all patients include the underlying IBD (pan-UC, status post-colectomy), symptoms (vomiting, diarrhea, dehydration), endoscopy findings (UC-like pan-enteritis), the absence of infectious or Crohn’s like complications (lack of positive cultures, fistulas, abscesses), pathology findings (UC-like mucosal inflammation), and response to treatment (rapid improvement on IV corticosteroids within 24h).
No consistent data is available on the value of serology in UC-related pan-enteritis. In our series, three patients were tested for serology and they were all pANCA (+), ASCA (−), these results most likely reflect their diagnosis of UC. Additional sero-immunogenetic testing is required to explore whether a unique pattern of serology (e.g. ASCA, pANCA, anti-CBir1, outer membrane porins) and genetics (NOD-2) is associated with UC-related pan-enteritis.
Generally, once the correct diagnosis is established, patients rapidly respond to treatment with IV corticosteroids and typically improve within 24 hours.2–3, 8 Indeed, 4/5 patients (80%) described in this report responded within 24 hours to treatment with corticosteroids. Although most patients with UC-related pan-enteritis are treated with corticosteroids for induction of remission, the choice of maintenance therapy is less obvious. Review of all reported cases with well-defined treatment in literature showed that 4/9 patients (44%) without immunosuppressive maintenance therapy experienced a flare-up of UC-related pan-enteritis during follow-up and required repeat courses of corticosteroids. In the current case report, we described five patients, all of whom received standardized maintenance therapy with azathioprine. Eighty percent maintained steroid-free remission during a mean follow-up time of 27 months. Including the present case series, we identified reports of 10 UC-related pan-enteritis patients in the literature who received immunosuppressive maintenance therapy and 9/10 (90%) maintained remission during follow-up.2 Although one patient in this series required the addition of allopurinol to optimize her 6-thioguanine levels, azathioprine was well tolerated in our series and no adverse events were noted. Based on this experience, azathioprine is the preferred maintenance therapy for UC-related pan-enteritis at our IBD Center. It is interesting to note that two cases described in the current series were refractory to azathioprine prior to colectomy, but responded well to post-colectomy treatment with the same agent. The reason for this discrepancy is unclear, but one explanation would be that UC-related pan-enteritis could represent a different disease with favorable response to immunosuppression. Therefore, it seems reasonable to consider thiopurines for UC-related pan-enteritis, even if patients failed to respond to this treatment during their UC treatment.
The endoscopy findings in our cases were surprisingly similar within the GI tract as well as between patients. Consistent findings included friable erythematous mucosa with superficial, ulcerative inflammation. The inflammation was diffuse, continuous and involved stomach, duodenum, ileum and pouch in most of our cases. No skip lesions or strictures were noted, findings that could have indicated Crohn’s disease. These findings are consistent with previously reported cases of UC-related pan-enteritis.2–3 To our knowledge, case four as described in the current case report is the first patient with a reported VCE for UC-related pan-enteritis. This procedure allowed us to visualize the entire small bowel and confirmed that the inflammatory condition was present throughout the duodenum, jejunum and ileum. We only found one other report that was able to confirm the presence of inflammation throughout the entire small bowel. This was done in a fatal case of UC-related pan-enteritis where post-mortem pathology showed scattered ulcerations in the entire small bowel.7
Multiple studies in recent years suggested that duodenitis can be associated with UC. In adult UC patients, reported rates of duodenitis were 3–5%.19–20 Most patients with duodenitis had a history of pancolitis, were post colectomy and were at increased risk of developing chronic pouchitis.19–21 The majority of patients in the latter studies were outpatients that were either asymptomatic or experiencing mild symptoms as opposed to the patients in our case series, all of whom required hospitalization. Most likely, enteritis in UC patients represents a spectrum, with isolated asymptomatic duodenitis on the ‘mild’ side of the spectrum. In contrast, UC-related pan-enteritis can be characterized by fulminant pan-enteritis requiring corticosteroids, immunosuppressive maintenance therapy and sometimes has led to dehydration, malnutrition and death.
The pathogenesis of UC-related pan-enteritis is unknown. Given the low incidence, only case reports and small series have been published, and no mechanistic studies have been performed. One case report showed improvement of UC-related pan-enteritis in one patient after initiation of infliximab.4 The response in this patient was associated with an increased Th1/Th2 ratio in peripheral CD4+ T cells, suggesting an underlying inflammatory Th2 response that perpetuates UC-related pan-enteritis. Other studies in UC-related isolated duodenitis focused on CD8+ cells. For example, duodenal biopsies in 15 UC patients showed microscopic duodenitis in four patients, characterized by an increased number of CD3+ and CD8+ intra-epithelial lymphocytes.22 These findings confirmed conclusions from a previous report that found increased numbers of mucosal CD8+ cells in patients with UC-associated duodenitis.23 Further research is required to obtain a better understanding of the underlying immunological mechanisms of UC-related pan-enteritis.
In summary, UC-related pan-enteritis is a rare disease phenotype with distinct endoscopic and histological inflammation of the stomach and small bowel that resembles the inflammation in the colon in patients with UC. Novelties of our series include VCE images confirming pan-enteritis and successful steroid-free maintenance therapy with standardized azathioprine dosing even if patients were refractory prior to their colectomy for UC. Future research is required to obtain a better understanding of the pathogenesis and treatment of UC-related pan-enteritis.
Acknowledgments
Funding: No relevant funding reported.
Footnotes
Disclosures: No conflicts of interest exist.
References
- 1.Haboubi N. Small bowel inflammation in ulcerative colitis. Colorectal Dis. 2006;8(4):245–6. doi: 10.1111/j.1463-1318.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 2.Corporaal S, Karrenbeld A, van der Linde K, et al. Diffuse enteritis after colectomy for ulcerative colitis: two case reports and review of the literature. Eur J Gastroenterol Hepatol. 2009;21(6):710–5. doi: 10.1097/MEG.0b013e32831bc400. [DOI] [PubMed] [Google Scholar]
- 3.Valdez R, Appelman HD, Bronner MP, et al. Diffuse duodenitis associated with ulcerative colitis. Am J Surg Pathol. 2000;24(10):1407–13. doi: 10.1097/00000478-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Akitake R, Nakase H, Tamaoki M, et al. Modulation of Th1/Th2 balance by infliximab rescues postoperative occurrence of small-intestinal inflammation associated with ulcerative colitis. Dig Dis Sci. 2010;55(6):1781–4. doi: 10.1007/s10620-009-0910-5. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman SS, Vanderhoof JA, Young R, et al. Gastroenteric inflammation in children with ulcerative colitis. Am J Gastroenterol. 1997;92(7):1209–12. [PubMed] [Google Scholar]
- 6.Mitomi H, Atari E, Uesugi H, et al. Distinctive diffuse duodenitis associated with ulcerative colitis. Dig Dis Sci. 1997;42(3):684–93. doi: 10.1023/a:1018836218391. [DOI] [PubMed] [Google Scholar]
- 7.Annese V, Caruso N, Bisceglia M, et al. Fatal ulcerative panenteritis following colectomy in a patient with ulcerative colitis. Dig Dis Sci. 1999;44(6):1189–95. doi: 10.1023/a:1026688526551. [DOI] [PubMed] [Google Scholar]
- 8.Terashima S, Hoshino Y, Kanzaki N, et al. Ulcerative duodenitis accompanying ulcerative colitis. J Clin Gastroenterol. 2001;32(2):172–5. doi: 10.1097/00004836-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Ikeuchi H, Hori K, Nishigami T, et al. Diffuse gastroduodenitis and pouchitis associated with ulcerative colitis. World J Gastroenterol. 2006;12(36):5913–5. doi: 10.3748/wjg.v12.i36.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubenstein J, Sherif A, Appelman H, et al. Ulcerative colitis associated enteritis: is ulcerative colitis always confined to the colon? J Clin Gastroenterol. 2004;38(1):46–51. doi: 10.1097/00004836-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Gooding IR, Springall R, Talbot IC, et al. Idiopathic small-intestinal inflammation after colectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2008;6(6):707–9. doi: 10.1016/j.cgh.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki M, Okada K, Koyama S, et al. Ulcerative colitis complicated by gastroduodenal lesions. J Gastroenterol. 1996;31(4):585–9. doi: 10.1007/BF02355062. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JW, 3rd, Bargen JA. Ulcerative duodenitis and chronic ulcerative colitis: report of two cases. Gastroenterology. 1960;38:452–5. [PubMed] [Google Scholar]
- 14.Kawai K, Watanabe T, Nakayama H, et al. Images of interest. Gastrointestinal: small bowel inflammation and ulcerative colitis. J Gastroenterol Hepatol. 2005;20(11):1791. doi: 10.1111/j.1440-1746.2005.04162.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima M, Nakashima H, Kiyohara K, et al. Case with diffuse duodenitis and enteritis following total colectomy for ulcerative colitis. Nippon Shokakibyo Gakkai Zasshi. 2008;105(3):382–90. [PubMed] [Google Scholar]
- 16.Shimura T, Inukai M, Yoshioka N, et al. A case of diffuse gastritis and duodenitis associated with ulcerative colitis. Nippon Shokakibyo Gakkai Zasshi. 2006;103(1):30–6. [PubMed] [Google Scholar]
- 17.Nakayama S, Matsuoka M, Yoshida Y, et al. A case of pouchitis, duodenitis and pancreatitis showing diffuse irregular narrowing of main pancreatic duct after total colectomy for ulcerative colitis. Nippon Shokakibyo Gakkai Zasshi. 2004;101(4):389–96. [PubMed] [Google Scholar]
- 18.Kinekawa F, Minami A, Yoshida M, et al. A case of ulcerative colitis after total colectomy associated with diffuse duodenitis. Nippon Shokakibyo Gakkai Zasshi. 1997;94(4):272–7. [PubMed] [Google Scholar]
- 19.Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):39–44. doi: 10.1002/ibd.21356. [DOI] [PubMed] [Google Scholar]
- 20.Hori K, Ikeuchi H, Nakano H, et al. Gastroduodenitis associated with ulcerative colitis. J Gastroenterol. 2008;43(3):193–201. doi: 10.1007/s00535-007-2143-8. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, McKenna BJ, Appelman HD. Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study. Am J Surg Pathol. 2010;34(11):1672–7. doi: 10.1097/PAS.0b013e3181f3de93. [DOI] [PubMed] [Google Scholar]
- 22.Vidali F, Di Sabatino A, Broglia F, et al. Increased CD8+ intraepithelial lymphocyte infiltration and reduced surface area to volume ratio in the duodenum of patients with ulcerative colitis. Scand J Gastroenterol. 2010;45(6):684–9. doi: 10.3109/00365521003663662. [DOI] [PubMed] [Google Scholar]
- 23.Honma J, Mitomi H, Murakami K, et al. Nodular duodenitis involving CD8+ cell infiltration in patients with ulcerative colitis. Hepatogastroenterology. 2001;48(42):1604–10. [PubMed] [Google Scholar]


