Abstract
For nearly half a century, contact lenses have been proposed as a means of ocular drug delivery, but achieving controlled drug release has been a significant challenge. We have developed a drug-eluting contact lens designed for prolonged delivery of latanoprost for the treatment of glaucoma, the leading cause of irreversible blindness worldwide. Latanoprost-eluting contact lenses were created by encapsulating latanoprost–poly(lactic-co-glycolic acid) films in methafilcon by ultraviolet light polymerization. In vitro and in vivo studies showed an early burst of drug release followed by sustained release for one month. Contact lenses containing thicker drug–polymer films demonstrated released a greater amount of drug after the initial burst. In vivo, single contact lenses were able to achieve, for at least one month, latanoprost concentrations in the aqueous humor that were comparable to those achieved with topical latanoprost solution, the current first-line treatment for glaucoma. The lenses appeared safe in cell culture and animal studies. This contact lens design can potentially be used as a treatment for glaucoma and as a platform for other ocular drug delivery applications.
Keywords: Drug delivery, Contact lens, Sustained release, Latanoprost, Glaucoma, Ocular release
1. Introduction
Glaucoma is the leading cause of irreversible blindness worldwide [1]. The mainstay of preventive therapy is topical medications (drops) that reduce intraocular pressure (IOP). Unfortunately, only 1–7% of the medication in eye drops is absorbed, and the duration of effect is not sustained [2]. The excess medication typically washes down the nasolacrimal duct or spills onto the cheek, where it can potentially lead to side effects such as allergic blepharitis and contact dermatitis. Eye drops also can be difficult to administer and they can sting, burn, or cause a transient blurring of vision. All of these factors are believed to contribute to the notoriously poor patient adherence with glaucoma therapy, with an estimated adherence rate of less than 50% [3]. Poor adherence contributes to irreversible vision loss and is one of the biggest challenges facing eye care providers [4].
A noninvasive method of sustained ocular drug delivery could help improve adherence to glaucoma therapy by decreasing the frequency of drug administration. Controlling drug release from a contact lens has historically proven to be difficult [5]. Commercially available contact lenses can absorb and release drugs, but the duration of release tends to be limited to only several hours [5]. Recent research has focused on extending the duration of drug release through modification of the contact lens design [5,6]. However, a contact lens has yet to demonstrate the ability to elute a drug for weeks at a time in an animal model.
Here, we describe the formulation, characterization, and performance of a contact lens designed to elute a glaucoma medication for at least one month. The contact lens, composed of methafilcon, a high water content (55%) co-polymer of poly(hydroxyethylmethacrylate) and methacrylic acid [7,8], contains a drug–polymer film composed of poly(lactic-co-glycolic) acid (PLGA). PLGA is well-known for its biocompatibility in many settings, biodegradability, and ability to control drug release kinetics, and it is approved by the Federal Drug Administration (FDA) in both ocular and systemic drug delivery devices [9]. Latanoprost is a first-line treatment for glaucoma, it is the most commonly prescribed anti-glaucoma drug in the United States [10], and it demonstrates minimal systemic or local toxicity at therapeutic doses [11].
2. Materials and methods
2.1. Materials
High molecular weight (118 kDa) 65:35 PLGA (65 glycolide:35 l-lactide) and 85:15 PLGA (85 glycolide:15 l-lactide) were obtained from Surmodics (Birmingham, AL). Irgacure 2959 was purchased from Ciba Specialty Chemicals Corporation (Tarrytown, NY). Latanoprost for incorporation into lenses (“commercial latanoprost”) was obtained in an aqueous solution (50 µg/mL, Bausch and Lomb, Tampa, FL) and latanoprost standards were obtained in methyl acetate (50 mg/mL, Cayman Chemical, Ann Arbor, MI). Unpolymerized methafilcon was purchased in liquid form from Kontur Kontact Lens Company (Hercules, CA). Glucose, ethyl acetate, and all the other reagents were purchased from Sigma Aldrich (St. Louis, MO). Phosphate buffered saline (PBS, pH 7.4) was obtained from Invitrogen (Carlsbad, CA). Biopsy punches (3 mm) were obtained from Sklar Instruments (West Chester, PA).
2.2. Contact lens fabrication
PLGA (60 mg, 65:35 or 85:15) and 80 µL of latanoprost solution (50 mg/mL) were added to 920 µL ethyl acetate. 50 µL of the combined solution was then pipetted onto a concavity lathed into a cylinder of dry polymerized methafilcon (Kontur Kontact Lens Company). After 6 min of rotation on a spin coater (Model P6700, Speedline Technologies, Franklin, MA), the liquid ethyl acetate evaporated, leaving a drug–polymer film. Thinner 65:35 PLGA films were created by using a diluted solution that contained half the amount of polymer and latanoprost.
A 3 mm central aperture in the drug–polymer film was incised with a 3 mm biopsy punch. The hydrogel blanks containing the films were placed on a desiccator under vacuum for 3 days and then lyophilized for a day. The side of the films that was not yet in contact with methafilcon was then encapsulated in methafilcon by ultraviolet (UV) photopolymerization using a 400 W metal halide bulb (Loctite Zeta 7401, Loctite Corporation, Rocky Hill, CT). The methafilcon block was then lathed into a contact lens that consisted of the drug–PLGA film fully encapsulated in methafilcon (Table 1).
Table 1.
Polymer film characteristics.
| Name | Lactide/glycolide mole ratio | Film thickness (µm)a |
|---|---|---|
| CL65:35, 20 | 65:35 | 19.2 ± 4.4 |
| CL65:35, 40 | 65:35 | 38.5 ± 8.4 |
| CL85:15, 45 | 85:15 | 45.5 ± 6.4 |
Measurements are means ± one standard deviation.
2.3. Scanning electron microscopy
The morphologies of the drug polymer films and the latanoprost-eluting contact lenses (CLs) were examined under scanning electron microscopy (SEM). Dry CLs and pre-encapsulated films were cross-sectioned and sputter coated with a gold–plutonium alloy under vacuum (Hummer 6.2, Anatech Limited, Union City, CA). Images were acquired with a JEOL 590 scanning electron microscope (JEOL JSM 5600 LV, USA Inc., Peabody, MA). The average thickness for each set of latanoprost–PLGA films was calculated by loading the respective SEM images, which contain a scale bar, into ImageJ and measuring the thickness of the film cross-section at the center and at each end.
2.4. In vitro drug release
Three sets of CLs were submerged in 5 mL of PBS solution that was pre-heated to 37 °C. The lenses and PBS were placed on a rotational shaker at 64 rpm. Each day, aliquots of the release media were sampled and the contact lenses were placed in fresh PBS. The aliquots of release media were stored at 4 °C, and latanoprost concentrations were quantified using an enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI).
2.5. Latanoprost molecular analysis
During lens construction and characterization, latanoprost was exposed to various environmental factors that could potentially result in drug degradation. To determine if latanoprost was altered, we analyzed release media (day 1) from CL85:15, 45 (n = 4) by high-performance liquid chromatography in combination with high-resolution mass spectrometry (LC-MS). We monitored the presence of latanoprost and its most abundant degradation product, latanoprost free acid. We also analyzed commercial latanoprost solution (0.005%, Bausch and Lomb, Tampa, FL), latanoprost standard solution and latanoprost acid standard solution (both 50 mg/mL in methyl acetate, ≥98% purity, Cayman Chemical) by LC-MS. Latanoprost and latanoprost acid standards were used to form standard curves, which were used to quantify the concentrations of latanoprost and latanoprost acid in experimental samples.
Data were acquired on a Maxis Impact q-TOF mass spectrometer (Bruker Corporation, Billerica, MA) in combination with an Agilent 1200 HPLC, using LC-MS. An isocratic elution of water:acetonitrile:formic acid (45:55:0.05% v/v/v) was used at 200 µL/min flow rate. A Gemini Phenomenex 2.0 mm (5 µm particle size, 80 E pore size, 10 cm length; Phenomenex Inc., Torrance, CA) in combination with a 5 cm pre-column was used for the high-performance liquid chromatography (HPLC) separation. 0.1% formic acid was added to the sample solution to facilitate chromatographic elution, resulting in an acidic mobile phase (pH 4.0). Samples were run in positive ion mode to detect latanoprost and the same HPLC method was run in negative ion mode to monitor for latanoprost free acid [12]. The extracted ion currents with a threshold of ±0.005 Da for expected ions were used to monitor for the presence of the expected ions of unmodified latanoprost and latanoprost free acid.
2.6. Residual solvent analysis
When an organic solvent is used in the production product of a drug product, the FDA recommends weight loss analysis for initially measuring the mass of the residual solvent [13]. We sought to determine the percent weight of residual ethyl acetate in the drug–polymer films using thermogravimetric analysis (TGA). Films at various stages of production (solvent evaporation, desiccation, or lyophilization) were removed from the contact lens material and cut into quadrants. Under a flow of nitrogen at 20 L per minute, film sections were heated from 30 °C to 350 °C at an increase of 10 °C/min and analyzed using a thermogravimetric analyzer (Pyris 1, Perkin Elmer, Waltham, MA). Four samples were run for each time point.
Gas chromatography (GC) was used to determine the amount of residual solvent released from the CLs at various time points. CLs were incubated in PBS as described previously. Release media was collected daily for analysis and it was combined with dichloromethane in a 1:1 weight ratio to extract ethyl acetate. A constant amount of dodecane (2000 parts per million) was added to each extraction sample to correct for any injection volume variability. Extraction samples were analyzed using an Agilent 7890A gas chromatograph with 10 m DB-1 column. One µL was injected and heated at 35 °C for 2 min and then raised to 250 °C at a rate of 50 °C/min. Retention time was measured using a flame ionization detector. Ethyl acetate in release media was calculated by comparing the ratio of the area under the curve (AUC) for the ethyl acetate peak to the AUC for dodecane in the sample and comparing this ratio to that of reference standards. Latanoprost solution (0.005%) was extracted and analyzed in the same manner.
2.7. Cytotoxicity
Latanoprost, residual solvent, or polymer breakdown products could potentially result in local toxicity. Therefore, cell culture studies were conducted to investigate this possibility prior to performing animal studies. Immortalized human corneal limbal epithelial (HCLE) cell lines were a generous gift provided by Dr. Ilene Gipson of Harvard Medical School. As previously described [14], the HCLE cells were grown in T75 flasks and maintained in keratinocyte serum-free medium supplemented with 1% penicillin–streptomycin, 25 µg/mL bovine pituitary extract, 0.2 ng/mL epidermal growth factor, and 0.4 mm calcium chloride dihydrate (Life Technologies, Grand Island, NY).
HCLE were plated on the bottom surface of 3.0 µm pore size Transwell® plates (Corning Life Sciences, Corning, NY). Cell density in all experiments was 1 × 105 cells/mL. CL85:15, 45 were sterilized by UV light and placed in the Transwell® inserts. Commercial contact lenses (Kontur Kontact Lens Company, Hercules, CA) composed of the same hydrogel (methafilcon) as the CL85:15, 45 were purchased in a sterile condition and also placed in Transwell® inserts. After 24 h of exposure, cell viability was assessed using a commercially-available colorimetric assay (MTT viability assay kit, CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay, Promega Corporation, Madison, WI) after 24 h of exposure. Results were reported as means ± standard deviations of measured absorbance normalized to the absorbance for non-treated control cells (% normalized cell viability = 100 × absorbance for cells treated with a CL/absorbance for non-treated cells).
2.8. In vivo drug absorption and biocompatibility studies
Because in vitro drug release studies can be poorly correlated with in vivo drug release performance, we investigated the ability of the contact lens to elute latanoprost safely and effectively in New Zealand white rabbit eyes. This species is commonly used to study the safety of contact lenses given that the size and structure of the animals’ eyes are similar to that of human eyes [15]. Since latanoprost does not induce a reduction in IOP in rabbits [11], we studied the drug flux from the CL into the aqueous humor of the eye.
The study was approved by the animal care committee of the Massachusetts Eye and Ear Infirmary and the procedures conformed to principles of animal treatment described in the Statement for Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology. In all animals, only the left eye was studied.
The concentration of latanoprost in the aqueous humor was quantified using the same EIA employed in the in vitro study. Given that the EIA is based on competitive binding of anti-rabbit immunoglobin, the manufacturer of the assay recommended filtration of the rabbit fluid samples to remove rabbit antibodies prior to EIA analysis. Filtration was performed using a microelution plate (Oasis HLB 96-well µElution Plate 5 µm, Waters Corporation). To validate our filtration process, we analyzed pristine aqueous humor samples before and after filtration. On EIA analysis, the microelution plate was found to remove approximately 97% of the rabbit antibodies. The remaining rabbit antibodies resulted in a background signal that corresponded to 0.24 ± 0.13 ng/mL of latanoprost. This background proved to be negligible in our study (see Results). To ensure adequate retention of a contact lens throughout the four-week study period, a partial permanent lateral tarsorrhaphy was performed and the 3rd eyelid, known as the nictitating membrane, was kept intact. The CLs were hydrated in sterile saline for about 30 min prior to placement. Under anesthesia (intramuscular injection of ketamine [35 mg/kg] combined with xylazine [10 mg/kg]), a CL was placed onto the cornea, under the nictitating membrane. We studied 3 different formulations of CLs (Table 2). One of the formulations underwent pre-conditioning to reduce the initial burst release that has been reported in analogous drug delivery systems [16]. Preconditioning was done by submerging the CLs in 5 mL of PBS solution at 37 °C under gentle rotation for 1 or 3 days with daily changes in PBS.
Table 2.
Aqueous humor latanoprost concentrations after application of latanoprost solution or latanoprost-eluting contact lenses.
| Solution | Contaact lens formulations | ||||||
|---|---|---|---|---|---|---|---|
| No pre-conditioning | After pre-conditioning | ||||||
| 1 Day | 3 Days | ||||||
| CL65:35, 20 | CL65:35, 40 | CL85:15, 45 | CL85:15, 45 | CL85:15, 45 | |||
| Cmax (ng/mL) | 54 ± 19 | Cmax (ng/mL) | 970 ± 1028 | 854 ± 387* | 1473 ± 892* | 74 ± 48 | 46 ± 24 |
| Cave (ng/mL) | 12.8 ± 3.4 | Css (ng/mL) | 5.6 ± 8.2 | 39.6 ± 24.2* | 21.0 ± 2.4* | 5.5 ± 2.4* | 5.7 ± 1.9* |
Cave = average hourly concentration, Css = average steady state concentration, Cmax = maximum concentration, data are means ± standard deviations.
p < 0.05, compared to latanoprost solution by t-test.
During one month of contact lens wear, the eyes were assessed each day for tearing, discharge, blepharospasm (twitchy and forceful blinking of the eyelids), ptosis (eyelid drooping), and conjunctival redness, which are all signs of ocular infection or discomfort. At predetermined time periods, the eyes of anesthetized (see above) rabbits were examined under the operating microscope. While under anesthesia, 100 µL of aqueous humor was sampled to study the drug flux into the eye. To accomplish this, the contact lens was slid to the side of the cornea and a 30-gauge needle was inserted through the superior cornea in a manner that created a self-sealing wound. Aqueous humor samples were filtered, and quantified by the EIA method described above.
We sought to compare the aqueous humor concentrations achieved by the CL and that of topically applied 0.005% latanoprost solution, which is used clinically once a day. Therefore, we applied a drop of 0.005% latanoprost solution to the left eye and then sampled the aqueous humor 1, 3, 6, 12, and 24 h post-administration. The measured latanoprost concentration was plotted against time, and the area under the curve to any given time point (e.g. AUC0–24 h), was calculated using the trapezoid rule [17], using the equation:
AUC = § (Ci + Ci+1)(tx+1 − tx)
where tx+1 and tx are the times of aqueous humor sampling and Ci + Ci+1 are the corresponding sample concentrations. The average hourly concentration (Cave) achieved in the aqueous humor latanoprost drop was calculated by dividing the AUC0–24 h by 24.
2.9. Statistical analysis
Data from drug-release studies and cytotoxicity studies are presented as means ± standard deviations. Differences between groups in the thermogravimetric analysis were determined using a one-way ANOVA and Student’s unpaired t-test. Cytotoxicity data were analyzed using a Student’s unpaired t-test.
3. Results
3.1. The latanoprost-eluting contact lens
Latanoprost-eluting contact lenses (CLs) were produced as described in Methods. They contained blank or drug–polymer films with a range of PLGA compositions and thicknesses (Table 1). Lenses are described as CLxx:yy, nn, where xx:yy is the ratio of lactide to glycolide in the drug–polymer film, and nn is the film thickness in micrometers. Films in the CL65:35, 20 were thinner than in CL65:35, 40 and CL85:15, 45, as they were made with a solution that contained half the amount of drug and polymer. The CL65:35, 20 films contained approximately 89 µg of latanoprost and the thicker films, CL65:35, 40 and CL85:15, 45, contained approximately 178 µg. All of the films were translucent and had a 9 mm outer diameter and a 3 mm central aperture. They were entirely encapsulated in methafilcon and then lathed into the shape of contact lenses (Fig. 1). In the dry state, the lenses measured 12.0 mm in diameter and approximately 300 µm in central thickness. The dimensions of the polymeric films were unaffected by incorporation into the lenses. After immersion in PBS for 20 min, the outer diameter of the CL increased to 15 mm, the outer diameter of the drug–polymer film to 12 mm, and the film’s central aperture to 4 mm.
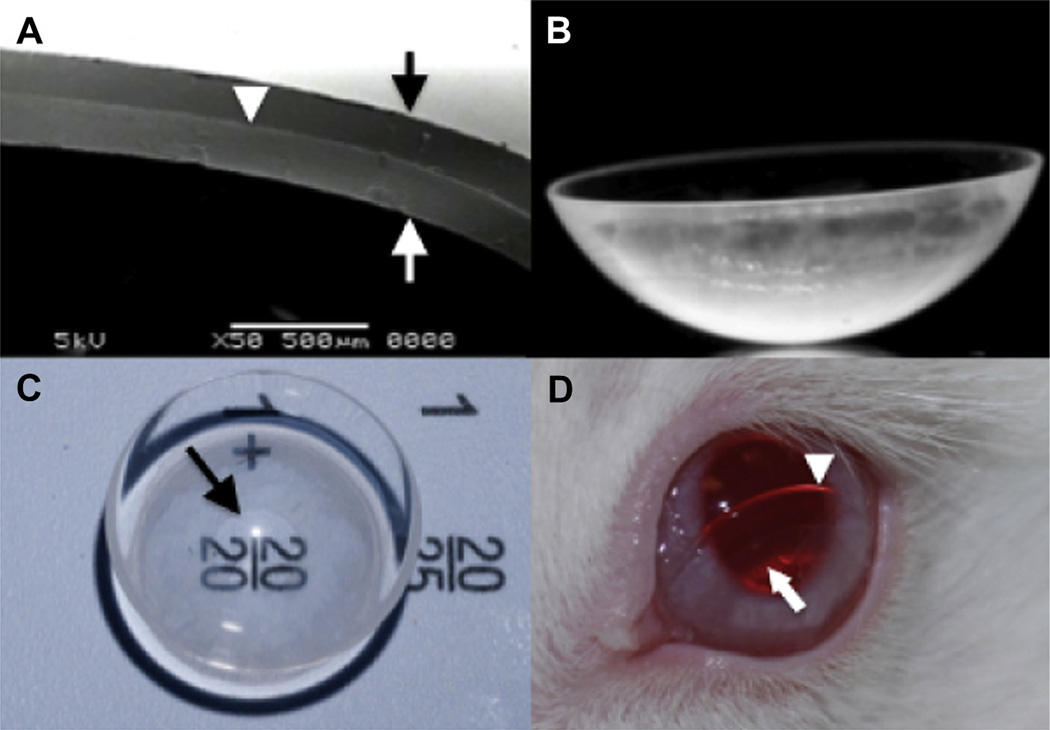
Fig. 1.
The latanoprost-eluting contact lens. (A) Representative scanning electron microscope (SEM) image of a cross-section of the lens demonstrated the drug–polymer film (white arrowhead) located between the methafilcon hydrogel’s outer surface (black arrow) and inner surface (white arrow). (B) Photograph from side view. (C) Top view shows a clear central aperture surrounded by a translucent ring of drug–polymer film. Arrow points to the inner margin of the drug polymer film. (D) The contact lens on the surface of a rabbit’s eye. The lens was intentionally displaced inferiorly for better visualization of the lens edge (white arrowhead) and inner diameter of the drug–polymer film (white arrow).
3.2. In vitro drug release studies
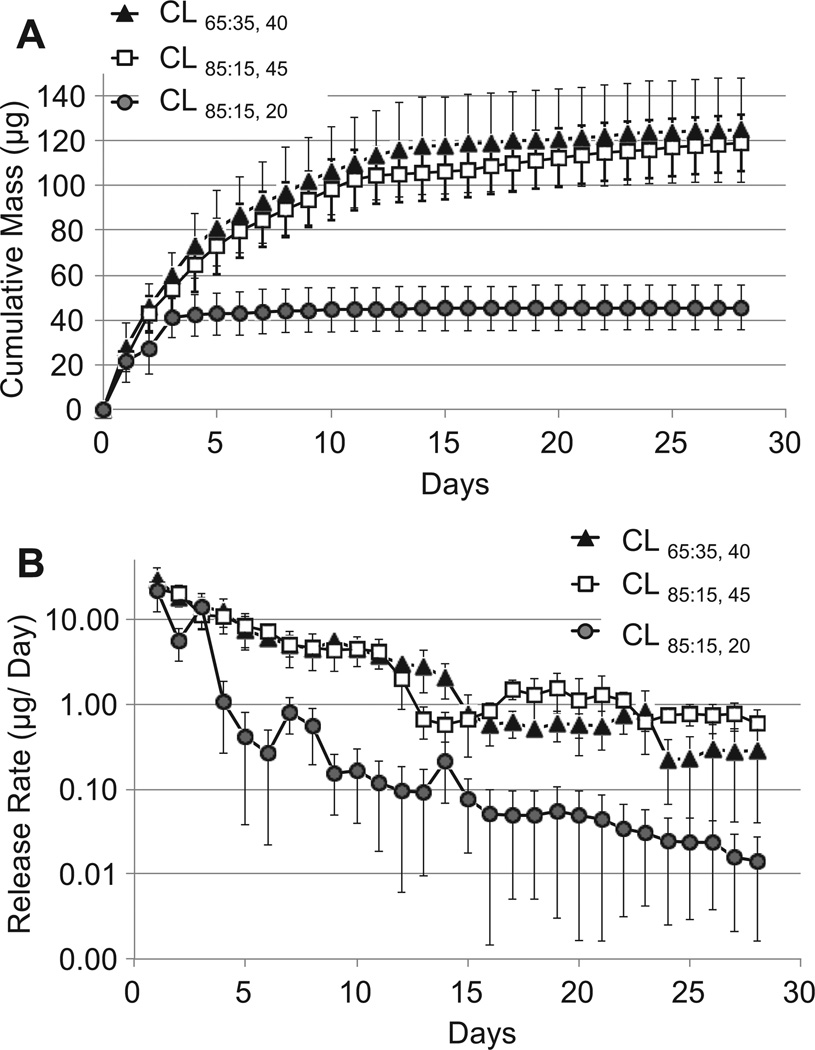
CLs with drug–polymer films of differing compositions and thicknesses (CL65:35, 20, CL65:35, 40, CL85:15, 45) were individually immersed in PBS incubated at 37 °C under constant agitation. The PBS was changed daily and the concentration of latanoprost in the removed PBS was measured by an enzyme immunoassay (EIA). All lenses demonstrated an initial burst release followed by slower release during the 4-week study period (Fig. 2). In the CL65:35, 20 group, 90% of the total amount of drug was released during the first 3 days. In comparison, lenses containing a thicker film (CL65:35, 40 and CL85:15, 45) demonstrated a smaller burst (48% and 45%, respectively) during the same period. Following the initial burst, CL65:35, 40 and CL85:15, 45 achieved a daily release rate that was, on average, more than ten times that of the CL65:35, 20 group (Fig. 2b), which contained only half as much latanoprost. The release profiles of the CL65:35, 40 and CL85:15, 45 were similar.
Fig. 2.
In vitro release from latanoprost-eluting contact lenses. (A) Cumulative mass released. (B) Semi-log plot of the release per day, using the same data as in panel A. Data are means ± standard deviations (n = 4).
3.3. Drug stability
The contact lenses were designed to release latanoprost, an isopropyl ester prodrug that is hydrolyzed to its biologically active form, latanoprost acid, as it passes through the cornea into the aqueous humor [11]. Clinically, latanoprost is administered topically instead of latanoprost acid since the latter is irritating, causes redness of the conjunctiva, and has difficulty penetrating the cornea [11]. Environmental conditions, such as heat and UV light exposure, have been shown to degrade latanoprost into latanoprost acid, the most abundant degradation product of latanoprost [12,18,19]. Here, we analyzed the stability of latanoprost incorporated into and eluted from a CL85:15, 45 (over 24 h), and compared it to unencapsulated commercial latanoprost solution (50 µg/mL), using high-performance liquid chromatography-mass spectrometry (LC-MS). In particular, we wished to determine whether the production process resulted in the conversion of latanoprost to latanoprost acid.
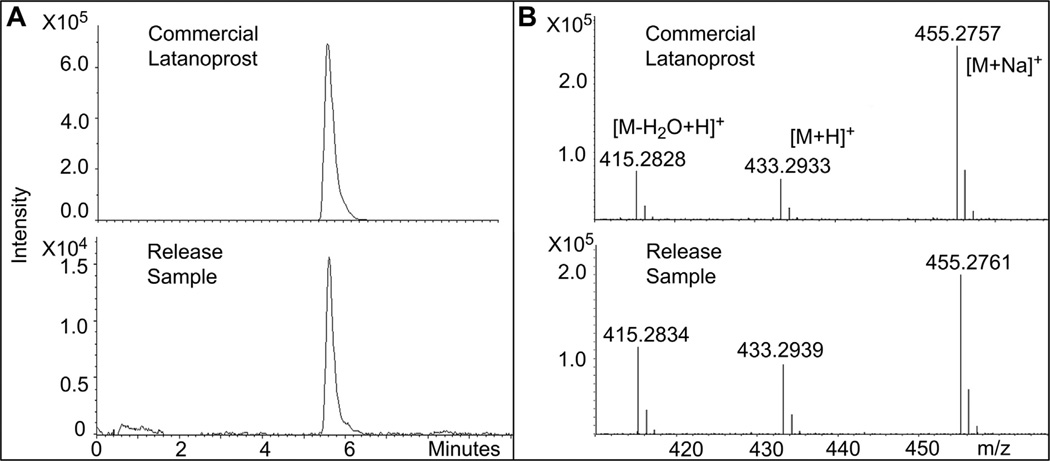
Commercial latanoprost solution (Bausch and Lomb), CL85:15, 45 release media (day 1), and latanoprost standard (98% purity, Cayman Chemical) demonstrated substantially similar high-resolution spectra (Fig. 3). The concentrations of latanoprost and latanoprost acid in commercial latanoprost and in the release media were quantified using standard curves derived from reference standards of latanoprost and latanoprost acid. The lower limits of detection of latanoprost and latanoprost acid were 10 ng/mL and 1 ng/mL, respectively. The measured latanoprost concentration in the day 1 release media from four CL85:15, 45 lenses was 1.743 ± 0.815 µg/mL (range 0.637–2.593) and was similar to the amount measured by EIA (0.991 ± 0.046, range 0.928–1.012; p = 0.11). The concentration of latanoprost measured in commercial latanoprost solution (0.005%; 50 µg/mL) was 57.52 µg/mL. Other researchers have obtained similar measurements with commercial latanoprost (e.g. 57.7 ± 3.2 µg/mL) [20]. Latanoprost acid was detected in both release media and commercial latanoprost (0.037 ± 0.008 µg/mL and 1.03 µg/mL, respectively), but at concentrations that were far below those of latanoprost. The ratio of latanoprost to latanoprost acid was similar in the release media (3.0% ± 2.0%, range 0.9–6.7%) and commercial latanoprost (1.7%). The LC-MS results suggest that the active ingredient eluted from the CLs was latanoprost, as found in commercial latanoprost solution.
Fig. 3.
High-performance liquid chromatography-mass spectrometry (LC-MS) of latanoprost samples. Media containing latanoprost released over 1 day from a CL85:15, 45 demonstrated ion chromatograms (A) and annotated high-resolution mass spectra (B) that were substantially similar to those of unencapsulated commercial latanoprost. The mass spectra of both samples generated the same fragmentation pattern of the protonated ion [M + H]+ at m/z 433.29 for latanoprost, and produced abundant fragment ions at m/z 415.28 and 455.27, corresponding to a loss of water [M-H2O + H]+ and a sodium adduct [M + Na]+, respectively. m/z = mass to charge ratio.
3.4. Residual solvent analysis
Given the intended use of the CL for extended wear, it was also important to study the residual solvent in the formed lenses, and its elution. The quantity of residual solvent was assessed by thermogravimetric analysis of PLGA films with and without latanoprost, made with the same mass of polymer (85:15 PLGA) and volume of ethyl acetate. The mass of both sets of PLGA films decreased in the temperature range of 40 °C–120 °C, then decreased sharply to a small percentage of the original mass at 250 °C. The initial decrease in mass was not seen in pellets of 85:15 PLGA that had not been exposed to solvents, suggesting that the weight loss was attributable to the evaporation of residual ethyl acetate (boiling point of 77.1 °C). Both the latanoprost–PLGA films and the PLGA pellets exhibited the loss of mass at 250 °C, which could be attributed to degradation of the polymer [21].
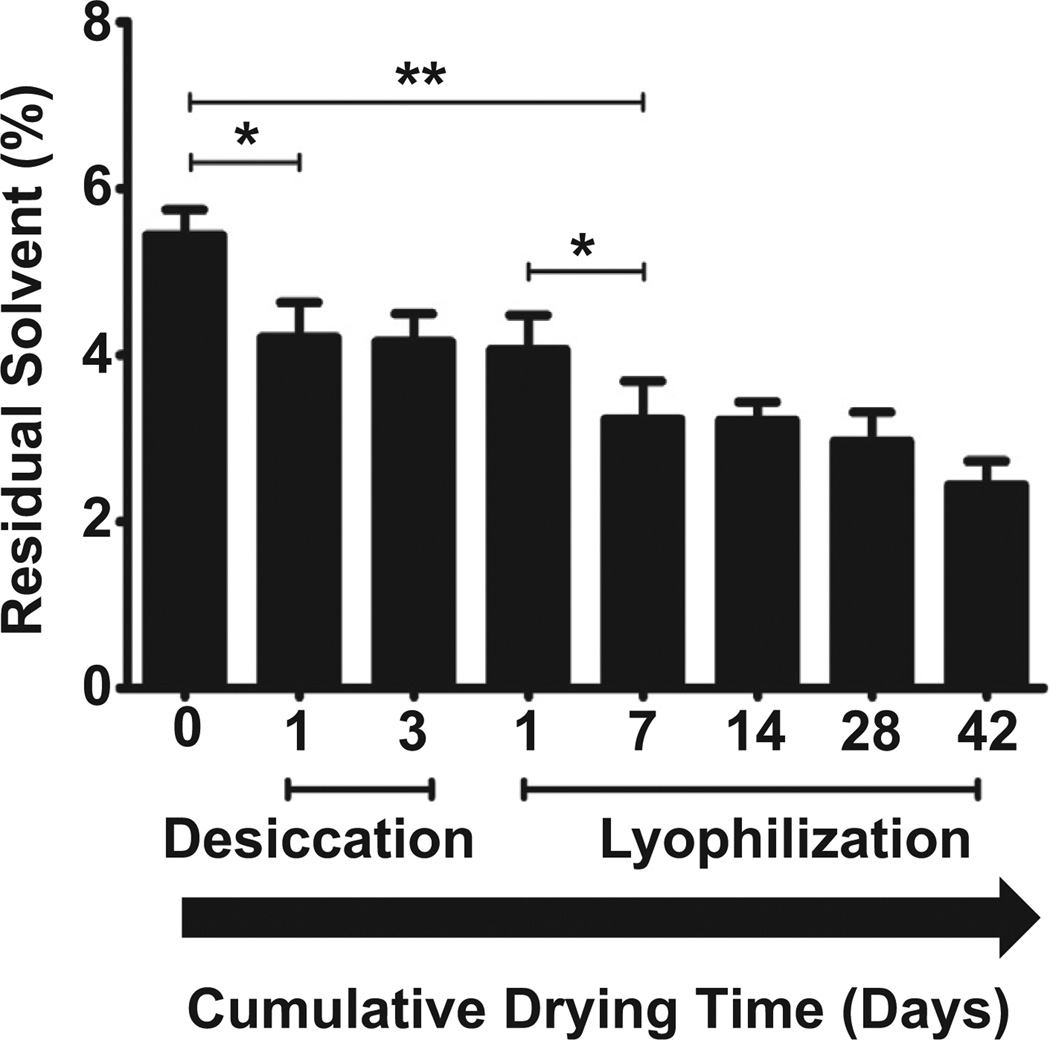
The amount of residual solvent in latanoprost–PLGA films was determined by measuring the loss of mass from 40 °C to 120 °C. Latanoprost–PLGA films were analyzed immediately after formation, or after one or three days of desiccation, or after 3 days of desiccation followed by lyophilization for 1 day to 42 days (Fig. 4). Thermogravimetric analysis revealed that residual solvent accounted for 5.4 ± 0.3% of the drug–polymer films’ masses after formation. Films that underwent desiccation for one day demonstrated significantly less residual solvent (3.2 ± 0.5%, p < 0.05). There was little change in solvent content after desiccating for 3 days or with only one subsequent day of lyophilization. Additional lyophilization resulted in further solvent loss.
Fig. 4.
Residual solvent (as % of total film mass) determined by thermogravimetric analysis of CL85:15, 45 lenses, before and after desiccation with or without subsequent lyophilization. Data are means ± standard deviations (n = 4). *p < 0.05, **p < 0.005.
The amount of residual ethyl acetate eluted from the was measured by gas chromatography. CL85:15, 45 were fabricated containing latanoprost–polymer films that were desiccated for three days after solvent casting, and then lyophilized for one day prior to encapsulation in methafilcon. They were submerged in PBS, which was changed daily. Aliquots from each day (n = 4) were stored in a sealed container at 4 °C until the time of analysis by gas chromatography. (To ensure that latanoprost did not interfere with the measurement of ethyl acetate, we also analyzed an unmodified commercial latanoprost solution [50 µg/mL] that lacked ethyl acetate. We found that latanoprost did not demonstrate a peak at the retention time that corresponded to ethyl acetate [1.1 min] and therefore would not influence the measurement of the solvent.) Gas chromatography detected ethyl acetate in the CL85:15, 45 release media at just above the limit of detection (100 parts per million [ppm]) in 50%, 50%, 25%, 25%, and 0% of the samples from release days 1–5, respectively. When detected, the average concentration measured was 120 ± 20 ppm (range 103–160).
3.5. Cytotoxicity
Cytotoxicity could result from eluted residual solvent, a burst of latanoprost, or other factors. Immortalized human corneal limbal epithelial (HCLE) cells were used for the cytotoxicity assay; that cell line has previously been used to evaluate the biomaterial toxicity of ocular devices [22] and chemicals intended for ocular exposure [23]. Cytotoxicity was assessed by incubating HCLE cells in media that contained a Transwell® insert, which held a commercial methafilcon contact lens (Kontur 55, Kontur Kontact Lens Company), a CL85:15, 45, or vehicle (media only, no contact lens). After 24 h of exposure, cellular viability was assessed using an MTS colorimetric assay. Compared to cells that were exposed to media only, commercial contact lenses and CL85:15, 45 demonstrated cell viabilities of 115.9 ± 4.9% (p < 0.05 compared to media only, Student t-test) and 111.27 ± 9.47% (p = 0.09), respectively. The viability of cells exposed to the commercial contact lens or CL85:15, 45 was not statistically significantly different.
3.6. In vivo drug absorption and biocompatibility studies
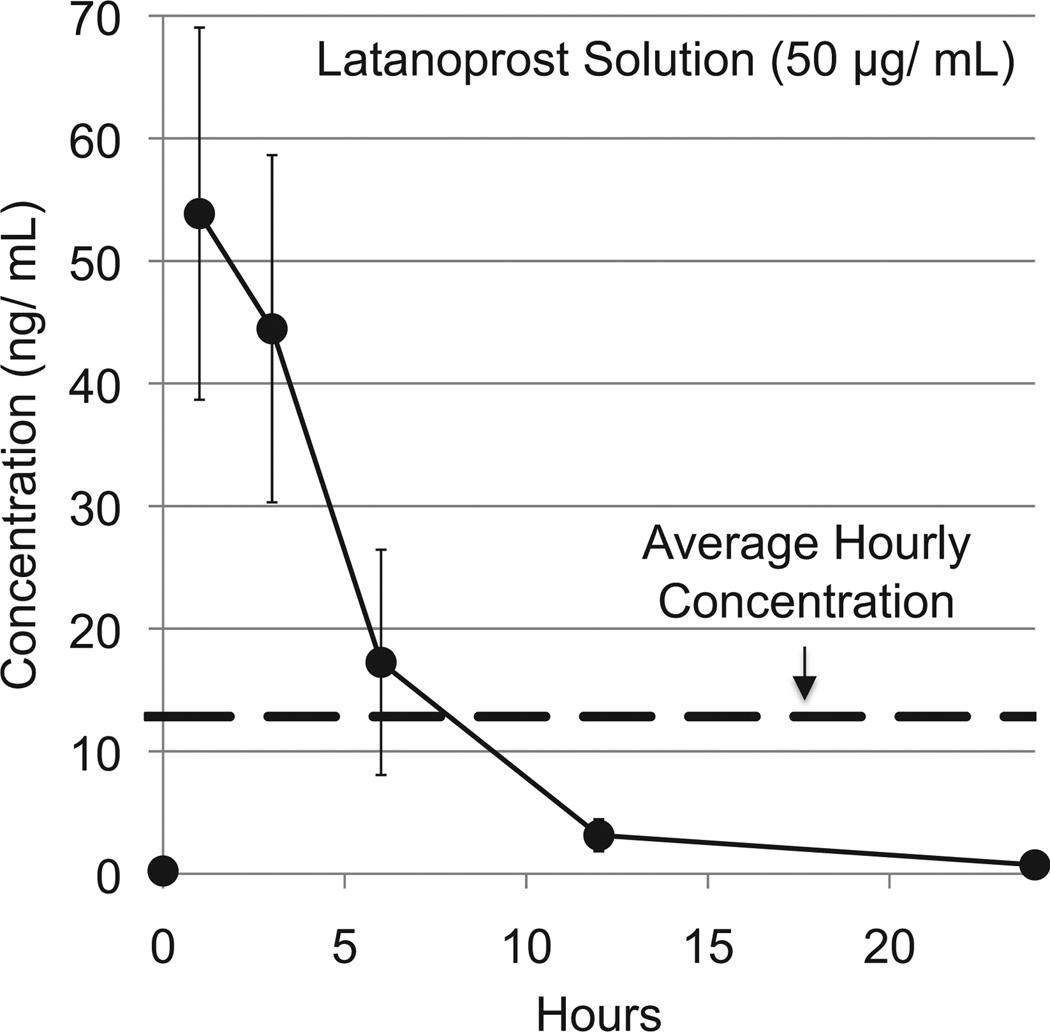
We compared the aqueous humor concentrations achieved by continuous wear of CLs and by the current clinical therapy of one drop of 50 µg/mL latanoprost solution once per day. After a 30 µL drop of latanoprost solution was applied to one eye of a New Zealand white rabbit, we measured the aqueous humor concentrations at predetermined time points over a 24-h period (Fig. 5). A maximum concentration (Cmax) of 54 ± 19 ng/mL was measured 1 h after drop administration. The concentration vs. time curve was found to be consistent with previous studies that demonstrated rapidly declining concentrations over the first 12 h and little, if any, drug present after 24 h [24,25]. The area under the curve (AUC0–24) was calculated by the trapezoidal rule (see Methods). Dividing the AUC0–24 by 24 provided an average hourly concentration (Cave) of 12.8 ± 3.4 µg/mL of latanoprost from a single drop (Table 2).
Fig. 5.
Concentration of latanoprost in aqueous humor after topical application of a drop of latanoprost solution (50 µg/mL). Data are means ± standard deviations (n = 4).
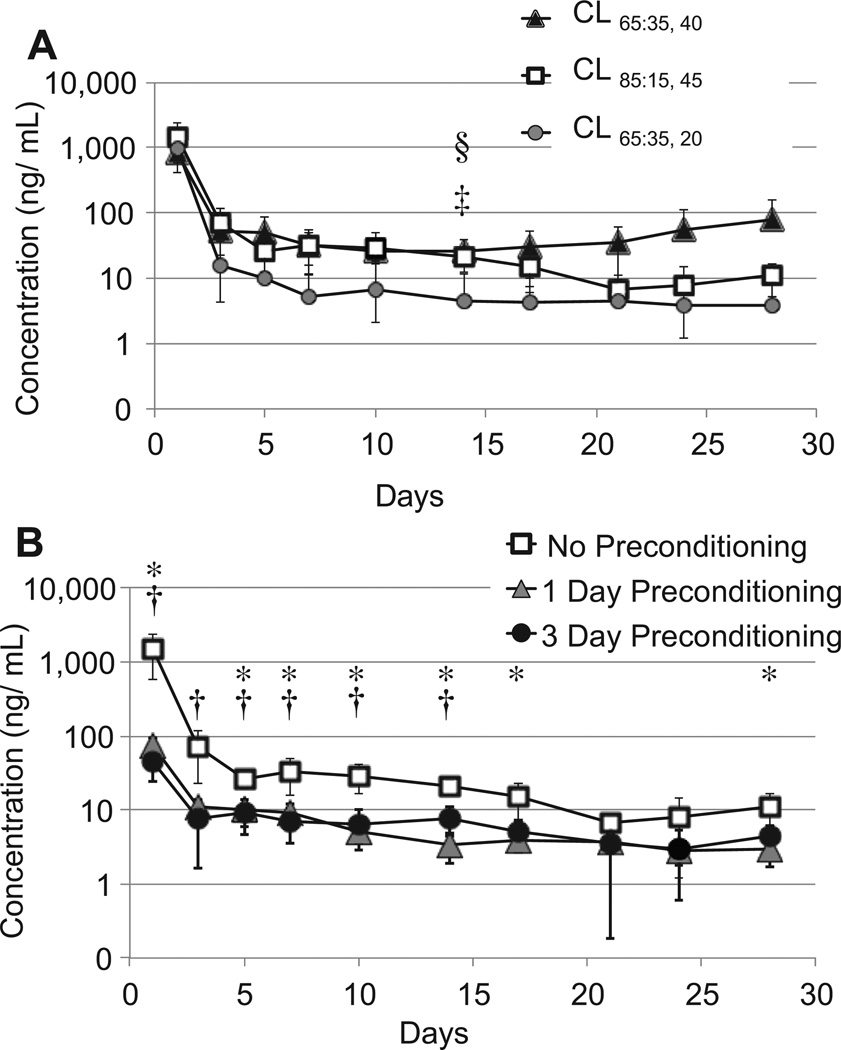
Three types of CLs (CL65:35, 20, CL65:35, 40, CL85:15, 45) were worn continuously by rabbits for one month (Fig. 1). Care was taken to insert the lenses under the rabbits’ 3rd eyelid, or nictitating membrane. The aqueous humor was sampled on predetermined days throughout 4 weeks of continuous lens wear. The concentration of latanoprost in processed aqueous humor (see Methods) was measured with an enzyme immunoassay (EIA; Fig. 6).
Fig. 6.
Concentrations of latanoprost in aqueous humor in rabbits wearing latanoprost-eluting contact lenses (CL). A. Latanoprost concentrations from CLs without pre-conditioning. B. Latanoprost concentrations from CL85:15, 45 with and without pre-conditioning for 1 or 3 days. Statistically significantly differences between groups were detected by unpaired Student’s t-tests. In panel A, §p < 0.05, CL65:35, 40 vs. CL65:35, 20, ‡p < 0.05; CL85:15, 45 vs. CL65:35, 20, in panel B, *p < 0.05, no preconditioning vs. 1 day preconditioning; †p < 0.05, no preconditioning vs. 3 day preconditioning. Data are means ± standard deviations (n = 4).
All 3 sets of lenses released a burst of latanoprost into the aqueous humor on day 1 that was significantly higher than the Cmax of latanoprost solution (Table 2). CL65:35, 40 and CL85:15, 45 exhibited substantially similar release profiles (Fig. 6). CL65:35, 20, which contained thinner drug–polymer films, delivered less drug to the aqueous humor following the initial burst, but this difference was only statistically significant on day 14. All of the lenses achieved steady state from day 3–28 (Table 2). Over this period, the steady state aqueous humor concentrations (Css) of latanoprost from CL85:15, 45 and CL65:35, 40 were significantly greater (p < 0.05) than the Cave of latanoprost solution (Table 2).
To eliminate the initial burst, CLs (CL85:15, 45) were preconditioned by submerging the lenses in 5 mL of PBS for 1 or 3 days prior to the in vivo study. Preconditioned lenses demonstrated a significantly smaller initial burst in the aqueous humor than lenses without pre-conditioning (p < 0.05, Fig. 6B). CLs that were preconditioned for 1 and 3 days demonstrated similar release kinetics with no statistically significant differences in the burst or steady state concentrations (Table 2). In general, lenses that were not preconditioned exhibited a higher steady state concentration than those that were preconditioned (Fig. 6B).
None of the study eyes demonstrated any tearing, discharge, blepharospasm, ptosis, or other signs of discomfort. The majority of the CLs appeared to be well-fitting and were centered over the cornea. The lenses used here moved slightly with each blink. In approximately 25% of the eyes, the fit of the contact lenses may not have been ideal as the lenses moved inferiorly away from the center. Under microscopic examination, some of those eyes developed a temporary indentation line along the surface of the corneal epithelium where the edge of the contact lens apposed the cornea. Some of these same eyes eventually developed 1–2 mm of corneal neovascularization. No abrasions or breaks in the epithelium were noted in any of the eyes.
4. Discussion
We have developed a drug-eluting contact lens capable of delivering a glaucoma drug to the eye in a sustained manner for at least four weeks. Drug release is controlled by encapsulation of a thin drug–polymer film within a commonly used contact lens material [7]. In our previous publications, we demonstrated that this design can be used to release an antibiotic or an antifungal agent for weeks to months at a time [7,8]. Here, we incorporated a glaucoma drug, latanoprost, into the contact lens and demonstrated that a therapeutic amount of the drug can be released for 4 weeks in vivo.
The amount of drug delivered to the eye exceeded or was comparable to that delivered by topical drops. CLs with polymer–drug films 40–45 µm in thickness demonstrated an initial burst of latanoprost in the aqueous humor followed by a steady state concentration (Css) that was comparable to the average hourly concentration (Cave) delivered from a drop of commercially-available latanoprost solution (50 µg/mL). For example, the Css for CL85:15, 45 lenses was between the Cave and the Cmax for a latanoprost drop (Table 2). The Css of the CLs depended on the type of polymer–drug film they contained.
All of the CLs demonstrated an initial burst in vitro and in vivo. Although latanoprost has a high therapeutic index with respect to systemic and local side effects, conjunctival hyperemia (redness) and local toxicity are seen at high doses in humans [11]. Therefore, we attempted to reduce the burst by pre-conditioning the CLs in vitro in PBS for 1 or 3 days prior to applying the lenses in vivo. The smallest burst was seen in CLs that were preconditioned for the longest time. However, the preconditioned CLs exhibited Css that were lower than those from non-preconditioned CLs (Fig. 6). While commercial latanoprost solution is only available in the United States at concentrations of 50 micrograms per mL, it bears mentioning that latanoprost has been found to be clinically effective at lower doses in animals [26] and in humans [27]; and the Cmax, Css, and/or Cave needed for latanoprost drops to be optimally effective is not known. For example, a study comparing the ocular pharmacokinetics and pharmacodynamics from two different topical formulations found both to be equally efficacious clinically despite the fact that they differed considerably in Cmax and AUC [28]. It was not possible to determine the therapeutic efficacy of our lenses in these experiments because latanoprost does not cause a reduction in intraocular pressure in rabbits [11]. Therefore, efficacy studies would have to be conducted in other animals such as canines or non-human primates [11].
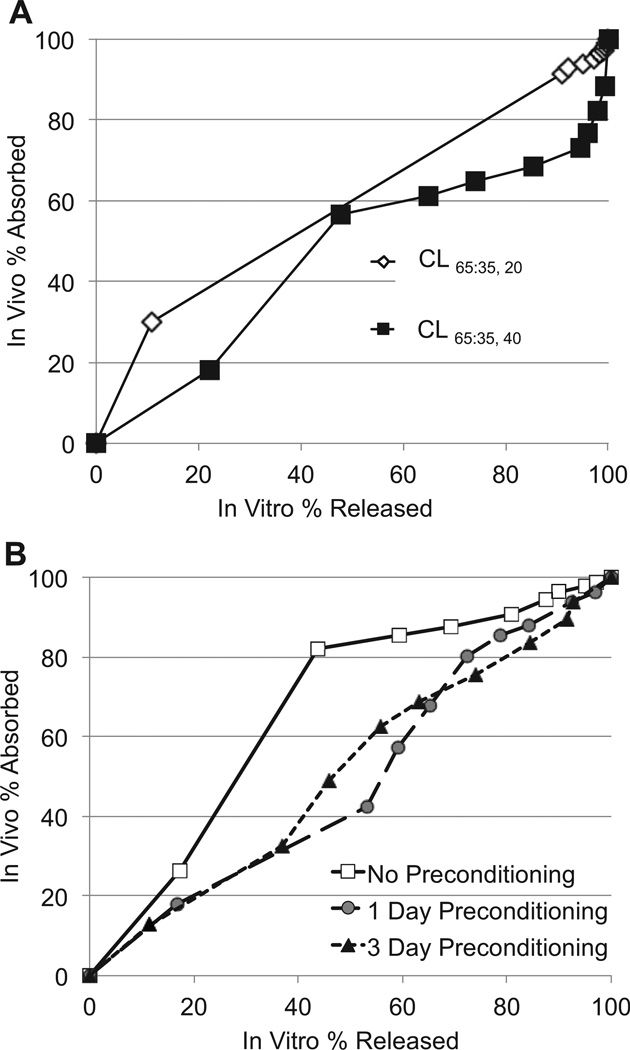
It is interesting to note that in these experiments the in vitro drug release correlated well with aqueous humor absorption in vivo. The in vitro–in vivo (IVIV) relationship is shown in a Levy plot [29,30] which shows, for each time point for 28 days, the percentage of total drug release in vitro against the percentage of total drug absorption in vivo (percentage of AUC0–28 days) (Fig. 7). All lenses had high correlation coefficients between drug release in vitro and drug absorption in vivo. When we analyzed the IVIV relationship of the preconditioned CLs, we found that the CLs that were preconditioned for the longest duration (3 days) demonstrated the most linear relationship between in vitro and in vivo data (R2 = 0.98). The linear IVIV relationship observed here suggests that the in vitro drug release study was predictive – at least in trend – of the in vivo absorption of latanoprost into the aqueous humor from the CL.
Fig. 7.
Levy plots for the relationship between the percentage of latanoprost absorbed into aqueous humor in vivo over 28 days and the percentage of drug released in vitro over 28 days. A. Levy plots for CL65:35, 20 (R2 = 0.98) and CL65:35, 40 (R2 = 0.93) without pre-conditioning. B. Levy plots for CL85:15, 45 without pre-conditioning (R2 = 0.0875) and with pre-conditioning for 1 day (R2 = 0.979) or 3 days (R2 = 0.982). R2 = correlation coefficients.
As is the case with many drug delivery systems [9], an organic solvent was used in the production of the CLs. A latanoprost–PLGA film was formed through evaporation of ethyl acetate and then encapsulated in methafilcon. The Occupational Health and Safety Administration indicates that eye irritation can occur in the presence of ethyl acetate vapors above 400 ppm [31]. Therefore, we quantified the amount of residual ethyl acetate contained within and released from the CL. Thermogravimetric analysis revealed that residual solvent was present in the drug polymer film and that the amount present could be reduced by lyophilization. With a detection limit of 100 ppm, gas chromatography failed to detect any ethyl acetate in most of the release media over the first 4 days of release, and no ethyl acetate was found in any of the samples after 4 days of release. When detected, the solvent levels were well below the Occupational Health Safety Administration exposure limits.
The CLs appeared safe in cell culture and on rabbit eyes. The CL and its eluted products did not demonstrate any evidence of cytotoxicity in human corneal–limbal epithelial cells over 24 h. Our in vivo studies were performed in New Zealand white rabbits, which are commonly used for contact lens toxicology studies [15]. While worn for 28 days continuously, the eyes did not develop any signs of toxicity, such as conjunctival redness. We did note that the fit of approximately 25% of the contact lenses was not ideal and they became inferiorly displaced. This is not surprising given that there are subtle differences in the dimensions of human and rabbit corneas that can make placement and retention of the contact lens on the rabbit eye difficult [32]. Compared to humans, adult rabbit corneas have a steeper curvature (average radius of curvature is 7.2 mm in rabbits vs. 7.7–7.8 mm in humans), and a greater diameter (13.0–13.4 mm in rabbits vs. 10.5–11.8 mm in humans) [33]. In our study, we employed CLs that had outer curvatures, optical centers (inner curvatures), and diameters typical of commercial contact lenses intended for human use.
Since their introduction, soft contact lenses have been proposed as a means for ocular drug delivery [34], but controlling the drug release has been challenging. The drug-eluting contact lenses described here could expand the treatment options for glaucoma and other ocular diseases. Given that these contact lenses can release latanoprost for at least 4 weeks, they could be administered once a month instead of daily administration of latanoprost drops. This simplified treatment regimen could reduce the treatment burden of glaucoma therapy and help address the problem of patient non-adherence, which is notoriously problematic with glaucoma drops [3]. Difficulty with self-administration of glaucoma eye drops contributes to non-adherence [35]. About 20% of patients overcome the challenge of self-administration by relying on others to install their eye drops [36]; this patient population may benefit from a sustained-release contact lens since they would require assistance only once a month. Like drops, a contact lens could be also administered by a non-health care professional, such as a patient’s family member or friend.
5. Conclusion
We have developed a latanoprost-eluting contact lens that can deliver a therapeutic amount of drug into the eye for at least one month. Eluted latanoprost did not appear to be altered by the production and release processes. The contact lens appeared safe in both cell culture and animal studies. This contact lens design may offer an alternative to the current treatment of glaucoma and may serve as an ocular drug delivery platform capable of treating other eye diseases.
Acknowledgment
The authors wish to thank Professor Stephen L. Buchwald for use of gas chromatograph equipment and Pedro L. Arrechea for assistance with gas chromatography experiments. This research was funded by NEI 1K08EY019686-01 (JBC), Massachusetts Lions Eye Research Fund (JBC), New England Cornea Transplant Fund (JBC), NIGMS GM073626 (DSK), Eleanor and Miles Shore Foundation (JBC), and by a Career Development Award from Research to Prevent Blindness, Inc., NY, NY (JBC).
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Ghate D, Edelhauser H. Barriers to glaucoma drug delivery. J Glaucoma. 2008;17(2):147. doi: 10.1097/IJG.0b013e31814b990d. [DOI] [PubMed] [Google Scholar]
- 3.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 4.Sleath B, Blalock S, Covert D, Stone JL, Skinner AC, Muir K, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengani LC, Hsu K-H, Gause S, Chauhan A. Contact lenses as a platform for ocular drug delivery. Expert Opin Drug Deliv. 2013 Jul 22; doi: 10.1517/17425247.2013.821462. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Ciolino JB, Dohlman CH, Kohane DS. Contact lenses for drug delivery. Semin Ophthalmol. 2009;24(3):156–160. doi: 10.1080/08820530902802161. [DOI] [PubMed] [Google Scholar]
- 7.Ciolino JB, Hoare TR, Iwata NG, Behlau I, Dohlman CH, Langer R, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50(7):3346–3352. doi: 10.1167/iovs.08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciolino JB, Hudson SP, Mobbs AN, Hoare TR, Iwata NG, Fink GR, et al. A prototype antifungal contact lens. Invest Ophthalmol Vis Sci. 2011;52(9):6286–6291. doi: 10.1167/iovs.10-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 10.Berenson KL, Kymes SK, Hollander DA, Fiscella R, Burk C, Patel V. Cost-offset analysis: bimatoprost versus other prostaglandin analogues in open-angle glaucoma. Am J Manag Care. 2011;17(9):e365–e374. [PubMed] [Google Scholar]
- 11.Stjernschantz J. From PGF(2alpha)-isopropyl ester to latanoprost: a review of the development of xalatan: the Proctor lecture. Invest Ophthalmol Vis Sci. 2001;42(6):1134. [PubMed] [Google Scholar]
- 12.Mehta J, Patel V, Kshatri N, Vyas N. A versatile LC method for the simultaneous quantification of latanoprost, timolol and benzalkonium chloride and related substances in the presence of their degradation products in ophthalmic solution. Anal Methods. 2010;2(11):1737–1744. [Google Scholar]
- 13.Convention USP. Chemical tests/chapter (467) organic volatile impurities. Rockville, MD: United States Pharmacopeia and National Formulary; 2007. [Google Scholar]
- 14.Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44(6):2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 15.ISO. 9394Ophthalmic optics – contact lenses and contact lens care products – Determination of biocompatibility by ocular study with rabbit eyes. 2nd ed. Geneva: 1998. [Google Scholar]
- 16.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2–3):121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 17.Riviere JE. Comparative pharmacokinetics. Wiley-Blackwell; 2011. [Google Scholar]
- 18.Morgan PV, Proniuk S, Blanchard J, Noecker RJ. Effect of temperature and light on the stability of latanoprost and its clinical relevance. J Glaucoma. 2001;10(5):401–405. doi: 10.1097/00061198-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Ochiai A, Danjo K. The stabilization mechanism of latanoprost. Int J Pharm. 2011;410(1–2):23–30. doi: 10.1016/j.ijpharm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Johnson TV, Gupta PK, Vudathala DK, Blair IA, Tanna AP. Thermal stability of bimatoprost, latanoprost, and travoprost under simulated daily use. J Ocul Pharmacol Ther. 2011;27(1):51–59. doi: 10.1089/jop.2010.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jose MV, Thomas V, Dean DR, Nyairo E. Fabrication and characterization of aligned nanofibrous PLGA/collagen blends as bone tissue scaffolds. Polymer. 2009;50(15):3778–3785. [Google Scholar]
- 22.Ament JD, Spurr-Michaud SJ, Dohlman CH, Gipson IK. The Boston Keratoprosthesis: comparing corneal epithelial cell compatibility with titanium and PMMA. Cornea. 2009;28(7):808–811. doi: 10.1097/ICO.0b013e31819670ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konynenbelt BJ, Mlnarik DS, Ubels JL. Effects of peroxide-based contact lens-disinfecting systems on human corneal epithelial cells in vitro. Eye Contact Lens. 2011;37(5):286–297. doi: 10.1097/ICL.0b013e3182182d02. [DOI] [PubMed] [Google Scholar]
- 24.Sjöquist B, Basu S, Byding P, Bergh K, Stjernschantz J. The pharmacokinetics of a new antiglaucoma drug, latanoprost, in the rabbit. Drug Metab Dispos. 1998;26(8):745–754. [PubMed] [Google Scholar]
- 25.Sjöquist B, Stjernschantz J. Ocular and systemic pharmacokinetics of latanoprost in humans. Surv Ophthalmol. 2002;47(Suppl. 1):S6–S12. doi: 10.1016/s0039-6257(02)00302-8. [DOI] [PubMed] [Google Scholar]
- 26.Friström B, Nilsson S. A double masked comparison of the intraocular pressure reducing effect of latanoprost 0.005% and 0.001% administered once daily in open angle glaucoma and ocular hypertension. Br J Ophthalmol. 1997;81(10):867. doi: 10.1136/bjo.81.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alm A, Villumsen J. PhXA34, a new potent ocular hypotensive drug: a study on dose-response relationship and on aqueous humor dynamics in healthy volunteers. Arch Ophthalmol. 1991;109(11):1564. doi: 10.1001/archopht.1991.01080110100045. [DOI] [PubMed] [Google Scholar]
- 28.Daull P, Buggage R, Lambert G, Faure M-O, Serle J, Wang R-F, et al. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther. 2012;28(5):515–523. doi: 10.1089/jop.2011.0245. [DOI] [PubMed] [Google Scholar]
- 29.Levy G. Effect of dosage form on drug absorption a frequent variable in clinical pharmacology. Arch Int Pharmacodyn Ther. 1964;152:59–68. [PubMed] [Google Scholar]
- 30.Levy G, Hollister LE. Inter- and intrasubject variations in drug absorption kinetics. J Pharm Sci. 1964;53:1446–1452. doi: 10.1002/jps.2600531203. [DOI] [PubMed] [Google Scholar]
- 31.Occupational health guidelines for ethyl acetate. Available from: http://www.cdc.gov/niosh/docs/81-123/pdfs/0260.pdf.
- 32.Green K, Edelhauser HF, Hackett RB. Advances in ocular toxicology. Springer; 1997. [Google Scholar]
- 33.Bozkir G, Bozkir M, Dogan H, Aycan K, Güler B. Measurements of axial length and radius of corneal curvature in the rabbit eye. Acta Med Okayama. 1997;51(1):9–11. doi: 10.18926/AMO/30804. [DOI] [PubMed] [Google Scholar]
- 34.Wichterle O, Lim D. Cross-linked hydrophilic polymers and articles made there from. 3,220,960. US Patent No. 1965
- 35.Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol. 2006;17(2):190–195. doi: 10.1097/01.icu.0000193078.47616.aa. [DOI] [PubMed] [Google Scholar]
- 36.Kass MA, Hodapp E, Gordon M, Kolker AE, Goldberg I. Patient administration of eyedrops: observation. Part II. Ann Ophthalmol. 1982;14(9):889–893. [PubMed] [Google Scholar]