Abstract
The palladium(II) bis-chelate complexes of the type [Pd(TSC1-5)2] (6–10), with their corresponding ligands 4-phenyl-1-(acetone)-thiosemicarbazone, HTSC1 (1), 4-phenyl-1-(2′-chloro-benzaldehyde)-thiosemicarbazone, HTSC2 (2), 4-phenyl-1-(3′-hydroxy-benzaldehyde)-thiosemicarbazone, HTSC3 (3), 4-phenyl-1-(2′-naphthaldehyde)-thiosemicarbazone, HTSC4 (4), and 4-phenyl-1-(1′-nitro-2′-naphthaldehyde)-thiosemicarbazone, HTSC5 (5), were synthesized and characterized by elemental analysis and spectroscopic techniques (IR and 1H- and 13C-NMR). The molecular structure of HTSC3, HTSC4, and [Pd(TSC1)2] (6) have been determined by single crystal X-ray crystallography. Complex 6 shows a square planar geometry with two deprotonated ligands coordinated to PdII through the azomethine nitrogen and thione sulfur atoms in a cis arrangement. The in vitro cytotoxic activity measurements indicate that the palladium(II) complexes (IC50 = 0.01–9.87 μM) exhibited higher antiproliferative activity than their free ligands (IC50 = 23.48–70.86 and >250 μM) against different types of human tumor cell lines. Among all the studied palladium(II) complexes, the [Pd(TSC3)2] (8) complex exhibited high antitumor activity on the DU145 prostate carcinoma and K562 chronic myelogenous leukemia cells, with low values of the inhibitory concentration (0.01 and 0.02 μM, resp.).
1. Introduction
In recent years, sulfur containing ligands such as dithiocarbamates and thiosemicarbazones and their transition metal complexes have received more attention in the area of medicinal chemistry, due to their pharmacological properties, such as antiviral [1–3], antibacterial [4–7], antifungal [8–10], antiparasitic [11, 12], and antitumor [13–19] activities.
The synthesis of thiosemicarbazones (R–CH=N–NH–CS–NHR1) has been developed due to the facility to replace the R and R1 substituent groups by alkyl, aryl, or heterocyclic derivative and thus leading to a broad spectrum of new bidentate (N,S or N,N) and tridentate (N,N,N or N,N,S) and also tetra- and pentadentate ligands, capable of coordinating to metal centres [6, 20–22].
It has been shown that the α-(N)-heterocyclic carbaldehyde thiosemicarbazones act as chelating agents of the transition metals and some of them exhibit antitumor activity by inhibiting the biosynthesis of DNA, possibly by blocking the enzyme ribonucleotide diphosphate reductase [23–25]. On the other hand, the ligand 6-methylpyridine-2-carbaldehyde-N(4)-ethylthiosemicarbazone (HmpETSC) and its complexes [Zn(HmpETSC)Cl2] and [Pd(mpETSC)Cl] exhibit antineoplastic activity against colon cancer human cell lines (HCT 116) with IC50 values of 14.59, 16.96, and 20.65 μM, respectively [26].
In previous articles, we have reported the cytotoxic activity of the ligands derived from benzaldehyde and furaldehyde thiosemicarbazone and their palladium(II) bis-chelate complexes. In vitro antitumor studies against different human tumor cell lines revealed that these metal complexes (IC50 = 0.21–12.46 μM) were more cytotoxic than their corresponding ligands (IC50 > 60 μM). On the other hand, the platinum(II) tetranuclear, [Pt4L4] (HL = 4-phenyl-1-benzaldehyde thiosemicarbazone), exhibits higher antiproliferative activity with IC50 values in the range of 0.07–0.12 μM [27].
The present work describes the synthesis, characterization, and antitumor activity of palladium(II) bis-chelate complexes of the type [Pd(TSC1–5)2] (6–10) with the ligands 4-phenyl-1-(acetone)-thiosemicarbazone, HTSC1 (1), 4-phenyl-1-(2′-chloro-benzaldehyde)-thiosemicarbazone, HTSC2 (2), 4-phenyl-1-(3′-hydroxy-benzaldehyde)-thiosemicarbazone, HTSC3 (3), 4-phenyl-1-(2′-naphthaldehyde)-thiosemicarbazone, HTSC4 (4), and 4-phenyl-1-(1′-nitro-2′-naphthaldehyde)-thiosemicarbazone, HTSC5 (5).
2. Experimental
2.1. Materials and Measurements
Chemicals were reagent grade and were used without further purification. Palladium(II) bis(acetylacetonate), potassium tetrachloropalladate, acetone, 4-phenyl-thiosemicarbazide, o-chloro-benzaldehyde, m-hydroxy-benzaldehyde, naphthaldehyde, and 1-nitro-2-naphthaldehyde were purchased from Aldrich. Elemental analyses were determined on a Fisons-Carlo Erba Elemental Microanalyzer. Infrared spectra were recorded as KBr pellets (4000–400 cm−1) on a Bruker FT-IR IFS 55 Equinox spectrophotometer. The FAB(+) mass spectra were recorded on a ZAB-HSQ (V.G. Analytical Ltd. Floats Roads, Wythenshawe, Manchester, UK) spectrometer, using 3-nitrobenzyl alcohol as the matrix. NMR spectra were recorded on a Bruker Avance DRX 300 spectrometer in DMSO-d6, operating at 300 and 75.5 MHz (1H, 13C). The chemical shifts were measured in ppm relative to tetramethylsilane (SiMe4).
2.2. Synthesis of the Ligands
2.2.1. General Method
To a hot solution of 4-phenyl thiosemicarbazide (3.34 g, 20 mmol) in methanol (100 mL) was added a solution of acetone (1.47 mL, 20 mmol) in 40 mL of methanol with a few drops of glacial acetic acid. The reaction mixture was refluxed for 2-3 h and stirred for 24 h at room temperature. The solid product was filtered, washed several times with ethanol, and dried in vacuo. A similar procedure was applied using o-chloro-benzaldehyde (2.25 mL, 20 mmol) in 60 mL of methanol, m-hydroxy-benzaldehyde (2.44 g, 20 mmol) in 60 mL of methanol, naphthaldehyde (2.72 mL, 20 mmol) in 40 mL of methanol, or 1-nitro-2-naphthaldehyde (4.02 g, 20 mmol) in 70 mL of methanol. Single crystals suitable for X-ray crystallography for both HTSC3 and HTSC4 were obtained by slow evaporation of the solvent at room temperature.
2.2.2. 4-Phenyl-1-acetone Thiosemicarbazone, HTSC1 (1)
Colorless solid. Yield 78%. Anal. for C10H13N3S (207.30 g/mol): calcd. C 57.94, H 6.32, N 20.27, S 15.47; found C 58.07, H 6.48, N 20.09, S 15.40. FAB(+)-MS: m/z 207.3 (M+, 100%). IR (KBr): ν = 3251 (NHPh), 3182 (NHCS), 1600 (C=N), 820, 1078 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 2.0 (s, CH3); 7.16 (t, 1Hpara, NHPh, J = 7.5 Hz), 7.33 (t, 2Hmeta, NHPh, J = 8.1 Hz), 7.61 (d, 2Hortho, NHPh, J = 7.5 Hz), 9.83 (s, 1H, NHPh); 10.35 (s, 1H, =N–NH). 13C NMR (DMSO-d6): δ = 18.39, 25.54 (CH3), 125.36, 128.48, 130.48, 139.52 (NHPh); 153.21 (HC=N); 176.78 (C=S).
2.2.3. 4-Phenyl-1-(2′-chlorobenzaldehyhe) Thiosemicarbazone, HTSC2 (2)
Yellow solid. Yield 72%. Anal. for C14H12N3ClS (289.79 g/mol): calcd. C 58.03, H 4.17, N 14.50, Cl 12.23, S 11.07; found C, 57.92, H 4.04, N 14.73, Cl 12.15, S 11.21. FAB(+)-MS: m/z 290.70 (MH+, 100%). IR (KBr): ν = 3305 (NHPh), 3166 (NHCS), 1600 (C=N), 835, 1065 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 8.46 (d, H3′, J = 7.5 Hz), 7.50 (m, H4′), 7.22 (t, H5′, J = 7.2 Hz), 7.33 (d, H6′, J = 8.0 Hz); 7.58 (d, 2Hortho, NHPh, J = 8.7 Hz), 7.38 (t, 2Hmeta, NHPh, J = 8.4 Hz), 7.16 (t, 1Hpara, NHPh, J = 7.5 Hz); 8.59 (s, 1H, HC=N); 10.22, 9.83 (s, 1H, NHPh); 12.03, 10.35 (s, 1H, =N–NH). 13C NMR (DMSO-d6): δ = 124.78, 126.47, 128.29, 129.17, 130.98, 133.78 (Ph–CH=N–); 125.37, 128.48, 130.29, 139.38 (NHPh); 153.22 (HC=N); 176.92 (C=S).
2.2.4. 4-Phenyl-1-(3′-hydroxybenzaldehyde) Thiosemicarbazone, HTSC3 (3)
Colorless crystals. Yield 87%. Anal. for C14H13N3OS (271.34 g/mol): calcd. C 61.97, H 4.83, N 15.49, S 11.82; found C 60.65, H 4.95, N 15.16, S 11.64. FAB(+)-MS: m/z 272.25 (MH+, 100%). IR (KBr): ν = 3290 (NHPh), 3140 (NHCS), 1598 (C=N), 825, 1020 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 7.36 (m, H2′), 7.21 (m, H4′), 7.09 (m, H5′), 7.41 (d, H6′, J = 7.8 Hz); 7.57 (d, 2Hortho, NHPh, J = 7.5 Hz), 7.33 (t, 2Hmeta, NHPh, J = 8.1 Hz), 7.15 (t, 1Hpara, NHPh, J = 7.2 Hz); 8.07 (s, 1H, HC=N); 9.56 (s, 1H, OH); 10.34, 9.91 (s, 1H, NHPh), 11.77, 10.07 (s, 1H, =N–NH). 13C NMR (DMSO-d6): δ = 114.68, 118.93, 125.80, 130.05, 135.32, 158.02 (Ph–CH=N–); 121.84, 125.31, 128.06, 139.1 (NHPh); 152.79 (HC=N); 193.17 (C=S).
2.2.5. 4-Phenyl-1-naphthaldehyde Thiosemicarbazone, HTSC4 (4)
Rectangular-shaped yellow crystals. Yield 75%. Anal. for C18H15N3S (305.39 g/mol): calcd. C 70.79, H 4.95, N 13.76, S 10.50; found: C 70.93, H 4.80, N 13.85, S 10.38. FAB(+)-MS: m/z 305.40 (M+, 100%). IR (KBr): ν = 3327 (NHPh), 3165 (NHCS), 1600 (C=N), 815, 1088 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 7.33 (d, H2′, 7.8 Hz), 7.67 (t, H3′, 7.2 Hz), 8.34 (d, H4′, 8.4 Hz), 8.47 (d, H5′, H8′, J = 6.0 Hz), 7.67 (t, H6′, J = 7.2 Hz), 8.02 (t, H7′, J = 7.2 Hz); 7.39 (t, 2Hmeta, NHPh, J = 7.8 Hz), 7.16 (t, 1Hpara, NHPh, J = 7.2 Hz), 7.61 (d, 2Hortho, NHPh, J = 7.2 Hz); 9.08 (s, 1H, HC=N); 9.84, 10.35 (s, 1H, NHPh); 10.20, 11.89 (s, 1H, =N–NH). 13C NMR (DMSO-d6): δ = 122.94, 125.83, 126.05, 126.62, 127.69, 129.37, 130.87, 131.15, 133.83, 141.45 (Naphthoyl); 125.38, 126.25, 128.57, 139.54 (NHPh), 153.25 (HC=N); 176.38 (C=S).
2.2.6. 4-Phenyl-1-(1′-nitro-2′-naphthaldehyde) Thiosemicarbazone, HTSC5 (5)
Yellow solid. Yield 85%. Anal. for C18H14N4O2S (350.39 g/mol): calcd. C 61.69, H 4.03, N 16.00, S 9.15; found: C 61.54, H 4.10, N 15.82, S 8.94. FAB(+)-MS: m/z 350.40 (M+, 100%). IR (KBr): ν = 3250 (NHPh), 3174 (NHCS), 1713, 1626 (C=N), 820, 1047 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 8.40 (d, H3′, J = 8.5 Hz), 8.09 (d, H4′, J = 8.5 Hz), 8.22 (d, H5′, H8′, J = 6.0 Hz), 7.86 (t, H6′, H7′, J = 6.0 Hz); 7.61 (d, 2Hortho, NHPh, J = 8.5 Hz), 7.33 (t, 2Hmeta, NHPh, J = 6.5 Hz), 7.15 (t, 1Hpara, NHPh, J = 7.5 Hz); 10.15 (s, 1H, NHPh), 9.83, 10.34 (s, 1H, =N–NH). 13C NMR (DMSO-d6): δ = 118.28, 123.71, 126.01, 129.17, 130.84, 132.22, 136.47, 137.96, 153.24, 163.47 (Naphthoyl); 122.65, 125.36, 128.49, 139.5 (NHPh); 176.76 (HC=N); 189.98 (C=S).
2.3. Synthesis of the Palladium(II) Complexes
2.3.1. General Method
A solution of K2[PdCl4] (0.163 g, 0.5 mmol) in ethanol (60 mL) or a solution of [Pd(acac)2] (0.153 g, 0.5 mmol) in dichloromethane/ethanol (2 : 1, 45 mL) was added dropwise to a stirred hot solution of the corresponding thiosemicarbazone (1.0 mmol) in 70 mL of methanol. Then, sodium acetate (0.082 g, 1 mmol) in 5 mL of water was added. The solution was refluxed for 2-3 h and stirred for 24 h at room temperature. The precipitate was collected by filtration, washed three times with ethanol (30 mL), and dried under vacuum. For the complex [Pd(TSC1)2] (6), single crystals suitable for X-ray diffraction studies were grown by slow evaporation from an acetone solution.
2.3.2. Bis[4-phenyl-1-(acetone) Thiosemicarbazonato]palladium(II), [Pd(TSC1)2] (6)
Square-shaped orange crystals. Yield 65%. Anal. for C20H24N6S2Pd (518.99 g/mol): calcd. C 46.28, H 4.66, N 16.19, S 12.36; found C 46.05, H 4.74, N 16.21, S 12.23. FAB(+)-MS: m/z 518.50 (M+, 75%). IR (KBr): ν = 3375 (NHPh), 1590 (C=N), 800, 964 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 2.18, 2.35 (s, 12H, 4CH3); 7.62 (d, 4Hortho, J = 7.9 Hz, NHPh), 7.26 (t, 4Hmeta, J = 7.7 Hz, NHPh), 6.93 (t, 2Hpara, J = 7.5 Hz, NHPh); 9.34 (s, 2H, NHPh). 13C NMR (DMSO-d6): δ = 19.65, 22.08 (CH3), 119.06, 123.15, 129.01, 141.80 (NHPh); 154.50 (HC=N); 176.20 (C=S).
2.3.3. Bis[4-phenyl-1-(2′-chlorobenzaldehyde) Thiosemicarbazonato]palladium(II), [Pd(TSC2)2] (7)
Red solid. Yield 60%. Anal. for C28H22N6Cl2S2Pd (683.97 g/mol): calcd. C 49.17, H 3.24, N 12.29, Cl 10.37, S 9.38; found C, 49.26, H 3.12, N 12.36, Cl 10.45, S 9.25. FAB(+)-MS: m/z 648.5 (M+-Cl, 65%). IR (KBr): ν = 3375 (NHPh), 1580 (C=N), 795, 927 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 8.29 (d, H3′, J = 8.1 Hz), 7.52 (m, H4′), 7.28 (t, H5′, J = 7.8 Hz), 7.42 (d, H6′, J = 8.1 Hz); 7.59 (d, 4Hortho, NHPh, J = 8.1 Hz), 7.19 (t, 4Hmeta, NHPh, J = 8.1 Hz), 6.98 (t, 2Hpara, NHPh, J = 7.2 Hz); 8.0 (s, 2H, HC=N); 9.34, 9.92 (s, 1H, NHPh). 13C NMR (DMSO-d6): δ = 119.05, 120.83, 125.01, 126.72, 127.38, 138.77 (Ph–CH=N–); 121.94, 127.90, 129.0, 141.81 (NHPh); 166.48 (HC=N); 169.19 (C=S).
2.3.4. Bis[4-phenyl-1-(3′-hydroxybenzaldehyde) Thiosemicarbazonato]palladium(II), [Pd(TSC3)2] (8)
Orange solid. Yield 68%. Anal. for C28H24N6O2S2Pd (647.08 g/mol): calcd. C 51.97, H 3.74, N 12.99, S 9.91; found C 52.05, H 3.66, N 13.04, S 9.83. FAB(+)-MS: m/z 646.95 (M+, 55%). IR (KBr): ν = 3300 (NHPh), 1585 (C=N), 800, 930 (C=S) cm−1. 1H NMR (DMSO-d6): δ = 7.02 (d, 2H4′, 2H6′, J = 8.4 Hz) 7.48 (t, 2H5′, J = 7.5 Hz); 7.60 (d, 4Hortho, NHPh, J = 7.8 Hz), 7.28 (t, 4Hmeta, NHPh, J = 8.4 Hz), 6.92 (t, 2Hpara, NHPh, J = 7.5 Hz); 7.81 (s, 2H, HC=N), 8.45 (d, OH, J = 7.5 Hz); 9.46 (s, 2H, NHPh, J = 7.5 Hz). 13C NMR (DMSO-d6): δ = 110.71, 121.75, 130.69, 133.47, 155.19 (Ph–CH=N–); 119.04, 120.0, 128.62, 141.23 (NHPh); 158.37 (HC=N); 167.06 (C=S).
2.3.5. Bis[4-phenyl-1-naphthaldehyde Thiosemicarbazonato]palladium(II), [Pd(TSC4)2] (9)
Orange solid. Yield 61%. Anal. for C36H28N6S2Pd (715.20 g/mol): calcd. C 60.46, H 3.95, N 11.75, S 8.97; found: C 60.32, H 4.05, N 11.81, S 8.84. FAB(+)-MS: m/z 716.30 (MH+, 58%). IR (KBr): ν = 3373 (NHPh), 1588 (C=N), 780, 1032 (C=S) cm−11H NMR (DMSO-d6): δ = 8.44 (d, 2H2′, J = 9.0 Hz), 7.85 (m, 2H3′), 8.24 (d, 2H4′, 2H5′, J = 9.0 Hz), 7.85 (m, 2H6′, 2H7′), 8.14 (d, 2H8′, J = 6.0 Hz); 7.60 (d, 4Hortho, NHPh, J = 6.0 Hz), 7.33 (t, 4Hmeta, NHPh, J = 8.0 Hz), 7.15 (t, 2Hpara, NHPh, J = 8.5 Hz); 9.83, 10.15, 10.33 (s, 2H, NHPh). 13C NMR (DMSO-d6): δ = 118.05, 121.04, 123.64, 124.34, 126.12, 129.09, 132.50, 134.30, 136.47, 139.52 (Naphthoyl); 125.14, 126.77, 128.71, 141.04 (NHPh), 165.62 (HC=N); 175.72 (C=S).
2.3.6. Bis[4-phenyl-1-(1′-nitro-2′-naphthaldehyde) Thiosemicarbazonato]palladium(II), [Pd(TSC4)2] (10)
Orange solid. Yield 50%. Anal. for C36H26N8O4S2Pd (805.19 g/mol): calcd. C 53.70, H 3.25, N 13.92, S 7.96; found: C 53.64, H 3.15, N 13.86, S 7.78. FAB(+)-MS: m/z 787.10 (M+-H2O, 100%). IR (KBr): ν = 3374 (NHPh), 1578 (C=N), 790, 1017 (C=S) cm−1. 1H NMR (DMSO, d6): δ = 7.80 (t, 2H3′, J = 9.0 Hz), 8.38 (d, 2H4′, 2H5′, J = 9.0 Hz), 7.80 (t, 2H6′, 2H7′, J =6.0 Hz), 7.31 (m, 2H8′); 7.62 (d, 4Hortho, NHPh, J = 9.0 Hz), 7.26 (t, 4Hmeta, NHPh, J = 9.0 Hz), 6.92 (t, 2Hpara, NHPh, J = 6.0 Hz); 9.33, 9.59 (s, 2H, NHPh). 13C NMR (DMSO-d6): δ = 113.57, 123.44, 128.20, 129.38, 130.13, 132.36, 133.25, 137.41, 152.57, 157.61 (Naphthoyl); 121.22, 124.54, 127.75, 138.45 (NHPh); 178.83 (HC=N); 189.50 (C=S).
2.4. Crystal Structure Determinations
Crystallographic measurements were made using an IPDS1 diffractometer (graphite monochromated Mo-Kα radiation (λ = 0.71073 Å)). Data were collected using Φ scan technique with a scan width of 0.7°. The structures were solved by direct methods using the program SIR2004 [28] and were refined using anisotropic approximation for the nonhydrogen atoms using SHELXL-97 software [29].
2.5. Biological Activity
2.5.1. Cell Culture
The H460 (human lung large cell carcinoma), M-14 (human amelanotic melanoma), DU145 (human prostate carcinoma), MCF-7 (human breast adenocarcinoma), HT-29 (human colon adenocarcinoma), and K562 (human chronic myelogenous leukemia) cell lines were obtained from the research laboratory of the Faculty of Sciences and Philosophy, Universidad Peruana Cayetano Heredia. All the cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum and 50 μg/mL gentamycin in humidified 5% CO2/95% air at 37°C.
2.6. Assessment of Cytotoxicity
The assay was performed as described previously [30]. Briefly, 3000–5000 cells were inoculated in each well of 96-well tissue culture plates and incubated at 37°C with their corresponding culture medium during 24 h. The ligands HTSC1–5 (10–250 μM), palladium(II) complexes (0.01–10 μM), or cisplatin (1–10 μM) in DMSO were then added and incubated for 48 h at 37°C with a highly humidified atmosphere, 5% CO2 and 95% air. After the incubating period, cell monolayers were fixed with 10% trichloroacetic acid and stained for 20 minutes using the sulforhodamine B dye. Then, the excess dye was removed by washing repeatedly with 1% acetic acid. The protein-bound dye was solubilized with 10 mM Tris buffer (pH 10.5) and the absorbance values were obtained at 510 nm using a microplate reader. The IC50 value was defined as the concentration of a test sample resulting in a 50% reduction of absorbance as compared with untreated controls and was determined by linear regression analysis.
3. Results and Discussion
3.1. Synthesis and Characterization
The ligands HTSC1–5 were prepared according to the literature [31–33], as shown in Scheme 1. The ligands were obtained in good yields (72–87%) and characterized by elemental analysis and FT-IR, FAB(+)-mass, and NMR (1H, 13C) spectroscopy.
Scheme 1.
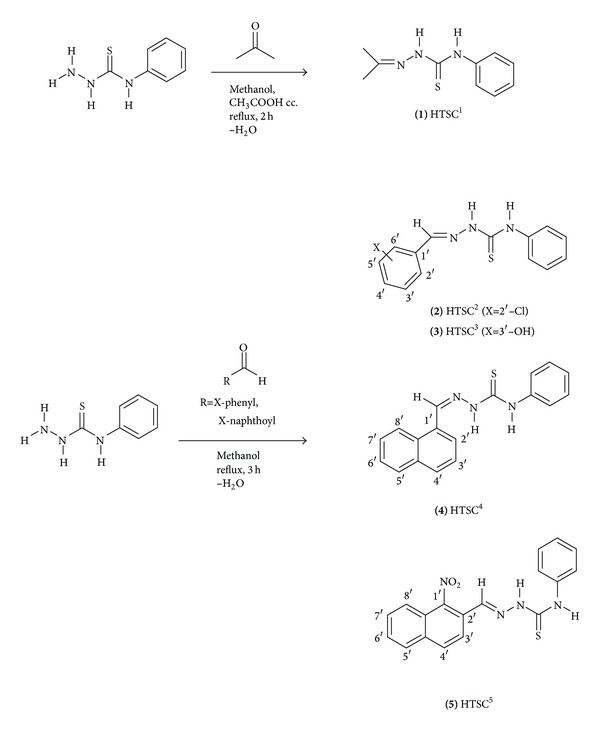
Synthesis of 4-phenyl-1-acetone thiosemicarbazone, 4-phenyl-1-benzaldehyde thiosemicarbazone, and 4-phenyl-1-naphthaldehyde thiosemicarbazone ligands.
The palladium(II) complexes (Scheme 2) were obtained in satisfactory yield (50–68%) and characterized by elemental analysis and FT-IR, FAB(+)-mass, and NMR(1H, 13C) spectroscopy.
Scheme 2.
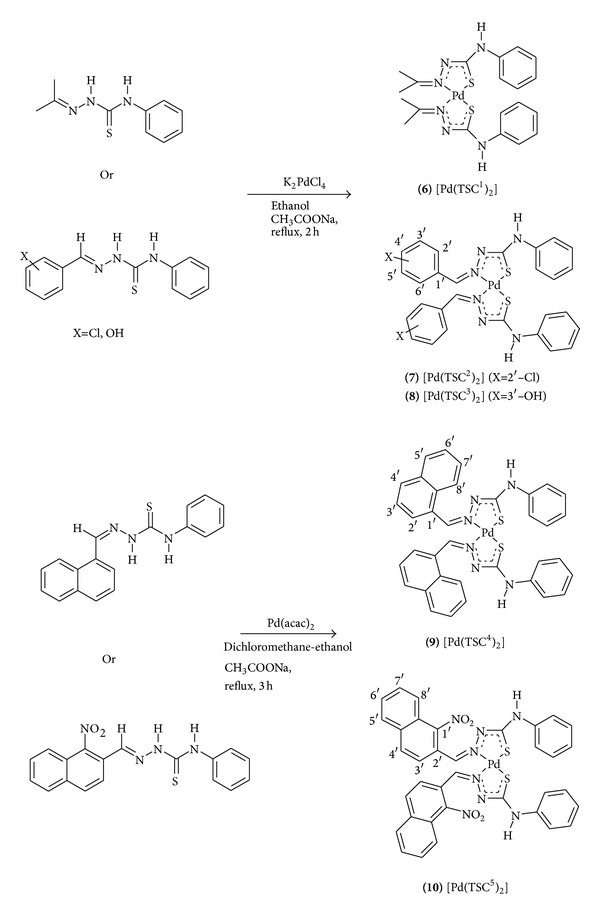
Synthesis of palladium(II) bis-chelate complexes of acetone, benzaldehyde, and naphthaldehyde thiosemicarbazone derivatives.
Analytical and spectroscopy data obtained for the thiosemicarbazone ligands and their palladium(II) complexes are in agreement with the proposed structures.
The ligand HTSC3 (3) and the complex [Pd(TSC1)2] (6) were recrystallized from acetone, and single crystals suitable for X-ray crystallography were obtained, while single crystals of the ligand HSTC4 (4) were obtained by slow evaporation of the solvent from the final reaction mixture.
3.2. Infrared Spectra
The broad bands of the –NH group observed at 3140–3182 cm−1 in the spectra of the free ligands disappeared in the spectra of the corresponding complexes, thus indicating the deprotonation of the =N–NH– group. The strong bands observed in the range of 1598–1626 cm−1 were assigned to (C=N) stretching vibrations of the free thiosemicarbazones. These bands were shifted to lower frequencies (10–22 cm−1) after coordination, which is in agreement with the observed behaviour of other bis-chelate complexes [26, 34–39]. These results indicate the coordination of the azomethine nitrogen to the metal ion. The ν(C=S) vibrations observed at 815–1088 cm−1 in the spectra of the free ligands shift 20–138 cm−1 towards lower frequencies upon complexation, indicating the involvement of the thione sulphur in the bond formation to the metal ion [40, 41].
3.3. NMR Spectra
The 1H NMR and 13C NMR spectra of the ligands and their metal complexes were recorded in DMSO-d6. In the 1H NMR spectra of the ligands HTSC1–5, the signal of the =N–NH proton appears as a singlet at δ 10.07–12.03, while on complexation these signals disappeared, thus indicating the deprotonation of the =N–NH group [25, 33, 42–46]. In the 1H NMR spectra of the ligands HTSC2–5, the signal of the HC=N proton appeared as a singlet at δ = 8.07–9.08. These signals are shifted by 0.26–0.59 ppm upfield for [Pd(TSC2-3)2] complexes (7, 8). These results are consistent with the IR spectral data and suggest the coordination of palladium to the imine nitrogen [24, 25, 43–45]. For all ligands, the resonance lines found at δ = 9.83–10.35 were assigned to the proton of the NHPh group. The presence of the phenyl group on the terminal amine induces the shift of these signals by 1.9 ppm downfield, as compared to the resonance lines of the –NH2 terminal group found for other thiosemicarbazone derivatives [33, 44]. On the other hand, the aromatic proton signals of the phenyl amine group in all the ligands were observed at δ = 7.15–7.61, and these resonance lines show the expected calculated multiplicity. For the ligands HTSC2 (2) and HTSC3 (3) the aromatic proton signals of the phenyl fragment bound to the −CH=N group were affected by the presence of the chloro and hydroxy substituents in the C-2′ and C-3′positions, respectively, of the phenyl moiety. For the HTSC2 (2) ligand, these signals are shifted downfield for the protons in the positions C-3′ (1 ppm) and C-4′ (0.1 ppm), while for HTSC3 (3) ligand they are shifted upfield for the protons in the positions C-2′ (0.55 ppm) and C-4′ (0.21 ppm), with respect to the unsubstituted phenyl moiety [33]. For the HTSC5 (5) ligand, the presence of the nitro substituent group in the naphthoyl moiety affected the resonance signals of the aromatic protons. These signals are shifted downfield for the protons in the positions C-3′ (0.73 ppm) and C-6′ (0.19 ppm), while for the protons in the positions C-4′, C-5′, C-7′, and C-8′ these are shifted upfield by 0.16–0.25 ppm, relative to the HTSC4 (4) ligand with the unsubstituted naphthoyl moiety. Thus, the aromatic protons signals in all the ligands do not suffer relevant changes in their chemical shifts after complexation.
In the 13C NMR spectra, the carbon resonance signals of the C=N group appear at δ = 152.8–176.8. These results are similar to the chemical shifts found for other ligands derived from benzaldehyde thiosemicarbazone [33, 40]. The C=S signals observed at δ = 176.4–193.2 are characteristic for the thiocarbonyl group present in all the ligands. For [Pd(TSC1–5)2] complexes (6–10), the C=N and C=S signals are shifted downfield by 1.3–13.3 ppm and upfield by 0.5–26.1 ppm, respectively, with respect to their ligands. These results confirm the coordination of the thiocarbonyl sulphur and azomethine nitrogen atoms to the palladium(II) ion [36, 47]. For all ligands, the aromatic carbons of the NHPh group were observed at δ = 121.8–134.6, and these chemical shifts are in agreement with those found for other thiosemicarbazone ligands [33, 44].
3.4. Structural Data
Crystal data, data collection procedure, structure determination methods, and refinement results for compounds HTSC3, HTSC4, and [Pd(TSC1)2] are summarized in Table 1, whereas selected bond lengths and bond angles are presented in Tables 2 and 3.
Table 1.
Crystal data and structure refinement for HTSC3, HTSC4, and [Pd(TSC1)2].
| Compound | HTSC3 | HTSC4 | [Pd(TSC1)2] |
|---|---|---|---|
| Empirical formula | C14H13N3OS | C18H15N3S | C20H24N6S2Pd |
| Formula weight | 271.33 | 305.39 | 518.97 |
| Temperature (K) | 213 | 213 | 213 |
| Crystal system | Triclinic | Orthorhombic | Monoclinic |
| Space group | P-1 | P212121 | C2/c |
| a (Å) | 6.3202(7) | 5.3471(3) | 23.456(2) |
| b (Å) | 10.357(1) | 15.7563(9) | 7.7080(4) |
| c (Å) | 11.506(1) | 16.456(2) | 12.3813(10) |
| α (°) | 65.95(1) | 90 | 90 |
| β (°) | 80.14(1) | 90 | 97.96(1) |
| γ (°) | 85.32(1) | 90 | 90 |
| Volume (Å3) | 677.56(13) | 1554.91(18) | 2216.9(3) |
| Z | 2 | 4 | 4 |
| Density (g/cm3) | 1.33 | 1.305 | 1.555 |
| Absorption coeff. (mm−1) | 0.234 | 0.208 | 1.044 |
| Crystal size (mm) | 0.7 × 0.3 × 0.2 | 0.7 × 0.05 × 0.05 | 0.4 × 0.4 × 0.4 |
| θ range for data collect. (°) | 3–28 | 2–26 | 3–28 |
| Index ranges | −7 ≤ h ≤ 8 | − 6 ≤ h ≤ 6 | − 30 ≤ h ≤ 30 |
| −13 ≤ k ≤ 13 | − 18 ≤ k ≤ 19 | −9 ≤ k ≤ 10 | |
| −15 ≤ l ≤ 15 | − 22 ≤ l ≤ 22 | −16 ≤ l ≤ 16 | |
| Reflections collected | 7186 | 10232 | 10366 |
| Independent reflections | 2997 (R int = 0.024) |
3005 (R int = 0.050) |
2662 (R int = 0.042) |
| Max./min. transmission | 0.8534/0.9547 | 0.8683/0.9897 | 0.7171/0.9674 |
| Data/parameters | 2997/224 | 3005/259 | 2662/180 |
| Goodness-of-fit on F 2 | 0.803 | 0.804 | 0.841 |
| Final R indices [I > 2σ(I)] | R 1 = 0.0289 | R 1 = 0.0322 | R 1 = 0.0235 |
| ωR 2 = 0.0685 | ωR 2 = 0.0624 | ωR 2 = 0.0442 | |
| R indices (all data) | R 1 = 0.0478 | R 1 = 0.0486 | R 1 = 0.0317 |
| ωR 2 = 0.0717 | ωR 2 = 0.0653 | ωR 2 = 0.0454 | |
| Lgst diff. peak/hole (eÅ−3) | 0.19/−0.16 | −0.1/0.03 | −0.31/0.07 |
Table 2.
Bond length (Å) and torsion angles (°) for HTSC3 and HTSC4.
| HTSC3 | HTSC4 |
|---|---|
| C2-N2 1.280(2) | C8-N3 1.278(2) |
| N2-N1 1.382(2) | N3-N2 1.380(2) |
| N1-C1 1.352(2) | N2-C7 1.351(2) |
| C1-N3 1.347(2) | C7-N1 1.351(2) |
| C1-S1 1.689(1) | C7-S1 1.688(2) |
| N3-C9 1.430(2) | N1-C1 1.414(2) |
| C2-N2-N1-C1 173.8(1) | C8-N3-N2-C7 180.0(2) |
| N2-N1-C1-N3 5.33(2) | N3-N2-C7-N1 1.0(2) |
| N1-C1-N3-C9 −177.1(1) | N2-C7-N1-C1 −176.8(2) |
Table 3.
Selected bond lengths (Å) and angles in (°) for [Pd(TSC1)2] (6).
| Distances | Angles |
|---|---|
| Pd1-S1 2.270(1) | N1-Pd1-S1 82.21(4) |
| Pd1-N1 2.099(1) | Pd1-S1-C4 95.20(6) |
| N1-N2 1.421(2) | S1-C4 -N2 125.7(1) |
| N2-C4 1.290(2) | C4-N2-N1 112.6(2) |
| C4-S1 1.773(2) | N2-N1-Pd1 117.3(1) |
The molecular structures of HTSC3, HTSC4, and [Pd(TSC1)2] are shown in Figures 1, 2, and 3, respectively. The thiocarbazone fragments in the two structures of ligands HTSC3 and HTSC4 are very similar. Structurally significant is the cis-arrangement between the atoms N2-N1-C1-N3 (HTSC3) and N3-N2-C7-N1 (HTSC4), the torsion angles being 5.33(2)° and 1.0(2). All bond lengths in the thiosemicarbazone fragment are identical for the two ligands (Table 2).
Figure 1.
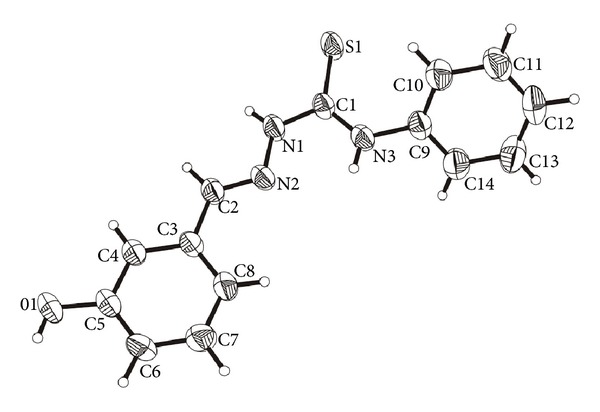
Molecular structure of HTSC3 (3). The displacement ellipsoids are drawn at the 50% probability.
Figure 2.
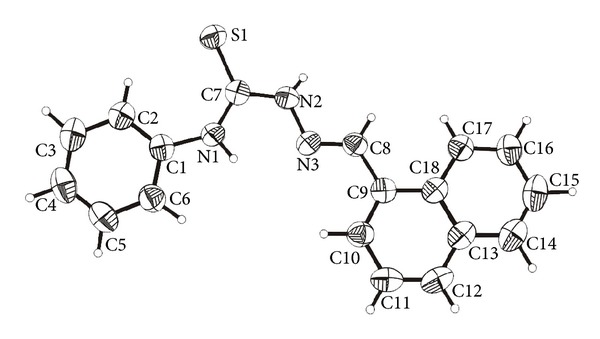
Molecular structure of HTSC4 (4). The displacement ellipsoids are drawn at the 50% probability.
Figure 3.
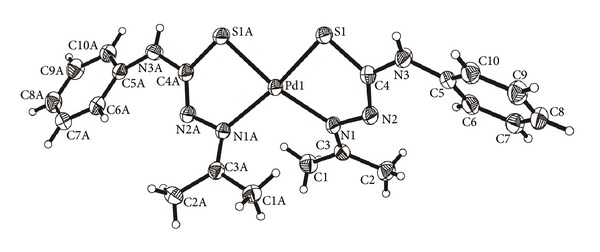
Molecular structure of [Pd(TSC1)2] (6). The displacement ellipsoids are drawn at the 50% probability.
The crystal structure of ligand HTSC3 is stabilized by intermolecular O–H⋯ S [O⋯S 3.169 Å, H⋯S 2.548 Å, and O–H⋯S 136.46°] hydrogen bonds which lead to a double chain along the a-axis, as shown in Figure 4. On the other hand, the crystal structure of ligand HTSC4 is also stabilized by a N–H⋯ S hydrogen bond with the bond parameters as follows: N⋯S 3.523 Å, H⋯S 3.523 Å, and N–H–S 158.82°. We found along of a 21-screw axis a typical helix structure, as shown in Figure 5.
Figure 4.
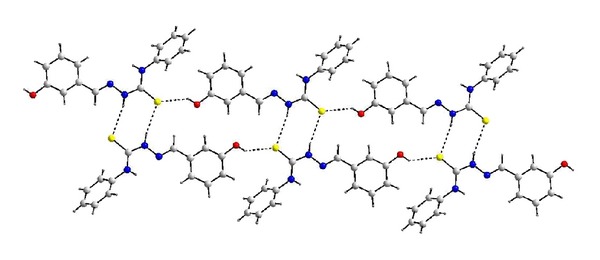
Double chain structure of HTSC3 in the crystal.
Figure 5.
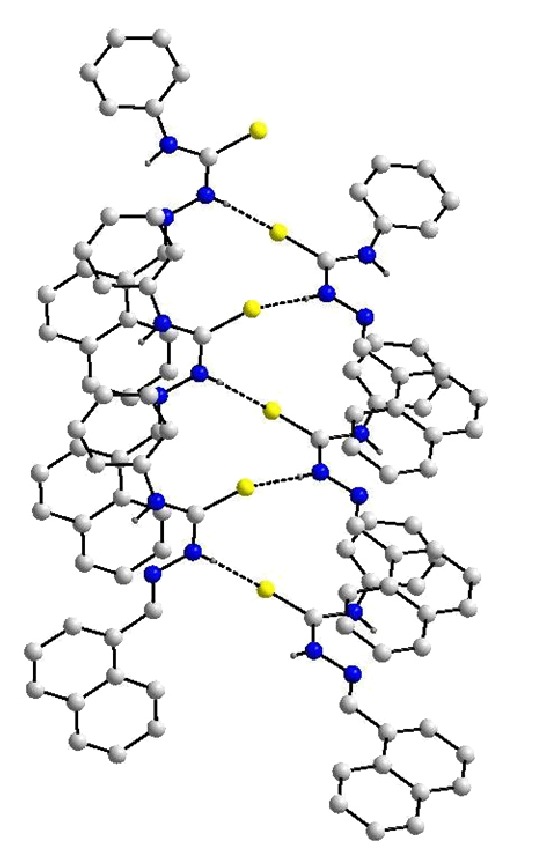
Helix structure of HTSC4 in the crystal.
The complex [Pd(TSC1)2] (6) (Figure 3) crystallizes in the monoclinic space group C2/c with four molecules in the unit cell and with a C2 molecular symmetry. The sulfur and nitrogen donor atoms are in a cis arrangement. The deprotonated ligand coordinates bidentately to PdII ion through S and N. It leads to lengthening of the C4–S1 bond (1.773 Å) and shortening of the N2–C4 bond (1.29 Å) and these results are in agreement with those found for other palladium(II) bis-chelate complexes of the type [PdL2] with thiosemicarbazone ligands [27, 42].
The chelate ring with the atoms Pd1, N1, N2, C4, and S1 has an envelope configuration. For the plane formed by the atoms N1, N2, S1, and C4, the average deviation is 0.003 Å, while the deviation of the Pd atom from this plane is 0.664 Å; this distortion indicates a pseudo square planar coordination geometry.
3.5. Antitumor Evaluation
The cytotoxic potential of the ligands derived from thiosemicarbazones and their respective palladium(II) complexes were investigated in the following six human tumor cell lines: H460, DU145, MCF-7, M14, HT-29, and K562. For comparison purposes, the cytotoxicity of cisplatin was evaluated under the same experimental conditions.
The results of the cytotoxic activity of the ligands, palladium(II) complexes, and cisplatin are expressed as IC50 values (micromolar concentration inhibiting 50% cell growth), and these compounds were evaluated in vitro against the different human tumor cell lines, as shown in Table 4. In general, the palladium(II) complexes (IC50 = 0.01–9.87 μM) exhibited higher antiproliferative activity than their free ligands (IC50 = 23.48–70.86 and >250 μM). Figure 6 shows the antiproliferative activity of the ligands HTSC1–5 and their palladium(II) complexes [Pd(TSC1–5)2] against H460 and K562 human tumor cell lines after 48 h incubation time. These results indicate that the cytotoxicity is enhanced when the ligands are coordinated to the Pd(II) ion. Probably, the palladium(II) bis-chelate complexes of square planar geometry act as intercalating agents between the pyrimidine and guanine bases of the DNA tumor cells, inducing conformational changes on the DNA double helix specific that finally produce tumor cell death [33, 44, 48].
Table 4.
IC50 (µm) valuesa of the ligands HTSC1–5, palladium(II) complexes [Pd(TSC1–5)2], and cisplatin against the different human tumor cell linesb.
| Human tumor cell lines | H460 | DU145 | MCF-7 | M14 | HT-29 | K562 |
|---|---|---|---|---|---|---|
| HTSC1 | >250 | >250 | >250 | >250 | >250 | 70.86 |
| HTSC2 | >250 | 31.55 | 38.05 | >250 | >250 | 32.89 |
| HTSC3 | 23.48 | 26.64 | 34.00 | 28.67 | 25.73 | 35.05 |
| HTSC4 | 39.65 | 26.45 | 29.94 | >250 | >250 | 24.66 |
| HTSC5 | 24.95 | 31.60 | 25.46 | 27.31 | 26.72 | 27.76 |
| [Pd(TSC1)2] | 9.40 | 8.27 | 6.95 | 9.87 | 8.20 | 9.43 |
| [Pd(TSC2)2] | 2.26 | 2.05 | 1.61 | 2.14 | 1.87 | 1.95 |
| [Pd(TSC3)2] | 0.23 | 0.01 | 0.13 | 0.05 | 0.05 | 0.02 |
| [Pd(TSC4)2] | 2.05 | 2.39 | 2.14 | 2.27 | 2.37 | 1.84 |
| [Pd(TSC5)2] | 0.68 | 0.84 | 0.78 | 1.06 | 1.04 | 0.65 |
| cisplatin | 2.85 | 6.50 | 7.20 | 2.95 | 7.60 | 3.20 |
aIC50 corresponds to the concentration required to inhibit 50% of the cell growth when the cells are exposed to the compounds during 48 h. Each value is the average of two independent experiments.
bLung large cell carcinoma (H460), prostate carcinoma (DU145), breast adenocarcinoma (MCF-7), amelanotic melanoma (M-14), colon adenocarcinoma (HT-29), and chronic myelogenous leukemia (K562).
Figure 6.
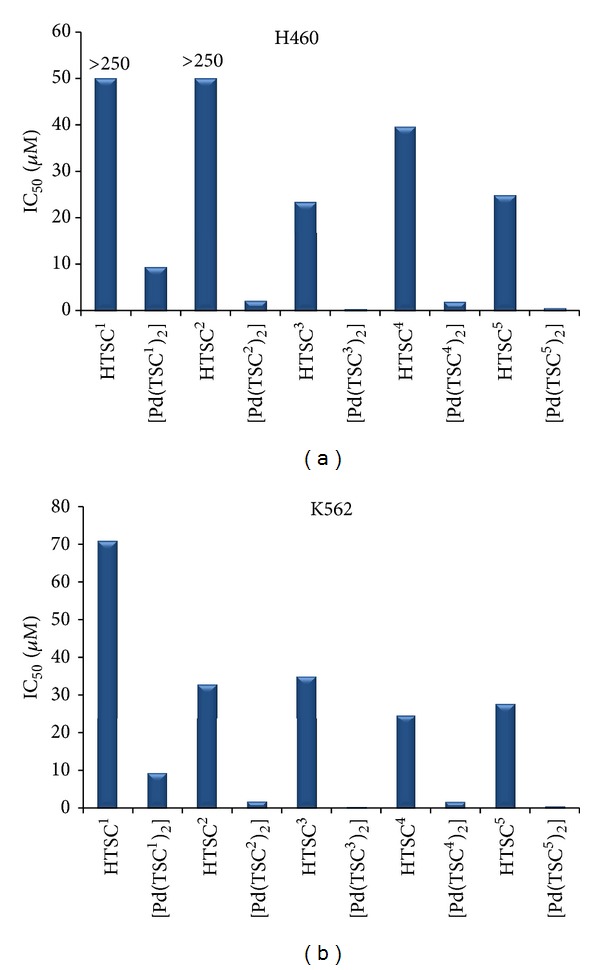
The antiproliferative activity in vitro expressed as IC50 (μM) values of the ligands HTSC1–5 and palladium(II) complexes [Pd(TSC1–5)2] against (a) H460 and (b) K562 human tumor cell lines after 48 h incubation time.
All palladium(II) complexes except [Pd(TSC1)2] (6) were more cytotoxic than cisplatin (IC50 = 2.85−7.60 μM) against all the investigated human tumor cell lines. Figure 7 shows a comparison of the magnitude of the IC50 values of the palladium(II) complexes and cisplatin against human breast adenocarcinoma MCF-7 cell line. On the other hand, between all the tested palladium(II) complexes, [Pd(TSC3)2] (8) and [Pd(TSC5)2] (10) complexes showed greater cytotoxic activity against all human tumor cell lines, with IC50 values of 0.01–0.23 and 0.65–1.06 μM, respectively. Therefore, the presence of the 3-hydroxy and 1-nitro substituents groups in the benzene and naphthalene aromatic rings plays an important role in the enhancement the antiproliferative activity [1, 16, 27, 42]. The effect of these substituents may be related to their hydrogen-bonding ability compared with the chloro substituent in complex (7). Following this reasoning, the [Pd(TSC3)2] (8) complex, with the 4-phenyl-1-(3′-hydroxy-benzaldehyde) thiosemicarbazone ligand, was also more active than the palladium(II) bis-chelate complex of 3′-cyano-benzaldehyde thiosemicarbazone (IC50 = 0.45–3.53 μM) against all human tumor cell lines tested [27]. In addition, complex (8) was found to be about thirteen times more cytotoxic than the gold(I) complex from 4-methyl-1-(2′-acetylpyridine) thiosemicarbazone ligand (IC50 = 1.65 μM) against the (MCF-7) human breast adenocarcinoma tumor cell line [49]. With respect to the cytotoxic activity shown by the ruthenium(II) complex of the [Ru(Phen)2(L)]Cl2 type, with L being a 3-methoxy, or 4-hydroxy-benzaldehyde thiosemicarbazone ligand (IC50 = 3.60 μM) assayed on the (CEM) human leukemia cell line [24], complex (8) presented higher cytotoxicity at low micromolar concentrations (IC50 = 0.02 μM) tested in vitro against the (K562) chronic myelogenous leukemia cell line. Since the ruthenium complex is octahedral and complex (8) presents a pseudo-planar geometry, the larger cytotoxicity of (8) is in agreement with the proposed intercalation mechanism as the intercalation is favored for a planar moiety.
Figure 7.
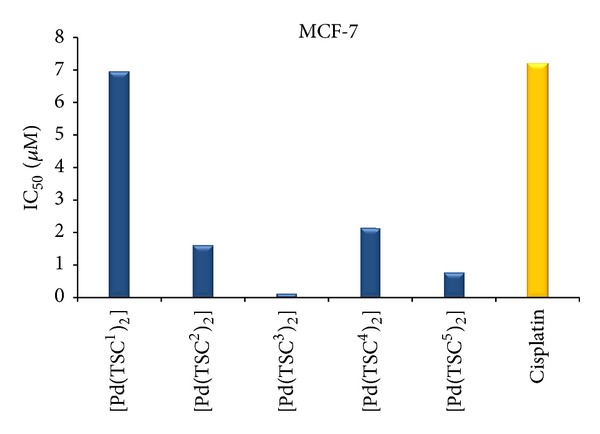
The antiproliferative activity in vitro expressed as IC50 (μM) values of the ligands HTSC1–5, palladium(II) complexes [Pd(TSC1–5)2], and cisplatin against MCF-7 human tumor cell line after 48 h incubation time.
Complex [Pd(TSC5)2] (10) (IC50 = 0.78 μM) tested against the MCF-7 tumor cell line resulted to be more cytotoxic than the palladium(II) monochelate complexes with 2-acetylpyridine thiosemicarbazone derivatives (IC50 = 4.9–5.5 μM) when being tested on the MDA-MB231 human breast cancer cell line [32]. Furthermore, complex (10) tested in vitro against the HT29 colon adenocarcinoma tumor cell line exhibited higher cytotoxicity (IC50 = 1.04 μM) than that of the [Pd(mpETSC) Cl] (HmpETSC = 4-ethyl-1-(6′-methylpyridine-2′-carbaldehyde) thiosemicarbazone) monochelate complex (IC50 = 20.65 μM) assayed on the HCT 116 human colon tumor cell line [26].
In summary, we have synthesized palladium(II) bis-chelate complexes with ligands derived from acetone, benzaldehyde, and naphthaldehyde thiosemicarbazone. The molecular structure of [Pd(TSC1)2] (6) shows a square-planar geometry with deprotonated ligands coordinated to Pd(II) through the azomethine nitrogen and thione sulfur atoms in a cis arrangement.
Of all the studied complexes, the hydroxy-substituted [Pd(TSC3)2] (8) complex resulted to be more cytotoxic in all tumor cell lines at low micromolar concentrations, compared to the other complexes and the free ligands.
3.6. Extra Material
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, numbers CCDC 894930 for HTSC3, 894931 for [Pd(TSC1)2], and 894932 for HTSC4. Copies of this information can be obtained free of charge from the Cambridge Crystallographic Data Center (CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; email: deposit@ccdc.cam.ac.uk).
Supplementary Material
Supplementary Information (figures containing IR, 1H, COSY and 13C NMR spectra).
Acknowledgments
Wilfredo Hernández gratefully thanks the Universidad de Lima Scientific Research Institute for financial support to carry out the research work. The authors also thank the Development and Research Laboratory of the Faculty of Sciences and Philosophy, Universidad Peruana Cayetano Heredia, for the evaluation of the cytotoxic activity of the compounds. Evgenia Spodine and Jorge Manzur thank Financiamiento Basal Program, FB0807 Project (CEDENNA).
References
- 1.Pelosi G, Bisceglie F, Bignami F, et al. Antiretroviral activity of thiosemicarbazone metal complexes. Journal of Medicinal Chemistry. 2010;53(24):8765–8769. doi: 10.1021/jm1007616. [DOI] [PubMed] [Google Scholar]
- 2.Karaküçük-Iyidoğan A, Taşdemir D, Oruç-Emre EE, Balzarini J. Novel platinum(II) and palladium(II) complexes of thiosemicarbazones derived from 5-substitutedthiophene-2-carboxaldehydes and their antiviral and cytotoxic activities. European Journal of Medicinal Chemistry. 2011;46(11):5616–5624. doi: 10.1016/j.ejmech.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genova P, Varadinova T, Matesanz AI, Marinova D, Souza P. Toxic effects of bis(thiosemicarbazone) compounds and its palladium(II) complexes on herpes simplex virus growth. Toxicology and Applied Pharmacology. 2004;197(2):107–112. doi: 10.1016/j.taap.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Thota S, Karki SS, Bhukya BR. Synthesis, characterization and antibacterial activity of some novel mononuclear Ru(II) complexes. International Journal of Pharmacy and Pharmaceutical Sciences. 2009;1(2):62–70. [Google Scholar]
- 5.Kizilcikh I, Kurt YD, kkurt BA, et al. Antimicrobial activity of a series of thiosemicarbazones and their ZnII and PdII Complexes. Folia Microbiologica. 2007;52:15–25. doi: 10.1007/BF02932132. [DOI] [PubMed] [Google Scholar]
- 6.Rosu T, Gulea A, Nicolae A, Georgescu R. Complexes of 3dn metal ions with thiosemicarbazones: synthesis and antimicrobial activity. Molecules. 2007;12(4):782–796. doi: 10.3390/12040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Er M, Ünver Y, Sancak K, Dügdü E. Synthesis and characterizations of some new tetra-thiosemicarbazones and their cyclization reactions; tetra-4-methyl-5-etoxycarbonyl-2,3-dihydro-1,3-thiazole and tetra-2-acetylamino-4-acetyl-4,5-dihydro-1,3,4-thiodiazole derivatives. Arkivoc. 2008;15:99–120. [Google Scholar]
- 8.Chandra S, Tyagi M. Ni(II), Pd(II) and Pt(II) complexes with ligand containing thiosemicarbazone and semicarbazone moiety: synthesis, characterization and biological investigation. Journal of the Serbian Chemical Society. 2008;73(7):727–734. [Google Scholar]
- 9.Konstantinović SS, Radovanović BC, Sovilj SP, Stanojević S. Antimicrobial activity of some isatin-3-thiosemicarbazone complexes. Journal of the Serbian Chemical Society. 2008;73(1):7–13. [Google Scholar]
- 10.Singh RV, Fahmi N, Biyala MK. Coordination behavior and biopotency of N and S/O donor ligands with their palladium(II) and platinum(II) complexes. Journal of the Iranian Chemical Society. 2005;2:40–46. [Google Scholar]
- 11.Otero L, Vieites M, Boiani L, et al. Novel antitrypanosomal agents based on palladium nitrofurylthiosemicarbazone complexes: DNA and redox metabolism as potential therapeutic targets. Journal of Medicinal Chemistry. 2006;49(11):3322–3331. doi: 10.1021/jm0512241. [DOI] [PubMed] [Google Scholar]
- 12.Chellan P, Stringer T, Shokar A, et al. Synthesis and in vitro evaluation of palladium(II) salicylaldiminato thiosemicarbazone complexes against Trichomonas vaginalis . Journal of Inorganic Biochemistry. 2011;105(12):1562–1568. doi: 10.1016/j.jinorgbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Lewis NA, Liu F, Seymour L, et al. Synthesis, characterisation, and preliminary in vitro studies of vanadium(IV) complexes with a Schiff base and thiosemicarbazones as mixed ligands. European Journal of Inorganic Chemistry. 2012;(4):664–677. doi: 10.1002/ejic.201100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran E, Kalaivani P, Prabhakaran R, et al. Synthesis, X-ray crystal structure, DNA binding, antioxidant and cytotoxicity studies of Ni(II) and Pd(II) thiosemicarbazone complexes. Metallomics. 2012;4(2):218–227. doi: 10.1039/c1mt00143d. [DOI] [PubMed] [Google Scholar]
- 15.Kulandaivelu U, Padmini VG, Suneetha K, et al. Synthesis, antimicrobial and anticancer activity of new thiosemicarbazone derivatives. Archiv der Pharmazie. 2011;344(2):84–90. doi: 10.1002/ardp.201000201. [DOI] [PubMed] [Google Scholar]
- 16.Atasever B, Ülküseven B, Bal-Demirci T, Erdem-Kuruca S, Solakoğlu Z. Cytotoxic activities of new iron(III) and nickel(II) chelates of some S-methyl-thiosemicarbazones on K562 and ECV304 cells. Investigational New Drugs. 2010;28(4):421–432. doi: 10.1007/s10637-009-9272-2. [DOI] [PubMed] [Google Scholar]
- 17.Li MX, Chen CL, Zhang D, Niu JY, Ji BS. Mn(II), Co(II) and Zn(II) complexes with heterocyclic substituted thiosemicarbazones: synthesis, characterization, X-ray crystal structures and antitumor comparison. European Journal of Medicinal Chemistry. 2010;45(7):3169–3177. doi: 10.1016/j.ejmech.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Vrdoljak V, Dilović I, Rubčić M, et al. Synthesis and characterisation of thiosemicarbazonato molybdenum(VI) complexes and their in vitro antitumor activity. European Journal of Medicinal Chemistry. 2010;45:38–48. doi: 10.1016/j.ejmech.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Ferraz KSO, Ferandes L, Carrilho D, et al. 2-Benzoylpyridine-N(4)-tolyl thiosemicarbazones and their palladium(II) complexes: cytotoxicity against leukemia cells. Bioorganic and Medicinal Chemistry. 2009;17(20):7138–7144. doi: 10.1016/j.bmc.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 20.Khan H, Badshah A, Zia-Ur-Rehman Z-U, et al. New dimeric and supramolecular mixed ligand Palladium(II) dithiocarbamates as potent DNA binders. Polyhedron. 2012;39(1):1–8. [Google Scholar]
- 21.Khan H, Badshah A, Murtaz G, et al. Synthesis, characterization and anticancer studies of mixed ligand dithiocarbamate palladium(II) complexes. European Journal of Medicinal Chemistry. 2011;46(9):4071–4077. doi: 10.1016/j.ejmech.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Lobana TS, Sharma R, Bawa G, Khanna S. Bonding and structure trends of thiosemicarbazone derivatives of metals-An overview. Coordination Chemistry Reviews. 2009;253(7-8):977–1055. [Google Scholar]
- 23.Suvarapu LN, Reddy AV, Kumar GS, Ok Baek S. Spectral characterization and antibacterial activities of benzyloxybenzaldehydethiosemicarbazone 3,4-Dihydroxybenzaldehydeisonicotinoylhydrazone and their transitional metal complexes. European Journal of Chemistry. 2011;8(4):1848–1858. [Google Scholar]
- 24.Thota S, Karki SS, Jayaveera KN, Balzarini J, Clercq ED. Synthesis and cytotoxic activity of some mononuclear Ru(II) Complexes. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2010;1(3):704–713. [Google Scholar]
- 25.Sampath N, Ponnuswamy MN, Nethaji M. Crystal structure and conformation study of N-methyl-t-3-methyl-r-2, c-6-diphenylpiperidin-4-one thiosemicarbazone. Crystal Research and Technology. 2006;41(2):192–197. doi: 10.1107/s010827010300917x. [DOI] [PubMed] [Google Scholar]
- 26.Butler IS, Elsayed SA, El-Hendawy AM, Mostafa SI, Jean-Claude BJ, Todorova M. Antineoplastic activity of new transition metal complexes of 6-methylpyridine-2-carbaldehyde-n(4)-ethylthiosemicarbazone: X-Ray crystal structures of [VO2(mpETSC)] and [Pt(mpETSC)Cl] Bioinorganic Chemistry and Applications. 2010;2010:11 pages. doi: 10.1155/2010/149149.149149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernándeza W, Paz J, Vaisberg A, Spodine E, Richter R, Beyer L. Synthesis, characterization, and in vitro cytotoxic activities of benzaldehyde thiosemicarbazone derivatives and their palladium (II) and platinum (II) complexes against various human tumor cell lines. Bioinorganic Chemistry and Applications. 2008;2008:9 pages. doi: 10.1155/2008/690952.690952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrugia LJ. WinGX suite for small-molecule single-crystal crystallography. Journal of Applied Crystallography. 1999;32(4):837–838. [Google Scholar]
- 29.Sheldrick GM. A short history of SHELX. Acta Crystallographica A. 2007;64(1):112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 30.Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 31.Glinma B, Kpoviessi DSS, Gbaguidi FA, et al. Synthesis, caracterization, trypanosomal activities on Trypanosoma bruceibrucei and toxicity against Artemia salina leach of N(4)-aryl semicarbazones and thiosemicarbazones. Journal of Chemical and Pharmaceutical Research. 2012;4(2):1016–1021. [Google Scholar]
- 32.da Maia PIS, Graminha A, Pavan FR, et al. Palladium(II) complexes with thiosemicarbazones: syntheses, characterization and cytotoxicity against breast cancer cells and anti-Mycobacterium tuberculosis activity. Journal of the Brazilian Chemical Society. 2010;21(7):1177–1186. [Google Scholar]
- 33.Lobana TS, Sánchez A, Casas JS, et al. Symmetrisation, isomerism and structural studies on novel phenylmercury(II) thiosemicarbazonates: correlation of the energy barrier to rotation of the amino group with the bonding parameters of the thioamide group. Journal of the Chemical Society. 1997;(22):4289–4299. [Google Scholar]
- 34.El-Shazly RM, Al-Hazmi GAA, Ghazy SE, El-Shahawi MS, El-Asmy AA. Synthesis and spectroscopic characterization of cobalt(II) thiosemicarbazone complexes. Journal of Coordination Chemistry. 2006;59(8):845–859. [Google Scholar]
- 35.Agarwal RK, Singh L, Sharma DK. Synthesis, spectral, and biological properties of copper(II) complexes of thiosemicarbazones of Schiff bases derived from 4-aminoantipyrine and aromatic aldehydes. Bioinorganic Chemistry and Applications. 2006;2006:10 pages. doi: 10.1155/BCA/2006/59509.59509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey OP, Sengupta SK, Mishra MK, Tripathi CM. Synthesis, spectral and antibacterial studies of binuclear titanium(IV)/zirconium(IV) complexes of piperazine dithiosemicarbazones. Bioinorganic Chemistry and Applications. 2003;1(1):35–44. doi: 10.1155/S1565363303000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Asmy AA, Khalifa ME, Hassanian MM. Synthesis of mono and binuclear complexes of α-oximinoacetoacetanilide-4-phenylthiosemicarbazone. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 2001;31(10):1787–1801. [Google Scholar]
- 38.Zidan ASA, El-Said AI. Studies of mixed ligand complexes from dialkyldithiophosphate and thiosemicarbazide or thiosemicarbazones with nickel(II) Phosphorus, Sulfur and Silicon and the Related Elements. 2004;179(7):1293–1305. [Google Scholar]
- 39.Affan MA, Wan FS, Ngaini Z, Shamsuddin M. Synthesis, characterization and biological studies of organotin(IV) complexes of thiosemicarbazone ligand derived from pyruvic acid: X-ray crystal structure of [Me2Sn(PAT)] The Malaysian Journal of Analytical Sciences. 2009;13:63–72. [Google Scholar]
- 40.Yakuphanoglu F, Balaban A, Dagdelen F, Aydogdu Y, Sekerci M, Erk B. Synthesis, characterization, and electrical properties of metal complexes of 2-pyridinecarbaldehyde thiosemicarbazone. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 2002;32(10):1865–1878. [Google Scholar]
- 41.Rajendran G, Amritha CS, Anto RJ, Cheriyan VT. Synthesis, thermal and antitumour studies of Th(IV) complexes with furan-2-carboxaldehyde4-phenyl-3-Thiosemicarbazone. Journal of the Serbian Chemical Society. 2010;75(6):749–761. [Google Scholar]
- 42.Hernández W, Paz J, Carrasco F, et al. Synthesis and characterization of new palladium(II) complexes with ligands derived from furan-2-carbaldehyde and benzaldehyde thiosemicarbazone and their in vitro cytotoxic activities against various human tumor cell lines. Zeitschrift für Naturforschung B. 2010;65(10):1271–1278. [Google Scholar]
- 43.Kumar AP, Reddy PR, Reddy VK. Spectrophotometric determination of nickel(II) with 2-hydroxy-3-methoxy-benzaldehyde thiosemicarbazone. Indian Journal of Chemistry A. 2007;46(10):1625–1629. doi: 10.1155/2007/48768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stringer T, Chellan P, Therrien B, Shunmoogam-Gounden N, Hendricks DT, Smith GS. Synthesis and structural characterization of binuclear palladium(II) complexes of salicylaldimine dithiosemicarbazones. Polyhedron. 2009;28(14):2839–2846. [Google Scholar]
- 45.Kizilcikli I, Ülküseven B, Daşdemir Y, Akkurt B. Zn(II) and Pd(II) complexes of thiosemicarbazone-S-alkyl esters derived from 2/3-formylpyridine. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 2004;34(4):653–665. [Google Scholar]
- 46.Al-Amiery AA, Al-Majedy YK, Abdulreazak H, Abood H. Synthesis, characterization, theoretical crystal structure, and antibacterial activities of some transition metal complexes of the thiosemicarbazone (Z)-2-(pyrrolidin-2-ylidene)hydrazinecarbothioamide. Bioinorganic Chemistry and Applications. 2011;2011:6 pages. doi: 10.1155/2011/483101.483101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovala-Demertzi D, Wiecek J, Ciunik Z, Zervou M, Demertzis MA. Diorganotin complexes of a thiosemicarbazone, synthesis: properties, X-ray crystal structure, and antiproliferative activity of diorganotin complexes. Bioinorganic Chemistry and Applications. 2010;2010:9 pages. doi: 10.1155/2010/867195.867195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yousef TA, Badria FA, Ghazy SE, El-Gammal OA, El-Reash GMA. In vitro and in vivo antitumor activity of some synthesized 4-(2-pyridyl)-3-Thiosemicarbazides derivatives. International Journal of Medicine and Medical Sciences. 2011;3:37–46. [Google Scholar]
- 49.Lessa JA, Guerra JC, De Miranda LF, et al. Gold(I) complexes with thiosemicarbazones: cytotoxicity against human tumor cell lines and inhibition of thioredoxin reductase activity. Journal of Inorganic Biochemistry. 2011;105(12):1729–1739. doi: 10.1016/j.jinorgbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (figures containing IR, 1H, COSY and 13C NMR spectra).


