Abstract
Spirometra erinaceieuropaei cysteine protease (SeCP) in sparganum ES proteins recognized by early infection sera was identified by MALDI-TOF/TOF-MS. The aim of this study was to predict the structures and functions of SeCP protein by using the full length cDNA sequence of SeCP gene with online sites and software programs. The SeCP gene sequence was of 1 053 bp length with a 1011 bp biggest ORF encoding 336-amino acid protein with a complete cathepsin propeptide inhibitor domain and a peptidase C1A conserved domain. The predicted molecular weight and isoelectric point of SeCP were 37.87 kDa and 6.47, respectively. The SeCP has a signal peptide site and no transmembrane domain, located outside the membrane. The secondary structure of SeCP contained 8 α-helixes, 7 β-strands, and 20 coils. The SeCP had 15 potential antigenic epitopes and 19 HLA-I restricted epitopes. Based on the phylogenetic analysis of SeCP, S. erinaceieuropaei has the closest evolutionary status with S. mansonoides. SeCP was a kind of proteolytic enzyme with a variety of biological functions and its antigenic epitopes could provide important insights on the diagnostic antigens and target molecular of antisparganum drugs.
1. Introduction
Sparganosis is a serious parasitic zoonosis caused by infection with spargana, the plerocercoid larvae of some Diphyllobothrium tapeworms that belong to the genus Spirometra [1]. The most important species of the genus Spirometra tapeworms with plerocercoids that can produce sparganosis in human include Spirometra erinaceieuropaei (syn. Spirometra erinacei or Spirometra mansoni) which is the most common in Asia, and Spirometra mansonoides which is mainly distributed in North America [2]. The adults are intestinal parasites of some species of Canidae and Felidae; the first intermediate hosts are freshwater copepods (cyclops), whereas the second intermediate or paratenic hosts belong to different species of vertebrates (frogs, snakes, pigs, etc.) [3, 4]. Human is an accidental host. Human infection results mainly from drinking raw water contaminated with cyclops harboring procercoid, ingesting raw fleshes of frogs and snakes infected with plerocercoids, or placing frog or snake flesh on open wound for treatment of skin ulcers or eye inflammations [5, 6].
Human sparganosis is reported in many countries of the world but is most common in Eastern Asia and the Far East [7]. Sparganosis poses a serious threat to human health; the plerocercoids usually lodge in the subcutaneous tissues and muscles but sometimes invade the abdominal cavity, eye, and central nervous system causing blindness, seizures, headache, epilepsy, paralysis, and even death [8]. Ocular sparganosis is especially prevalent in China and Vietnam [9]. The clinical diagnosis of sparganosis is rather difficult and often misdiagnosed because the larvae have no predilection site in humans and the specific signs or symptoms are lacking. A definite diagnosis of subcutaneous sparganosis can be achieved by detection of the larvae in a biopsy specimen from the lesion, but the confirmative diagnosis is very difficult for visceral and cerebral sparganosis since the larva is found only by surgical removal [10]. The ELISA using the crude or excretory-secretory (ES) antiagens of plerocercoids has high sensitivity for the detection of sparganum infection in humans, but the main disadvantage is the false negative results during the early stage of infection and the cross-reactions with serum samples from patients with other parasitic diseases (cysticercosis, paragonimiasis, clonorchiasis, etc.) [11, 12].
In order to separate the early specific diagnostic antiagens, the ES proteins of S. erinaceieuropaei sparganum were analyzed by two-dimensional electrophoresis (2DE) and Western blot probed with early sera from infected mice at 14 days after infection. Three immunoreactive protein spots were successfully identified by MALDI-TOF/TOF-MS and characterized as the S. erinaceieuropaei cysteine protease (SeCP) [13]. In this paper, the full-length cDNA sequence of SeCP (GenBank accession no. 1834307) was analyzed; its structure and function were predicted by using bioinformatics techniques.
2. Materials and Methods
The full-length cDNA sequence of SeCP (GenBank accession no. 1834307) was used in this study. The structure domain and function domain were predicted by online analysis http://smart.embl-heidelberg.de/. The amino acid sequence was submitted to http://www.expasy.org/tools/protparam.html and its physical and chemical properties were predicted. Signal peptide was predicted by a web-based tool (http://www.cbs.dtu.dk/services/SignalP/), and subcellular localization was predicted using http://psort.nibb.ac.jp/form2.html. Hydrophilic prediction was predicted at http://www.expasy.org/cgi-bin/protscale.pl. Transmembrane domain was predicted through http://www.cbs.dtu.dk/services/TMHMM-2.0/. The secondary structures were constructed using the software PSIPRED v3.0 http://bioinf.cs.ucl.ac.uk/psipred/ [14, 15]. The 3D models of proteins were constructed by I-TASSER, a protein structure server on the website http://zhanglab.ccmb.med.umich.edu/I-TASSER/, which is considered to predict protein 3D structures that have more than 100 amino acids [16–18]. Visual molecular dynamics (VMD) was used to read standard Protein Data Bank (PDB) files and display the contained structure [19–21]. VMD is a molecular visualization software for displaying, animating, and analyzing large biomolecular systems using 3D graphics and built-in scripts http://www.ks.uiuc.edu/Research/vmd/. Amino acid sequence was submitted to http://www.cbs.dtu.dk/services/BepiPred/ in order to predict its antigen epitopes. Conserved HLA-restricted CD8+ T cells epitopes were also predicted using the software from IEDB http://www.immuneepitope.org/ which could identify novel HLA-class I restricted CD8+T cell epitopes.
Other cysteine protease amino sequences of model organisms of other parasites used in this study were obtained from GenBank (http://www.ncbi.nlm.nih.gov/Genbank/index.html) and listed as follows: Clonorchis sinensis (AAD29130.1), Homo sapiens (CAB42883.1), Spirometra mansonoides (AAB17051.1), Taenia solium (BAH03395.1), Paragonimus westermani (AAF21457.2), Schistosoma japonicum (CAX71578.10), Schistosoma mansoni (P25792.1), Taenia pisiformis (AEE69034.1), Haemonchus contortus (ACS36090.1), Entamoeba histolytica (CAA62836.1), Trichinella spiralis (XP_003377240.1), Plasmodium vivax (AAA60368.1), Brugia malayi (XP_001896823.1), Echinococcus multilocularis (BAF02516.1), Arabidopsis thaliana (AAB67626.1), Mus musculus (AAA37445.1), Drosophila melanogaster (AAB18345.1), Caenorhabditis elegans (AAA98785.1), Haemaphysalis longicornis (BAH86062.1), and Aedes aegypti (ABE72970.1). The multiple sequence alignment of SeCP and the above-mentioned sequences were carried out by Clustal X; then, molecular evolutionary tree was constructed by MEGA4.1 [22]. Phylogenies were estimated under the neighbor-joining (N-J) method [23].
3. Results
3.1. The Basic Properties of SeCP Sequence
The SeCP sequence was of 1 053 bp length with a 1011 bp biggest OFR from 9 bp (ATG) to 1019 bp (TAA), which encoded 336-amino acid protein with 3′UTR locating at the positions 1020–1053 bp. Nucleotide sequence and deduced amino acid sequence were shown in Figure 1.
Figure 1.

Sequences and amino acid residues of SeCP. The SeCP sequence was of 1 053 bp length with a 1011 bp biggest OFR from 9 bp (ATG) to 1019 bp (TAA), which encoded 336-amino acid protein with 3′UTR locating at the positions 1020–1053 bp.
3.2. Physical and Chemical Properties of SeCP
The SeCP had the molecular weight of 37.87 kDa and theoretical isoelectric point (pI) of 6.47. Extinction coefficients are 74300 M−1 cm−1, at 280 nm measured in water, assuming all pairs of Cys residues form cysteines. The half-life was 30 h, >20 h, and >10 h in mammalian reticulocytes (in vitro), yeast (in vivo), and Escherichia coli (in vivo), respectively. The instability index (II) was computed to be 32.11. This classifies the protein as stable. Aliphatic index is 75.74. Grand average of hydropathicity (GRAVY) is −0.321.
3.3. Structural Domain, Hydrophobicity, Signal Peptide, Subcellular Localization, and Transmembrane Domain
The confidently predicted SeCP structure domains contained a complete cathepsin propeptide inhibitor domain (I29) located at 32aa–92aa and a peptidase_C1A with an active site located at 39aa–303aa and a S2 subsite 189aa–330aa, which has the function of cysteine-type peptidase activity (Figure 2). Using the scale Hphob./Kyte and Doolittle, the SeCP protein has an obvious hydrophobic regions at 5′ (Figure 3).
Figure 2.
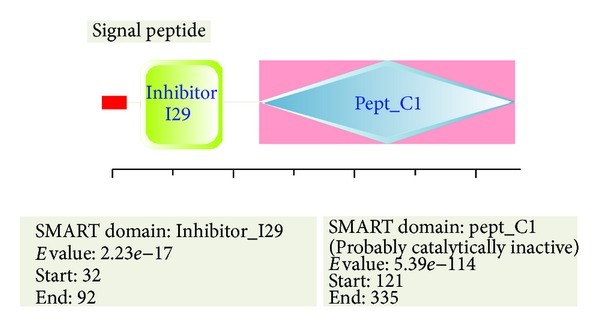
Prediction of structure domains of SeCP by SMART servers. The confidently predicted SeCP structure domains contained a complete cathepsin propeptide inhibitor domain (I29) located at 32aa–92aa and a peptidase_C1A with an active site located at 39aa–303aa and a S2 subsite 189aa–330aa, which has the function of cysteine-type peptidase activity.
Figure 3.
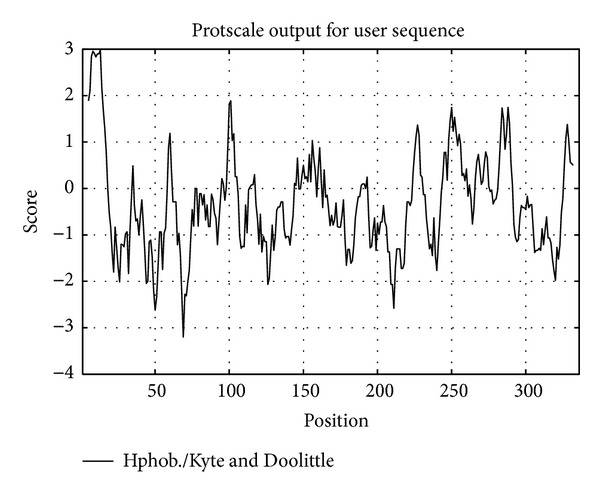
Hydrophobicity of SeCP. The SeCP protein has an obvious hydrophobic regions at 5′ predicted by using the scale Hphob./Kyte and Doolittle.
The prediction results of SeCP signal by Signal P-4.1 showed that there was a peak fraction at 19aa residue position (Figure 4). The score was 3.87 which was high enough with split site. So, the SeCP protein had a cleavable signal peptide (from 1 to 19) and with possible cleavage site between 19aa and 20aa.
Figure 4.
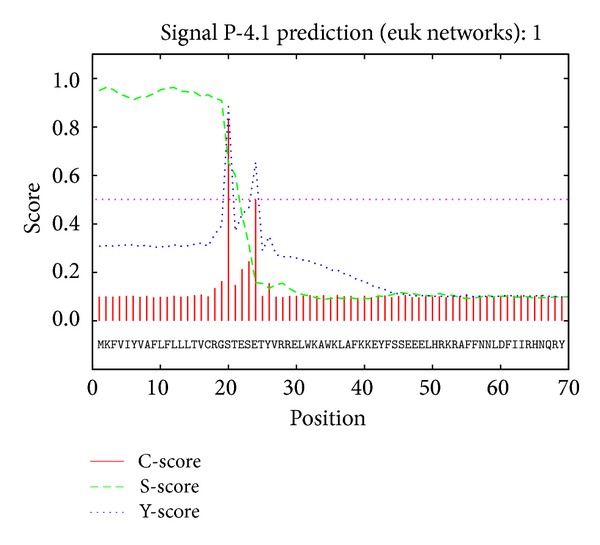
Prediction of SeCP signal peptide. There was a peak fraction at 19aa residue position and the score was 3.87 which was high enough with split site. The SeCP protein had a cleavable signal peptide (1 to 19) with possible cleavage site between 19aa and 20aa.
Results of the k-NN prediction of SeCP suggested that the peptide chain was located in the extracellular (including cell wall), vacuolar, mitochondrial, and endoplasmic reticulum, with the possibility of 55.6%, 22.2%, 11.1%, and 11.1%, respectively. The maximum possible location was in the extracellular (k = 23).
Prediction of transmembrane domain of SeCP with TMHMM Server v. 2.0 suggested that the SeCP had no transmembrane domain, located outside the membrane.
3.4. 2D Structure Alignment for SeCP
PSIPRED v. 3.3 was used to predict the secondary structures of SeCP which had 8 α-helixes, 7 β-strands, and 20 coils (Figure 5).
Figure 5.
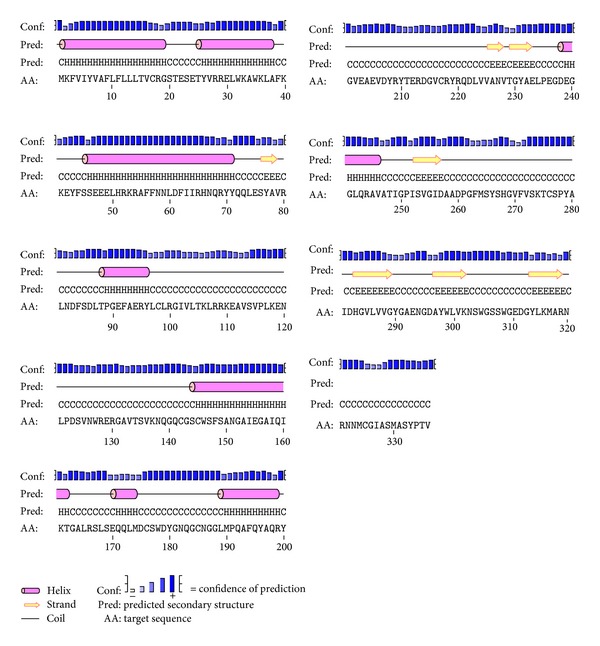
The predicted secondary structure of SeCP by using PSIPRED. There were 8 α-helixes, 7 β-strands, and 20 coils of the predicted secondary structure of SeCP.
3.5. Construction of 3D Model and Enzyme Activity Predicting
Five models were set up for each protein by Dr. Zhang's lab [16]. We selected the model with the highest confidence C-score (Figure 6), which estimates the quality of predicted models by I-TASSER. It was calculated based on the significance of threading template alignments and the convergence parameters of the structure assembly simulations. C-score is typically in the range of [−5, 2], and a model with a C-score above 2 suggested a high confidence. Enzyme homologs in PDB predicted by I-TASSER showed that the most reliable enzyme classification (EC) number prediction was 3.4.22.43, with the highest CscoreEC (0.664). CscoreEC is the confidence score for the EC number prediction. CscoreEC values range in between [0-1], where a higher score indicates a more reliable EC number prediction. The recombinant enzyme hydrolyzes proteins (serum albumin, collagen) and CA synthetic substrates (Z-Phe-Arg-NHMec > Z-Leu-Arg-NHMec > Z-Val-Arg-NHMec). The maximum activity of the enzyme was at pH 5.7 and was unstable at neutral pH. Compound E-64, leupeptin, and chicken cystatin are inhibitors of cysteine protease which belongs to peptidase family C1 (http://enzyme.expasy.org/EC/3.4.22.43).
Figure 6.
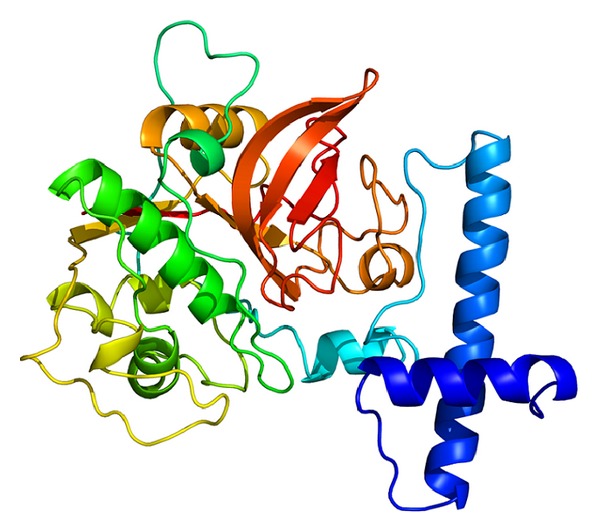
The 3D model of SeCP with highest confidence C-score, which estimates the quality of predicted models by I-TASSER.
3.6. Antigenic Epitope of SeCP
The sequence of SeCP was compared with the host's homologous sequences corresponding regional sequence by BepiPred 1.0b Server. The SeCP had 15 potential antigen epitopes (19aa–26aa, 44aa–50aa, 86aa–92aa, 118aa–128aa, 130aa–143aa, 151aa–155aa, 179aa–189aa, 199aa–205aa, 207aa–214aa, 231aa–243aa, 254aa–264aa, 276aa–280aa, 291aa–296aa, 305aa–312aa, and 332aa–336aa). Epitope prediction algorithm consensus was used to predict peptides that could stimulate human to induce effective and protective immune response against S. erinaceieuropaei, when the conserved HLA-restricted CD8+ T cells, epitopes of SeCP were predicted. The SeCP had 19 conserved peptides based on a high HLA allele binding score (percentile rank < 1) (Table 1).
Table 1.
The predicted HLA restricted CD8+ T cell epitopes for SeCP.
| Allele | Start | End | Peptide | Method | Percentile_rank |
|---|---|---|---|---|---|
| HLA-A*02:01 | 3 | 16 | FVIYVAFLFLLLTV | Ann | 0.3 |
| HLA-A*02:01 | 263 | 272 | FMSYSHGVFV | Consensus (ann/smm) | 0.4 |
| HLA-A*02:01 | 194 | 206 | FQYAQRYGVEAEV | Ann | 0.4 |
| HLA-A*02:01 | 194 | 202 | FQYAQRYGV | Consensus (ann/smm/comblib_sidney 2008) | 0.5 |
| HLA-A*02:01 | 188 | 197 | GLMPQAFQYA | Consensus (ann/smm) | 0.55 |
| HLA-A*02:01 | 6 | 16 | YVAFLFLLLTV | Consensus (ann/smm) | 0.65 |
| HLA-A*02:01 | 3 | 14 | FVIYVAFLFLLL | Ann | 0.8 |
| HLA-A*02:01 | 9 | 16 | FLFLLLTV | Consensus (ann/smm) | 0.9 |
| HLA-A*11:01 | 124 | 137 | SVNWRERGAVTSVK | Ann | 0.3 |
| HLA-A*11:01 | 33 | 40 | KAWKLAFK | Consensus (ann/smm) | 0.45 |
| HLA-A*11:01 | 307 | 316 | SSWGEDGYLK | Consensus (ann/smm) | 0.5 |
| HLA-A*11:01 | 262 | 274 | GFMSYSHGVFVSK | Ann | 0.6 |
| HLA-A*11:01 | 33 | 41 | KAWKLAFKK | Consensus (ann/smm) | 0.6 |
| HLA-A*11:01 | 266 | 274 | YSHGVFVSK | Consensus (ann/smm) | 0.6 |
| HLA-A*11:01 | 264 | 274 | MSYSHGVFVSK | Consensus (ann/smm) | 0.7 |
| HLA-B*07:02 | 277 | 286 | SPYAIDHGVL | Consensus (ann/smm) | 0.35 |
| HLA-B*07:02 | 317 | 330 | MARNRNNMCGIASM | Ann | 0.5 |
| HLA-B*07:02 | 277 | 288 | SPYAIDHGVLVV | Ann | 0.6 |
| HLA-B*07:02 | 106 | 117 | KLRRKEAVSVPL | Ann | 0.7 |
3.7. Multiple Sequence Alignment and Molecular Evolution of SeCP
Multiple sequence alignment and phylogenetic analysis of SeCP with the cysteine protease of other species were displayed in Figure 7. Based on the phylogenetic analysis of SeCP, Spirometra erinaceieuropaei has the closest evolutionary status with Spirometra mansonoides.
Figure 7.
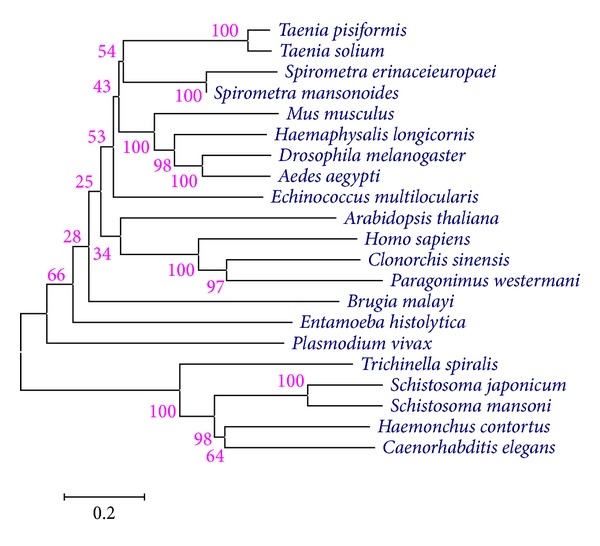
Neighbor-joining phylogenetic tree referred from cysteine protease amino acid sequence of Spirometra erinacei. Bootstrap values are indicated on branches.
4. Discussion
Cysteine protease is a kind of proteolytic enzyme, which contains cysteine residues in the center of enzyme activity. It has been shown that the cysteine protease of many parasites acts extracellularly to help invade tissues and cells, to uptake nutrient, to hatch, or to evade the host immune system [24–26]. Cysteine protease is the key factor in the parasitic pathogenicity, either by inducing tissue damage and facilitating invasion or by empowering the parasites to salvage metabolites from host proteins [27, 28]. Cysteine protease has been detected in S. erinacei [29, 30]. The plerocercoids of S. erinacei is also known to secrete a large amount of cysteine proteases [31]. The cysteine protease from S. erinacei can hydrolyze collagen, hemoglobin, and immunoglobulin G (IgG) in vitro and may be concerned with digestion of host tissue in pathogenesis [32, 33]. Our previous study on 2DE analysis showed that the ES proteins of S. erinacei plerocercoids had a total of approximately 149 proteins spots with molecular weight varying from 20 to 115 kDa and isoelectric point (pI) from 3 to 7.5. When probed with sera from infected mice at 14 days after infection, seven protein spots with molecular weight of 23–31 kDa were recognized and analyzed by MALDI-TOF/TOF-MS. Three of seven spots were successfully identified and characterized as the same protein SeCP [13]. The SeCP might come from the excretory and secretory products and the cuticles (membrane proteins) and are directly exposed to the host's immune system and are the main target antiagens which induce the immune responses.
Based on the construction of full-length cDNA library of SeCP, the sequence of SeCP gene was of 1 053 bp length with a 1011 bp biggest ORF encoding 336-amino acid protein with a complete cathepsin propeptide inhibitor domain and a peptidase_C1A conserved domain. The predicted molecular weight and isoelectric point of the deduced SeCP protein were 37.87 Da and 6.47, respectively. Based on the phylogenetic analysis of SeCP, Spirometra erinaceieuropaei has the closest evolutionary status with Spirometra mansonoides. The secondary structure of SeCP contained has 8 α-helixes, 7 β-strands, and 20 coils. The SeCP had 15 potential antigenic epitopes and 19 HLA-I restricted epitopes. These predicted antigenic epitopes could provide important insights on the diagnostic antiagens and target molecular of antiparasitic drugs for sparganosis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81172612) and the Zhengzhou University Scientific Research Grant for the Postgraduates (no. 12Y03001).
References
- 1.Bogitsch BJ, Carter CE, Oeltmann TN. Human Parasitology. 3rd edition. New York, NY, USA: Academic Press; 2005. [Google Scholar]
- 2.Roberts LS, Schmidt GD, Janovy J. Foundations of Parasitology. 8th edition. New York, NY, USA: McGraw-Hill; 2009. [Google Scholar]
- 3.Nithiuthai S, Anantaphruti MT, Waikagul J, Gajadhar A. Waterborne zoonotic helminthiases. Veterinary Parasitology. 2004;126(1-2):167–193. doi: 10.1016/j.vetpar.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Shin EH, Guk SM, Kim HJ, Lee SH, Chai JY. Trends in parasitic diseases in the Republic of Korea. Trends in Parasitology. 2008;24(3):143–150. doi: 10.1016/j.pt.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima T, Yamane Y. How does the sparganosis occur? Parasitology Today. 1999;15(3):p. 124. doi: 10.1016/s0169-4758(99)01405-2. [DOI] [PubMed] [Google Scholar]
- 6.Magnino S, Colin P, Dei-Cas E, et al. Biological risks associated with consumption of reptile products. International Journal of Food Microbiology. 2009;134(3):163–175. doi: 10.1016/j.ijfoodmicro.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Anantaphruti MT, Nawa Y, Vanvanitchai Y. Human sparganosis in Thailand: an overview. Acta Tropica. 2011;118(3):171–176. doi: 10.1016/j.actatropica.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Shirakawa K, Yamasaki H, Ito A, Miyajima H. Cerebral sparganosis: the wandering lesion. Neurology. 2010;74(2):p. 180. doi: 10.1212/WNL.0b013e3181c91a15. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Lin XM, Zhang HW, Xu BL, Wang ZQ. Sparganosis, Henan province, central China. Emerging Infectious Diseases. 2011;17(1):146–147. doi: 10.3201/eid1701.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata K, Abe T, Gohda M, et al. Difficulty in diagnosing a case with apparent sequel cerebral sparganosis. Surgical Neurology. 2007;67(4):409–411. doi: 10.1016/j.surneu.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 11.Nishiyama T, Ide T, Himes SR, Jr., Ishizaka S, Araki T. Immunodiagnosis of human sparganosis mansoni by micro-chemiluminescence enzyme-linked immunosorbent assay. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88(6):663–665. doi: 10.1016/0035-9203(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Li N, Wang ZQ, Jiang P, Lin XM. Serodiagnosis of experimental sparganum infections of mice and human sparganosis by ELISA using ES antigens of Spirometra mansoni spargana. Parasitology Research. 2011;108(6):1551–1556. doi: 10.1007/s00436-010-2206-2. [DOI] [PubMed] [Google Scholar]
- 13.Hu DD, Cui J, Wang L, Liu LN, Wei T, Wang ZQ. Immunoproteomic analysis of the excretory-secretory proteins from Spirometra mansoni Sparganum. Iranian Journal of Parasitology. 2013;8(3):408–416. [PMC free article] [PubMed] [Google Scholar]
- 14.Sadowski MI, Jones DT. The sequence-structure relationship and protein function prediction. Current Opinion in Structural Biology. 2009;19(3):357–362. doi: 10.1016/j.sbi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Buchan DW, Ward SM, Lobley AE, Nugent TCO, Bryson K, Jones DT. Protein annotation and modelling servers at University College London. Nucleic Acids Research. 2010;38:W563–W568. doi: 10.1093/nar/gkq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9(article 40) doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nature Protocols. 2010;5(4):725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy A, Yang J, Zhang Y. COFACTOR: an accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Research. 2012;40:W471–W477. doi: 10.1093/nar/gks372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. Journal of Molecular Graphics. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 20.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Research. 2003;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30(supplement 1):S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 22.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 23.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 24.Salas F, Fichmann J, Lee GK, Scott MD, Rosenthal PJ. Functional expression of falcipain, a Plasmodium falciparum cysteine proteinase, supports its role as a malarial hemoglobinase. Infection and Immunity. 1995;63(6):2120–2125. doi: 10.1128/iai.63.6.2120-2125.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasilewski MM, Lim KC, Phillips J, McKerrow JH. Cysteine protease inhibitors block schistosome hemoglobin degradation in vitro and decrease worm burden and egg production in vivo. Molecular and Biochemical Parasitology. 1996;81(2):179–189. doi: 10.1016/0166-6851(96)02703-x. [DOI] [PubMed] [Google Scholar]
- 26.Olson JE, Lee GK, Semenov A, Rosenthal PJ. Antimalarial effects in mice of orally administered peptidyl cysteine protease inhibitors. Bioorganic and Medicinal Chemistry. 1999;7(4):633–638. doi: 10.1016/s0968-0896(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 27.Chung YB, Kong Y, Joo IJ, Cho SY, Kang SY. Excystment of Paragonimus westermani metacercariae by endogenous cysteine protease. Journal of Parasitology. 1995;81(2):137–142. [PubMed] [Google Scholar]
- 28.Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP. Proteinases and associated genes of parasitic helminths. Advances in Parasitology. 1999;43:161–266. doi: 10.1016/s0065-308x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- 29.Fukase TM, Akihama Y, Itagaki H. Purification and some properties of cysteine protease of Spirometra erinacei plerocercoid (Cestoda: Diphyllobothriidae) Japanese Journal of Parasitology. 1985;35(5):351–360. [Google Scholar]
- 30.Song CY, Choi DH, Kim TS, Lee SH. Isolation and partial characterization of cysteine proteinase from sparganum. Kisaengchunghak Chapchi. 1992;30(3):191–199. doi: 10.3347/kjp.1992.30.3.191. [DOI] [PubMed] [Google Scholar]
- 31.Chung YB, Yang HJ. Partial purification and characterization of a cysteine protease Inhibitor from the plerocercoid of Spirometra erinacei . Korean Journal of Parasitology. 2008;46(3):183–186. doi: 10.3347/kjp.2008.46.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song CY, Chappell CL. Purification and partial characterization of cysteine proteinase from Spirometra mansoni plerocercoids. Journal of Parasitology. 1993;79(4):517–524. [PubMed] [Google Scholar]
- 33.Kong Y, Chung YB, Cho SY, Kang SY. Cleavage of immunoglobulin G by excretory-secretory cathepsin S-like protease of Spirometra mansoni plerocercoid. Parasitology. 1994;109(part 5):611–621. doi: 10.1017/s0031182000076496. [DOI] [PubMed] [Google Scholar]


