Abstract
We describe the unique properties of low-molecular-weight heparins and review studies of the use of these agents in the catheterization laboratory for percutaneous coronary intervention. Recent data regarding bedside monitoring of low-molecular-weight heparin activity are also discussed, as are ongoing and future studies that should be of assistance to clinicians as they consider expanding the use of this newer form of antithrombotic therapy.
Key words: Angioplasty, transluminal, percutaneous coronary; anticoagulants; antithrombin therapy; myocardial revascularization; platelet glycoprotein GP IIb/IIIa complex/antagonists & inhibitors
During the past few years, significant advances have been made in the treatment of patients presenting with acute coronary syndromes (ACS). Medical therapy for patients with unstable angina and non-ST-elevation myocardial infarction (MI) now includes a variety of antiplatelet and antithrombotic agents. In addition, percutaneous coronary intervention (PCI) is an increasingly important tool in the management of high-risk patients. Clinicians now recognize that “optimal” medical management of patients with ACS has to allow for a smooth, effective transition to the catheterization laboratory, should that option be selected.
The use of low-molecular-weight heparins (LMWHs) as 1st-line antithrombotic therapy for the management of patients presenting with ACS is beginning to gain widespread acceptance. In the context of standard-of-care medical management, the LMWH enoxaparin was compared with unfractionated heparin (UFH) in 2 large, randomized, double-blinded trials in patients with ACS: 1,2 the Thrombolysis in Myocardial Infarction (TIMI) 11B study 1 and the Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events (ESSENCE) trial. 2 Pooled data from these trials showed an approximately 20% reduction in the composite of death or non-fatal MI at all time points in the group that received enoxaparin. 3 Clinical trials in patients with ACS have also shown the efficacy of glycoprotein (GP) IIb/IIIa antagonists when added to conventional therapy. 4,5 More recently, attempts have been made to combine the newer LMWHs and GP IIb/IIIa antagonists. The ACUTE II study, 6 published in 2002, compared the use of enoxaparin versus UFH in patients with ACS treated with the GP IIb/IIIa antagonist tirofiban and aspirin. In those patients, the use of enoxaparin as antithrombotic therapy was associated with a low rate of bleeding events similar to that found with UFH, and carried a significantly lower incidence of refractory ischemia requiring urgent revascularization and rehospitalization because of unstable angina. 6
Major questions have yet to be answered, however, such as how to handle the transition of patients to the catheterization laboratory after they have been given LMWHs, and whether LMWHs are truly better than UFH in invasively managed patients. Although the short- and long-term benefits of PCI are becoming increasingly apparent, optimal antiplatelet and antithrombotic therapies have the potential to improve outcomes further. We present this review in order to describe the unique properties of LMWH, to discuss studies that have used LMWH in the cardiac catheterization laboratory, to present some of the recent data regarding bedside monitoring of LMWH activity, and to call attention to ongoing and future studies that will guide clinicians as they consider using LMWHs.
Antithrombotic Therapy: UFH and LMWH
Heparin is a glycosaminoglycan composed of a heterogeneous mixture of polysaccharide chains ranging in molecular weight from 3,000 to 30,000 daltons. 7 Low-molecular-weight heparins are fragments of commercially available UFH prepared by either chemical or enzymatic depolymerization processes that yield chains with mean molecular weights of approximately 5,000 daltons. The anticoagulant activities of UFH and LMWH occur via activation of antithrombin. This activation depends on a specific pentasaccharide sequence randomly dispersed within the heparin chain, which has a high affinity for antithrombin. Heparin of any length containing this unique sequence can bind to antithrombin, cause a conformational change in antithrombin, and enhance the inactivation of factor Xa. Inhibition of thrombin (IIa) activation, however, requires the formation of a ternary complex between heparin, antithrombin, and thrombin. This complex can be formed only by heparin molecules with <18 saccharide units. Unfractionated heparin is composed primarily of chains with <18 units; LMWHs are enriched in shorter-chain versions. Hence, UFH preparations exhibit an anti-Xa:anti-IIa potency of approximately 1:1, whereas LMWHs preferentially inhibit factor Xa, since the actions of the heparin-antithrombin complex on factor Xa are not chain-length dependent, as are the actions on factor IIa. A consequence of this difference is that LMWHs have more activity upstream in the coagulation cascade and therefore act more efficiently (Fig. 1).
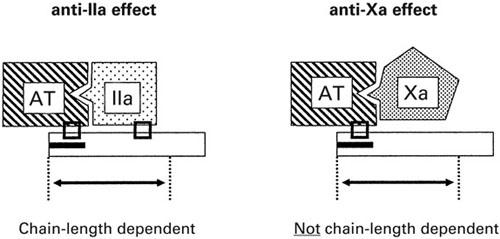
Fig. 1 Heparin works by binding to antithrombin (AT) and facilitating the inhibition of thrombin (factor IIa). In order for this to happen, thrombin needs to bind to the heparin-antithrombin complex; hence, the inhibition of thrombin is chain-length dependent. The heparin-antithrombin complex also works upstream in the coagulation cascade, at the level of factor X. The inhibition of factor X does not require additional binding; hence, it is not chain-length dependent. Therefore, it is possible to modify heparin by shortening the chain length (thereby reducing protein binding, platelet activation, and risk of heparin-induced thrombocytopenia) to have more specific activity at the level of factor X. The low-molecular-weight heparins are enriched in exactly such shorter-chain heparin fragments.
The longer chain lengths of UFH underlie a number of substantial limitations. Standard UFH binds nonspecifically to a variety of plasma proteins, thereby limiting the amount available to interact with antithrombin, and consequently leading to an unreliable degree of anticoagulation. Both ex vivo and in vitro findings have shown that the shorter-chain LMWHs bind significantly less to plasma proteins. 8 Other features of LMWHs that are of particular clinical importance are decreased neutralization by plasma regulatory proteins (such as platelet factor 4) and lower rates of the unwanted side effects of heparin, such as platelet activation, heparin-induced thrombocytopenia, and osteoporosis. Unfractionated heparin has an unpredictable anticoagulant response, which makes frequent laboratory monitoring and dose adjustments essential. The LMWHs have increased bioavailability, dose-dependent clearance, and decreased affinity for plasma proteins, resulting in a more predictable anticoagulant response; consequently, routine laboratory monitoring and dose adjustments are unnecessary to achieve therapeutic efficacy.
Postprocedural Use of LMWH (Table I)
TABLE I. Clinical Studies of LMWH as Procedural and Postprocedural Anticoagulation for PCI
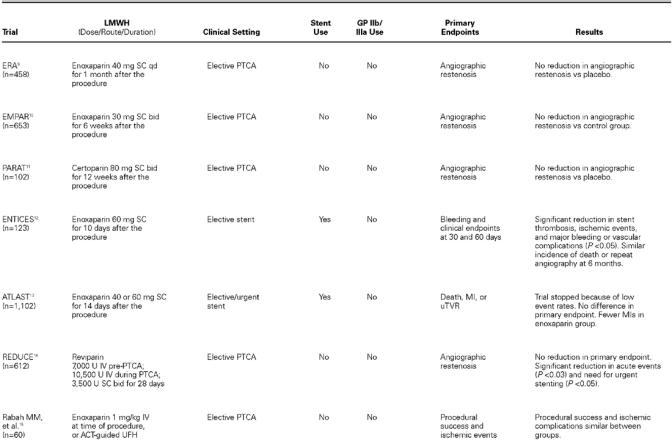
TABLE I. Continued.
ERA. The Enoxaparin Restenosis (ERA) trial 9 was a small study investigating the effect on restenosis of enoxaparin administered subcutaneously (SC) once daily for 1 month after successful angioplasty. A total of 458 patients were randomized to receive either 40 mg enoxaparin (n = 227) or placebo (n = 231). The primary endpoints of clinical or angiographic restenosis were no different between the 2 groups. The only difference found was the incidence of minor bleeding complications, which were more common in the group receiving enoxaparin. 9
EMPAR. The EMPAR (Enoxaparin MaxEPA Prevention of Angioplasty Restenosis) study 10 also examined the postprocedural use of enoxaparin in reducing restenosis after percutaneous transluminal coronary angioplasty (PTCA). In this study, 814 patients were randomized to treatment with fish oils or placebo for a median of 6 days before PTCA. Of these, 653 patients with at least 1 successfully dilated lesion were further randomized to receive enoxaparin (30 mg SC twice a day) or control for 6 weeks. No reduction in restenosis was found with sustained LMWH therapy. 10
PARAT. PARAT (Prophylaxis Against Restenosis Angioplasty Trial) 11 evaluated the safety and efficacy of the LMWH certoparin in preventing restenosis after PTCA. In this randomized placebo-controlled study, 118 patients with at least 1 lesion were enrolled (102 completed the study). Patients were randomized to twice daily SC injections of either placebo or cetoparin (80 mg) for 3 months. At the 3-month follow-up, patients underwent quantitative coronary angiography and analysis of safety. The safety of the certoparin cohort was confirmed by low rates of hematuria, by fecal occult blood positivity, and by bone-density analysis. The primary endpoint of post-PTCA restenosis, however, was similar between the LMWH and placebo cohorts. 11
ENTICES. The pilot study Enoxaparin and Ticlopidine after Elective Stenting (ENTICES) 12 examined the use of enoxaparin, ticlopidine, and aspirin after elective stent placement and compared the results with those of the then-conventional therapy, consisting of warfarin, UFH, dextran, dipyridamole, and aspirin. Those patients assigned to enoxaparin, ticlopidine, and aspirin had a significantly lower composite rate of in-hospital bleeding and vascular complications, a significantly lower composite endpoint rate at 30 days, and a significantly lower rate of stent thrombosis. However, the incidence of death or repeat angioplasty at 6 months was found to be similar between the 2 groups. 12
ATLAST. The Antiplatelet Therapy alone versus Lovenox plus Antiplatelet therapy in patients at increased risk of Stent Thrombosis (ATLAST) trial 13 randomized 1,102 patients to receive either enoxaparin (60 mg SC twice a day) or placebo for 2 weeks after stent placement. All patients received aspirin (325 mg daily for 6 months) and ticlopidine (250 mg twice daily for 14 days) after stent placement. The primary endpoint of death, MI, or urgent revascularization at 30 days occurred in 1.8% of enoxaparin-treated patients and in 2.7% of those treated with placebo (P = 0.30), but the difference did not reach statistical significance. (The trial was halted early when an interim analysis revealed an overall event rate far lower than predicted.) Enoxaparin was, however, associated with significantly fewer MIs at both 14 and 30 days (P < 0.05). The secondary endpoint of minor bleeding was increased with use of enoxaparin in comparison with placebo (P < 0.001), but the rate of major bleeding was not statistically significant between the 2 groups (P = 0.08). 13
Peri- and Postprocedural Use of LMWH (Table I)
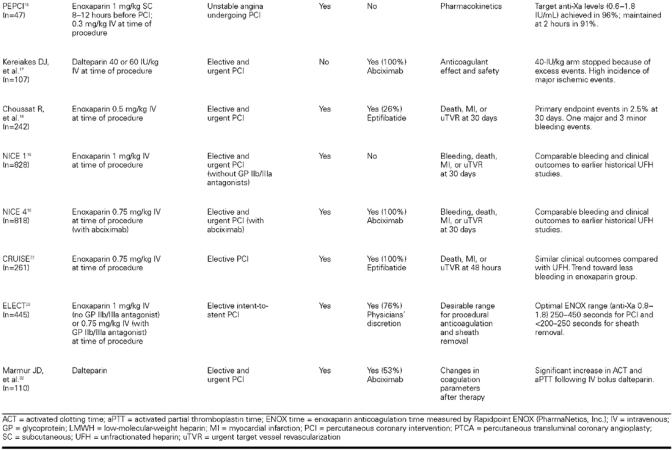
REDUCE. In the REDUCE study (Reduction of Restenosis after PTCA, Early Administration of Reviparin in a Double-Blind Unfractionated Heparin and Placebo-Controlled Evaluation), Karsch and colleagues 14 examined the efficacy of peri- and postprocedural reviparin on major clinical events and angiographic restenosis. A total of 612 qualifying patients were randomized; 306 patients received a reviparin bolus before PTCA followed by an infusion for 24 hours, then SC twice daily for 28 days. The control arm received heparin at the time of PCI and for the next 24 hours. This group then received SC placebo injections for 28 days. At 30 weeks, the incidence of death, MI, or repeat revascularization was comparable between the 2 groups. Reviparin administration was also similar to UFH with regard to angiographic restenosis. The study did, however, show that acute events during or immediately after the procedure were significantly lower in the reviparin group (P = 0.027). The need for emergency stent placement was also significantly reduced with reviparin (2.0%), compared with UFH (6.9%; P = 0.003). 14
LMWH as Procedural Anticoagulation for PCI (Table I)
Rabah, et al. Rabah and coworkers 15 examined the safety and efficacy of intravenous (IV) enoxaparin used for elective PCI compared with UFH. A total of 60 patients received either a single IV bolus of enoxaparin (1 mg/kg) or UFH (control group), with subsequent boluses as necessary to maintain an activated clotting time (ACT) <300 seconds before angioplasty. Procedural success, bleeding events, and vascular events were similar between the 2 groups. In addition, comparable levels of factor Xa inhibition were achieved in both groups of patients. Although this study was limited by the small number of patients and the exclusion of patients presenting with ACS, it was the 1st trial comparing an IV LMWH versus an IV UFH used for sole anticoagulation in the catheterization laboratory. 15
PEPCI. The Pharmacokinetics of Enoxaparin in Patients undergoing PCI (PEPCI) study 16 examined anticoagulation levels in 47 patients undergoing PCI 8 to 12 hours after receiving a 1.0-mg/kg SC enoxaparin dose. All patients were given a supplemental dose of enoxaparin (0.3 mg/kg IV) at the beginning of the procedure. Forty-five out of 47 patients achieved therapeutic anti-Xa levels (0.6–1.8 IU/mL) after the IV enoxaparin dose. Anti-Xa activity remained within the therapeutic range 2 hours after IV bolus in 40 of 44 (91%) patients that were studied. 16
Kereiakes, et al. Kereiakes and associates 17 examined the efficacy and safety of intravenous dalteparin in 107 patients undergoing PCI. All patients received adjunctive abciximab, aspirin, and clopidogrel. Four patients who had received prior subcutaneous dalteparin were treated with a modified dosing regimen. Patients without prior SC dalteparin therapy or those for whom it had been <12 hours since their last SC dose were randomized to receive either 40 or 60 IU/kg IV dalteparin at the time of PCI. Early in the study, 3 episodes of thrombus formation prompted unblinding, which revealed all 3 patients to be in the 40-IU/kg group. After those 3 episodes, the 40-IU/kg arm was discontinued in the initial 56 randomized patients, and an additional 48 patients were enrolled in an open-label fashion in the 60-IU/kg arm. In this group of 76 patients, outcome events included death, 1.3%; Q-wave MI, 3.9%; urgent PCI, 0; urgent coronary artery bypass grafting (CABG), 1.3%; creatine kinase-MB, <3 × upper limits of normal, 15.8%; and major bleeding, 2.6%. The mean anti-Xa activity in the 60-IU/kg group was 0.9 + 0.30 U/mL at 30 min and 0.2 + 0.14 U/mL at 4 hours. 17
Choussat, et al. Choussat and co-authors 18 reported the use of low-dose IV enoxaparin (0.5 mg/kg) in 242 patients undergoing elective PCI, 64 (26%) of whom were also given eptifibatide. In the patients given enoxaparin alone, sheaths were removed immediately after the procedure; in the patients given both enoxaparin and eptifibatide, sheaths were removed 4 hours later. Peak anti-Xa levels were <0.5 U/mL in 97.5% of the patients. At 30 days, the composite incidence of death, MI, or urgent revascularization was 2.5%. There were 1 major and 3 minor bleeding events; none of these were associated with excessive anticoagulation levels. 18
NICE 1 and NICE 4. Two recent studies have examined the use of enoxaparin for procedural anticoagulation in the catheterization laboratory. In NICE 1 (National Investigators Collaborating on Enoxaparin), 19 828 patients received 1 mg/kg enoxaparin intravenously at the time of PCI without GP IIb/IIIa inhibitor. Of these patients, 85% had stents implanted. Major bleeding events at 30 days were observed in 1.1% of the patients. Major bleeding events not related to CABG at 30 days were found in 0.5%. The incidence of bleeding, transfusion, and major adverse clinical events in NICE 1 and NICE 4 (Figs. 2 and 3) were compared with results from the EPISTENT 20 and EPILOG 21 trials and showed, at least in comparison with these historical controls, similar safety and efficacy.
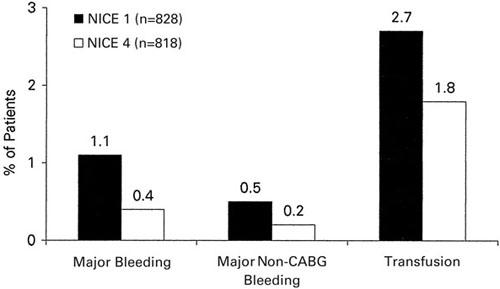
Fig. 2 Thirty-day bleeding events in NICE 1 and NICE 4.
CABG = coronary artery bypass grafting
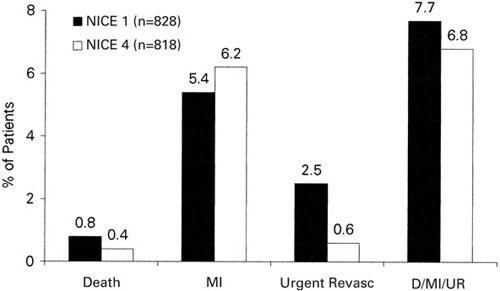
Fig. 3 Thirty-day clinical events in NICE 1 and NICE 4.
D = death; MI = myocardial infarction; revasc = revascularization; UR = urgent revascularization
In NICE 4, 19 818 patients undergoing elective or urgent PCI received a low dose of enoxaparin (0.75 mg/kg) intravenously in combination with abciximab (bolus and 12-hour infusion). Anti-factor Xa levels measured 5 minutes and 4 hours after the IV bolus were 1.5 and 0.6 IU/mL, respectively. The incidence of major bleeding was 0.4%, non-CABG bleeding was 0.2%, and requirement of transfusions was 1.8%. The incidence of death, MI, or urgent revascularization at 30 days was 6.8% and compared favorably with results from previous trials.
CRUISE. In the Coronary Revascularization Utilizing Integrilin and Single-Bolus Enoxaparin (CRUISE) trial, 22 261 patients undergoing PCI were treated with the GP IIb/IIIa antagonist eptifibatide and were subsequently randomized to either standard IV UFH or IV enoxaparin (0.75 mg/kg bolus) at the time of intervention. Clinical outcomes in the enoxaparin/eptifibatide arm were similar to those in the UFH/eptifibatide arm. There was a trend toward fewer bleeding events in the group receiving enoxaparin and eptifibatide (4.1% vs 10.5%), but the difference was not significant (P = 0.08). 22
Enoxaparin: Transition to the Catheterization Laboratory (Table II)
TABLE II. Clinical Studies of LMWH in Patients with ACS Brought Forward to the Catheterization Laboratory
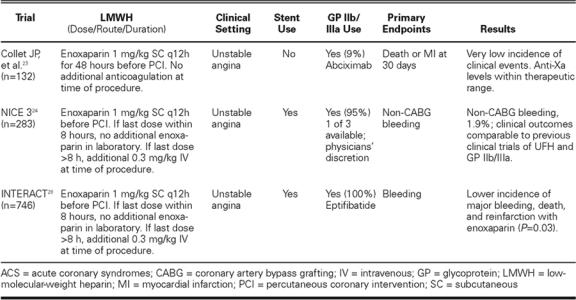
Collet, et al. In the early ACS trials of LMWHs, coronary interventions were performed using UFH. Concerns regarding the safe transition of ACS pa-tients treated “upstream” with subcutaneous LMWHs prompted discontinuation of the LMWHs before catheterization. Collet and associates 23 examined 132 patients undergoing PCI within 8 hours of SC enoxaparin administration (1 mg/kg every 12 hours). These patients were part of a larger group of 451 patients with ACS who had been pre-treated with enoxaparin and other standard-of-care therapies for a minimum of 48 hours. Percutaneous coronary intervention was performed without any additional anticoagulation or monitoring. Anti-Xa activity was measured in all patients and was found to be adequate (<0.5 IU/mL) in 97.6% of them, regardless of the timing of the last dose of enoxaparin. The incidence of death or MI in the PCI group at 30 days was 3.0%, and the incidence of major bleeding was 0.8%. 23 These results again compared favorably with those of earlier, similarly designed studies using UFH. 9
NICE 3. In patients undergoing PCI, the safety of enoxaparin alone and of enoxaparin and abciximab combined was demonstrated in the NICE 1 and NICE 4 trials, respectively. The NICE-3 trial 24 sought to examine the safety of a strategy of SC enoxaparin and a GP IIb/IIIa antagonist (abciximab, eptifibatide, or tirofiban) in the initial management of patients presenting with ACS, including those subsequently brought to the catheterization laboratory. In NICE-3, 628 patients were treated with SC enoxaparin at 1 mg/kg every 12 hours, along with one of the 3 commercially available GP IIb/IIIa antagonists. Of these patients, 283 (45%) were brought forward to the catheterization laboratory for PCI. Patients who underwent PCI within 8 hours of their last dose of enoxa-parin were continued on a GP IIb/IIIa antagonist with no additional anticoagulation. Patients brought to the catheterization laboratory 8 to 12 hours after their last enoxaparin dose were continued on a GP IIb/IIIa inhibitor but received an additional 0.3 mg/ kg IV enoxaparin at the time of PCI. Of those 283 patients, the incidence of non-CABG bleeding was, on average, <2% (Fig. 4). The incidences of death, MI, and major bleeding events were comparable with those found in earlier randomized studies using UFH and GP IIb/IIIa antagonists. 4,5,20,21,25–27
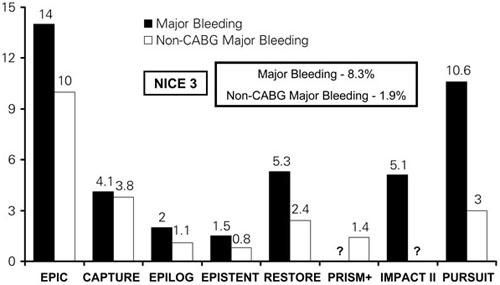
Fig. 4 Major bleeding and non-CABG major bleeding in recent trials that served as comparators for NICE 3.
CABG = coronary artery bypass grafting
INTERACT. INTERACT 28 was a randomized study of 746 ACS patients treated with eptifibatide and aspirin and randomized to receive either enoxaparin 1 mg/kg SC or standard-dose UFH. In the patients given enoxaparin, a transition strategy was applied, whereby no additional enoxaparin was given to patients who came to the catheterization laboratory within 8 hours of the last subcutaneous dose, and an adjunctive IV bolus of 0.3 mg/kg of enoxaparin was given to patients who came to the catheterization laboratory between 8 and 12 hours after the last subcutaneous dose, which was similar to the strategy used in NICE 3. Invasive management was not precluded. Approximately 63% of the patients underwent coronary angiography, and about half of those proceeded to PCI. Of the overall study population, the enoxaparin group had a significantly lower incidence of major bleeding at 48 hours (1.1% vs 3.8% with UFH; P = 0.014) and 96 hours (1.8% vs 4.6% with UFH; P = 0.03), a lower incidence of recurrent ischemia within the first 48 hours (14.3% vs 25.4% with UFH; P = 0.0002) and from 48 to 96 hours (12.7% vs 25.9 with UFH%; P <0.0001), and a lower composite of death and reinfarction at 30 days (5.0% vs 9.0% with UFH; P = 0.031). 28
Monitoring of LMWH Therapy
One major difficulty with the use of LMWHs, particularly in the catheterization laboratory, is that there is no readily available bedside assay—such as the use of ACTs with UFH—to measure the anticoagulant effect of LMWH. Although LMWHs may have a more predictable anticoagulant dose response (which might obviate the need for monitoring in most cases), a rapid point-of-care monitoring system could improve the safety or efficacy of LMWH administration. For instance, in cases of weight extremes or renal insufficiency, such a system might be used to determine the timing safety of vascular sheath removal or to guide protamine reversal of LMWH in the setting of clinical bleeding. A card-based technology has been developed, which incorporates a dry reagent embedded with paramagnetic iron oxide particles. A drop of whole blood is placed on the card, an oscillating magnetic field is activated, and light reflected from the card is measured by a sensor. Thus, an LMWH “clotting time” is determined, and this result may be analogous to the ACT measured after UFH administration. The LMWH clotting time appears to most closely relate to the anti-factor Xa activity. This rapid point-of-care monitoring technology could obviate the need for arbitrary and empiric dose reduction in cases of renal insufficiency or low body mass.
The recently completed ELECT (EvaLuating Enoxaparin Clotting Times) study 29 highlights the potential utility of just such a bedside device (the Rapidpoint ENOX test, PharmaNetics, Inc.; Morrisville, NC) for measuring anti-Xa activity. The ELECT study was a nonrandomized, multicenter, observational trial assessing the predictive value of the ENOX test in 445 patients undergoing elective PCI. In that study, Moliterno and colleagues proposed an ENOX clotting time of 250 to 450 seconds as a target range for PCI. Event rates in patients who had ENOX times within this range were 4.0%, as opposed to 7.2% for those outside this range (P = 0.134). The lower limit of 250 seconds appears to reliably predict an anti-Xa activity of <1.0 IU/mL (a level thought to be adequate for PCI). 29 The exact clinical utility of this test remains to be demonstrated in prospective studies.
Other bedside devices have been investigated for monitoring the efficacy of LMWHs. Henry and coworkers 30 retrospectively examined peak and trough ACTs and anti-Xa levels in 26 patients who had been enrolled in the TIMI (Thrombolysis In Myocardial Infarction) 11A study, which examined the safety and tolerability of SC enoxaparin in ACS. Henry's group noted no significant change in ACTs by either the HemoTec® (Medtronic HemoTec; Englewood, Colo) or the Hemochron® (ITC; Edison, NJ) measurement system, despite adequate dosing of enoxaparin and significant increases in anti-Xa levels. Of note, the exact measurement cartridges or tubes used in the study were not specified. 30 Rabah and co-authors 15 reported moderate increases in Hemochron ACTs (130 + 19 seconds to 188 + 29 seconds) 5 minutes after the initial bolus in 30 patients receiving IV enoxaparin (1 mg/kg) for PCI. Other investigators 31 have also noted changes in ACTs and activated partial thromboplas-tin times (aPTTs) (with use of special cartridges) that have correlated relatively well with anti-Xa activity in patients receiving dalteparin.
Marmur and associates 32 examined the utility of ACTs (Hemochron [CA 510 tubes]) by using blood samples from the following groups: 10 volunteers to whose samples were added increasing concentrations of dalteparin or UFH; 15 patients who were sequentially treated with IV dalteparin and then UFH; and 110 patients undergoing PCI who were given either 60 or 80 IU/kg dalteparin intravenously, with or without abciximab (dosing was modified in a small number of patients on prior dalteparin). The ACT appeared to be sensitive to increasing concentrations of dalteparin, albeit in a different range than that seen with UFH. There were no deaths or urgent repeat revascularizations in the PCI population; 2 patients experienced procedural MIs. 32
A similar study examined the effect of IV procedural enoxaparin in 45 patients undergoing PCI. 33 A total of 36 patients were treated with IV enoxaparin alone (1 mg/kg); 9 received a lower dose of enoxaparin (0.75 mg/kg) with eptifibatide. The mean post-enoxaparin ACT was 207 seconds in the low-dose group (mean increase, 74 + 20 seconds) and 212 seconds in the 1.0-mg/kg group (mean increase, 92 + 28 seconds). 33
Transition to the Catheterization Laboratory
Many of the modern-day concerns about the use of LMWHs in patients presenting with ACS pertain to the transition to the catheterization laboratory. A recently convened expert panel has set forth guidelines for the use of LMWHs in such patients, including those managed invasively. 34 The panel concluded that patients receiving LMWH can safely undergo catheterization and PCI, and laid out a number of management algorithms. Similar guidelines for the handling of patients receiving LMWH who are brought forward to the catheterization laboratory have been published recently. 35 In addition, data from the ENTIRE-TIMI 23 36 and ASSENT-3 37 trials suggest that the use of enoxaparin in patients with thrombolytic-treated MI does not preclude subsequent urgent or elective revascularization. The recently published RITA 3 study 38 compared an invasive strategy with a conservative strategy in medium-risk patients who were receiving enoxaparin therapy. However, the transition to the catheterization laboratory in RITA 3 was largely accomplished by withholding the morning dose of enoxaparin and using UFH in the catheterization laboratory.
What Have We Learned?
Since the publication of TIMI 11B1 and ESSENCE, 2 we have found convincing clinical evidence that supports the use of LMWHs over UFH in patients presenting with an ACS. When these patients are managed invasively, however, the use of LMWHs in the catheterization laboratory is still in question. Both observational and randomized prospective clinical studies have evaluated the use of LMWHs in the cardiac catheterization laboratory (Table I) and in patients being transitioned to the catheterization laboratory (Table II); more studies are in progress.
Data from ERA, 9 EMPAR, 10 PARAT, 11 and other such studies did not show any convincing evidence that supported the use of LMWHs after PCI. The ENTICES 12 and ATLAST 13 trials examined LMWH use after PCI and stenting in patients at high risk for stent thrombosis, and the results were encouraging. However, improving stent technology and newer adjunctive pharmacologic alternatives (for example, thienopyridines and GP IIb/IIIa antagonists) caused the incidence of subacute thrombosis to fall to a point where the ATLAST trial was stopped for futility.
Collet and coworkers 23 were the first to examine the use of LMWH as a sole anticoagulant in patients undergoing PCI. The study was limited by the lack of a control group, but it nonetheless provided evidence that subcutaneous pretreatment of ACS patients with enoxaparin allows for safe and effective PCI without the need of additional anticoagulation in the catheterization laboratory. Rabah and coworkers 15 showed that IV enoxaparin used for elective PCI was both safe and effective in comparison with IV UFH. The early NICE trials 19 evaluated the use of enoxaparin (NICE 1) and reduced-dose enoxaparin in combination with a GP IIb/IIIa antagonist (NICE 4) in patients managed by early PCI. Those 2 studies found similar incidences of bleeding and efficacy when examined alongside older, comparably designed cohorts (EPISTENT 20 and EPILOG 21) that made use of UFH. In patients receiving the GP IIb/IIIa antagonist eptifibatide, the CRUISE trial 22 compared treatment with UFH or enoxaparin and found similar rates of bleeding and vascular complications, not only in patients undergoing elective or urgent PCI, but also in those patients receiving closure devices. Clinical outcomes were similar in the 2 cohorts as well.
Moving outside the catheterization laboratory, the NICE 3 study 24 demonstrated the safety of medically managing ACS patients with an LMWH and a GP IIb/IIIa antagonist and then allowing the patients to be brought forward to the catheterization laboratory without the addition of UFH. The INTERACT trial 28 demonstrated improved safety and efficacy outcomes in ACS patients who were treated with enoxaparin and eptifibatide compared with those treated with UFH and eptifibatide; all patients underwent a relatively slow transition strategy to the catheterization laboratory.
These trials have provided valuable safety and efficacy data but have not yet answered a number of questions. Are LMWHs truly better than UFH in patients who require coronary intervention? Furthermore, are LMWHs better in patients presenting with ACS who are aggressively managed by early intervention? How can LMWHs best be used in conjunction with GP IIb/IIIa antagonists? How can we best monitor anticoagulation levels and induce reversibility if need be? Finally, which LMWHs are the best? Although 7 LMWHs are currently available, there are differences in these compounds, including molecular weight, relative factor Xa/IIa activity, and biological properties. 39 Experience with LMWHs in ACS is derived mainly from clinical trials of enoxaparin and dalteparin. The recent EVET (Enoxaparin versus tinzaparin in non-ST-segment elevation acute coronary syndromes) trial, 40 the only head-to-head compari-son published thus far, randomized 438 patients with ACS to SC enoxaparin or tinzaparin. The primary endpoint of angina, MI, or death at 7 days was significantly lower in the enoxaparin cohort, and the relative benefit was sustained for at least 30 days. 40
Future Studies
The SYNERGY (Superior Yield of the New Strategy of Enoxaparin, Revascularization and GlYcoprotein IIb/IIIa inhibitors) study 41 is designed to provide a definitive comparison of enoxaparin and UFH in those high-risk ACS patients presenting with unstable angina or non-ST-elevation MI in whom an early invasive treatment strategy is planned. The study treatment strategies will allow “upstream” and/or procedural use of thienopyridines and GP IIb/IIIa antagonists at the operator's discretion. The primary endpoint of the study is the incidence of death or MI at 30 days. 41
The ExTRACT-TIMI 25 trial is a large-scale study (21,000 patients) that compares the LMWH enoxaparin versus UFH in patients with acute MI receiving a thrombolytic agent for reperfusion. Patients with ST-elevation MI who present within 6 hours of chest pain will be randomized to treatment with enoxaparin (if <75 years, 30-mg IV bolus + 1 mg/kg SC twice a day through hospital discharge; if ≥75 years, no bolus + 0.75 mg/kg SC twice a day through hospital discharge) or UFH (60-U/kg bolus, 12-U/kg/hr infusion for at least 48 hours, aPTT adjusted). The choice of reperfusion therapy is at the discretion of the treating physician and can include tenecteplase, tissue plasminogen activator, reteplase, or streptokinase. The primary efficacy endpoint is the composite incidence of death or MI at 30 days. The primary safety endpoint is the incidence of TIMI major hemorrhage.
The ACUITY trial is an ACS study consisting of 5 arms: these will compare initial therapy with enoxaparin and a GP IIb/IIIa antagonist, initial therapy with enoxaparin and in-laboratory GP IIb/IIIa antagonists, initial therapy with bivalrudin and a GP IIb/ IIIa antagonist, initial therapy with bivalrudin and in-laboratory GP IIb/IIIa antagonists, and initial therapy with bivalrudin and “bailout” use of GP IIb/IIIa antagonists in the catheterization laboratory. Interestingly, there is no UFH arm in the trial: the “control” arms are the enoxaparin groups.
The OASIS 5 study is a direct comparison of the Xa inhibitor pentasaccharide with enoxaparin in patients with unstable angina; again, the “control” arm is enoxaparin. The overall study will enroll approximately 16,000 patients; a substudy of 4,000 patients will focus on routine early (<24-hour) versus delayed (<48-hour) angiography; another substudy will focus on women, comparing an invasive strategy (early catheterization and revascularization within 7 days) to a noninvasive strategy in 1,600 female patients. However, in OASIS 5, patients in the enoxaparin group who come to the catheterization laboratory will be switched to UFH.
Another major trial involving acute MI and LMWH treatment is FINESSE. This study will prospectively examine combination thrombolytic and GP IIb/IIIa antagonist therapy followed by PCI versus emergent PCI for acute MI. In FINESSE, at least 1 arm will include the initial use of enoxaparin rather than UFH.
With regard to future studies of LMWH in the catheterization laboratory, STEEPLE is a recently initiated, controlled study of approximately 2,000 patients undergoing PCI who are randomized either to standard UFH doses or to 1 of 2 doses of IV enoxaparin (0.5 or 0.75 mg/kg IV, with or without a GP IIb/IIIa antagonist) for procedural anticoagulation. This is primarily designed as a safety study, with bleeding as the primary endpoint.
Summary
The message that consistently emerges from both the older and the more recent ACS trials is that the old standard of aspirin and UFH can be improved considerably. Low-molecular-weight heparins (most notably enoxaparin) are emerging as a broad replacement for UFH in the management of unstable angina and non-ST-elevation MI. The LMWHs are easily administered, have a more reliable degree of anticoagulation, and have fewer associated side effects compared with UFH. A recently published meta-analysis advocated treatment with LMWHs as a superior strategy across the broad spectrum of ACS and a reasonable alternative strategy for procedural anticoagulation for PCI. 42 These agents may also synergize better with GP IIb/IIIa antagonists than does UFH. Future studies will provide additional important information on low-molecular-weight heparins in high-risk invasively managed patients with unstable angina (SYNERGY 41), in patients undergoing primary PCI for acute myocardial infarction (FINESSE), and in comparison with other antithrombotic alternatives in patients with acute coronary syndromes (ACUITY and OASIS 5).
Clinical Trial Acronyms
ACUITY – Acute catheterization and urgent intervention triage strategy
ACUTE II – Antithrombotic combination using tirofiban and enoxaparin
ASSENT – Assessment of the safety and efficacy of new thrombolytic regimens
ATLAST – Antiplatelet therapy alone versus Lovenox plus antiplatelet therapy in patients at increased risk of stent thrombosis
CAPTURE – Chimeric 7E3 antiplatelet therapy in unstable angina refractory to standard treatment
CRUISE – Coronary revascularization utilizing Integrilin and single-bolus enoxaparin
ELECT – Evaluating enoxaparin clotting times
EMPAR – Enoxaparin MaxEPA prevention of angioplasty restenosis
ENTICES – Enoxaparin and ticlopidine after elective stenting
ENTIRE – Enoxaparin and tenecteplase t-Pa with or without GP IIb/IIIa inhibitor as reperfusion strategy in ST-elevation myocardial infarction
EPIC – Evaluation of c7E3 directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty
EPILOG – Evaluation of PTCA to improve long-term outcome with abciximab GP IIb/IIIa receptor blockade
EPISTENT – Evaluation of platelet IIb/IIIa inhibitor in stenting
ERA – Enoxaparin restenosis trial
ESSENCE – Efficacy and safety of subcutaneous enoxaparin in non-Q-wave coronary events
EVET – Enoxaparin versus tinzaparin in non-ST-segment elevation acute coronary syndromes
EXTRACT – Enoxaparin and thrombolysis reperfusion for acute myocardial infarction treatment
FINESSE – Facilitated intervention with enhanced reperfusion speed to stop events
INTERACT – Integrilin and enoxaparin randomized assessment of acute coronary syndrome treatment
NICE – National investigators collaborating on enoxaparin
OASIS – Organization to assess strategies for ischemia syndromes
PARAT – Prophylaxis against restenosis angioplasty trial
PEPCI – Pharmacokinetics of enoxaparin in PCI
PRISM-PLUS – Platelet receptor inhibition in ischemic syndrome management in patients limited by unstable signs and symptoms
PURSUIT – Platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using Integrilin therapy
REDUCE – Reduction of restenosis after PTCA, early administration of reviparin in a double-blind unfractionated heparin and placebo-controlled evaluation
RESTORE – Randomized efficacy study of tirofiban for outcomes and restenosis
RITA – Randomized intervention trial of unstable angina
STEEPLE – Safety and efficacy of enoxaparin in PCI patients; an international, randomized evaluation
SYNERGY – Superior yield of the new strategy of enoxaparin, revascularization and glycoprotein IIb/ IIIa inhibitors
TIMI – Thrombolysis in myocardial infarction
Footnotes
Address for reprints: James J. Ferguson, MD, Cardiology Research, MC 1-191, St. Luke's Episcopal Hospital, P.O. Box 20269, Houston, TX 77225-0269
E-mail: jferguson@heart.thi.tmc.edu
The authors have provided a key to acronyms at the end of the paper.
References
- 1.Antman EM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, Salein D, et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) 11B trial. Circulation 1999;100:1593–601. [DOI] [PubMed]
- 2.Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med 1997;337(7):447–52. [DOI] [PubMed]
- 3.Antman EM, Cohen M, Radley D, McCabe C, Rush J, Premmereur J, Braunwald E. Assessment of the treatment effect of enoxaparin for unstable angina/non-Q-wave myocardial infarction. TIMI 11B-ESSENCE meta-analysis. Circulation 1999;100(15):1602–8. [DOI] [PubMed]
- 4.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med 1998;339:436–43. [DOI] [PubMed]
- 5.Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators [published erratum appears in N Engl J Med 1998;339:415]. N Engl J Med 1998;338:1488–97. [DOI] [PubMed]
- 6.Cohen M, Theroux P, Borzak S, Frey MJ, White HD, Van Mieghem W, et al. Randomized double-blind safety study of enoxaparin versus unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes treated with tirofiban and aspirin: the ACUTE II study. The Antithrombotic Combination Using Tirofiban and Enoxaparin. Am Heart J 2002;144(3):470–7. [DOI] [PubMed]
- 7.Weitz JI. Low-molecular-weight heparins [published erratum appears in N Engl J Med 1997;337:1567]. N Engl J Med 1997;337(10):688–98. [DOI] [PubMed]
- 8.Young E, Wells P, Holloway S, Weitz J, Hirsh J. Ex-vivo and in-vitro evidence that low molecular weight heparins exhibit less binding to plasma proteins than unfractionated heparin. Thromb Haemost 1994;71(3):300–4. [PubMed]
- 9.Faxon DP, Spiro TE, Minor S, Cote G, Douglas J, Gottlieb R, et al. Low molecular weight heparin in prevention of restenosis after angioplasty. Results of Enoxaparin Restenosis (ERA) Trial. Circulation 1994;90(2):908–14. [DOI] [PubMed]
- 10.Cairns JA, Gill J, Morton B, Roberts R, Gent M, Hirsh J, et al. Fish oils and low-molecular-weight heparin for the reduction of restenosis after percutaneous transluminal coronary angioplasty. The EMPAR Study. Circulation 1996; 94(7):1553–60. [DOI] [PubMed]
- 11.Grassman ED, Leya F, Fareed J, Lewis BE, Bacher P, Loeb HS, Moran JF. A randomized trial of the low-molecular-weight heparin certoparin to prevent restenosis following coronary angioplasty. J Invasive Cardiol 2001;13(11):723–8. [PubMed]
- 12.Zidar JP. Low-molecular-weight heparins in coronary stenting (the ENTICES trial). ENoxaparin and TIClopidine after Elective Stenting. Am J Cardiol 1998;82(5B):29L-32L. [DOI] [PubMed]
- 13.Batchelor WB, Mahaffey KW, Berger PB, Deutsch E, Meier S, Hasselblad V, et al. A randomized, placebo-controlled trial of enoxaparin after high-risk coronary stenting: the ATLAST trial. J Am Coll Cardiol 2001;38(6):1608–13. [DOI] [PubMed]
- 14.Karsch KR, Preisack MB, Baildon R, Eschenfelder V, Foley D, Garcia EJ, et al. Low molecular weight heparin (reviparin) in percutaneous transluminal coronary angioplasty. Results of a randomized, double-blind, unfractionated heparin and placebo-controlled, multicenter trial (REDUCE trial). Reduction of Restenosis After PTCA, Early Administration of Reviparin in a Double-Blind Unfractionated Heparin and Placebo-Controlled Evaluation. J Am Coll Cardiol 1996;28(6):1437–43. [DOI] [PubMed]
- 15.Rabah MM, Premmereur J, Graham M, Fareed J, Hoppensteadt DA, Grines LL, Grines CL. Usefulness of intravenous enoxaparin for percutaneous coronary intervention in stable angina pectoris. Am J Cardiol 1999;84:1391–5. [DOI] [PubMed]
- 16.Martin JL, Fry ET, Sanderink GJ, Atherley TH, Guimart CM, Chevalier PJ, et al. Reliable anticoagulation with enoxaparin in patients undergoing percutaneous coronary intervention: The pharmacokinetics of enoxaparin in PCI(PEPCI) study. Catheter Cardiovasc Interv 2004;61:163–70. [DOI] [PubMed]
- 17.Kereiakes DJ, Kleiman NS, Fry E, Mwawasi G, Lengerich R, Maresh K, et al. Dalteparin in combination with abciximab during percutaneous coronary intervention. Am Heart J 2001;141:348–52. [DOI] [PubMed]
- 18.Choussat R, Montalescot G, Collet JP, Vicaut E, Ankri A, Gallois V, et al. A unique, low dose of intravenous enoxaparin in elective percutaneous coronary intervention. J Am Coll Cardiol 2002;40:1943–50. [DOI] [PubMed]
- 19.Kereiakes DJ, Grines C, Fry E, Esente P, Hoppensteadt D, Midei M, et al. Enoxaparin and abciximab adjunctive pharmacotherapy during percutaneous coronary intervention. J Invasive Cardiol 2001;13(4):272–8. [PubMed]
- 20.Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. The EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. Lancet 1998;352:87–92. [DOI] [PubMed]
- 21.Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med 1997;336:1689–96. [DOI] [PubMed]
- 22.Bhatt DL, Lee BI, Casterella PJ, Pulsipher M, Rogers M, Cohen M, et al. Safety of concomitant therapy with eptifibatide and enoxaparin in patients undergoing percutaneous coronary intervention: results of the Coronary Revascularization Using Integrilin and Single bolus Enoxaparin Study. J Am Coll Cardiol 2003;41(1):20–5. [DOI] [PubMed]
- 23.Collet JP, Montalescot G, Lison L, Choussat R, Ankri A, Drobinski G, et al. Percutaneous coronary intervention after subcutaneous enoxaparin pretreatment in patients with unstable angina pectoris. Circulation 2001;103:658–63. [DOI] [PubMed]
- 24.Ferguson JJ, Antman EM, Bates ER, Cohen M, Every NR, Harrington RA, et al. The use of enoxaparin and IIb/IIIa antagonists in acute coronary syndromes, including PCI: final results of the National Investigators Collaborating on Enoxaparin-3 (NICE 3) study. Am Heart J 2003;146:628–34. [DOI] [PubMed]
- 25.Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med 1994;330:956–61. [DOI] [PubMed]
- 26.Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE study. Lancet 1997;349:1429–35. [PubMed]
- 27.Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Circulation 1997;96:1445–53. [DOI] [PubMed]
- 28.Goodman SG, Fitchett D, Armstrong PW, Tan M, Langer A; Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial Investigators. Randomized evaluation of the safety and efficacy of enoxaparin versus unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes receiving the glycoprotein IIb/IIIa inhibitor eptifibatide. Circulation 2003;107(2):238–44. [DOI] [PubMed]
- 29.Moliterno DJ, Hermiller JB, Kereiakes DJ, Yow E, Applegate RJ, Braden GA, et al. A novel point-of-care enoxaparin monitor for use during percutaneous coronary interven-tion. Results of the Evaluating Enoxaparin Clotting Times (ELECT) Study. J Am Coll Cardiol 2003;42(6):1132–9. [DOI] [PubMed]
- 30.Henry TD, Satran D, Knox LL, Iacarella CL, Laxson DD, Antman EM. Are activated clotting times helpful in the management of anticoagulation with subcutaneous low-molecular-weight heparin? Am Heart J 2001;142(4):590–3. [DOI] [PubMed]
- 31.Schooley CC, Gilbert JH, Harlan M, Bracey A, Coulter S, Wilson JM. Kinetics of intravenously administered dalteparin [abstract]. J Am Coll Cardiol 2003;41(6 Suppl A):25A.
- 32.Marmur JD, Anand SX, Bagga RS, Fareed J, Pan CM, Sharma SK, Richard MF. The activated clotting time can be used to monitor the low molecular weight heparin dalteparin after intravenous administration. J Am Coll Cardiol 2003;41(3):394–402. [DOI] [PubMed]
- 33.Lawrence M, Mixon T, Cross D, Dehmer G. Assessment of anticoagulation using activated clotting times in patients receiving intravenous enoxaparin during percutaneous coronary intervention [abstract]. J Am Coll Cardiol 2003;41(6 Suppl A):68A. [DOI] [PubMed]
- 34.Keriakes DJ, Montalescot G, Antman EM, Cohen M, Darius H, Ferguson JJ, et al. Low-molecular-weight heparin therapy for non-ST-elevation acute coronary syndromes and during percutaneous coronary intervention: an expert consensus. Am Heart J 2002;144(4):615–24. [DOI] [PubMed]
- 35.Kadakia RA, Ferguson JJ. Use of enoxaparin in patients undergoing percutaneous coronary intervention. Critical Pathways in Cardiology 2003;2(1):1–6. [DOI] [PubMed]
- 36.Antman EM, Louwerenburg HW, Baars HF, Wesdorp JC, Hamer B, Bassand JP, et al. Enoxaparin as adjunctive antithrombin therapy for ST-elevation myocardial infarction: results of the ENTIRE-Thrombolysis in Myocardial Infarction (TIMI) 23 Trial [published erratum appears in Circulation 2002;105:2799]. Circulation 2002;105:1642–9. [DOI] [PubMed]
- 37.Dubois CL, Belmans, Granger CB, Armstrong PW, Wallentin L, Fioretti PM, et al. Outcome of urgent and elective percutaneous coronary interventions after pharmacologic reperfusion with tenecteplase combined with unfractionated heparin, enoxaparin, or abciximab. J Am Coll Cardiol 2003;42:1178–85. [DOI] [PubMed]
- 38.Fox KA, Poole-Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet 2002;360(9335):743–51. [DOI] [PubMed]
- 39.Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol 1998;82(5B):3L-10L. [DOI] [PubMed]
- 40.Michalis LK, Katsouras CS, Papamichael N, Adamides K, Naka KK, Goudevenos J, Sideris DA. Enoxaparin versus tinzaparin in non-ST-segment elevation acute coronary syndromes: the EVET trial. Am Heart J 2003;146(2):304–10. [DOI] [PubMed]
- 41.SYNERGY Executive Committee. Superior Yield of the New strategy of Enoxaparin, Revascularization and GlYcoprotein IIb/IIIa inhibitors. The SYNERGY trial: study design and rationale. Am Heart J 2002;143(6):952–60. [DOI] [PubMed]
- 42.Wong G, Giugliano RP, Antman EM. Use of low-molecular-weight heparins in the management of acute coronary artery syndromes and percutaneous coronary intervention. JAMA 2003;289(3):331–42. [DOI] [PubMed]


