Abstract
C1q is the first subcomponent of the classical complement pathway that can interact with a range of biochemically and structurally diverse self and nonself ligands. The globular domain of C1q (gC1q), which is the ligand-recognition domain, is a heterotrimeric structure composed of the C-terminal regions of A (ghA), B (ghB), and C (ghC) chains. The expression and functional characterization of ghA, ghB, and ghC modules have revealed that each chain has specific and differential binding properties toward C1q ligands. It is largely considered that C1q–ligand interactions are ionic in nature; however, the complementary ligand-binding sites on C1q and the mechanisms of interactions are still unclear. To identify the residues on the gC1q domain that are likely to be involved in ligand recognition, we have generated a number of substitution mutants of ghA, ghB, and ghC modules and examined their interactions with three selected ligands: IgG1, C-reactive protein (CRP), and pentraxin 3 (PTX3). Our results suggest that charged residues belonging to the apex of the gC1q heterotrimer (with participation of all three chains) as well as the side of the ghB are crucial for C1q binding to these ligands, and their contribution to each interaction is different. It is likely that a set of charged residues from the gC1q surface participate via different ionic and hydrogen bonds with corresponding residues from the ligand, instead of forming separate binding sites. Thus, a recently proposed model suggesting the rotation of the gC1q domain upon ligand recognition may be extended to C1q interaction with CRP and PTX3 in addition to IgG1.
C1q is the first subcomponent of the classical complement pathway and its binding to IgG- or IgM-containing immune complexes leads to the autoactivation of C1r, which in turn, activates C1s. C1r and C1s, the two serine protease proenzymes, together with C1q constitute C1, the first component of the classical pathway. The activation of the C1 complex (C1q + C1r2 + C1s2) subsequently leads to the activation of the C2−C9 components of the classical pathway and the formation of the terminal-membrane-attack complex (MAC) (1, 2). C1q is a versatile innate immune molecule that can bind a diverse range of self and nonself ligands, ranging from proteins to lipids to nucleic acids, including envelope proteins of certain retroviruses, β-amyloid fibrils, lipopolysaccharides (LPS), porins from Gram-negative bacteria, phospholipids, apoptotic cells, and pentraxins such as C-reactive protein (CRP)11 and pentraxin 3 (PTX3) (3–5). The polyanionic nature of these ligands appears to suggest that C1q recognizes a pattern of charged residues or groups (3). The functional versatility of C1q is well matched by its structural modularity and complexity (4). The C1q molecule is a bouquet-of-tulip like structure composed of six C-terminal heterotrimeric globular head domains (gC1q) and an N-terminal triple-helical collagen region. Each gC1q domain is composed of C-terminal portions of A (ghA), B (ghB), and C (ghC) chains (3, 4, 6).
C1q interacts with a majority of its known ligands via its heterotrimeric gC1q domain. The expression and characterization of individual chains of the gC1q domain have revealed that ghA, ghB, and ghC modules can bind C1q ligands specifically and preferentially (3, 4, 6). This modular nature of the gC1q domain has been confirmed by the crystal structure solved at 1.9 Å resolution that has revealed a compact, spherical heterotrimer (50 Å diameter) with a noncrystallographic pseudo-3-fold symmetry (7). Each of the individual globular head modules, with their N- and C-termini emerging at the base of the trimer, has a jellyroll topology consisting of a 10-stranded β sandwich made up of two 5-stranded antiparallel β sheets. This topology is a characteristic feature of the members of a newly designated C1q and the tumor-necrosis-factor (TNF) superfamily (3, 8). The three modules within gC1q, ghA, ghB, and ghC, show clear differences in their electrostatic surface potentials, which in part explains their differing ligand recognition abilities (7). Thus, the modular organization of the heterotrimeric assembly, together with different surface charge patterns, and the spatial orientation of individual modules enable gC1q to interact with a diverse range of ligands (3, 4, 9)
Despite a number of studies carried out on the C1q–ligand interactions, the precise binding sites on the gC1q domain have not been defined because of the technical difficulties in expressing the heterotrimeric gC1q domain in a recombinant form. However, the availability of the recombinant forms of ghA, ghB, and ghC has allowed an opening into the mutational analyses of the individual modules. In the present study, a number of single-residue mutants of ghA, ghB, and ghC (ArgA162Ala/Glu, ArgB114Gln/Glu, HisB117Ala/Asp, ArgB129Ala/Glu, LysB136Glu, ArgB163Ala/Glu, TyrB175-Leu, HisC101 Ala, ArgC156Ala/Glu, and LysC170Glu) were examined for their binding to IgG1, CRP, and PTX3. Our selection of mutants was based on four major premises. (i) Recently, a number of amino acid residues (ArgA162, ArgB114, HisB117, ArgB129, ArgB163, and ArgC156) on gC1q have been identified as important for IgG binding via site-directed mutagenesis of ghA, ghB, and ghC (10). The importance of these residues have been previously shown by chemical modification studies (11). (ii) Molecular modeling based on the crystal structure of the gC1q of human C1q has revealed that within the ghB module ArgB129 and GluB162 seem central to C1q–IgG interaction, and additional ionic interactions are provided by ArgB114 and ArgB161 (7). The modeling has also proposed that TyrB175 (ghB) and LysA200 (ghA) can potentially form complementary CRP binding sites. (iii) An analysis of ghA, ghB, and ghC sequences individually, using a computer program called ConSurf, has identified a number of functionally critical residues that are highly variable and map within the potential binding area on the gC1q crystal structure. (iv) Recently, we have found that the exposed Ca2+ within the gC1q heterotrimer primarily influences the target recognition properties of C1q toward IgG, IgM, CRP, and PTX3 (12). At pH 7.4, the loss of Ca2+ leads to changes in the direction of the electric moment from a coaxial position in the calcium-saturated holo form (toward the gC1q apex) to one perpendicular to the molecular axis in the calcium-depleted apo form (toward equatorial side of the B chain). Thus, two planes, normal to the electric moment vectors in the holo form (the holo plane) and the apo form (the apo plane), can be defined with potential importance for target recognition. Some of the mutated residues as well as residues that have been demonstrated to be important for ligand binding belong to these two planes (4, 7, 13).
Our results suggest that charged residues from the apex of the heterotrimeric gC1q (with the participation of all three chains) and the sides of the B chain are important for ligand binding, and their contribution is different for the targets studied. Most likely they participate in the formation of complementary noncovalent bonds with corresponding residues from the target molecule, rather than forming separate binding sites. Thus, the model for the rotation of gC1q upon target binding, proposed for IgG1 (12), could be applied for CRP and PTX3 as well.
MATERIALS AND METHODS
Purified Proteins and Antibody Conjugates
C1q was purified from pooled human serum by affinity chromatography on IgG–Sepharose (14). The purity of C1q was assessed by SDS–PAGE (15% w/v) under reducing conditions. Monoclonal IgG1 was obtained from Mabtera and F. Hoffmann-La Roche. Human CRP was purified from ascitic fluid (15). Recombinant human PTX3 was expressed in Chinese hamster ovary cells (16). Goat anti-rabbit IgG-horseradish peroxidase (HRP) conjugate, rabbit anti-mouse IgG–HRP conjugate, mouse anti-MBP antibodies, and o-phenylenediamine dihydrochloride (OPD) were purchased from Sigma-Aldrich. Rabbit anti-human C1q polyclonal antibodies were purchased from DAKO.
Site-Directed Mutagenesis and Cloning of Single-Residue Mutants of LysB136Glu, TyrB175Leu, LysC170Glu, and HisC101Ala
The generation of mutants ArgA162Ala/Glu, ArgB114Gln/Glu, HisB117Ala/Asp, ArgB129Ala/Glu, LysB136Glu, ArgB163Ala/Glu, TyrB175Leu, HisC101Ala, ArgC156Ala/Glu, and LysC170Glu has already been published (10). Additional new mutants were generated via point mutations within the DNA sequences by site-directed mutagenesis using the overlapping PCR approach (17). The PCR products incorporating the mutations were digested with KpnI and HindIII and subcloned into the pMal-c2 expression vector (New England Biolabs, Beverly, MA). The following custom-made (DNA Technology A/S, Denmark) terminal primers were used to generate the mutations. The primers for the LysB136Glu mutant were FP: ACCTGCGAGGTGCCCGGTCTCTACTAC and RP: AGAC-CGGGCACCTCGCAGGTGAACT; for the TyrB175Leu mutant, FP: GTGACTATGCCCTCAACACCTT and RP: AAG-GTGTTGAGGGCATAGTCAC; for the LysC170Glu mutant, FP: GCCACACGTCCGAAACCAATCAGG and RP: CT-GATTGGTTTCGGACGTGT; and for the HisC101Ala mutant, FP: GGCAGACCGCCCAGCCCCCTGCA CCCAACA and RP: AGGGGGCTGGGCGGTCTGCCGAGT. The expression constructs containing mutant sequences were verified by DNA sequencing (ABI Prism 3100 analyzer; Applied Biosystems) using bacteriophage M13 and maltose-binding protein (MBP)-specific malE primers.
The recombinant wild type and mutant versions were expressed as maltose binding protein fusion proteins in E. coli and purified using amylose-affinity chromatography (18). The mutant variants were tested using ELISA and immu-noblot for their ability to bind anti-human C1q, chain specific antibodies, and anti-MBP monoclonal antibodies (data not included).
Detection of pH Dependence of the Interaction between C1q, ghA, ghB, and ghC with IgG1, CRP, or PTX3
The microtiter wells were coated for 1 h at 37 °C with either 1 µg/well heat-aggregated monoclonal IgG1, 1 µg/well human CRP, or 1µg/well recombinant human PTX3 in a carbonate buffer at pH 9.6. Any nonspecific binding sites were blocked using 200 µL/well 1% w/v BSA for 1 h at 37 °C. The wells were then washed with PBS containing 0.05% Tween-20 and were incubated with C1q, ghA, ghB, or ghC (1 µg/well), and diluted in a citrate–phosphate buffer (50 mM sodium citrate, 50 mM Na2HPO4, 140 mM NaCl, 0.05% v/v Tween-20) for a pH range between 3.5 and 8 or in a carbonate buffer (50 mM NaHCO3, 50 mM Na2CO3, 140 mM NaCl, 0.05% v/v Tween-20) for a pH range between 8.5 and 12. After washing, the microtiter wells were incubated with rabbit anti-C1q antibodies for C1q and mouse anti-MBP antibodies (1: 4000 dilutions) for 1 h at 37 °C for the recombinant modules. Bound C1q and recombinant globular head fragments were detected using HRP-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG (1:1000 dilutions in both cases). OPD was used as the substrate. The results for the pH dependence of target binding were presented in arbitrary units, where the minimal observed binding of C1q was taken as 0 and the maximal as 1. The pH dependence of C1q and its recombinant modules binding and the inflection points (representing the effective pKa) were analyzed by fitting the data plots with the closest sigmoid curve, using the data analysis software Microcal Origin 6.0. The data are given as an average of three experiments ± standard deviation.
Determination of the Dependence on NaCl Concentration of the Interaction between C1q, ghA, ghB, and ghC with IgG1, CRP, or PTX3
As described above, IgG1, CRP and PTX3 were coated on microtiter wells. After blocking, immobilized target molecules (1µg/well) were incubated for 1h at 37 °C with C1q and recombinant globular head modules diluted in a phosphate buffer containing 0.05% v/v Tween-20 and increasing amounts of NaCl (0, 0.019, 0.038, 0.075, 0.15, 0.3, 0.6, or 1.2 M NaCl). After washing, the detection was performed as described above. The values obtained for the absence of NaCl were considered as 100%, and those of representative increased concentrations of NaCl were used to calculate the percent reduction in target binding. The data is given as an average of three experiments ± standard deviation.
ELISA for Detecting the Interaction of Recombinant ghA, ghB, and ghC and Their Substitution Mutants with Target Proteins (IgG1, PTX3, or CRP)
Microtiter wells were coated for 1 h at 37 °C with 1 µg/well IgG1, CRP, or PTX3 in carbonate buffer at pH 9.6. Any nonspecific binding sites were blocked using 200 µL/well 1% w/v BSA for 1 h at 37 °C. The wells were then washed with PBS containing 0.05% Tween-20 and incubated with a serial dilution of recombinant ghA, ghB, or ghC and corresponding mutant forms (from 0.015 µg/well to 2 µg/well) in TPBS for 1 h at 37 °C. The amount of bound protein was detected with anti-MBP antibodies as described above. The data are given as an average of three experiments ± standard deviation. The plateau values for the wild-type recombinant modules were considered as 100%, and those of the mutant form were used to calculate the percent reduction in target binding.
RESULTS
Effect of NaCl Concentration (Ionic Strength) on the Interaction of C1q, ghA, ghB, or ghC with IgG1, CRP, or PTX3
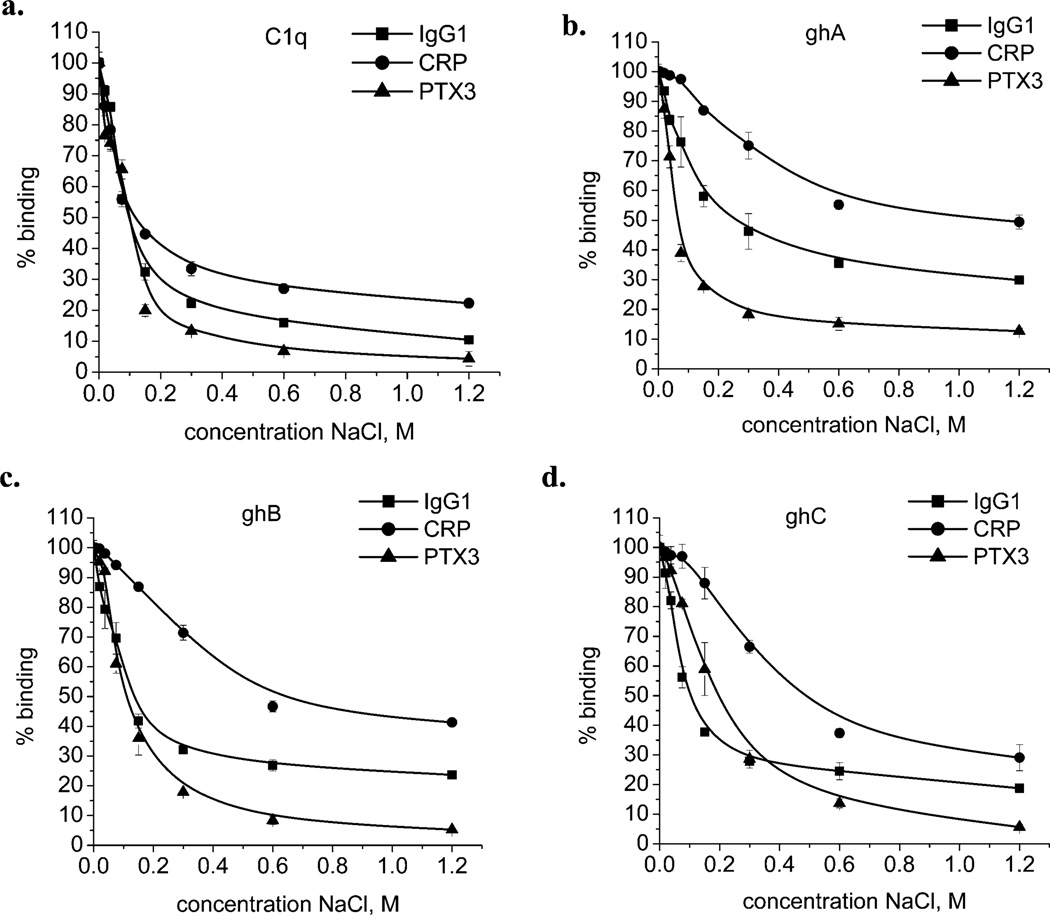
We examined the inhibitory effect of increasing NaCl concentrations on the interaction of C1q, ghA, ghB, or ghC with IgG1, CRP, and PTX3 by ELISA. In all cases studied, the interactions were highly dependent on the ionic strength of the binding buffer (Figure 1).The interaction between C1q (as well as recombinant modules) with PTX3 appeared to be the one most sensitive to ionic strength, followed by IgG1 and CRP. In the case of IgG1 and CRP, even 1.2 M NaCl was unable to completely disrupt binding. As shown in Table 1, the percentage residual binding in the presence of 1.2 M NaCl was in the range 10–30% for IgG1, 20–50% for CRP and 4–15% for PTX3. Among the recombinant modules, the ghC seemed most sensitive to the ionic strength of the binding buffer.
Figure 1.
Interaction of C1q, ghA, ghB, or ghC with IgG1, CRP, or PTX3 at various NaCl concentrations: (a) C1q, (b) ghA, (c) ghB, and (d) ghC. One µg/well of IgG1, CRP, or PTX3 was coated on the microtiter wells for 1 h at 37 °C. After blocking with PBS containing 1% w/v BSA and subsequent washing, the wells were incubated with 1 µg/well of C1q or recombinant-globular-head fragments (ghA, ghB, and ghC) in the assay buffer containing different concentrations of NaCl. The bound C1q was detected using rabbit anti-human C1q antibodies, followed by the goat anti-rabbit IgG–HRP conjugate. The recombinant-globular-head fragments were probed using mouse anti-MBP antibodies, followed by the rabbit anti-mouse IgG–HRP conjugate. The data shown is the mean ± SD of triplicate measurements. The result is presented in % residual binding. The value for 0% NaCl (only phosphate buffer) was taken as 100%.
Table 1.
Effective pKa Values and the Residual Percentage Binding for Native C1q and Recombinant-Globular-Head Modules (ghA, ghB, and ghC) in the Presence of 1.2 M NaCl
| protein | effective pKa |
residual binding in the presence of 1.2 M NaCl |
||||
|---|---|---|---|---|---|---|
| IgG1 | CRP | PTX3 | IgG1 | CRP | PTX3 | |
| C1q | 4.7 and 6.7 | 4 and 6.3 | 8.5 | 10% | 20% | 4% |
| ghA | 3.5 and 5.9 | 4.2 and 5.5 | 6 and 4.5 | 30% | 50% | 15% |
| ghB | 4.5 and 6.7 | 3.7, 6.1, and 9.0 | 6 and 4.5 | 24% | 40% | 5% |
| ghC | 4.1 and 6.2 | 4.3 and 6.5 | 6 and 4.5 | 19% | 30% | 6% |
Effect of pH on the Interaction of C1q, ghA, ghB, or ghC with IgG1, CRP, or PTX3
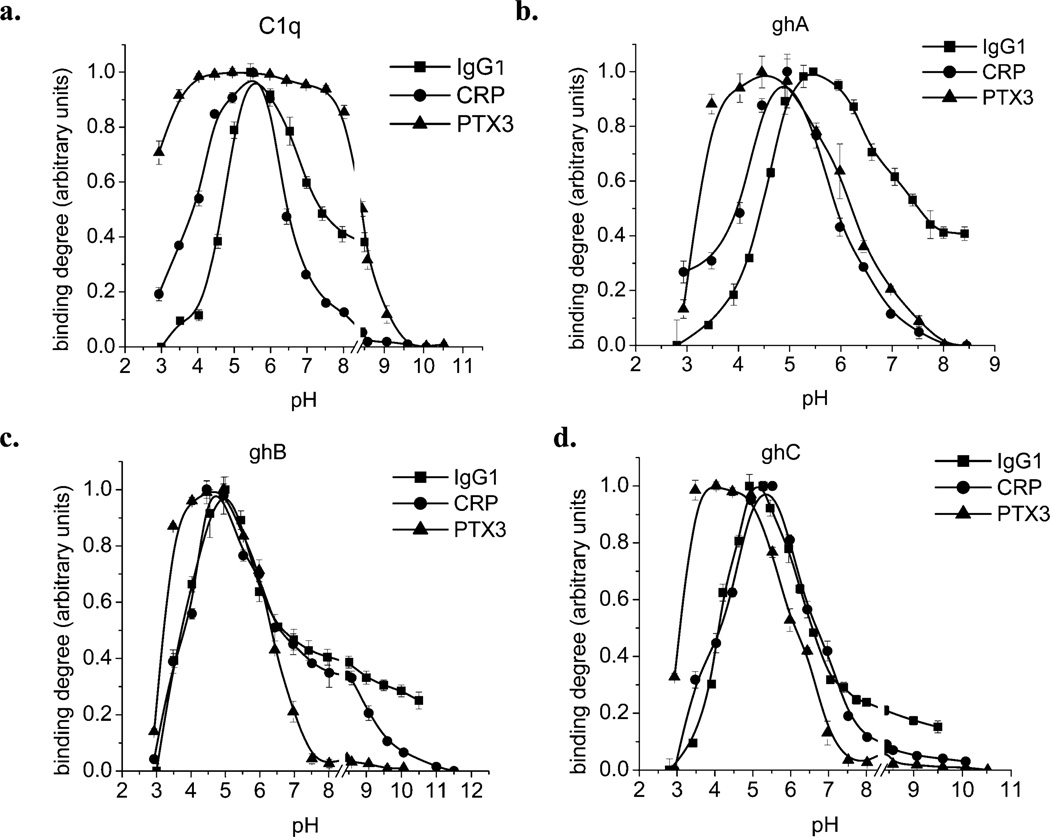
The results of the experiments to judge the effect of pH on the interaction between C1q, ghA, ghB, or ghC with IgG1, CRP, or PTX3 clearly demonstrate the differences between the behavior of C1q and its recombinant globular head modules in their binding to studied target molecules (Figure 2; Table 1). The data on the pH dependence of C1q (and its recombinant modules) binding to IgG1 (Figure 2a) is consistent with a previously published observation using rabbit IgG (19). The binding curves have maxima at pH between 5 and 5.5. In the neutral pH range, these interactions are pH independent. In the case for CRP (Figure 2b), the behavior of ghB is different than that of ghA, ghC, and C1q, which were generally comparable. The inflection points (effective pKa) for C1q, ghA, and ghC are two in number and similar in value. However, for ghB, there are three inflection points. The maxima for C1q and ghC are at pH 5.5 and for ghA and ghB about pH 5. The results of the C1q–PTX3 interaction differ greatly from other observed pH-dependence curves. The binding is pH independent over a wide pH range. The curves for ghA, ghB, and ghC are quite similar and have a maximum at pH 4.5. The effective pKa values, calculated for all curves, are given in Table 1.
Figure 2.
Effect of pH on the binding of C1q, ghA, ghB, or ghC to IgG1, CRP, or PTX3. One µg/well of heat-aggregated IgG1, CRP, or PTX3 was coated on the microtiter wells for 1 h at 37 °C. After blocking with PBS containing 1% w/v BSA and subsequent washing, the wells were incubated with 1 µg/well of C1q or recombinant-globular-head fragments in the assay buffer adjusted to different pH values. The bound C1q was detected using rabbit anti-human C1q antibodies, followed by the goat anti-rabbit IgG–HRP conjugate. The recombinant-globular-head fragments were probed using mouse anti-MBP antibodies, followed by the rabbit anti-mouse IgG–HRP conjugate. The data shown is the mean ± SD of triplicate measurements. The result is presented in arbitrary units, where the minimal observed binding was considered to be 0 and the maximal to be 1.
Bacterial Expression of the Point Mutants of ghA, ghB, and ghC Modules
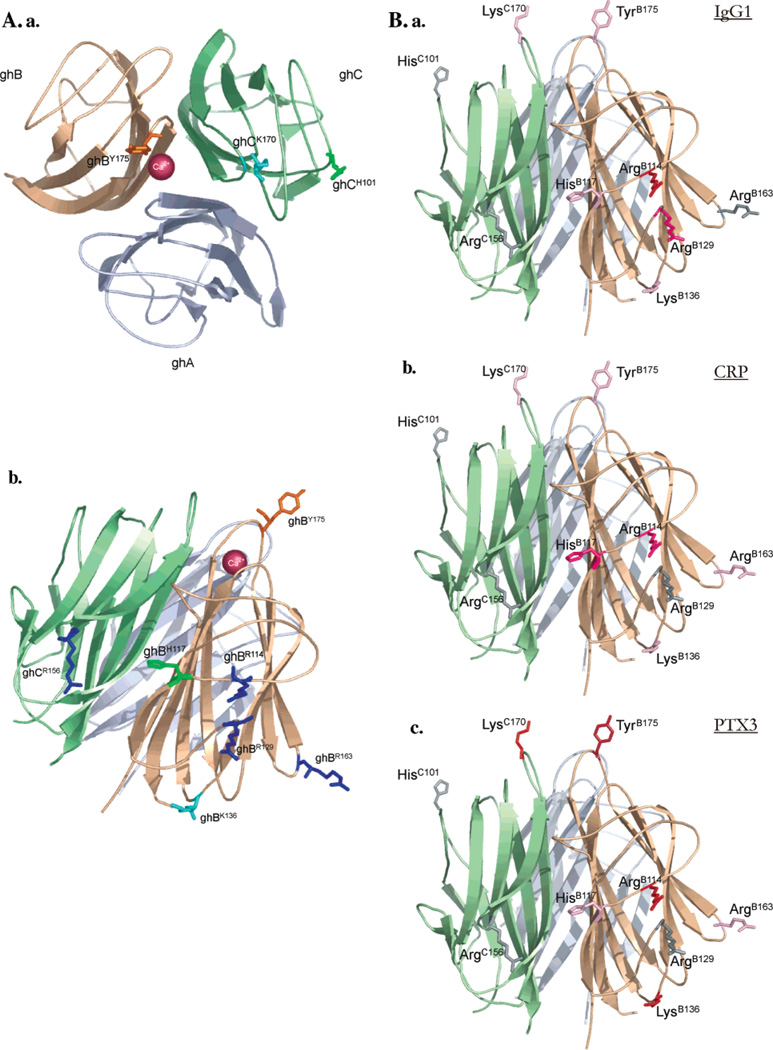
Using PCR-based site-directed mutagenesis, the alanine and glutamate variants of key arginine residues (ArgA162, ArgB114, ArgB129, ArgB163, and ArgC156) were generated. Another three mutants were also engineered that included the substitution of ArgB114 to glutamine and of HisB117 to either alanine or aspartate (10). Four other mutants (TyrB175Leu, LysB136Glu, HisC101Ala, and LysC170Glu) were generated to study the role of ghB and ghC in target binding in more detail. The relative positions of all residues within the gC1q heterotrimer that were subjected to site-directed mutagenesis are shown in Figure 3a. The incorporation of each mutation was confirmed by automated DNA sequencing. The PCR products were subcloned in the pMal-c2 vector and expressed in the soluble fraction as MBP fusion proteins in E. coli BL21 cells. Following induction with 0.4 mM IPTG for 3 h, each fusion protein accumulated intracellularly as an overexpressed protein band of ~60 kDa, as judged by SDS–PAGE under reducing conditions (gel not included). The majority of the MBP fusion proteins, extracted in the soluble fraction after cell lysis and sonication, bound to amylose resin and eluted as >95% pure soluble fractions. The mutants were expressed to levels comparable to those of their wild-type counterparts. When the fusion proteins were passed through a Q-Sepharose anion-exchange column to remove contaminating DNA, the fusion proteins bound at 0.1 M NaCl and eluted as a sharp peak at ~0.6 M NaCl, with the mutants behaving in a fashion very similar to that of the wild-type proteins. The factor Xa cleavage, used to separate the globular domain from the MBP, caused aggregation of the wild type as well as the mutants; therefore, the MBP fusions of each globular region were used for functional assays.
Figure 3.
Positions of the amino acid residues that were substituted using site-directed mutagenesis: (a) the apex of gC1q with TyrB175, LysC170, and HisC101; (b) the side surfaces of ghB and ghC with ArgB114, HisB117, ArgB129, LysB136, ArgB163, and ArgC156. Arginine residues are colored in dark blue, lysine residues are in light blue, histidine residues are in green, and the tyrosine residue is in orange. The calcium ion is represented as a dark-red ball. (B) Important residues for binding the three targets: (a) IgG1, (b) CRP, and (c) PTX3. The mutated residues are colored according to the reduction exhibited by them in their abilities to bind the tested target molecules. The following color scale is used: 0–10% gray, 11–20% light pink, 21–30% dark pink, 31–40% red, and 41–50% brown. The Figures were generated using PyMOL.
The point mutants of the ghA, ghB, and ghC modules were recognized by rabbit anti-human C1q antisera. All the point mutants retained the antigenic characteristic of their wild type forms because they were well recognized by module-specific antibodies, anti-human C1q polyclonal antibodies using ELISA and immunoblot (data not included).
Contribution of Single Residue Mutants of ghA, ghB, and ghC in the Interaction with IgG1, CRP, or PTX3
A number of single-residue mutants of ghA, ghB, and ghC (ArgA162-Ala/Glu, ArgB114Gln/Glu, HisB117Ala/Asp, ArgB129Ala/Glu, ArgB163Ala/Glu, and ArgC156Ala/Glu) were tested for their interactions with IgG1, CRP, and PTX3. Additional mutants (TyrB175Leu, LysB136Glu, HisC101Ala, and LysC170Glu) were also tested to narrow C1q–pentraxin binding. The importance of the mutated residues within the gC1q structure and their positions have been highlighted in Figure 3B.
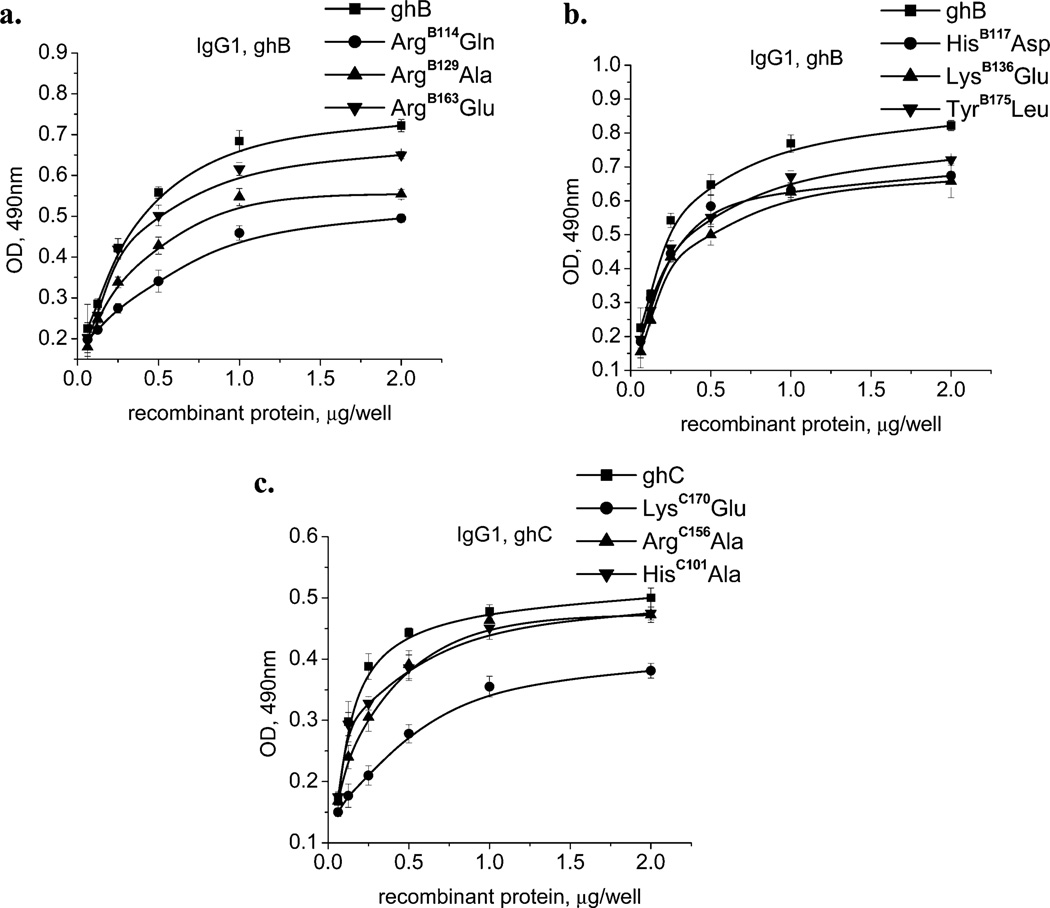
The binding of the wild type and their corresponding mutants to all of the three targets was significant and dose-dependent, as shown in Figure 4 (for IgG1), Figure 5 (for CRP), and Figure 6 (for PTX3). The reduction in binding has been summarized in Table 2 and Figure 7. In most instances, the nature of the substitution did not matter, and the binding of both variants was comparable. Interestingly, two of the mutants, HisC101Ala and ArgB129Ala, showed slightly increased binding for CRP and PTX3, respectively, as compared to their wild-type counterparts. All other mutants showed either reduced or unchanged abilities to bind to the tested targets.
Figure 4.
Interaction of the wild-type and mutant forms of the recombinant-globular-head modules with human IgG1. The microtiter wells were coated with 1 µg/well of heat-aggregated IgG1. After blocking and washing, different quantities (0.063, 0.125, 0.25, 0.5, 1.0, and 2 µg/well) of (a) ghB, ArgB114Glu, ArgB129Ala, and ArgB163Glu; (b) ghB, HisB117Asp, LysB136Glu, and TyrB175Leu; and (c) ghC, LysC170Glu, ArgC156Ala, and HisC101Ala were added to the wells and incubated. Mouse anti-MBP antibodies, followed by the rabbit anti-mouse IgG–HRP conjugate detected the amount of bound globular-head modules or their mutants. The data shown is the mean ± SD of triplicate measurements. The result is presented in % residual binding. The values for the wild-type modules were taken as 100%.
Figure 5.
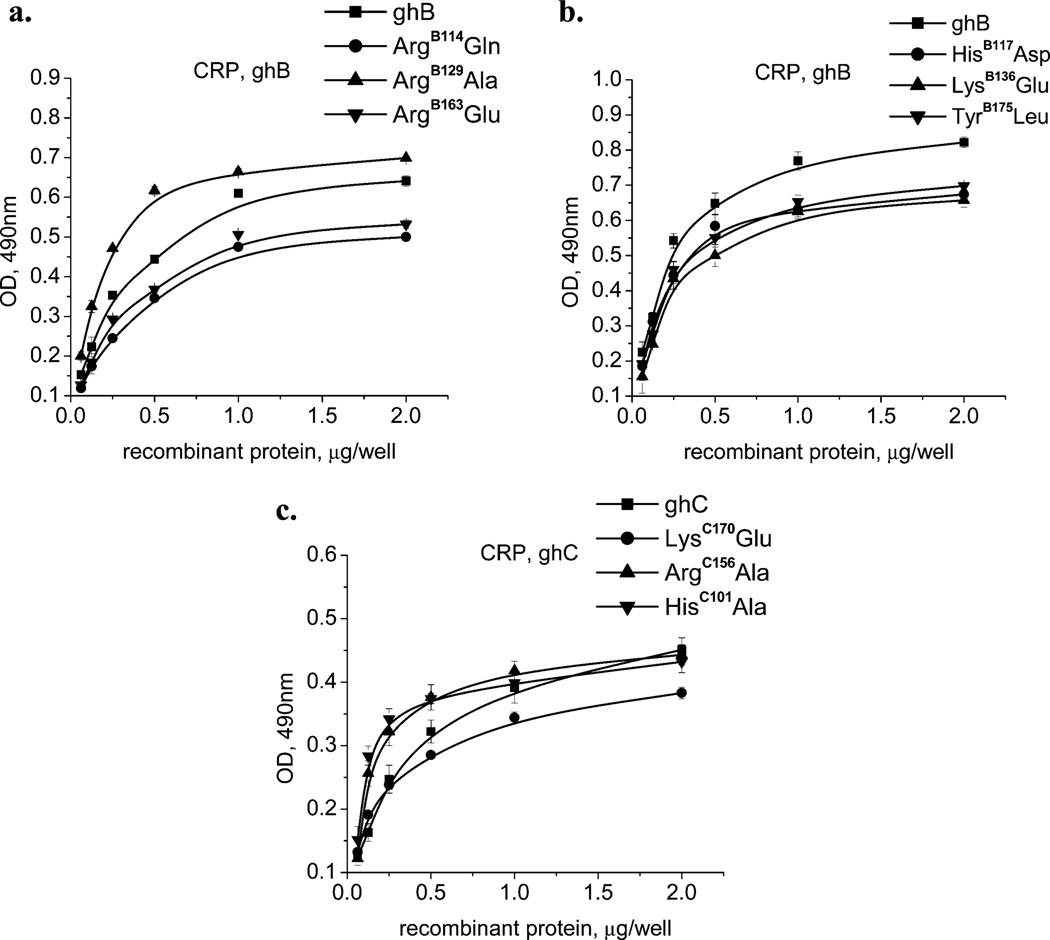
Interaction of wild-type and mutant forms of ghA, ghB, and ghC with human CRP. The microtiter wells were coated with 1 µg/well of CRP. After blocking and washing, different quantities (0.063, 0.125, 0.25, 0.5, 1.0, and 2 µg/well) of (a) ghB, ArgB114Glu, ArgB129Ala, and ArgB163Glu; (b) ghB, HisB117Asp, LysB136Glu, and TyrB175Leu; and (c) ghC, LysC170Glu, ArgC156Ala, and HisC101Ala were added to the wells and incubated. Mouse anti-MBP antibodies, followed by the rabbit anti-mouse IgG–HRP conjugate detected the amount of bound globular-head modules or their mutants. The data shown is the mean ± SD of triplicate measurements. The result is presented in % residual binding. The values for the wild-type modules were taken as 100%.
Figure 6.
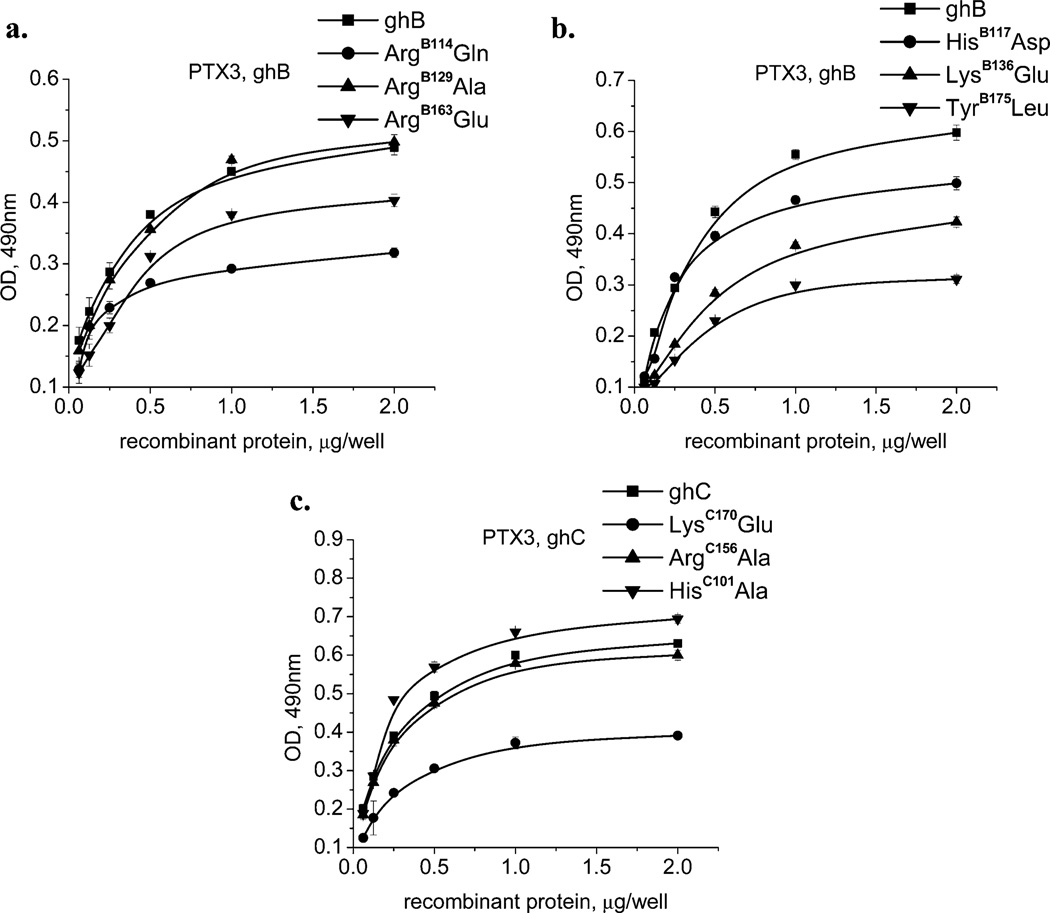
Interaction of wild-type and mutant forms of ghA, ghB, and ghC with recombinant-human PTX3. The microtiter wells were coated with 1 µg/well of PTX3. After blocking and washing, different quantities (0.063, 0.125, 0.25, 0.5, 1.0, and 2 µg/well) of (a) ghB, ArgB114Glu, ArgB129Ala, and ArgB163Glu; (b) ghB, HisB117Asp, LysB136Glu, and TyrB175Leu; and (c) ghC, LysC170Glu, ArgC156Ala, and HisC101-Al were added to the wells and incubated. Mouse anti-MBP antibodies, followed by the rabbit anti-mouse IgG–HRP conjugate, detected the amount of bound recombinant-globular-head modules or their mutants. The data shown is the mean ± SD of triplicate measurements. The result is presented in % residual binding. The values for the wild-type modules were taken as 100%.
Table 2.
Percent Reduction in the Binding of Substitution Mutants to IgG1, CRP, and PTX3a
| mutated residue | obtained decreases in target binding |
||
|---|---|---|---|
| IgG1 | CRP | PTX3 | |
| ArgA162Ala | 0% − |
0% − |
0% − |
| ArgA162Glu | 0% − |
0% − |
0% − |
| ArgB114Gln | 30% +++ |
20% ++ |
35% +++ |
| ArgB114Glu | 34% +++ |
22% ++ |
37% +++ |
| HisB117Ala | 15%+ | 25% ++ |
10% + |
| HisB117Asp | 20% ++ |
30% +++ |
15% + |
| ArgB129Ala | 20% ++ |
10% increase −− |
0% − |
| ArgB129Glu | 23% ++ |
5% ± |
0% − |
| LysB163Glu | 20% ++ |
20% ++ |
32% +++ |
| ArgB163Ala | 8% ± |
15% + |
18% + |
| ArgB163Glu | 10% + |
20% ++ |
20% ++ |
| TyrB175Leu | 15% + |
15% + |
50% +++++ |
| HisC101Ala | 0% − |
0% − |
10% increase −− |
| ArgC156Ala | 0% − |
0% − |
0% − |
| ArgC156Glu | 0% − |
0% − |
0% − |
| LysC170Glu | 20% ++ |
20% ++ |
40% ++++ |
Scoring has been made to rank the importance of mutated residues by their importance; one + means 10% decrease in binding. Native C1q protein has 100% binding, 0% decrease, and hence is indicated by one −. A 10% increase is indicated by −−. A decrease to 10% is indicated by ±; between 10 and 20% is +; between 20 and 30% is ++; between 30 and 40% is +++; between 40 and 50% is ++++; and between 50 and 60% is +++++.
Figure 7.
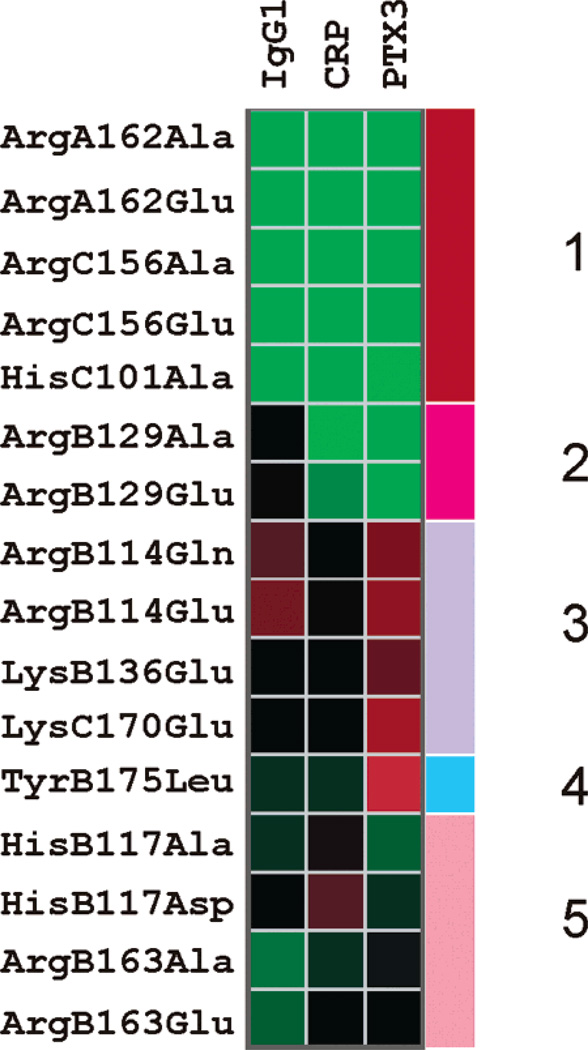
Cluster analysis of the observed reduction in the binding of mutants to the tested target molecules. The reduction in binding is exhibited via a color scale where red represents a higher reduction and green represents a lesser reduction. The data has been clustered by hierarchical clustering using the euclidean distance measure. The dendrogram is not shown. Five clusters are apparent and are indicated as Clusters 1, 2, 3, 4, and 5. Cluster 1 mutants do not show any dramatic changes in binding to any target. Cluster 2 shows some specific effects toward IgG1. Cluster 3 mutants show effects across all targets. Cluster 4 is similar to Cluster 3 in that it shows effects against all three targets but different in that it shows a maximum effect against PTX3. Cluster 5 shows somewhat mixed effects to IgG1 and PTX3 but remains consistent with regard to CRP.
Of the total of 16 mutants examined, 5 clusters of mutants can be identified (Figure 7). Cluster 1 mutants (ArgA162Ala, ArgA162Glu, ArgC156Glu, ArgC156Ala, and HisC101Ala) showed no significant change in binding any of the three targets (except for the small increase in HisC101Ala). Four of these mutants are different variants of two amino acids, ArgA162-Ala and ArgC156Glu, both being arginine residues. Cluster 2 contains only two variants of the same residue, ArgB129Ala and ArgB129Glu, both of which affect binding to IgG1. Cluster 3 comprises four mutants (ArgB114Glu, ArgB114Gln, LysB136-Glu, and LysC170Glu), all of which showed a decrease of at least 20% binding to all of the three targets examined. Cluster 4 comprises a single mutant, TyrB175Leu, which causes the maximum decrease in binding to PTX3 (50%). It is also the maximum decrease obtained for any mutant for binding to any target. Cluster 5 represents a mixed effect of substitution mutations when tested for IgG1 and PTX3 binding; however, they remained consistent in their properties with regard to CRP.
DISCUSSION
C1q recognizes a broad range of ligands via its heterotrimeric gC1q domain, which is composed of ghA, ghB, and ghC modules (3–5). The expression and functional characterization of individual modules have revealed structural and functional autonomy within the gC1q domain in terms of preferentially binding a diverse range of C1q ligands (9, 18). However, how C1q manages to specifically bind to a large number of structurally diverse self and nonself targets is still unexplained (4). C1q interacts with charged polyampholytic proteins (IgG, CRP, and gC1qR) as well as with hydrophobic ligands (β-amyloid peptide and fibrils and LPS and viral proteins such as gp41of HIV-1). A charge pattern recognition mechanism has been proposed for C1q, but a discrete pattern has not been conclusively established. It is also not clear how many binding sites exist on gC1q, whether they overlap, and whether conformational changes are required for specific binding. The availability of the recombinant forms of ghA, ghB, and ghC have allowed us to mutate a number of residues that have been considered important for C1q–target interactions by a variety of approaches, including chemical modification and mutational studies (10, 11), molecular modeling (7), bioinformatics (4), and in silico theoretical calculations of electric moment vectors (12). Thus, we examined their interactions with three well-known and physiologically relevant targets of C1q: IgG1, CRP, and PTX3 (4, 12, 16, 18, 20, 21). We also investigated the effect of ionic strength and pH on the interaction of C1q and recombinant ghA, ghB, and ghC with IgG1, CRP, and PTX3.
Binding of C1q to IgG1, CRP, and PTX3 is Highly Electrostatic in Nature
C1q recognizes several charged molecules, including IgG (22), IgM (23), spectrin (24), OmpK36 (25), Tamm-Horsfall protein (26), decorin (27), and fibromodulin (28). Many of these interactions have been shown to be sensitive to the ionic strength of the binding buffer. The results of this study reaffirm this point that the binding of C1q to IgG1, CRP, and PTX3 is highly electrostatic in nature because the presence of 1.2 M NaCl abolishes the interaction to a large extent (Figure 1). In all cases, ghA appeared to be less involved in charge interactions, especially with CRP. For this target, ghB also demonstrates its significant participation in hydrophobic contacts.
Hydrophobic Contacts within the gC1q Domain Have a Functional Relevance Even at a very Low pH
The presence of hydrophobic contacts in the case of C1q and the recombinant modules is amply demonstrated by the pH-dependent binding analysis, where even extreme acidic conditions fail to completely abolish the studied interaction. The pH dependence of C1q and the recombinant modules for binding to all targets showed different trends. The main differences were observed for C1q and ghA (Figure 2a and b), whereas the ghB and ghC modules showed similar properties (Figure 2c and d). The implication is that the binding sites on the gC1q domain for the three tested targets are not identical, but those located on ghB and ghC modules probably involve similar residues. It is also likely that the effective pKa values of the groups engaged as well as the apparent value of the electrostatic potential in the contact area are similar for IgG, CRP, and PTX3. The inflection points of the binding curves suggest participation of His and Glu residues for IgG1, Lys/Tyr, His, and Glu for CRP and Asp, and His and possibly Lys residues for PTX3. In all cases, maximal binding was achieved in slightly acidic pH. This supports the notion of maximal complement activation by CRP under mild acidic conditions (29). At low pH, the gC1q domain seems to be stable (12, 30) and binds more avidly to its targets, thereby initiating the classical complement pathway. Thus, the pH at the sites of inflammation could serve as a regulatory mechanism, influencing the magnitude of complement activation.
Certain Key Residues Belonging to the Apo and Holo Planes of the gC1q Domain Are Central to C1q–Ligand Interaction
We have recently shown that the exposed Ca2+ located near the apex of the gC1q heterotrimer primarily influences the target recognition properties of C1q toward IgG, CRP, and PTX3 (12). Thus, at pH 7.4, the loss of Ca2+ leads to changes in the direction of the electric moment from coaxial (where the putative CRP binding site is located) to perpendicular to the molecular axis (toward the most likely IgG-binding site), which appears important for target recognition by C1q and subsequent complement activation. This suggests a leading role for the electric moment in the target recognition properties of C1q. In the heterotrimeric gC1q apo form, the electric moment vector is perpendicular to the quasi-C3v molecular axis. In its holo form, the vector changes direction by 87° and stands parallel to the same axis, pointing toward the apex of the molecule. On the basis of the electrostatic properties of gC1q (12), we defined two planes that are important for C1q functions: the holo plane, which is normal to the electric moment vectors in the holo form; and the apo plane, which is normal to the electric moment vectors in the apo form. The apo plane contains the following positively charged surface residues: ArgB163, LysB136, ArgB129, ArgB108, ArgB109, ArgB114, and LysB132 as well as AsnB104, HisB117, GluB127, and GluB162. The holo plane consists of LysA173, ArgB108, ArgB109, ArgB150, and LysC170 as well as TyrB175 that protrude above the molecular surface TrpA147, AsnB176, AspB201, and GluA209. These residues on the gC1q domain are the potential candidates involved in the target binding. In view of apo and holo planes and the pH scanning experiments, the mutants ArgA162Ala/Glu, ArgB114Gln/Glu, HisB117Ala/Asp, ArgB129Ala/Glu, LysB136Glu, ArgB163Ala/Glu, TyrB175Leu, HisC101Ala, ArgC156Ala/Glu, and LysC170Glu were selected for their interactions with IgG1, CRP, and PTX3 (Figure 3A).
Most of these residues, which were subjected to site-directed mutagenesis, are conserved among the C1q in different species; however, among C1q family proteins, including collagen VIII and X, precerebellin, hibernation proteins, multimerin, ACRP30/adiponectin, saccular collagen, and elastin-microfibril-interface-located protein (EMILIN), they are highly variable as revealed by the ConSurf analysis (4). The Arg residues have been shown to be central to C1q–IgG interactions (10), and the mutated Arg residues from ghB belong to the apo plane. LysC170 and TyrB175, which belong to holo plane, have been proposed by molecular modeling to be involved in the interaction with CRP (7). In addition, the substitution of HisC101, which is located on the edge of the apex and side surface of ghC, may interfere with a rotation toward the side surface of ghC, as predicted previously for ghB (12). The pH-dependent binding and determined inflection points (corresponding to apparent pKa values, Table 1) likely suggest the participation of the His residue(s) in C1q–ligand interaction (with the exception of C1q–PTX3 binding). LysB136 was selected as a residue from the apo plane, which is located near the bottom side of ghB and far away from any proposed binding site.
ArgB114, ArgB129, and HisB117 are Central to C1q–IgG1 Interaction
A number of studies have addressed the C1q interaction with IgG1 and IgG3 and C1q binding sites on IgG molecules (31–35). Chemical modification, mutational analysis, and molecular modeling approaches have been used to dissect the complementary IgG binding sites on the gC1q domain (7, 10, 11, 19). Studies using point mutants of ghA, ghB, and ghC and their interactions with heat-aggregated IgG have highlighted that the ghB module has a dominant role in the gC1q–IgG interaction: ArgB114 being the key residue, and ArgB129, ArgB163, and HisB117 having subsidiary roles in the C1q–IgG interaction (10).
Molecular modeling based on the crystal structure of the heterotrimeric gC1q domain of human C1q has revealed that the ghB module is the most accessible of the three modules probably because of its position on the outer part of the C1q molecule (7). It has a predominantly positively charged outer surface distinguished by the presence of the three basic amino acids: Arg101, Arg114, and Arg129, two of which have already been described as important in the IgG–C1q interaction. The most attractive structural model positions the two molecules in such a way that Asp270 and Lys322 of IgG form salt bridges with Arg129 and Glu162 of ghB, respectively, with additional ionic interactions provided by Arg114 and Arg161 of ghB. In this orientation, the Arg129 appears to act like a wedge between the CH2 and the light-chain-constant domains.
Our data on C1q–IgG1 interactions are consistent with mutational analysis (10) and molecular modeling (7). The percentage contribution of residues ArgA162, ArgB114, HisB117, ArgB129, ArgB163, and ArgC156 in C1q–IgG1 binding is slightly different to those for heat-aggregated IgG, but the trend is comparable. For IgG1, ArgB114 is again the most important residue, followed by ArgB129 and HisB117 (Figures 3 and 4). The differences between the present data and those in ref 10 (10) are most likely due to the fact that heat-aggregated IgG is a mixture of all IgG subclasses. We show here that residues from the top of the gC1q domain are important for IgG1 recognition. Mutations of TyrB175 and LysC170 to Leu and Glu caused about 15 and 20% decrease, respectively, in the IgG1 binding. In addition, previous chemical modifications revealed the participation of tryptophan residues from gC1q (available only in A- and C-chains) in the IgG interaction. One possible candidate is TrpA147, which belongs to the holo plane and is quite exposed to the solvent.
TyrB175 and LysC170 Appear Important for the C1q–CRP Interaction
CRP is an antiinflammatory acute-phase protein of the innate immune system. Activation of the classical pathway initiated by the binding of C1q to ligand-bound CRP is considered a participant in several of the proposed antiinflammatory actions of CRP such as the phagocytosis of apoptotic cells and the protection from S. pneumoniae (4). The CRP molecule has five identical, 206-amino-acid-long subunits held together through noncovalent interactions and arranged with pentameric symmetry around a central pore. The crystal structure has revealed a flattened jellyroll appearance for each subunit (A, B, C, D, and E) that is made up of two antiparallel β-sheets and a single short α-helix (36, 37).
Site-directed mutagenesis initially identified residues Asp112 and Lys114 as important in the CRP–C1q interaction (38). The crystal structure revealed a striking extended cleft on the effector face of CRP, which starts at about the center of each subunit and extends its edge to the central pore of the pentamer. The wider and shallow end of the cleft, which is close to the pore and incorporates Asp112, provided accommodation for C1q (36, 38). Asp112 and Tyr175 appear to be the contact residues and participate directly in the CRP–C1q interactions. Thus, the starting point for the refinement of the structural model of the CRP–gC1q interaction aligned the basic residues of gC1q with the CRP cleft region and CRP Asp112 and Tyr175 in particular. In the resulting best-fit model, CRP Tyr175 (subunits A and D) is within the H-bond distance of gC1q residues Tyr175 (ghB) and Lys200 (ghA), with the CRP Tyr175 (subunit E) at 4 Å from gC1q Trp147 (ghA) (7). The proposed model has been found to be sterically restrained, consistent with the requirement for ligand-bound CRP and associated conformational change within either the protomer or the pentameric ring (7, 36, 38).
Mutagenesis results reported here (Table 1, Figures 3 and 5) highlight the importance of TyrB175 and LysC170. The mutation of TyrB175 to leucine probably impairs potential hydrogen bonding without disrupting its hydrophobic effect. A slight decrease observed in the case of this mutant (15%) may be interpreted as a prevalent role of stacking interactions of the aromatic ring of TyrB175 (potentially with TyrB175 from CRP) rather than hydrogen bonding. Some residues from the side of ghB, which were demonstrated to be involved in IgG binding, also appeared to be important for the CRP interaction. The negatively charged substitution of ArgB129 had only up to 5% decreased binding and the neutral one about a 10% increase in binding to CRP. The two mutants from the apex of ghC demonstrated clear differences in their behavior. The LysC170 mutant appeared to be involved in the interaction, and HisC101 did not show a significant difference.
TyrB175 and LysC170 Substitutions Have a Dramatic Effect on the C1q–PTX3 Interaction
C1q as well as recombinant ghA, ghB, and ghC modules interacts with PTX3, a long prototypical pentraxin (39). The C1q–PTX3 interaction probably has an important role in the removal of apoptotic and necrotic cells. The complementary interacting sites on the gC1q and PTX3 are not well defined. Interestingly, the residues on CRP that have been found to be involved in C1q binding (38) are not conserved in PTX3 (37). Furthermore, on the basis of molecular modeling, the PTX3 pentraxin domain has a similar structural fold to SAP because most of the β-strands and the α-helical regions are conserved. Our results suggest that residues from both the apex and the side surface of ghB are involved in the interaction with PTX3. Consistent with the NaCl-inhibition experiment data, mutation of charged residues caused considerable reduction in PTX3 binding, confirming the highly electrostatic nature of this interaction. As highlighted in Table 2 and Figure 7, ArgB129, which is important for the C1q−IgG1 interaction, does not contribute toward C1q binding to PTX3 and CRP. The substitution of TyrB175 to leucine caused nearly 50% reduction in binding, indicating the likely participation of this residue in a hydrogen bond and/or aromatic stacking effects (as opposed to IgG1 and CRP). LysC170Glu also showed a considerable reduction in binding PTX3 (about 40%); however, the HisC101 Ala mutation led to a slight increase (10%) in binding.
Cluster Analysis Reveals the Differential Contributions of the Residues in the Apo and Holo Planes to C1q–Target Interactions
In an attempt to define target-binding sites on the gC1q domain, we performed a cluster analysis on the basis of data available from target binding studies using wild-type and mutant ghA, ghB, and ghC modules (Figure 7). There are five clusters that throw a definite light on the overlapping nature of gC1q binding sites. It must be noted, however, that the clusters defined here do not indicate proximity in sequence or structure but only represent similarity in the behavior of mutants toward binding the target proteins. Cluster 1 mutants do not show any dramatic changes in binding to any target. Mutants belonging to Cluster 2 show specific effects toward IgG1 binding. Cluster 3 mutants show effects across all tested targets. Cluster 4 is similar to Cluster 3 in that it shows effects against all three targets but is different in exhibiting a maximum effect toward PTX3. Cluster 5 shows somewhat mixed effects of mutations when tested for IgG1 and PTX3 binding but remains consistent with regard to CRP. Thus, cluster analysis revealed that the contribution of residues examined in this study to IgG1, CRP, and PTX3 binding is not equal. In all cases, residues from the top of gC1q as well as from the equatorial region of ghB participate in interaction with targets. In addition, ArgA162 and ArgC156 mutants as well as the HisC101 mutant for IgG1 and CRP were indistinguishable from their wild-type counterparts. This indicates that the side surfaces of ghA and ghC most likely are not involved in interaction with these targets, and no rotation toward these surfaces occurs upon target recognition. Although LysB136 mutation caused a significant reduction in binding, it will be surprising if it formed a part of a binding site for IgG1, CRP, and PTX3 considering its position near the collagen arms. It is possible that this residue exerts a remote effect over the residues participating in target-binding sites. The binding data is also summarized in Figure 3B, where the mutated residues in the gC1q structure have been colored according to the percentage decrease obtained for binding.
Reichmann et al. (40) have recently suggested that protein-protein binding sites have a modular architecture made up of clusters of residues with both strong intracluster connections and weak intercluster connections. The deletion of a whole cluster of residues has no impact on the structure of the interface, whereas single mutations within a given cluster may lead to the structural rearrangement of their cluster. The present study emphasizes this issue in the context of C1q–target interactions. However, further structural studies would be required to ascertain the contribution of individual residues to C1q–target interactions in the context of the gC1q heterotrimer and the geometry of the binding site. This study highlights the differential importance of ArgB114, HisB117, ArgB129, LysB136 ArgB163, TyrB175, and LysC170 in C1q binding to IgG1, CRP, and PTX3 that probably participate in different combinations with respect to a specific ligand. It has been proposed that a specific formation of a complex of unrelated binding partners occurs through the use of alternative single-binding-site residues (41). This behavior is considered to be intrinsic to the fabric of protein binding sites that is highly specific. For the three tested target molecules in the present study, there are two common important binding surfaces on the gC1q domain, the apex of the heterotrimer with the participation of all three chains and the side of the B chain rather than discrete binding sites. We envisage that a set of charged residues from these apo and holo planes within the gC1q heterotrimer form different ionic and hydrogen bonds with complementary residues similar to polyspecific antibodies (41), a concept that can be extended to other versatile pattern-recognition proteins in innate immunity. Because hydrophobic patches are present on the gC1q domain, the recognition of the danger-alerting hydrophobic portions (acting as damage-associated molecular patterns) is also an interesting proposition (42).
ACKNOWLEDGMENT
We thank Dr. Boris P. Atanasov and Jordan D. Dimitrov for the fruitful discussions and for carefully reading the manuscript. Dr. T. Sakari Jokiranta (Helsinki, Finland) is acknowledged for his kind gift of IgG1. We are also thankful to Progetto Nobel by Fondazione Cariplo.
Abbreviations
- gC1q
globular domain of C1q
- CLR
collagen-like region of C1q
- ghA, ghB, ghC
the recombinant forms of the carboxyl-terminal, globular region of human C1q A, B, and C chains, respectively
- CRP
C-reactive protein
- PTX3
pentraxin 3
- HRP
horseradish peroxidase
- MBP
maltose-binding protein
- TPBS
phosphate-buffered saline, containing 0.05% v/v Tween-20
Footnotes
This study was supported by the National Science Foundation of Bulgaria, Grant MY–K-1303 to L.T.R. and L-1000 to M.S.K. R.G. is sponsored by the Deutsche Forschungsgemeinschaft through the Graduiertenkolleg GK370. U.K. is funded by the European Commission, University of Oxford, and the Alexander von Humboldt Foundation. A.M. and B.B. are funded by TELETHON, MIUR (FIRB Fund), the European Commission, and AIRC.
REFERENCES
- 1.Arlaud GJ, Gaboriaud C, Thielens NM, Rossi V, Bersch B, Hernandez JF, Fontecilla-Camps JC. Structural biology of C1: dissection of a complex molecular machinery. Immunol. Rev. 2001;180:136–145. doi: 10.1034/j.1600-065x.2001.1800112.x. [DOI] [PubMed] [Google Scholar]
- 2.Arlaud GJ, Gaboriaud C, Thielens NM, Budayova-Spano M, Rossi V, Fontecilla-Camps JC. Structural biology of the C1 complex of complement unveils the mechanisms of its activation and proteolytic activity. Mol. Immunol. 2002;39:383–394. doi: 10.1016/s0161-5890(02)00143-8. [DOI] [PubMed] [Google Scholar]
- 3.Kishore U, Gaboriaud C, Waters P, Shrive AK, Greenhough TJ, Reid KBM, Sim RB, Arlaud GJ. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 2004;10:551–561. doi: 10.1016/j.it.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Kishore U, Ghai R, Greenhough TJ, Shrive AK, Bonifati DM, Gadjeva MG, Waters P, Kojouharova MS, Chakraborty T, Agrawal A. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol. Lett. 2004;95:113–128. doi: 10.1016/j.imlet.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishore U, Reid KBM. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 6.Kishore U, Reid KBM. Modular organization of proteins containing C1q–like globular domain. Immunopharmacology. 1999;42:15–21. doi: 10.1016/s0162-3109(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 7.Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, Verger D, Fontecilla-Camps JC, Arlaud GJ. The crystal structure of the globular head of complement protein C1q provides a basis for Its versatile recognition properties. J. Biol. Chem. 2003;278:46974–46982. doi: 10.1074/jbc.M307764200. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 9.Kishore U, Kojouharova MS, Reid KBM. Recent progress in the understanding of the structure−function relationships of the globular head regions of C1q. Immunobiology. 2002;205:355–364. doi: 10.1078/0171-2985-00138. [DOI] [PubMed] [Google Scholar]
- 10.Kojouharova MS, Gadjeva MG, Tsacheva IG, Zlatarova A, Roumenina LT, Tchorbadjieva MI, Atanasov BP, Waters P, Urban BC, Sim RB, Reid KBM, Kishore U. Mutational analyses of the recombinant globular regions of human C1q A, B, and C chains suggest an essential role for arginine and histidine residues in the C1q–IgG interaction. J. Immunol. 2004;172:4351–4358. doi: 10.4049/jimmunol.172.7.4351. [DOI] [PubMed] [Google Scholar]
- 11.Marques G, Anton LC, Barrio E, Sanchez A, Ruiz S, Gavilanes F, Vivanco F. Arginine residues of the globular regions of human C1q involved in the interaction with immunoglobulin G. J. Biol. Chem. 1993;268:10393–10402. [PubMed] [Google Scholar]
- 12.Roumenina LT, Kantardjiev AA, Atanasov BP, Waters P, Gadjeva M, Reid KBM, Mantovani A, Kishore U, Kojouharova MS. Role of Ca2+ in the electrostatic stability and the functional activity of the globular domain of human C1q. Biochemistry. 2005;44:14097–14109. doi: 10.1021/bi051186n. [DOI] [PubMed] [Google Scholar]
- 13.Tissot B, Gonnet F, Iborra A, Berthou C, Thielens N, Arlaud GJ, Daniel R. Mass spectrometry analysis of the oligomeric C1q protein reveals the B chain as the target of trypsin cleavage and interaction with fucoidan. Biochemistry. 2005;44:2602–2609. doi: 10.1021/bi047802h. [DOI] [PubMed] [Google Scholar]
- 14.Reid KBM. C1q. Methods Enzymol. 1982;82:319–324. doi: 10.1016/0076-6879(82)82069-7. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal A, Simpson MJ, Black S, Carey MP, Samols D. A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide. J. Immunol. 2002;169:3217–3222. doi: 10.4049/jimmunol.169.6.3217. [DOI] [PubMed] [Google Scholar]
- 16.Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D’Ettorre C, Gianazza E, Tagliabue A, Salmona M, Tedesco F, Introna M, Mantovani A. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J. Biol. Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 17.Ausbel FM, Brent R, Kingston RE, Moore DD, Seidman JS, Struhl K. Mutagenesis of cloned DNA. In: Ausubel FM, editor. Current Protocols in Molecular Biology. Sons, NY: John Wiley; 1998. [Google Scholar]
- 18.Kishore U, Gupta SK, Perdikoulis MV, Kojouharova MS, Urban BC, Reid KBM. Modular organization of the carboxyl-terminal, globular head region of human C1q A, B, and C chains. J. Immunol. 2003;171:812–820. doi: 10.4049/jimmunol.171.2.812. [DOI] [PubMed] [Google Scholar]
- 19.Wines BD, Easterbrook-Smith SB. Carbodiimide cross-linking of human C1q and rabbit IgG. Mol. Immunol. 1990;27:221–226. doi: 10.1016/0161-5890(90)90133-k. [DOI] [PubMed] [Google Scholar]
- 20.Kishore U, Leigh LE, Eggleton P, Strong P, Perdikoulis MV, Willis AC, Reid KBM. Functional characterization of a recombinant form of the C-terminal, globular head region of the B-chain of human serum complement protein, C1q. Biochem. J. 1998;333:27–32. doi: 10.1042/bj3330027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojouharova MS, Panchev ID, Tchorbadjieva MI, Reid KBM, Hoppe H-J. Differential binding of IgG and of a HIV gp41 peptide by the B chain and A chain globular head sequences of C1q, respectively. J. Immunol. 1998;161:4325–4331. [PubMed] [Google Scholar]
- 22.Hughes-Jones NC, Gardner B. The reaction between the complement subcomponent C1q, IgG complexes and polyionic molecules. Immunology. 1978;34:459–463. [PMC free article] [PubMed] [Google Scholar]
- 23.Poon PH, Phillips ML, Schumaker VN. Immunoglobulin M possesses two binding sites for complement subcomponent C1q, and soluble 1:1 and 2:1 complexes are formed in solution at reduced ionic strength. J. Biol. Chem. 1985;260:9357–9365. [PubMed] [Google Scholar]
- 24.Comis A, Easterbrook-Smith SB. Binding of complement component C1q by spectrin. Biochim. Biophys. Acta. 1986;870:426–431. doi: 10.1016/0167-4838(86)90250-5. [DOI] [PubMed] [Google Scholar]
- 25.Alberti S, Marques G, Hernandez-Alles S, Rubires X, Tomas JM, Vivanco F, Benedi VJ. Interaction between complement subcomponent C1q and the Klebsiella pneumoniae porin OmpK36. Infect. Immun. 1996;64:4719–4725. doi: 10.1128/iai.64.11.4719-4725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes DC. Binding of Tamm-Horsfall protein to complement 1q measured by ELISA and resonant mirror biosensor techniques under various ionic-strength conditions. Immunol. Cell Biol. 2000;78:474–482. doi: 10.1111/j.1440-1711.2000.t01-3-.x. [DOI] [PubMed] [Google Scholar]
- 27.Groeneveld TW, Oroszlan M, Owens RT, Faber-Krol MC, Bakker AC, Arlaud GJ, McQuillan DJ, Kishore U, Daha MR, Roos A. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collec-tins. J. Immunol. 2005;175:4715–4723. doi: 10.4049/jimmunol.175.7.4715. [DOI] [PubMed] [Google Scholar]
- 28.Sjoberg A, Onnerfjord P, Morgelin M, Heinegard D, Blom AM. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 2005;280:32301–32308. doi: 10.1074/jbc.M504828200. [DOI] [PubMed] [Google Scholar]
- 29.Miyazawa K, Inoue K. Complement activation induced by human C-reactive protein in mildly acidic conditions. J. Immunol. 1990;145:650–654. [PubMed] [Google Scholar]
- 30.Liberti PA, Paul SM. Gross conformation of C1q: A subcomponent of the first component of complement. Biochemistry. 1978;17:1952–1958. doi: 10.1021/bi00603a023. [DOI] [PubMed] [Google Scholar]
- 31.Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. Mapping of the C1q binding site on Rituxan, a chimeric antibody with a human IgG1 Fc. J. Immunol. 2000;164:4178–4184. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- 32.Kojouharova MS, Tsacheva I, Tchorbadjieva M, Reid KBM, Kishore U. Localization of the ligand binding sites on the human C1q globular region using recombinant globular head fragments and single-chain antibodies. Biochim. Biophys. Acta. 2003;1652:64–74. doi: 10.1016/j.bbapap.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Thommesen JE, Michaelsen TE, Loset GA, Sandlie I, Brekke OH. Lysine 322 in the human IgG3 CH2 domain is crucial for antibody dependent complement activation. Mol. Immunol. 2000;37:995–1004. doi: 10.1016/s0161-5890(01)00010-4. [DOI] [PubMed] [Google Scholar]
- 34.Michaelsen TE, Thommesen JE, Ihle O, Gregers TF, Sandin RH, Brekke OH, Sandlie I. A mutant human IgG molecule with only one C1q binding site can activate complement and induce lysis of target cells. Eur. J. Immunol. 2006;36:129–138. doi: 10.1002/eji.200535178. [DOI] [PubMed] [Google Scholar]
- 35.Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 36.Shrive AK, Cheetham GM, Holden D, Myles DA, Turnell WG, Volanakis JE, Pepys MB, Bloomer AC, Greenhough TJ. Three-dimensional structure of human C-reactive protein. Nat. Struct. Biol. 1996;3:346–354. doi: 10.1038/nsb0496-346. [DOI] [PubMed] [Google Scholar]
- 37.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure Fold. Des. 1999;7:169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal A, Shrive AK, Greenhough TJ, Volanakis JE. Topology and structure of the C1q–binding site on C-reactive protein. J. Immunol. 2001;166:3998–4004. doi: 10.4049/jimmunol.166.6.3998. [DOI] [PubMed] [Google Scholar]
- 39.Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, Gingras AR, Tzima S, Vivanco F, Egido J, Tijsma O, Hack EC, Daha MR, Roos A. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 2003;33:465–473. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- 40.Reichmann D, Rahat O, Albeck S, Meged R, Dym O, Schreiber G. The modular architecture of protein-protein binding interfaces. Proc. Natl. Acad. Sci. U.S.A. 2005;102:57–62. doi: 10.1073/pnas.0407280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James LC, Tawfik DS. The specificity of cross-reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Sci. 2003;12:2183–2193. doi: 10.1110/ps.03172703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]