Abstract
Purpose
To investigate processing speed as a latent dimension in children with dyslexia and children and adults with typical reading skills.
Method
Exploratory factor analysis (FA) was based on a sample of multigenerational families, each ascertained through a child with dyslexia. Eleven measures—6 of them timed—represented verbal and nonverbal processes, alphabet writing, and motor sequencing in the hand and oral motor system. FA was conducted in 4 cohorts (all children, a subset of children with low reading scores, a subset of children with typical reading scores, and adults with typical reading scores; total N = 829).
Results
Processing speed formed the first factor in all cohorts. Both measures of motor sequencing speed loaded on the speed factor with the other timed variables. Children with poor reading scores showed lower speed factor scores than did typical peers. The speed factor was negatively correlated with age in the adults.
Conclusions
The speed dimension was observed independently of participant cohort, gender, and reading ability. Results are consistent with a unified theory of processing speed as a quadratic function of age in typical development and with slowed processing in poor readers.
Keywords: dyslexia, generalized slowed processing, latent dimension, motor sequencing, life span
How rapidly a person can write the alphabet, produce within-category word labels such as animals, read color names when the font is in a different color, or respond to a visual signal by pressing a button may depend on many conditions, including task type, familiarity with the task, focus, attentiveness, cognitive ability, working memory, access to information stored in long-term memory, language ability, and intact motor pathways. Two opposing hypotheses about processing speeds are (a) that they are highly domain specific, such that processing speed during a language task is completely independent from processing speed during a cognitive or motor task, and (b) that they are mutually associated across a wide variety of task types, sharing a common biologic substrate. In recent years, there has been increasing support for the latter view.
Evidence for a global speed factor includes the observation that processing speed across a variety of task types increases during childhood and adolescence and then declines again, possibly as a function of white matter volume and integrity across the life span. Kail and Salthouse (1994) showed this age effect with respect to cognitive tasks, using the terms information processing speed or processing speed to describe the speed of performance during a variety of different cognitive tasks. They reviewed an analysis of normative data from the visual match task in the Woodcock–Johnson Test of Cognitive Ability (Woodcock & Johnson, 1990), for which data from more than 6,000 individuals ages 6–80 years were available, and found that processing speed for this task peaked at approximately 18 years of age. The youngest children performed approximately 5 SDs below this young adult mean, and by age 75 years, performance had dropped again to 2 SDs below the young adult mean. For processing speed in motor tasks, Lin, Brown, and Walsh (1996) showed a similar increase during childhood and adolescence, in that speeds in alternating limb movements rapidly increased during childhood and were less variable than those found in adult participants, suggesting a rate-limiting envelope for all motor tasks during development. Processing speeds in senescent adulthood were not collected for this study. Bartzokis et al. (2010) evaluated maximal finger-tapping speeds in adults ages 23–80 years. Tapping speeds reached a plateau at around age 39 years and declined as a function of participant age. This quadratic trajectory was highly and significantly correlated with an increase and subsequent decline in frontal lobe myelin integrity.
A second body of evidence in support of a global processing speed factor is found in the literature of various disorders, where limits on speeded performance have been observed across a wide variety of tasks. These disorders include, but are not limited to, multiple sclerosis (MS; Arnett, Smith, Barwick, Benedict, & Ahlstrom, 2008; Bodling, Denney & Lynch, 2008; Macniven et al., 2008), language impairment (Johnston & Weismer, 1983; Lowe & Campbell, 1965; Miller, Kail, Leonard, & Tomblin, 2001; Leonard et al., 2007; Miller et al., 2006; Owen & McKinlay, 1997; Powell & Bishop, 1992; Tallal & Piercy, 1973a, 1973b, 1974, 1975; Tallal, Stark, Kallman, & Mellits, 1981; Tallal, Stark, & Mellits, 1985; Wright, Bowen, & Zecker, 2000), and dyslexia.
According to the International Dyslexia Association (Lyon, Shaywitz, & Shaywitz, 2003), dyslexia is a specific disability that interferes with the acquisition of written language at the word level, characterized by deficits in accurate and/or fluent word recognition, decoding, and spelling. These difficulties are not predicted by other cognitive skills or quality of reading instruction and are thought to be neurobiological in origin. The emerging consensus is that dyslexia involves three types of deficits: orthographic coding, phonological coding, and rapid automatic naming. A central impairment in dyslexia is difficulty with decoding written words by translating letter sequences into sound sequences (Berninger, Abbott, Thomson, & Raskind, 2001). There is new evidence to suggest that deficits in working memory may underlie problems with word decoding, spelling, and reading fluency in individuals with dyslexia (Berninger, Raskind, Richards, Abbott, & Stock, 2008). There is some debate regarding underlying processes. After being discredited over 25 years ago, a visual deficit has been repostulated in the form of the magnocellular hypothesis, which holds that dyslexia results from slowed processing of visually presented material (Stein & Walsh, 1997). The auditory hypothesis suggests that these difficulties are related to limited sensory processing of rapid changes in auditory material (Tallal, Miller, Jenkins, &Merzenich, 1997). The magnocellular, auditory, and rapid naming hypotheses of dyslexia all incorporate elements of limited processing speed.
Reduced processing speeds have been observed in children with dyslexia, with deficits similar to those seen in language impairment. Catts, Gillispie, Leonard, Kail, and Miller (2002) showed that 279 third graders with dyslexia were slower than controls in response times during motor, visual, lexical, grammatical, and phonologic measures and, additionally, in rapid naming. Rey, DeMartino, Espesser, and Habib (2002) showed that children with dyslexia were less accurate than controls in judging the order of phonemes in consonant clusters, and their accuracy improved with slowed auditory presentation of the stimuli. In a longitudinal study of 27 children, most of whom were at high familial risk for dyslexia, those who were diagnosed with dyslexia in grade school had substantially slower speaking rates at ages 2 and 3 years, compared with those who did not develop dyslexia (Smith, Smith, Locke, & Bennett, 2008).
At least three studies have investigated processing speed in dyslexia samples using exploratory factor analysis (FA) as a tool to cluster relevant variables and to investigate latent dimensions. In a large German study of dyslexia phenotypes (Schulte-Körne et al, 2006), 287 sibling pairs with at least one sibling affected with dyslexia participated. Proband children had been referred to the study because of difficulties with reading and spelling, and the diagnosis of dyslexia was based on spelling scores at least 1 SD below that predicted from the IQ score. Absolute cutoff spelling scores were not listed. An exploratory FA with orthogonal rotation was conducted with 11 input variables—five of which were timed—representing measures of), word identification (timed), phonological decoding (timed), orthographic processing (untimed), phoneme awareness (untimed), rapid naming (timed) of letters, rapid naming of numbers, and the average score of rapid naming of colors and objects, digit span (untimed), as well as two measures of mathematical ability (timed). Results showed that the first factor was characterized by variables related to reading ability, whereas all timed measures except the two measures of mathematical ability loaded heavily on the second factor; a third factor represented mathematical ability.
In an American study designed to evaluate whether reduced cognitive processing speed is a shared endophenotype in dyslexia and attention-deficit/hyperactivity disorder (ADHD), Shanahan et al. (2006) measured processing speeds in 105 participants with ADHD, 95 participants with dyslexia, 51 participants with ADHD and dyslexia, and 144 controls, all ages 8–18 years. Dyslexia criteria included a positive school history of difficulty with reading and a composite score—constructed from measures of word recognition, reading comprehension, and spelling—of −1.75 SD below the mean of a control sample. Tasks incorporated various aspects of processing speed and required either a verbal output (e.g., naming items) or a motor response (e.g., pressing a key on a keyboard). Specifically, the 12 FA input variables—all derived from timed tasks—represented two color word tasks (reading color words printed in black ink, naming colors in a series of solid color patches), four types of rapid automatic naming (colors, numbers, letters, pictures), sequentially tracing numbers (Condition A) and numbers and letters (Condition B)scattered across a sheet of paper, visual matching tasks based on letter strings that were phonetically similar/dissimilar and pronounceable nonword strings, a shape-coding task, and a picture-matching task. Untimed measures were not included in the model. Principal axis components analysis with oblimin rotation resulted in two factors. The first factor was characterized by speeded processing with respect to verbal output, and the second factor was characterized by speeded processing with motor output.
Vaessen, Gerretsen, and Blomert (2009) investigated the double-deficit hypothesis, which posits two separate and independent core deficits in dyslexia: slow naming speed and impaired phonological awareness. Participants were 162 primary school children with percentile rankings of 10 and/or lower in a standardized Dutch word reading test and/or a standardized Dutch spelling test. Exploratory FA, using an oblique rotation algorithm, was based on measures of phoneme deletion accuracy, phoneme deletion speed, rapid automatic naming of letters and digits, object naming speed, visual coding, and digit span. Three factors were extracted and rotated with an oblimin algorithm. The first factor was characterized by high loadings from rapid automatic naming of objects, rapid automatic naming of letters and digits, and visual coding, interpreted by the authors as nonalphabetic processing speed. The second factor showed high loadings from phoneme deletion accuracy and digit span, interpreted by the authors as a phonological accuracy factor, and the third factor showed high loadings from phoneme deletion speed and, additionally, rapid automatic naming of letters and digits, interpreted by the authors as a phonological decoding speed factor.
Suspected Biological Factors Associated With Processing Speed
White matter integrity appears to play an important role in processing speeds, as exemplified in typical populations (Bartzokis et al., 2010; Posthuma et al., 2003) as well as in populations with disorders such as multiple sclerosis (Benedict, Carone, & Bakshi, 2004; Benedict et al., 2007; Christodoulou et al., 2003), language impairment (Jaencke, Siegenthaler, Preis, & Steinmetz, 2007), and dyslexia (Deutsch et al., 2005; Klingberg et al., 2000; Richards et al., 2008). Given that white matter supports axonal speeds of action-potential propagation and that neuronal synchronization, as measured in high-frequency brain-wave coherence, supports memory and language function (“binding” of information facilitated by synchronous firing of divergent neuron populations to create associations between sources of sensory input; Freeman & Rogers, 2002; Weiss & Mueller 2003), it is not surprising that research has shown neural transmission speeds and synchrony to be impaired in language impairment (Marler & Champlin, 2005) and dyslexia (Gaab, Gabrieli, Deutsch, Tallal, & Temple, 2007; Kraus et al., 1996; Nagarajan et al., 1999, Schulte-Körne, Deimel, Bartling, & Remschmidt, 2004). Together, these findings are consistent with a unified theory of global processing speeds as a function of brain changes across the life span (Era, 1988; Hoyer, Stawski, Wasylyshyn & Verhaeghen, 2004; Salthouse, 2000; Schaie, Willis & Caskie, 2004), which is potentially related to breakdown of myelin integrity (Bartzokis et al., 2004, 2006; Braak & Braak, 1996; Marner, Nyengaard, Tang, & Pakkenberg, 2003; Peters et al., 1996; Peters, Sethares, & Killiany, 2001; Peters & Sethares, 2004; Sloane, Hinman, Lubonia, Hollander, & Abraham, 2003).
Measures of Processing Speed
Although there is no universally accepted definition of processing speed, one broad definition (e.g., Shanahan et al., 2006) is that it underlies cognitive efficiency when interpreting and acting upon external stimuli. Included in this definition is the integration of low-level perceptual speed, higher level cognitive speed, and output speed. Processing speed can be measured in a variety of ways, including response time, time-by-count (task completion time such as the time needed to say “pataka” 10 times, and count-by-time (number of items completed in a given amount of time, such as number of words read in 45 s). In addition, measures of processing accuracy as a function of stimulus speed capture the limits of processing speed (rapid temporal processing; Tallal & Piercy, 1973a). For instance, the accuracy of detection, discrimination, and sequencing of acoustic stimuli may decrease as the durations of the stimuli or the interstimulus intervals are shortened.
Processing Speeds in a Dyslexia Family Study
The purpose of this study was to evaluate the role of processing speed across a variety of task types in a family sample with dyslexia. The data for this study were originally collected at the University of Washington Learning Disabilities Center (UW LDC) to study the genetics of dyslexia. This extensive and multivariate dataset has been characterized in a number of publications, including studies evaluating familial aggregation (e.g, Raskind, Hsu, Berninger, Thomson, & Wijsman, 2000), segregation (e.g., Wijsman et al., 2000; Chapman, Raskind, Thomson, Berninger & Wijsman, 2003), and linkage (e.g., Chapman et al., 2004; Igo et al., 2006; Raskind et al., 2005) of various traits. Regarding the interactions among various phenotypic traits, several studies have addressed theory-driven models of dyslexia and/or writing disability. Working from conceptual platforms based on systems theory and connectionist theory, these studies have evaluated the role of language processes and working memory in the expression of reading and writing abilities. The statistical tools selected to test the theory-derived hypotheses included confirmatory FA and structural equation modeling (e.g., Berninger, Abbott, et al., 2001; Berninger, Nielsen, Abbott, Wijsman, & Raskind, 2008; Thomson et al., 2005).
We now report evidence of latent dimensions based on exploratory factor analytic models of the phenotypic data in the dyslexia family dataset. Research questions were designed to replicate and extend the results in Schulte-Körne et al.’s (2006) study of timed and untimed tasks in a German-speaking sample of children with and without dyslexia, Shanahan et al.’s (2006) study of timed tasks in an English-speaking sample with dyslexia and/or ADHD and controls, and Vaessen et al.’s (2009) study of timed and untimed tasks in a Dutch-speaking sample of children with dyslexia. Specifically, we asked (a) whether our sample showed evidence of a global processing speed factor in a sample that included children with a wide range of reading abilities; (b) whether a global processing speed factor could be observed in two subsets of these children with current evidence of low and of typical reading ability separately; (c) whether a global processing speed factor could be observed in adults with current evidence of typical reading ability; and (d) how age was associated with global processing speed.
Method
Participants
Selection criteria for the UW LDC study
The data for the UW LDC study of dyslexia genetics were obtained with the approval of the University of Washington’s Human Subjects Institutional Review Board. Procedures for recruitment and data collection have been described in detail elsewhere (Berninger, Abbott, et al., 2001; Berninger et al., 2006; Raskind et al., 2000). Participating families were recruited through a child in grades 1–9 who had lower-than-average reading and/or spelling ability as documented with at least one score below the population mean in a variety of measures assessing word reading speed and accuracy, nonword decoding speed and accuracy, reading speed and accuracy on the paragraph level, and spelling (Raskind et al., 2000). On average, the probands’ scores were more than 0.67 SD below the population, and they met inclusionary criteria on 6.0 of 10.0 measures of reading and spelling and on 4.1 of 6.0 measures of writing (Berninger et al., 2006). Hence, as a group, the UW LDC probands had substantially impaired written language abilities across a variety of measures.
To participate in the study, the probands were required to have a prorated verbal IQ (VIQ) of at least 90 on the Wechsler Intelligence Scale for Children—Third Edition (Wechsler, 1991) to screen out severe language comprehension deficits and comorbid neurologic or developmental disorders that are more prevalent below this cutoff (Berninger et al., 2006). As recently reviewed (Lewis et al., 2006; Pennington & Bishop, 2009; Peterson, McGrath, Smith & Pennington, 2007), many children who are diagnosed with language impairment in early childhood show difficulty learning to read and spell once they enter school. In these cases, however, not all observed reading problems fall under the dyslexia definition provided by the International Dyslexia Association (Lyon et al., 2003) that is restricted to impairments in word-level reading and spelling skills.
Another requirement for inclusion as proband in the UW LDC study was a discrepancy of at least 1 SD between reading performance and VIQ. This VIQ–reading discrepancy criterion is generally not appropriate for a clinical diagnosis of dyslexia (Berninger, Stage, Smith, & Hildebrand, 2001) but was used for the purposes of the UW LDC genetics study because several studies show that despite their phenotypic similarities, IQ-discrepant reading deficits are more likely to provide significant heritability estimates compared with reading deficits without such a discrepancy (Knopik et al., 2002; Olson, Datta, Gayán & DeFries, 1999; Olson, Rack, Conners, DeFries, and Fulker, 1991; Wadsworth, Olson, Pennington, & DeFries, 2000). In these studies, full-scale IQ scores were used to calculate the discrepancy criterion, where minimum verbal and performance scores varied among the studies. The UW LDC probands’ reading scores ranged, on average, from 1.5 SD to 1.8 SD below their VIQ (Berninger et al., 2006). Although the VIQ discrepancy criterion, in theory, would allow inclusion of probands with average reading ability in the presence of above-average VIQ scores, the UW LDC probands’ mean word reading and decoding skills on nationally normed measures were substantially impaired in multiple measures of written language, as mentioned above.
In the UW LDC study, children with neurological or psychiatric disorders or other medical conditions—which could have impaired their reading or writing for reasons other than dyslexia or dysgraphia—were excluded, except for ADHD. Both biological parents of each proband participated and, where available, additional family members also participated. A wide variety of behavioral data were collected, including measures of verbal and nonverbal reasoning, reading, spelling, handwriting, language processing, syntax, motor processes, and executive functioning.
Additional criteria for inclusion in factor analytic modeling
For the purposes of this analysis, behavioral data from 1,861 individuals representing 289 families in the UW LDC dataset were queried. To evaluate the role of processing speed in a sample that included children with and without dyslexia, as reported in Schulte-Körne et al. (2006) and Shanahan et al. (2006), FA was conducted in a subset of all individuals under age 18 years in the sample for whom the selected variables were available. This cohort (N = 388; 212 males and 176 females) included 155 children who met inclusionary criteria as probands in the UW LDC study as well as 233 other children who had low, average, or high reading scores.
To specifically observe the role of processing speed in children with current evidence of poor reading skills, as reported in Vaessen et al. (2009), a subset of the children under age 18 was selected on the basis of (a) a low score; (b) 1 SD or more below the population mean from a pseudoword decoding accuracy task, hereafter referred to as Word Attack (WATT; Woodcock, 1987); and/or (c) an equally defined low score from a pseudoword decoding efficiency task, hereafter referred to as Nonword Reading Efficiency (NWRE; Torgesen, Wagner, & Rashotte, 1999; norms are based on a prepublication version), where the WATT or NWRE score also fell at least 1 SD below the prorated VIQ score. In the original UW LDC dataset, these two measures, WATT and NWRE, were strongly and significantly correlated with each other (r = .77, p < .0001) and with many other measures of written language ability— for instance, spelling from the Wide Range Achievement Test—3—Spelling subtest (WRAT–3–SP; Wilkinson, 1993), which was correlated with WATT at r = .70 (p < .0001) and with NWRE at r = .71 (p < .0001). These strong and positive correlations are consistent with the fact that the UW LDC probands as a group had low reading scores on multiple measures of written language ability. In the present study, the cohort of children with stringent criteria of poor reading scores with −1 SD discrepancy from both the VIQ and the population mean (n = 105) consisted of 62 males and 43 females and included 71 of the original probands. The mean WATT score in this cohort was 78.0 (SD=9.4; population mean = 100,SD=15), and the mean NWRE was −1.1 (SD = 0.8; population mean = 0, SD = 1). Although this cohort was not defined on spelling criteria, mean spelling scores from the WRAT3–SP (M = 82.3, SD = 9.2) were also substantially below the population mean (M = 100, SD = 15).
To observe the role of processing speed in children with current evidence of average or high reading scores, a subset of the children under age 18 years was defined on the basis of a WATT and NWRE score above −1SD that did not fall more than 1 SD below the VIQ. All probands from the original UW LDC study were excluded. This cohort of children (n = 197) consisted of 98 males and 99 females. The cohort of all children, hence, contained 86 children who were not included in either of the two subcohorts because of their intermediate reading scores.
To observe the role of processing speeds in adults with current evidence of average or high reading scores, the same criteria regarding WATT and NWRE as those in the cohort of children with average or high reading scores were applied. Additionally, a score above −1 SD in a measure of spelling, WRAT3–SP (Wilkinson, 1993), not falling more than 1 SD below the VIQ, was required because poor spelling ability frequently persists in adults with a childhood history of dyslexia (Berninger, Nielsen, et al., 2008). This cohort of adults with typical reading skills (n = 441) consisted of 193 males and 248 females.
Tasks
Measures were selected from each of the following domains (see Table 1): (a) short-term and working memory: digit span (DIGI); (b) verbal reasoning: vocabulary knowledge (VOCA); (c) language processing: nonword memory (NWM), rapid automatic naming of colors (RAN-C), and formulating sentences (FSENT); (d) handwriting: alphabet writing accuracy (ALPH-ACC) and speed (ALPH-TIME); (e) executive functioning: rapid automatic naming requiring switching between the categories of colors, letters, and numbers (RAS-CLN), and inhibition/switching time during a task requiring rapidly switching between naming a word’s ink color and its label (IST); and (f) motor sequencing: finger succession speed in the dominant hand (FS-D) and rapid alternating place of articulation (RAPA). Six of these measures—RAN-C, ALPH-TIME, RAS-CLN, IST, FS-D, and RAPA—were collected under timed conditions and, hence, incorporated an element of rapid performance because high scores required not only accurate but also rapid performance. Whereas subgroups of poor and typical readers were defined on the basis of reading ability, measures of reading were not used as inputs into FA. In the original UW LDC dataset, the selected measures were only weakly or moderately correlated with WATT and NWRE, whereas WATT and NWRE were strongly and positively correlated with each other, as mentioned earlier (r = .77). For WATT, correlation coefficients ranged from .14 (RAPA) to .46 (RAS-CLN), with an average correlation coefficient of .32, and for NWRE, correlation coefficients ranged from .09 (ALPH-ACC) to .58 (RAS-CLN), with an average correlation coefficient of .34. It follows that whereas WATT and NWRE were efficient measures to separate poor readers from average readers, the FA input variables were allowed to vary widely within and across the four cohorts.
Table 1.
Input measures for factor analysis.
| Construct | Measure | Test | Norming sample | Timed? |
|---|---|---|---|---|
| Short-Term and Working Memory | Digit Span (DIGI): Short-term and working memory for digits (e.g., repeat numbers in reverse order) | WISC–III (Wechsler, 1991) WAIS–R (Wechsler, 1981) |
Ages 6–16 Ages 17–97 |
No No |
| Verbal Reasoning | Vocabulary (VOCA): Comprehension and expression of vocabulary terms | WISC–III (Wechsler, 1991) WAIS-R (Wechsler, 1981) |
Ages 6–16 Ages 17–97 |
No No |
| Language Processes | Nonword Memory (NWM): Ability to hold pseudowords in short-term working memory and repeat them correctly | Comprehensive Test of Phonological Processing (CTOPP; Wagner & Torgesen, 1999) | Ages 5–24 | No |
| Rapid Automatic Naming of Colors (RAN–C): Rapid color naming | Wolf Rapid Automatized Naming/ Switching (RAN/RAS; Wolf, 1986; Wolf & Denckla, 2004) | Ages 5–18 | Yes | |
| Formulating Sentences (FSENT): Generate sentences given a picture and target words or phrases | Clinical Evaluation of Language Fundamentals—Third Edition (CELF–3; Semel, Wiig, & Secord, 1995) | Ages 5–21 | No | |
| Handwriting | Total Alphabet Writing Accuracy (ALPH–ACC): Legibility and accuracy of alphabetic order from long-term memory | University of Washington (UW) Alphabet Task (Berninger & Rutberg, 1992) | Elementary school grades 1–6 | No |
| Total Alphabet Writing Time (ALPH–TIME): Speed of letter retrieval in alphabetic order from long-term memory | UW Alphabet Task (Berninger & Rutberg, 1992) | Elementary school grades 1–6 | Yes | |
| Executive Function | Rapid Automatic Switching of Colors, Letters, and Numbers (RAS–CLN) | Wolf Rapid Automatized Naming/ Switching (RAN/RAS; Wolf, 1986; Wolf & Denckla, 2004) | Ages 5–18 (prepublication version) | Yes |
| Inhibition/Switching Time (IST): Rapidly switching between naming a word’s ink color and its label | Delis–Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001) | Ages 8–89 | Yes | |
| Motor Processes | Finger Succession—Dominant Hand (FS–D): Imitative, sequential thumb-to-finger touches in the dominant hand | Process Assessment of the Learner (PAL; Berninger, 2001) | Elementary school grades K–6 | Yes |
| Rapid Alternating Place of Articulation (RAPA): Rapidly producing trisyllables with varied consonants (e.g., “pataka”) | pataka (Fletcher, 1978) | Ages 6–13 | Yes |
All variables were aligned with equal polarity, where a high score represented good performance. For instance, variables measured as time-by-count such as the oral motor task of saying “pataka” 10 times, capturing good performance in low z scores, were inverted. All measures were adjusted for participant age using the norms provided with the assessment instruments. Table 1 lists the measures along with their norming information. Where norms were based on child samples, standard scores for adults were computed using the norms for the highest available age or grade level (Berninger, Abbott, et al., 2001), which is commonly done in genetics studies of multigenerational family samples (e.g., Rice, Smith, & Gayán, 2009). For each of the four cohorts (all children, children with poor reading skills, children with good reading skills, and adults with good reading skills), Table 2 lists descriptive statistics of these measures as well as VIQ, WATT, and NWRE, which were used to differentiate between low and typical reading ability in children and adults,andWRAT3–SP, which was used for the same purpose in the adults.
Table 2.
Descriptive statistics for four groups: all children, children with poor reading skills, children with typical reading skills, and adults with typical reading skills.
| All children |
Children with poor reading scores |
Children with typical reading scores |
Adults with typical reading scores |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Var. | Pop. M | Pop. SD | Obs. | M | SD | Obs. | M | SD | Obs. | M | SD | Obs. | M | SD |
| Age | N/A | N/A | 390 | 145 | 32 | 105 | 140 | 28 | 198 | 150 | 37 | 444 | 542 | 139 |
| WATT | 100 | 15 | 390 | 92.4 | 13.1 | 105 | 78.0 | 9.4 | 198 | 99.4 | 10.4 | 444 | 102.8 | 9.0 |
| NWRE | 0 | 1 | 390 | 0.0 | 1.2 | 105 | −1.1 | 0.8 | 198 | 0.74 | 0.9 | 442 | 1.2 | 0.6 |
| WRAT3−SP | 100 | 15 | 390 | 93.9 | 14.1 | 105 | 82.3 | 9.2 | 198 | 101.8 | 12.9 | 444 | 101.6 | 9.1 |
| VIQ | 100 | 15 | 390 | 109.9 | 12.5 | 105 | 106.7 | 10.0 | 198 | 109.7 | 13.0 | 444 | 110.2 | 11.9 |
| DIGI | 10 | 3 | 390 | 9.4 | 2.7 | 105 | 7.9 | 2.4 | 198 | 10.1 | 2.5 | 444 | 10.6 | 2.4 |
| VOCA | 10 | 3 | 390 | 12.0 | 2.7 | 105 | 11.2 | 2.3 | 198 | 12.0 | 2.8 | 444 | 12.6 | 2.4 |
| NWM | 0 | 1 | 390 | 0.4 | 1.0 | 105 | −0.1 | 1.2 | 198 | 0.7 | 0.9 | 444 | 0.9 | 0.8 |
| RAN-C | 0 | 1 | 390 | −0.7 | 1.4 | 105 | −1.2 | 1.4 | 198 | −0.4 | 1.3 | 444 | 0.1 | 1.1 |
| FSENT | 10 | 3 | 390 | 10.4 | 2.7 | 105 | 9.6 | 2.8 | 198 | 10.7 | 2.7 | 444 | 11.2 | 2.4 |
| ALPH–ACC | 0 | 1 | 390 | −0.4 | 1.4 | 105 | −0.5 | 1.3 | 198 | −0.4 | 1.5 | 444 | −0.4 | 1.1 |
| ALPH–TIME | 0 | 1 | 390 | 0.3 | 1.6 | 105 | 0.8 | 2.2 | 198 | −0.1 | 1.1 | 444 | −0.1 | 0.8 |
| RAS–CLN | 0 | 1 | 390 | −2.0 | 2.2 | 105 | −3.2 | 2.7 | 198 | −1.2 | 1.7 | 444 | −0.6 | 1.2 |
| IST | 10 | 3 | 390 | 9.4 | 2.7 | 105 | 8.7 | 3.0 | 198 | 10.1 | 2.3 | 444 | 10.9 | 2.2 |
| FS–D | 0 | 1 | 388 | 0.3 | 1.2 | 105 | 0.0 | 1.3 | 197 | 0.5 | 1.2 | 442 | 0.7 | 1.0 |
| RAPA | 0 | 1 | 390 | −0.3 | 1.7 | 105 | −0.6 | 2.0 | 198 | −0.2 | 1.6 | 442 | −0.2 | 1.3 |
Note. Due to selection criteria, the two child subcohorts combined contain fewer participants than the cohort of all children. Pop. = population; Obs. = observation; WATT = Word Attack; NWRE = Nonword Reading Efficiency; WRAT3–SP = Wide Range Achievement Test—3—Spelling subtest; VIQ = Verbal IQ.
Statistical Analyses
Underlying dimensions in the multivariate dyslexia dataset were evaluated with exploratory FA. Factor extraction was specified for eigenvalues > 1.00. Following principal-components extraction, factors were rotated with the oblimin oblique algorithm. This algorithm was selected over orthogonal rotation because the orthogonal algorithm is based on the assumption that the factors are mutually completely independent. In the present dataset, however, this assumption may not be appropriate.
FA was completed in all four cohorts (all children, children with poor reading ability, children with average reading ability, and adults with average reading ability). Results were corroborated in the male and female participants within each cohort separately. This step was problematic only for the cohort of children with poor reading skills. We sought to maintain a minimal subject-to-item ratio of 5 (Bartholomew, Steele, Moustaki, & Galbraith, 2002; Costello & Osborne, 2005), and running 11 variables in 43 female subjects undercut this standard.
To compare children with low reading scores and their peers with typical reading scores with respect to their speed factor scores from the FA procedure in the cohort of all children, t tests with adjustments for unequal variances due to different sample sizes were used.
To evaluate the role of age in general speed, pairwise correlation coefficients were calculated between age in months and the factor scores related to speed. Statistical significance was determined based on Bonferroni corrections, where the traditional alpha of .05 was divided by the number of tests performed in the four cohorts, resulting in an adjusted alpha of .0125.
Results
In all four cohorts, processing speed characterized the first factor (F1). In each case, FA procedures in male and female subcohorts separately (results not shown) resulted in the same first factor and in loading structures that differed only slightly among the male and female subcohorts and the whole cohort. In general, the two motor sequencing variables had slightly lower F1 loadings, compared with the timed measures of language processes, executive functioning, and alphabet writing.
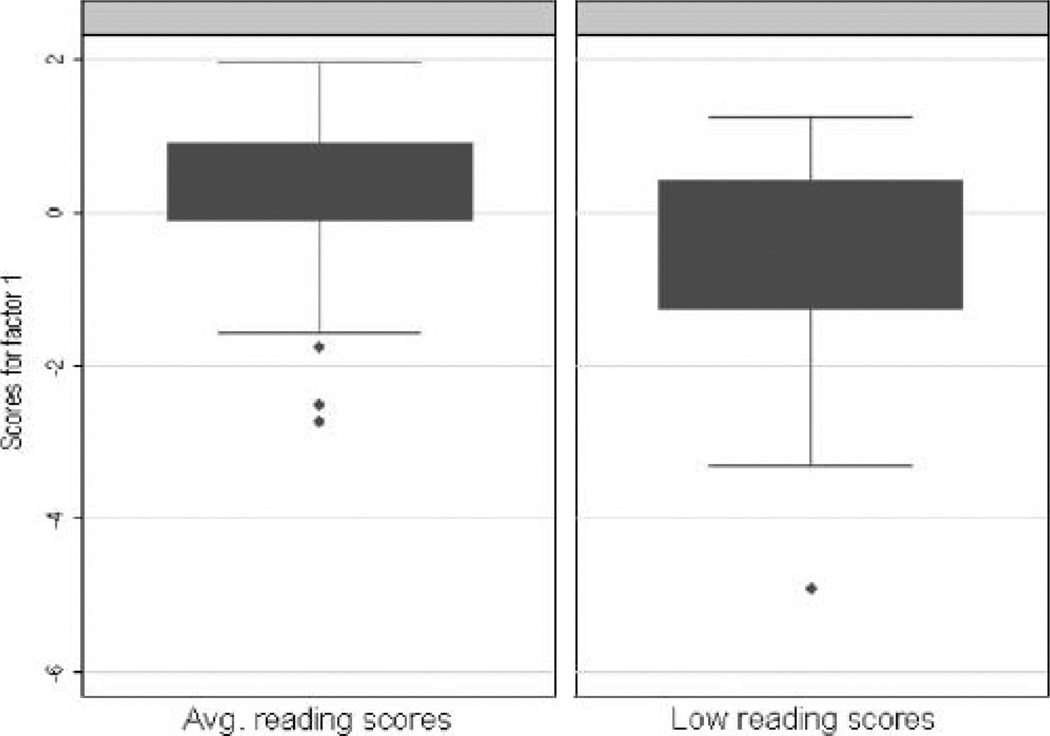
In the cohort of all children (n = 388; see Table 3), F1 had a rotated eigenvalue of 2.81 and was characterized by all timed measures as follows: RAS-CLN (loading = .83), RAN-C (loading = .81), IST (loading = .75), ALPH-TIME (loading = .53),FS-D(loading=.49),and RAPA(loading = .29). The second factor (F2) had a rotated eigenvalue of 2.21 and was characterized by VOCA (loading = .78), FSENT (loading = .72), NWM (loading = .63), and DIGI (loading = .55); this factor can be interpreted as an underlying dimension of language combined with short-term and working memory. The third factor (F3) had a rotated eigenvalue of 1.21 and was characterized by the two measures of alphabet writing, ALPH-ACC (loading = .83) and ALPH-TIME (loading = .27). This model accounted for 57% of the variance. Within this cohort, F1 scores for children with low reading ability (n = 105) were significantly lower than F1 scores for children with typical reading ability (n = 197), as calculated with a t test with unequal variances (t = 6.86, p < .0001; see Figure 1). Age was not significantly correlated with F1 factor scores (r = .05, p = .3143). Table 3 shows the FA results including uniqueness values. Figure 1 shows boxplots of F1 scores separately for children with evidence of low and typical reading ability.
Table 3.
Oblique oblimin factor loadings in the cohort of all children (n = 388; 212 males, 176 females).
| Variable | T | F1 | Var | T | F2 | Variable | T | F3 | Variable | Uniqueness |
|---|---|---|---|---|---|---|---|---|---|---|
| RAS–CLN | X | 0.83 | VOCA | 0.78 | ALPH–ACC | 0.83 | ALPH–ACC | 0.30 | ||
| RAN–C | X | 0.81 | FSENT | 0.72 | ALPH–TIME | X | 0.27 | ALPH–TIME | 0.56 | |
| IST | X | 0.75 | NWM | 0.63 | IST | X | 0.19 | DIGI | 0.61 | |
| ALPH–TIME | X | 0.53 | DIGI | 0.55 | FSENT | 0.15 | FS–D | 0.51 | ||
| FS–D | X | 0.49 | RAPA | X | 0.21 | VOCA | 0.03 | FSENT | 0.47 | |
| RAPA | X | 0.29 | ALPH–TIME | X | 0.18 | DIGI | 0.01 | IST | 0.42 | |
| DIGI | 0.20 | FS–D | X | 0.12 | RAS–CLN | X | 0.00 | NWM | 0.53 | |
| ALPH–ACC | 0.10 | ALPH–ACC | 0.06 | RAN–C | X | −0.09 | RAN–C | 0.36 | ||
| NWM | 0.03 | RAS–CLN | X | 0.01 | NWM | −0.24 | RAPA | 0.73 | ||
| FSENT | 0.00 | RAN–C | X | −0.02 | RAPA | X | −0.33 | RAS–CLN | 0.30 | |
| VOCA | −0.11 | IST | X | −0.08 | FS–D | X | −0.46 | VOCA | 0.42 |
Note. Loadings are sorted by magnitude in each factor. Uniqueness is shown in alphabetical order. T = timed; F = factor.
Figure 1.
Boxplots of the F1 (speed) scores run in all children, shown separately for children with average and low reading scores (t with unequal variances = 6.86, p < .0001).
Within the cohort of children with evidence of poor reading ability (n = 105) separately (see Table 4), F1 had a rotated eigenvalue of 2.65 and was characterized by IST (loading = .83), RAS-CLN (loading = .82), RAN-C (loading = .70), FS-D (loading = .48), RAPA (loading = .43), and ALPH-TIME (loading = .33). Unlike the FA results in the cohort of all children, language and memory characterized two separate factors. F2 had a rotated eigenvalue of 1.95 and was characterized by FSENT (loading = .82) and VOCA (loading = .76) and, additionally, ALPH-TIME (loading = .49) and RAPA (loading = .45). F3 had a rotated eigenvalue of 1.46 and was characterized by NWM (loading = .77) and DIGI (loading = .63) and, additionally, FS-D (loading = .40) and VOCA (loading = .31). A fourth factor (F4) with a rotated eigenvalue of 1.27 was characterized by ALPH-ACC (loading = .80) and, additionally, DIGI (loading = .37). This model accounted for 67% of the variance. In this cohort, the correlation between age and F1 scores did not reach statistical significance (r = −.17, p = .0918). Table 4 summarizes the FA results in this cohort.
Table 4.
Oblique oblimin factor loadings from in the cohort of children with poor reading scores (n = 105; 62 males, 43 females).
| Variable | T | F1 | Variable | T | F2 | Variable | T | F3 | Variable | T | F4 | Variable | Uniqueness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IST | X | 0.83 | FSENT | 0.82 | NWM | 0.77 | ALPH–ACC | 0.80 | ALPH–ACC | 0.31 | |||
| RAS–CLN | X | 0.82 | VOCA | 0.76 | DIGI | 0.63 | DIGI | 0.37 | ALPH–TIME | 0.43 | |||
| RAN–C | X | 0.70 | ALPH–TIME | X | 0.49 | FS–D | X | 0.40 | ALPH–TIME | X | 0.26 | DIGI | 0.38 |
| FS–D | X | 0.48 | RAPA | X | 0.45 | VOCA | 0.31 | RAN–C | X | 0.12 | FS–D | 0.46 | |
| RAPA | X | 0.43 | NWM | 0.13 | ALPH–ACC | 0.06 | IST | X | 0.06 | FSENT | 0.33 | ||
| ALPH–TIME | X | 0.33 | RAS–CLN | X | 0.11 | RAN–C | X | 0.04 | RAS–CLN | X | 0.06 | IST | 0.31 |
| DIGI | 0.21 | DIGI | 0.10 | IST | X | 0.00 | VOCA | 0.04 | NWM | 0.35 | |||
| ALPH–ACC | 0.15 | RAN–C | X | −0.02 | FSENT | −0.02 | FSENT | −0.06 | RAN–C | 0.47 | |||
| FSENT | 0.01 | ALPH–ACC | −0.03 | RAS–CLN | X | −0.04 | NWM | −0.07 | RAPA | 0.52 | |||
| NWM | −0.09 | FS–D | X | −0.10 | RAPA | X | −0.07 | RAPA | X | −0.32 | RAS–CLN | 0.28 | |
| VOCA | −0.16 | IST | X | −0.12 | ALPH–TIME | X | −0.33 | FS–D | X | −0.43 | VOCA | 0.28 |
Note. Loadings are sorted by magnitude in each factor. Uniqueness is shown in alphabetical order.
Within the cohort of children with evidence of typical reading ability (n = 197) separately (see Table 5), F1 had a rotated eigenvalue of 2.57 and was characterized by RAN-C (loading = .84), RAS-CLN (loading = .78), IST (loading = .65), ALPH-TIME (loading = .52), FS-D (loading = .50), and RAPA (loading = .21). Similar to the cohort of all children, F2, with a rotated eigenvalue of 2.20, was characterized by VOCA (loading = .76), FSENT (loading = .73), NWM (loading = .71), and DIGI (loading = .53), showing an underlying dimension of language processes combined with aspects of short-term and working memory. Also similar to the cohort of all children, F3, with a rotated eigenvalue of 1.41, was characterized by ALPH-ACC (loading = .79), IST (loading = .34), and ALPH-TIME (loading = .31). This model accounted for 56% of the variance. The correlation between age and F1 scores did not reach statistical significance (r = .13, p = .0690). Table 5 summarizes the FA results in this cohort.
Table 5.
Oblique oblimin factor loadings from FA1 in the cohort of children with typical reading scores (n = 197; 98 males, 99 females).
| Variable | T | F1 | Variable | T | F2 | Variable | T | F3 | Variable | Uniqueness |
|---|---|---|---|---|---|---|---|---|---|---|
| RAN–C | X | 0.84 | VOCA | 0.76 | ALPH–ACC | 0.79 | ALPH–ACC | 0.37 | ||
| RAS–CLN | X | 0.78 | FSENT | 0.73 | IST | X | 0.34 | ALPH–TIME | 0.59 | |
| IST | X | 0.65 | NWM | 0.71 | ALPH–TIME | X | 0.31 | DIGI | 0.64 | |
| ALPH–TIME | X | 0.52 | DIGI | 0.53 | FSENT | 0.16 | FS–D | 0.53 | ||
| FS–D | X | 0.50 | ALPH–TIME | X | 0.15 | VOCA | 0.00 | FSENT | 0.45 | |
| RAPA | X | 0.21 | FS–D | X | 0.15 | RAS–CLN | X | −0.04 | IST | 0.47 |
| DIGI | 0.13 | RAPA | X | 0.13 | RAN–C | X | −0.12 | NWM | 0.48 | |
| ALPH–ACC | 0.05 | IST | X | 0.05 | NWM | −0.13 | RAN–C | 0.29 | ||
| NWM | 0.01 | ALPH–ACC | 0.04 | DIGI | −0.14 | RAPA | 0.57 | |||
| FSENT | −0.01 | RAS–CLN | X | −0.01 | FS–D | X | −0.38 | RAS–CLN | 0.39 | |
| VOCA | −0.09 | RAN–C | X | −0.07 | RAPA | X | −0.58 | VOCA | 0.45 |
Note. Loadings are sorted by magnitude in each factor. Uniqueness is shown in alphabetical order.
In the adults with evidence of typical reading ability (n = 441; see Table 6), the factor sequence and the loading structures resembled those from the cohort of all children and, within that, the cohort of children with evidence of typical reading ability. F1 had a rotated eigenvalue of 2.84 and was characterized by RAN-C (loading = .80), RAS-CLN (loading = .74), FS-D (loading = .65), IST (loading = .58), ALPH-TIME (loading = .58), and RAPA (loading = .52). F2 had a rotated eigenvalue of 2.01 and was characterized by VOCA (loading = .79), DIGI (loading = .63), NWM (loading = .59), and FSENT (loading = .51), consistent with a latent dimension of language combined with aspects of short-term and working memory. F3, with a rotated eigenvalue of 1.21, was characterized by ALPH-ACC (loading = .84), FSENT (loading = .32), and ALPH-TIME(loading=.29). This model accounted for 55% of the variance. The correlation between age and F1 scores was negative and statistically significant (r = −.34, p < .0001). The correlation was examined separately in the three generational subcohorts of adults 59 years or older (n = 45, r = −.39, p = .0080); those between 33 and 58 years (n = 357, r = −.16, p = .0018); and those younger than 32 years (n = 39, r = −.18, p = .2818). In the adults with typical reading scores, the timed variables were correlated with age as follows: RAN-C: r = −.40, p < .0001; RAS-CLN: r = −.24, p < .0001; FS-D: r = −.47, p < .0001; IST: r = .05, p < .2959; ALPH-TIME: r = −.39, p < .0001; RAPA: r= −.16, p = .0004. Table 6 summarizes the FA results in this cohort.
Table 6.
Oblique oblimin factor loadings from FA1 in the cohort of adults with typical reading scores (n = 441; 193 males, 248 females).
| Variable | T | F1 | Variable | T | F2 | Variable | T | F3 | Variable | Uniqueness |
|---|---|---|---|---|---|---|---|---|---|---|
| RAN–C | X | 0.80 | VOCA | 0.79 | ALPH–ACC | 0.84 | ALPH–ACC | 0.29 | ||
| RAS–CLN | X | 0.74 | DIGI | 0.63 | FSENT | 0.32 | ALPH–TIME | 0.53 | ||
| FS–D | X | 0.65 | NWM | 0.59 | ALPH–TIME | X | 0.29 | DIGI | 0.56 | |
| IST | X | 0.58 | FSENT | 0.51 | NWM | 0.17 | FS–D | 0.56 | ||
| ALPH–TIME | X | 0.58 | IST | X | 0.14 | FS–D | X | 0.15 | FSENT | 0.60 |
| RAPA | X | 0.52 | RAS–CLN | X | 0.12 | RAN–C | X | 0.10 | IST | 0.57 |
| NWM | 0.16 | ALPH–TIME | X | 0.08 | VOCA | −0.04 | NWM | 0.53 | ||
| DIGI | 0.08 | ALPH–ACC | 0.02 | DIGI | −0.13 | RAN–C | 0.37 | |||
| FSENT | 0.05 | RAPA | X | 0.00 | RAS–CLN | X | −0.13 | RAPA | 0.60 | |
| ALPH–ACC | 0.03 | FS–D | X | −0.03 | IST | X | −0.23 | RAS–CLN | 0.38 | |
| VOCA | −0.10 | RAN–C | X | −0.11 | RAPA | X | −0.39 | VOCA | 0.41 |
Note. Loadings are sorted by magnitude in each factor. Uniqueness is shown in alphabetical order.
Discussion
The purpose of this study was to evaluate latent dimensions in the UW LDC dataset with a special focus on the role of processing speed. We asked whether this dataset corroborated findings in the recent literature regarding a dimension of processing speed in mixed samples of children with and without low reading skills, whether this dimension can be observed separately in children with and without low reading skills, whether it can be observed in adults with current evidence of typical reading skills as well, and how age was associated with performance speed.
The first research question regarding the role of processing speed in children with a variety of reading abilities was answered affirmatively with a general dimension of processing speed in this cohort that emerged as the first factor with high loadings from all timed variables. One set of variables, ALPH-ACC and ALPH-TIME, measured aspects of accuracy and speed of the same task. The speed factor dissociated these two variables, showing a greater loading for the timed variable than for the untimed one. The heaviest contributors to the speed factor were the two measures of rapid automatic naming, where one (RAS-CLN) additionally taxed the executive functioning system due to the category switches during the naming task. F2 was characterized by aspects of language processing and short term and working memory, and F3 was dominated by alphabet writing accuracy.
These findings can be compared with those reported by Schulte-Körne et al. (2006) as follows: Their first factor was characterized by measures of spelling and reading, which were excluded from our study. Their second factor represented speed in a variety of tasks including word reading, pseudoword reading, and rapid automatic naming of letters, numbers, and pictures/colors. Speed emerged as the first factor in our study and included similar measures of rapid automatic naming in addition to the other measures of executive functioning, alphabet writing speed, and motor sequencing. In their study, mathematical ability was represented by the third factor, whereas our FA procedures resulted in additional factors characterized by language/memory scores and alphabet writing ability. This difference is due to the nature of the input variables, as our model did not include any variables related to mathematical ability, and the Schulte-Körne et al. (2006) model did not include measures of verbal processing and handwriting. The American study (Shanahan et al., 2006) in children with dyslexia and/or ADHD and typical controls only included timed measures of cognitive processing, differentiated by motor output versus verbal output. Their first factor was dominated by measures of processing speed with verbal outputs, including four measures of rapid automatic naming and two measures of word and color naming, whereas their second factor was characterized by timed measures with motor output. This result is similar to ours in that the first factor in our study also showed highest loadings from the two measures of rapid automatic naming and the measure of executive functioning, all of which required verbal output, whereas the measure of alphabet writing speed and the two measures of motor sequencing, all of which require motor output, had loadings of lower magnitude on this factor.
A latent dimension of processing speed was found in the cohort of children with low reading scores and peers with typical reading scores in separate FA procedures, emerging as the first factor in both cases. The cohort of children with evidence of typical reading ability produced a second factor characterized by measures representing language processes as well as short-term and working memory, whereas the cohort of children with evidence of poor reading ability produced a second factor led by measures of language processes and a third factor led by measures incorporating short-term and working memory. In both cohorts, alphabet writing accuracy formed the smallest retained factor. Regarding the cohort with low reading scores, the FA results compare to those reported by Vaessen et al. (2009), who conducted FA in 162 Dutch children with dyslexia, as follows: Their study used only six input variables, none of which represented handwriting, but their model resembled ours in that it included two timed measures of rapid automatic naming and an untimed measure of digit span, whereas measures of reading were not included. Their first factor, interpreted as nonalphabetic processing speed, resembled our first factor in that it was characterized by high loadings from the measures of rapid automatic naming in addition to another cognitive measure of processing speed. Their second factor represented phonological accuracy, and their third represented phonological decoding speed based on input variables that were not used in the present study.
The finding that processing speed formed a prominent latent dimension in separate child cohorts with low and typical reading ability, respectively, is consistent with a general rate limit that is expressed across modalities regardless of reading ability. More generally, this coincidence of findings across three languages (German, Dutch, and English), different dyslexia criteria, different sample compositions, and different sets of input variables strengthens our hypothesis that processing speed underlies abilities across a wide variety of domains in children.
FA results in the cohort of all children showed that the poor readers within this cohort had lower speed factor scores than their peers with typical reading scores. These two subcohorts were defined using measures of reading, whereas the FA input variables did not include measures of reading, although it is acknowledged that some of these measures were weakly to moderately correlated with measures of reading. The difference in speed factor scores is consistent with a generalized slowed processing rate in children with low reading scores, compared with their typical peers, as described in the literature (e.g., Catts et al., 2002). It is possible that the deficits in white matter observed in children with dyslexia (Deutsch et al., 2005; Klingberg et al., 2000; Richards et al., 2008) contribute to this failure to develop an increase in processing and motor speeds with age, although we did not directly measure white matter volume or integrity. It is possible that limits in perceptual resolution of acoustic stimuli also explain aspects of dyslexia. As mentioned, children with dyslexia have difficulty with auditory perception at rapid speeds (Rey et al., 2002). A central substrate of reading is grapheme/phoneme association. It is theoretically possible that children with deficits in processing rapid auditory stimuli may have difficulty segmenting speech streams into component phonemes and developing the requisite phonemic awareness for phoneme/grapheme association.
A global dimension of processing speed was also found in adults with typical reading scores. As in all other cohorts, speed emerged as the first factor. As in the cohort of all children and that with only those children who showed typical reading ability, the second factor was characterized by aspects of language processing and short-term and working memory. As in all other cohorts, the smallest retained factor was an alphabet writing factor. The similarities with all child cohorts regarding the prominent presence of a latent speed dimension are consistent with the hypothesis that processing speed acts as a global rate limit throughout the life span.
Age showed a statistically nonsignificant, weakly positive correlation with the speed factor in the cohort of all children; a statistically nonsignificant, weakly positive correlation in the cohort of children with typical reading scores; a statistically nonsignificant, weakly negative correlation in the child cohort with low reading scores; and a statistically significant, moderately negative correlation in the adults with typical reading scores. This negative correlation was expressed most strongly in the grandparental generation. It is of note that several of the variables contributing to the speed factor were normed on samples of children and young adults (see Table 1). Specifically, the measures of rapid automatic naming were normed on samples up through age 18 years, the alphabet writing and finger motor sequencing task up through grade 6, and RAPA up through age 13 years. Hence, in young, typically developing children, an increase of the speed factor would not necessarily be expected because increases in raw performance speeds are already accounted for in the norming processes and the speed factor scores are linear combinations of standard scores. However, such an increase in standard scores would be possible for children beyond grade 6 or age 13 if their average raw speed scores continue to increase as a function of age beyond the highest normed age, and a decrease would be possible in adults if their average raw speed scores decrease as a function of age. For instance, a raw score of 30 s in the RAN-C task translates into a standard score of 100 (M = 100, SD = 15) for age 18 years, the highest age for which norming was provided by the test publisher. At age 12 years, however, a standard score of 100 is equivalent to a longer color-naming duration of 37 s because children have slower naming speeds than young adults in general, and the norms took this into account. If older adults, on average, have slower naming speeds than 18-year-olds as well, their raw scores, when standardized against the norms at age 18 years instead of norms determined at their own ages, would automatically receive lowered standard scores. Our results are consistent with this pattern of declining standard scores in adults, as evident in speed factor scores and their individual component traits, especially RAN-C, FS-D, and ALPH-TIME, that declined as a function of age in this cohort.
Regarding the timed measures of linguistic and executive functioning, the increasing speed factor trend in children with typical reading scores and the significant decrease in adults as a function of age is consistent with Kail and Salthouse’s (1994) finding of increasing and decreasing processing speed as a function of age in a cognitive task, with maximal speeds occurring around age 18 years. With respect to the motor sequencing speeds, the increasing speed factor trend in children with typical reading scores and the significant decrease in adults as a function of age is consistent with Lin et al.’s (1996) finding that motor speeds increase in childhood and adolescence and with Bartzokis et al.’s (2010) finding that motor speeds decline with increasing age. Together, these results are consistent with a quadratic trajectory of processing speeds in children and adults with typical reading scores as a function of age, and they point out the need to develop age-adjusted norms for timed measures of cognitive and motor tasks in adults of all ages, rather than relying on norms from adolescents or young adults.
In all cohorts, the two measures of motor sequencing speed—one in an oral and one in a hand task— patterned with other measures incorporating speed of performance, although generally with lower loadings. The finger succession task had higher loadings than the oral motor task and, hence, was correlated with global processing speed to a greater extent. Nonetheless, these findings are consistent with the hypothesis that motor sequencing tasks, whether in the motor speech system or in the hand motor system, carry inherent rate limits that are associated with rate limits in tasks with higher cognitive loads. In this context, it is of interest that the two measures of rapid automatic naming, RAN-C and RAS-CLN, generally were among the leading variables in the speed factors, regardless of cohort. As mentioned, RAS-CLN incorporates an additional element of executive functioning compared with RAN-C because the task involves category switching during a rapid naming task. The fact that these two variables patterned closely with each other on the speed factor in the various models suggests that the added element of executive functioning inherent in RAS-CLN is itself rate-limited and falls under a global dimension of processing speed. Hence, the results from this study are consistent with an extensive scope of processing speed, ranging from tasks with low cognitive loads such as motor sequencing to tasks that tax the executive functioning system. As mentioned, the observation that the three timed tasks requiring motor output (ALPH-TIME, FS-D, and RAPA) had lower factor loadings than those requiring a verbal output (RAN-C, RAS-CLN, and IST) is consistent with the findings by Shanahan et al. (2006), where the variables with verbal output formed the first factor and those with motor output formed the second factor with a lower eigenvalue and, hence, had less weight in accounting for the variability in the data.
Conclusion and Future Studies
This study confirms previous reports that children with low reading ability have slower global processing speed than do their peers with typical reading ability. It extends our understanding of processing speed by showing that processing speed underlies performance in separate cohorts of children with low reading scores, children with typical reading scores, and adults with typical reading scores. It contributes new insights into the breadth of tasks affected by processing speed, ranging from motor sequencing to tasks that involve higher cognitive and executive loads. The observation that in adults with typical reading scores, the latent processing speed dimension and several of its component traits decline as a function of age confirms previous reports of such a decline in single component traits. Overall, results are consistent with a unified theory of global processing speed, and they point to a biological origin of limits in processing speeds that, when perturbed by a neurodevelopmental disorder such as dyslexia or by advancing age in adults, has a slowing effect on performance speed in a variety of task types. Because evidence suggests that dyslexia is highly heritable, we hypothesize a genetic cause underlying this type of structural central nervous system abnormality. Future studies will evaluate processing speed as a possible endophenotype and address genetic substrates in terms of familial aggregation, patterns of inheritance, and involvement of specific genetic regions.
Acknowledgments
These studies were supported by National Institute of Child Health and Development Grants P50 HD33812 and R01 HD054562 (Wendy H. Raskind, P. I.) and by National Institute on Deafness and Other Communication Disorders Grant 05 T32 DC000033-18 (Beate Peter). We appreciate the expert help of many Department of Educational Psychology graduate students in administering the test battery. Hiep Nguyen and Ted Holzman provided computer support. Richard Wagner and Joseph Torgesen gave permission to use the prepublication measures of the Comprehensive Test of Phonological Processing (CTOPP). Ellen Wijsman, Elena Erosheva, and Hil Lyons provided helpful comments on statistical procedures. We thank the families for their willingness to devote the time necessary to participate in these studies.
References
- Arnett PA, Smith MM, Barwick FH, Benedict RH, Ahlstrom BP. Oralmotor slowing in multiple sclerosis: Relationship to neuropsychological tasks requiring an oral response. Journal of the International Neuropsychological Society. 2008;14:454–462. doi: 10.1017/S1355617708080508. [DOI] [PubMed] [Google Scholar]
- Bartholomew DJ, Steele F, Moustaki I, Galbraith JI. The analysis and interpretation of multivariate data for social scientists. Boca Raton, FL: Chapman & Hall/CRC; 2002. [Google Scholar]
- Bartzokis G, Lu PH, Geschwind DH, Edwards N, Mintz J, Cummings JL. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Archives of General Psychiatry. 2006;63:63–72. doi: 10.1001/archpsyc.63.1.63. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tinguis K, Mendez MF, Richard A, Peters DG, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiology of Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings J. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Bruce J, Dwyer MG, Weinstock-Guttman B, Tjoa C, Tavazzi E, Zivadinov R. Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Multiple Sclerosis. 2007;13:722–730. doi: 10.1177/1352458507075592. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Carone DA, Bakshi R. Correlating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosis. Journal of Neuroimaging. 2004;14(3) Suppl 1:36S–45S. doi: 10.1177/1051228404266267. [DOI] [PubMed] [Google Scholar]
- Berninger VW. Process assessment of the learner: Test battery for reading and writing. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Berninger VW, Abbott RD, Thomson J, Raskind WH. Language phenotype for reading and writing disability: A family approach. Scientific Studies of Reading. 2001;5:59–106. [Google Scholar]
- Berninger VW, Abbott RD, Thomson J, Wagner R, Swanson HL, Wijsman E, Raskind W. Modeling phonological core deficits within a working memory architecture in children and adults with developmental dyslexia. Scientific Studies of Reading. 2006;10:165–198. [Google Scholar]
- Berninger VW, Nielsen KH, Abbott RD, Wijsman E, Raskind WH. Writing problems in developmental dyslexia: Under-recognized and under-treated. Journal of School Psychology. 2008;46:1–21. doi: 10.1016/j.jsp.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger VW, Raskind W, Richards T, Abbott R, Stock P. A multidisciplinary approach to understanding developmental dyslexia within working-memory architecture: Genotypes, phenotypes, brain, and instruction. Developmental Neuropsychology. 2008;33:707–744. doi: 10.1080/87565640802418662. [DOI] [PubMed] [Google Scholar]
- Berninger VW, Rutberg J. Relationship of finger function to beginning writing: Application to diagnosis of writing disabilities. Developmental Medicine and Child Neurology. 1992;34:198–215. doi: 10.1111/j.1469-8749.1992.tb14993.x. [DOI] [PubMed] [Google Scholar]
- Berninger V, Stage S, Smith D, Hildebrand D. Assessment for reading and writing intervention: A 3-tier model for prevention and intervention. In: Andrews J, Saklofske HD, Janzen H, editors. Handbook of psycho-educational assessment. New York, NY: Academic Press; 2001. pp. 195–223. [Google Scholar]
- Bodling AM, Denney DR, Lynch SG. Rapid serial processing in patients with multiple sclerosis: The role of peripheral deficits. Journal of the International Neuropsychological Society. 2008;14:646–650. doi: 10.1017/S1355617708080739. [DOI] [PubMed] [Google Scholar]
- Braak E, Braak H. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropatholica. 1996;92:197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- Catts HW, Gillispie M, Leonard LB, Kail RV, Miller CA. The role of speed of processing, rapid naming, and phonological awareness in reading achievement. Journal of Learning Disability. 2002;35:509–524. doi: 10.1177/00222194020350060301. [DOI] [PubMed] [Google Scholar]
- Chapman NH, Igo RP, Thomson JB, Matsushita M, Brkanac Z, Holzman T, Raskind WH. Linkage analyses of four regions previously implicated in dyslexia: Confirmation of a locus on chromosome 15q. American Journal of Medical Genetics: Part B, Neuropsychiatric Genetics. 2004;131B:67–75. doi: 10.1002/ajmg.b.30018. [DOI] [PubMed] [Google Scholar]
- Chapman NH, Raskind WH, Thomson JB, Berninger VW, Wijsman EM. Segregation analysis of phenotypic components of learning disabilities. II. Phonological decoding. American Journal of Medical Genetics: Part B, Neuropsychiatric Genetics. 2003;121B:60–70. doi: 10.1002/ajmg.b.20068. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, Krupp LB, Liang Z, Huang W, Melville P, Roque C, Peyster R. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology. 2003;60:1793–1798. doi: 10.1212/01.wnl.0000072264.75989.b8. [DOI] [PubMed] [Google Scholar]
- Costello AB, Osborne JW. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical Assessment, Research & Evaluation. 2005;10:1–9. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis– Kaplan executive function system. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Era P. Sensory, psychomotor, and motor functions in men of different ages. Scandinavian Journal of Social Medicine. 1988;39(Suppl.):1–77. [PubMed] [Google Scholar]
- Fletcher SG. The Fletcher Time-by-Count Test of Diadochokinetic Syllable Rate. Austin, TX: Pro-Ed; 1978. [Google Scholar]
- Freeman WJ, Rogers LJ. Fine temporal resolution of analytic phase reveals episodic synchronization by state transitions in gamma EEGs. Journal of Neurophysiology. 2002;87:937–945. doi: 10.1152/jn.00254.2001. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JD, Deutsch G, Tallal K, Temple P. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Restorative Neurology & Neuroscience. 2007;25(3/4):295–310. [PubMed] [Google Scholar]
- Hoyer WJ, Stawski RS, Wasylyshyn C, Verhaeghen P. Adult age and digit symbol substitution performance: A meta-analysis. Psychology and Aging. 2004;19:211–214. doi: 10.1037/0882-7974.19.1.211. [DOI] [PubMed] [Google Scholar]
- Igo RP, Jr., Chapman NH, Berninger VW, Matsushita M, Brkanac Z, Rothstein JH, Wijsman EM. Genomewide scan for real-word reading subphenotypes of dyslexia: Novel chromosome 13 locus and genetic complexity. American Journal of Medical Genetics: Part B, Neuropsychiatric Genetics. 2006;141B:15–27. doi: 10.1002/ajmg.b.30245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaencke L, Siegenthaler T, Preis S, Steinmetz H. Decreased white-matter density in a left-sided fronto-temporal network in children with developmental language disorder: Evidence for anatomical anomalies in a motor-language network. Brain and Language. 2007;102:91–98. doi: 10.1016/j.bandl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Johnston JR, Weismer SE. Mental rotation abilities in language-disordered children. Journal of Speech and Hearing Research. 1983;26:397–403. doi: 10.1044/jshr.2603.397. [DOI] [PubMed] [Google Scholar]
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychologica. 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Micro-structure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Smith SD, Cardon L, Pennington B, Gayan J, Olson RK, DeFries J. Differential genetic etiology of reading component processes as a function of IQ. Behavior Genetics. 2002;33:181–198. doi: 10.1023/a:1016069012111. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996 Aug 16;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Leonard LB, Ellis Weismer S, Miller CA, Francis DJ, Tomblin JB, Kail RV. Speed of processing, working memory, and language impairment in children. Journal of Speech, Language, and Hearing Research. 2007;50:408–428. doi: 10.1044/1092-4388(2007/029). [DOI] [PubMed] [Google Scholar]
- Lewis BA, Shriberg LD, Freebairn LA, Hansen AJ, Stein CM, Taylor HG, Iyengar SK. The genetic bases of speech sound disorders: evidence from spoken and written language. Journal of Speech, Language and Hearing Research. 2006;49:1294–1312. doi: 10.1044/1092-4388(2006/093). [DOI] [PubMed] [Google Scholar]
- Lin JP, Brown JK, Walsh EG. The maturation of motor dexterity: Or why Johnny can’t go any faster. Developmental Medicine and Child Neurology. 1996;38:244–254. doi: 10.1111/j.1469-8749.1996.tb15086.x. [DOI] [PubMed] [Google Scholar]
- Lowe AD, Campbell RA. Temporal discrimination in aphasoid and normal children. Journal of Speech and Hearing Research. 1965;8:313–314. doi: 10.1044/jshr.0803.313. [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz S, Shaywitz B. A definition of dyslexia. Annals of Dyslexia. 2003;53:1–14. [Google Scholar]
- Macniven JA, Davis C, Ho MY, Bradshaw CM, Szabadi E, Constantinescu CS. Stroop performance in multiple sclerosis: Information processing selective attention, or executive functioning? Journal of the International Neuropsychological Society. 2008;14:805–814. doi: 10.1017/S1355617708080946. [DOI] [PubMed] [Google Scholar]
- Marler JA, Champlin CA. Sensory processing of backward-masking signals in children with language learning impairment as assessed with the auditory brain-stem response. Journal of Speech, Language, and Hearing Research. 2005;48:189–203. doi: 10.1044/1092-4388(2005/014). [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. Journal of Comparative Neurology. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Miller CA, Kail R, Leonard LB, Tomblin JB. Speed of processing in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2001;44:416–433. doi: 10.1044/1092-4388(2001/034). [DOI] [PubMed] [Google Scholar]
- Miller CA, Leonard LB, Kail R, Zhang X, Tomblin JB, Francis DJ. Response time in 14-year-olds with language impairment. Journal of Speech, Language, and Hearing Research. 2006;49:712–728. doi: 10.1044/1092-4388(2006/052). [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich MM. Cortical auditory signal processing in poor readers. Proceedings of the National Academy of Sciences of the United States of America. 1999;97:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson RK, Datta H, Gayán J, DeFries J. A behavioral genetic analysis of reading disabilities and component processes. In: Klein RM, McMullen PA, editors. Converging methods for understanding reading and dyslexia. Cambridge, MA: MIT Press; 1999. pp. 133–151. [Google Scholar]
- Olson RK, Rack JP, Conners FA, DeFries JC, Fulker DW. Genetic etiology of individual differences in reading disability. In: Feagans LV, Short EJ, Meltzer LJ, editors. Subtypes of learning disabilities: Theoretical perspectives and research. Hillsdale, NJ: Erlbaum; 1991. pp. 113–135. [Google Scholar]
- Owen SE, McKinlay IA. Motor difficulties in children with developmental disorders of speech and language. Child: Care, Health and Development. 1997;23:315–325. doi: 10.1046/j.1365-2214.1997.864864.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Bishop DVM. Relations among speech, language, and reading disorders. Annual Review of Psychology. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. Journal of Neuropathology and Experimental Neurology. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cerebral Cortex. 2004;14:995–1007. doi: 10.1093/cercor/bhh060. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. Journal of Neuropathology and Experimental Neurology. 2001;435:241–248. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- Peterson RL, McGrath LM, Smith SD, Pennington BF. Neuropsychology and genetics of speech, language, and literacy disorders. Pediatric Clinics in North America. 2007;54:543–561. doi: 10.1016/j.pcl.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Baaré WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Research. 2003;6:131–139. doi: 10.1375/136905203321536254. [DOI] [PubMed] [Google Scholar]
- Powell RP, Bishop DVM. Clumsiness and perceptual problems in children with specific language impairment. Developmental Medicine and Child Neurology. 1992;34:755–765. doi: 10.1111/j.1469-8749.1992.tb11514.x. [DOI] [PubMed] [Google Scholar]
- Raskind WH, Hsu L, Berninger VW, Thomson JB, Wijsman EM. Familial aggregation of dyslexia phenotypes. Behavior Genetics. 2000;30:385–396. doi: 10.1023/a:1002700605187. [DOI] [PubMed] [Google Scholar]
- Raskind WH, Igo RP, Chapman NH, Berninger VW, Thomson JB, Matsushita M, Wijsman EM. A genome scan in multigenerational families with dyslexia: Identification of a novel locus on chromosome 2q that contributes to phonological decoding efficiency. Molecular Psychiatry. 2005;10:699–711. doi: 10.1038/sj.mp.4001657. [DOI] [PubMed] [Google Scholar]
- Rey V, DeMartino S, Espesser R, Habib M. Temporal processing and phonological impairment in dyslexia: Effect of phoneme lengthening on order judgment of two consonants. Brain and Language. 2002;80:576–591. doi: 10.1006/brln.2001.2618. [DOI] [PubMed] [Google Scholar]
- Rice ML, Smith SD, Gayán J. Convergent genetic linkage and associations to language, speech and reading measures in families of probands with specific language impairment. Journal of Neurodevelopmental Disorders. 2009;1:264–282. doi: 10.1007/s11689-009-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T, Stevenson J, Crouch J, Johnson LC, Maravilla K, Stock P, Berninger V. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. American Journal of Neuroradiology. 2008;29:1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biological Psychology. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Caskie G. The Seattle longitudinal study: Relationship between personality and cognition. Aging, Neuropsycholology and Cognition. 2004;11:304–324. doi: 10.1080/13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. Neurophyiological correlates of word recognition in dyslexia. Journal of Neural Transmission. 2004;111:971–984. doi: 10.1007/s00702-004-0141-z. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G, Ziegler A, Deimel W, Schulacher J, Plume E, Bachmann C, König IR. Interrelationship and familiality of dyslexia related quantitative measures. Annals of Human Genetics. 2006;71:160–175. doi: 10.1111/j.1469-1809.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals. Third Edition. San Antonio, TX: Psychological Corporation; 1995. [Google Scholar]
- Shanahan MA, Pennington BF, Yeris BE, Scott A, Boada R, Willcutt EG, Olson RK. Processing speed deficits in attention deficit/hyperactivity disorder and reading disability. Journal of Abnormal Child Psychology. 2006;34:585–602. doi: 10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Hinman JD, Lubonia M, Hollander W, Abraham CR. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. Journal of Neurochemistry. 2003;84:157–168. doi: 10.1046/j.1471-4159.2003.01541.x. [DOI] [PubMed] [Google Scholar]
- Smith AB, Smith SL, Locke JL, Bennett J. A longitudinal study of speech timing in young children later found to have reading disability. Journal of Speech, Language, and Hearing Research. 2008;51:1300–1314. doi: 10.1044/1092-4388(2008/06-0193). [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read: The magnocellular theory of dyslexia. Trends in Neuroscience. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller SL, Jenkins WM, Merzenich MM. The role of temporal processing in developmental language-based learning disorders: Research and clinical implications. In: Bachman BA, editor. Foundations of reading acquisition and dyslexia: Implications for early intervention. Hillsdale, NJ: Erlbaum; 1997. pp. 49–66. [Google Scholar]
- Tallal P, Piercy M. Defects of non-verbal auditory perception in children with developmental aphasia. Nature. 1973a Feb 16;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: Impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia. 1973b;11:389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: Rate of auditory processing and selective impairment of consonant perception. Neuropsychologia. 1974;12:83–93. doi: 10.1016/0028-3932(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: The perception of brief vowels and extended stop consonants. Neuropsychologia. 1975;13:69–74. doi: 10.1016/0028-3932(75)90049-4. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark R, Kallman C, Mellits D. A reexamination of some nonverbal perceptual abilities of language-impaired and normal children as a function of age and sensory modality. Journal of Speech and Hearing Research. 1981;24:351–357. doi: 10.1044/jshr.2403.351. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE, Mellits ED. Identification of language-impaired children on the basis of rapid perception and production skills. Brain and Language. 1985;25:314–322. doi: 10.1016/0093-934x(85)90087-2. [DOI] [PubMed] [Google Scholar]
- Thomson J, Chennault B, Abbott R, Raskind W, Richards T, Aylward E, Berninger VW. Converging evidence for attentional influences on the orthographic word form in child dyslexics. Journal of Neurolinguistics. 2005;18:93–126. [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Vaessen A, Gerretsen P, Blomert L. Naming problems do not reflect a second independent core deficit in dyslexia: Double deficits explored. Journal of Experimental Child Psychology. 2009;103:202–221. doi: 10.1016/j.jecp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Olson RK, Pennington BF, DeFries JC. Differential genetic etiology of reading disability as a function of IQ. Journal of Learning Disabilities. 2000;33:192–199. doi: 10.1177/002221940003300207. [DOI] [PubMed] [Google Scholar]
- Wagner R, Torgesen J. Comprehensive Test of Phonological Processing. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale— Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—. Third Edition. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Weiss S, Mueller HM. The contribution of EEG coherence to the investigation of language. Brain and Language. 2003;85:325–343. doi: 10.1016/s0093-934x(03)00067-1. [DOI] [PubMed] [Google Scholar]
- Wijsman EM, Peterson D, Leutenegger A-L, Thomson JB, Goddard KAB, Berninger VW, &Raskind WH. Segregation analysis of phenotypic components of learning disabilities: I. Nonword memory and digit span. American Journal of Human Genetics. 2000;67:631–646. doi: 10.1086/303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. The Wide Range Achievement Test: Manual. 3rd ed. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- Wolf M. Rapid alternating stimulus naming in the developmental dyslexias. Brain and Language. 1986;27:360–379. doi: 10.1016/0093-934x(86)90025-8. [DOI] [PubMed] [Google Scholar]
- Wolf M, Denckla M. Test of Rapid Automatic Naming. Austin, TX: Pro-Ed; 2004. [Google Scholar]
- Woodcock R. Woodcock Reading Mastery Tests— Revised. Circle Pines, MN: AGS; 1987. [Google Scholar]
- Woodcock R, Johnson B. Woodcock–Johnson Psychoeducational Battery—Revised Tests of Achievement. Chicago, IL: Riverside; 1990. [Google Scholar]
- Wright BA, Bowen RW, Zecker SG. Nonlinguistic perceptual deficits associated with reading and language disorders. Current Opinion in Neurobiology. 2000;10:482–486. doi: 10.1016/s0959-4388(00)00119-7. [DOI] [PubMed] [Google Scholar]