Abstract
PURPOSE
The relationship of race/ethnicity with outcomes of umbilical cord blood transplantation (UCBT) is not well known. We analyzed the association between race/ethnicity and outcomes of unrelated single UCBT for leukemia and myelodysplastic syndromes.
PATIENTS AND METHODS
Our retrospective cohort study consisted of 885 adults and children (612 Whites, 145 Blacks, and 128 Hispanics) who received unrelated single UCBT for leukemia and myelodysplastic syndromes between 1995 and 2006 and were reported to the Center for International Blood and Marrow Transplant Research.
RESULTS
A 5–6/6 HLA-matched unit with a total nucleated cell count infused of ≥2.5 × 107/kg was given to 40% White and 42% Hispanic, but only 21% Black patients. Overall survival (OS) at 2-years was 44% for Whites, 34% for Blacks and 46% for Hispanics (P=0.008). In multivariate analysis adjusting for patient, disease, and treatment factors (including HLA-match and cell dose), Blacks had inferior OS (RR of death=1.31, P=0.02) while OS of Hispanics was similar (RR=1.03, P=0.81) to that of Whites. For all patients, younger age, early-stage disease, use of units with higher cell dose, and performance status ≥80 were independent predictors of improved survival. Black patients and White patients infused with well-matched cords had comparable survival; similarly, Black and White patients receiving units with adequate cell dose had similar survival.
CONCLUSIONS
These results suggest that Blacks have inferior survival to Whites after single UCBT, but outcomes are improved when units with higher cell dose are used.
Keywords: umbilical cord blood, leukemia, myelodysplastic syndrome, race, ethnicity, transplantation
INTRODUCTION
Umbilical cord blood (UCB) is an alternative stem-cell source for patients without HLA-matched related or unrelated donors.(1–3) Recent results in children and adults suggest that outcomes with UCB transplantation (UCBT) are similar to those seen in patients receiving fully HLA-matched unrelated donor bone marrow transplantation, and may approximate the results seen in HLA-matched related donor transplantation.(4,5) Many of the published studies have predominantly included White recipients, despite increased ability to find donors for racial/ethnic minorities being a purported advantage of UCB over adult unrelated bone marrow or peripheral blood stem-cell donors.
Analysis of hematopoietic cell transplantation (HCT) is complex as variables such as economic status, delay in treatment, donor issues, matching criteria, and biologic issues related to disease or treatment may all be important contributing factors to outcomes. Previous studies have addressed racial disparities after HLA-matched related and unrelated donor HCT.(6–9) However, association between race/ethnicity and UCBT outcomes has not been well described. Two recent reports have suggested that the CD34+ count of the cord blood unit, an important prognostic factor for transplant outcome, may be lower in units from Black mothers than from White mothers.(10,11) While limited by the small number of patients, retrospective data from 122 patients transplanted with cord blood units from the American Red Cross cord blood banks showed no difference in survival between Whites and other groups.(12)
In this study, we describe the outcomes of over 800 patients from different racial/ethnic backgrounds receiving single-unit UCBT. These results may have important implications for allocation of precious resources towards UCB banks providing sufficient inventory for patients of diverse backgrounds.
MATERIALS AND METHODS
DATA SOURCE
The Center for International Blood and Marrow Transplant Research (CIBMTR) comprises a voluntary working group of more than 500 transplantation centers worldwide that contribute detailed data on consecutive HCT to a statistical center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. The Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin approve observational studies conducted by the CIBMTR with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations as determined.
PATIENTS
The study included patients who received an unrelated single UCBT for acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), myelodysplastic syndromes (MDS), and chronic myeloid leukemia (CML) between 1995 and 2006 and were reported to the CIBMTR. Only patients transplanted in the United States were included. Pediatric and adult patients, and those treated with myeloablative, reduced intensity (RIC), or non-myeloablative (NMA) regimens were all included. Patients receiving multiple UCB units were excluded, as there were too few recipients of double cord blood transplantation with diverse racial/ethnic backgrounds and sufficient follow up. Patient and donor race/ethnicity were reported by transplant centers and cord blood banks, respectively, according to the US Office of Management and Budget Classification as White, Black, or Hispanic.(13) Due to their relatively small numbers (N=22), patients belonging to other (e.g., Asian, Native Hawaiian/Pacific Islander) and multiple race groups were excluded from this analysis, All surviving recipients included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program. Informed consent was waived by the NMDP Institutional Review Board for all deceased recipients. Approximately 10% of surviving patients would not provide consent for use of research data. To adjust for the potential bias introduced by exclusion of non-consenting, surviving patients, a corrective action plan modeling process randomly excluded the same percentage of deceased patients.(14) The final study cohort consisted of 885 patients. The follow up completeness index from time of HCT, which is the ratio of observed person-time and the potential person-time of follow up in a study, was 84% for Whites, 84% for Blacks, and 73% for Hispanics at three years after transplantation.(15)
OUTCOMES AND STUDY DEFINITIONS
Our primary objective was to evaluate the association of race/ethnicity with three year overall survival (OS), leukemia-free survival (LFS), relapse, and treatment-related mortality (TRM). Secondary endpoints included neutrophil recovery (absolute neutrophil count of ≥0.5 × 109/L sustained for three consecutive days), platelet recovery (platelet count of ≥20 × 109/L independent of platelet transfusions for three consecutive days), and acute and chronic graft-versus-host disease (GVHD) as assessed by standard criteria.(16,17) All outcomes were assessed from the date of HCT. Disease status was classified as early, intermediate, or advanced.(7,9,18) Preparative regimens were classified as myeloablative, RIC, or NMA.(19,20) HLA matching was performed at low resolution for Class I and high resolution for Class II, consistent with prevalent typing of UCB HCT during the time period covered by this study.
STATISTICAL ANALYSIS
Patient-, disease- and treatment-related factors were compared using the Chi-square test and the Kruskal-Wallis test, as appropriate. Univariate probabilities of OS and LFS were calculated using the Kaplan-Meier estimator.(21) Probabilities of neutrophil and platelet recovery, acute and chronic GVHD, TRM and relapse were generated using cumulative incidence estimates. Survival curves were compared using the log-rank test.
The transplant groups were compared using proportional hazards regression models. All factors were examined for proportional hazards using a time dependent covariate approach. The model was stratified on any non-proportional hazards factor. A model was built for the factors using a stepwise regression technique. Each model included a factor for race of the patient. First order interactions between race and each factor were included in the model. The probabilities of neutrophil recovery at 60 days and platelet engraftment at 6 months were modeled using the pseudo-value approach.(22) The interaction between time and race was also examined. Risk factors with P<0.05 in univariate analyses were included in the model. Patient-related variables included age, gender, Karnofsky or Lansky performance score, recipient cytomegalovirus (CMV) status, and weight at HCT. Disease-related variables included diagnosis, disease stage at HCT, and time from diagnosis to transplantation. Treatment-related variables included cell dose infused, HLA match, conditioning regimen intensity, year of HCT and donor race. For OS, LFS, TRM and relapse, we performed pair-wise comparisons of race and cell dose and race and HLA-match; pair-wise comparisons were done adjusting for other patient, disease and treatment-related variables. We tested for center effects using a random effects scores test in the final regression model for each outcome.(23)
All computations were performed using the statistical package SAS Version 9.1 (SAS Institute, Cary, NC).
RESULTS
PATIENT CHARACTERISTICS
Patient characteristics are outlined in Table 1. Our cohort included 612 White patients, 145 Black patients, and 128 Hispanic patients. The median age at transplant was 8 (range <1–78), 8 (<1–57), and 6 (<1–56) years, respectively for the three racial/ethnic groups, as the majority of patients receiving UCBT in this time period were children.
Table 1.
Characteristics of patients with AML, ALL, CML or MDS receiving a single unrelated umbilical cord blood transplant in the United States between 1995 and 2006 and reported to the CIBMTR
| White | tBlack/African- American |
Hispanic/ Latino |
P-value | |
|---|---|---|---|---|
| Variables | N (%) | N (%) | N (%) | |
| Number of patients | 612 | 145 | 128 | |
| Number of centers * | 77 | 45 | 48 | |
| Patient-related | ||||
| Age at HCT, years, median (range) | 8 (<1–78) | 8 (<1–57) | 6 (<1–56) | 0.02 |
| Age | 0.02 | |||
| 0–9 | 334 (55) | 81 (56) | 88 (69) | |
| 10–17 | 141 (23) | 34 (23) | 24 (19) | |
| 18–54 | 113 (18) | 29 (20) | 15 (12) | |
| ≥ 55 | 24 (4) | 1 (1) | 1 (1) | |
| Male gender | 336 (55) | 90 (62) | 80 (63) | 0.12 |
| Karnofsky/Lansky Performance Score | 0.07 | |||
| ≥ 80 | 553 (90) | 129 (89) | 115 (90) | |
| < 80 | 43 (7) | 14 (10) | 3 (2) | |
| Missing | 16 (3) | 2 (1) | 10 (8) | |
| Weight at transplant, kg, median (range) | 29 (5–133) | 29 (7–118) | 22 (5–114) | 0.12 |
| Missing | 1 | 0 | 3 | |
| Recipient CMV status | 0.01 | |||
| Negative | 328 (54) | 64 (44) | 54 (42) | |
| Positive | 273 (45) | 79 (54) | 74 (58) | |
| Missing | 11 (2) | 2 (1) | 0 (.) | |
| Disease-related | ||||
| Disease | 0.15 | |||
| AML | 255 (42) | 55 (38) | 46 (36) | |
| ALL | 253 (41) | 56 (39) | 60 (47) | |
| CML | 30 (5) | 14 (10) | 11 (9) | |
| MDS | 74 (12) | 20 (14) | 11 (9) | |
| Disease stage at HCT † | 0.47 | |||
| Early | 184 (30) | 38 (26) | 38 (30) | |
| Intermediate | 262 (43) | 70 (48) | 63 (49) | |
| Advanced | 166 (27) | 37 (26) | 27 (21) | |
| Transplant-related | ||||
| Time from diagnosis to transplant | 0.07 | |||
| ≤ 12 months | 346 (57) | 67 (46) | 67 (52) | |
| > 12 months | 266 (43) | 78 (54) | 61 (48) | |
| Cell dose infused | 0.08 | |||
| < 2.5 × 107 NC/kg | 157 (26) | 50 (34) | 26 (20) | |
| ≥ 2.5 × 107 NC/kg | 420 (69) | 90 (62) | 93 (73) | |
| Missing | 35 (6) | 5 (3) | 9 (7) | |
| CD34+ dose infused | <0.0001 | |||
| ≤ 3.0 × 105 /kg | 171 (28) | 57 (39) | 17 (13) | |
| > 3.0 × 105 /kg | 87 (14) | 17 (12) | 12 (9) | |
| Missing | 354 (58) | 71 (49) | 99 (77) | |
| HLA Match ‡ | <0.0001 | |||
| 6/6 match | 96 (16) | 6 (4) | 12 (9) | |
| 5/6 match | 230 (38) | 37 (26) | 50 (39) | |
| ≤ 4/6 match | 281 (46) | 98 (68) | 60 (47) | |
| Missing | 5 (1) | 4 (3) | 6 (5) | |
| Gender match (Donor/Recipient) | 0.43 | |||
| Male/Male | 171 (28) | 39 (27) | 39 (30) | |
| Male/Female | 148 (24) | 25 (17) | 24 (19) | |
| Female/Male | 153 (25) | 45 (31) | 36 (28) | |
| Female/Female | 112 (18) | 25 (17) | 24 (19) | |
| Missing | 28 (5) | 11 (8) | 5 (4) | |
| Year of transplant | 0.20 | |||
| 1995–2000 | 207 (34) | 49 (34) | 33 (26) | |
| 2001–2006 | 405 (66) | 96 (66) | 95 (74) | |
| Race of cord blood donor | <0.0001 | |||
| White | 384 (63) | 34 (23) | 34 (27) | |
| African-American | 15 (2) | 43 (30) | 2 (2) | |
| Asian / Pacific Islander | 10 (2) | 2 (1) | 3 (2) | |
| Hispanic | 36 (6) | 13 (9) | 42 (33) | |
| Other § / Unknown / Missing | 167 (28) | 53 (37) | 47 (37) | |
| Conditioning regimen intensity | 0.04 | |||
| Myeloablative | 431 (70) | 98 (68) | 103 (80) | |
| Non-myeloablative / Reduced intensity | 181 (30) | 47 (32) | 25 (20) | |
| GVHD prophylaxis | 0.43 | |||
| Cyclosporine ± other | 411 (67) | 96 (66) | 77 (60) | |
| Methotrexate + Cyclosporine ± other | 72 (12) | 18 (12) | 15 (12) | |
| FK506 ± other | 125 (20) | 30 (21) | 33 (26) | |
| Other | 4 (0) | 1 (1) | 3 (3) | |
| Median follow-up, months, median (range) | 52 (3–149) | 37 (4–87) | 36 (3–102) | |
Total numbers of centers: 90
Early disease included AML and ALL in first complete remission, CML in first chronic phase, and MDS with refractory anemia or refractory anemia with ringed sideroblasts, or bone marrow blasts < 5% at HCT; intermediate disease included AML and ALL in second or greater remission or CML in accelerated phase or second or greater chronic phase; advanced disease included AML and ALL in relapse or primary induction failure, CML in blast phase, or MDS with refractory anemia with excess blasts or bone marrow blasts ≥ 5% at HCT.
HLA Match is defined by low-resolution typing at HLA-A and -B, and high-resolution typing at -DRB1.
Includes multiple race and Native American.
CORD BLOOD CHARACTERISTICS
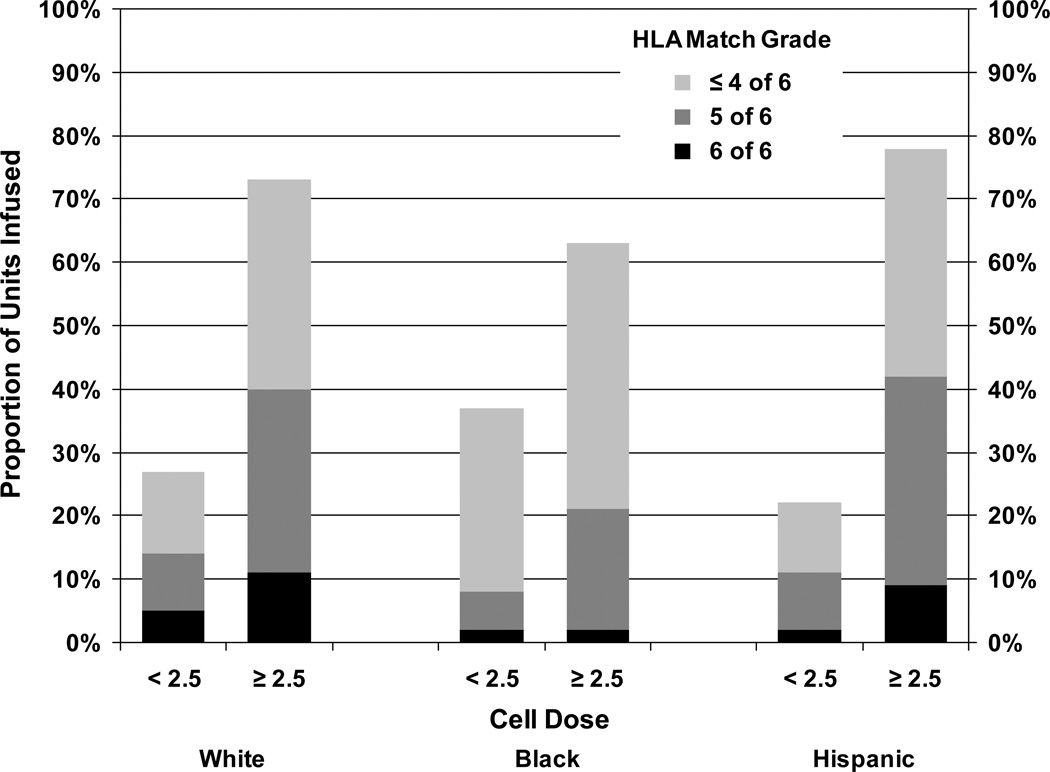
Fewer Blacks received well matched UCB units with a good cell dose compared to Whites and Hispanics (Table 1). Sixty-nine percent of Whites, 62% of Blacks, and 73% Hispanics received a cell dose ≥2.5 × 107 NC/kg (P=0.08). HLA match differed among the racial/ethnic groups; a higher proportion of Whites (54%) received 5/6 or 6/6 HLA-A, -B, and -DR matched UCB units compared to 30% for Blacks and 48% for Hispanics (P<0.0001). More Whites (40%) and Hispanics (42%) than Blacks (21%) received UCB units that were well-matched (5/6 or 6/6 HLA matched) and had a cell dose ≥2.5 × 107 NC/kg (P=0.0002; Figure 1). The racial distribution of the UCB units indicates that 63% White, 30% Black, and 33% Hispanic patients received UCB units from donors of the same race, although race/ethnicity information was missing for a large proportion of donors.
Figure 1.
Distribution of UCB units based on race, cell dose, and HLA match
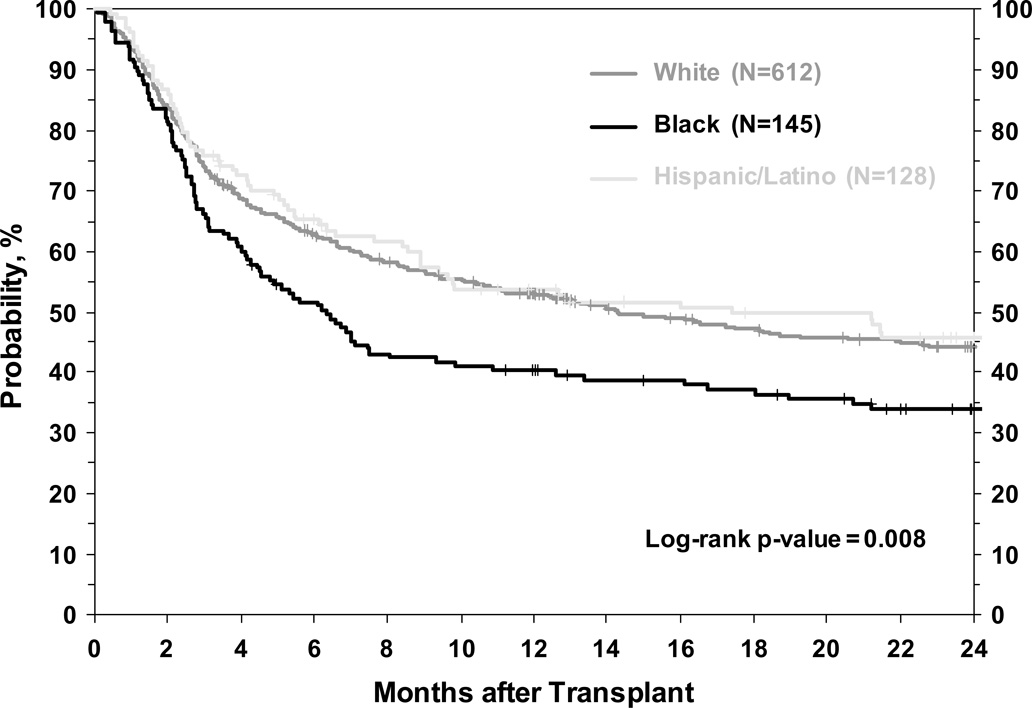
ASSOCIATION OF RACE/ETHNICITY WITH OVERALL SURVIVAL
Three-year OS rates for Whites, Blacks, and Hispanics were 41%, 29%, and 45% respectively (P=0.008) (Table 2, Figure 2). In multivariate analyses that considered patient, disease and transplant related variables (see Methods), Blacks had worse OS while Hispanic patients had similar OS compared to Whites (Table 3).
Table 2.
Univariate outcomes of patients with AML, ALL, CML or MDS receiving a single unrelated cord blood transplant between 1995 and 2006 and reported to the CIBMTR
| Outcome Event | White | Black | Hispanic | P-value | |||
|---|---|---|---|---|---|---|---|
| N | Probability (95%CI) |
N | Probability (95%CI) |
N | Probability (95%CI) |
||
| Overall survival, 3 years | 612 | 41 (37–45)% | 145 | 29 (22–38)% | 128 | 45 (35–54)% | 0.008† |
| Leukemia-free survival, 3 years | 607 | 38 (34–42)% | 144 | 29 (22–37)% | 127 | 39 (31–49)% | 0.04† |
| Relapse, 3 years | 607 | 27 (23–30)% | 144 | 29 (22–37)% | 127 | 31 (23–40)% | 0.60* |
| Treatment related mortality, 3 years | 607 | 35 (31–39)% | 144 | 41 (33–50)% | 127 | 30 (22–38)% | 0.15* |
| Neutrophil engraftment, 60 days | 605 | 86 (83–88)% | 145 | 81 (74–87)% | 127 | 86 (79–91)% | 0.40* |
| Platelet engraftment, 6 months | 602 | 62 (58–66)% | 139 | 52 (44–61)% | 126 | 67 (58–75)% | 0.05* |
| Acute GVHD (grades 2–4), 100 days | 608 | 50 (46–54)% | 145 | 50 (42–59)% | 127 | 56 (47–65)% | 0.42* |
| Chronic GVHD, 2 years | 598 | 24 (20–27)% | 143 | 21 (14–28)% | 125 | 30 (22–39)% | 0.24* |
Log-rank p-value.
Cumulative incidence estimate with pointwise p-value
Figure 2.
Unadjusted overall survival of White, Black and Hispanic patients receiving single umbilical cord blood transplant
Table 3.
Multivariate analysis for outcomes of single unrelated umbilical cord blood transplant for AML, ALL, CML or MDS
| Endpoint | N | Relative Risk | 95% CI | P-value | |
|---|---|---|---|---|---|
| Overall Survival* | |||||
| Race/Ethnicity† | |||||
| White | 612 | 1.00 | |||
| Black | 145 | 1.31 | 1.04 | 1.66 | 0.02 |
| Hispanic | 128 | 1.03 | 0.79 | 1.35 | 0.80 |
| Cell dose infused† | |||||
| > 2.5 × 107 NC/kg | 603 | 1.00 | |||
| < 2.5 × 107 NC/kg | 233 | 1.44 | 1.15 | 1.80 | 0.001 |
| Missing | 49 | 1.22 | 0.83 | 1.80 | 0.31 |
| HLA match‡ | |||||
| 6/6 | 114 | 1.00 | |||
| 5/6 | 317 | 1.01 | 0.74 | 1.37 | 0.95 |
| 4/6 | 439 | 1.25 | 0.93 | 1.67 | 0.13 |
| Missing | 15 | 1.77 | 0.88 | 3.49 | 0.11 |
| Leukemia-free Survival§ | |||||
| Race/Ethnicity† | |||||
| White | 607 | 1.00 | |||
| Black | 144 | 1.25 | 0.99 | 1.58 | 0.06 |
| Hispanic | 127 | 1.01 | 0.78 | 1.31 | 0.95 |
| Cell dose infused† | |||||
| >2.5 × 107 NC/kg | 600 | 1.00 | |||
| < 2.5 × 107 NC/kg | 229 | 1.32 | 1.11 | 1.73 | 0.004 |
| Missing | 56 | 1.08 | 1.09 | 1.59 | 0.66 |
| HLA match‡ | |||||
| 6/6 | 113 | 1.00 | |||
| 5/6 | 314 | 1.01 | 0.75 | 1.35 | 0.89 |
| 4/6 | 436 | 1.21 | 0.92 | 1.61 | 0.18 |
| Missing | 22 | 1.69 | 0.85 | 3.37 | 0.14 |
| Relapse‖ | |||||
| Race/Ethnicity† | |||||
| White | 607 | 1.00 | |||
| Black | 144 | 1.40 | 0.96 | 2.03 | 0.08 |
| Hispanic | 127 | 1.13 | 0.77 | 1.63 | 0.53 |
| Cell dose infused† | |||||
| > 2.5 × 107 NC/kg | 600 | 1.00 | |||
| < 2.5 × 107 NC/kg | 229 | 1.18 | 0.67 | 2.08 | 0.56 |
| Missing | 49 | 1.00 | 0.68 | 1.46 | 0.99 |
| HLA match‡ | |||||
| 6/6 | 113 | 1.00 | |||
| 5/6 | 314 | 0.71 | 0.48 | 1.04 | 0.08 |
| 4/6 | 436 | 0.77 | 0.52 | 1.14 | 0.19 |
| Missing | 15 | 1.40 | 0.50 | 4.17 | 0.54 |
| Treatment-related mortality¶ | |||||
| Race/Ethnicity† | |||||
| White | 607 | 1.00 | |||
| Black | 144 | 1.18 | 0.87 | 1.58 | 0.29 |
| Hispanic | 127 | 0.92 | 0.64 | 1.31 | 0.63 |
| Cell dose infused† | |||||
| > 2.5 × 107 NC/kg | 600 | 1.00 | |||
| < 2.5 × 107 NC/kg | 229 | 1.67 | 1.26 | 2.19 | 0.0003 |
| Missing | 56 | 0.92 | 0.56 | 1.53 | 0.75 |
| HLA match‡ | |||||
| 6/6 | 113 | 1.00 | |||
| 5/6 | 314 | 1.52 | 0.96 | 2.39 | 0.07 |
| 4/6 | 436 | 2.05 | 1.33 | 3.17 | 0.001 |
| Missing | 22 | 2.52 | 1.00 | 6.325 | 0.05 |
Chi-square test with 2 d.f.
Chi-square test with 3 d.f.
Other variables significantly associated with overall survival were: diagnosis, disease status, age, and performance status score (see Appendix Table 1)
Other variables significantly associated with leukemia-free survival were: diagnosis, disease status, age, and performance status score (see Appendix Table 1)
Other variables significantly associated with relapse were: diagnosis, disease status, age, performance status score, gender and time from diagnosis to transplantation (see Appendix Table 1)
Other variables significantly associated with transplant-related mortality were: disease status, and age (see Appendix Table 1)
In multivariate analysis, we also examined factors other than race for their association with OS. Cell dose was significantly associated with OS while HLA match did not affect OS (Table 3). We tested for and found no significant interaction between race and cell dose and race and HLA match. In addition to cell dose, as expected, younger age, less advanced disease, and performance status ≥ 80 were independent predictors of improved OS for all patients (Appendix Table 1). Donor race did not impact OS; however, information regarding race of the cord blood donor was missing in a substantial proportion of patients in all three race groups. Data from 90 centers was included in our study. However, four centers contributed >40 transplants each and collectively contributed to 45% of patients included in our analysis. Hence, we tested for center effect and did not find a significant center effect in our analyses.
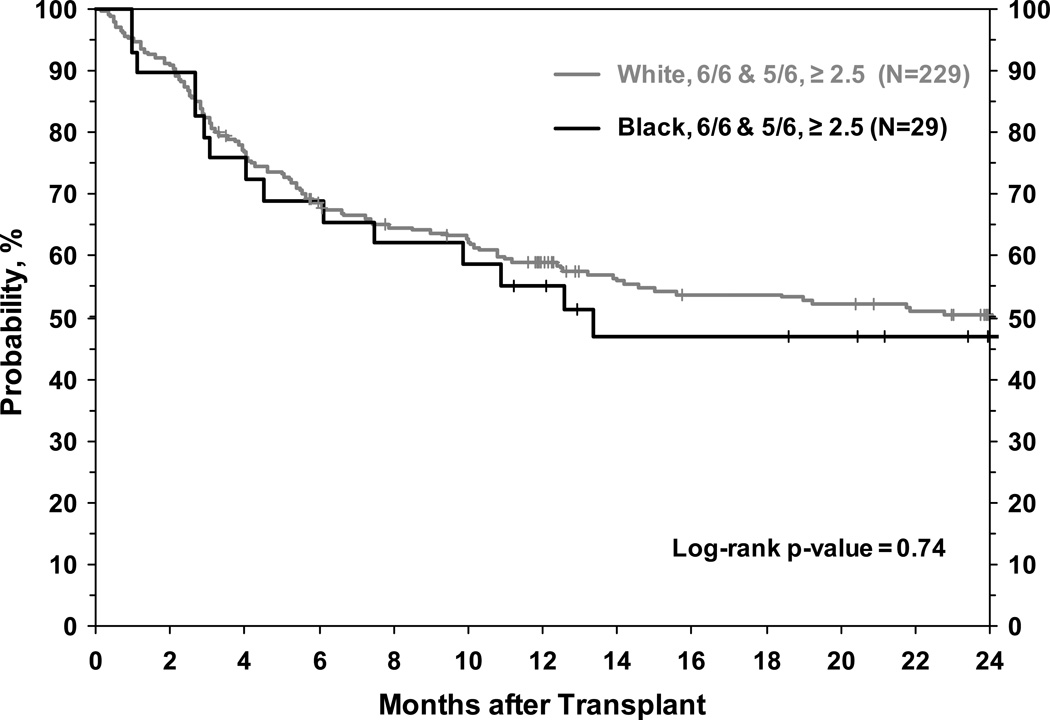
We were also interested in evaluating whether patients from the three race groups who received UCB units with good cell dose and well matched units had comparable OS. In pair-wise comparisons that adjusted for other patient, disease, and transplant factors (including HLA-match status) we found no significant differences in OS by race among patients receiving the same cell dose. White, Black and Hispanic patients receiving units with cell dose ≥2.5 × 107 NC/kg had comparable OS (Table 4). Similarly, in pair-wise comparisons (that also adjusted for cell dose) we found no significant differences in OS by race among patients receiving well-matched (5/6 or 6/6 HLA-A, -B, -DR match) UCB units (Table 4). Due to the relatively small number of Black and Hispanic patients in our cohort, we could not perform multivariate analyses in the subgroup of patients who received well-matched units with adequate cell dose. However in unadjusted analyses, Black patients receiving HLA 5/6 or 6/6 matched UCB unit with a cell dose ≥ 2.5 × 107 NC/kg (N=26) had similar OS compared to White patients receiving units of similar cell dose and HLA match (Figure 3).
Table 4.
Pair-wise comparisons of (1) race and cell dose and (2) race and HLA match for White, Hispanic and Black patients receiving single unrelated umbilical cord blood units with adequate cell dose and with adequate HLA match. Analyses were adjusted for other patient, disease and transplant related factors (analysis by cell dose also adjusted for HLA match and analysis by HLA match also adjusted for cell dose)
| Cell dose | HLA Match* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Race/cell dose | N | Relative Risk | 95% CI | P-value | Race/HLA match | N | Relative Risk | 95% CI | P-value | ||
| Overall Survival | |||||||||||
| White, ≥ 2.5 × 107 NC/kg | 420 | 1.00 | White, 5/6 match | 230 | 1.00 | ||||||
| Hispanic, ≥ 2.5 × 107 NC/kg | 93 | 0.92 | 0.65 | 1.30 | 0.63 | White, 6/6 match | 96 | 0.84 | 0.59 | 1.35 | 0.32 |
| Black, ≥ 2.5 × 107 NC/kg | 90 | 1.26 | 0.93 | 1.70 | 0.13 | Hispanic, 5/6 match | 49 | 0.86 | 0.55 | 1.35 | 0.49 |
| Black, 5/6 match | 37 | 1.22 | 0.76 | 1.97 | 0.41 | ||||||
| Leukemia-free Survival | |||||||||||
| White, ≥ 2.5 × 107 NC/kg | 417 | 1.00 | White, 5/6 match | 228 | 1.00 | ||||||
| Hispanic, ≥ 2.5 × 107 NC/kg | 93 | 0.93 | 0.68 | 1.29 | 0.67 | White, 6/6 match | 95 | 0.84 | 0.60 | 1.13 | 0.31 |
| Black, ≥ 2.5 × 107 NC/kg | 90 | 1.20 | 0.89 | 1.61 | 0.24 | Hispanic, 5/6 match | 49 | 0.84 | 0.55 | 1.29 | 0.43 |
| Black, 5/6 match | 37 | 1.10 | 0.68 | 1.76 | 0.70 | ||||||
| Relapse | |||||||||||
| White, ≥ 2.5 × 107 NC/kg | 417 | 1.00 | White, 5/6 match | 228 | 1.00 | ||||||
| Hispanic, ≥ 2.5 × 107 NC/kg | 93 | 0.98 | 0.63 | 1.53 | 0.94 | White, 6/6 match | 95 | 1.27 | 0.81 | 1.96 | 0.28 |
| Black, ≥ 2.5 × 107 NC/kg | 90 | 1.30 | 0.85 | 2.00 | 0.23 | Hispanic, 5/6 match | 49 | 1.02 | 0.58 | 1.79 | 0.93 |
| Black, 5/6 match | 37 | 1.14 | 0.58 | 2.24 | 0.71 | ||||||
| Treatment-related mortality | |||||||||||
| White, ≥ 2.5 × 107 NC/kg | 417 | 1.00 | White, 5/6 match | 228 | 1.00 | ||||||
| Hispanic, ≥ 2.5 × 107 NC/kg | 93 | 0.89 | 0.55 | 1.42 | 0.62 | White, 6/6 match | 95 | 0.46 | 0.27 | 0.80 | 0.005 |
| Black, ≥ 2.5 × 107 NC/kg | 90 | 1.16 | 0.77 | 1.74 | 0.47 | Hispanic, 5/6 match | 49 | 0.65 | 0.33 | 1.25 | 0.20 |
| Black, 5/6 match | 37 | 1.08 | 0.55 | 2.10 | 0.82 | ||||||
Because of a relatively small number of patients, data on Black patients (N=6) and Hispanic patients (N=12) who received 6/6 HLA matched units is not included in this table
Figure 3.
Unadjusted overall survival of White and Black patients receiving umbilical cord blood units with 5/6 or 6/6 HLA match and cell dose ≥ 2.5 × 107 NC/kg
LEUKEMIA FREE SURVIVAL
Three-year LFS for Whites, Blacks, and Hispanics was 38%, 29%, and 39%, respectively (P=0.04) (Table 2). In multivariate analyses, there was no significant difference in LFS among racial groups (Table 3). As with OS, cell dose influenced LFS while HLA-match status did not (Table 3). As with OS, in pair-wise comparisons there was no difference in LFS among White, Hispanic and Black patients receiving UCB units with similar cell dose and among patients receiving well-matched units (Table 4). For all patients, transplantation for advanced disease, older age at transplant and performance status of <80 adversely impacted LFS, while HCT for MDS was associated with better LFS (Appendix Table 1).
TRANSPLANT-RELATED MORTALITY AND RELAPSE
Table 2 highlights cumulative incidences of TRM and relapse. In multivariate analysis adjusting for other important variables including cell dose and HLA match, race had no influence on risk of relapse or TRM (Table 3).
Cell dose and HLA-match status did not significantly affect the risks of relapse (Table 3). There was no difference in relapse risks when we compared White, Hispanic and Black patients who had received units with good cell dose and units with good HLA-match (Table 4).
In multivariate analyses, there was no difference in the relative risks of TRM by race (Table 3). Both cell dose and HLA-match were significant predictors of TRM. On pair-wise comparisons, White, Hispanic and Black patients receiving units with cell dose ≥2.5 × 107 NC/kg had comparable TRM (Table 4). Similarly, White, Hispanic and Black patients who received 5/6 HLA-matched units also had similar risks of TRM (Table 4).
ENGRAFTMENT AND GVHD
The median time to neutrophil recovery was 23 (range 1–104) days, and there was no difference among racial/ethnic groups (Table 2). The median time to platelet recovery was 59 (range 1–241) days, again with no difference among racial/ethnic groups. In multivariate analysis, race had no independent association with the likelihood of neutrophil engraftment or platelet engraftment. As expected, cell dose was an independent predictor of neutrophil and platelet engraftment. In multivariate analysis, race was not associated with risks of acute GVHD or chronic GVHD.
CAUSES OF DEATH
Causes of death differed slightly among the different groups. For all patients, relapse was a major cause of death; 37% of White patients (N=129), 46% of Hispanics (N=31), and 38% of Black patients (N=38) who died succumbed to relapsed disease. For White (N=86, 25%) and Hispanic (N=17, 25%) patients, infection was the second most common cause of death after relapse. For Black patients, organ failure (N=15, 15%) was the second most common cause of death. Death from GVHD was rare in all groups (Whites, N=22 (6%), Hispanics, N=1 (1%), Blacks, N=8 (8%)).
DISCUSSION
This report examines the association of racial/ethnic background of the recipient with outcomes after UCBT. Results indicate that Black patients have inferior OS compared to White patients after single UCB transplantation. This disparity in outcomes is partially a result of the inability to find a well-matched, appropriately sized UCB unit for Black patients, as fewer Black than White patients received UCB that were 5/6 or 6/6 HLA matched and had cell dose of >2.5 × 107 NC/kg. In contrast, when the analysis was restricted to patients receiving well-matched units and units with adequate cell dose, there was no significant difference in survival by race. These results suggest that the increased difficulty in finding appropriately matched cord units of sufficient cell dose may be a major obstacle to better outcomes in Black patients. Furthermore, the poorer outcomes in Black patients may be improved by infusing UCB units with sufficient cell dose. As reported by other investigators, outcomes were better among all patients when transplanted with a higher cell dose. Death from GVHD was low in all groups.(24)
This study has some limitations. The majority of patients included in this analysis were children. Geographic variation in distribution of racial/ethnic groups in the United States and extensive UCB transplantation experience could lead to strong center effects. However, transplant center was not a significant correlate of OS in our analysis. Although use of double cord blood HCT has been increasingly used in adults, too few transplants in a racially/ethnically diverse population with adequate follow-up were available during the study time period. A future study of racial disparities after double cord blood transplantation is planned. Small patient numbers limited the ability to understand differences in the causes of death. We could not robustly evaluate the impact of using race matched vs. mismatched UCB units on outcomes because these data were missing for a large proportion of donor units. Race/ethnicity was reported by transplant centers and cord blood banks and was not verified. Furthermore, there may have been differences in access to post transplant care or post transplant medications among racial/ethnic groups that could not be addressed in this study.(3,25) We could not evaluate the effect of patient socioeconomic status in our study, as Zip Code data was not available for a large proportion of patients. In a previous study, socioeconomic status estimated by Zip Code of residence was found to be an independent predictor of OS and TRM following unrelated donor HCT for acute and chronic leukemia.(9)
Although there was a difference in OS by race, the risks of LFS, relapse and TRM were not significantly different among White and Black patients. Because of the relatively small number of Black patients available for our analysis, our study may have lacked the power to detect any association of race with outcomes other than OS. Studies with larger number of minority patients and with additional information (e.g., socioeconomic status) remain necessary to better understand the reason for differences in survival between White and Black patients after UCBT. Nevertheless, an important finding from our study was that White and Black patients who received units with adequate cell dose had similar risks of mortality. Similarly, White and Black patients who received HLA well-matched units had comparable survival. Hence, survival disparities by race/ethnicity after UCBT could partly be overcome by availability of UCB units with adequate cell dose and HLA-match.
Despite extensive recruiting efforts, it remains more difficult for Blacks and other racial minorities to find matched adult unrelated donor volunteers via the NMDP. Donor selection using antigen level typing for Class I and allele level typing for Class II uncovers at least one fully matched adult volunteer donor for 79% of White patients, 40% of Black patients, and 57% of Hispanic patients.(26) With more stringent contemporary criteria for HLA-matching, the likelihood of finding a well-matched donor for racial minorities is likely even lower. A prospective analysis from Memorial Sloan-Kettering Cancer Center revealed that a 10/10 allele level matched adult unrelated donor was identified for 53% of patients with European ancestry and 21% of patients with non-European ancestry.(27) In contrast, 56% of patients receiving an UCB transplant were of non-European ancestry. However, an early study of five different cord blood banks found no increase in cord blood collections from minorities when compared to bone marrow collections from minorities in the same geographic area.(28) The American Red Cross cord blood banks attempted to increase diversity by establishing banks in different areas of the United States which resulted in a cord blood donor pool of 64% Whites, 16% Blacks, 12% Hispanics, 4% Asians, 1% Native Americans and 3% other.(10) While the proportion of minority donors may match the distribution of the US population, greater HLA diversity in the Black population and a higher proportion of UCB units of low cell dose, may limit the number of high-quality UCB units available to Black patients.(10,11) Extensive resources have been allocated in the United States and worldwide to create a large, diverse cord blood donor pool.
Black patients have been reported to have a higher mortality than White patients in both sibling and unrelated donor transplants.(9,29) However, other studies restricted to HLA matched sibling transplants showed that Hispanics had a higher risk of overall mortality after transplant, while Whites, Asians, and Blacks had similar outcomes.(7,30) Black patients are known to have greater genetic diversity and HLA polymorphisms, making matching for minor antigens less likely.(31) Increasing genetic diversity at minor transplantation antigens may affect survival after transplantation, and these differences may be even more important in UCBT given the greater degree of mismatch compared to unrelated donor transplantation.(32) Although this cord blood study has fewer patients than the transplant studies using sibling or unrelated donors cited above, we have been able to identify a potential solution to the discrepancy in outcomes, with the use of larger and better matched cord blood units. In addition, since the growth of UCB banks and transplantation was specifically designed to aid populations underrepresented in the traditional transplant registries, the new information presented here on race/ethnicity and UCB transplant outcomes is of importance to transplant physicians, cord blood banks, and policy makers.
This report examines for the first time the outcomes of UCB transplantation for the different racial/ethnic groups. These results suggest that Blacks have inferior survival to Whites after single UCB transplantation. Outcomes for Black and White patients are improved when units with higher cell doses are infused. The resources to collect a diverse UCB donor pool and to ensure UCB of high cell dose are extensive, but this report suggests the need to redouble these efforts in order to better meet the needs of the diverse population of transplant patients.
ACKNOWLEDGMENTS
We would like to acknowledge Mary Eapen, MD, MS, Associate Scientific Director, Center for International Blood and Marrow Transplant Research for her helpful review of the manuscript.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
APPENDIX TABLE 1
Factors other than race, cell dose and HLA-match (shown in Table 3) that were significantly associated with outcomes of single unrelated umbilical cord blood transplant for AML, ALL, CML or MDS
| Endpoint/Variable | Relative Risk | 95% CI | P-value | |
|---|---|---|---|---|
| OVERALL SURVIVAL | ||||
| Diagnosis | ||||
| AML | 1.00 | <0.01 | ||
| ALL | 0.90 | 0.73 | 1.10 | 0.29 |
| CML | 0.66 | 0.44 | 1.00 | 0.05 |
| MDS | 0.61 | 0.45 | 0.83 | <0.01 |
| Disease status at HCT | ||||
| Early | 1.00 | <0.01 | ||
| Intermediate | 1.01 | 0.80 | 1.27 | 0.95 |
| Late | 1.80 | 1.43 | 2.26 | <0.01 |
| Age | ||||
| < 10 years | 1.00 | <0.01 | ||
| 10–17 years | 1.07 | 0.84 | 1.35 | 0.59 |
| 18–54 years | 1.47 | 1.13 | 1.91 | <0.01 |
| ≥ 55 years | 2.00 | 1.24 | 3.22 | <0.01 |
| KPS score at HCT | ||||
| ≥ 80 | 1.00 | <0.01 | ||
| < 80 | 1.60 | 1.17 | 2.19 | <0.01 |
| Missing | 0.81 | 0.47 | 1.42 | 0.45 |
| LEUKEMIA FREE SURVIVAL | ||||
| Diagnosis | ||||
| AML | 1.00 | <0.01 | ||
| ALL | 0.88 | 0.72 | 1.07 | 0.19 |
| CML | 0.76 | 0.53 | 1.10 | 0.15 |
| MDS | 0.60 | 0.44 | 0.81 | <0.01 |
| Disease status at HCT | ||||
| Early | 1.00 | <0.01 | ||
| Intermediate | 0.98 | 0.78 | 1.22 | 0.83 |
| Late | 1.80 | 1.43 | 2.26 | <0.01 |
| Age | ||||
| < 10 years | 1.00 | <0.01 | ||
| 10–17 years | 0.94 | 0.75 | 1.19 | 0.62 |
| 18–54 years | 1.41 | 1.10 | 1.82 | <0.01 |
| ≥ 55 years | 1.85 | 1.15 | 2.98 | 0.01 |
| KPS score at HCT | ||||
| ≥ 80 | 1.00 | <0.01 | ||
| < 80 | 1.55 | 1.14 | 2.10 | <0.01 |
| Missing | 0.97 | 0.57 | 1.62 | 0.88 |
| RELAPSE | ||||
| Diagnosis | ||||
| AML | 1.00 | <0.01 | ||
| ALL | 0.78 | 0.57 | 1.07 | 0.13 |
| CML | 0.88 | 0.50 | 1.54 | 0.64 |
| MDS | 0.34 | 0.21 | 0.56 | <0.01 |
| Disease status at HCT | ||||
| Early | 1.00 | <0.01 | ||
| Intermediate | 1.19 | 0.80 | 1.77 | 0.39 |
| Late | 2.87 | 2.02 | 4.09 | <0.01 |
| Age | ||||
| < 10 years | 1.00 | <0.01 | ||
| 10–17 years | 0.47 | 0.31 | 0.73 | 0.06 |
| 18–54 years | 0.88 | 0.59 | 1.31 | 0.52 |
| ≥ 55 years | 1.93 | 0.98 | 3.82 | 0.06 |
| KPS score at HCT | ||||
| ≥ 80 | 1.00 | <0.01 | ||
| < 80 | 2.10 | 1.34 | 3.30 | <0.01 |
| Missing | 0.92 | 0.41 | 2.10 | 0.84 |
| Sex | ||||
| Male | 1.00 | <0.01 | ||
| Female | 1.56 | 1.20 | 2.04 | <0.01 |
| Time from DX to TX | ||||
| > 12 months | 1.00 | 0.03 | ||
| ≤ 12 months | 0.68 | 0.48 | 0.96 | 0.03 |
| TRM | ||||
| Disease status at HCT | ||||
| Early | 1.00 | 0.04 | ||
| Intermediate | 1.08 | 0.82 | 1.41 | 0.60 |
| Late | 1.45 | 1.07 | 1.95 | 0.02 |
| Age | ||||
| < 10 years | 1.00 | <0.01 | ||
| 10–17 years | 1.41 | 1.05 | 1.90 | 0.02 |
| 18–54 years | 1.91 | 1.39 | 2.63 | <0.01 |
| ≥ 55 years | 1.86 | 0.95 | 3.61 | 0.06 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP AND CONFLICT OF INTEREST DISCLOSURES
Conception and design: Karen K. Ballen, Navneet S. Majhail, John P. Klein
Provision of study materials or patients: Deepika Bhatla, Reggie Duerst, Joanne Kurtzberg, Hillard Lazarus, Charles F. LeMaistre, Phillip McCarthy, Paulette Mehta, Jeanne Palmer, John R. Wingard
Collection and assembly of data: Tanya L. Pedersen, Navneet S. Majhail
Data analysis and interpretation: Karen K. Ballen, Navneet S. Majhail, John P. Klein, Tanya L. Pedersen, Deepika Bhatla, Reggie Duerst, Joanne Kurtzberg, Hillard Lazarus, Charles F. LeMaistre, Phillip McCarthy, Paulette Mehta, Jeanne Palmer, Michelle Setterholm, John R. Wingard, Steven Joffe, Susan K. Parsons, Galen Switzer, Stephanie J. Lee, J. Douglas Rizzo Manuscript writing: Karen K. Ballen, Navneet S. Majhail, John P. Klein, Tanya L. Pedersen, Deepika Bhatla, Reggie Duerst, Joanne Kurtzberg, Hillard Lazarus, Charles F. LeMaistre, Phillip McCarthy, Paulette Mehta, Jeanne Palmer, Michelle Setterholm, John R. Wingard, Steven Joffe, Susan K. Parsons, Galen Switzer, Stephanie J. Lee, J. Douglas Rizzo
Final approval of manuscript: Karen K. Ballen, Navneet S. Majhail, John P. Klein, Tanya L. Pedersen, Deepika Bhatla, Reggie Duerst, Joanne Kurtzberg, Hillard Lazarus, Charles F. LeMaistre, Phillip McCarthy, Paulette Mehta, Jeanne Palmer, Michelle Setterholm, John R. Wingard, Steven Joffe, Susan K. Parsons, Galen Switzer, Stephanie J. Lee, J. Douglas Rizzo
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005;105(10):3786–3792. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351(22):2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 4.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haematopoietic stem-cell transplantation in adults with acute leukemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies SM, Kollman C, Anasetti C, et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the National Marrow Donor Program. Blood. 2000;96(13):4096–4102. [PubMed] [Google Scholar]
- 7.Serna DS, Lee SJ, Zhang MJ, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21(20):3756–3760. doi: 10.1200/JCO.2003.03.133. [DOI] [PubMed] [Google Scholar]
- 8.Oh H, Loberiza FR, Jr, Zhang MJ, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105(4):1408–1416. doi: 10.1182/blood-2004-06-2385. [DOI] [PubMed] [Google Scholar]
- 9.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(12):1543–1554. doi: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballen KK, Kurtzberg J, Lane TA, et al. Racial diversity with high nucleated cell counts and CD34 counts achieved in national network of cord blood banks. Biol Blood Marrow Transplant. 2004;10(4):269–275. doi: 10.1016/j.bbmt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Cairo MS, Wagner EL, Fraser J, et al. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, type of delivery: a Cord Blood Transplantation (COBLT) study report. Transfusion. 2005;45(6):856–866. doi: 10.1111/j.1537-2995.2005.04429.x. [DOI] [PubMed] [Google Scholar]
- 12.Ballen KK, Haley NR, Kurtzberg J, et al. Outcomes of 122 diverse adult and pediatric cord blood transplant recipients from a large cord blood bank. Transfusion. 2006;46(12):2063–2070. doi: 10.1111/j.1537-2995.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 13.Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. [Accessed February 25, 2011];Office of Management and Budget. http://www.whitehouse.gov/omb/fedreg_1997standards/.
- 14.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the Center for International Blood and Marrow Transplant Research, the European Blood and Marrow Transplant Registry, and the Dutch Registry. Biol Blood Marrow Transplant. 2006;12(8):876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359(9314):1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 16.Prezpiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28(3):250–259. [PubMed] [Google Scholar]
- 18.Ringdén O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113(13):3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Bone Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables (with discussion) J Royal Statist Soc, Ser B (Stat Method) 1972;34:187–220. [Google Scholar]
- 22.Klein JP. Modelling competing risks in cancer studies. Stat Med. 2006;25(6):1015–1034. doi: 10.1002/sim.2246. [DOI] [PubMed] [Google Scholar]
- 23.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1(2):145–156. doi: 10.1007/BF00985764. [DOI] [PubMed] [Google Scholar]
- 24.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16(8):1070–1075. doi: 10.1016/j.bbmt.2009.12.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollman C, Abella E, Baitty RL, et al. Assessment of optimal size and composition of the U.S. National Registry of hematopoietic stem cell donors. Transplantation. 2004;78(1):89–95. doi: 10.1097/01.tp.0000132327.40702.97. [DOI] [PubMed] [Google Scholar]
- 27.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16(11):1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballen KK, Hicks J, Dharan B, et al. Racial and ethnic composition of volunteer cord blood donors: comparison with volunteer unrelated marrow donors. Transfusion. 2002;42(10):1279–1284. doi: 10.1046/j.1537-2995.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- 29.Mielcarek M, Gooley T, Martin PJ, et al. Effects of race on survival after stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(3):231–239. doi: 10.1016/j.bbmt.2004.12.327. [DOI] [PubMed] [Google Scholar]
- 30.Baker KS, Loberiza FR, Jr, Yu H, et al. Outcome of ethnic minorities with acute or chronic leukemia treated with hematopoietic stem-cell transplantation in the United States. J Clin Oncol. 2005;23(28):7032–7042. doi: 10.1200/JCO.2005.01.7269. [DOI] [PubMed] [Google Scholar]
- 31.Lohmueller KE, Indap AR, Schmidt S, et al. Proportionally more deleterious genetic variation in European than in African populations. Nature. 2008;451(7181):994–997. doi: 10.1038/nature06611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson AM, Harrold JL, Cullup H. Haematopoietic stem cell transplantation: can our genes predict clinical outcome? Expert Rev Mol Med. 2007;9(29):1–19. doi: 10.1017/S1462399407000488. [DOI] [PubMed] [Google Scholar]