Abstract
In the opportunistic fungal pathogen Candida albicans, up-regulation of MDR1, encoding an efflux transporter, leads to increased resistance to the antifungal drug fluconazole. Antifungal resistance has been linked to several types of genetic change in C. albicans, including changes in genome structure, genetic alteration of the drug target, and overexpression of transporters. High-level over-expression of MDR1 is commonly mediated by mutation in a trans-acting factor, Mrr1p. This report describes a second mechanism that contributes to up-regulation of MDR1 expression. By analyzing the sequence of the MDR1 promoter region in fluconazole-resistant and fluconazole-susceptible strains, we identified sequence polymorphisms that defined two linkage groups, corresponding to the two alleles in the diploid genome. One of the alleles conferred higher MDR1 expression compared with the other allele. Strains in which both alleles were of the higher activity type were common in collections of clinically isolated strains while strains carrying only the less active allele were rare. As increased expression of MDR1 confers higher resistance to drugs, strains with the more active MDR1 promoter allele may grow or survive longer when exposed to drugs or other selective pressures, providing greater opportunity for mutations that confer high-level drug resistance to arise. Through this mechanism, higher activity alleles of the MDR1 promoter could promote the development of drug resistance.
Keywords: Candida albicans, Drug resistance, MDR1, Fluconazole, Polymorphism, Promoter
Introduction
The diploid yeast Candida albicans is a commensal in the human gastrointestinal tract and genitourinary tract. While the organism does not usually cause serious disease in immune-competent hosts, immunodeficient hosts are susceptible to infections ranging from superficial mucosal infections to invasive life-threatening diseases (Odds 1987; Nucci and Anaissie 2001). Fluconazole, an azole that inhibits biosynthesis of the major sterol component in the fungal membrane (Kowalsky and Dixon 1991), is commonly used for treatment of candidiasis (Pienaar et al. 2010; Chalmers and Bal 2011). However, in recent years, fungal resistance to fluconazole has emerged as a problem (White et al. 2002; Denning and Hope 2010). Antifungal resistance is often conferred by mutation of the gene encoding the target of fluconazole, lanosterol 14-[alpha]-demethylase, or by up-regulation of efflux transporters such as Mdr1p, Cdr1p, or Cdr2p (recently reviewed in Akins 2005; Sanglard et al. 2009; Morschhauser 2010). A number of molecular mechanisms that allow high-level expression of transporters have been described including genetic rearrangements or mutation of trans-acting factors (Sanglard et al. 2009; Morschhauser 2010). The studies described in this report focus on the contributions of the MDR1 promoter region to the expression of MDR1 in fluconazole-susceptible and fluconazole-resistant strains of C. albicans.
Mdr1p is a major facilitator superfamily transporter and transports drugs and other compounds across the fungal plasma membrane (Fling et al. 1991; Pinjon et al. 2005). CDR1 and CDR2 encode drug-transporting ABC transporters (Sanglard et al. 1997; Krishnamurthy et al. 1998) that are overexpressed in some azole-resistant isolates (Akins 2005; Sanglard et al. 2009; Morschhauser 2010). Mutations that result in overexpression of the transporters due to alteration of transcriptional regulators such as Tac1p (for CDR; Coste et al. 2004, 2006) or Mrr1p (for MDR1; Morschhauser et al. 2007; Dunkel et al. 2008; Schubert et al. 2011) promote drug resistance. Deletion of the MDR1 gene in drug-resistant, Mdr1p over-expressing strains compromises drug resistance (Wirsching et al. 2000b).
Alterations in chromosome structure represent another important mechanism that contributes to the evolution of drug resistance. Aneuploidies such as the presence of an isochromosome are common in fluconazole-resistant strains (Selmecki et al. 2008, 2009). In a well-characterized example, an isochromosome composed of a centromere flanked by two copies of the left arm of chromosome 5 carries the gene encoding lanosterol 14-[alpha]-demethylase (ERG11) and TAC1 (Selmecki et al. 2008). The presence of the isochromosome increases the copy number of these important genes.
Fluconazole-susceptible, WT C. albicans isolates show very low levels of MDR1 gene expression when grown in laboratory conditions; however, expression can be increased by treatment of cells with some toxic agents (Gupta et al. 1998; Harry et al. 2005; Rognon et al. 2006). On the other hand, many C. albicans fluconazole-resistant isolates constitutively express MDR1 at high levels (Franz et al. 1998; Lopez-Ribot et al. 1998; Lyons and White 2000; Wirsching et al. 2000a; Hiller et al. 2006; Riggle and Kumamoto 2006; Morschhauser et al. 2007). MDR1 expression is controlled by a transcription factor of the zinc cluster family, termed Mrr1p (multi-drug-resistance regulator) (Morschhauser et al. 2007). Other transcription factors such as Cap1p and Mcm1p have also been implicated in the control of MDR1 expression (Schubert et al. 2011). Cap1p and Mcm1p both bind to sites within the MDR1 promoter (Harry et al. 2005; Riggle and Kumamoto 2006; Rognon et al. 2006).
C. albicans is a diploid organism that reproduces mainly by clonal propagation. However, it is capable of true mating and karyogamy between strains of opposite mating type (Hull et al. 2000; Magee and Magee 2000; Lockhart et al. 2003). Clinically isolated strains of C. albicans exhibit a significant degree of natural heterozygosity (Whelan et al. 1980; Whelan and Magee 1981). In some cases, one allele is functional, while the other carries a recessive mutation (Whelan et al. 1980; Whelan and Magee 1981; Defever et al. 1982; Whelan and Soll 1982; Gomez-Raja et al. 2008). In the MDR1 gene, polymorphisms within the promoter region and in the open reading frame (ORF) have been found (Gupta et al. 1998; Wirsching et al. 2000a). Mutations in the ORF of the MDR1 gene were associated with enhanced induction by benomyl, methotrexate, and several other unrelated drugs (Gupta et al. 1998). However, high-level MDR1 gene over-expression in fluconazole-resistant strains is associated with trans-activating mutations rather than cis-acting mutations (Wirsching et al. 2000a; Riggle and Kumamoto 2006).
In this communication, we studied polymorphisms in the MDR1 promoter region that are present in numerous C. albicans clinical isolates. The results showed that some of the polymorphisms defined two linkage groups. Alleles from one of the linkage groups showed higher MDR1 expression compared with alleles from the other group. Strains carrying only the allele with higher promoter activity were significantly more common in collections of clinically isolated strains in comparison with strains carrying only the less active allele. These findings and the results of previous studies showing homozygosis of point mutations in ERG11 (White 1997), MRR1 (Dunkel et al. 2008), and TAC1 (Coste et al. 2006) show that homozygosis is a common mechanism for genetic change in C. albicans organisms that are associated with humans.
Materials and methods
Strains
Genotypes of all strains and fluconazole MICs (Minimal Inhibitory Concentration) are listed in Table S1 and Table 1. The clinical strains used in this study were collected from blood and oral mucosa specimens submitted to the clinical microbiology laboratories of Tufts Medical Center, or Beth Israel Deaconess Medical Center, Boston, MA, USA (courtesy of Susan Hadley, MD) or University of Alabama Medical Center, Birmingham, AL, USA (courtesy of John Baddley, MD). The strains were subcultured at the Clinical Microbiology Laboratory, Tufts-New England Medical Center onto Sabouraud’s Dextrose agar plates and stored in vials of sterile skimmed milk at −70°C. Five clinically isolated strains were kind gifts of Dr. Michael Pfaller, M.D. and Richard Hollis (Molecular Epidemiology & Fungus Lab Pathology Department University of Iowa Hospitals & Clinics) and were isolated before fluconazole was in widespread clinical use.
Table 1.
Summary of strains
| Name | Alleles | O/E genea | MIC (mg/L) | Number/total |
|---|---|---|---|---|
| Strain Group 1 Clinical isolates, pre-1990, Fluconazole susceptible | ||||
| 978-14, 979-15, 979-33 | A/A | NA | <0.25 | 3/5 |
| 978-28 | A/G | NA | <0.25 | 1/5 |
| 979-05 | G/G | NA | <0.25 | 1/5 |
| Strain Group 2 Strains used in laboratories, Fluconazole susceptible | ||||
| SC5314, WO-1, SGY-243, 981, ATCC10261 | A/G | NA | <0.25 | 5/5 |
| Strain Group 3 Clinical isolates, Boston, MA, Fluconazole susceptible | ||||
| CI 072, CI 085, CI 159, CI 200, CI 210, CI 234, CI 237, CI 245, CI 247, CI 260, CI 285, CI 895, CI 898 | A/A | NA | <0.25 | 13/27 |
| CI 015, CI 050, CI 091, CI 105, CI 108, CI 116, CI 165, CI 183, CI 220, CI, 259, CI 267 | A/G | NA | <0.25 | 11/27 |
| CI 097, CI 138, CI 221 | G/G | NA | <0.25 | 3/27 |
| Strain Group 4 Clinical isolates, Boston, MA, Fluconazole resistant | ||||
| CI 158 | A/A | MDR1 | >128 | 11/22 |
| CI 122, CI 190 | A/A | CDR | >128 | |
| CI 113, CI 166, CI 167, CI 168, CI 177, CI 185, CI 199, CI 202 | A/A | unknown | >128 | 8/22 |
| CI 124 | A/G | CDR | >128 | |
| CI 125, CI 126, CI 128, CI 138, CI 172, CI 176, CI 189 | A/G | unknown | >128 | |
| CI 127, CI 187, CI 196 | G/G | unknown | >128 | 3/22 |
| Strain group 5 clinical isolates, Birmingham, AL, Fluconazole resistant | ||||
| AL 864, AL 868 | A/A | CDR | >128 | 10/11 |
| SH 855, AL 860, AL 861, AL 862, AL 863, AL 865, AL 866, AL 867 | A/A | unknown | >128 | |
| AL 859 | A/G | unknown | >128 | 1/11 |
NA not applicable
Overexpressed gene
Growth media
Yeast extract/peptone/dextrose (YPD) medium, synthetic defined (SD) medium, and complete medium (CM) were as described previously (Rose et al. 1990). RPMI medium 1,640/20 mM MOPS (pH 7.0) was used for MIC determination (Barchiesi et al. 1994). For culture of Ura− strains, uridine was added to 60 μg ml−1. Fluconazole was added to 8 μg ml−1 final concentration.
MIC determination
Susceptibility to fluconazole was analyzed using the standard CLSI (formerly NCCLS) microdilution protocol M27-A (Barchiesi et al. 1994). Briefly, cells at 1 ×103 to 5 × 103 cells/mL were incubated in increasing concentrations of fluconazole (0.0635–128 μg ml−1) in a 96-well plate. The plate was incubated for 46–50 h at 35°C without agitation. The MIC was determined by a lack of growth in the well at certain fluconazole concentrations. For some strains, fluconazole resistance was also confirmed by the semi-solid agar antifungal susceptibility (SAAS) screening method (Provine and Hadley 2000).
DNA analysis
PCR, restriction digestion and gel electrophoresis were performed by standard methods as described previously (Riggle and Kumamoto 2006; Bruzual et al. 2007). Automated DNA sequencing was performed by Michael Berne and coworkers at the Tufts University Core Facility.
Cloning and sequence analysis of the MDR1 promoter region
The MDR1 promoter region of each strain was amplified by PCR from genomic DNA prepared as previously described (Riggle and Kumamoto 2006). The product of each PCR reaction was cloned into the pCR2.1-TOPO vector (TOPO TA Cloning Kit, Invitrogene). For each strain, 5–10 independent plasmid clones containing the PCR-amplified MDR1 upstream region were sequenced to obtain the sequence of both MDR1 alleles and to exclude PCR artifacts.
Screening alleles by AseI digestion and Southern blotting
Upstream of the MDR1 ORF there is an AseI restriction site. In addition, in one allele of the MDR1 promoter, there is another AseI restriction site which includes one of the polymorphic residues. The extra AseI restriction site present in one allele makes it possible to identify the two alleles by Southern blotting or by digestion of PCR products.
Methods for chromosomal DNA isolation and Southern blot hybridization were performed by standard methods as described previously (Riggle and Kumamoto 2006). Genomic DNA was digested with AseI (New England Biolabs). DNA probes were labeled with the Prime-It II Random Primer Labeling Kit from Stratagene and [α-32P]-dATP (New England Nuclear). PCR products from the 1100-bp MDR1 gene promoter region (Riggle and Kumamoto 2006) were used for probing Southern blots.
Measurement of gene expression by quantitative real-time reverse transcriptase PCR (qRT-PCR)
The 1,100-bp promoter regions from several clinical isolates were cloned upstream of yEGFP in the vector pLIB1 (Riggle and Kumamoto 2006) and transformed into the Ura− C. albicans strain CAPR514 (Riggle and Kumamoto 2006), a fluconazole-resistant, MDR1 over-expressing strain. After growth of the cells in medium containing fluconazole (8 μg ml−1), total RNA was extracted from these strains by mechanical disruption using glass beads and a Mini BeadBeater (BioSpecs Products, Inc., Bartlesville, OK, USA) and the RNeasy Mini Kit (Qiagen Inc., Chatsworth, CA, USA) according to the manufacturers’ protocols. Strains from Strain group 1 were grown as above and extracted in the same way.
qRT-PCR was performed by standard methods as described previously (White et al. 2007). Briefly, 10 μg of total RNA was converted to cDNA by incubation with Superscript II Reverse Transcriptase (Invitrogen) using an oligo dT primer. After incubation for 1 h at 42°C, RNA was hydrolyzed and the reaction was stopped by addition of NaOH and EDTA to 0.16 N NaOH, 0.08 M EDTA, final concentrations. Following neutralization, cDNA was purified using Qiaquick columns (Qiagen) as described by the manufacturer, except that sodium acetate (pH 5.2) was added to the PB buffer to ensure an acidic pH. cDNA was quantitated by absorbance. Purified cDNA was stored frozen.
The following primer pairs were used to detect the indicated genes: YEGFP primers F: CTCCAATTGGTGAT GGTCCAGTCT and R: ACC ATGGGTAATACCAGCAG CAGT; for MDR1 primers F: TCTCGGTGGATTCTTTGC TAAT and R: AATGGACCAAAACTAGGACCAC and for ACT1 primers F: TATCATGGGTTGGTATGGG and R: TGTGGTGAACAATGGATG.
qRT-PCR was performed using SYBR green Mastermix and a Stratagene instrument, according to manufacturer’s protocols. All reactions were performed in triplicate. Melting curve analysis and/or agarose gel electrophoresis was performed following the reverse transcriptase PCR amplification to verify the presence of a single product. When RNA preparations not treated with reverse transcriptase were used as template, the primers failed to amplify products.
Results
Sequence analysis of the MDR1 promoter region
Polymorphisms in the MDR1 gene in clinically isolated strains have been previously detected (Gupta et al. 1998; Wirsching et al. 2000a). Since the promoter sequence may have been influenced by the use of fluconazole in the clinic, we first undertook an investigation of the sequence of the MDR1 promoter region in strains collected prior to 1990 when fluconazole was approved for patient treatment. Five fluconazole-naïve clinically isolated strains were kindly provided by Dr. Michael Pfaller, M.D. and Richard Hollis (Molecular Epidemiology & Fungus Lab, Pathology Department, University of Iowa Hospitals & Clinics). These strains, referred to as strain group 1 (Table 1), were highly susceptible to fluconazole (MIC of fluconazole, <0.25 μg ml−1) (Table 1).
The regulatory regions of the MDR1 gene, 1,100 bp upstream from the start codon, from these five strains were cloned as described in “Materials and methods”. This region is important for MDR1 gene overexpression, in fluconazole-resistant strains (Hiller et al. 2006; Riggle and Kumamoto 2006; Rognon et al. 2006). Multiple clones from each strain were sequenced.
Heterozygosities were detected between the two MDR1 alleles in strains 978–28, 979–15, and 979–33 (Figure S1). Strain 978–28 showed 20 differences within the 1,100-bp region between the two alleles. Strains 979–15 and 979–33 had 13 and 10 polymorphisms, respectively. The polymorphisms included single-base deletions and different single-base exchanges. For the remaining two strains (978–14 and 979–05), no differences were detected between any of the clones sequenced, suggesting that either the strains contained two identical alleles or that one of the two alleles was not amplified or cloned.
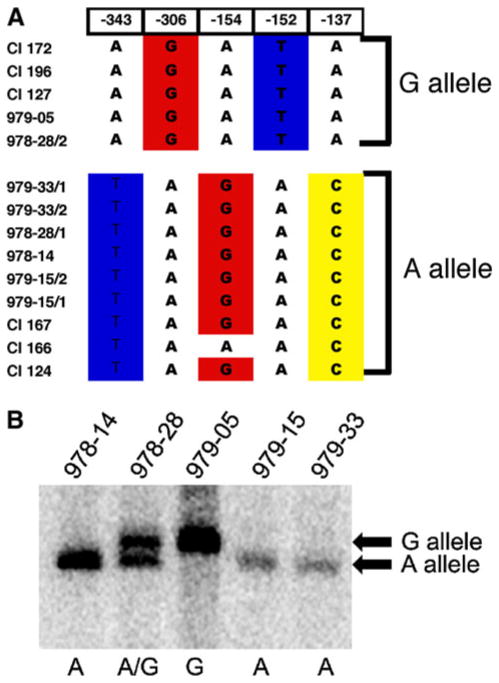
Interestingly, at positions −137, −152, −154, −306, and −343 [numbered relative to the start of the MDR1 open reading frame using the sequence from the reference strain SC5314 (Jones et al. 2004)], particular bases frequently occurred together (Fig. 1). Thus, the sequence in this region of the promoter defined two linkage groups, corresponding to two alleles of the MDR1 promoter. These polymorphisms were located near a functionally important sequence termed the MDRE (CGGTAAAATCCTAATTG GGAAAAATACCGAGAATGACACA, located at −261 to −295), which contains a binding site for Mcm1p and contributes to MDR1 expression in over-expressing strains (Riggle and Kumamoto 2006; Rognon et al. 2006). These polymorphisms thus could directly or indirectly affect promoter activity or they may be linked to other changes that influence promoter activity. Therefore, the occurrence and activity of these 2 MDR1 promoter alleles in C. albicans strains was analyzed.
Fig. 1.
Sequences of alleles of the MDR1 promoter region in several strains show polymorphisms. Panel a Strain names are given at left. For heterozygotes, the two alleles are denoted 1 and 2. The position of the residue relative to the start of the MDR1 open reading frame using the sequence from SC5314 is shown above. The sequence at each position in the allele indicated is shown. The nucleotides at these five positions were used to define two alleles of this MDR1 promoter region, termed the A allele and the G allele. Panel b Twenty micrograms of genomic DNA extracted from the indicated strains was digested with AseI and probed in a Southern blot. The mobilities of the bands produced from the G allele and A allele are indicated at right. The letters shown below indicate the alleles present in each strain
One set of polymorphisms was termed the A allele based on the polymorphism at position −306. This allele occurred in strains 978–14, 979–15, 979–33, and 978–28 (one of two alleles; Table 1). The other set of polymorphisms was termed the G allele based on the same polymorphism, and included the alleles from strain 979-05 and the second allele from 978-28. In addition to the polymorphisms noted in Fig. 1, a variety of other polymorphisms (base substitutions, additions or deletions) occurred throughout the promoter region in these strains, as shown in Figure S1. Wirsching et al. previously described several of these additional polymorphisms (Wirsching et al. 2000a). In addition, some of the polymorphisms were found in the two alleles present in the sequenced reference strain SC5314 (Jones et al. 2004), or its derivative, CAI-4 (data not shown).
The polymorphism at position −306 caused the formation of a restriction site polymorphism. When the residue A was present (as in the A allele) an AseI restriction site was created, whereas when G was present, the sequence was not a site for AseI. Therefore, the G allele yielded a large AseI fragment of 1492 bp on a Southern blot while the A allele yielded a 1,351-bp AseI fragment. This polymorphism allowed us to analyze the alleles by Southern blotting. Consistent with the results of sequencing, this analysis showed that strain 978-28 was heterozygous in this region of the promoter, strains 978-14, 978-15, and 979-33 carried only A alleles and strain 979-05 carried only G alleles (Fig. 1b).
Strains commonly used in laboratories represent another group of strains that were collected before the extensive use of fluconazole. We analyzed the alleles in a collection of five different fluconazole-susceptible strains (SC5314 (Gillum et al. 1984), WO-1 (Slutsky et al. 1985), SGY-243 (Kelly et al. 1987), ATCC10261 (Odds and Hierholzer 1973) and 981 (Goshorn and Scherer 1989) (Goshorn and Scherer 1989) (referred to as strain group 2, Table 1) and strains derived from them (Table S1). Although these laboratory strains have been cultured under non-physiological laboratory conditions and in some cases, manipulated genetically through transformation, all strains tested were heterozygous for the polymorphism at position −306. Fluconazole-resistant strains selected from SC5314-derived strains by laboratory growth in fluconazole (Riggle and Kumamoto 2006) were also heterozygotes. Thus, strains collected before fluconazole was widely used for patient treatment (strain groups 1 and 2) included 6 heterozygotes, 3 A/A strains, and 1 G/G strain; the frequency of A/A strains was 3/10.
A/A strains are more common than G/G strains in recently collected C. albicans clinically isolated strains
To determine whether there was a difference in MDR1 promoter genotypes among more recent isolates, the MDR1 promoter was studied in a collection of strains isolated from patients in two different cities. Twenty-seven clinically isolated, fluconazole-susceptible strains (strain group 3, Table 1) and 22 highly resistant strains (fluconazole MIC >128 mg/L; strain group 4, Table 1) from Tufts Medical Center (formerly New England Medical Center; Boston, MA, USA) or Beth Israel Deaconess Medical Center (Boston, MA, USA) were kindly provided by Dr. Susan Hadley. An additional 11 fluconazole-resistant strains (strain group 5, Table 1) were isolated at the University of Alabama Medical Center (Birmingham, AL, USA) and were kindly provided by Dr. John Baddley and Dr. Susan Hadley.
Genomic DNA was isolated from each strain and characterized by Southern blotting as described in Materials and methods. The 1,100-bp PCR amplified MDR1 promoter region from laboratory strain CAI4 was used as probe. Results showed that among the fluconazole-susceptible strains from group 3, 11 were heterozygous in this region of the MDR1 promoter, yielding both a 1.5 and 1.3 Kb band (Fig. 2a). Thirteen isolates were A/A strains and three isolates were G/G strains (Table 1).
Fig. 2.
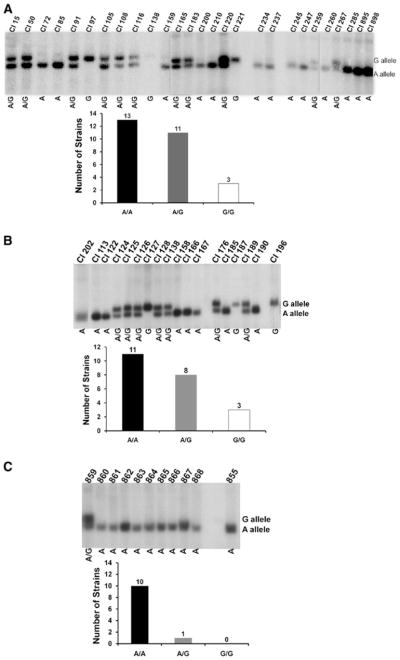
Southern blot analysis of genomic DNA from clinically isolated strains of C. albicans. Panel a The gel at the top shows 20 μg of genomic DNA extracted from the indicated fluconazole-susceptible strains (strain group 3), digested with AseI and probed in a Southern blot. The mobilities of the bands produced from the G allele and A allele are indicated at right. The letters shown below indicate the alleles present in each strain. The graph shows the numbers of strains of each genotype. Panel b The same analysis for fluconazole-resistant strains isolated in Boston, MA, USA (strain group 4). Panel c The same analysis for fluconazole-resistant strains isolated at Alabama Medical Center (strain group 5)
The isolates that were highly resistant to fluconazole were screened by the same methods (Fig. 2b). From the 22 isolates from Boston (strain group 4), 8 were heterozygous, 11 were A/A strains, and 3 were G/G strains. The 11 fluconazole-resistant clinically isolated strains from the University of Alabama Medical Center (strain group 5) contained 10 isolates that were A/A strains and one heterozygous strain (Fig. 2c). In this group of isolates, we did not identify any G/G strains.
Thus, among recently collected clinical isolates (strain groups 3, 4 and 5), the frequency of A/A strains was 34 out of 60 (Fig. 3). Among the strains collected recently in Boston, MA, fluconazole-susceptible (strain group 3) and fluconazole-resistant strains (strain group 4) gave similar frequencies of heterozygotes and A/A strains. The proportion of A/A strains among the recent isolates (34/60) (strain groups 3, 4 and 5) was not statistically significantly different from the proportion seen in older isolates (3/10) (strain groups 1 and 2; p <0.18, Fisher’s exact test). However, there was a difference between strains that have been propagated under laboratory conditions (5 out of 5 heterozygotes; strain group 2) and the clinically isolated strains (37 A/A strains out of 64 isolates; strain groups 1, 3, 4 and 5) (p <0.02, Fisher’s exact test). Although strains used in the laboratory were originally isolated from clinical samples, at least some of these strains were collected many years ago when environmental exposures to compounds and clinical practices were different. In addition, laboratory strains are commonly stored under conditions intended to minimize genetic changes (e.g. frozen), unlike strains in nature which have the opportunity to evolve. These facts may explain why all of these strains were A/G heterozygotes.
Fig. 3.
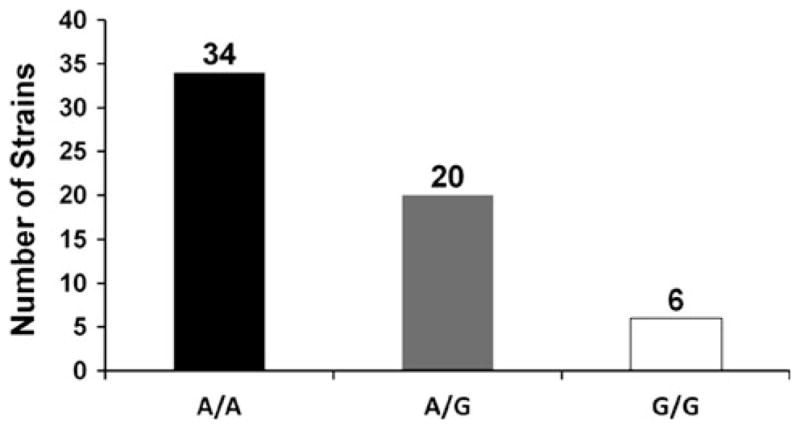
Summary of all recently isolated clinical strains studied. The graph includes fluconazole-susceptible and -resistant strains isolated in Boston, MA, USA or at Alabama Medical Center (strain group 3, 4 and 5)
Among all of the homozygous strains, there were significantly more A/A strains (37 out of 44 total) than G/G strains (7 out of 44 total) (p <0.0001, sign test). This observation suggests that, in some settings, the A/A genotype provides a selective advantage relative to the G/G genotype.
Functional differences in MDR1 gene expression from A alleles and G alleles
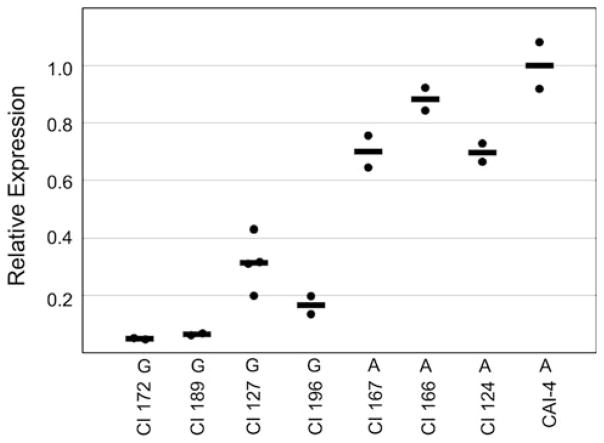
To determine whether A alleles and G alleles differed in expression of the MDR1 gene, we analyzed MDR1 expression in the fluconazole-susceptible strains isolated before fluconazole was widely used. Strains 978-14, 979-15, and 979-33 (A/A strains), and 979-05 (G/G strain) were analyzed. MDR1 expression in fluconazole-susceptible strains is very low, and we detected basal MDR1 gene expression using quantitative real-time reverse transcriptase PCR (qRT-PCR). For the analysis, RNA was prepared from C. albicans cells cultured in the presence of fluconazole (8 μg ml−1). cDNA prepared from the RNA was used as the template for qRT-PCR amplification, as described in Materials and Methods. Results were normalized using C. albicans ACT1 (encoding actin) and are shown relative to expression in a reference strain, the laboratory strain CAI-4.
As shown in Fig. 4, strain 978-14 (A/A strain) showed more than tenfold higher levels of MDR1 expression compared with strain 979-05 (G/G strain). The other two strains, 979-15 and 979-33 (A/A strains), showed more than fivefold higher levels of MDR1 expression in comparison with 979-05 (G/G strain). These results showed that strains carrying the A allele expressed higher levels of MDR1 transcript than the strain carrying the G allele. Expression in a drug-resistant A/G strain, CAPR514A, that overproduces Mdr1p is shown for comparison.
Fig. 4.
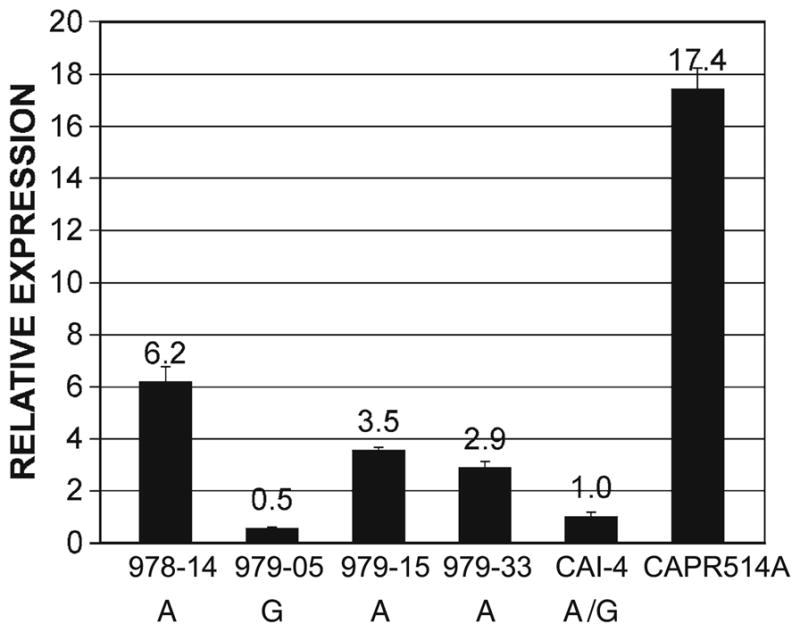
Higher levels of MDR1 transcript in A allele homozygotes. Expression of the MDR1 gene in strains indicated at bottom was analyzed by qRT-PCR as described in “Materials and methods”. Results were normalized using actin expression and are expressed relative to expression in the laboratory reference strain CAI-4, grown in the presence of fluconazole. The average of results from three experiments, each run in triplicate, is shown, with the standard deviation. Strains 978-14, 979-05, 979-15, 979-33, and CAI-4 are fluconazole susceptible (MIC < 0.25 μg ml−1). Alleles present in these strains are shown. Strain CAPR514A is a fluconazole-resistant, MDR1-overexpressing A/G strain (MIC >64 μg ml−1), shown for comparison
To eliminate other differences in the strain background of various clinical strains, the promoter regions of the alleles carried in several strain group 4 strains including G alleles from CI 127, 172, 189, and 196 and A alleles from CI 124, 166, and 167, were cloned upstream of the promoterless yEGFP gene in the vector pLIB1 (Riggle and Kumamoto 2006). The sequences of several of these MDR1 promoter alleles were also determined and showed that the alleles belonged to either the A or G allele group (Fig. 1 and Fig. S1). The PMDR1–yEGFP fusions were integrated onto the chromosome of the drug-resistant, MDR1-over-expressing laboratory strain CAPR514 (Riggle and Kumamoto 2006). Under these conditions, GFP fluorescence in the strains carrying either the A or G allele was high (data not shown), presumably due to the trans-activating MRR1 mutation (Morschhauser et al. 2007; Dunkel et al. 2008; Schubert et al. 2008). In order to quantify expression from the MDR1 promoters, RNA was isolated from cells grown in fluconazole (8 μg ml−1) and yEGFP expression was measured by quantitative real-time RT PCR (Fig. 5). ACT1 was used to normalize expression. Results showed that strains carrying A alleles expressed higher levels of the reporter gene yEGFP than strains carrying G alleles (p <0.001, t test). The average expression of A alleles was fivefold higher than the average expression of G alleles. Thus, in an isogenic strain background, A alleles were more active in promoting expression from the MDR1 promoter than G alleles.
Fig. 5.
Expression of yEGFP from A or G alleles of the MDR1 promoter. CAPR514-derived cells carrying fusions of the yEGFP reporter gene under control of MDR1 promoter A or G alleles from the indicated strains were grown in the presence of fluconazole and RNA was isolated from the cells. Expression of the yEGFP gene was analyzed by qRT-PCR as described in “Materials and methods”. Results were normalized using actin expression and are expressed relative to expression of yEGFP from an A allele from reference strain CAI-4. Each filled circle shows the average of a measurement performed in triplicate. Black bar indicates the mean of the two or four measurements
Discussion
With the approval of fluconazole by the FDA in 1990, the drug became the most popular antifungal to treat candidiasis. The extensive use of fluconazole was mainly due to its effectiveness and low toxicity (Graybill 1989; Larsen 1990). Recently, widespread use of fluconazole in candidiasis patients has led to a higher frequency of treatment failures due to drug resistance (Ruhnke et al. 1994; Chakrabarti et al. 2009). Because of this observation, there has been strong interest in understanding how drug resistance in C. albicans evolves. Our results show that recently isolated clinical strains commonly carry a more active allele of the MDR1 promoter, often on both homologues. Because two copies of the higher activity MDR1 promoter allele would result in higher basal expression of MDR1 due to increased gene dosage, we propose as a model that higher basal expression of Mdr1p allows strains to grow or survive longer in the presence of fluconazole or another compound. As a result, spontaneous, advantageous mutations have a greater opportunity to arise in a strain carrying higher activity MDR1 promoter alleles. Over evolutionary time, the more active MDR1 promoter alleles thus contribute to the evolution of drug resistance.
Fluconazole resistance in clinical isolates of C. albicans has been associated with a combination of several distinct mechanisms (White 1997; Franz et al. 1998; Lopez-Ribot et al. 1999; Morschhauser 2002, 2010; Anderson 2005; Sanglard et al. 2009). The order of the events that gives rise to a drug-resistant phenotype does not seem to be fixed. Rather, C. albicans cells can generate a partially resistant phenotype through different mechanisms, and the partially resistant mutant cells then overgrow the more susceptible cells in the population in the presence of drug (White 1997; Franz et al. 1998; Anderson 2005).
The results described in this communication add to the growing literature demonstrating the importance of alterations in genome structure during the evolution of drug resistance. Selmecki et al. showed that growth in the presence of an antifungal drug led to selection for a specific isochromosome that increased the copy number of EFG11 and TAC1 (Selmecki et al. 2009). Other chromosomal rearrangements were also noted during the evolutionary process. However, A/A strains were not isolated from A/G strains during laboratory selection of fluconazole-resistant strains or following passage through a mouse (data not shown). The length of a laboratory selection experiment may be insufficient for selection of A/A strains or the relevant selective pressure may not be replicated in a laboratory experiment.
The observation that several of the fluconazole-naive strains collected prior to the widespread use of fluconazole were A/A strains shows that the selective pressure leading to the high frequency of A/A strains was not related to fluconazole use. Older antifungals such as ketoconazole or itraconazole are unlikely sources of selection pressure because increased expression of MDR1 does not increase the MIC for these drugs (White 1997; Franz et al. 1998; Lopez-Ribot et al. 1999; Wirsching et al. 2000b; Harry et al. 2005; Cheng et al. 2007). There may be natural compounds produced by a host or therapeutic compounds other than azoles that require efflux by Mdr1p for detoxification. Exposure of C. albicans to such compounds during host colonization may have provided the selective pressure that resulted in the frequent occurrence of A/A strains. In addition, some compounds, such as the antibacterial drug rifampicin, induce C. albicans MDR1 expression (Vogel et al. 2008). Exposure to such a drug could amplify the effect of the A allele because higher MDR1 expression could give an A/A strain a greater advantage over a G/G strain. Since antibacterial drugs have been in use far longer than fluconazole, the use of such compounds may have increased the effect of the selection pressure that favored A/A strains as opposed to G/G strains.
Our results show that in fluconazole-susceptible and fluconazole-resistant strain backgrounds, the A alleles had increased promoter activity relative to the G alleles. In fluconazole-susceptible strains, MDR1 expression was much lower than the expression in a strain carrying a trans-activating MRR1 mutation such as strain CAPR514. Nevertheless, differential expression between A and G alleles was observed under these basal expression conditions. In an MRR1 mutant strain, expression from the MDR1 promoter was much higher. When clones carrying an A allele or a G allele were introduced into this mutant background, a difference in the level of expression was also observed. Therefore, the A allele leads to higher MDR1 expression in strains carrying either a WT or mutant allele of MRR1. Increased MDR1 expression, however, is not sufficient to produce a measurable increase in fluconazole MIC in an A/A strain relative to a G/G strain.
Differences in the promoter activity of two homologs have been observed previously in C. albicans. Staib and coworkers (Staib et al. 2002) showed that an increase or a reduction in the copy number of pentameric repeats (R1 and/or R2) in the promoter of the SAP2 gene affected expression from the promoter within infected organs but not during laboratory culture. The SAP2-2 promoter allele, containing five copies of R1 and five copies of R2, was induced more easily than the SAP2-1 promoter, which contains four copies of R1 and six copies of R2. In the CHS7 promoter, polymorphisms are also associated with differences in promoter activity (Sanz et al. 2007).
The five residues that segregated together and defined the A and G alleles (−137, −152, −154, −306, and −343) lie on either side of an element in the promoter termed the MDRE, composed of bases −261 to −295. This region is important for expression from the MDR1 promoter in an MRR1 mutant strain (Riggle and Kumamoto 2006). Therefore, it is possible that the particular bases that make up either the A or G alleles play a direct role in determining the activity of the promoter. Alternatively, these bases may be linked to other bases that play a direct functional role. As there are many polymorphisms in the MDR1 promoter region, the precise residues that confer the differences in activity of the A and G alleles are currently unknown. Given the intricate regulation of MDR1 expression with several trans-acting factors that bind to the MDR1 promoter and many regions that are important for expression (Harry et al. 2005; Hiller et al. 2006; Riggle and Kumamoto 2006; Rognon et al. 2006; Morschhauser et al. 2007; Mogavero et al. 2011), it is likely that the differences in activity will involve multiple, co-segregating residues.
Supplementary Material
Acknowledgments
We thank Dr. Susan Hadley, Dr. John Baddley and Dr. Michael Pfaller for the kind gifts of strains. We are also grateful to Dr. Perry Riggle, Dr. Paul Lephart and other members of the Kumamoto laboratory for helpful discussion and to Enrique Durand, Ivana Petrovska, Brian Meehan and Marcelo Vinces for careful review of the manuscript. This research was supported by grant AI 052805 (to C.A.K.) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00438-011-0650-z) contains supplementary material, which is available to authorized users.
References
- Akins RA. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med Mycol. 2005;43(4):285–318. doi: 10.1080/13693780500138971. [DOI] [PubMed] [Google Scholar]
- Anderson JB. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat Rev Microbiol. 2005;3(7):547–556. doi: 10.1038/nrmicro1179. [DOI] [PubMed] [Google Scholar]
- Barchiesi F, Colombo AL, McGough DA, Rinaldi MG. Comparative study of broth macrodilution and microdilution techniques for in vitro antifungal susceptibility testing of yeasts by using the National Committee for Clinical Laboratory Standards’ proposed standard. J Clin Microbiol. 1994;32(10):2494–2500. doi: 10.1128/jcm.32.10.2494-2500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzual I, Riggle P, Hadley S, Kumamoto CA. Biofilm formation by fluconazole-resistant Candida albicans strains is inhibited by fluconazole. J Antimicrob Chemother. 2007;59(3):441–450. doi: 10.1093/jac/dkl521. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Chatterjee SS, Rao KL, Zameer MM, Shivaprakash MR, Singhi S, Singh R, Varma SC. Recent experience with fungaemia: change in species distribution and azole resistance. Scand J Infect Dis. 2009;41(4):275–284. doi: 10.1080/00365540902777105. [DOI] [PubMed] [Google Scholar]
- Chalmers CM, Bal AM. Management of fungal infections in the intensive care unit: a survey of UK practice. Br J Anaesth. 2011;106(6):827–831. doi: 10.1093/bja/aer089. [DOI] [PubMed] [Google Scholar]
- Cheng S, Clancy CJ, Nguyen KT, Clapp W, Nguyen MH. A Candida albicans petite mutant strain with uncoupled oxidative phosphorylation overexpresses MDR1 and has diminished susceptibility to fluconazole and voriconazole. Antimicrob Agents Chemother. 2007;51(5):1855–1858. doi: 10.1128/AAC.00182-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste AT, Karababa M, Ischer F, Bille J, Sanglard D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell. 2004;3(6):1639–1652. doi: 10.1128/EC.3.6.1639-1652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste A, Turner V, Ischer F, Morschhauser J, Forche A, Selmecki A, Berman J, Bille J, Sanglard D. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 2006;172(4):2139–2156. doi: 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defever KS, Whelan WL, Rogers AL, Beneke ES, Veselenak JM, Soll DR. Candida albicans resistance to 5-fluorocytosine: frequency of partially resistant strains among clinical isolates. Antimicrob Agents Chemother. 1982;22(5):810–815. doi: 10.1128/aac.22.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DW, Hope WW. Therapy for fungal diseases: opportunities and priorities. Trends Microbiol. 2010;18(5):195–204. doi: 10.1016/j.tim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Dunkel N, Blass J, Rogers PD, Morschhauser J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol Microbiol. 2008;69(4):827–840. doi: 10.1111/j.1365-2958.2008.06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling ME, Kopf J, Tamarkin A, Gorman JA, Smith HA, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227(2):318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- Franz R, Kelly SL, Lamb DC, Kelly DE, Ruhnke M, Morschhauser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42(12):3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(1):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Gomez-Raja J, Andaluz E, Magee B, Calderone R, Larriba G. A single SNP, G929T (Gly310Val), determines the presence of a functional and a non-functional allele of HIS4 in Candida albicans SC5314: detection of the non-functional allele in laboratory strains. Fungal Genet Biol. 2008;45(4):527–541. doi: 10.1016/j.fgb.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshorn AK, Scherer S. Genetic analysis of prototrophic natural variants of Candida albicans. Genetics. 1989;123(4):667–673. doi: 10.1093/genetics/123.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill JR. New antifungal agents. Eur J Clin Microbiol Infect Dis. 1989;8(5):402–412. doi: 10.1007/BF01964056. [DOI] [PubMed] [Google Scholar]
- Gupta V, Kohli A, Krishnamurthy S, Puri N, Aalamgeer SA, Panwar S, Prasad R. Identification of polymorphic mutant alleles of CaMDR1, a major facilitator of Candida albicans which confers multidrug resistance, and its in vitro transcriptional activation. Curr Genet. 1998;34(3):192–199. doi: 10.1007/s002940050385. [DOI] [PubMed] [Google Scholar]
- Harry JB, Oliver BG, Song JL, Silver PM, Little JT, Choiniere J, White TC. Drug-induced regulation of the MDR1 promoter in Candida albicans. Antimicrob Agents Chemother. 2005;49(7):2785–2792. doi: 10.1128/AAC.49.7.2785-2792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller D, Stahl S, Morschhauser J. Multiple cis-acting sequences mediate upregulation of the MDR1 efflux pump in a fluconazole-resistant clinical Candida albicans isolate. Antimicrob Agents Chemother. 2006;50(7):2300–2308. doi: 10.1128/AAC.00196-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289(5477):307–310. doi: 10.1126/science.289.5477.307. pii: 8669. [DOI] [PubMed] [Google Scholar]
- Jones T, Federspiel NA, Chibana H, Dungan J, Kalman S, Magee BB, Newport G, Thorstenson YR, Agabian N, Magee PT, Davis RW, Scherer S. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci USA. 2004;101(19):7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Miller SM, Kurtz MB, Kirsch DR. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987;7(1):199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalsky SF, Dixon DM. Fluconazole: a new antifungal agent. Clin Pharm. 1991;10(3):179–194. [PubMed] [Google Scholar]
- Krishnamurthy S, Gupta V, Snehlata P, Prasad R. Characterisation of human steroid hormone transport mediated by Cdr1p, a multidrug transporter of Candida albicans, belonging to the ATP binding cassette super family. FEMS Microbiol Lett. 1998;158(1):69–74. doi: 10.1111/j.1574-6968.1998.tb12802.x. pii: S0378-1097(97)00502-8. [DOI] [PubMed] [Google Scholar]
- Larsen RA. Azoles and AIDS. J Infect Dis. 1990;162(3):727–730. doi: 10.1093/infdis/162.3.727. [DOI] [PubMed] [Google Scholar]
- Lockhart SR, Daniels KJ, Zhao R, Wessels D, Soll DR. Cell biology of mating in Candida albicans. Eukaryot Cell. 2003;2(1):49–61. doi: 10.1128/EC.2.1.49-61.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ribot JL, McAtee RK, Lee LN, Kirkpatrick WR, White TC, Sanglard D, Patterson TF. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42(11):2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ribot JL, McAtee RK, Perea S, Kirkpatrick WR, Rinaldi MG, Patterson TF. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1999;43(7):1621–1630. doi: 10.1128/aac.43.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons CN, White TC. Transcriptional analyses of antifungal drug resistance in Candida albicans. Antimicrob Agents Chemother. 2000;44(9):2296–2303. doi: 10.1128/aac.44.9.2296-2303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289(5477):310–313. doi: 10.1126/science.289.5477.310. pii: 8662. [DOI] [PubMed] [Google Scholar]
- Mogavero S, Tavanti A, Senesi S, Rogers PD, Morschhauser J. Differential requirement of the transcription factor Mcm1 for activation of the Candida albicans multidrug efflux pump MDR1 by its regulators Mrr1 and Cap1. Antimicrob Agents Chemother. 2011;55(5):2061–2066. doi: 10.1128/AAC.01467-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhauser J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta. 2002;1587(2–3):240–248. doi: 10.1016/s0925-4439(02)00087-x. pii: S092544390200087X. [DOI] [PubMed] [Google Scholar]
- Morschhauser J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol. 2010;47(2):94–106. doi: 10.1016/j.fgb.2009.08.002. 10.1016/j.fgb. 2009.08.002. [DOI] [PubMed] [Google Scholar]
- Morschhauser J, Barker KS, Liu TT, Bla BWJ, Homayouni R, Rogers PD. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 2007;3(11):e164. doi: 10.1371/journal.ppat.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33(12):1959–1967. doi: 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- Odds FC. Candida infections: an overview. Crit Rev Microbiol. 1987;15(1):1–5. doi: 10.3109/10408418709104444. [DOI] [PubMed] [Google Scholar]
- Odds FC, Hierholzer JC. Purification and properties of a glycoprotein acid phosphatase from Candida albicans. J Bacteriol. 1973;114(1):257–266. doi: 10.1128/jb.114.1.257-266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar ED, Young T, Holmes H. Interventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and children. Cochrane Database Syst Rev. 2010;11:CD003940. doi: 10.1002/14651858.CD003940.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinjon E, Moran GP, Coleman DC, Sullivan DJ. Azole susceptibility and resistance in Candida dubliniensis. Biochem Soc Trans. 2005;33(Pt 5):1210–1214. doi: 10.1042/BST20051210. [DOI] [PubMed] [Google Scholar]
- Provine H, Hadley S. Preliminary evaluation of a semisolid agar antifungal susceptibility test for yeasts and molds. J Clin Microbiol. 2000;38(2):537–541. doi: 10.1128/jcm.38.2.537-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggle PJ, Kumamoto CA. Transcriptional regulation of MDR1, encoding a drug efflux determinant, in fluconazole-resistant Candida albicans strains through an Mcm1p binding site. Eukaryot Cell. 2006;5(12):1957–1968. doi: 10.1128/EC.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognon B, Kozovska Z, Coste AT, Pardini G, Sanglard D. Identification of promoter elements responsible for the regulation of MDR1 from Candida albicans, a major facilitator transporter involved in azole resistance. Microbiology. 2006;152(Pt 12):3701–3722. doi: 10.1099/mic.0.29277-0. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1990. [Google Scholar]
- Ruhnke M, Eigler A, Tennagen I, Geiseler B, Engelmann E, Trautmann M. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J Clin Microbiol. 1994;32(9):2092–2098. doi: 10.1128/jcm.32.9.2092-2098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143(Pt 2):405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- Sanglard D, Coste A, Ferrari S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 2009;9(7):1029–1050. doi: 10.1111/j.1567-1364.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- Sanz M, Valle R, Roncero C. Promoter heterozygosity at the Candida albicans CHS7 gene is translated into differential expression between alleles. FEMS Yeast Res. 2007;7(6):993–1003. doi: 10.1111/j.1567-1364.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- Schubert S, Rogers PD, Morschhauser J. Gain-of-function mutations in the transcription factor MRR1 are responsible for overexpression of the MDR1 efflux pump in fluconazole-resistant Candida dubliniensis strains. Antimicrob Agents Chemother. 2008;52(12):4274–4280. doi: 10.1128/AAC.00740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S, Barker KS, Znaidi S, Schneider S, Dierolf F, Dunkel N, Aid M, Boucher G, Rogers PD, Raymond M, Morschhauser J. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob Agents Chemother. 2011;55(5):2212–2223. doi: 10.1128/AAC.01343-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68(3):624–641. doi: 10.1111/j.1365-2958.2008.06176.x. [DOI] [PubMed] [Google Scholar]
- Selmecki AM, Dulmage K, Cowen LE, Anderson JB, Berman J. Acquisition of aneuploidy provides increased fitness during the evolution of antifungal drug resistance. PLoS Genet. 2009;5(10):e1000705. doi: 10.1371/journal.pgen.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B, Buffo J, Soll DR. High-frequency switching of colony morphology in Candida albicans. Science. 1985;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Staib P, Kretschmar M, Nichterlein T, Hof H, Morschhauser J. Host versus in vitro signals and intrastrain allelic differences in the expression of a Candida albicans virulence gene. Mol Microbiol. 2002;44(5):1351–1366. doi: 10.1046/j.1365-2958.2002.02967.x. pii: 2967. [DOI] [PubMed] [Google Scholar]
- Vogel M, Hartmann T, Koberle M, Treiber M, Autenrieth IB, Schumacher UK. Rifampicin induces MDR1 expression in Candida albicans. J Antimicrob Chemother. 2008;61(3):541–547. doi: 10.1093/jac/dkm513. [DOI] [PubMed] [Google Scholar]
- Whelan WL, Magee PT. Natural heterozygosity in Candida albicans. J Bacteriol. 1981;145(2):896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan WL, Soll DR. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol Gen Genet. 1982;187(3):477–485. doi: 10.1007/BF00332632. [DOI] [PubMed] [Google Scholar]
- Whelan WL, Partridge RM, Magee PT. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]
- White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41(7):1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46(6):1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, Whiteway M, Mecsas J, Kumamoto CA. Self-regulation of Candida albicans population size during GI colonization. PLoS Pathog. 2007;3(12):e184. doi: 10.1371/journal.ppat.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsching S, Michel S, Kohler G, Morschhauser J. Activation of the multiple drug resistance gene MDR1 in fluconazole-resistant, clinical Candida albicans strains is caused by mutations in a trans-regulatory factor. J Bacteriol. 2000a;182(2):400–404. doi: 10.1128/jb.182.2.400-404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsching S, Michel S, Morschhauser J. Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol Microbiol. 2000b;36(4):856–865. doi: 10.1046/j.1365-2958.2000.01899.x. pii: mmi1899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.