Abstract
Net-negatively-charged heterospecific A:B:C collagen peptide heterotrimers were designed using an automated computational approach. The design algorithm considers both target stability and the energy gap between the target states and misfolded competing states. Structural characterization indicates the net-negative charge balance on the new designs enhances the specificity of the target state at the expense of its stability.
Keywords: Protein design, Collagen, Triple-helix, Electrostatics
1. Introduction
Protein design has potential applications in many fields of biology from molecular recognition to cell signaling to biomaterials. The design of novel proteins tests our understanding of the fundamental principles underlying molecular structure and energetics. Computational methods are playing an increasingly important role in molecular design. Most progress in computational design has focused on globular, single-chain proteins (Dahiyat and Mayo, 1997; Kuhlman et al., 2003) and the self-assembly of oligomeric α-helical complexes (Nautiyal et al., 1995; Reinke et al., 2010). The collagen triple helix has been given relatively less attention for protein design despite being the most abundant protein in higher animals, accounting for one-third of total mass.
There are twenty-nine known collagens in humans (Heino, 2007). Collagen is characterized by extended repeats of Gly–Xxx–Yyy triplets, where Xxx and Yyy are often proline and hydroxyproline respectively. Individual collagen chains trimerize into triplehelices, which further self-assemble into higher order structures such as long fibers and mesh-like networks (Kadler et al., 1996; Ramachandran and Kartha, 1955; Rich and Crick, 1961). These higher order structures provide tensile strength and flexibility to tissues. Several natural collagens are known to associate as heterotrimers. Model peptide systems have been essential tools in exploring the molecular bases for the stability, specificity, and stoichiometry of collagen assembly. Until recently most peptide model systems have focused on the homotrimers.
A promising strategy for engineering stable heterotrimeric collagen self-assembly exploits the complimentary pairing of acid and basic amino acids, forming a network of electrostatic interactions on the surface of the triple-helix (Fallas and Hartgerink, 2012; Fallas et al., 2012b; Gauba and Hartgerink, 2007a,b; Xu et al., 2011). This approach was developed on α-helical coiled-coils to modulate helix orientation, oligomerization state and heterospecificity of assembly (Bryson et al., 1998; Burkhard et al., 2002; O’Shea et al., 1991). The complexity and selectivity of intermolecular associations has been enhanced through the use of computational methods (Havranek and Harbury, 2003; Reinke et al., 2010; Summa et al., 2002).
Collagen peptide heterotrimers have been designed by both rational and computational approaches (Fallas and Hartgerink, 2012; Fallas et al., 2012b; Gauba and Hartgerink, 2007b; Xu et al., 2011). In each of these designs, an equal number of acidic and basic triplets were maintained across the three peptides. Although a net balance of positive and negative charges maximizes the number of possible favorable charge-pair interactions, there are several reasons to pursue designs that are net cationic or anionic. A net charge on a protein will increase its stability in solution allowing it to maintain solubility at high concentrations (Lawrence et al., 2007) – an important consideration in the development of protein therapeutics (Parmar and Muschol, 2009a). It also tests the extent to which specifying a net charge constrains the stability of the collagen trimer. Computational models indicated favoring acidic or basic groups in an ABC heterotrimer would decrease the energy gap between the target and the next most stable states and lower the number of possible favorable charge pairs in the target (Nanda et al., 2011). The prediction is that deviations from a net zero charge balance might adversely impact target stability and specificity.
2. Methods
2.1. Computational design
The overall interaction energies of all 27 possible species were computed considering interactions between Y–X and Y–X′ residues (Table S1). Energy scores were tabulated based on a scoring function adapted from α-helical coiled-coil designs as previously described (Summa et al., 2002). For each proline in the X position or hydroxyproline in the Y position, −3.8 kcals/mole were added to the total energy to account for collagen backbone propensities (Xu et al., 2010). Sequences were optimized with a Simulated Evolution protocol (Hellinga and Richards, 1994). Details are described in (Xu et al., 2011). Briefly, three collagen sequences of length thirty amino acids were initially randomly constructed from: POG, PKG, KOG, PDG, and DOG. This protocol consisted of one million iterations, each of which included the mutation of a randomly selected acidic, basic, or neutral triplet to another group. Mutations were accepted if they led to the formation of a collagen with an improved stability and greater energy gap. Ten groups of three sequences were generated (see Supplemental information for sequence details).
2.2. Sample preparation
Ten mixtures, A, B, C, A:2B, 2A:B, B:2C, 2B:C, A:2C, 2A:C, and A:B:C at the total concentration of 0.2 mM in 10.0 mM pH 7.0 phosphate buffer were prepared. Peptide concentration was monitored by measuring absorbance at 214 nm using ε214 = 2200 M−1 - cm−1 per peptide bond on an AVIV Model 14DS UV–Vis Spectrophotometer.
2.3. Circular dichroism (CD)
Samples were incubated at 50 °C for 15 min, and then annealed at 4 °C for 48–72 h. Experiments were performed on an AVIV Model 400 Spectrophotometer. Optically matched 0.1 cm path length quartz cuvettes (Model 110-OS, Hellma USA) were used. Wavelength scans were conducted from 190 to 260 nm at 4 °C. Ellipticity was monitored at 223 nm. Temperatures were sampled from 0 to 50 °C at 0.33 °C/step, 2 min equilibration time. In order to calculate an apparent melting temperature, Tm, we estimated the fraction folded using
where θ(T) is the observed ellipticity and θF(T) and θU(T) are estimated ellipticities derived from linear fits to the folded and unfolded baselines. The melting temperature Tm was estimated where F(T) = 0.5.
2.4. Dynamic light scattering
Samples were filtered with Millex-GV Syringe-driven filter units with pore size 0.22 µm (Millipore). Dynamic light scattering (DLS) measurements were carried out at 0 °C using a Zetasizer Nano ZS (Malvern Instruments, UK). Data was collected using a 3mW He-Ne laser light at a 633 nm wavelength back scattered light at an angle of 173°. Autocorrelation functions were determined from the average of 3 correlation functions, with an acquisition time of 2 min per correlation function. Reported viscosity values (Parmar and Muschol, 2009b) were used for the hydrodynamic radius calculation. Refer previous study (Parmar and Muschol, 2009a) for a detailed description of DLS.
3. Results and discussion
3.1. Sequence design
Three peptides can form a potential of twenty-seven triple-helical structures. The triple-helix is stabilized primarily by backbone-backbone hydrogen bonds, which are not modulated directly by sequence changes. Therefore, stability is controlled by incorporating networks of surface charge-pair interactions along the length of the triple-helix (Chan et al., 1997; Persikov et al., 2002, 2005; Yang et al., 1997). In order to form one species, ABC, instead of the twenty-six competing states, we had previously implemented a computational scheme that maximized the charge-pair network for the target while intentionally introducing destabilizing networks into competing states (Xu et al., 2011). The result is an energy gap, which is calculated as the difference in stability between the target and best stability for one of the competing states. We used this design scheme to create an ABC-type heterotrimer, which formed a stable triple-helix only when all three peptides were mixed in an equimolar 1:1:1 stoichiometry. This design was one of ten, which showed both optimal target stability and a large predicted energy gap. Despite the inclusion of a gap term, B + C mixtures of this design formed weak triple-helices which had marginal stabilities under low ionic strength conditions.
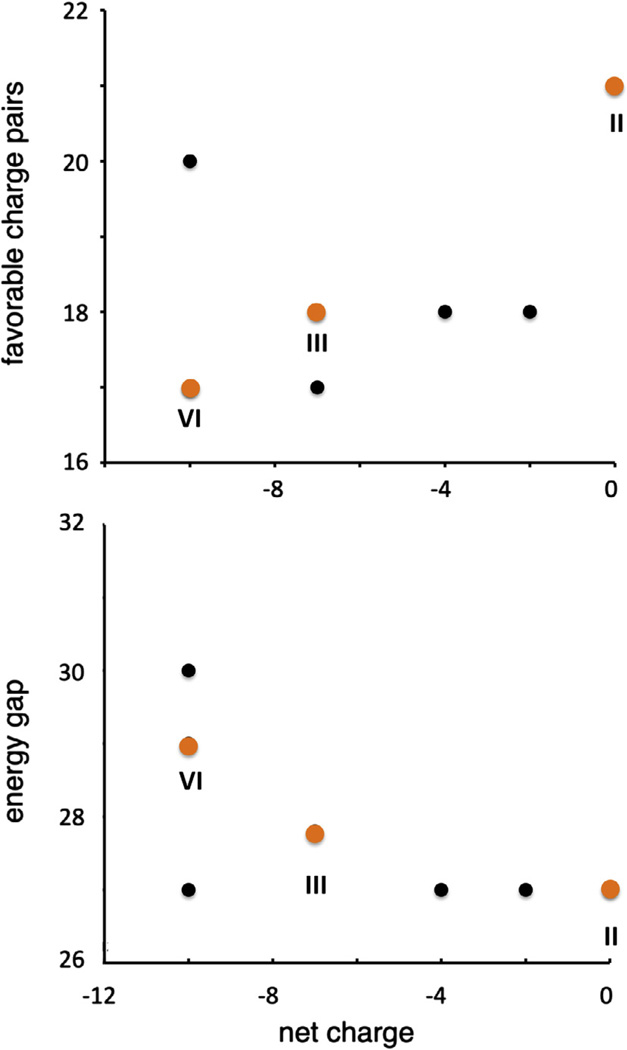
An examination of the ten designs shows that all meet the criteria of high computed target stability and energy gap, despite notable variation in the total amino acid composition (Table 1, sequences in Supplemental information). Candidate II was the originally studied system. II included fifteen lysines and fifteen aspartates, resulting in a net formal charge of zero. Equal numbers of acidic and basic residues allows the program to maximize the number of predicted charge pair interactions in the target state. Other designs showed a bias toward acidic amino acid compositions, ranging from a net charge of −2 to −10. While a net charge reduces the capacity of the designs to develop optimal charge pair networks in the target state, it allows for a greater energy gap by favoring charge-charge repulsions in competing states (Nanda et al., 2011) (Fig. 1). The charge asymmetry over the ten designs (i.e. no occurrence of net-basic systems) was due to the larger penalty originally assigned to repulsive interactions between acidic amino acids. A −/− interaction was scored +3, while a +/+ interaction was scored +2. This assumption was heuristically based on the longer and more flexible side chains of basic amino acids lysine and arginine over acidic amino acids aspartate and glutamate (Summa et al., 2002), allowing basic amino acids to more easily resolve repulsive electrostatic forces through side chain motions rather than main chain unfolding.
Table 1.
Experimentally determined melting temperature (Tm), and computationally predicted Energy gap, Energy score, Net charge, No. of favorable and unfavorable interactions for candidates II, III, and VI.
| Candidate | Tm (°C) | Energy gap | Net charge | No. of favorable interactions in ABC |
|---|---|---|---|---|
| II | 29 | −27 | 0 | 21 |
| III | 19 | −27.8 | −7 | 18 |
| VI | 18.5 | −29 | −10 | 17 |
Fig. 1.
Computed properties of the ten ABC heterotrimer designs. Favorable charge pairs are defined as Lys-Asp interactions at Y–X or Y–X′ positions in adjacent chains. The energy gap is computed using the collagen scoring function (Xu et al., 2011) to estimate the difference between ABC and the most stable competing binary composition heterotrimer or homotrimer. Experimentally synthesized and characterized ABC heterotrimers designs are shown in orange whereas others are in black.
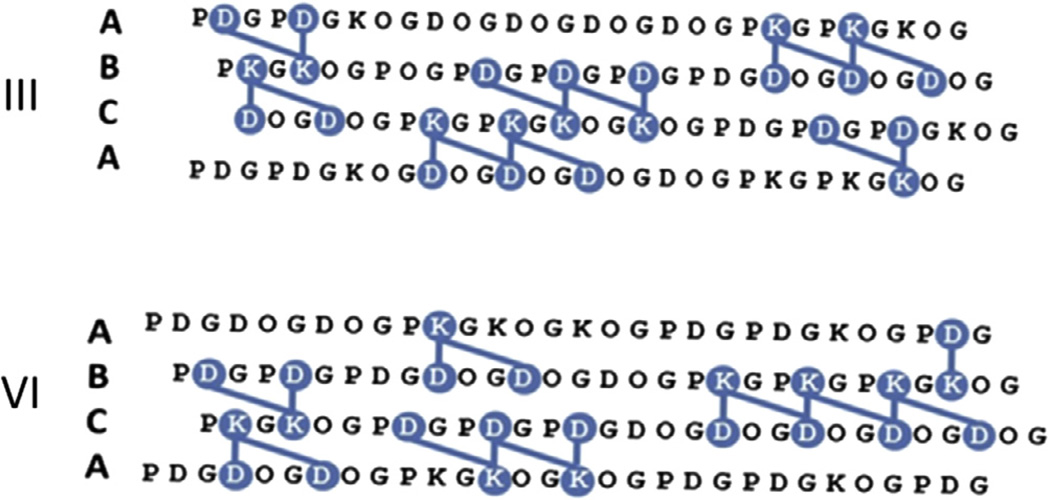
There is evidence that long-range electrostatics can influence the stability of both globular proteins (Lee et al., 2002) and collagen mimetic peptides (Giddu et al., 2013). These forces are not included in the original scoring function. Therefore, we revisited the original ten designs and chose two with significant net charges to assess their stability and heterotrimer specificity. Candidates III and VI had net charges of −7 and −10 respectively (Fig. 2). Both III and VI had fewer charge-pair interactions than II (Xu et al., 2011) and thus were predicted to have lower stabilities (Fig. 2). The computed energy gaps were similar in magnitude for all three species. As such, it would be expected that heterospecifity would be maintained. Candidate III also contained one POG triplet, a motif that significantly stabilizes the collagen triple helix (Engel et al., 1977).
Fig. 2.
Sequences and modeled charge pair interactions between Y–X (vertical lines) and Y–X′ (diagonal lines) positions in adjacent chains for candidates III and VI.
3.2. Experimental characterization
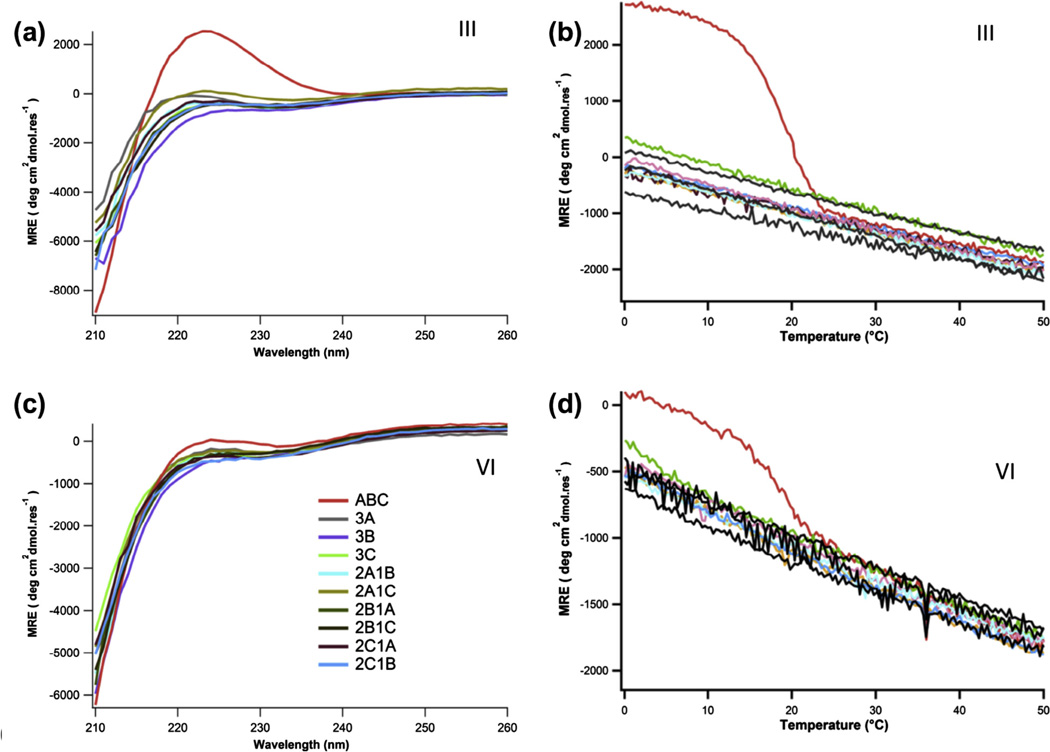
For each candidate, ten combinations of the peptides (A, B, C, A:2B, 2A:B, A:2C, 2A:C, B:2C, 2B:C, and A:B:C) were studied for triple- helix structure and thermal stability by CD spectroscopy as previously described (Xu et al., 2011). A:B:C formed a stable triple helix for both candidates III and VI (Fig. 3). The experimental stabilities were nearly identical, with a Tm of 19 °C and 18.5 °C for A:B:C mixtures of III and VI respectively. Compared to candidate II (Xu et al., 2011), III and VI exhibited lower mean residue ellipticities and melting temperature (Table 1, Fig. 3). An increase in net charge and a reduction in the number of predicted favorable interactions correlates with decreased stability and lower mean residue ellipticity of A:B:C heterotrimer. To confirm that favorable charge pair interactions promoted assembly of A:B:C, structure and stability of the peptides were measured under the same buffer conditions with the addition of 150 mM NaCl. Under these conditions, stability and structure of Candidate III was reduced (Figure S1). For candidate VI, the marginal secondary structure and cooperative thermal denaturation was completely lost upon increasing the ionic strength. Aggregation was not observed for any of the species as assessed by Dynamic Light Scattering (Figure S2). Only species with a hydrodynamic radius of around 2 nm consistent with a folded triple helix was observed (Kar et al., 2006).
Fig. 3.
Structure and stability characterization of candidate III (a,b) and VI (c,d) by circular dichroism spectroscopy.
The reduced stabilities of III and VI relative to II are expected based on the computed number of charge pair interactions for the ABC target. It is challenging to quantitatively relate the computed and experimental stabilities of these three designs based on circular dichroism data alone, as it is not possible to specify whether the observed species are the target ABC, or competing BCA, CAB, ACB, BAC and CBA, or some mixture of several of these. However, given the discrete nature of the scoring function, these results indicate the design process is qualitatively robust. These designs do not allow us to discriminate the contribution of Y–X versus Y–X′ interactions which are structurally unique (Fallas et al., 2012a), as II, III and VI all have equivalent numbers of both interaction types (Table S2).
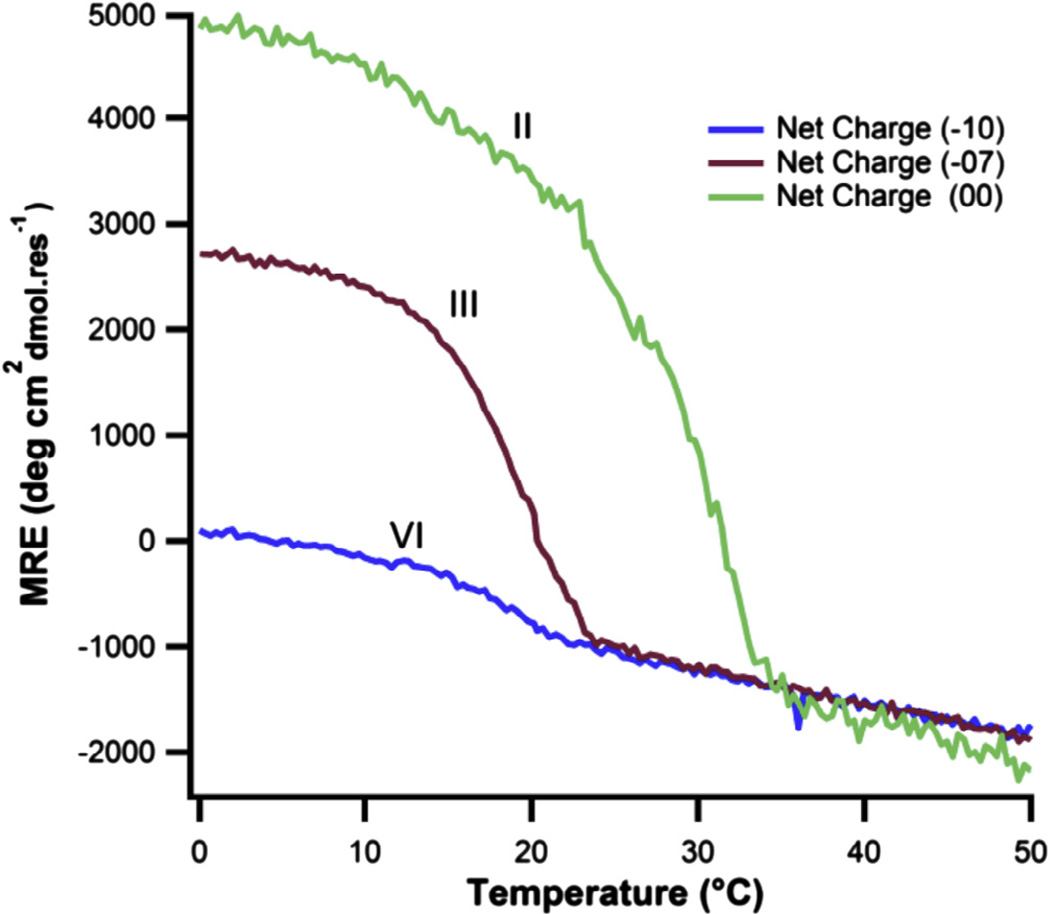
An interesting feature of the circular dichroism spectrum is the significantly lower ellipticity of VI relative to III, despite their equivalent stabilities (Fig. 4). The magnitude of the positive ellipticity band near 223 nm is in part a function of the fraction folded triple-helix. Given that both designs show a transition around 19 °C, the disparate signals at 4 °C suggest there are intrinsic structural differences in the folded state that account for the differences in ellipticity. It has been previously shown that the poly-proline II-like circular dichroism spectrum found in collagen can be recreated in charged homo-polymers, suggesting that intra-chain repulsions stabilize this chain conformation (Rucker and Creamer, 2002; Tiffany and Krimm, 1972). It is plausible that the combination of amino acids and intra-chain electrostatic interactions affect chain supercoiling in the triple helix, resulting in the disparate circular dichroism spectra. This is why mean residue ellipticity alone is not a good measure of triple helix stability when comparing different collagen-like sequences.
Fig. 4.
A notable difference in the magnitude of the MRE at 223 nm at temperatures below the unfolding transition is observed across the three designs.
Candidates III and V exhibit lower stability and higher specificity. While it was previously observed that II formed weakly stable B:C binary heterotrimeric species under low ionic strength conditions (Xu et al., 2011), III and VI only showed triple-helical structure and a cooperative denaturation profile when all three peptides were present (Fig. 3). The lower target stabilities and higher energy gaps in designs III and VI would additively contribute to the destabilization of competing states. If competing states are formed, their thermal stabilities would be below the freezing point of water, which are challenging to study with biophysical methods presented here. A stability/specificity tradeoff is predicted theoretically for collagen mimetic peptides (Nanda et al., 2011; Xu et al., 2010). Thus, a distinction must be made between a practical and theoretical definition of specificity. Species with similar energy gaps may exhibit different observed specificities when the competing state stabilities are below the freezing point of water. It is interesting to consider a recent computational design (Fallas and Hartgerink, 2012), where an ABC heterotrimer was generated with high target stability of 58 °C. In a 1:1:1 mixture, this was the only state formed, but for binary composition mixtures, heterotrimers formed stable triple helices with moderate Tm values, and one weak homotrimer was observed. Together these designs show a clear trend of achieving specificity at the expense of stability both theoretically and experimentally.
4. Conclusions
The computational approach for designing collagen mimetic sequences that self-assemble into abc-type heterotrimers has been validated on three cases to date. The introduction of charge asymmetry into the final design reduces the number of possible interactions and increases the computed energy gap between target and competing states. The results are peptide systems that do heterospecifically assemble, but with lower stability.
There appears to be a stability/specificity tradeoff where increasing the thermodynamic stability of the ABC target also necessitates the formation of competing states in other peptide mixtures. Lowering target stability is expected to prevent competing state formation at temperatures above the freezing point. Strategies that enhance native state stability, such as the use of Lys/Asp over Arg/Glu charge pairs can simultaneously improve specificity (Gauba and Hartgerink, 2007a,b; Xu et al., 2010, 2011). The ability to engineer a net charge A:B:C heterotrimer is expected to be useful down the road in controlling properties of higher-order structures, particularly for controlling the tendency to aggregate in high-concentration colloidal suspensions.
Supplementary Material
Acknowledgments
We gratefully acknowledge support from National Science Foundation DMR-0907273 and National Institutes of Health Office of the Director DP2-OD-006478-1 to carry out this work.
Abbreviation
- CD
circular dichroism
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsb.2013.04.006.
References
- Bryson JW, Desjarlais JR, Handel TM, DeGrado WF. From coiled coils to small globular proteins: design of a native-like three-helix bundle. Protein Sci. 1998;7:1404–1414. doi: 10.1002/pro.5560070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard P, Ivaninskii S, Lustig A. Improving coiled-coil stability by optimizing ionic interactions. J. Mol. Biol. 2002;318:901–910. doi: 10.1016/S0022-2836(02)00114-6. [DOI] [PubMed] [Google Scholar]
- Chan VC, Ramshaw JA, Kirkpatrick A, Beck K, Brodsky B. Positional preferences of ionizable residues in Gly-X-Y triplets of the collagen triple-helix. J. Biol. Chem. 1997;272:31441–31446. doi: 10.1074/jbc.272.50.31441. [DOI] [PubMed] [Google Scholar]
- Dahiyat BI, Mayo SL. De novo protein design: fully automated sequence selection. Science. 1997;278:82–87. doi: 10.1126/science.278.5335.82. [DOI] [PubMed] [Google Scholar]
- Engel J, Chen HT, Prockop DJ, Klump H. The triple helix in equilibrium with coil conversion of collagen-like polytripeptides in aqueous and nonaqueous solvents. Comparison of the thermodynamic parameters and the binding of water to (l-Pro-l-Pro-Gly)n and (l-Pro-l-Hyp-Gly)n. Biopolymers. 1977;16:601–622. doi: 10.1002/bip.1977.360160310. [DOI] [PubMed] [Google Scholar]
- Fallas JA, Hartgerink JD. Computational design of self-assembling register-specific collagen heterotrimers. Nat. Commun. 2012;3:1087. doi: 10.1038/ncomms2084. [DOI] [PubMed] [Google Scholar]
- Fallas JA, Dong J, Tao YJ, Hartgerink JD. Structural insights into charge pair interactions in triple helical collagen-like proteins. J. Biol. Chem. 2012a;287:8039–8047. doi: 10.1074/jbc.M111.296574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallas JA, Lee MA, Jalan AA, Hartgerink JD. Rational design of single-composition ABC collagen heterotrimers. J. Am. Chem. Soc. 2012b;134:1430–1433. doi: 10.1021/ja209669u. [DOI] [PubMed] [Google Scholar]
- Gauba V, Hartgerink JD. Surprisingly high stability of collagen ABC heterotrimer: evaluation of side chain charge pairs. J. Am. Chem. Soc. 2007a;129:15034–15041. doi: 10.1021/ja075854z. [DOI] [PubMed] [Google Scholar]
- Gauba V, Hartgerink JD. Self-assembled heterotrimeric collagen triple helices directed through electrostatic interactions. J. Am. Chem. Soc. 2007b;129:2683–2690. doi: 10.1021/ja0683640. [DOI] [PubMed] [Google Scholar]
- Giddu S, Xu F, Nanda V. Sequence recombination improves target specificity in a redesigned collagen peptide abc-type heterotrimer. Proteins. 2013;81:386–393. doi: 10.1002/prot.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek JJ, Harbury PB. Automated design of specificity in molecular recognition. Nat. Struct. Biol. 2003;10:45–52. doi: 10.1038/nsb877. [DOI] [PubMed] [Google Scholar]
- Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- Hellinga HW, Richards FM. Optimal Sequence Selection in Proteins of Known Structure by Simulated Evolution. Proc. Natl. Acad. Sci. USA. 1994;91:5803–5807. doi: 10.1073/pnas.91.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem. J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar K, Amin P, Bryan MA, Persikov AV, Mohs A, et al. Self-association of collagen triple helic peptides into higher order structures. J. Biol. Chem. 2006;281:33283–33290. doi: 10.1074/jbc.M605747200. [DOI] [PubMed] [Google Scholar]
- Kuhlman B, Dantas G, Ireton GC, Varani G, Stoddard BL, et al. Design of a novel globular protein fold with atomic-level accuracy. Science. 2003;302:1364–1368. doi: 10.1126/science.1089427. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Phillips KJ, Liu DR. Supercharging proteins can impart unusual resilience. J. Am. Chem. Soc. 2007;129:10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Fitch CA, Garcia-Moreno EB. Distance dependence and salt sensitivity of pairwise, coulombic interactions in a protein. Protein Sci. 2002;11:1004–1016. doi: 10.1110/ps.4700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda V, Zahid S, Xu F, Levine D. Computational design of intermolecular stability and specificity in protein self-assembly. Methods Enzymol. 2011;487:575–593. doi: 10.1016/B978-0-12-381270-4.00020-2. [DOI] [PubMed] [Google Scholar]
- Nautiyal S, Woolfson DN, King DS, Alber T. A designed heterotrimeric coiled coil. Biochemistry. 1995;34:11645–11651. doi: 10.1021/bi00037a001. [DOI] [PubMed] [Google Scholar]
- O’Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- Parmar AS, Muschol M. Hydration and hydrodynamic interactions of lysozyme: effects of chaotropic versus kosmotropic ions. Biophys. J. 2009a;97:590–598. doi: 10.1016/j.bpj.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar AS, Muschol M. Lysozyme as diffusion tracer for measuring aqueous solution viscosity. J. Colloid Interface Sci. 2009b;339:243–248. doi: 10.1016/j.jcis.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Peptide investigations of pairwise interactions in the collagen triple-helix. J. Mol. Biol. 2002;316:385–394. doi: 10.1006/jmbi.2001.5342. [DOI] [PubMed] [Google Scholar]
- Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Electrostatic interactions involving lysine make major contributions to collagen triple-helix stability. Biochemistry. 2005;44:1414–1422. doi: 10.1021/bi048216r. [DOI] [PubMed] [Google Scholar]
- Ramachandran GN, Kartha G. Structure of collagen. Nature. 1955;176:593–595. doi: 10.1038/176593a0. [DOI] [PubMed] [Google Scholar]
- Reinke AW, Grant RA, Keating AE. A synthetic coiled-coil interactome provides heterospecific modules for molecular engineering. J. Am. Chem. Soc. 2010;132:6025–6031. doi: 10.1021/ja907617a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A, Crick FH. The molecular structure of collagen. J. Mol. Biol. 1961;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- Rucker AL, Creamer TP. Polyproline II helical structure in protein unfolded states: lysine peptides revisited. Protein Sci. 2002;11:980–985. doi: 10.1110/ps.4550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa CM, Rosenblatt MM, Hong JK, Lear JD, DeGrado WF. Computational de novo design, and characterization of an A(2)B(2) diiron protein. J. Mol. Biol. 2002;321:923–938. doi: 10.1016/s0022-2836(02)00589-2. [DOI] [PubMed] [Google Scholar]
- Tiffany ML, Krimm S. Effect of temperature on the circular dichroism spectra of polypeptides in the extended state. Biopolymers. 1972;11:2309–2316. doi: 10.1002/bip.1972.360111109. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang L, Koder RL, Nanda V. De novo self-assembling collagen heterotrimers using explicit positive and negative design. Biochemistry. 2010;49:2307–2316. doi: 10.1021/bi902077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Zahid S, Silva T, Nanda V. Computational design of a collagen a:B:C-type heterotrimer. J. Am. Chem. Soc. 2011;133:15260–15263. doi: 10.1021/ja205597g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chan VC, Kirkpatrick A, Ramshaw JA, Brodsky B. Gly-Pro-Arg confers stability similar to Gly-Pro-Hyp in the collagen triple-helix of host-guest peptides. J. Biol. Chem. 1997;272:28837–28840. doi: 10.1074/jbc.272.46.28837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.