Abstract
Epichloae (Epichloë and Neotyphodium species; Clavicipitaceae) are fungi that live in systemic symbioses with cool-season grasses, and many produce alkaloids that are deterrent or toxic to herbivores. The epichloae colonize much of the aerial plant tissues, and most benignly colonize host seeds to transmit vertically. Of their four chemical classes of alkaloids, the ergot alkaloids and indole-diterpenes are active against mammals and insects, whereas peramine and lolines specifically affect insects. Comparative genomic analysis of Clavicipitaceae reveals a distinctive feature of the epichloae, namely, large repeat blocks in their alkaloid biosynthesis gene loci. Such repeat blocks can facilitate gene losses, mutations, and duplications, thus enhancing diversity of alkaloid structures within each class. We suggest that alkaloid diversification is selected especially in the vertically transmissible epichloae.
Ecology and agricultural implications of the epichloae
Epichloae (Epichloë and Neotyphodium species; Clavicipitaceae) are symbiotic fungi (endophytes) that grow within, and have apparent co-evolutionary relationships with, cool season grasses (Poaceae, subfamily Poöideae) [1,2•• ]. Epichloae are typically host-specific, and grow in intercellular spaces in a highly regulated fashion without eliciting responses from, or damage to, adjacent host cells [3• ,4•,5••]. They are best known for their production of various combinations of alkaloids in four different classes, which help protect their host plants by deterring vertebrate and invertebrate herbivores (Figure 1; Table 1).
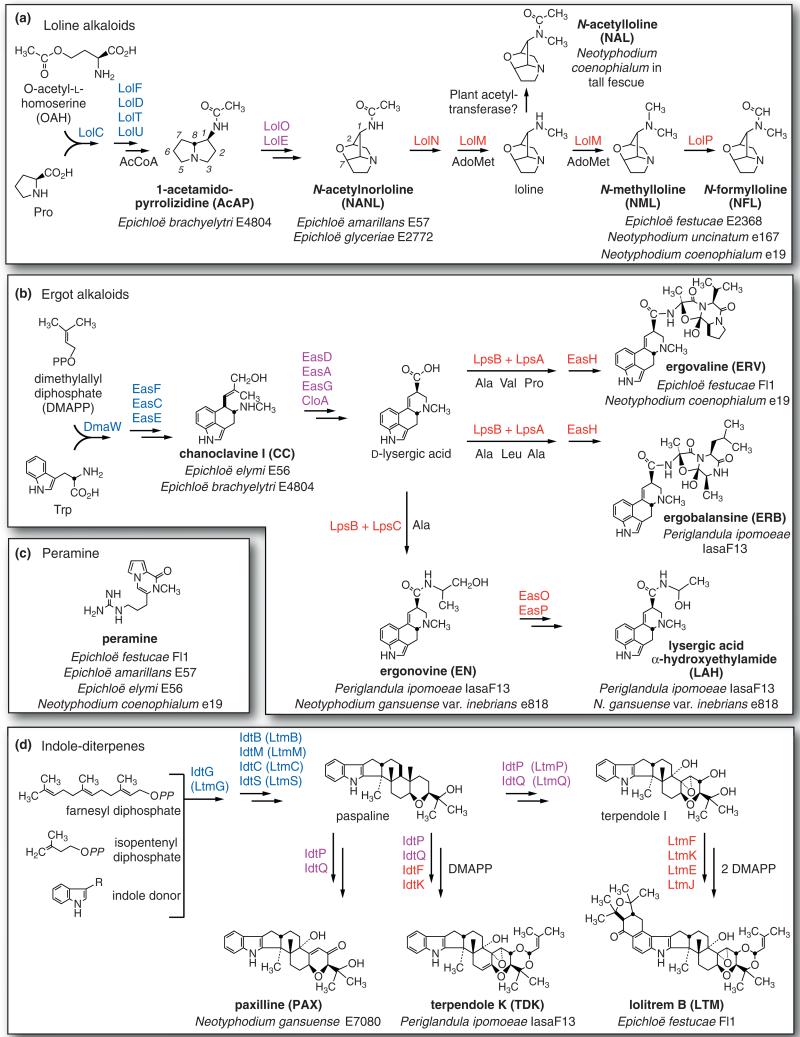
Figure 1.
Structures of alkaloids and proposed pathways for their biosynthesis in Epichoë, Neotyphodium and Periglandula species. Panel A: Proposed pathway for lolines. Panel B: Proposed pathway for ergot alkaloids. Panel C: Structure of peramine. Panel D: Proposed pathway for indole-diterpenes. Enzymes are designated according to names of genes that encode them. Enzyme designations are in blue type for early steps, purple type for intermediate steps, and red type for late steps. Substrates and cofactors indicated beneath or to the right of arrows are abbreviated as follows: AcCoA = acetyl-CoA, AdoMet = S-adenosylmethionine, Ala = l-alanine, DMAPP = dimethylallyl diphosphate, Leu = l-leucine, Pro = l-proline, Trp = l-tryptophan, Val = l-valine. Major pathway end products are designated in bold, and species and strains that produce them in symbio are given beneath each.
Table 1. Epichloë and Neotyphodium species with sequenced genomes, and their inferred alkaloid pathway end-products.
| Species | Isolatea | Host | Transmission | EAb | IDTb | LOLb | PERb |
|---|---|---|---|---|---|---|---|
| Epichloë amarillans | E57 = ATCC 200744 | Agrostis hyemalis | Mixed | – | – | NANL | PER |
| E. amarillans | E4668 | Agrostis hyemalis | Mixed | ERV | – | – | PER |
| E. baconii | E1031, = ATCC 200745 | Calamagrostis villosa | Horizontal | – | – | – | PER |
| E. brachyelytri | E4804 | Brachyelytrum erectum | Mixed | CC | – | AcAP | PER |
| E. bromicola | E502 = ATCC 200750 | Bromus erectus | Horizontal | – | – | – | – |
| E. elymi | E56 = ATCC 201551 | Elymus virginicus | Mixed | CC | – | – | PER |
| E. festucae | E2368 | Festuca rubra and Lolium | Mixed | ERV | – | NFL | (−) |
| giganteum (ascospore isolate) | |||||||
| E. festucae | Fl1 | Festuca trachyphylla | Mixed | ERV | LTB | – | PER |
| E. glyceriae | E277 | Glyceria striata | Horizontal | ERV | – | AcAP | (−) |
| E. “mollis” | E3601 = AL9924 | Holcus mollis | Mixed | ERV | – | – | PER |
| E. poae | E4646 | Poa nemoralis | Mixed | – | – | – | (−) |
| E. poae | E5819 | Poa nemoralis | Mixed | ERV | – | – | (−) |
| E. typhina | E8 = ATCC 200736 | Lolium perenne | Horizontal | – | – | – | PER |
| Neotyphodium aotearoae | e899 = MYA-1229 | Echinopogon ovatus | Vertical | – | TD | NFL | – |
| N. chisosum | e3609 = ATCC 64037 | Achnatherum eminens | Vertical | – | – | NFL | PER |
| N. coenophialum | e19 = ATCC 90664 | Lolium arundinaceum | Vertical | ERV | – | NFL | PER |
| N. coenophialum | e4163 | Lolium sp. (4×) | Vertical | ERV | TD | NFL | PER |
| N. funkii | e4096 | Achnatherum robustum | Vertical | CC | TD | – | PER |
| N. gansuense var. inebrians | e818 = MYA-1228 | Achnatherum inebrians | Vertical | LAH | – | – | – |
| N. gansuense | e7080 | Achnatherum inebrians | Vertical | – | PAX | – | – |
| N. uncinatum | e167 = CBS 102646 | Lolium pratense | Vertical | – | – | NFL | – |
Strains listed with ATCC and MYA prefixes are in the American Type Culture Collection. CBS = Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
Abbreviations: AcAP = 1-acetamidopyrrolizidine, CC = chanoclavine, EN = ergonovine, ERV = ergovaline, LAA = lysergic acid amide, LAH = lysergic acid α-hydroxyethylamide, LTB = lolitrem B, NANL = N-acetylnorloline, NFL = N-formylloline, nt=not tested, PAX = paxilline, PER = per-amine, TD = terpendoles. – = none detected. (−) indicates perA-ΔR* locus (see text).
Grass-epichloë symbiota consist of individual plants systemically harboring single clones of their Epichloë or Neotyphodium species. As such, a symbiotum is a genetic entity with phenotypes that are manifested under specific environmental conditions. Documented phenotypes of grass plants with symbiotic epichloae vary among species and genotypes, and include improved survival and growth, enhanced tolerance to stresses such as water deficit, and increased resistance to vertebrate or invertebrate herbivores [1,6-9]. Most epichloae are vertically transmitted in host maternal lineages by virtue of their abilities to grow benignly in seeds [3 •,10•]. However, some can produce fruiting bodies (stromata) around inflorescence primordia, and actually suppress maturation of host inflorescences (choke disease). In the culmination of sexual development, mature stromata produce contagious meiotic spores. With one possible exception described recently [11], sexual Epichloë species are haploid. In contrast, most asexual, strictly seed-borne epichloae described to date are polyploid interspecific hybrids [12].
The vast majority of studies on grass-epichloë interactions have focused on ryegrasses (Lolium spp.) and fescues (Festuca, Lolium and Schedonorus spp.) that are important pasture and forage grasses in temperate climates, and have been motivated by episodes of toxicosis suffered by livestock grazing some of those grasses. For example, Neotyphodium coenophialum, an asexual, strictly seed-transmitted symbiont of tall fescue (Lolium arundinaceum = Schedonorus arundinaceus = Festuca arundinacea), is responsible for fescue toxicosis, generally believed to be an effect of its ergot alkaloids [13,14•] (Figure 1b). Additionally, N. coenophialum produces peramine [15 •] (Figure 1c) and lolines [16] (Figure 1a), which are more specifically active against insects. Also, strains of Neotyphodium lolii in perennial ryegrass (Lolium perenne) produce tremorgenic indolediterpenes implicated in ryegrass staggers (Figure 1d) [17,18•], as well as peramine and ergot alkaloids. Although it has been suggested that alkaloids are an agronomically selected feature of epichloae in forage grasses [19], the widespread capability of epichloae in wild grasses to produce various combinations and often very high levels implies that they have important ecological roles in defensive mutualisms [12,20•,21••].
Activities against grazing mammals
Structural similarities of ergot alkaloids to neurotransmitters that control motility, body temperature, and appetite enable the alkaloids to interact with the dopamine, serotonin and noradrenaline receptors in central and peripheral nervous systems (reviewed in [22]). Symptoms of fescue toxicosis in cattle can include poor conception rates, agalactia, poor weight gain, increased respiration rate and salivation, and loss of blood flow to the extremities resulting in necrosis of the hooves and tail (dry gangrene). In horses, reproductive problems include placental abnormalities and fetal asphyxia [13,23]. Toxic effects of ergot alkaloids are exacerbated by heat or cold stress to the animal [13,23,24]. Also, because concentrations of the major ergot alkaloid, ergovaline, are highest in the crown area of vegetative tall fescue plants, toxicosis appears to be particularly common when the animals overgraze infested fields [23].
Effects of ergot alkaloids on rabbits have been tested with perennial ryegrass symbiotic with each of three epichloae — the natural N. lolii × Epichloëtyphina hybrid strain Lp1, a marker-exchange dmaW mutant lacking ergot alkaloids, and an lpsA disruption mutant that lacks ergovaline but produces simpler ergot alkaloids — as well as endophyte-free perennial ryegrass [25••]. Rabbits preferred plants symbiotic with the dmaW knockout to the plants with wild-type Lp1, the lpsA knockout, and endophyte-free plants, suggesting that simple ergot alkaloids counteract an endophyte enhancement of herbivore preference.
The various ergot alkaloids have distinct pharmacological properties. Hence, ergotamine is used for migraines, brominated ergocryptine is used for parkinsonism, and ergonovine has been used as an aid for parturition [22]. Therefore, it is reasonable to expect that, at higher doses, they also have different spectra of activity against natural herbivores. Much less information is available about the biological spectra and relative activities of different indole-diterpenes, which have a huge range of structural diversity [18•]. Though their tremorgenic activities are not directly lethal, affected animals lose their balance and can suffer accidental trauma or drowning. Published studies to date are limited, but so far this class of alkaloids has been demonstrated to affect neurotransmitter release and to block smooth muscle high-conductance calcium-activated potassium (maxi-K) channels in vertebrates [26].
Activities against insects
Among the four classes of alkaloids produced by epichloae, lolines and peramine are best documented to provide protection against insects, and neither alkaloid class exhibits appreciable activity against livestock [27•,28]. Peramine was identified as a feeding deterrent that is crucial for protection of perennial ryegrass pastures from Argentine stem weevil (Listronotus bonariensis; Coleoptera) in New Zealand pastures [29]. Loline alkaloids (reviewed in [16]) show a particularly broad spectrum of activity, and are approximately as potent as nicotine against insects. Lolines have been shown to be active against Japanese beetle (Popillia japonica; Coleoptera) [30], fall armyworm (Spodoptera frugiperda; Lepidoptera), European corn borer (Ostrinia nubilalis; Lepidoptera) [31•], Argentine stem weevil [32], birdcherry oat aphid (Rhopalosiphum padi; Homoptera), and greenbug aphid (Schizapus graminis; Homoptera) [33]. In vitro tests have also shown that N-acetylloline (NAL) and N-formylloline (NFL) deter feeding of Japanese beetle larvae (P. japonica, Coleoptera) at concentrations comparable to those measured in roots of tall fescue-N. coenophialum symbiota [34].
Different lolines may vary in specificity to different insect species and on certain development stages, and the decorations of the 1-amine are important in the spectrum of anti-insect activity of lolines. Significant effects of NFL and NAL have been observed in tests with S. frugiperda [31•]. Both affected mean larva weight in a no-choice test, and NFL also had a deterrent effect in a feeding choice experiment. In contrast, the two N-methyl forms, loline and N-methylloline (NML), had no significant activity in similar tests. When sprayed onto greenbug aphids, NAL and NML exhibited greater insecticidal activity than NFL and loline [31•]. Meadow fescue (Lolium pratense) with N. uncinatum, a symbiotum with high levels of NFL and NAL, deterred oviposition by Argentine stem weevil [35]. Interestingly, N-acetylnorloline (NANL) and NFL had different effects in deterrence and mortality of weevil larvae [32,35]. At high concentrations NFL reduced their growth, development and survival, whereas NANL caused high mortality but little effect on larval growth and development.
The pharmacological properties of lolines and peramine on insects remain to be investigated. Furthermore, several grass-epichloë symbiota accumulate 1-acetamidopyrrolizidine (AcAP; Figure 1a) instead of fully cyclized lolines, suggesting that AcAP may have a biological activity yet to be determined [21••]. Also relatively under-studied are the mechanisms of anti-insect activities of ergot alkaloids and indole-diterpenes (reviewed in [36]). Tests with dmaW and lpsA knockout endophytes indicate an effect of ergot alkaloids, probably ergovaline, against the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae) [37]. Also, the indole-diterpene, nodulisporic acid, is a potent and broad-spectrum insecticide, and has been shown to modulate glutamate-gated chloride channels in insects [38].
Genetic basis for alkaloid diversity
Much of the basis for diversity within each class of alkaloids (Table 1) is due to presence-absence polymorphism of middle-pathway or late-pathway genes. In addition, variations in certain genes for steps in ergot alkaloid and indole-diterpene biosynthesis affect the activity or specificity of certain biosynthetic enzymes, thereby contributing additional chemotypic diversity [21••].
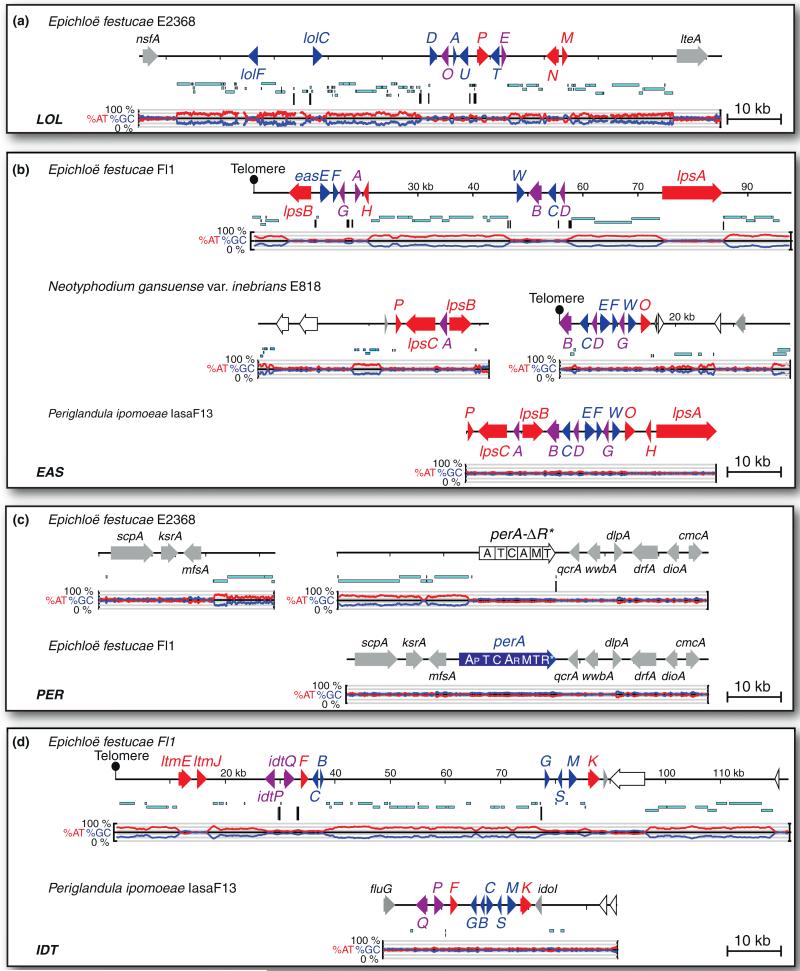
So far, variation in loline alkaloids is strictly associated with presence or absence of late-pathway genes. Epichloae with all 11 LOL genes, such as Epichloë festucae E2368 (Figure 2a), make loline, NML, and NFL (and when symbiotic with certain plants, NAL is also produced). Strains lacking functional lolO accumulate 1-acetamidopyrrolizidine (AcAP), and strains lacking lolN, lolM, and lolP accumulate NANL [21••].
Figure 2.
Structures of biosynthesis gene loci for four classes of alkaloids in several sequenced genomes of Epichoë, Neotyphodium and Periglandula species. Panel A: Loline alkaloid biosynthesis gene loci (LOL). Tracks from top to bottom of each map represent genes (arrows and letters), repeats (cyan bars), miniature inverted-repeat transposable elements (MITEs) (vertical bars), and graphs of AT (red) and GC (blue) contents. Genes are color coded according to Figure 1. Additionally, gray arrows indicate housekeeping and other genes not known to be involved in alkaloid biosynthesis, and open arrows indicate pseudogenes. Gene names are abbreviated as follows: A = lolA, C = lolC, D = lolD, E = lolE, F = lolF, M = lolM, N = lolN, O = lolO, P = lolP, T = lolT, and U = lolU. Panel B: Ergot alkaloid biosynthesis gene loci (EAS). Gene names are abbreviated as follows: A = easA, B = cloA, C = easC, D = easD, E = easE (= ccsA), F = easF, G = easG, H = easH, O = easO, P = easP, and W = dmaW. Tracks and color coding are as in panel A. Panel C: The peramine synthetase gene (perA) and flanking genes. Domains of the perA gene product are indicated, and are discussed in references [15•,21••]. The perA gene of E. festucae E2368 lacks the 3′ region encoding the reductase domain (R*), thought to be required for peramine biosynthesis, but the possibility that it may encode an enzyme for biosynthesis of a related alkaloid has not been explored. Tracks and color coding are as in panel A. Panel D: Indole-diterpene biosynthesis gene loci (IDT). Gene names are abbreviated as follows: B = idtB/ltmB, C = idtC/ltmC, F = idtF/ltmF, G = idtG/ltmG, K = idtK/ltmK, M = idtM/ltmM, P = idtP/ltmP, Q = idtQ/ltmQ, S = idtS/ltmS. Tracks and color coding are as in panel A.
Ergot alkaloids include clavines, lysergic acid and simple lysergic acid amides, as well as the complex ergopeptines derived from lysergic acid and three common amino acids by a series of condensations and cyclizations (Figure 1b) [22]. Considerable diversity in ergot alkaloid profiles is associated with variation in organization, gene content and gene sequences in the EAS clusters. Figure 2b compares EAS loci between E. festucae Fl1, which makes the same ergopeptine (ergovaline) found in toxic tall fescue, Neotyphodium gansuense var. inebrians, a seed-borne symbiont of drunken horse grass (Achnatherum inebrians) that makes ergonovine but no ergopeptine, and Periglandula ipomoeae, a related seed-transmitted symbiont of morning glories that makes another ergopeptine (ergobalansine) as well as ergonovine and lysergic acid α-hydroxyethylamide [21••]. The EAS cluster of N. gansuense var. inebrians resembles that of Periglandula ipomoeae, except that it lacks lpsA and easH, and the cluster is divided into two with a telomere immediately downstream of cloA. All other epichloae characterized to date have EAS clusters that more closely resemble that of E. festucae, though they vary considerably in content. Some lack EAS genes completely, some have all genes for ergopeptine biosynthesis, and some lack all but the four genes required for chanoclavine biosynthesis [21••]. The structural differnces between ergopeptines, such as ergobalansine and ergovaline, are determined by sequence variations in lpsA, which determine which amino acids are specified by each of the three modules of the LpsA subunit in the nonribosomal peptide synthetase (NRPS) [22,39•] (Figures 1b and 2b). So far, ergopeptines have been identified that are derived from 20 different permutations of amino acids [22,40]. However, ergovaline is the only ergopeptine definitively identified as a product of symbiotic epichloae.
Peramine biosynthesis also involves a multifunctional NRPS, the product of the perA gene [15•]. Intriguingly, two forms of perA have been found, one that specifies seven enzymatic domains, and a variant (perA-ΔR*) that lacks the sequence for the C-terminal reductase domain (Figure 2c) [21••]. Symbiotic E. festucae E2368 has, and expresses, the perA-ΔR* variant [21••], raising the possibility that it may play a role in synthesis of a heretofore unknown metabolite.
The extreme diversity of indole-diterpenes, similarly to ergot-alkaloid diversity, is determined by presence-absence polymorphism as well as sequence variations in idtP and idtQ that affect specificity of enzymes encoded by those genes (Figure 2c) [18•]. Furthermore, ltmE and ltmJ, which specify late steps in lolitrem B biosynthesis, are derived by duplication and neofunctionalization of other IDT genes [21••].
Is alkaloid diversity selectively favored?
Clearly the Clavicipitaceae, and particularly the epichloae, have the capability to produce a wide variety of alkaloids, both among and within chemical classes. The facts that various forms of ergot alkaloids, indole-diterpenes and lolines are produced by different strains and exhibit different biological specificities suggest that diversity of chemical profiles with many structural variants may be inherently beneficial. Several lines of evidence suggest that chemotypic variation is selectively favored. First, there is considerable variation between and even within Epichloë/Neotyphodium species for alkaloids in the various classes [12,41]. For example, of the two E. festucae strains with sequenced genomes, Fl1 produces ergot alkaloids (end-product, ergovaline), peramine, and indole-diterpenes (end-product, lolitrem B), whereas E2368 produces only lolines (end-product, NFL)[21••]. Even different E. festucae isolates from Festuca rubra subsp. commutata differ in production of ergot alkaloids [12] or indole-diterpenes [42•]. In a survey of E. festucae from different host grasses [42•], there was considerable variation in the end-product indole-diterpenes (terpendole C, lolitriol, and various lolitrems) based on presence or absence of functional ltmE, ltmF, and ltmJ genes.
Second, the EAS, IDT and LOL gene clusters in the epichloae contain large blocks of transposon-derived repeat sequences, which would be expected to promote rearrangements and partial or complete deletions of the genes. Comparisons of the alkaloid loci among various species and strains [21••] indicates that the lack of some or all of the genes in a cluster is most commonly due to deletions, because gene remnants are often present. An exception is in the E. festucae IDT cluster, where ltmE and ltmJ are uniquely found, and no remnants of those genes have been found in the other sequenced genomes. The abundance of repeats within each cluster is not indicative of other secondary metabolism gene clusters [21••], suggesting that unstable alkaloid clusters may be a selected trait.
Third, as described above, different forms of alkaloids within each class exhibit differences in biological activity. Therefore, it may be expected that different alkaloid profiles are selected according to the context of prevailing herbivores on the different host grasses and in different environments. It has been suggested that ecological and life-cycle variation among the epichloae has selected for alkaloid loci imbued with blocks of transposon-derived repeats that facilitate both gene losses and, as in the case of ltmE and ltmJ, gene duplication and neofunctionalization [21••].
Fourth, those symbionts that produce extremely high levels of certain alkaloids are strictly vertically transmitted, asexual species. Loline alkaloids in plants with strictly seed-borne epichloae range from 500 to nearly 20 000 μg g −1 of plant dry mass [43•], whereas levels in grasses with horizontally transmissible, sexual Epichloë species accumulate less than 500 μg g −1 plant dry mass [12]. Levels of the other alkaloids tend to be comparable between plants with sexual and asexual species, although alkaloids are less frequently found in plants with sexual epichloae [44,45]. However, levels of ergot alkaloids in drunken horse grass (A. inebrians) with strictly seed-borne N. gansuense var. inebrians are 2–3 orders of magnitude higher than in most other grasses with ergot-alkaloid-producing epichloae [46]. The high levels of alkaloids may simply relate to the importance of host protection for vertically transmitted symbionts, and might also compensate for the relatively low potential for asexual species to evolve diverse alkaloid profiles.
Fifth, an unexpected pattern emerged when characterizing alkaloid gene clusters in hybrid epichloae [47]; those that had received redundant copies of alkaloid gene clusters consistently lacked a subset of genes from one of the gene clusters. Neotyphodium chisosum and N. uncinatum had independently obtained two LOL clusters, and in both, one cluster was complete and the other lacked lolN, lolM, and a functional lolP. One of the two IDT clusters in the hybrid designated Neotyphodium sp. FaTG-2 lacked ltmE and ltmJ. Finally, two strains of N. coenophialum, probably both originating from an ancestor with complete, redundant EAS clusters, had independent gene losses. Strain e19 had an inactivating frame-shift in lpsB of the EAS2 cluster (lpsB2), whereas strain e4163 had an apparently functional lpsB2, but had lost lpsB1 and the adjacent easE1 gene. We speculate that these gene losses and mutations may reduce efficiency of the biosynthetic pathways such that intermediates as well as end-products accumulate to substantial levels. Panaccione [48••] has suggested that such a situation may be selectively favored based on the diversity of biological activities of different structures in each alkaloid class.
Conclusions
Two features of the epichloae are of paramount importance in establishment of mutualistic symbioses with grasses: their capability of, and sometimes dependence upon, vertical transmission via seeds, and their production of alkaloids that protect host plants from vertebrate and invertebrate herbivores. Both characteristics are variable in the epichloid clade. Epichloë/Neotyphodium species in some hosts are incapable of vertical transmission, and rely on spores formed in their fruiting structures for contagious (horizontal) transmission, others can use both transmission methods, and others, including a large number of interspecific hybrids, are asexual and mainly or solely seed-transmitted. On the basis of reports to date, alkaloids are most frequently associated with strictly seed-transmitted epichloae, less often with mixed-transmission strains, and rarest among those that only transmit horizontally. Also, strictly seed-borne epichloae can produce much higher levels of alkaloids — particularly lolines — than horizontally transmissible epichloae. Chemotypes of epichloae vary widely both with respect to presence or absence of each alkaloid class, and structural variation within each class, with implications for their spectra of biological activity. Also, selection may favor alkaloid profiles that include substantial levels of intermediates over those dominated by pathway endproducts [48••]. Evidence for selection on alkaloid levels and chemotypic diversification is particularly strong for symbiota with vertically transmissible epichloae.
Acknowledgements
Work by the authors was supported by USDA-NIFA grant 2012-67013-19384, USDA-CSREES grant 2010-34457-21269; National Institutes of Health grants R01GM086888 and 2 P20 RR-16481. Genome sequence analysis was conducted in the University of Kentucky Advanced Genetic Technologies Center. This is publication number 13-12-069 of the Kentucky Agricultural Experiment Station, published with approval of the director.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. http://dx.doi.org/10.1146/annurev.arplant.55.031903.141735. [DOI] [PubMed] [Google Scholar]
- 2••.Schardl CL, Craven KD, Speakman S, Stromberg A, Lindstrom A, Yoshida R. A novel test for host-symbiont codivergence indicates ancient origin of fungal endophytes in grasses. Syst. Biol. 2008;57:483–498. doi: 10.1080/10635150802172184. http://dx.doi.org/10.1080/10635150802172184. A pattern of diffuse codivergence, with exceptions, was detected in phylogenies of Epichloë and Neotyphodium species and their hosts, indicating that these heritable symbionts originated approximately con-currently with the highly speciose grass subfamily, Poöideae.
- 3•.Christensen MJ, Bennett RJ, Ansari HA, Koga H, Johnson RD, Bryan GT, Simpson WR, Koolaard JP, Nickless EM, Voisey CR. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008;45:84–93. doi: 10.1016/j.fgb.2007.07.013. As plant cells elongate, the adjacent Epichloë hyphae also exhibit intercallary growth, a novel growth characteristic for fungi that accounts for the ability of the symbiont to keep pace with plant growth.
- 4•.Christensen MJ, Simpson WR, Al Samarrai T. Infection of tall fescue and perennial ryegrass plants by combinations of different Neotyphodium endophytes. Mycol. Res. 2000;104:974–978. Host specificity of Epichloë (Neotyphodium) species was demonstrated. Strains elicit no apparent defensive response in their natural hosts, but when they are exchanged between even closely related grass species, the grasses respond and even exhibit necrosis.
- 5••.Tanaka A, Takemoto D, Chujo T, Scott B. Fungal endophytes of grasses. Curr. Opin. Plant Biol. 2012;15:462–468. doi: 10.1016/j.pbi.2012.03.007. http://dx.doi.org/10.1016/j.pbi.2012.03.007. Epichloë festucae employs reactive oxygen species as part of a self-regulatory system to maintain a mutualistic symbiosis with host plants.
- 6.Malinowski DP, Belesky DP. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci. 2000;40:923–940. [Google Scholar]
- 7.Hamilton C, Dowling T, Faeth S. Hybridization in endophyte symbionts alters host response to moisture and nutrient treatments. Microb. Ecol. 2010;59:768–775. doi: 10.1007/s00248-009-9606-9. http://dx.doi.org/10.1007/s00248-009-9606-9. [DOI] [PubMed] [Google Scholar]
- 8.Saari S, Faeth SH. Hybridization of Neotyphodium endophytes enhances competitive ability of the host grass. New Phytol. 2012;195:231–236. doi: 10.1111/j.1469-8137.2012.04140.x. http://dx.doi.org/10.1111/j.1469-8137.2012.04140.x. [DOI] [PubMed] [Google Scholar]
- 9.Clay K, Marks S, Cheplick GP. Effects of insect herbivory and fungal endophyte infection on competitive interactions among grasses. Ecology. 1993;74:1767–1777. [Google Scholar]
- 10•.Freeman EM. The seed fungus of Lolium temulentum L., the darnel. Phil. Trans. R. Soc. Lond. B. 1904;196:1–27. The joint plant-fungus life cycle is detailed, demonstrating that the fungus colonizes the host embryo, suggesting a mechanism for vertical transmission.
- 11.Yan K, Yanling J, Kunran Z, Hui W, Huimin M, Zhiwei W. A new Epichloë species with interspecific hybrid origins from Poa pratensis ssp. pratensis in Liyang, China. Mycologia. 2011;103:1341–1350. doi: 10.3852/10-352. http://dx.doi.org/10.3852/10-352. [DOI] [PubMed] [Google Scholar]
- 12.Schardl CL, Young CA, Faulkner JR, Florea S, Pan J. Chemotypic diversity of epichloae, fungal symbionts of grasses. Fungal Ecol. 2012;5:331–344. http://dx.doi.org/10.1016/j.funeco.2011.04.005. [Google Scholar]
- 13.Evans TJ, Blodgett DJ, Rottinghaus GE. Fescue toxicosis. In: Gupta RC, editor. Veterinary Toxicology: Basic and Clinical Principles. Second ed. Academic Press; Boston: 2012. pp. 1166–1177. [Google Scholar]
- 14•.Lyons PC, Plattner RD, Bacon CW. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science. 1986;232:487–489. doi: 10.1126/science.3008328. This discovery of the symbiont species, later named Neotyphodium coenophialum, and its association with toxicity of a very common pasture grass, launched decades of intensive research into the ecology, agroecology, toxicology, biochemistry, molecular biology and genomics of the epichloae.
- 15•.Tanaka A, Tapper BA, Popay A, Parker EJ, Scott B. A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol. Microbiol. 2005;57:1036–1050. doi: 10.1111/j.1365-2958.2005.04747.x. The perA gene encodes a multifunctional enzyme with two NRPS modules, an N-methylation domain and a C-terminal reductase domain, which in total appear sufficient for peramine biosynthesis.
- 16.Schardl CL, Grossman RB, Nagabhyru P, Faulkner JR, Mallik UP. Loline alkaloids: currencies of mutualism. Phytochemistry. 2007;68:980–996. doi: 10.1016/j.phytochem.2007.01.010. http://dx.doi.org/10.1016/j.phytochem.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Young CA, Felitti S, Shields K, Spangenberg G, Johnson RD, Bryan GT, Saikia S, Scott B. A complex gene cluster for indole-diterpene biosynthesis in the grass endophyte Neotyphodium lolii. Fungal Genet. Biol. 2006;43:679–693. doi: 10.1016/j.fgb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18•.Saikia S, Takemoto D, Tapper BA, Lane GA, Frazer K, Scott B. Functional analysis of an indole-diterpene gene cluster for lolitrem B biosynthesis in the grass endosymbiont Epichloë festucae. FEBS Lett. 2012;586:2563–2569. doi: 10.1016/j.febslet.2012.06.035. http://dx.doi.org/10.1016/j.febslet.2012.06.035. The tremorgen, lolitrem B, is the product of a complex metabolic network in which LtmP (IdtP) and LtmQ (IdtQ) have promiscuous and variable substrate specificities.
- 19.Faeth SH. Asexual fungal symbionts alter reproductive allocation and herbivory over time in their native perennial grass hosts. Am. Nat. 2009;173:554–565. doi: 10.1086/597376. http://dx.doi.org/10.1086/597376. [DOI] [PubMed] [Google Scholar]
- 20••.Crawford K, Land J, Rudgers J. Fungal endophytes of native grasses decrease insect herbivore preference and performance. Oecologia. 2010;164:431–444. doi: 10.1007/s00442-010-1685-2. http://dx.doi.org/10.1007/s00442-010-1685-2. Epichloae in several wild grasses are shown to protect against insect herbivores, countering longstanding assertions by some that biological protection by seedborne symbionts is mainly a result of artificial selection in agronomic plant species.
- 21••.Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, Fleetwood DJ, Haws DC, Moore N, Oeser B, Panaccione DG, Schweri KK, Voisey CR, Farman ML, Jaromczyk JW, Roe BA, O’Sullivan DM, Scott B, Tudzynski P, An Z, Arnaoudova EG, Bullock CT, Charlton ND, Chen L, Cox M, Dinkins RD, Florea S, Glenn AE, Gordon A, Güldener U, Harris DR, Hollin W, Jaromczyk J, Johnson RD, Khan AK, Leistner E, Leuchtmann A, Li C, Liu J, Liu J, Liu M, Mace W, Machado C, Nagabhyru P, Pan J, Schmid J, Sugawara K, Steiner U, Takach J, Tanaka E, Webb JS, Wilson EV, Wiseman JL, Yoshida R, Zeng Z. Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013;9:e1003323. doi: 10.1371/journal.pgen.1003323. http://dx.doi.org/10.1371/journal.pgen.1003323. Comparisons of genome sequences from 15 Clavicipitaceae, including 10 Epichloë and Neotyphodium species, indicated that alkaloid loci in epichloae are destabilized by large blocks of transposon-derived repeats, thereby facilitating chemotypic diversification.
- 22.Schardl CL, Panaccione DG, Tudzynski P. Ergot alkaloids — biology and molecular biology. Alkaloids (San Diego, CA, U.S.) 2006;63:45–86. doi: 10.1016/s1099-4831(06)63002-2. http://dx.doi.org/10.1016/S1099-4831(06)63002-2. [DOI] [PubMed] [Google Scholar]
- 23.Cross DL, White JF, Jr, Bacon CW, Hywel-Jones NL, Spatafora JW. Clavicipitalean Fungi: Evolutionary Biology, Chemistry, Biocontrol and Cultural Impacts. Vol. 19. Marcel-Dekker, Inc.; New York/Basel: 2003. Ergot alkaloid toxicity; pp. 475–494. [Google Scholar]
- 24.Al Tamimi HJ, Eichen PA, Rottinghaus GE, Spiers DE. Nitric oxide supplementation alleviates hyperthermia induced by intake of ergopeptine alkaloids during chronic heat stress. J. Therm. Biol. 2007;32:179–187. [Google Scholar]
- 25•.Panaccione DG, Cipoletti JR, Sedlock AB, Blemings KP, Schardl CL, Machado C, Seidel GE. Effects of ergot alkaloids on food preference and satiety in rabbits, as assessed with gene-knockout endophytes in perennial ryegrass (Lolium perenne) J. Agric. Food Chem. 2006;54:4582–4587. doi: 10.1021/jf060626u. Simple ergot alkaloids apparently counteract an endophyte effect that increases the palatability of perennial ryegrass.
- 26.Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms L, Sanchez M, Giangiacomo K, Reuben JP, Smith A, III, Kaczorowski GJ, Garcia ML. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calciumactivated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 27•.Bluett SJ, Thom ER, Clark DA, Waugh CD. Effects of a novel ryegrass endophyte on pasture production, dairy cow milk production and calf liveweight gain. Aust. J. Exp. Agric. 2005;45:11–19. Natural strains of N. coenophialum that produce little or no ergot alkaloids do not exhibit the toxicity to livestock that is associated with ergot alkaloids produced by other strains in cultivars widely distributed in the USA.
- 28.Hopkins AA, Young CA, Simpson WR, Panaccione DG, Mittal S, Bouton JH. Agronomic performance and lamb safety of tall fescue novel endophyte combinations in the south central USA. Crop Sci. 2010;50:1552–1561. [Google Scholar]
- 29.Rowan DD, Hunt MB, Gaynor DL. Peramine, a novel insect feeding deterrent from ryegrass infected with the endophyte Acremonium loliae. J. Chem. Soc. Chem. Commun. 1986;12:935–936. doi: 10.1007/BF01012099. [DOI] [PubMed] [Google Scholar]
- 30.Potter DA, Patterson CG, Redmond CT. Influence of turfgrass species and tall fescue endophyte on feeding ecology of Japanese beetle and southern masked chafer grubs (Coleoptera: Scarabaeidae) J. Econ. Entomol. 1992;85:900–909. [Google Scholar]
- 31•.Riedell WE, Kieckhefer RE, Petroski RJ, Powell RG. Naturally occurring and synthetic loline alkaloid derivatives: insect feeding behavior modification and toxicity. J. Entomol. Sci. 1991;26:122–129. Natural loline alkaloid forms differing in substituents on the 1-amine have different specificities in actions against insects.
- 32.Jensen J, Popay A, Tapper B. Argentine stem weevil adults are affected by meadow fescue endophyte and its loline alkaloids. N. Z. Plant Prot. 2009;62:12–18. [Google Scholar]
- 33.Wilkinson HH, Siegel MR, Blankenship JD, Mallory AC, Bush LP, Schardl CL. Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. Mol. Plant Microbe Interact. 2000;13:1027–1033. doi: 10.1094/MPMI.2000.13.10.1027. [DOI] [PubMed] [Google Scholar]
- 34.Patterson CG, Potter DA, Fannin FF. Feeding deterrency of alkaloids from endophyte-infected grasses to Japanese beetle grubs. Entomol. Exp. Appl. 1991;61:285–289. [Google Scholar]
- 35.Popay AJ, Tapper BA, Podmore C. Endophyte-infected meadow fescue and loline alkaloids affect argentine stem weevil larvae. N. Z. Plant Prot. 2009;62:19–27. [Google Scholar]
- 36.Panaccione DG, Beaulieu WT, Cook D. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol. 2013 http://dx.doi.org/10.1111/1365-2435.12076. [Google Scholar]
- 37.Potter DA, Stokes JT, Redmond CT, Schardl CL, Panaccione DG. Contribution of ergot alkaloids to suppression of a grass-feeding caterpillar assessed with gene-knockout endophytes in perennial ryegrass. Entomol. Exp. Appl. 2008;126:138–147. http://dx.doi.org/10.1111/j.1570-7458.2007.00650.x. [Google Scholar]
- 38.Smith MM, Warren VA, Thomas BS, Brochu RM, Ertel EA, Rohrer S, Schaeffer J, Schmatz D, Petuch BR, Tang YS, Meinke PT, Kaczorowski GJ, Cohen CJ. Nodulisporic acid opens insect glutamate-gated chloride channels: Identification of a new high affinity modulator. Biochemistry. 2000;39:5543–5554. doi: 10.1021/bi992943i. http://dx.doi.org/10.1021/bi992943i. [DOI] [PubMed] [Google Scholar]
- 39•.Haarmann T, Lorenz N, Tudzynski P. Use of a nonhomologous end joining deficient strain (Δku70) of the ergot fungus Claviceps purpurea for identification of a nonribosomal peptide synthetase gene involved in ergotamine biosynthesis. Fungal Genet. Biol. 2008;45:35–44. doi: 10.1016/j.fgb.2007.04.008. The EAS locus of Claviceps purpurea P1 has two lpsA genes encoding enzyme subunits that differ in specificity for the amino acid precursors that, together with lysergic acid, are incorporated into ergopeptine alkaloids to give ergotamine and ergocryptine.
- 40.Cvak L, Jegorov A, Sedmera P, Cisarova I, Cejka J, Kratochvil B, Pakhomova S. Norleucine, a natural occurrence in a novel ergot alkaloid gamma-ergokryptinine. Amino Acids. 2005;29:145–150. doi: 10.1007/s00726-005-0180-2. [DOI] [PubMed] [Google Scholar]
- 41.Christensen MJ, Leuchtmann A, Rowan DD, Tapper BA. Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis), and perennial rye-grass (Lolium perenne) Mycol. Res. 1993;97:1083–1092. [Google Scholar]
- 42•.Young CA, Tapper BA, May K, Moon CD, Schardl CL, Scott B. Indole-diterpene biosynthetic capability of epichloë endophytes as predicted by ltm gene analysis. Appl. Environ. Microbiol. 2009;75:2200–2211. doi: 10.1128/AEM.00953-08. http://dx.doi.org/10.1128/AEM.00953-08. The chemotypic diversity of indole-diterpene-producing epichloae was discerned by analysis of IDT/LTM cluster genes.
- 43•.Zhang D-X, Nagabhyru P, Schardl CL. Regulation of a chemical defense against herbivory produced by symbiotic fungi in grass plants. Plant Physiol. 2009;150:1072–1082. doi: 10.1104/pp.109.138222. http://dx.doi.org/10.1104/pp.109.138222. Levels of loline alkaloids produced by Neotyphodium uncinatum and Neotyphodium siegelii are elevated in young leaves, apparently due to host mobilization of amino acid precursors to growing tissues.
- 44.Siegel MR, Latch GCM, Bush LP, Fannin FF, Rowan DD, Tapper BA, Bacon CW, Johnson MC. Fungal endophyte-infected grasses: alkaloid accumulation and aphid response. J. Chem. Ecol. 1990;16:3301–3315. doi: 10.1007/BF00982100. [DOI] [PubMed] [Google Scholar]
- 45.Leuchtmann A, Schmidt D, Bush LP. Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloë/Neotyphodium endophytes. J. Chem. Ecol. 2000;26:1025–1036. [Google Scholar]
- 46.Miles CO, Lane GA, Di Menna ME, Garthwaite I, Piper EL, Ball OJP, Latch GCM, Allen JM, Hunt MB, Bush LP, Min FK, Fletcher I, Harris PS. High levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompany infection by an Acremonium-like endophytic fungus. J. Agric. Food Chem. 1996;44:1285–1290. [Google Scholar]
- 47.Schardl CL, Young CA, Pan J, Florea S, Takach JE, Panaccione DG, Farman ML, Webb JS, Jaromczyk J, Charlton ND, Nagabhyru P, Chen L, Shi C, Leuchtmann A. Currencies of mutualisms: sources of alkaloid genes in vertically transmitted epichloae. Toxins. 2013;5:1064–1088. doi: 10.3390/toxins5061064. http://dx.doi.org/10.3390/toxins5061064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Panaccione DG. Origins and significance of ergot alkaloid diversity in fungi. FEMS Microbiol. Lett. 2005;251:9–17. doi: 10.1016/j.femsle.2005.07.039. The hypothesis is presented that inefficiency in alkaloid biosynthetic pathways may be selectively favored such that pathway intermediates as well as end-products accumulate to levels at which they can exert various biological effects.