Abstract
This study investigated the differentiation of human amniotic fluid-derived stem cells (hAFSCs) into insulin-producing clusters in vitro. Adenovirally-delivered mouse Pdx1 (Ad-Pdx1) induced human Pdx1 expression in hAFSCs and enhanced the coordinated expression of downstream b-cell markers. When Ad-Pdx1-transduced hAFSCs were sequentially treated with activin A, bFGF and nicotinamide and the culture plate surface coated with poly-L-ornithine, the expression of islet-associated human mRNAs for Pdx1, Pax6, Ngn3 and insulin was increased. C-peptide ELISA confirmed that Ad-Pdx1-transduced hAFSCs processed and secreted insulin in a manner consistent with that pathway in pancreatic b-cells. To sustain the b-cell-like phenotype and investigate the effect of three-dimensional (3D) conformation on the differentiation of hAFSCs, Pdx1-transduced cells were encapsulated in alginate and cultured long-term under serum-free conditions. Over 2 weeks, partially differentiated hAFSC clusters increased in size and increased insulin secretion. Taken together, these data demonstrate that ectopic Pdx1 expression initiates pancreatic differentiation in hAFSCs and that a b-cell-like phenotype can be augmented by culture conditions that mimic the stromal components and 3D geometry associated with pancreatic islets.
Keywords: amniotic fluid-derived stem cells, Pdx1, differentiation, diabetes, cell therapy, growth factors, extracellular matrix components
1. Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by destruction of the insulin-producing b-cells of the islets of Langerhans in the endocrine pancreas (Gepts, 1965). Current treatment regimens for T1DM combine blood glucose monitoring with frequent insulin injections. However, even careful application of this therapy does not achieve full metabolic control. As a result, many patients suffer from severe long-term complications, including neuropathy, vascular disease, retinopathy and renal failure (Tripathi and Srivastava, 2006). Islet transplantation to replace lost b-cell mass is a promising approach to treat T1DM and has allowed some patients to achieve full metabolic control and insulin independence (Shapiro et al., 2000, 2006; Truong and Shapiro, 2006). Unfortunately, the disadvantages of islet transplantation include a shortage of donors and side-effects associated with life-long immunosuppression (Shapiro et al., 2000; Lechner and Habener, 2003). These significant drawbacks have led to the investigation of alternative cell sources for b-cell replacement.
Stem and progenitor cells with the ability to differentiate into lineages of the endocrine pancreas have become a promising, renewable source of transplantable cells for T1DM therapy. Insulin-producing cells can be generated from pluripotent ES and iPS cells, using a multi-stage approach that mimics the signalling pathways necessary for embryonic pancreatic development (D’Amour et al., 2006; Jiang et al., 2007a, 2007b). However, upon transplantation, pluripotent stem cells have the potential to form teratomas (Fujikawa et al., 2005) and may be subject to immune rejection (Drukker et al., 2002; Drukker and Benvenisty, 2004; Draper et al., 2002). Bone marrow mesenchymal stromal cells (BM-MSCs) are an attractive candidate for b-cell replacement therapy because they do not form teratomas and can be patient-matched (Krause et al., 2001; Jiang et al., 2002b; Fausto et al., 2004; Ong et al., 2006). BM-MSCs have been reported to produce insulin in vitro and to restore normoglycaemia in streptozoticin (STZ)-treated mice (Ianus et al., 2003; Tang et al., 2004: Xie et al., 2009; Sun et al., 2007; Oh et al., 2004; Ai et al., 2007).
Despite significant progress in differentiating various stem cell populations into cells resembling b-cells, the amount of insulin produced is typically far below the levels required for sustained physiological impact. Therefore, several methods have been employed to enhance insulin production in adult stem or progenitor cell populations, including overexpression of pancreatic genes. The pancreatic master transcription factor pancreatic duodenal homeobox 1 (PDX1) is indispensible in pancreatic development and maintenance of b-cell function (Guz et al., 1995; Hui et al., 2002). Ectopic expression of Pdx1 has been shown to lead to insulin production in vitro in BM-MSCs and hepatic cells (Zalzman et al., 2005; Ferber et al., 2000; Colter et al., 2000; Itkin-Ansari et al., 2000; Li et al., 2007) and reduced hyperglycaemia upon transplantation in STZ-treated mice (Karnieli et al., 2007).
The microenvironment is a critical factor in the differentiation and survival of b-cells and directly impacts islet function. The pancreatic microenvironment is made up of extracellular matrix (ECM) proteins that maintain organ integrity and facilitate sensing and signalling. Pancreatic ECM contains fibronectin, which is associated with endothelial cells and epithelial ducts, and laminin, which is found in the interface between the epithelia and connective tissue, while the basement membrane is primarily composed of laminin and collagen IV (Cirulli et al., 2000; Jiang et al., 2002a). Inclusion of ECM proteins has proved useful in generating insulin-producing cells from stem cells in vitro. In addition to ECM mimicry, maturation can be further improved by imitating the native conformation of b-cells within an islet by forcing the cells to aggregate into clusters. To achieve this type of three-dimensional (3D) culture, cells are embedded in a polymeric material (such as alginate), which allows free diffusion of oxygen, nutrients and biologicals (Risbud and Bhonde, 2001).
Fetal stem cell sources have also been of great interest as a result of their broad potency and lack of ethical concerns. C-kit-selected amniotic fluid-derived stem cells (hAFSCs), originally isolated in our laboratory, are a particularly attractive therapeutic cell source because of their extensive capacity for self-renewal in culture, broad multipotency and lack of teratoma formation (De Coppi et al., 2007). AFSCs have been shown to differentiate efficiently into mesenchymal lineages, such as bone, cartilage, fat and muscle, and, when cultured under specific inductive conditions, will also differentiate into endodermal and ectodermal lineages, albeit less efficiently (De Coppi et al., 2007). AFSCs express most of the MSC-associated markers (CD29, CD44, CD73, CD90 and CD105) but also express markers typically associated with ES cells (Oct-4, Sox2, SSEA-4 and Tra-1-60), which suggests that they lie somewhere between ES cells and adult somatic stem cells on the developmental continuum. Additionally, hAFSCs do not express cell surface markers associated with rejection, including CD80, CD86 and CD40, and they exhibit immunomodulatory activity (Perin et al., 2010; Moorefield et al., 2011).
Here we report a novel, step-wise protocol that induces differentiation of hAFSCs toward the endocrine pancreatic lineage. Initially, mouse Pdx1 was overexpressed in hAFSCs using an adenoviral vector system. In the first step of differentiation, transduced hAFSCs were subjected to high levels of activin A to initiate differentiation toward endodermal lineages (D’Amour et al., 2005). Next, endodermally committed AFSCs were cultured on a coating of poly-L-ornithine, along with bFGF, which promoted further proliferation (Li et al., 2010; Ogneva and Martinova, 2002; Le Bras et al., 1998). Thereafter, nicotinamide was included to induce b-cell maturation and enhance insulin production from the resulting clusters (Ye et al., 2006). Finally, Ad-Pdx1 hAFSC-derived insulin-producing clusters were encapsulated in alginate to prolong survival and sustain insulin production. Insulin-expressing hAFSC clusters may serve as a novel, renewable cell therapy option for the long-term treatment of T1DM.
2. Materials and methods
2.1. Isolation and culture of hAFSCs
hAFSCs were isolated as previously reported (De Coppi et al., 2007). Briefly, amniotic fluid was provided by consenting pregnant women undergoing routine amniocentesis between 15 and 19 weeks of fetal gestation. Total amniotic fluid was cultured in AminoMAX™ II (Gibco-Invitrogen, Grand Island, NY, USA) on a coverglass for at least 2 weeks, until the cell density had reached approximately 70%. This population was then immuno-selected using a rabbit polyclonal antibody against c-kit (CD117; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Selected hAFSCs were then expanded in Chang medium: a-MEM (Invitrogen, Carlsbad, CA, USA) containing 15% ------------ (ES)–fetal bovine serum (FBS), 1% glutamine and 1% penicillin/streptomycin (Invitrogen), supplemented with 18% Chang B and 2% Chang C (Irvine Scientific, Irvine, CA, USA) at 37° C in a 5% CO2 atmosphere.
2.2. Adenoviral vector amplification and titre
An adenovirus serotype 5 vector (Wu et al., 2002) was chosen to induce pancreatic differentiation of hAFSCs because it can be produced at high titre and can efficiently infect a wide range of cell types. The mouse Pdx1 gene was chosen for adenovirus-mediated ectopic expression, so that mouse Pdx1 mRNA could be distinguished from endogenous expression of human Pdx1 using species-specific PCR primers. Adenoviruses containing the mouse Pdx1 (Ad-Pdx1) or LacZ (Ad-LacZ) genes under the control of the cytomegalovirus (CMV) promoter were kindly provided by Dr Jeng-Shin Lee (Harvard Gene Therapy Initative, Harvard University, Boston, MA, USA) (Figure 1A). Adenoviruses were amplified by infecting 70–80% confluent dishes of human embryonic kidney cell line 293 at a multiplicity of infection (MOI) of 10. After 3–4 days, when the majority of cells exhibited cytopathic effects, the cell suspension was collected and centrifuged at 1000 rpm for 5 min. The resulting pellets were lysed by three consecutive freeze–thaw cycles, the cellular debris was removed by centrifugation at 1000 rpm for 5 min and the viral lysate was collected and stored at −80° C. Adenovirus preparations were titred by transducing human cervical carcinoma cells (HeLa) with the virus and subsequent immunostaining for viral particles. HeLa cells were plated at a density of 5 × 104 cells/well of a six-well plate in triplicate; 24 h after plating, the cells were infected in Eagle’s minimum essential medium (MEM) with 0, 8, 40, 200 or 1000 ml of adenovirus. Two hours later the medium was changed to Eagle’s MEM and the cells were cultured for 24 h. The cells were then fixed with 4% paraformaldehyde (PFA) for 30 min, permeabilized with 3% hydrogen peroxidase for 30 min and blocked with 10% rabbit serum for 1 h. The transduced cells were then stained with anti-Pdx1 antibody (1:300 dilution; Santa Cruz) for 1 h and incubated with a FITC-conjugated a-bungarotoxin secondary antibody (1:300 dilution; Sigma-Aldrich, St. Louis, MO, USA) for 30 min. The transduction efficiency of adenovirus preparations was also tested on hAFSCs in a similar manner. hAFSCs were plated at a density of 5 × 103 cells/well of a six-well plate in growth medium supplemented with 15% fetal bovine serum to form a highly proliferative culture. Upon reaching 80% confluence, the hAFSCs were transduced with 1 × 105 IU/ml adenovirus (Ad-Pdx1 or Ad-LacZ) in serum-free medium for 24 h. The cells were then fixed, permeabilized, blocked and stained as above.
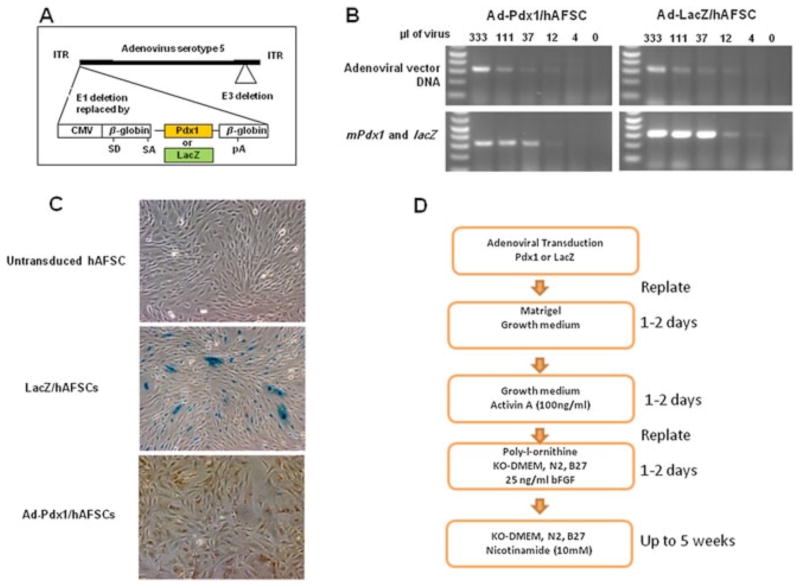
Figure 1.
AFSCs transduction with a Pdx1-expressing adenovirus and the transgene expressed. (A) Schematic representation of the adenoviral vector (35 840 bp) containing the mouse Pdx1 cDNA. CMV, cytomegalovirus promoter. (B) (top) RT–PCR of adenoviral vector DNA at different amounts (ml) used to transduce AFSCs; (bottom) RT–PCR of mPdx1 and lacZ genes at different amounts (ml) used to transduce AFSCs. (C) LacZ and PDX1 protein expression in transduced AFSCs, as determined by hydrolysis of X-Gal substrate (middle) and immunostaining of mPdx1 (right). (D) A scheme showing the sequence of hAFSCs viral transduction and culture conditions during pancreatic differentiation
2.3. Induction of insulin-producing cells
hAFSCs were transduced with adenovirus (Ad-Pdx1 or Ad-LacZ) at an MOI of 10 and cultured under normal growth conditions for 48 h. Transduced cells were then replated on Matrigel™-coated dishes and cultured for 24–48 h in growth medium. High concentrations of activin A (100 ng/ml) were then added to the culture for 24–48 h. Finally, the cells were transferred to plates coated with either poly-L-ornithine (15 mg/ml, 4° C, 24 h; BD Biosciences, San Jose, CA, USA), poly-L-lysine (15 mg/ml, 4° C, 24 h; Sigma), Matrigel™ (0.5 mg/ml, 37° C, 1 h; BD Biosciences), laminin (20 mg/ml, 37° C, 3 h; BD Biosciences), collagens type-I and -IV (1 mg/ml, 20° C, 1 h; BD Biosciences) or fibronectin (50 mg/ml, 37° C, 2 h; Chemicon, Temecula, CA, USA) in knockout-DMEM with 0.1 mM 2-mercaptoethanol, 1% antibiotics, 1% L-glutamine, 1% N2, 2% B27, 1% non-essential amino acids (Gibco–Invitrogen) and 25 ng/ml bFGF (R&D Systems, Minneapolis, MN, USA). After 24–48 h, 10 mM nicotinamide (Sigma) was added and the cells were cultured for up to 5 additional weeks.
2.4. 3D culture system
Ad-Pdx1-transduced hAFSCs or clusters were combined with sodium alginate (1–1.5% w/v in 0.85% NaCl solution) at a density of about 106 cells or 100 clusters/ml. Droplets of the suspension were formed by extrusion through a 22-gauge needle and collected in 1.5% CaCl2 solution to induce gel formation. The cell-containing alginate capsules were washed with saline and coated with 0.5% poly-L-ornithine solution (Sigma; 5000 g/mol). After an additional saline wash, the poly-L-ornithine-coated capsules were suspended for 7 min in 0.12% sodium alginate solution to form an outer membrane layer and washed twice with saline. The final capsules were transferred into b-cell differentiation medium and maintained in cultures for up to 5 weeks.
2.5. Sequences of primers and probes
These are shown in Table 1.
Table 1.
Sequences of primers and probes
| Gene | Primer sequences and probe |
|---|---|
| mPdx1 | tgtaggcagtacgggtcctc |
| ccaccccagtttacaagctc | |
| hPdx1 | ccacgcagctttacaaggac |
| tgtaggccgtgcgcgtccgc | |
| hPax6 | gccaaatggagaagagaagaaaaac |
| gttgaagtggtgcccgagg | |
| Human insulin | ccagccgcagcctttgtga |
| ggtacagcattgttccacaat | |
| Adenoviral DNA | gagcgccacttctttttgtc |
| cccaactgcgacttcaagat | |
| LacZ | gagaatccgacgggttgtta |
| cagcagcagaccattttcaa | |
| mGAPDH | tgt gtccgtcgtggatctga |
| cctgcttcaccaccttcttga | |
| hGAPDH | cctgcttcaccaccttcttg |
| ccactggcgtcttcaccac | |
| mPdx1 | Mm00435565_m1 |
| hPdx1 | Hs00236830_m1 |
| hNgn3 | Hs01875204_s1 |
| hNkx6 | Hs00332012_m1 |
| hInsulin | Hs0035573_m1 |
| mGAPDH | Mm99999915_g1 |
| hGAPDH | Hs99999905_m1(from ABI) |
2.6. Enzyme-linked immunosorbent assay (ELISA)
De novo insulin production by hAFSCs transduced with Ad-Pdx1 and cultured under differentiation conditions was confirmed by measuring human c-peptide by ELISA (Mercodia, Uppsala, Sweden). Medium was collected from final stage differentiation cultures and the ELISA was performed following the manufacturer’s instructions. Reactivity was measured at 450 nm, using a plate reader (BioTek, Winooski, VT, USA). Values were converted to c-peptide concentrations using references supplied by the manufacturer.
2.7. Statistical analysis
The results are presented as average value ± standard deviation (SD). Data corresponding to each experimental condition were compared with control values in a two-tailed, unpaired Student’s t-test (p < 0.05).
3. Results
3.1. Transduction of human AFSCs with an adenovirus encoding for murine Pdx1 induces endogenous Pdx1 expression and cell cluster formation
To test whether AFSCs, ectopically expressing Pdx1, can be differentiated toward the pancreatic lineage, we transduced hAFSCs with Ad-Pdx1 (Ad-Pdx1/AFSCs), or Ad-LacZ (LacZ/AFSCs) as a control (Figure 1A). At 48 h post-transduction, the presence of adenoviral vector DNA and expression of Pdx1 and LacZ transgenes was determined by quantitative RT–PCR. A direct correlation was observed between the amounts of adenovirus used for infection and the vector DNA present in AFSCs, as well as the transgene expression level (Figure 1B). Immunostaining of AFSCs at the same time after infection was used to determine protein expression of the transgene and to confirm the transduction efficiency. After transduction of 1 × 105 hAFSCs with 1 × 106 IU Ad-Pdx1 or Ad-LacZ (MOI = 10), the efficiency was approximately 50% (Figure 1C).
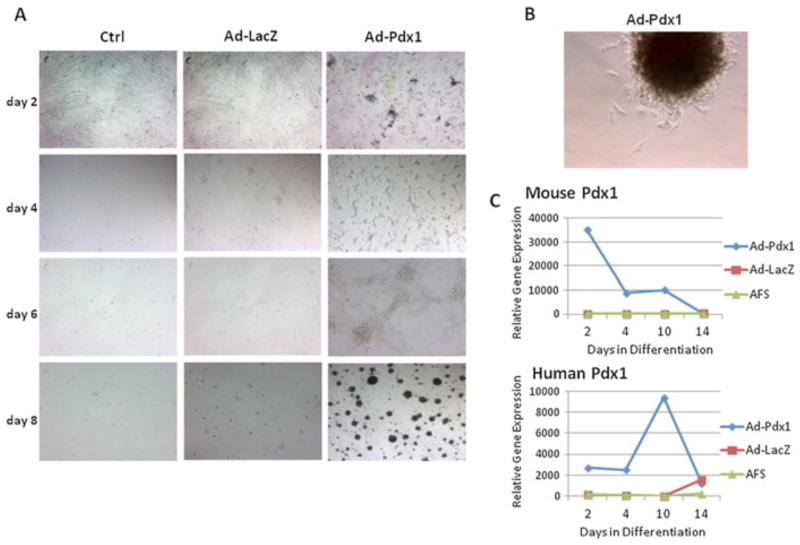
In the initial stages of differentiation, after transfer to Matrigel™, similar survival and proliferation rates were seen between the experimental Ad-Pdx1-transduced hAFSCs and the Ad-LacZ and untransduced control cells (not shown). Several growth factors were then added to the cultures to induce pancreatic differentiation; Activin A drives stem cells towards the endodermal lineage; bFGF further differentiates progenitor cells toward the pancreatic lineage and nicotinamide promotes b-cell maturation. At this stage, a clear difference in cell numbers and morphology was noticed between the groups. Under the differentiation conditions, Ad-Pdx1/AFSCs self-assembled into rounded colonies resembling islet-like clusters, while Ad-LacZ/AFSCs and untransduced hAFSCs showed poor survival and no colony formation under the same conditions (Figure 2A). Colonies derived from the Ad-Pdx1/AFSCs culture under differentiation conditions were manually isolated and replated onto tissue culture plates in knockout DMEM medium. The colonies reattached to the plastic surface and individual cells were observed migrating and proliferating, confirming that the Ad-Pdx1/AFSCs colonies are viable (Figure 2B). Colonies at each stage of differentiation were also assessed for Pdx1 gene expression by quantitative RT–PCR, using mouse and human specific probes. Expression of adenoviral-derived mouse Pdx1 peaks 2 days after transduction and decreases steadily during culture under the differentiation conditions, and becomes undetectable after 2 weeks (Figure 2C). In contrast, endogenous human Pdx1 is present at low levels early after viral transduction, peaks at day 10 and then drops again at day 14 (Figure 2C). This pattern of endogenous Pdx1 expression is consistent with normal b-cell development, with a peak in expression at the pancreatic progenitor stage and lower levels in the final insulin-producing cells.
Figure 2.
hAFSCs transduced with Ad-Pdx1 form proliferating clusters and upregulate endogenous human Pdx1. (A) Phase-contrast microscopy showing Pdx1-dependent cluster formation over 8 days after viral transduction (B) High-magnification view of one AFSC cluster replated on tissue culture plastic. (C) RT–PCR analysis showing relative fold change increase in the expression of exogenously introduced mPdx1 and the resulting endogenous hPDX1 expression over a 2 week time course
3.2. Combination of Ad-Pdx1 transduction and growth factor supplementation induces pancreatic development in hAFSCs
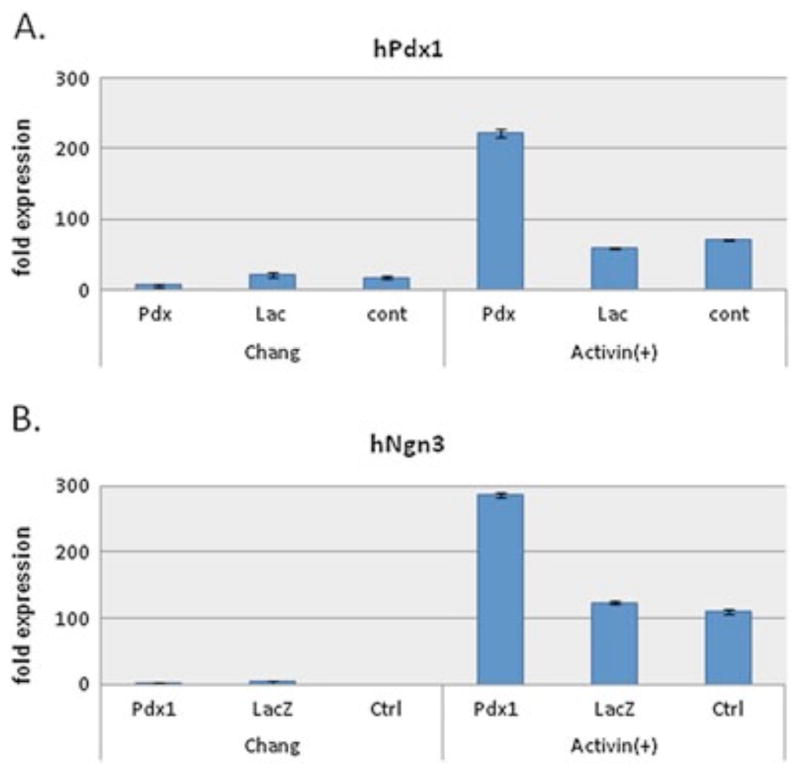
Ectopic expression of Pdx1 is required to initiate pancreatic differentiation but additional inducers are required for the subsequent steps in the differentiation sequence. Therefore, the effect of individual growth factors on the differentiation of hAFSCs was examined. AFSCs were plated on Matrigel™-coated dishes and transduced with Ad-Pdx1, Ad-LacZ or untransduced (control), then cultured in the presence or absence of 100 ng/ml activin A for 2 days. The effect of activin A was analysed by harvesting cells at this early stage in lineage specification, extracting RNA and performing quantitative RT–PCR for human Pdx1 and Ngn3. As expected, in the presence of activin A, endogenous human Pdx1 was induced four-fold above Ad-LacZ and untransduced controls (Figure 3A). More interestingly, Ngn3 expression, which is important for pancreatic differentiation, was also upregulated approximately three-fold in Ad- Pdx1/AFSCs cultured with activin A compared to controls (Figure 3B). However, higher than baseline Ngn3 expression was also observed in activin A-containing cultures that were not transduced with Ad-Pdx1. Since Ngn3 is critical in b-cell specification, but is not expressed in mature b-cells, these results suggest that, in the presence of activin-A, AFSCs cells can differentiate along the pancreatic lineage but are immature at this point.
Figure 3.
Activin A upregulates endogenous PDX1 and NGN3 expression in Ad-Pdx1-transduced AFSCs. RT–PCR analysis showing expression of human PDX1 (A) and NGN3 (B) after Ad-Pdx1 transduction and exposure to activin A-containing induction medium compared to transduced cultures grown in Chang medium alone
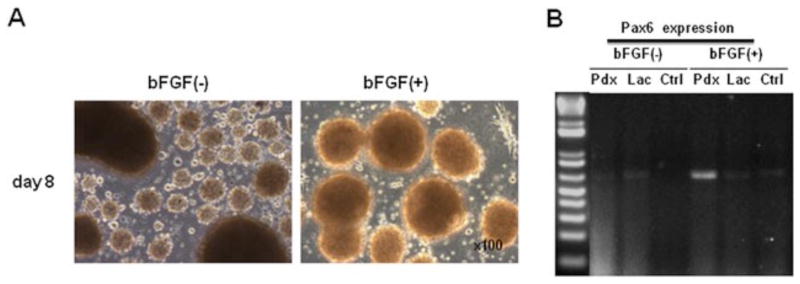
The FGF pathway cooperates with activin A and BMP to promote endoderm and pancreatic differentiation. Transduced AFSCs grown in the presence of activin A were replated onto poly-L-ornithine and cultured in the presence or absence of 25 ng/ml bFGF for 2 days. Morphological analyses of these cultures revealed that bFGF induced large, uniform islet-like cluster formation at this stage (Figure 4A). Gene expression analysis of these clusters revealed specific Ad-Pdx1-induced upregulation of Pax6 expression only in cells cultured in the presence of bFGF (Figure 4B).
Figure 4.
Basic FGF sustains cluster formation of Ad-Pdx1/AFSCs and induces PAX6 gene expression. (A) Phase-contrast microscopy showing Ad-Pdx1/AFSC clusters cultured in the presence and absence of bFGF for 8 days. (B) RT–PCR showing Pax6 expression, dependent on Pdx1 transduction and bFGF
3.3. Pdx1 transduction and matrix proteins induce insulin gene expression and c-peptide release in hAFSCs
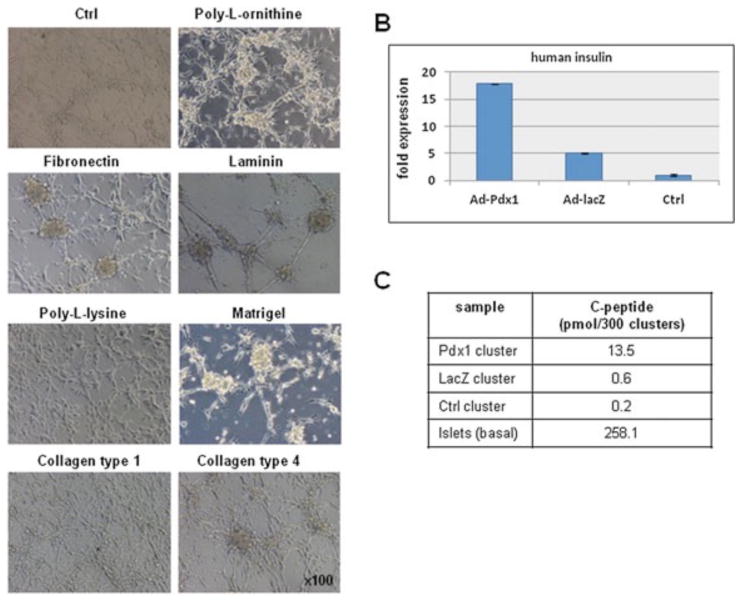
The effects of cell–matrix interactions on the formation islet-like clusters, and their further differentiation along the endocrine pancreatic lineage, was investigated plating Ad-Pdx1/AFSCs, which have been exposed to activin A, onto the following matrices: poly-L-ornithine, fibro nectin, laminin, poly-L-lysine, Matrigel™, collagen type collagen type 4 and uncoated tissue culture dishes. The cells were then exposed to bFGF for 2 days and visible cluster formation was observed on several of the matrices at this stage, including poly-L-ornithine, fibronectin, laminin, Matrigel™ and collagen 4, but not on poly-L-lysine or collagen type 1 (Figure 5A).
Figure 5.
The effect of extracellular matrix proteins on Ad-Pdx1/AFSCs cluster formation and insulin expression. (A) Phase-contrast microscopy demonstrating the effect of extracellular matrix proteins on the morphology of Ad-Pdx1/AFSCs clusters. (B) Insulin-specific RT–PCR gene expression analysis on AFSCs clusters cultured on poly-L-ornithine then transferred to nicotinamide-containing induction medium. (C) Quantitation of c-peptide protein expression from culture media of the cells described in (B), using human-specific ELISA
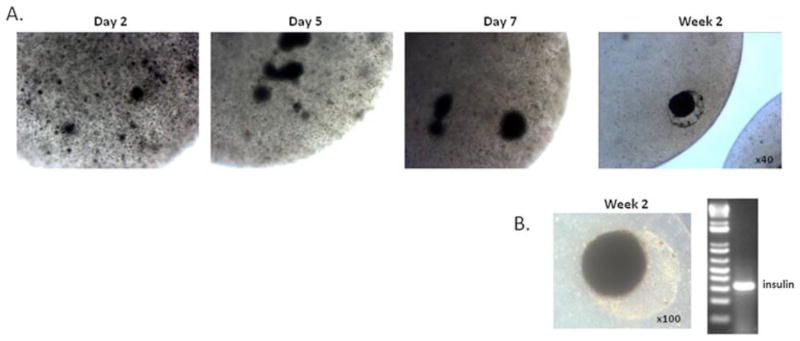
AFSC clusters, on each of these matrix conditions, were cultured in the presence of nicotinamide in order to further their differentiation toward insulin-producing cells. After 3 weeks under these conditions, RNA was extracted from the clusters and gene expression analysis revealed low levels of insulin mRNA only in Ad-Pdx1/AFSCs cultured on poly-L-ornithine coating (Figure 5B). To further confirm insulin production, medium was collected and a c-peptide ELISA was performed. The ELISA results showed that, after more than 3 weeks under differentiation conditions, Ad-Pdx1/AFSCs clusters produced 13.5 ± 0.8 pM c-peptide/300 clusters, Ad-LacZ/AFSCs produced 0.6 ± 0.09 pM/300 clusters and untransduced AFSCs produced 0.2 ± 0.07 pM/300 clusters (n = 3 for each condition) (Figure 5C). These results indicate de novo insulin synthesis in Ad-Pdx1/AFSCs. Alginate encapsulation of hAFSC-derived insulin-producing clusters was performed to enhance the 3D organization of the cells and to confirm the ability of the clusters to secrete insulin long-term for future therapeutic use. Alginate-encapsulated Ad-Pdx1/AFSC clusters showed a visible increase in size during the first week after encapsulation, indicating active cell proliferation (Figure 6A). Insulin mRNA expression 2 weeks after the encapsulation revealed a strong insulin signal by RT–PCR (Figure 6B).
Figure 6.
Alginate encapsulation maintains AFSCs clusters and insulin expression for 2 weeks in culture. (A) Phase-contrast microscopy of alginate capsules containing partially differentiated AFSCs clusters over a 2 week time course. (B) Representative encapsulated cluster and its corresponding insulin expression, as detected by RT–PCR
4. Discussion
This study investigated whether introducing a key transcription factor, Pdx1, was sufficient to initiate the differentiation of hAFSCs toward the pancreatic lineage and adopt a phenotype resembling mature b-cells. Here we have described pancreatic lineage differentiation of AFSCs transiently overexpressing exogenous Pdx1 and cultured in inductive medium containing sequential addition of activin A, bFGF and nicotinamide. Under inductive conditions, Pdx1-expressing AFSCs assembled to form uniform clusters expressing endogenous Pdx1 and the pancreatic lineage markers Ngn3 and Pax6. AFSC-derived clusters expressed insulin in combination with c-peptide release and were able to be maintained in alginate for several weeks.
Pdx1 is indispensible in pancreatic development and the maintenance of b-cell function and is expressed in all pancreatic precursors; however, its expression is limited to b-cells in the mature pancreas (Guz et al., 1995; Hui et al., 2002). In b-cells, the target phenotype for this study, PDX1 regulates the expression of insulin, somatostatin, islet amyloid polypeptide (IAPP), glucokinase (GK) and the glucose transporter, GLUT-2 (Hui et al., 2002). hAFSCs transduced with mouse Pdx1 transiently expressed the transgene and responded by spontaneously forming islet-like clusters that expressed other downstream markers of pancreatic differentiation (such as Ngn3 and Pax6) after being transferred to media containing activin A, bFGF and nicotinamide. Under the same conditions, the uninfected or Ad-LacZ-infected hAFSCs survived poorly and cluster number and size were reduced significantly.
Growth factor interactions and are a critical part of pancreatic lineage specification and development. Recent results demonstrate that ES-derived embryoid bodies (EBs) exposed to the nodal mimetic activin A alone do not express pancreatic-specific markers, while EBs exposed to activin A, BMP4 and bFGF resulted in cells resembling pancreatic progenitors (Xu et al., 2011). Our results are consistent with the combinatorial effect of activin A and bFGF, although we did not include BMP4 as part of our induction medium. Instead we chose to include nicotinamide, a vitamin supplement which has also been shown to induce fetal pancreatic cells into insulin-positive cells in vitro (Szukiewicz et al., 2010). The combination of Pdx1 transduction and exposure to activin A induced endogenous Ngn3 expression. However, activin A treatment alone also induced a low level of Ngn3 expression. This result is somewhat surprising, given that ES cells treated with activin A only differentiate into definitive endoderm expressing Sox17 and FoxA2, but will not reach the pancreatic endoderm or endocrine progenitor stage, as evidenced by the lack of Ngn3 expression (Kroon et al., 2008).
Zhou et al. (2007) recently proposed a tip-trunk model of pancreatic development to explain how a multipotent progenitor pool, located in the tip of the branching pancreatic tree, can give rise to both endocrine and exocrine pancreas. This model suggests that carboxypeptidase A1 (Cpa1)-expressing tip cells make up a progenitor pool and, upon division, leave behind elongated branches (trunks) containing NGN3-positive cells that differentiate into islets. Ngn3 expression is necessary for the induction of the endocrine lineage and is dependent on Notch signalling in the tip progenitor cells (Jensen et al., 2000). Ngn3 is transiently expressed in endocrine progenitors and it is never co-expressed with endocrine hormones in the mature islets (Schwitzgebel et al., 2000). Overexpression of Ngn3 can induce all four endocrine lineages (a-, b-, d- and e-cells) (Apelqvist et al., 1999; Schwitzgebel et al., 2000; Grapin-Botton et al., 2001). Therefore, expression of Ngn3 downstream of Pdx1 in partially differentiated AFSC clusters is consistent with pancreatic endoderm differentiation. However, Pdx1 has been shown to occupy an evolutionarily conserved transcriptional enhancer sequence in the Ngn3 gene during pancreatic development (Oliver-Krasinski et al., 2009), so it remains possible that ectopic Pdx1 expression in AFSCs is driving Ngn3 expression directly. In this case, evidence of Pax6 expression after Pdx1 transduction, in the presence of bFGF, suggests that the partially differentiated AFSCs most closely resemble an endocrine progenitor, prior to the a-cell/b-cell lineage split. PAX6, along with several of transcription factors like cMaf and MafB, is involved with glucagon biosynthesis and secretion, specific to the a-cell lineage (Gosmain et al., 2011). In addition, it plays a critical role in regulation of islet number, morphology and hormone gene expression in the adult but is not involved in b-cell neogenesis during late embryonic pancreatic development (Ashery-Padan et al., 2004).
Several studies have shown that interactions between cells and their ECM improve the efficiency of b-cell differentiation and preserve islet function in vitro (Rosenberg et al., 1999; Daoud et al., 2010a, 2010b, 2011). The primary matrix components of the basement membrane that surrounds an islet are collagens-I and -IV, fibronectin and laminin. Integrins mediate adherence and signalling between islets and the components of the peri-insular basement membrane and have a direct impact on survival and function of the b-cells. Certain islet functions can be separated in terms of how they interact with their extracellular matrix. For example, aVb3 and aVb5 regulate adhesion and differentiation, while a3b1 and a6b1 participate in the regulation of insulin secretion (Cirulli et al., 2000; Kantengwa et al., 1997; Ris et al., 2002). The goal of incorporating ECM components into our long-term culture conditions was to determine whether re-establishing cell–matrix and, by extension, cell–cell interactions would have any effect on survival and further differentiation of the AFSC clusters. Ad-Pdx1/AFSC clusters that were exposure to activin A, bFGF and nicotinamide survived for a prolonged time (21 days) on poly-L-ornithine and secreted insulin into the medium, as determined using an ultra-sensitive ELISA kit from Mercodia to avoid a false assessment of insulin secretion due to uptake of exogenous insulin from the medium (Hansson et al., 2004; Paek et al., 2005). This observation is consistent with the role the ECM plays in an intact islet and justifies further investigation into the role of ECM in the development of artificial culture systems for b-cell-directed differentiation from stem cells.
In the present study, we have achieved in vitro insulin-production from AFSCs transiently expressing exogenous Pdx1. In our pancreatic differentiation protocol we observed induction of pancreatic lineage genes, including endogenous Pdx1, Ngn3 and Pax6. In addition, we observed the formation of uniform cell clusters capable of producing insulin and secreting c-peptide. Partially differentiated AFSC clusters not only survived but proliferated for an additional 2 weeks after encapsulation in 1.5% alginate/poly-L-ornithine and continued to secrete insulin at levels higher than before encapsulation. The ability of AFSCs to respond to ectopic Pdx1 expression and spontaneously form islet-like clusters that can survive for weeks on synthetic matrix and secrete insulin demonstrates that AFSCs could serve as a renewable source of b-cells to treat T1DM.
References
- Ai C, Todorov I, Slovak ML, et al. ; Human marrow-derived mesodermal progenitor cells generate insulin-secreting islet-like clusters in vivo. Stem Cells Dev. 2007;16:757–770. doi: 10.1089/scd.2007.0038. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, et al. ; Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Zhou X, Marquardt T, et al. ; Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev Biol. 2004;269:479–488. doi: 10.1016/j.ydbio.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Cirulli V, Beattie GM, Klier G, et al. ; Expression and function of avb3 and avb5 integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150:1445–1460. doi: 10.1083/jcb.150.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, et al. ; Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. ; The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials. 2010a;31:1676–1682. doi: 10.1016/j.biomaterials.2009.11.057. [DOI] [PubMed] [Google Scholar]
- Daoud J, Rosenberg L, Tabrizian M. ; Pancreatic islet culture and preservation strategies: advances, challenges, and future outlook. Cell Transplant. 2010b;19:1523–1535. doi: 10.3727/096368910X515872. [DOI] [PubMed] [Google Scholar]
- Daoud JT, Petropavlovskaia MS, Patapas JM, et al. ; Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds. Biomaterials. 2011;32:1536–1542. doi: 10.1016/j.biomaterials.2010.10.036. [DOI] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. ; Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Draper JS, Pigott C, Thomson JA, Andrews PW. ; Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M, Benvenisty N. ; The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136–141. doi: 10.1016/j.tibtech.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Drukker M, Katz G, Urbach A, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, et al. ; Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Oh SH, Pi L, et al. ; Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. 2005;166:1781–1791. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepts W. ; Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Gosmain Y, Cheyssac C, Heddad Masson M, et al. ; Glucagon gene expression in the endocrine pancreas: the role of the transcription factor Pax6 in a-cell differentiation, glucagon biosynthesis and secretion. Diabetes Obes Metab. 2011;13(suppl 1):31–38. doi: 10.1111/j.1463-1326.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA. ; Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444–454. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz Y, Montminy MR, Stein R, et al. ; Expression of murine STF-1, a putative insulin gene transcription factor, in b-cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- Hansson M, Tonning A, Frandsen U, et al. ; Artifactual insulin release from differentiated embryonic stem cells. Diabetes. 2004;53:2603–2609. doi: 10.2337/diabetes.53.10.2603. [DOI] [PubMed] [Google Scholar]
- Hui H, Perfetti R. ; Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002a;146:129–141. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- Hui H, Perfetti R. ; Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002b;146:129–141. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- Ianus A, Holz GG, Theise ND, Hussain MA. ; In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkin-Ansari P, Demeterco C, Bossie S, et al. PDX-1 and cell–cell contact act in synergy to promote d-cell development in a human pancreatic endocrine precursor cell line. Mol Endocrinol. 2000;14:814–822. doi: 10.1210/mend.14.6.0476. [DOI] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, et al. ; Independent development of pancreatic a- and b-cells from neurogenin 3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Jiang FX, Stanley EG, Gonez LJ, Harrison LC. ; Bone morphogenetic proteins promote development of fetal pancreas epithelial colonies containing insulin-positive cells. J Cell Sci. 2002a;115:753–760. doi: 10.1242/jcs.115.4.753. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. ; Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002b;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jiang J, Au M, Lu K, et al. ; Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007a;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- Jiang W, Shi Y, Zhao D, et al. In vitro derivation of functional insulin-producing. Cell Res. 2007b;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- Kantengwa S, Baetens D, Sadoul K, et al. ; Identification and characterization of a3b1 integrin on primary and transformed rat islet cells. Exp Cell Res. 1997;237:394–402. doi: 10.1006/excr.1997.3803. [DOI] [PubMed] [Google Scholar]
- Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. ; Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–2844. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, et al. ; Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, et al. ; Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Le Bras S, Miralles F, Basmaciogullari A, et al. ; Fibroblast growth factor 2 promotes pancreatic epithelial cell proliferation via functional fibroblast growth factor receptors during embryonic life. Diabetes. 1998;47:1236–1242. [PubMed] [Google Scholar]
- Lechner A, Habener JF. ; Stem/progenitor cells derived from adult tissues: potential for the treatment of diabetes mellitus. Am J Physiol Endocrinol Metab. 2003;284:E259–E266. doi: 10.1152/ajpendo.00393.2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang R, Qiao H, et al. ; Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol. 2007;211:36–44. doi: 10.1002/jcp.20897. [DOI] [PubMed] [Google Scholar]
- Li G, Huang LS, Jiang MH, et al. ; Implantation of bFGF-treated islet progenitor cells ameliorates streptozotocin-induced diabetes in rats. Acta Pharmacol Sin. 2010;31:1454–1463. doi: 10.1038/aps.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang S, Liu H, et al. ; Neuronal restrictive silencing factor silencing induces human amniotic fluid-derived stem cells differentiation into insulin-producing cells. Stem Cells Dev. 2011;20:1223–1231. doi: 10.1089/scd.2010.0195. [DOI] [PubMed] [Google Scholar]
- Moorefield EC, McKee EE, Solchaga L, et al. ; Cloned, CD117-selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS One. 2011;6:e26535. doi: 10.1371/journal.pone.0026535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogneva V, Martinova Y. ; The effect of in vitro fibroblast growth factors on cell proliferation in pancreas from normal and streptozotocin-treated rats. Diabetes Res Clin Pract. 2002;57:11–16. doi: 10.1016/s0168-8227(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Oh SH, Muzzonigro TM, Bae SH, et al. ; Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84:607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- Oliver-Krasinski JM, Kasner MT, Yang J, et al. ; The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. J Clin Invest. 2009;119:1888–1898. doi: 10.1172/JCI37028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SY, Dai H, Leong KW. ; Hepatic differentiation potential of commercially available human mesenchymal stem cells. Tissue Eng. 2006;12:3477–3485. doi: 10.1089/ten.2006.12.3477. [DOI] [PubMed] [Google Scholar]
- Paek HJ, Morgan JR, Lysaght MJ. ; Sequestration and synthesis: the source of insulin in cell clusters differentiated from murine embryonic stem cells. Stem Cells. 2005;23:862–867. doi: 10.1634/stemcells.2004-0288. [DOI] [PubMed] [Google Scholar]
- Perin L, Sedrakyan S, Giuliani S, et al. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse. 2010;5:e9357. doi: 10.1371/journal.pone.0009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris F, Hammar E, Bosco D, et al. ; Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human b-cells in vitro. Diabetologia. 2002;45:841–850. doi: 10.1007/s00125-002-0840-7. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Bhonde RR. ; Suitability of cellulose molecular dialysis membrane for bioartificial pancreas: in vitro biocompatibility studies. J Biomed Mater Res. 2001;54:436–444. doi: 10.1002/1097-4636(20010305)54:3<436::aid-jbm180>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Wang R, Paraskevas S, Maysinger D. ; Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126:393–398. [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, et al. ; Expression of neurogenin 3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, et al. ; Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering BJ, et al. ; International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen L, Hou XG, et al. ; Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007;120:771–776. [PubMed] [Google Scholar]
- Szukiewicz D, Pyzlak M, Stangret A, et al. ; Decrease in expression of histamine H2 receptors by human amniotic epithelial cells during differentiation into pancreatic b-like cells. Inflamm Res. 2010;59(suppl 2):S205–S207. doi: 10.1007/s00011-009-0131-6. [DOI] [PubMed] [Google Scholar]
- Tang DQ, Cao LZ, Burkhardt BR, et al. ; In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006;12:RA130–RA147. [PubMed] [Google Scholar]
- Truong W, Shapiro AM. Progress in islet transplantation in patients with type 1 diabetes mellitus. Treat Endocrino. 2006;15:147–158. doi: 10.2165/00024677-200605030-00003. [DOI] [PubMed] [Google Scholar]
- Wu H, Dmitriev I, Kashentseva E, et al. ; Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J Virol. 2002;76:12775–12782. doi: 10.1128/JVI.76.24.12775-12782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QP, Huang H, Xu B, et al. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation. 2009;77:483–491. doi: 10.1016/j.diff.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Xu X, Browning VL, Odorico JS. ; Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev. 2011;128:412–427. doi: 10.1016/j.mod.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye DZ, Tai MH, Linning KD, et al. ; MafA expression and insulin promoter activity are induced by nicotinamide and related compounds in INS-1 pancreatic, 8-cells. Diabetes. 2006;55:742–750. doi: 10.2337/diabetes.55.03.06.db05-0653. [DOI] [PubMed] [Google Scholar]
- Zalzman M, Anker-Kitai L, Efrat S. ; Differentiation of human liver-derived, insulin-producing cells toward the, 8-cell phenotype. Diabetes. 2005;54:2568–2575. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, et al. ; A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]