Abstract
Background
HIV-1 establishes a life-long infection in the human body, but host factors that influence viral persistence remain poorly understood. Cell-intrinsic characteristics of CD4 T cells, the main target cells for HIV-1, may impact the composition of the latent viral reservoir by altering the susceptibility to CD8 T cell mediated killing.
Results
We observed that susceptibilities of CD4 T cells to CD8 T cell-mediated killing, as determined in direct ex-vivo assays, were significantly higher in persons with natural control of HIV-1 (elite controllers) than in individuals effectively treated with antiretroviral therapy. These differences were most pronounced in naïve and in terminally-differentiated CD4 T cells, and corresponded to a reduced viral reservoir size in elite controllers. Interestingly, the highest susceptibility to CD8 T cell-mediated killing and lowest reservoirs of cell-associated HIV-1 DNA was consistently observed in elite controllers expressing the protective HLA class I allele B57.
Conclusion
These data suggest that the functional responsiveness of host CD4 T cells to cytotoxic effects of HIV-1-specific CD8 T cells can contribute to shaping the structure and composition of the latently infected CD4 T cell pool.
Keywords: HIV-1, viral reservoir, cytotoxic T cells, viral persistence
Introduction
Although active replication of HIV-1 can be effectively suppressed with antiretroviral combination therapy, HIV-1 persists lifelong in the human body by establishing a latent, transcriptionally silent infection in CD4 T cells [1, 2]. Central-memory and effector-memory CD4 T cells may represent the predominant reservoir for these latently infected cells [3], however, naïve CD4 T cells, a long-lasting, antigen-inexperienced quiescent T cell population, also contribute to the latently infected cell pool [4]. Current efforts focus on mobilizing this reservoir by pharmaceutical agents that can reactivate HIV-1 replication in latently infected cells [5], however, reactivation of HIV-1 replication itself may not necessarily eliminate these cells, and combinations of immune-mediated and pharmaceutical interventions may ultimately be necessary to reduce viral persistence.
HIV-1-specific CD8 T cells represent an important component of the natural immune response to HIV-1, and can kill HIV-1 infected cells by MHC class I restricted cytolysis. Such cells are observed in the vast majority of HIV-1 infected patients, but their functional properties and antiviral effectiveness vary. The most powerful HIV-1-specific CD8 T cells can be detected in elite controllers, a small group of HIV-1 infected patients who, in the absence of antiretroviral therapy, maintain undetectable levels of HIV-1 replication [6–8]. HIV-1-specific CD8 T cells from elite controllers can effectively inhibit active HIV-1 replication in autologous CD4 T cells in vitro [9–11], and can also facilitate elimination of autologous latently infected CD4 T cells after viral reactivation with pharmaceutical agents [12].
Such superior antiviral effects of HIV-1-specific CD8 T cells from elite controllers have mainly been attributed to specific functional characteristics of these cells. For instance, prior work demonstrated a “polyfunctional” profile of HIV-1-specific T cells from controllers with improved antigen-specific proliferation and cytokine secretion [6, 8], optimized affinity/avidity to the target epitope [13, 14] and enhanced intracellular expression of the cytotoxic molecules perforin and granzyme B [7, 15]. However, killing of HIV-1-infected CD4 T cells by CD8 T cells involves a bidirectional cellular interaction that, in addition to CD8 T cell functionality, critically depends on the susceptibility of CD4 T cells to CD8 T cell mediated killing. Previous studies have indeed shown that leukocellular subsets differ substantially with regard to their responsiveness to cytotoxic effects of HIV-1-specific CD8 T cells [16]. Whether such differences influence the cellular composition and structure of the latent HIV-1 reservoir in persons with durable suppression of active HIV-1 replication is not known.
To better understand host factors that shape the size and cellular composition of the latent HIV-1 reservoir, we here systematically investigated the susceptibility of CD4 T cell subsets to cytotoxic effects of HIV-1-specific CD8 T cells in individuals with spontaneous viral control, or HAART-mediated suppression of viral replication. Our results show that the responsiveness of CD4 T cells to CD8 T cell-mediated killing is significantly elevated in elite controllers, and inversely correlated to the size of the viral reservoir, particularly in naïve CD4 T cells. Together these data suggest that cell-intrinsic functional characteristics in host CD4 T cells modulate the dynamics of viral persistence in these cells.
Patients and Methods
Patients
PBMC samples from HIV-infected individuals and HIV-1–negative persons were used for this study according to protocols approved by the Institutional Review Board of Massachusetts General Hospital. All study participants gave written informed consent. Demographic and clinical characteristics of the study cohorts are summarized in Table 1.
Table 1.
Clinical and demographical characteristics of study patients.
| HIV negative persons | HAART-treated patients | Elite Controllers | |
|---|---|---|---|
| Age (years) (median, range) | 42 (23–68) | 44 (21–67) | 47 (22–70) |
| Females (%) | 10 | 4 | 33 |
| CD4 count (cells μl−1) (median, range) | 870 (658–1257) | 765 (372–1337) | 825 (112–1583) |
| Viral load (copies ml−1) | NA | <50 | <50 |
| Duration of undetectable HIV-1 viremia (years) (median, range) | NA | 8 (4–10) | 9 (5–14) |
| HLA class I types | A2 (n=8) B8 (n=4) B57 (n=10) B57/A2 (n=1) B57/B8/A2 (n=1) |
A2 (n=10) B8 (n=5) B57 (n=12) B57/A2 (n=1) |
A2 (n=10) B8 (n=6) B57 (n=13) |
Generation of CD8 T cell clones
CTL clones targeting the HLA-B57-restricted epitopes KF11 (KAFSPEVIPMF) and TW10 (TSTLQEQIGW), the HLA-A2-restricted epitope SL9 (SLYNTVATL) and the HLA-B8-restricted epitope EI8 (EIYKRWII) (all epitopes in HIV-1 Gag) were generated by limiting dilution as previously described [17].
Flow cytometry
For phenotypic characterization of CD4+ T cells, cells were stained with a viability dye and antibodies directed against CD4 and other surface markers, as indicated. After 20 minutes, cells were washed, fixed in paraformaldehyde, and acquired on a LSRII flow cytometer (BD Biosciences). Data were analyzed using FlowJo software.
Ex vivo cytotoxicity assay
Susceptibilities to CD8 T cell mediated killing were investigated using a previously-described cytotoxicity assay [16]. Target CD4 T cells were pulsed with the indicated epitopic peptides at 10 μg/ml for 1 hr at 37°C. CD4 T cells were then stained with fluorescently-conjugated antibodies against CD4, CCR7 and CD45RA (all antibodies from Becton Dickinson) and FITC-labeled Annexin V at room temperature for 15 min. HLA-matched effector CD8 T cell clones were added and co-cultured with target cells at the E:T ratios of 1:1 for 60 minutes, followed by surface staining with APC-labeled Annexin V. Unpulsed and peptide-pulsed target cells without exposure to CD8 T effector cells and CD8 T effector cells mixed with unpulsed target cells were used as controls. Cells were acquired on a LSR-II flow cytometer (BD Biosciences, CA), using DiVA software. Data were analyzed using FlowJo software (TreeStar). To calculate the susceptibility of cells to epitope-specific cytotoxicity, the proportions of Annexin V-positive cells were normalized to proportions of Annexin V-positive cells prior to co-incubation with effector cells, and the background from negative controls was subtracted.
Analysis of HIV-1-specific CD8 T cells
ELISPOT assays were performed as described previously to quantify the magnitude of HIV-1-specific CD8 T cells [17, 18]. PBMCs were plated in 96-well polyvinylidene plates that had been precoated with 0.5 μg/ml of an anti–human interferon γ mAb (Mabtech). PBMCs were added at a concentration of 50,000–100,000 cells per well in a volume of 100μl. Plates were incubated overnight at 37°C, 5% CO2, and developed on the following day. To calculate the number of specific T cells, the number of spots in the negative control wells was subtracted from the counted number of spots in each well. Responses were considered positive if there were >50 spot-forming cells (SFCs)/106 PBMCs and at least three times the mean number of SFCs of the three control wells.
Quantification of HIV-1 DNA
Total, naïve and memory CD4 T cells were isolated by immunomagnetic selection using commercial kits according to the manufacturer’s protocol (Miltenyi). Purity of cells was confirmed by flow cytometry and was >95%. Isolated CD4 T cells were digested as previously described [3] to extract cell lysates. We amplified total HIV-1 DNA with primers and probes previously described [19]. As a standard curve, we amplified serial dilutions of chronically infected 293T cells (kindly provided by Dr. Bushman, University of Pennsylvania). Proviral HIV-1 DNA copy numbers were calculated relative to CCR5 gene copy number previously quantified with the same standards.
Statistics
Pearson’s correlation coefficient was calculated to analyze correlations. Differences between nominal data were tested for statistical significance by 2-tailed Student’s t test, Mann-Whitney U test, or paired Wilcoxon test as appropriate.
Results
Higher susceptibility of CD4 T cells from elite controllers to cytotoxic effects of CD8 T cells
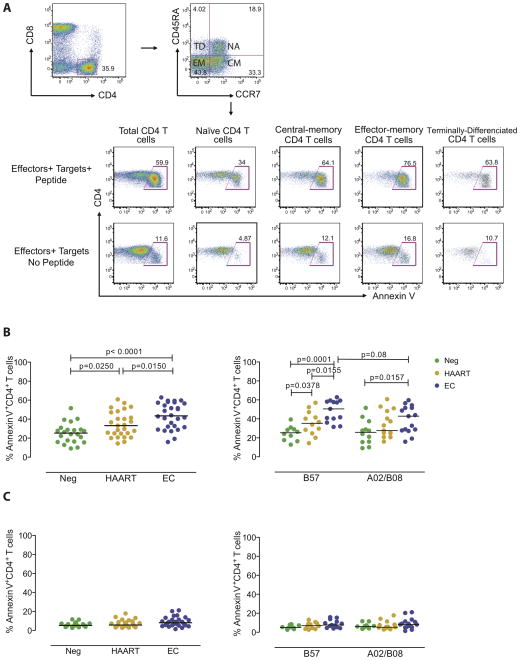
To analyze the susceptibility of target cells to HIV-1-specific CD8 T cell killing, we pulsed CD4 T cells from HIV-1 negative individuals, HAART-treated persons and elite controllers with antigenic peptides corresponding to HLA-B8-, HLA-B57- and HLA-A2-restricted immunodominant CD8 T cell epitopes in HIV-1 Gag (B8-EI8, B57-TW10, B57-KF11, A2-SL9), followed by co-culture with HIV-1-specific CD8 T cell clones targeting these epitopes. Antigen-specific killing of target cells was then detected in CD4 T cells by flow cytometric detection of Annexin V, as described in a previously-published protocol [16]. An example for the flow-cytometric assessment of CD4 T cell susceptibility to cytotoxic effects of CD8 T cells is demonstrated in Figure 1A, and the demographic and clinical characteristics of the three different study cohorts are summarized in Table 1.
Figure 1. Increased susceptibility of CD4 T cells from elite controllers to CD8 T cell-mediated killing.
(A) Representative dot plots reflecting the proportions of Annexin V-positive CD4 T cells following exposure to A2-SL9-specific CD8 T cells, with or without prior pulsing of target cells with the epitopic peptide. Data from bulk CD4 T cells and indicated CD4 T cells subsets are shown. (B–C) Proportions of Annexin V-positive CD4 T cells from HIV-1 negative persons (Neg), HAART-treated subjects (HAART) or elite controllers (EC) after co-culture with identical immunodominant HIV-1-specific CD8 T cell populations (B), or without exposure to HIV-1-specific CD8 T cell clones (C). Left panels reflect data from all individuals in each study cohort, right panels indicated data from subgroups of patients expressing HLA-B57 or HLA-A2/HLA-B8. Significance was tested using Mann-Whitney U tests.
Overall, we observed that the susceptibility of CD4 T cells to HIV-1-specific CD8 T cell mediated killing was substantially higher in elite controllers, compared to CD4 T cells from HAART-treated persons or HIV-1 negative individuals (Figure 1B). These differences were most significant after exposure to CD8 T cell clones restricted by the protective HLA class I allele HLA-B57. Susceptibilities to the HLA-A2 or –B8 restricted CD8 T cells were not statistically significantly different between elite controllers and HAART-treated persons, although there was a trend for higher levels of susceptibility in elite controllers (Figure 1B). Since spontaneous cell death rates can influence the susceptibility of CD4 T cells to CD8 T cell mediated killing, we simultaneously analyzed Annexin V expression in CD4 T cells from the study subjects in the absence of CD8 T effector cells; however, these did not substantially differ among the different study cohorts (Figure 1C). Because the level of cellular activation may influence the susceptibility to CD8 T cell mediated killing, we analyzed the expression of activation surface markers, including HLA class I, HLA-DR and CD38 on CD4 T cells from the different study cohorts. In line with previous reports, expression of these cell surface markers was slightly higher in HAART-treated persons compared to elite controllers and HIV-1 negative persons, but there was no correlation between these markers and corresponding levels of susceptibility to CD8 T cell killing, neither within elite controllers nor HAART-treated patients or HIV-1 negative persons (data not shown); this suggests that possible differences between the levels of HLA class I-mediated CTL epitope presentation in the different CD4 T cell subsets were not responsible for the observed effects. Overall, these experiments indicate elevated susceptibilities of CD4 T cells from elite controllers to CD8 T cell-mediated killing, specifically in the context of restriction by the protective HLA class I allele B57.
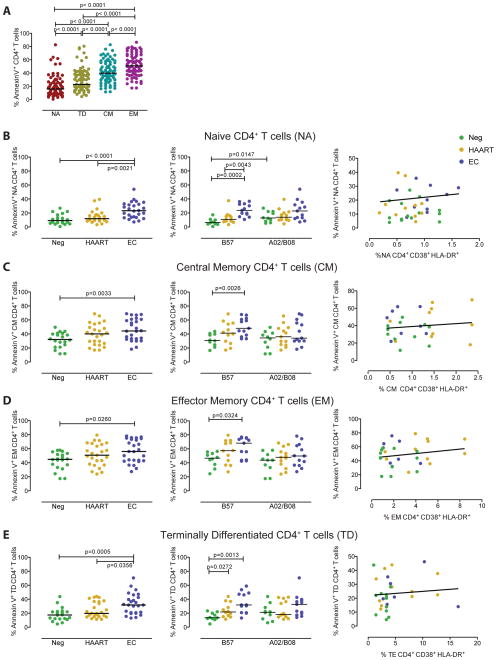
Cell subset-specific differences in susceptibility to CD8 T cell killing
CD4 T cells consist of distinct subsets which may differ with regard to their susceptibility to CD8 T cell mediated killing. To investigate this, we selectively analyzed the susceptibility of CCR7+ CD45RA+ naïve, CCR7+ CD45RA− central-memory, CCR7− CD45RA− effector-memory and CCR7− CD45RA+ terminally-differentiated CD4 T cells to cytotoxic effects of the described four HIV-1-specific CD8 T cell clones. Overall, we observed that the susceptibility to HIV-1-specific CD8 T cell-mediated killing was highest in effector-memory CD4 T cells, followed by central-memory CD4 T cells, terminally-differentiated CD4 T cells and naïve CD4 T cells; this hierarchy was seen across all analyzed patient populations (Figure 2A). Interestingly, we observed that among naïve CD4 T cells, susceptibility to CD8 T cell killing was highest in elite controllers and significantly exceeded corresponding levels in HIV-1 negative persons and HAART-treated individuals. Again, this difference was most strongly detectable in the context of epitope restriction by HLA-B57, but a similar trend was also seen for the epitopes restricted by HLA-A2 and –B8 (Figure 2B). Similar, but less pronounced effects were observed for central-memory and terminally-differentiated CD4 T cells from elite controllers, which both had higher susceptibilities to CD8 T cell killing compared to the two alternative study cohorts; again, such differences were more obvious in the context of restriction by the protective HLA class I allele B57 (Figure 2C/E). Differences between the study cohorts were least pronounced for effector-memory CD4 T cells, but a trend for higher susceptibility to HLA-B57-restricted CD8 T cells remained also visible within this subset (Figure 2D). To test whether increased susceptibilities of CD4 T cell subsets to CD8 T cell-mediated killing were related to an altered activation status of the cells, we analyzed the phenotypic expression of HLA class I, HLA-DR and CD38 in the different T cell subsets. Yet, none of these markers correlated with susceptibility to CD8 T cell-mediated killing of the respective CD4 T cell subsets (Figure 2B–E). These data indicate that naïve CD4 T cells, and to a lesser extent terminally-differentiated, central-memory and effector-memory CD4 T cells from elite controllers have higher susceptibilities to cytotoxic effects of CD8 T cells, particularly in context of restriction by the protective HLA class I allele B57.
Figure 2. CD4 T cell subset-specific susceptibility to cytotoxic effects of HIV-1-specific CD8 T cells.
(A): Proportions of Annexin V-positive CD4 T cells with naïve (NA), terminally-differentiated (TE), central-memory (CM) and effector-memory (EM) phenotype, after exposure to four different HIV-1-specific CD8 T cells. Data from all three study cohorts are included. (B–E): Susceptibility of naïve (B), central-memory (C), effector-memory (D) or terminally-differentiated CD4 T cells from the three study cohorts to HIV-1-specific CD8 T cells. Left panels indicate data from all individuals within each study group, middle panels indicate data stratified by expression of HLA-B57 vs. HLA-A2/B8. Significance was tested using Mann-Whitney U tests. Right panels indicate Spearman’s correlations between susceptibilities to CD8 T cell mediated killing and corresponding levels of immune activation in indicated CD4 T cell subsets.
Viral reservoirs in naïve CD4 T cells correlate with susceptibility to CD8 T cell mediated killing
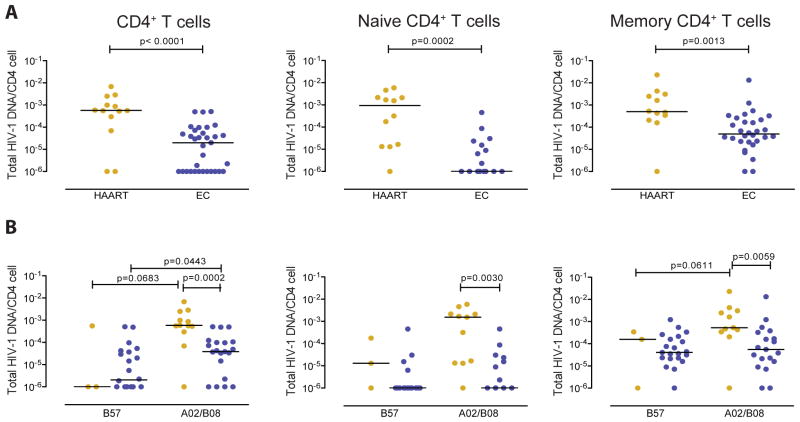
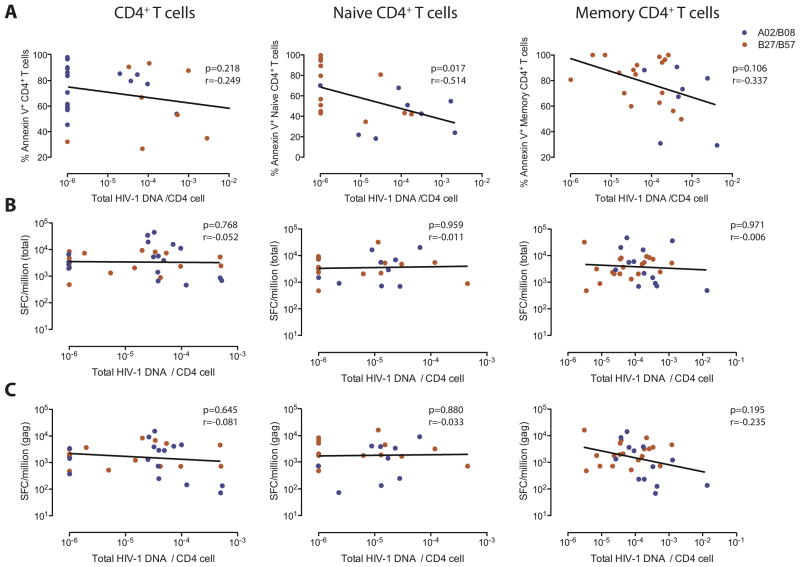
We subsequently analyzed whether altered susceptibility of CD4 T cells to CD8 T cell mediated killing influences the size and composition of the HIV-1 reservoir in CD4 T cells from elite controllers and HAART-treated persons. For this purpose, we isolated CD4 T cells from our study subjects and determined their amount of cell-associated HIV-1 DNA. We observed that in total, unselected CD4 T cells as well as naïve and memory CD4 T cells, viral reservoirs were substantially lower in elite controllers compared to HAART-treated persons (Figure 3A). In addition, within elite controllers, amounts of cell-associated HIV-1 DNA in total CD4 T cells were significantly lower in carriers of HLA-B57 compared to elite controllers expressing alternative HLA class I alleles (Figure 3B). To investigate mechanisms that may influence the size of the viral reservoir, we calculated correlations between quantities of cell-associated HIV-1 DNA and corresponding susceptibilities of CD4 T cells to CD8 T cell-mediated killing (Figure 4A) or numbers of HIV-1-specific CD8 T cells determined by interferon-γ elispots (Figure 4B–C) in elite controllers. Overall, we observed that the amount of cell-associated HIV-1 DNA in bulk CD4 T cells was neither related to the susceptibility to CD8 T cell mediated killing, nor to the magnitude of total or gag-specific HIV-1-specific CD8 T cells. Yet, within isolated naïve CD4 T cells, a statistically significant inverse correlation was observed between reservoir sizes and respective susceptibilities to CD8 T cell-mediated killing; a trend for such an inverse correlation was also noted in isolated memory CD4 T cells. No correlation was observed between reservoirs sizes in naïve or memory CD4 T cells and corresponding frequencies of total or Gag-specific HIV-1-specific CD8 T cells. These data suggest that susceptibilities to cytotoxic effects of CD8 T cells influence the size of the latent viral reservoir in naïve and, to a lesser degree, in memory CD4 T cells.
Figure 3. Latent viral reservoirs in CD4 T cell subsets from elite controllers and HAART-treated patients.
(A–B): Cell-associated HIV-1 DNA in total, naïve and memory CD4 T cells from all HAART-treated persons and elite controllers (A) and following stratification according to expression of HLA-B57 or expression of HLA-A2 or B8 (B). (A–B): Data from each patient are shown as individual symbols. The lower limit of detection with minimum input DNA of 20,000 cell equivalents was 1 copy/1 million cells. Data are not shown for samples with insufficient DNA. Significance was calculated using Mann-Whitney U tests. Orange symbols=HAART treated patients, blue symbols=elite controllers.
Figure 4. Latent viral reservoirs in naïve CD4 T cells are associated with susceptibilities to CD8 T cell-mediated killing.
(A): Data reflect Spearman’s Correlations between susceptibility of indicated CD4 T cell populations to CD8 T cell mediated killing, and corresponding levels of cell-associated HIV-1 DNA. (B–C): Spearman’s correlation between magnitude of total (B) or Gag-specific (C) interferon-γ secreting HIV-1-specific CD8 T cells and corresponding levels of cell-associated HIV-1 DNA.
Discussion
HIV-1 persists for the entirety of a person’s life, even when active viral replication is effectively suppressed with currently-existing pharmaceutical agents. Moreover, lifelong viral persistence is also observed in the rare group of patients who are able to suppress HIV-1 to undetectable levels in the absence of antiretroviral therapy. HIV-1-specific cytotoxic T cells, an essential component of the adaptive immune response against HIV-1, seem to play a critical role for limiting active viral replication in elite controllers [7, 20], and recent studies suggest that they can also influence the size of the latently infected CD4 T cell pool [21, 22]. In contrast, the impact of functional characteristics of CD4 T cells on the size and composition of the latent viral reservoir is less certain. Here, we used a recently-described assay to measure the susceptibility of CD4 T cells to HIV-1-specific CD8 T cell mediated killing, a functional parameter in CD4 T cells that has not systematically been assessed in prior studies. In comparison to viral inhibition assays in which the abilities of HIV-1-specific CD8 T cells to block activate viral replication are evaluated [23], our assay measures the responsiveness of directly ex-vivo isolated, peptide-pulsed CD4 T to CD8 T cell mediated killing without being biased by different susceptibilities of CD4 T cells to HIV-1 infection [24–26], differences in cell-intrinsic antigen presentation or processing [27] or artifacts resulting from in vitro culture. Using this experimental system, we show that the susceptibility of CD4 T cells from elite controllers to CD8 T cell-mediated killing is significantly enhanced in comparison to alternative patient populations, and that this difference is associated with a lower HIV-1 reservoir size in corresponding CD4 T cell populations. Moreover, we observed that within elite controllers, higher susceptibility to CD8 T cell mediated killing in combination with lower viral reservoirs were more visible in carriers of the protective HLA class I allele HLA-B57, as compared to individuals expressing alternative HLA class I alleles. Together, our findings indicate that the susceptibility of CD4 T cells to CD8 T cell-mediated killing may represent an important factor for shaping and structuring the size and cellular composition of the latent HIV-1 reservoir, and likely needs to be considered in future clinical strategies that aim at reducing HIV-1 persistence.
CD4 T cells represent a highly heterogeneous cell population that includes different subsets with individual developmental programs and functional characteristics [28], which influence the ability of these cells to serve as long-term reservoirs for HIV. In this study, we observed that inverse correlations between the latent viral reservoir size and the corresponding susceptibility to CD8 T cell-mediated killing were most pronounced in naïve CD4 T cells, a quiescent, antigen-inexperienced cell subset with critical functions for replenishing the memory T cell pool. Although naïve HIV-1-infected CD4 T cells in our study were difficult to distinguish from terminally-differentiated CD4 T memory cells due to co-expression of CD45RA, they typically represent a detectable component of the long-lasting viral reservoir that decays very slowly [4, 29, 30]. Our work shows that naïve CD4 T cells have comparatively weak susceptibilities to cytotoxic effects of CD8 T cells, which may contribute to long-term viral persistence in these cells. Interestingly, we observed that the susceptibility of naïve CD4 T cells to CD8 T cell mediated killing was markedly elevated in elite controllers as compared to HAART-treated persons, which appeared to translate into a lower reservoir size in this cell compartment in elite controllers. Together, these findings suggest that the responsiveness of naïve CD4 T cells to cytotoxic effects of CD8 T cells represents an important factor for shaping the viral reservoir size in the naïve CD4 T cell compartment.
Although a correlation between cell-associated viral DNA and susceptibility to HIV-1-specific CD8 T cells was also detected in memory CD4 T cells, this association was relatively weak, suggesting that the reservoir size in this cell compartment is influenced by a combination of multiple factors. For instance, previous work suggested that homeostatic and transitional proliferation of memory CD4 T cell subsets may have a critical role for structuring the latent CD4 T cell reservoir [3]. In addition, susceptibility of memory CD4 T cells to HIV-1 infection varies considerably among different individuals [25, 26, 31], which may also influence the size of the latently infected CD4 T cell pool. Notably, despite evidence from previous studies indicating that quantities of Gag-specific CD8 T cells influence the viral reservoir size in memory CD4 T cells [22], our work only showed a moderate correlation between those parameters, that was weaker than correlations between reservoir sizes and susceptibility of CD4 T cells to CD8 T cell mediated killing. Overall, these findings suggest that the presence of Gag-specific CD8 T cells by itself may not necessarily limit viral reservoirs in CD4 T cells, and that the functional responsiveness of CD4 target cells to HIV-1-specific CD8 T cells may be more critical for influencing the size and composition of latently infected CD4 T cells. However, in all of these studies, we analyzed the viral reservoir in CD4 T cells by measuring HIV-1 DNA, a large proportion of which is not encoding for replication-competent virus; future studies will therefore be necessary to analyze how HIV-1-specific CD8 T cell-mediated immune activity may influence the reservoir of cells harboring replication-competent virus.
Despite numerous detailed investigations, the ability of elite controllers to spontaneously control HIV-1 replication remains difficult to explain, likely due to the substantial heterogeneity of patients who develop this specific clinical phenotype [32]. Our data shows that CD4 T cell susceptibility to CD8 T cell killing and viral reservoir size in CD4 T cells vary greatly among elite controllers, and tend to be associated with expression of the protective HLA class I allele HLA-B57. This allele restricts specific immunodominant CD8 T cell epitopes in HIV-1 Gag, and HIV-1 infected CD4 T cells from elite controllers may be more responsive to CD8 T cell killing when recognized in the context of this HLA allotype. Recently, it was shown that HLA-B57-restricted cytotoxic T cells from elite controllers are highly potent in killing latently infected CD4 T cells after reactivation of active viral replication with histone deacetylase inhibitors (HDACi)[12]. These observations have mostly been attributed to superior functional properties of HIV-1-specific CD8 T cells from elite controllers. However, our data suggest that an increased responsiveness of CD4 T cells from elite controllers to CD8 T cell mediated killing may also facilitate elimination of latently infected cells in this specific patient population.
The reasons for the elevated susceptibility of CD4 T cells from elite controllers to cytotoxic effects of HIV-1-specific CD8 T cells are unclear at present. Notably, CD4 T cells from these patients were more responsive to HIV-1-specific CD8 T cells irrespectively of their antigenic specificity, suggesting that our findings reflect a general constitutive characteristic of CD4 T cells from elite controllers. Importantly, a considerable proportion of elite controllers paradoxically develops declining CD4 T cell counts in the absence of detectable viral replication [33, 34] and higher sensitivities to apoptosis-inducing processes may in part be responsible for that. As such, increased susceptibility of CD4 T cells from elite controllers to CD8 T cell mediated killing may contribute to lowering the reservoir of latently HIV-1 infected cells, but in some cases be associated with higher risks for more pronounced CD4 T cell losses.
Acknowledgments
MJB is supported by a fellowship award from the European Molecular Biology Organization (EMBO). ML is supported by the U.S. National Institutes of Health (AI093203, AI098487), by the Clinical Scientist Development Award from the Doris Duke Charitable Foundation (grant number 2009034), and by the American Foundation for AIDS Research (grant 108302-51-RGRL). Recruitment of HIV-1 controllers was supported by the Bill and Melinda Gates Foundation, the Mark and Lisa Schwartz Foundation and the International HIV Controller Study.
Footnotes
None of the authors declare a conflict of interest related to this work.
References
- 1.Trono D, Van Lint C, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, Chomont N. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329:174–180. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- 2.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 3.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wightman F, Solomon A, Khoury G, Green JA, Gray L, Gorry PR, et al. Both CD31(+) and CD31(-) naive CD4(+) T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J Infect Dis. 2010;202:1738–1748. doi: 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- 5.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 9.Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saez-Cirion A, Sinet M, Shin SY, Urrutia A, Versmisse P, Lacabaratz C, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol. 2009;182:7828–7837. doi: 10.4049/jimmunol.0803928. [DOI] [PubMed] [Google Scholar]
- 11.O’Connell KA, Han Y, Williams TM, Siliciano RF, Blankson JN. Role of natural killer cells in a cohort of elite suppressors: low frequency of the protective KIR3DS1 allele and limited inhibition of human immunodeficiency virus type 1 replication in vitro. J Virol. 2009;83:5028–5034. doi: 10.1128/JVI.02551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger CT, Frahm N, Price DA, Mothe B, Ghebremichael M, Hartman KL, et al. High-functional-avidity cytotoxic T lymphocyte responses to HLA-B-restricted Gag-derived epitopes associated with relative HIV control. J Virol. 2011;85:9334–9345. doi: 10.1128/JVI.00460-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Roederer M. Differential susceptibility of leukocyte subsets to cytotoxic T cell killing: implications for HIV immunopathogenesis. Cytometry A. 2007;71:94–104. doi: 10.1002/cyto.a.20363. [DOI] [PubMed] [Google Scholar]
- 17.Lichterfeld M, Kavanagh DG, Williams KL, Moza B, Mui SK, Miura T, et al. A viral CTL escape mutation leading to immunoglobulin-like transcript 4-mediated functional inhibition of myelomonocytic cells. J Exp Med. 2007;204:2813–2824. doi: 10.1084/jem.20061865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 19.Liszewski MK, Yu JJ, O’Doherty U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods. 2009;47:254–260. doi: 10.1016/j.ymeth.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, et al. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat Immunol. 2012;13:691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatano H, Hayes TL, Dahl V, Sinclair E, Lee TH, Hoh R, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203:960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Descours B, Avettand-Fenoel V, Blanc C, Samri A, Melard A, Supervie V, et al. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis. 2012;54:1495–1503. doi: 10.1093/cid/cis188. [DOI] [PubMed] [Google Scholar]
- 23.Spentzou A, Bergin P, Gill D, Cheeseman H, Ashraf A, Kaltsidis H, et al. Viral inhibition assay: a CD8 T cell neutralization assay for use in clinical trials of HIV-1 vaccine candidates. J Infect Dis. 2010;201:720–729. doi: 10.1086/650492. [DOI] [PubMed] [Google Scholar]
- 24.Ciuffi A, Bleiber G, Munoz M, Martinez R, Loeuillet C, Rehr M, et al. Entry and transcription as key determinants of differences in CD4 T-cell permissiveness to human immunodeficiency virus type 1 infection. J Virol. 2004;78:10747–10754. doi: 10.1128/JVI.78.19.10747-10754.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Li C, Huang J, Cung T, Seiss K, Beamon J, et al. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J Clin Invest. 2011;121:1549–1560. doi: 10.1172/JCI44539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saez-Cirion A, Hamimi C, Bergamaschi A, David A, Versmisse P, Melard A, et al. Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood. 2011;118:955–964. doi: 10.1182/blood-2010-12-327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazaro E, Kadie C, Stamegna P, Zhang SC, Gourdain P, Lai NY, et al. Variable HIV peptide stability in human cytosol is critical to epitope presentation and immune escape. J Clin Invest. 2011;121:2480–2492. doi: 10.1172/JCI44932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012;24:297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambotte O, Demoustier A, de Goer MG, Wallon C, Gasnault J, Goujard C, et al. Persistence of replication-competent HIV in both memory and naive CD4 T cell subsets in patients on prolonged and effective HAART. AIDS. 2002;16:2151–2157. doi: 10.1097/00002030-200211080-00007. [DOI] [PubMed] [Google Scholar]
- 30.Baldanti F, Paolucci S, Gulminetti R, Maserati R, Migliorino G, Pan A, et al. Higher levels of HIV DNA in memory and naive CD4(+) T cell subsets of viremic compared to non-viremic patients after 18 and 24 months of HAART. Antiviral Res. 2001;50:197–206. doi: 10.1016/s0166-3542(01)00142-5. [DOI] [PubMed] [Google Scholar]
- 31.Elahi S, Niki T, Hirashima M, Horton H. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood. 2012;119:4192–4204. doi: 10.1182/blood-2011-11-389585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migueles SA, Connors M. Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. JAMA. 2010;304:194–201. doi: 10.1001/jama.2010.925. [DOI] [PubMed] [Google Scholar]
- 33.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamya P, Tsoukas CM, Boulet S, Routy JP, Thomas R, Cote P, et al. T cell Activation does not drive CD4 decline in longitudinally followed HIV-infected Elite Controllers. AIDS Res Ther. 2011;8:20. doi: 10.1186/1742-6405-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]