Abstract
Several studies have reported reduced cerebral gray matter (GM) volume/density in chronic pain conditions, but there is limited research on plasticity of the human cortex in response to psychological interventions. We investigated GM changes after cognitive behavioral therapy (CBT) in patients with chronic pain. We used voxel based morphometry (VBM) to compare anatomical MRI scans of 13 patients with mixed chronic pain types before and after an 11-week CBT treatment and to 13 healthy control participants. CBT led to significant improvements in clinical measures. Patients did not differ from healthy controls in GM anywhere in the brain. After treatment, patients had increased GM in bilateral dorsolateral prefrontal (DLPFC), posterior parietal (PPC), subgenual anterior cingulate (ACC)/orbitofrontal, and sensorimotor cortices, as well as hippocampus, and reduced GM in supplementary motor area. In most of these areas showing GM increases, GM became significantly higher than in controls. Decreased pain catastrophizing was associated with increased GM in left DLPFC and ventrolateral prefrontal (VLPFC), right PPC, somatosensory cortex, and pregenual ACC. While future studies with additional control groups will be needed to determine the specific roles of CBT on GM and brain function, we propose that increased GM in the PFC and PPC reflects greater top-down control over pain and cognitive reappraisal of pain, and that changes in somatosensory cortices reflect alterations in the perception of noxious signals.
Perspective
An 11-week CBT intervention for coping with chronic pain resulted in increased gray matter volume in prefrontal and somatosensory brain regions, as well as increased dorsolateral prefrontal volume associated with reduced pain catastrophizing. These results add to mounting evidence that CBT can be a valuable treatment option for chronic pain.
Keywords: dorsolateral prefrontal cortex, neuroimaging, Voxel-Based Morphometry, pain catastrophizing, CBT
Accumulating evidence of structural brain changes in chronic pain patients indicates neuroplasticity in areas implicated in the experience and anticipation of pain 46. Some studies reported a correlation between reduced gray matter (GM) (which could include changes in GM volume (GMV) or GM density (GMD)) in these regions and the duration or intensity of symptoms1, 37, 39, 64, 65. The degree to which these structural abnormalities are reversible with surgical treatment was the subject of several recent investigations. After hip replacement therapy, increases in GM were found in the anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), amygdala, brainstem and insular cortex61. Another study in the same patient population reported increased gray matter volume in the thalamus after surgery23. Most recently, Seminowicz and colleagues68 examined chronic low back pain (CLBP) patients before and after surgical intervention and reported a normalization of cortical thickness within the left DLPFC after effective treatment.
Building on previous research of structural brain changes following surgery for chronic pain and the documented structural changes following longer term (six months to two years) cognitive interventions in clinical populations of chronic fatigue syndrome13 and schizophrenia14, we aimed to examine cerebral GM changes in patients with chronic pain after an 11-week course of CBT (a typical clinical practice). CBT for chronic pain is designed to decrease maladaptive coping and improve self-regulatory skills and attention diversion abilities. Practicing these skills improves coping with pain and often reduces pain in clinical trials4, 5, 35, 36, 47, 51.
We hypothesized that prefrontal brain regions involved in the experience and modulation of pain, that have documented structural abnormalities in chronic pain patients, will normalize following CBT and that the changes in these prefrontal regions will correlate with clinical improvement. Since CBT is likely to reduce the anticipatory fear and the emotional impact of pain, we hypothesized that regions implicated in the cognitive regulation of pain and emotion (particularly, the DLPFC, the ventrolateral prefrontal cortex (VLPFC) and the ACC) will be affected the most. Additionally, we tested how the clinical aspects of chronic pain showing post-CBT improvement were related to the measures of structural neuroplasticity.
Materials and Methods
Participants and study design
The study sample included in the analyses consisted of 13 patients with chronic pain (3 male, mean age 51.4, S.D. 11.8, range 30 to 70) and 13 healthy age-matched controls (3 male, mean age 51.6, S.D. 11.9, range 31 to 68). Patients were scanned before and after 11 weeks of CBT (mean time elapsed 3.58 months, SD 1.06). Controls were scanned only once. An additional 10 healthy controls (4 male, mean age 36.0, S.D. 9.73, range 22 to 54) from another study 68 who were scanned at two time points separated by six months were also included to show that gray matter levels are stable over time, as described below. The University of Vermont Institutional Review Board approved the research protocol, and informed consent was obtained from each participant. All procedures were in compliance with the Declaration of Helsinki.
Inclusion criteria were defined as: at least 6 months of chronic pain; a subjective pain rating of at least 4 out of 10 on a 0–10 point pain scale for the last month; ability to perform usual self-care; and ongoing care of a physician. Exclusion criteria included: malignancy; pending pain-related surgery; involvement in pain-related litigations; psychosis; uncontrolled Axis I disorder or a severe personality disorder interfering with participation in group therapy; and typical MRI contraindications. Of the 24 patients originally recruited, three dropped out of treatment, and six were unwilling/unable to complete the second scan. The remaining fifteen patients completed both scanning sessions and CBT group therapy; however, two patients were excluded because of late disclosure of exclusion criteria. The primary pain diagnosis in the patients included in this study was low back (n=6), myofacial (n =2), headache (n=2), fibromyalgia, upper body, pelvic floor. Self-reported duration of chronic pain ranged from 1 to 23 years (mean/s.d. 9.15/7.16).
Clinical Assessment Measures
The following measures of pain, function/disability, depression, and coping were used to assess patients with chronic pain. All the clinical measures have been validated by previous research. All were self-administered at each evaluation. More details about the clinical measures can be found in Naylor et al.54
Pain
Two measures were used to assess pain: 1) the Short Form McGill Pain Questionnaire (SFMPQ)49, and 2) the Pain Symptoms subscale from the Treatment Outcomes in Pain Survey (TOPS)63. In addition to the SFMPQ descriptors, two questions were added at the end of the questionnaire to assess typical (Pain Typical) and present (Pain Now) levels of pain on a 0 to 10 scale, with 0 labeled as “no pain” and 10 labeled as “worst pain.”
Function/Disability
The TOPS provides three measures of patients’ functioning and disability: 1) the SF-36 Mental Health Composite 2) the SF-36 Physical Health Composite, 3) The TOPS Total Pain Experience scale62, 63, 75.
Depression
All participants completed the Beck Depression Inventory (BDI) 6 at all evaluations. BDI scores below 13 were interpreted as minimal depression, 14 to 19 - mild depression, 20 to 28 - moderate depression, and 29 to 63 - severe depression.
Pain Coping Strategies
Coping Strategies Questionnaire (CSQ) was used to measure the degree to which patients perceive themselves as able to use coping strategies to control and decrease pain and the degree of pain catastrophizing (Catastrophizing Subscale)34, 42.
Control participants completed a demographics questionnaire and the BDI.
CBT intervention
CBT was delivered in eleven, 90-minute weekly group sessions. Our CBT intervention for pain management was designed to: 1) change cognitions and decrease maladaptive coping (pain catastrophizing), 2) enhance patients’ ability to use attention diversion to control pain, and 3) change activity patterns to better control pain. The curriculum was comprised of five major components: self-regulatory skills, including relaxation techniques including progressive relaxation training; cognitive coping strategies, such as cognitive restructuring and methods for reducing catastrophizing; attention diversion methods including imagery; changing activity patterns, including activity pacing, pleasant activity scheduling, and regular exercise; and, lastly, methods for enhancing social support. An in-depth description of the program has been previously reported53, 54.
MRI
Imaging parameters
Anatomical MRI scanning was performed on a Philips Achieva 3T system with an 8-channel head coil. The scan sequence was a 3D T1-weighted TFE (turbo field echo), TR = 9.9ms, TE= 4.6ms, flip angle 8, field of view 256x256, with 140 1mm slices for a resolution of 1x1x1mm. Five pre-CBT scans in the patient group and two scans in the control group took place on an identical scanner, using identical imaging parameters but in a different physical location. High reliability of structural neuroimaging methods has been established in several recent investigations24, 33, provided identical hardware is used17. An axial T2-weighted gradient spin echo (GRASE) sequence was also obtained for radiological reading to rule out neurologically significant abnormalities.
Image processing
We performed voxel based morphometry (VBM) analysis on the anatomical MRI data. For VBM, data preprocessing was performed in VBM8 toolbox (version r435) http://dbm.neuro.uni-jena.de/vbm/ implemented through SPM8 http://www.fil.ion.ucl.ac.uk/spm/. All data underwent bias correction, segmentation, DARTEL normalization, modulation for non-linear effects, and smoothing of 8mm FWHM. Since modulation accounts for total brain volume, no adjustments for total intracranial, brain or ventricle volume were used in subsequent analyses. For patients, data additionally underwent longitudinal preprocessing, in which there was a realignment step prior to all other steps, where each patient’s pre- and post-CBT scan was aligned to an aligned average of the two scans, and no modulation was performed. In longitudinal analyses, it is expected that overall intracranial volume does not change in the short period between scans, and thus the images are not modulated by this volume adjustment (further details on longitudinal preprocessing can be found at http://dbm.neuro.uni-jena.de/vbm8/VBM8-Manual.pdf). When no modulation was performed, results are referred to as changes in GMD (all comparisons involving both pre-CBT and post-CBT scans), while GMV is used to refer to changes when the modulated data were used (patients vs. controls, correlation between duration and GMV in patients pre-CBT). Difference maps were created by subtracting each patient’s pre-CBT scan from the post-CBT scan. VBM results are reported as increased or decreased GMV/GMD. For all whole-brain analyses we used an initial threshold of p<0.001 to identify clusters. To correct for effects of nonstationary smoothing (NS)27, we used the NS toolbox http://fmri.wfubmc.edu/cms/software#NS implemented in SPM5. Results reported in the tables are corrected for family-wise error (FWE) and NS at p<0.05.
Whole-brain analyses
Statistical analyses were performed in SPM8. We conducted separate t-tests comparing GMV in patients pre-CBT and controls and patients post-CBT and controls, removing the effects of age. Additionally, a paired t-test was performed for GMD in patients pre- vs. post-CBT. We performed further analyses to test the relationship between changes in pain, depression, pain catastrophizing, and quality of life measures with changes in GMD by subtracting each patient’s pre-CBT GMD from the post-CBT GMD, then running a separate one-sample t-test with the composite scores of each of the clinical measures as a covariate. We deemed a total of six measures highly relevant to this investigation and tested them as covariates. The Physical Health and Mental Health composites of SF-36 and the Total Pain Experience composite were included from the TOPS questionnaire. The Ability to Control Pain item and the Pain Catastrophizing subscale were included from CSQ. Ability to Control Pain consistently improved with this type of CBT in the past53, 54, while catastrophizing modulates brain activity during acute pain and is proportional to structural changes in chronic pain8, 9, 21, 66. We also examined the effects of pain duration on GMV in patients pre-CBT.
Because of the high co-morbidity of chronic pain with depressive symptoms29, 67, we included additional analyses to rule out the potential dependence of GMD changes on depression. We compared whole brain one sample t-test results with and without the BDI composite score as a covariate and compared t-values in the peak coordinates identified in the repeated measures analysis. Furthermore, for regions that were identified as having an association with change in clinical outcome, we performed partial correlations controlling for BDI to rule out the dependence on depression scores.
Region of interest (ROI) analyses and plots
In order to perform comparisons across all the conditions (controls, pre-CBT, post-CBT), we performed separate analyses on the clusters that showed significant differences in the above whole-brain analyses. Thus, each significant cluster became an ROI. For each ROI detected in the whole-brain analyses, we extracted the GMD or GMV values for each participant and performed separate t-tests (pre-CBT vs. post-CBT and pre-CBT vs. controls) for each ROI and controlling for age. Since our sample did not show any whole-brain differences between patients and controls, only values for significant clusters from the whole-brain pre-CBT vs. post-CBT analysis were extracted and submitted to the analysis described above. Plots and data analysis on the ROI data were performed in SPSS version 19 (IBM SPSS Statistics). ROI data were extracted using MarsBar http://marsbar.sourceforge.net/. We also qualitatively examined effects of pain type and individual differences on the ROI data, and we examined the effect of duration on these ROIs. The purpose of these ROI analyses was to test for subtler differences in the regions identified in whole brain analyses, rather than using ROIs defined a priori based on the literature. Since only a few longitudinal studies on chronic pain interventions have been published and the types of chronic pain and interventions vary across studies, we find there is currently insufficient evidence for the latter approach.
Pain type
The patients in the current study had varying primary pain diagnoses. Because of our low sample size, we pooled patients into a single group. Our primary interest was on the effects of CBT on brain GM in patients with chronic pain, regardless of type. Nonetheless, we ran all the above whole-brain and ROI analyses again with pain type included as a covariate. We included 6 categories for pain type: low back (n=6), myofacial (n =2), headache (n=2), fibromyalgia (n=1), upper body (n=1), pelvic floor (n=1).
Longitudinal effects in healthy participants
While the healthy control participants in the current study were only scanned at one time point, we were able to examine longitudinal changes in a separate sample of healthy control participants from a previously reported study 68. We used MRI scans (3T Siemens Tim Trio scanner, 8 channel head coil resolution 1 x 1 x 1 mm) from 10 healthy controls (4 male, mean age 36.0, S.D. 9.73, range 22 to 54), each scanned at two time points separated by 6 months. Data were pre-processed the exact same way as described above (non-modulated). We used a 2 (group) x 2 (time point) repeated measures ANOVA on the ROIs that showed significant changes in patients to determine whether effects could be related to natural time-dependent changes in brain structure. Age was included as a covariate. Although the use of datasets acquired on different scanners and at different time points is not ideal, the within group design nevertheless allows us to compare changes over time between the two groups.
Results
Clinical Characteristics
Patients rated their pre-CBT typical pain as 6.3, S.D. 1.7, on a scale of 0–10, with the mean length of pain of 9.7, S.D. 7.5, years. Patients pre-CBT had significantly higher BDI scores than controls (patients: 19.85, S.D. 10.07; controls: 2.5, S.D. 2.91; t=5.74, p < 0.001). After CBT, patients showed significant improvements in six out of nine clinical measures. A summary of all clinical change scores are given in Table 1.
Table 1.
Clinical Measures (including post-CBT minus pre-CBT change scores)
| mean pre-(SE) | mean post-(SE) | post-pre (SE) | t | p* | |
|---|---|---|---|---|---|
| CSQ Catastrophizing | 16.23 (2.59) | 8 (1.61) | −8.24 (2.28) | −3.607 | 0.004 |
| CSQ Ability to Control Pain | 2.46 (0.33) | 3.65 (0.25) | 1.19 (0.34) | 3.533 | 0.004 |
| TOPS Total Pain Experience | 59.59 (3.61) | 43.75 (3.48) | −15.2 (2.85) | −5.338 | <0.001 |
| SF-36 Mental Composite | 38.83 (3.45) | 51.48 (2.37) | 12.65 (3.19) | 3.97 | 0.002 |
| SF-36 Physical Composite | 29.10 (2.34) | 32.42 (2.55) | 3.32 (1.85) | 1.792 | ns |
| TOPS Pain Symptoms | 68.17 (3.81) | 56.23 (5.44) | −11.94 (4.95) | −2.412 | 0.033 |
| MPQ Pain Now | 5.85 (0.55) | 4.62 (0.95) | −0.62 (0.87) | −0.71 | ns |
| MPQ Pain Typical | 6.31 (0.47) | 5.23 (0.72) | −1.08 (0.52) | −2.05 | 0.063 |
| Beck Depression Inventory | 19.85 (2.79) | 10.77 (1.63) | −9.08 (1.95) | −4.656 | 0.001 |
significant after Bonferroni correction for multiple comparisons (p=0.0056 is the threshold)
Gray Matter VBM
Patients vs. controls
Patients pre-CBT and controls had no significant differences in GM. Even at a very liberal initial height threshold of p < 0.05 uncorrected, there were no clusters where controls had greater or less GM than patients.
Patients pre- vs. post-CBT
Ten clusters showed significantly increased GM post-CBT compared to pre-CBT. The clusters included left inferior PPC, right premotor/M1/S1, right hippocampus (HC), right DLPFC, left S1, left subgenual ACC/orbitofrontal cortex (sACC/OFC), left superior PPC, left inferior temporal cortex, S2/M1, right premotor/IFG, left inferior temporal. Only one region, a right medial wall cluster that included SMA/pre-SMA (referred to as juxtapositional lobule cortex in the Harvard-Oxford cortical atlas), had decreased GM after CBT. Clusters are shown in Fig 1 and statistics for each cluster are shown in Table 2.
Figure 1.

All significant clusters from post-CBT vs. pre-CBT repeated measures analysis. The order (left to right) matches the order of clusters shown in Table 2. Note that the SMA cluster on the far right is pre-CBT > post-CBT. MNI coordinates (x,y,z) for each crosshair location are shown for each cluster. DLPFC, dorsolateral prefrontal cortex; M1, primary motor cortex; PPC, posterior parietal cortex; S1, primary somatosensory cortex; S2, second somatosensory cortex; sACC/OFC, subgenual ACC/oribotofrontal cortex; SMA, supplementary motor area. ¥ cluster is not significant when a covariate for pain type is included.
Table 2.
VBM significant results
| Region | Left/ Right | p-value for cluster* | volume of cluster (mm3) | Peak MNI coordinates | Peak T-value | ||
|---|---|---|---|---|---|---|---|
| Patients post-CBT > patients pre-CBT | |||||||
| PPC (inferior) | L | 0.008 | 1195 | −56 | −58 | 18 | 8.50 |
| −54 | −54 | 8 | 5.69 | ||||
| −52 | −68 | 15 | 4.65 | ||||
| Premotor/M1/S1¥ | R | 0.040 | 2413 | 66 | 9 | 15 | 7.43 |
| 69 | −9 | 28 | 6.97 | ||||
| 68 | −20 | 38 | 6.91 | ||||
| Hippocampus | R | 0.027 | 793 | 28 | −42 | −12 | 7.16 |
| 33 | −52 | −3 | 5.17 | ||||
| 27 | −52 | −12 | 4.61 | ||||
| DLPFC | R | 0.001 | 8414 | 26 | 32 | 56 | 7.10 |
| 16 | 36 | 52 | 6.32 | ||||
| 21 | 45 | 44 | 6.25 | ||||
| S1 | L | 0.013 | 1225 | −45 | −32 | 62 | 7.05 |
| −39 | −39 | 62 | 5.10 | ||||
| −33 | −40 | 56 | 4.17 | ||||
| sACC/OFC¥ | L | 0.016 | 996 | −8 | 20 | −18 | 6.92 |
| −15 | 14 | −22 | 5.75 | ||||
| PPC (superior) | L | 0.006 | 2133 | −30 | −56 | 60 | 6.35 |
| −34 | −60 | 42 | 5.81 | ||||
| −34 | −51 | 52 | 5.68 | ||||
| Inferior temporal | L | 0.040 | 1232 | −54 | −60 | −18 | 6.17 |
| −46 | −78 | −12 | 4.65 | ||||
| −60 | −54 | −12 | 4.61 | ||||
| S2/M1 | L | 0.009 | 2400 | −60 | −26 | 14 | 6.12 |
| −66 | −20 | 33 | 5.79 | ||||
| −60 | −30 | 40 | 5.03 | ||||
| DLPFC¥ | L | 0.047 | 2008 | −26 | 22 | 51 | 5.70 |
| −27 | −3 | 64 | 5.38 | ||||
| −26 | 15 | 57 | 5.32 | ||||
| Patients post-CBT < patients pre-CBT | |||||||
| SMA/pre-SMA | R | 0.002 | 2204 | 15 | 30 | 42 | 6.96 |
| 16 | 14 | 54 | 6.72 | ||||
| 15 | 21 | 50 | 6.13 | ||||
| DECREASED pain catastrophizing correlated with increased GMD | |||||||
| S2/S1/PPC | R | 0.0001 | 12477 | 54 | −18 | 30 | 8.70 |
| 62 | −34 | 38 | 7.53 | ||||
| 46 | −26 | 22 | 7.32 | ||||
| ACC | L | 0.0030 | 2373 | −4 | 52 | 0 | 6.45 |
| −3 | 50 | 22 | 6.41 | ||||
| −6 | 48 | 10 | 5.81 | ||||
| DLPFC | L | 0.0005 | 5755 | −52 | 14 | 22 | 6.28 |
| −42 | 18 | 46 | 6.23 | ||||
| −46 | 12 | 38 | 6.20 | ||||
| IFG | L | 0.0060 | 1796 | −56 | 6 | 4 | 5.77 |
| −54 | 22 | −3 | 5.72 | ||||
| −60 | −8 | 6 | 5.65 | ||||
| INCREASED pain catastrophizing correlated with increased GMD | |||||||
| Hippocampus¥ | R | 0.0040 | 1438 | 24 | −38 | −3 | 9.12 |
| 28 | −42 | −9 | 6.57 | ||||
| DLPFC¥ | R | 0.0200 | 824 | 38 | 40 | 16 | 7.57 |
| 42 | 34 | 24 | 5.75 | ||||
| INCREASED ability to control pain correlated with increased GMD | |||||||
| Motor cortex¥ | R | 0.0170 | 1137 | 48 | −8 | 48 | 7.14 |
| 46 | 3 | 45 | 5.39 | ||||
| 52 | −4 | 33 | 4.89 | ||||
| DECREASED physical health (SF-36 subscale) correlated with increased GMD | |||||||
| Middle temporal gyrus, inferior parietal¥ | R | 0.0040 | 824 | 39 | −54 | 14 | 9.30 |
| 40 | −58 | 4 | 5.86 | ||||
| 34 | −66 | 18 | 4.10 | ||||
FWE corrected at cluster level, non-stationarity corrected
cluster is not significant when a covariate for pain type is included.
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; M1, primary motor cortex; PPC, posterior parietal cortex; S1, primary somatosensory cortex; S2, second somatosensory cortex; sACC/OFC, subgenual ACC/oribotofrontal cortex; SMA, supplementary motor area.
Data from each of the clusters were extracted and these ROI data were used for subsequent analyses. Three ROIs and their plotted GM values for each group are shown in Fig 2. We chose to show the right and left DLPFC based on previous findings in the literature, and the SMA/pre-SMA because it was the only region that showed a decrease in GM. Fig 2 shows that for ROIs where GM increased, the GM level in patients after CBT was significantly greater than that of controls. This was true for 3 other clusters that are not shown, ventral and dorsal left PPC and right HC. None of the changes in GM in the ROIs extracted from the post-pre analysis correlated with change scores for catastrophizing, BDI, pain control, or physical component summary of the SF-36.
Figure 2.
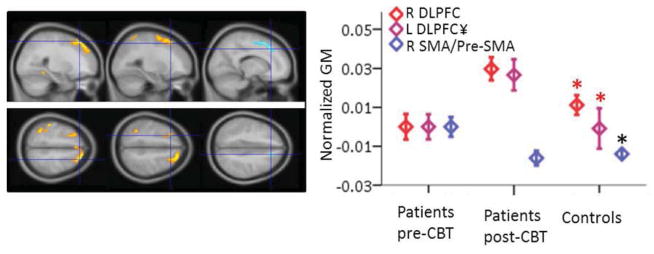
VBM results showing three regions where GM increased (right and left DLPFC) or decreased (Pre-SMA/SMA) in patients post-CBT compared to pre-CBT. The plot to the right shows the means and standard errors for each region shown in the panels to the left. Data for all three groups (patients pre-and post-CBT and controls) are normalized to patients pre-CBT. See Table 2 for details on the SPM statistics. Red asterisks indicate ROIs where controls had significantly less GM than patients post-CBT, and the black asterisk indicates the ROI where controls had less GM than patients pre-CBT, as determined by t-tests on mean values of the ROIs. DLPFC, dorsolateral prefrontal cortex; SMA, supplementary motor area. ¥ cluster is not significant when a covariate for pain type is included.
Clinical Measures and GM
In a whole-brain analysis, pain catastrophizing was significantly negatively correlated with increased GM following CBT in four clusters: a very large right PPC cluster that included parts of S2 and S1, a very large left DLPFC cluster and a smaller inferior frontal gyrus (IFG) or ventrolateral prefrontal (VLPFC) cluster, and a cluster in the bilateral pACC/MPFC (Fig 3, Table 2). That is, as pain catastrophizing went down after CBT, GM in these regions increased. There were also two clusters that were positively correlated with pain catastrophizing, right HC and right DLPFC. Extracted data from the HC cluster were also negatively correlated with the change in perceived pain control as measured by the CSQ (r = −0.619, p<0.05). All significant clusters are shown in Fig 3. Fig 4 shows the scatter plots for change in GM of three clusters against the change in catastrophizing score (larger negative numbers indicate greater improvement).
Figure 3.
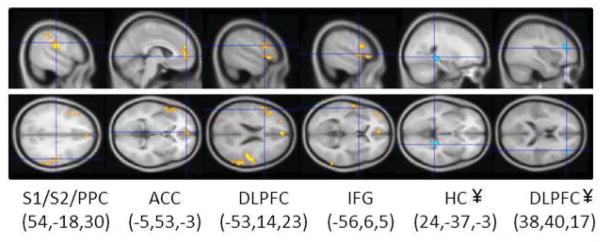
All significant clusters from regression of change in catastrophizing and change in GM. The order (left to right) matches the order of clusters shown in Table 2. Note that the first four clusters from left to right were negative correlations between GM and catastrophizing, while the 2 right clusters (HC, DLPFC) were positive correlations. MNI coordinates (x,y,z) for each crosshair location are shown for each cluster. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; PPC, posterior parietal cortex; S1, primary somatosensory cortex; S2, second somatosensory cortex. ¥ cluster is not significant when a covariate for pain type is included.
Figure 4.
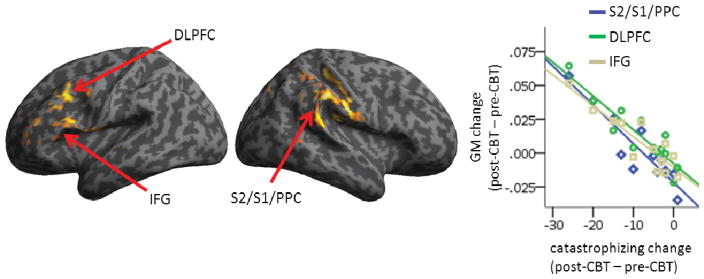
Three of the four clusters where increased gray matter volume after CBT correlated with decreased pain catastrophizing. The left DLPFC and IFG and the right S2/S1/PPC clusters are shown. Results are cluster corrected at pFWE<0.05. See Table 2 for details. Scatterplot shows change in GM as a function of change in catastrophizing. DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; PPC, posterior parietal cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex.
Pain control as measured by the CSQ was significantly correlated with increased GMD in the right motor cortex (Table 2). Extracted data from this ROI identified in the pain control analysis were also significantly negatively correlated with pain catastrophizing (r = −0.627, p<0.05). Improvement on the physical component summary of the SF-36 correlated with increased GM in the right middle temporal gyrus. No other clinical measures tested, including BDI depression scores, mental component summary of the SF-36, and total pain experience, reached our significance criteria.
Depression
To rule out the possibility that the treatment changes in GM were driven by changes in depression, we ran two models using one-sample t-tests with the subtraction maps (post-CBT – pre-CBT) with and without BDI scores as a covariate. We then extracted the t-values for each coordinate identified in the repeated measures analysis. The t-values for the model with BDI included as a covariate were higher in 27 of the 32 coordinates, and in the remaining 5 coordinates, t-values were no more than 0.12 higher in the model without the covariate compared to the model with the covariate. This finding suggests that depression did not influence GM changes in the identified areas.
We tested whether the correlation between change in pain catastrophizing and change in the GMD was influenced by change in depression by controlling for BDI in partial correlations. BDI did not decrease the r-value or significance in any of the ROIs that correlated with pain catastrophizing. Of note, pain catastrophizing and depression scores were significantly correlated pre-CBT (r= 0.734, p < 0.005), but not post-CBT (r=0.441, p=0.131), and there was also no significant correlation between pain catastrophizing and depression change scores (r = 0.523, p=0.0668). Thus, there was a clear dissociation between treatment-related changes in pain catastrophizing and depression, and GM changes associated with improvement in pain catastrophizing are independent of changes in depression.
Duration
Duration of chronic pain ranged from 1 to 23 years (mean/s.d. 9.15/7.16). There were no significant correlations between duration and any of the post-pre differences in the clinical measures or in the ROIs. Similarly, age also did not correlate with the change in any of the variables.
There were two significant GMV clusters that were negatively correlated with duration in pre-CBT patient scans (i.e., the longer they have had pain, the less GMV in these areas): pMCC (peak 3, −6, 28, T = 9.41, p<0.000001, cluster k = 959, pFWE < 0.01), and left temporoparietal junction (TPJ; peak −58, −51, 6, T = 6.03, p<0.00001, cluster k = 531, pFWE < 0.05).
We also examined the relationship between pain duration and the average GMD of the ROIs from the pre-CBT vs post-CBT analysis. Duration negatively correlated with the left PPC ROI pre-CBT (r = −0.777, p<0.005) and post-CBT (r = −0.750, p < 0.005), and with the left ITG ROI pre-CBT (r = −0.582, p < 0.05), but not post-CBT (r = −0.422, p = 0.151).
Pain type
Upon examination of individual effects within the 11 ROIs that showed altered GMD post-CBT, 10 of the 13 patients always had an effect consistent with the overall group effect, and of the remaining three patients, one had low back pain, and the other two headache. We re-ran the whole-brain and ROI analyses with pain type included as a covariate. The inclusion of pain type as a covariate had no effect on the ROI analyses: significant differences remained. For whole-brain analyses, inclusion of pain type had some effect, although most of the significant clusters reported in the two main analyses remained significant. Clusters that were not significant after controlling for pain type are reported in table 2 and figures 1 to 3.
Longitudinal effects in healthy participants
For the 11 ROIs showing altered GMD after CBT, we performed at 2(group) x 2(time) ANOVA, with age as a covariate, comparing patients and a set of healthy controls from another study who were scanned at two time points separated by six months. For every ROI, there was a significant group-by-time interaction. There were very minimal and non-significant changes in GMD in these ROIs in the healthy control group, suggesting that GMD in these regions is stable over time in a pain-free group that has not undergone CBT.
Discussion
CBT resulted in increased GM in bilateral DLPFC and PPC, and several other sensory, motor, and affective areas, and decreased GM in the left SMA. Furthermore, pain catastrophizing negatively correlated with changes in left DLPFC, ACC, and right parietal cortical regions, and positively correlated with right DLPFC and hippocampus. The type of CBT implemented in this study provided training in several coping strategies, ranging from relaxation techniques and attention diversion away from pain to active engagement with and restructuring of the maladaptive cognitions leading to negative emotions associated with chronic pain. As predicted, CBT in this study was associated with GM increases in bilateral DLPFC. GM increases in the DLPFC are consistent with normalization of functional activity during performance of a cognitive task and concurrent cortical thickening in patients having undergone surgery or facet joint block for CLBP68. We propose that changes in prefrontal regions reflect compensatory mechanisms to increase descending modulation of pain7, 25, 38, 43, 73, 76–78 or freeing of cognitive resources for cognitive or affective reappraisal of pain and pain-related challenges28, 48, 55, 56, while changes in sensory and motor regions reflect adaptive responses to the repetitive input of noxious signals.
There have now been many studies reporting GM changes in chronic pain compared with healthy controls12 and we therefore hypothesized that patients would have reduced regional GM compared to controls. While the majority of these studies reported decreased GM in patients, some reported increased GM50. Furthermore, some studies reported relatively few regional differences in GM between patients and controls12, and at least one reported no differences at all16. In the current study, patients did not have significantly different GM than controls. This might not be surprising for a few reasons. First, it has been suggested that different chronic pains might be associated with unique brain “signatures” 3. Second, the specific regions that have different GM in patients and controls vary across studies and patient groups79. Third, there have been suggestions that patient age might be an important factor in GM changes50, 65. Importantly, in spite of the heterogeneous patient group in the current study and lack of difference from healthy controls, we still observed the expected regional changes in GM following CBT.
While previous studies reported reversal of GM or cortical thickness changes in patients with effective treatment23, 61, 68, the GM changes reported in the current study are likely adaptive, rather than a return to normal levels. None of the regions that had increased GM in patients post-CBT compared to pre-CBT were significantly different from controls at the pre-CBT time-point (based on t-tests for the average GM in the ROIs). However, after CBT the GM in most of these regions increased beyond the GM level of controls. Thus, it is possible that these regions have gained function beyond normal levels to support pain coping mechanisms. Such a finding of increase in GM beyond the normal levels of healthy controls has been reported in recovery from addiction 11, and a recent review of the literature suggests that the increase in PFC GM and functional activity reported in several studies is related to an increase in cognitive control required to maintain the new cognitive state 19. It is also worth noting that the one area that had reduced GM after CBT treatment – the SMA/pre-SMA – was the only region that had significantly greater GM in patients pre-CBT compared to controls (Fig 1), and after CBT the GM level decreased to a level more similar to controls, making this region the only one that showed normalization. The SMA/preSMA plays a role in generation of voluntary movement 30 and has been implicated in motor dysfunction in chronic regional pain syndrome 44. In addition, SMA/preSMA is frequently activated in various pain and cognitive task paradigms 18, 58, it is activated by arthritic pain 40, and its activity increases with increasing intensity of spontaneous chronic pain 2. Thus, the normalization of GM in this region could be related to motor function or more directly to chronic pain. Disambiguating between these possibilities is an important avenue for future investigations.
In previous longitudinal studies on surgical treatment effects on GM in chronic pain, all of23, 61 or the majority of68 patients had reduced pain after treatment. Here, in response to an 11-week cognitive-behavioral intervention, patients had relatively minor decreases in pain compared to the changes in coping and affect. In fact, of all the clinical measures, pain and physical health were the only ones that did not show significant reductions after treatment (after Bonferroni correction; see Table 1). However, improvement in pain beliefs, including control over pain, and catastrophizing were achieved, and these changes can predict later improvements in pain32, 72, so it is possible that later (e.g. one year post-CBT) patients would show reduced pain as well as pain-related GM changes. In fact, previous work showed that patients enrolled in a 4-month relapse prevention program after CBT had a significant decrease of pain and improved coping compared to immediately after CBT54.
Several studies have examined the effects of CBT for various conditions and have reported changes in PFC activity. For example, Jensen and colleagues reported increased pain-related activity in the left VLPFC in fibromyalgia patients after they underwent CBT31. The authors suggested that the increased VLPFC activity reflected reappraisal of the pain with resources freed up through cognitive coping. In schizophrenia, CBT led to increased DLPFC and frontopolar activation during a working memory task26, and bilateral DLPFC and ACC activation during a memory task predicted improvement of symptoms following CBT41. Generalized anxiety disorder patients also had increased VLPFC in response to threat stimuli after CBT45. Finally, in major depression, CBT led to increased activity in ventromedial PFC during an arousal task60. Therefore, one interpretation of the increased GM in prefrontal areas we report here is that they reflect increased activity related to changes in ongoing cognitive and affective appraisals.
Improvement in pain catastrophizing was the only clinical measure that significantly correlated with structural changes post-CBT in multiple regions (control of pain and physical ability each correlated with GM change in one region). Pain catastrophizing is known to be a particularly salient feature of chronic pain states70, 71, and a significant portion of our current CBT program is devoted to developing skills to reduce catastrophizing and restructure patients’ perception of pain. Functional neuroimaging studies of acute pain paradigms in healthy control participants and in chronic pain populations have reported catastrophizing-related activations primarily within networks engaged in anticipation of and attention to pain21, 66. Furthermore, a recent diffusion tensor imaging study revealed negative correlations between pain catastrophizing and fractional anisotropy (a measure of white matter tract integrity) in the cingulum in IBS9. Similarly, in the present study pain catastrophizing was associated with changes in pACC/MPFC, regions of the brain implicated in direct control of emotional circuits20 as well as in placebo analgesia57, 73, 74. Consistent with the anticipation and attention to pain hypothesis of pain catastrophizing, GM also increased in the PPC after CBT. Interestingly, pain catastrophizing correlated negatively with GM change in the left DLPFC, but positively correlated with the right DLPFC. In depression, high frequency rTMS is generally more effective on the left side22. Likewise, previous work showed functional and structural changes primarily in the left DLPFC after peripheral interventions to relieve CLBP68. Evidence of pain catastrophizing-related structural neuroplasticity presented here highlights the importance of teaching methods to reduce pain catastrophizing for effective control of chronic pain. In general, the structural changes in the prefrontal cortex were consistent with the theoretical models of CBT that generally implicate top-down mechanisms10, 15, 59, 69.
Including depression scores as a covariate in the pre-CBT versus post-CBT analysis did not decrease significance levels in the great majority of significant peaks and where it did decrease the significance it was by marginal amounts. Furthermore, while pain catastrophizing and depression scores were correlated before treatment, after CBT there was no significant correlation between the two. Controlling for depression has had large effects in other VBM studies in chronic pain populations29, 67, but in the current study, the reported changes in GM appear to be independent of depression.
One final interesting finding involved the right hippocampus. This region had increased GM in patients post-CBT compared to pre-CBT, and GM in an almost identical cluster in the hippocampus was positively correlated with change in pain catastrophizing. The hippocampus was recently shown to altered neurogenesis and short-term plasticity in a mouse model of neuropathic pain, and its volume was also decreased in chronic back pain and chronic regional pain syndrome patients52. Thus, the increased GM in this structure in the present study could indicate normalization of hippocampal function in learning, memory, and emotion. However, the inverse relationship between increased hippocampal GM and improvement in pain catastrophizing is unclear.
There are some limitations to our study. First, this is non-randomized study with healthy participants serving as a control group. However, as our patients’ average length of pain was 10 years we do not think the structural brain changes could be attributed to the simple passage of time. Moreover these changes correlated with clinical improvement. Furthermore, we compared the GMD in the ROIs that showed significant changes in patients pre- versus post-CBT to a set of healthy controls from another study who were scanned at two time points, and the group-by-time interaction was significant for every region, suggesting that the effect was specific to CBT, since brain GMD is stable in these areas in healthy individuals. It is also possible that the changes we report herein are an effect of participating in the CBT intervention and are not specific to the cognitive therapy itself. For these reasons, future studies should include scans at multiple time-points for all participants to rule out non-specific brain structural changes over time, and should include control groups such as an education session in which time spent in therapy is matched, to determine CBT-specific effects. Second, the pain diagnoses in our patients were heterogeneous. However, the goal of the current study was not to determine a treatment effect that is specific for one type of chronic pain (which has been shown in prior studies34–36), but rather to determine brain changes associated with CBT irrespective of chronic pain type. Despite a lack of GM differences between patients pre-CBT and controls, possibly owing to the mixed sample of pain diagnoses, we can assume that the brain changes we report in the short time-frame of the study are related to the one manipulation – i.e. CBT intervention – that is common to all patients, rather than due to a change in the variability between chronic pain types. To clarify whether CBT is reversing abnormal GM in some subgroups, while causing overcompensation in GM in others, a large study involving multiple chronic pain conditions would be required.
To conclude, we have shown that treating chronic pain with CBT leads to increased GM in several brain areas including prefrontal and parietal regions, and that decreased pain catastrophizing is associated with increased GM in prefrontal and parietal areas. Our data suggest that the GM changes following standard 11-week group CBT parallels clinical improvements in coping with pain and overall mental health.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number R01 AR052131 and R21 AR055716. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The Authors thank Dr. Trevor Andrews, Jay Gonyea and Scott Hipko from the UVM MRI Center for Biomedical Imaging.
Footnotes
Disclosures: The authors have no conflicts of interest to declare. This research was supported by grants from the National Institute of Arthritis, Musculoskeletal Diseases (NIAMS)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic Back Pain Is Associated with Decreased Prefrontal and Thalamic Gray Matter Density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic Pain and the Emotional Brain: Specific Brain Activity Associated with Spontaneous Fluctuations of Intensity of Chronic Back Pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain Morphological Signatures for Chronic Pain. PLoS One. 2011;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basler HD. Group treatment for pain and discomfort. Patient Educ Couns. 1993;20:167–175. doi: 10.1016/0738-3991(93)90130-o. [DOI] [PubMed] [Google Scholar]
- 5.Basler HD, Jakle C, Kroner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: a controlled randomized study in German pain treatment centers. Patient Educ Couns. 1997;31:113–124. doi: 10.1016/s0738-3991(97)00996-8. [DOI] [PubMed] [Google Scholar]
- 6.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, Carlino E, Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in IBS. potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 10.Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends Cogn Sci. 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated Grey Matter Changes with Prolonged Addiction and Extended Abstinence in Cocaine Users. PLoS One. 2013;8:e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis KD, Moayedi M. Central Mechanisms of Pain Revealed Through Functional and Structural MRI. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- 13.de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JW, Toni I. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- 14.Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. 2010;67:674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etkin A, Pittenger C, Polan HJ, Kandel ER. Toward a neurobiology of psychotherapy: basic science and clinical applications. J Neuropsychiatry Clin Neurosci. 2005;17:145–158. doi: 10.1176/jnp.17.2.145. [DOI] [PubMed] [Google Scholar]
- 16.Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Focke NK, Helms G, Kaspar S, Diederich C, Toth V, Dechent P, Mohr A, Paulus W. Multi-site voxel-based morphometry -- Not quite there yet. Neuroimage. 2011;56:1164–1170. doi: 10.1016/j.neuroimage.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garavan H, Brennan K, Hester R, Whelan R. The neurobiology of successful abstinence. Curr Opin Neurobiol. 2013;10 doi: 10.1016/j.conb.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 22.Gross M, Nakamura L, Pascual-Leone A, Fregni F. Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta-analysis comparing the recent vs. the earlier rTMS studies. Acta Psychiatr Scand. 2007;116:165–173. doi: 10.1111/j.1600-0447.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 23.Gwilym SE, Fillipini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty; a longitudinal voxel-based-morphometric study. Arthritis Rheum. 2010 doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 24.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 25.Hardy SG, Haigler HJ. Prefrontal influences upon the midbrain: a possible route for pain modulation. Brain Res. 1985;339:285–293. doi: 10.1016/0006-8993(85)90094-0. [DOI] [PubMed] [Google Scholar]
- 26.Haut KM, Lim KO, MacDonald A., III Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35:1850–1859. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli - An fMRI study. Psychiatry Research: Neuroimaging. 2007;154:1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143:262–267. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda A, Luders HO, Burgess RC, Shibasaki H. Movement-related potentials recorded from supplementary motor area and primary motor area. Role of supplementary motor area in voluntary movements. Brain. 1992;115:1017–1043. doi: 10.1093/brain/115.4.1017. [DOI] [PubMed] [Google Scholar]
- 31.Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgeia. Pain. 2012;153:1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP, Turner JA, Romano JM. Changes after multidisciplinary pain treatment in patient pain beliefs and coping are associated with concurrent changes in patient functioning. Pain. 2007;131:38–47. doi: 10.1016/j.pain.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keefe FJ, Caldwell DS, Martinez S, Nunley J, Beckham J, Williams DA. Analyzing pain in rheumatoid arthritis patients. Pain coping strategies in patients who have had knee replacement surgery. Pain. 1991;46:153–160. doi: 10.1016/0304-3959(91)90070-E. [DOI] [PubMed] [Google Scholar]
- 35.Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, Martinez S, Nunley J, Beckham JC, Crisson JE, Helms M. Pain coping skills training in the management of osteoarthritic knee pain: A comparative study. Behavior Therapy. 1990;21:49–62. [Google Scholar]
- 36.Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, Martinez S, Nunley J, Beckham JC, Helms M. Pain coping skills training in the management of osteoarthritic knee pain-II: Follow-up results. Behavior Therapy. 1990;21:435–447. [Google Scholar]
- 37.Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, Park KW, Koh SB. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28:598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 38.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated Brain Gray Matter Loss in Fibromyalgia Patients: Premature Aging of the Brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El-Deredy W, Jones AK. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–1354. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- 41.Kumari V, Peters ER, Fannon D, Antonova E, Premkumar P, Anilkumar AP, Williams SC, Kuipers E. Dorsolateral prefrontal cortex activity predicts responsiveness to cognitive-behavioral therapy in schizophrenia. Biol Psychiatry. 2009;66:594–602. doi: 10.1016/j.biopsych.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawson K, Reesor KA, Keefe FJ, Turner JA. Dimensions of pain-related cognitive coping: cross-validation of the factor structure of the Coping Strategy Questionnaire. Pain. 1990;43:195–204. doi: 10.1016/0304-3959(90)91073-R. [DOI] [PubMed] [Google Scholar]
- 43.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 44.Maihofner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, Handwerker HO, Schattschneider J. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–2687. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- 45.Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, Monk CS. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20:105–111. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May A. Structural Brain Imaging: A Window into Chronic Pain. Neuroscientist. 2011;17:209–220. doi: 10.1177/1073858410396220. [DOI] [PubMed] [Google Scholar]
- 47.McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine (Phila Pa. 1976;27:2564–2573. doi: 10.1097/00007632-200211150-00033. [DOI] [PubMed] [Google Scholar]
- 48.McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 50.Moayedi M, Weissman-Fogel I, Salomons TV, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Abnormal gray matter aging in chronic pain patients. Brain Res. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 51.Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 52.Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in Hippocampal Functioning with Persistent Pain. The Journal of Neuroscience. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naylor MR, Helzer JE, Naud S, Keefe FJ. Automated telephone as an adjunct for the treatment of chronic pain: a pilot study. J Pain. 2002;3:429–438. doi: 10.1054/jpai.2002.129563. [DOI] [PubMed] [Google Scholar]
- 54.Naylor MR, Keefe FJ, Brigidi B, Naud S, Helzer JE. Therapeutic Interactive Voice Response for chronic pain reduction and relapse prevention. Pain. 2008;134:335–345. doi: 10.1016/j.pain.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gabrieli JD, Gross JJ. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20:1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 58.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 59.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain Gray Matter Decrease in Chronic Pain Is the Consequence and Not the Cause of Pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogers WH, Wittink H, Wagner A, Cynn D, Carr DB. Assessing individual outcomes during outpatient multidisciplinary chronic pain treatment by means of an augmented SF-36. Pain Med. 2000;1:44–54. doi: 10.1046/j.1526-4637.2000.99102.x. [DOI] [PubMed] [Google Scholar]
- 63.Rogers WH, Wittink HM, Ashburn MA, Cynn D, Carr DB. Using the “TOPS,” an outcomes instrument for multidisciplinary outpatient pain treatment. Pain Med. 2000;1:55–67. doi: 10.1046/j.1526-4637.2000.99101.x. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 65.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional Gray Matter Density Changes in Brains of Patients With Irritable Bowel Syndrome. Gastroenterology. 2010;139:48–57. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective Treatment of Chronic Low Back Pain in Humans Reverses Abnormal Brain Anatomy and Function. The Journal of Neuroscience. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WH. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan MJ, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77:253–260. doi: 10.1016/S0304-3959(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 74.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. PNAS. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 76.Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, Dolan RJ. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005;27:59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 77.Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral Prefrontal Cortex Mediates the Analgesic Effect of Expected and Perceived Control over Pain. J Neurosci. 2006;26:11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in Cognitive Sciences. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Wood PB. Variations in brain gray matter associated with chronic pain. Curr Rheumatol Rep. 2010;12:462–469. doi: 10.1007/s11926-010-0129-7. [DOI] [PubMed] [Google Scholar]


