Abstract
Pancreatic β-cell survival remains poorly understood despite decades of research. GATA transcription factors broadly regulate embryogenesis and influence survival of several cell types, but their role in adult β-cells remains undefined. To investigate the role of GATA factors in adult β-cells, we derived β-cell-inducible Gata4- and Gata6-knockout mice, along with whole-body inducible Gata4 knockouts. β-Cell Gata4 deletion modestly increased the proportion of dying β-cells in situ with ultrastructural abnormalities suggesting endoplasmic reticulum (ER) stress. Notably, glucose homeostasis was not grossly altered in Gata4- and Gata6-knockout mice, suggesting that GATA factors do not have essential roles in β-cells. Several ER stress signals were up-regulated in Gata4 and Gata6 knockouts, most notably CHOP, a known regulator of ER stress-induced apoptosis. However, ER stress signals were not elevated to levels observed after acute thapsigargin administration, suggesting that GATA deficiency only caused mild ER stress. Simultaneous deletion of Gata4 and CHOP partially restored β-cell survival. In contrast, whole-body inducible Gata4 knockouts displayed no evidence of ER stress in other GATA4-enriched tissues, such as heart. Indeed, distinct GATA transcriptional targets were differentially expressed in islets compared with heart. Such β-cell-specific findings prompted study of a large meta-analysis dataset to investigate single nucleotide polymorphisms harbored within the human GATA4 locus, revealing several variants significantly associated with type 1 diabetes mellitus. We conclude that GATA factors have important but nonessential roles to promote ER integrity and β-cell survival in a tissue-specific manner and that GATA factors likely contribute to type 1 diabetes mellitus pathogenesis.
Pancreatic β-cell death is a central but enigmatic component of the pathogenesis of type 1 and type 2 diabetes mellitus (T1DM and T2DM). Surprisingly, little is currently known about the cell-specific mechanisms that govern β-cell survival. Although several studies have established roles for caspases (1, 2) and Bcl-2 family members (3, 4), the upstream signals that regulate programmed β-cell death remain poorly understood. Turnover of β-cells, the balance between renewal and death, is a tightly controlled process (5). Indeed, β-cell turnover is exceedingly low in mature mammals (5), suggesting that tissue-specific factors might act within β-cells to tonically suppress apoptosis. Dysregulation of β-cell-specific survival signals could tip this balance in favor of apoptosis, reducing β-cell mass below a critical threshold such that residual β-cells are unable to compensate. Impaired β-cell survival could also lead to excessive release of β-cell antigens in T1DM, which could provoke immune attack of islets and accelerate β-cell loss (6).
β-Cells are potently regulated by the unfolded protein response (UPR), a highly dynamic set of adaptive signals that preserve endoplasmic reticulum (ER) homeostasis to ensure proper protein folding and cell viability (7). β-Cells appear to be uniquely susceptible to malfunctions in the protein-processing machinery, perhaps due to their low turnover rate, or the life-sustaining hormone they secrete. Although the UPR is primarily viewed as a homeostatic stress response, UPR signals can also drive β-cell apoptosis if ER stress cannot be resolved (8). The signals that mediate apoptosis in response to ER stress are largely unknown. However, the CCAAT enhancer binding protein homologous protein CHOP (Gadd153/Ddit3) has been shown to direct ER stress-induced apoptosis and is responsible for β-cell death in several diabetic models (9). Notably, ER stress has also been shown to be a prominent feature of prediabetic nonobese diabetic mouse islets, suggesting that ER perturbation and the UPR might, in fact, initiate autoimmune cell destruction in T1DM (10). To our knowledge, PDX1 is the only β-cell transcription factor known to regulate ER stress responses (11). Notably, PDX1 also regulates β-cell survival (12). Taken together, these studies hint that other major β-cell survival factors could remain undiscovered, some of which might act to promote ER homeostasis. Thus, elucidating the transcriptional regulation of ER integrity and the signals that maintain it represents a key area of investigation to understand how β-cell homeostasis is preserved.
GATA transcription factors powerfully influence the development of many tissue types but have no known role in adult β-cells. GATA4 and GATA6 have been best studied as cardiac transcription factors, where they coordinate tissue-specific gene expression programs that influence morphogenesis by a range of mechanisms. GATA factors have also been implicated in embryonic development of the endocrine and exocrine pancreas (13, 14). Recent studies suggest not only a requirement for GATA4 and 6 in embryonic pancreas formation but that GATA4 and GATA6 directly activate Pdx1 in the embryonic pancreas, thus assuming a critical role upstream of this essential regulator of pancreatic cell survival and organogenesis (15–18). We now show that GATA4 and GATA6 have important but nonessential roles to promote adult β-cell survival by promoting ER integrity. β-Cell inducible deletion of Gata4 or Gata6 led to increased β-cell death. Gata4-knockout β-cells exhibited striking ultrastructural abnormalities and increased CHOP expression, consistent with models of unresolvable ER stress. Simultaneous CHOP deletion partially restored β-cell survival, suggesting that it contributes to apoptosis in Gata4-deficient β-cells. Finally, we show that several single nucleotide polymorphisms (SNPs) within the human GATA4 locus are significantly associated with T1DM occurrence. Taken together, these findings introduce GATA factors as novel mediators of ER homeostasis and β-cell survival and implicate them in the pathogenesis of human diabetes.
Materials and Methods
Mice
Animal experiments were performed at Children's Hospital of Philadelphia in accordance with the IACUC. Gata4 loxP mice (19) were crossed with Ins2-CreERT mice (20), generously provided by Doug Melton of Harvard University, to yield β-cell-inducible Gata4-knockout mice (Gata4 loxP/loxP Ins2-CreERT). Gata6 loxP mice were obtained from The Jackson Laboratory (JAX 008196) (21) and crossed with Ins2-CreERT mice to yield β-cell-inducible Gata6-knockout mice (Gata6 loxP/loxP Ins2-CreERT). Gata4- and Gata6-knockout mice were further crossed to generate β-cell-inducible Gata4 Gata6 double-knockout mice (Gata4 loxP/loxP Gata6 loxP/loxP Ins2-CreERT. β-Cell-inducible Gata4-knockout mice were additionally crossed with CHOP null mice from The Jackson Laboratory (JAX 005530) to generate β-cell-inducible Gata4-knockout CHOP null mice (Gata4 loxP/loxP Ins2-CreERT CHOP null/null). Gata4 loxP mice were lastly crossed with Ubc-CreERT2 mice (22), a generous gift from Eric Brown of the University of Pennsylvania, to generate whole-body-inducible Gata4-knockout mice (Gata4 loxP/loxP Ubc-CreERT2). Pups were genotyped by the REDExtract-N-Amp kit (Sigma-Aldrich) using specific genotyping primers (Supplemental Table 1). Intraperitoneal glucose tolerance tests were performed on mice fasted for 16 hours with 2 g d-glucose per kg body weight. Mice were gavaged with tamoxifen as previously described (23), labeled continuously with 5-bromo-2-deoxyuridine or 5-iodo-2-deoxyuridine prior to being humanely destroyed as previously reported (24). Low-dose streptozotocin (30 mg/kg) was administered to mice on 5 consecutive days as previously reported (5). High-fat diet (60%; Research Diets; D12492) was fed ad libitum in indicated experiments. All experiments were performed on adult mice between 2 and 2.5 months of age unless otherwise noted.
Cell lines
βHC9 cells (25) were a generous gift from the Clinical and Translational Research Center at Children's Hospital of Philadelphia and were originally obtained via Dr Franz Matchinsky (26).
Islet isolation
Islets were isolated as described elsewhere (27). Islets were grouped in biological replicates from individual mice. Islet preparations were assessed for purity by confirming minimal contribution of exocrine RNA contamination.
Real-time quantitative PCR
RNA was isolated and converted to cDNA as previously reported (28). Fluorescent-labeled fluorescence resonance energy transfer PCR or SYBR Green PCR was performed to amplify triplicate samples, and expression was normalized to cyclophilin (Primers [Supplemental Table 1]).
Microarray
Islet RNA samples were amplified via Ovation Pico WTA System (NuGen Technologies). Labeling and hybridization with Gene Arrays (Affymetrix) were performed by the Nucleic Acid and Protein Core at Children's Hospital of Philadelphia.
Immunohistochemistry and pancreatic morphometry
Paraffin sections were prepared as previously described (24). Frozen pancreas sections were dehydrated in a sucrose gradient, cut with a cryostat, and incubated with primary antisera in 0.2% Triton-X. Primary antisera comprised guinea pig anti-INSULIN (Zymed/Invitrogen) and mouse anti-GATA4 (Santa Cruz Biotechnology). Slides were imaged to quantify β-cell morphometry as previously reported (27).
Apoptosis analysis
Apoptosis analysis was performed as described elsewhere (27). At least 10 000 β-cells per condition were analyzed for the total intra-islet terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)-positive cells.
Electron microscopy
Pancreas samples were dissected from mice and immediately fixed in glutaraldehyde, processed, and imaged.
Statistics
All results are reported as mean ± SE unless noted otherwise. Results were compared with independent t tests (unpaired and two-tailed) reported as P values.
Results
GATA factors are expressed in adult β-cells
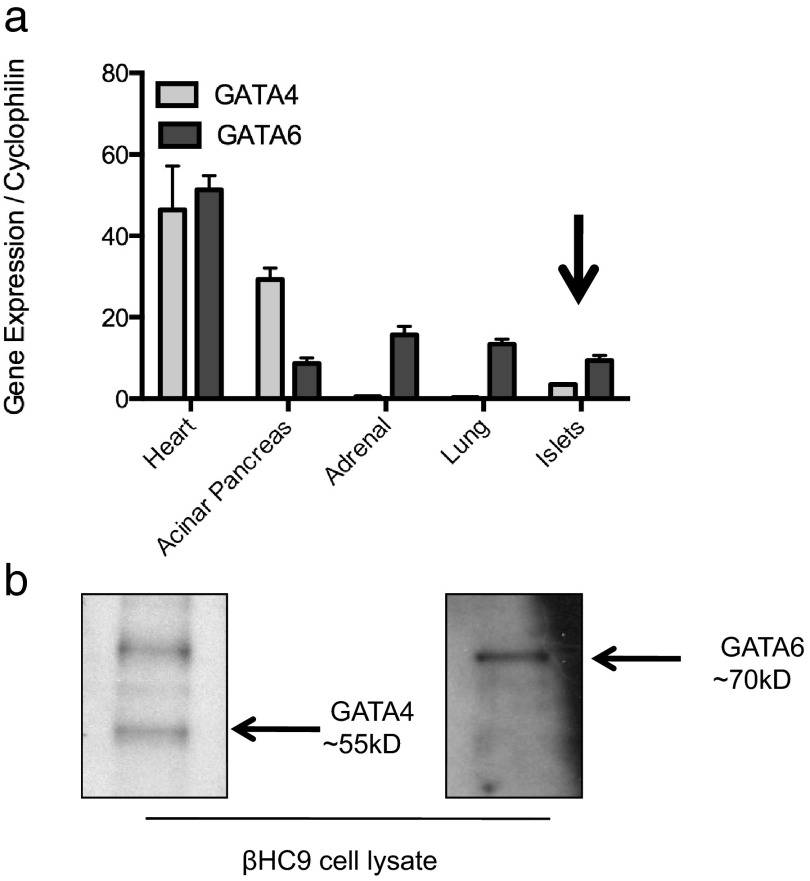
Presence of GATA factors in mature β-cells remains controversial. We analyzed expression of Gata4 and Gata6 in several adult mouse tissues. We detected Gata4 and Gata6 mRNAs by RT-PCR of islet cDNA isolated from adult mice (Figure 1a). Gata6 mRNAs were present in islets at higher levels than Gata4, consistent with previous observations (13, 29). Nevertheless, Gata4 and Gata6 mRNAs were expressed at much lower levels in islets than in several other adult somatic tissues (Figure 1a).
Figure 1.
GATA transcription factors are expressed at low levels in adult pancreatic β-cells a, Quantitative real-time PCR of cDNA isolated from various crude tissue preparations from mice 2.5 months of age. Data are reported as mean ± SE for 3–4 biological replicates per condition. b, Western blots of whole-cell protein lysates from βHC9 cells using GATA4 (left) and GATA6 (right)-specific antisera.
We additionally analyzed expression of GATA factors in βHC9 cells, an established mouse β-cell line. GATA4 and GATA6 were readily detected by Western blotting of whole-cell protein lysates (Figure 1b).
GATA4 immunostaining in adult mice revealed nuclear localization in exocrine acini and unexpectedly demonstrated cytoplasmic staining within islets in several trials (Supplemental Figure 1a). However, staining did not consistently detect significant nuclear localization of GATA4 in adult mouse islets, likely reflective of low expression in adult β-cells.
Genetic deletion of Gata4 impairs β-cell survival
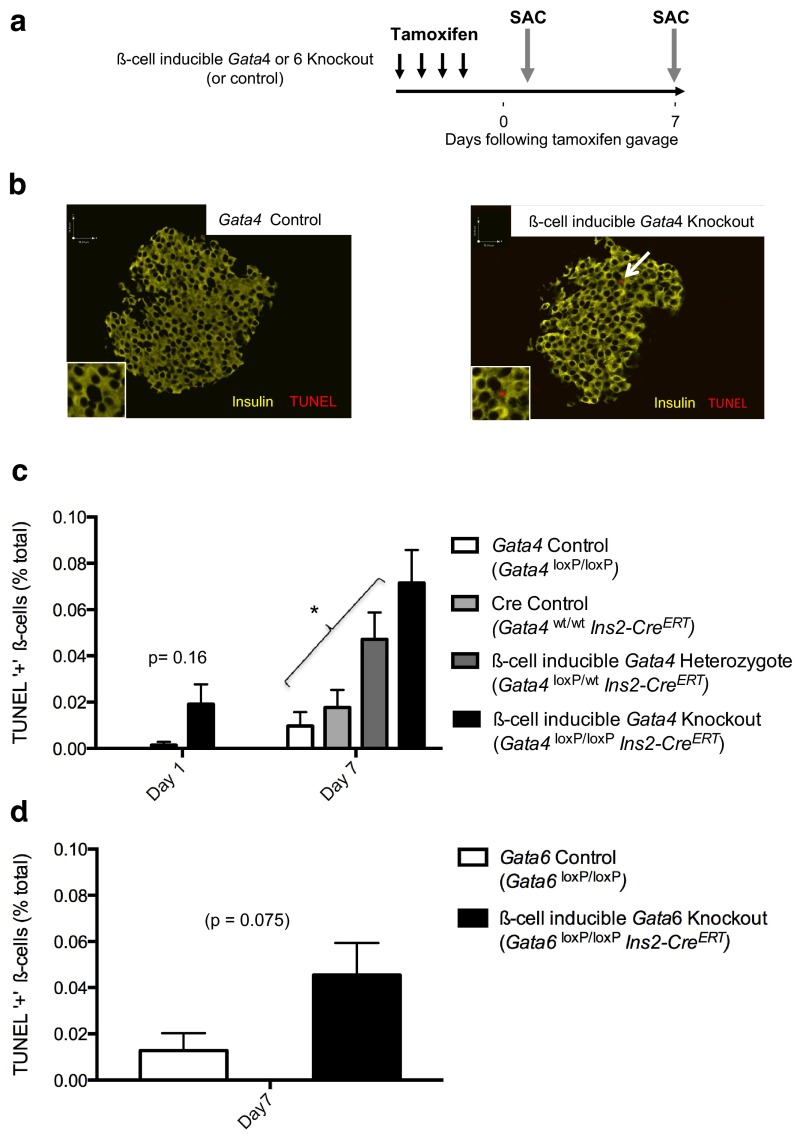
To study the role of GATA factors specifically in adult β-cells, we derived β-cell-inducible Gata4-knockout mice expressing Ins2-CreERT and Gata4 loxP alleles. Tamoxifen treatment reduced Gata4 genomic DNA, mRNA, and protein levels in adult islets. Notably, cre-mediated recombination did not appear completely efficient. This is not unexpected given the previously demonstrated subtotal efficiency of Ins2-CreERT in β-cells with a highly efficient loxP reporter (23) and the crude nature of the islet preparations, which contain other islet endocrine cell types, nonendocrine cells, and exocrine cells (Supplemental Figures 1, a and b, and 2, b and c). Acute β-cell Gata4 deletion did not severely impact glucose homeostasis: fed glucose levels were equivalent in knockouts and controls, and glucose tolerance tests revealed intact glucose tolerance (Supplemental Figures 3 and 4).
We characterized islet histology from β-cell-inducible Gata4-knockout mice. As expected, given mostly normal glucose homeostasis, islet histology was grossly normal and β-cell mass was preserved (Supplemental Figure 3). TUNEL labeling of pancreas sections from knockouts and controls confirmed characteristic low raw levels of apoptotic β-cells, but even by 1 day following deletion, Gata4-knockout β-cells had increased rates of apoptosis (Figure 2c). By 1 week following deletion, β-cell-inducible Gata4-knockout mice exhibited significantly increased β-cell apoptosis compared with controls (Figure 2c). Increased β-cell death could not be attributed to tamoxifen or transgenic CreERT expression (Figure 2c). Moreover, heterozygous animals containing only one Gata4 loxP allele exhibited an intermediate phenotype of increased β-cell death, indicating a dose-dependent effect of Gata4 deficiency to reduce β-cell survival (Figure 2c). These studies suggest that GATA4 has an important, but nonessential role that contributes to β-cell survival. Notably, the absolute amount of β-cell death remained quite low following Gata4 deletion (TUNEL increased from ∼1 in 10 000 β-cells to 1 in 1000 β-cells). There was no compensatory β-cell proliferation, as measured by ki67 or by thymidine incorporation (Supplemental Figure 3). The lack of a proliferative response is not unexpected given the low level of β-cell death.
Figure 2.
β-Cell-inducible Gata4 or Gata6 deletion impairs β-cell survival a, Schema indicating timing of β-cell-inducible Gata4 or Gata6 deletion. b, Pancreatic immunostaining for TUNEL (red) and insulin (yellow) in control (left) and β-cell-inducible Gata4-knockout (right) islets. Scale bars, 51 μm. c, Quantification of intra-islet TUNEL-positive cells in Gata4-deficient islets and controls humanely destroyed 1 or 7 days following deletion. *, P < 0.05. d, Quantification of intra-islet TUNEL-positive cells in Gata6-deficient islets and controls humanely destroyed 7 days following deletion. For both experiments pancreata from at least 6 mice per condition (>10 000 β-cells) were machine counted and curated by hand.
Genetic deletion of Gata6 impairs β-cell survival
Low-levels of β-cell death in Gata4-deficient islets could imply that GATA4 is a minor player within β-cells compared with GATA6. To test this we derived β-cell-inducible Gata6-knockout mice. Glucose homeostasis in these mice was preserved, with normal random-fed glucose levels as well as normal glucose tolerance testing, even following high-fat challenge (Supplemental Figures 3 and 4d). These studies indicate that GATA6, like GATA4, is not essential to regulate glucose homeostasis in adult mice.
We then studied β-cell apoptosis in pancreata from β-cell-inducible Gata6-knockout mice. Loss of Gata6 led to increased β-cell death after 1 week (Figure 2d), mirroring our studies in Gata4-deficient β-cells. Taken together, these studies indicate that GATA4 and GATA6 have similar roles in adult β-cells.
Compound deletion of Gata4 and Gata6 impairs β-cell survival
The modest phenotype observed following Gata4 or Gata6 deletion could imply that Gata6 compensates for Gata4 deficiency and vice versa, because overlapping roles for GATA4 and GATA6 have been described in endocrine pancreas development (15–18). To directly rule out such compensation, we derived β-cell inducible Gata4 Gata6 double-knockout mice. As a further challenge, we weaned a cohort of these mice onto a 60% high-fat diet, which has been shown to potentiate β-cell apoptosis due to increased metabolic demand and ER stress (30). After treatment with tamoxifen, we followed this cohort for several weeks. As expected, TUNEL labeling revealed higher raw levels of β-cell apoptosis in mice challenged by high-fat feeding compared with those fed normal diet (Supplemental Figure 5b and Figure 2c). β-Cell-inducible deletion of Gata4 and Gata6 exacerbated β-cell apoptosis with double knockouts exhibiting significantly more apoptotic β-cells than controls (Supplemental Figure 5b). However, even despite such additional insults to β-cell survival, inducible Gata4 Gata6 double-knockout mice still exhibited normal glucose tolerance even after 8 weeks with only a slight nonsignificant reduction in β-cell mass (Supplemental Figure 5, c and d). Taken together, these data further support a role for Gata4 and Gata6 as important mediators of β-cell survival; however, they again suggest that inducible Gata deletion, although tipping the balance of β-cell survival toward apoptosis, does not reduce β-cell mass severely enough to alter glucose homeostasis.
GATA factors are not transcriptional regulators of programmed cell death pathways in β-cells
β-Cell death in Gata knockouts could imply that GATA factors directly regulate programmed cell death pathways in a manner equivalent to GATA4, which directs expression of Bcl-xL, and Bcl-2 in smooth muscle cells and ovarian cells (31–33) However, the absolute amount of measurable β-cell death in any Gata-knockout model, although significantly higher than controls, was nevertheless quite low at an absolute level (TUNEL+ were ∼0.08% of β-cells in Gata4 knockouts). Low-level β-cell death in Gata knockouts hints that apoptosis could be a secondary adaptive response to Gata deficiency rather than a direct perturbation of programmed cell death pathways. To test this idea, we carried out gene expression studies and observed that mRNAs of gene products related to programmed cell death were not increased in either Gata4- or Gata6-knockout islets compared with controls (Supplemental Figure 6b). We then treated β-cell-inducible Gata4-knockout mice with a multiple low-dose streptozotocin to augment β-cell death. Streptozotocin increased apoptosis in control and Gata4-knockout islets to a similar degree (Supplemental Figure 6d). Taken together, these studies suggest that Gata deficiency likely does not directly impair programmed cell death pathways in β-cells but rather may secondarily decrease β-cell survival by influencing other cellular stressors.
GATA factors promote ER integrity
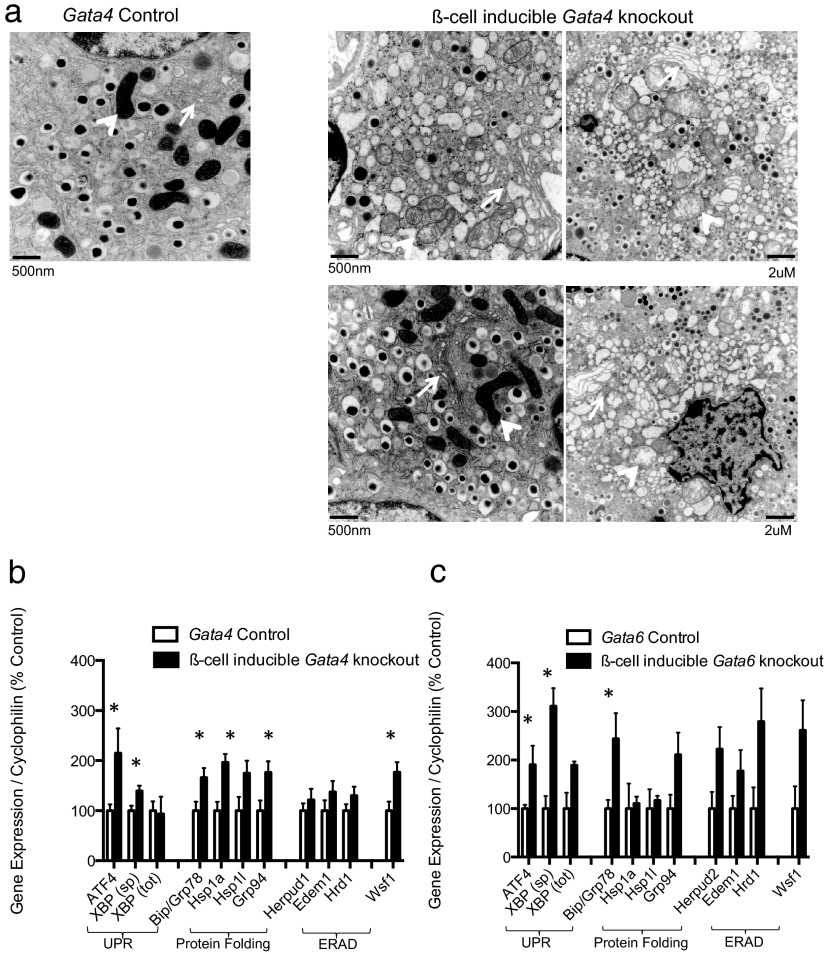
Given that Gata deficiency might exacerbate cellular stress pathways, we performed electron microscopy (EM) on Gata4-deficient islets to elucidate the ultrastructural mechanisms mediating apoptosis. Acute Gata4 deletion resulted in striking ultrastructural abnormalities characterized by distended ER and dilated mitochondria (Figure 3a), features consistent with previous reports of ER stress in β-cells (11, 34). Such distressed β-cells were prominent and were present in nearly all knockout images analyzed. However, there was a high degree of heterogeneity in the severity of abnormalities, and several β-cells retained normal subcellular morphology (Figure 3a and Supplemental Figure 9). Other pancreatic cell types remained undisturbed and contained intact subcellular organelles.
Figure 3.
GATA factors promote ER integrity in β-cells a, Representative electron micrographs of β-cells from Gata4 control (left) and knockout (right) islets. Roughly 20 images per mouse, 4 mice per condition were qualitatively analyzed. Arrows indicate ERs; arrowheads indicate mitochondria. See supplemental figures for more images. RT-PCR of islet cDNA from β-cell-inducible Gata4 (panel b), and Gata6 (panel c) knockouts. Several common ER stress response genes were chosen for analysis, including transcriptional regulators of the UPR, chaperones involved in protein folding, and genes involved in the ER-associated protein degradation (ERAD) pathway. Data are reported as mean ± SE for 4–5 biological replicates per condition. *, P < 0.05.
We examined expression of common ER stress response genes in order to confirm our observations indicating ER perturbation. Indeed, several ER stress signals, including chaperones Bip/Grp78, Hsp1a, and the stress-induced transcription factor ATF-4, were up-regulated in Gata4-deficient islets (Figure 3b). Given that cellular responses to ER stress are dynamic and potently regulated, we exposed a subset of wild-type islets to a thapsigargin (TG) time course in order to provide comparison of gene expression profiles of Gata4-deficient islets to those of maximally stressed islets. Notably, the response to acute Gata4 deletion was fairly mild compared with acute treatment of wild-type islets with thapsigargin, and instead more closely resembled chronic TG-induced ER stress in islets after 48 hours (Supplemental Figure 7). This experiment illustrates that acute Gata4 deletion only causes mild ER stress and thus provides a possible explanation for the mild β-cell death phenotype in Gata4-deficient mice. In further support of this notion, Gata4-knockout islets exhibited normal calcium flux, suggesting that the observed ER perturbations are not severe enough to disrupt calcium homeostasis (Supplemental Figure 8).
Given that Gata6 mRNAs are expressed at higher levels in primary islets than Gata4 mRNAs, we questioned whether GATA6 may serve a similar role in ER integrity. Gata6-knockout islets exhibited a highly similar gene expression program with up-regulation of several of the same chaperones and ER-associated targets genes, supporting a role for GATA6 in ER fidelity (Figure 3c).
CHOP contributes to β-cell death in Gata4-deficient β-cells
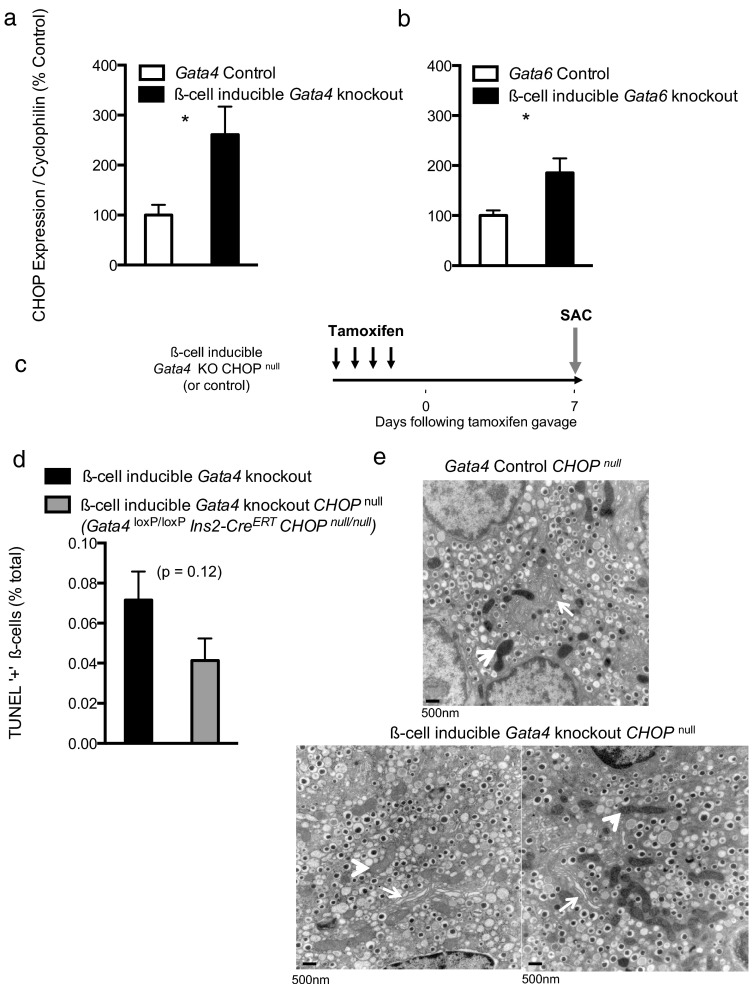
CHOP is a stress-inducible transcription factor known to direct apoptosis in the event of unresolvable ER stress. We questioned whether the distressed ER observed in Gata4-deficient islets might trigger CHOP gene expression. Increased CHOP expression indeed accompanied β-cell death in Gata4- and Gata6-knockout islets (Figure 4, a and b). Notably, CHOP expression roughly mirrored counts of TUNEL-positive nuclei, with Gata4 knockouts exhibiting higher levels of expression relative to Gata6 knockouts.
Figure 4.
CHOP contributes to β-cell death in Gata4-deficient β-cells CHOP expression measured by RT-PCR of islet cDNA from Gata4 (panel a) and Gata6 (panel b) knockouts. Data are reported as mean ± SE for 4–5 biological replicates per condition. c, Schema indicating timing of Gata4 deletion in β-cell-inducible Gata4-knockout CHOP null mice. d, Quantification of intra-islet TUNEL-positive cells in β-cell-inducible Gata4-knockout CHOP null islets and controls 1 week following deletion. Pancreata from 5 mice (>10 000 β-cells) were machine counted and curated by hand. e, Representative electron micrographs of control (top) and β-cell-inducible Gata4-knockout CHOP null β-cells (bottom). Roughly 30 images were analyzed by hand from one animal per condition. Arrows indicate ERs; arrowheads indicate mitochondria.
To test whether CHOP directly influenced β-cell death in Gata4-knockout islets, we derived β-cell inducible Gata4-knockout CHOP null mice (Gata4 loxP/loxP Ins2-CreERT CHOP null/null). TUNEL labeling of Gata4-knockout CHOP null pancreas sections harvested 1 week following deletion revealed substantially fewer TUNEL-positive nuclei (Figure 4d), suggesting that CHOP does act as a mediator of apoptosis in Gata4-deficient β-cells. Notably, CHOP deletion only partially restored β-cell survival, with Gata4-knockout CHOP null islets still exhibiting rates of apoptosis greater than Gata4 controls.
CHOP deletion additionally improved some of the ultrastructural abnormalities associated with Gata4-deficient β-cells (Figure 4e), consistent with previous reports citing improvement in β-cell morphology following CHOP deletion (9). Mildly distressed cells exhibiting dilated mitochondria were still present in Gata4-knockout CHOP null β-cells, although in scarce quantity, again supporting the observation that CHOP deletion reflects a partial rescue. Taken together, these data suggest that Gata-deficient β-cells may experience unresolvable ER stress, necessitating a switch from an adaptive to self-destructive transcriptional response involving CHOP protein expression.
GATA4 directs a tissue-specific gene expression program in β-cells
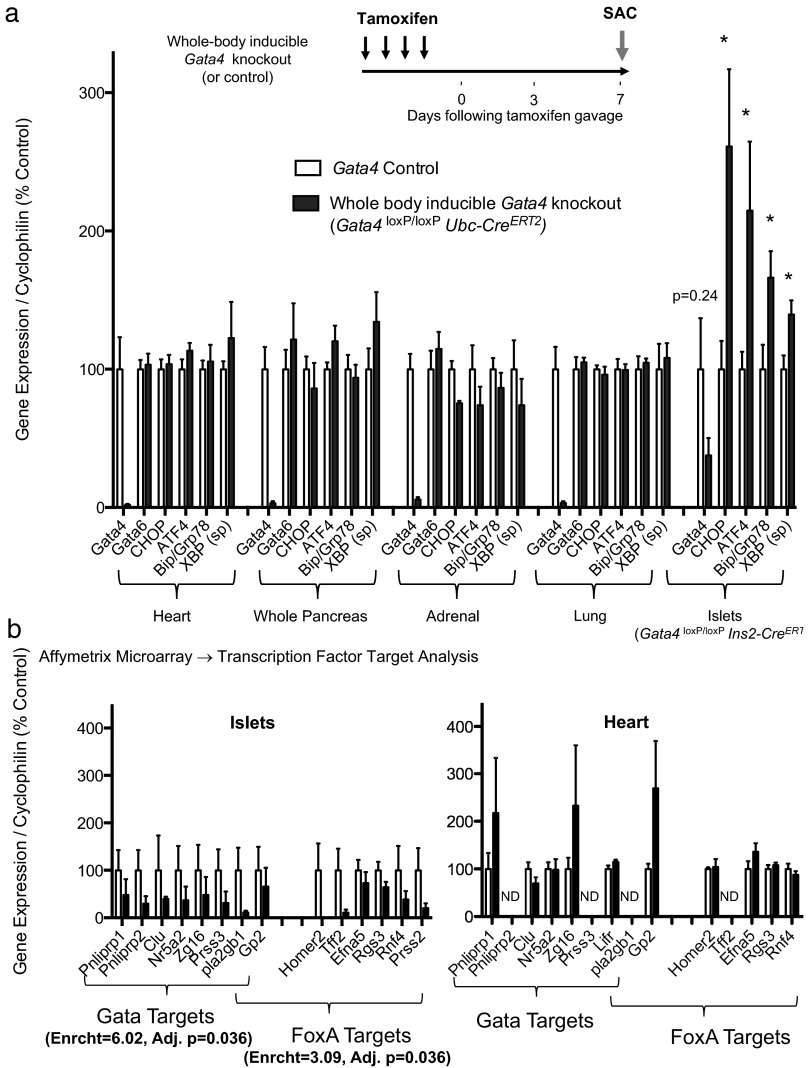
We next asked whether our observations in β-cells could be extended to other GATA-enriched tissues. We generated whole-body inducible Gata4-knockout mice (Gata4 loxP/loxP Ubc-CreERT2) that yielded efficient Gata4 deletion in multiple tissues (Figure 5a). In stark contrast to Gata4-deficient islets, Gata4 deletion did not result in activation of ER stress signals in the heart, adrenal, or lung measured 1 week following deletion (Figure 5a). Given that secretory cells are uniquely susceptible to ER stress, we measured expression of analogous targets in the acinar pancreas, a major secretor of digestive pancreatic enzymes. Again, no activation of such targets was detected, suggesting that GATA4's transcriptional activity in pancreatic β-cells is not universal to other GATA-enriched tissues, including other secretory cell types.
Figure 5.
GATA4 directs a tissue-specific gene expression program in pancreatic β-cells a, RT-PCR of cDNA isolated from various Gata4-knockout tissues 1 week following deletion. Several common ER stress-responsive genes were chosen for analysis. Note that gene expression data from islets were replotted from Figure 3b for reference. Data are reported as mean ± SE for 4 biological replicates per condition. b, Genes down-regulated following Gata4 deletion reflect enrichment for GATA and FoxA transcriptional targets. Gene expression profiles of such candidate GATA and FoxA targets in islet (left) and heart (right) cDNA preparations. Affymetrix genome-wide microarray targets were validated by RT-PCR. Data are reported as mean ± SE for 3–4 biological replicates per condition. ND, not determined. *, P < 0.05.
We performed genome-wide microarray studies using Gata4-knockout islet cDNA in order to help identify putative GATA4 transcriptional targets in β-cells. Using the WebGestalt analysis toolkit provided by Vanderbilt University, we found that a subset of genes down-regulated following Gata4 deletion was significantly enriched for canonical WGATAR target sequences (Figure 5b). To test whether these candidate β-cell GATA targets were common to other GATA-enriched cell types, we also measured expression of such targets in Gata4-knockout hearts by RT-PCR. RT-PCR validated down-regulation of several candidate GATA target genes in β-cell-specific Gata4 knockouts (Figure 5b). However, no such changes were observed in Gata4-knockout heart, suggesting that GATA4 might occupy these targets specifically in β-cells.
GATA4 has been shown to coordinate with other transcription factors to drive tissue-specific gene expression (35, 36). We asked whether genome-wide expression data might reveal a β-cell transcription factor that serves as a candidate GATA4 cofactor. We found that genes, following Gata4 deletion, also showed significant enrichment for targets of the FOXA subfamily, which include β-cell transcription factors known to play diverse roles in metabolism and insulin secretion (Figure 5b). Such FOXA targets were not differentially expressed in Gata4-knockout heart indicating that potential cooperativity is not conserved across tissues (Figure 5b). These data suggest that GATA4 may coordinate with FOXA to drive a nonstereotypic transcriptional program that results in a β-cell-specific phenotype.
Genetic variation at the human GATA4 locus is associated with T1DM risk
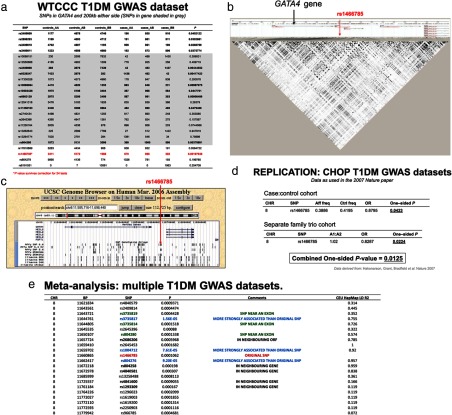
GATA factors have not previously been implicated in diabetes pathogenesis; however, the tissue-restricted gene expression program of GATA4 and knockout phenotype prompted us to evaluate the possibility of an association between human GATA4 SNPs and type 1 diabetes risk. We examined a dataset of T1DM patients and controls from the Wellcome Trust Case Control Consortium, testing only those SNPs within the GATA4 coding region and flanking 200 kb (37). Of the 24 SNPs tested, several yielded odd's ratios that were significantly associated with the trait, including one (rs1466785) that remained significant following correction for multiple testing (Figure 6). The common risk-conferring allele has a frequency of 60% in patients compared with 57% for controls, yielding a hemizygous odds ratio of 1.27 (95% confidence interval: 1.08–1.52). The homozygous odds ratio was 1.30 (95% confidence interval: 1.09–1.59). We then tested for GATA4 associations across multiple independent cohorts of type 1 persons with diabetes, totaling 9934 patients and 16 956 controls (38). Eigenvectors were used as covariates to control for population stratification in a logistic regression analysis. Several SNPs in the GATA4 genetic region revealed affected frequencies significantly different from control frequencies (Figure 6). Taken together, these studies extend our findings in mouse models to risk of diabetes in man, thus implicating GATA factors as mediators of β-cell survival and strongly suggesting that mutations in GATA4 influence type 1 diabetes risk.
Figure 6.
Genetic variation at the human GATA4 locus is associated with T1DM risk a, WTCCC T1D GWAS dataset. b, Linkage disequilibrium map. c, GATA4 genetic region. d, Replication in separate cohorts. e, Meta-analysis of T1DM datasets.
Discussion
GATA transcription factors have been shown to broadly coordinate expression of cardiac, pulmonary, and pancreatic genes during development and adulthood but play no known role in adult pancreatic β-cells. Using β-cell-specific inducible knockout models we demonstrate a novel role for GATA4 and GATA6 as regulators of β-cell survival. Our results indicate that Gata4 and Gata6 mRNAs are expressed at low levels in β-cells, validating previously described observations (14, 29). Gata4 and Gata6 mRNAs were reliably detected in islets from adult mice whereas more robust expression in heart and acini confirmed expected expression patterns. Reduction of Gata4 and Gata6 mRNA in crude islet preparations from β-cell-specific floxed mice confirmed our observations and limits the possibility that Gata detection was attributed to exocrine contamination or nonspecific staining.
β-Cell death is an extremely rare event and is thought to be tightly regulated (39). Our observation of increased apoptosis following β-cell deletion of Gata4 and Gata6 suggest that GATA proteins influence the regulation of β-cell survival. Nevertheless, the raw level of β-cell death in Gata knockouts is quite low and is not deleterious enough to affect total β-cell mass, even following compound deletion. This reflects a nonessential role for β-cell GATA factors to regulate glucose homeostasis. Although most knockout β-cells appeared to lack Gata4 or Gata6, we cannot rule out incomplete gene deletion as a cause of the mild metabolic phenotype in our mice. We note that several other factors could also contribute to the mild phenotype of Gata4- and Gata6-deficient β-cells: 1) Adult inducible knockouts have fully formed endocrine pancreata at the time of Gata deletion, thus requiring massive amounts of cell death to appreciably reduce total β-cell mass; 2) Minimal residual β-cells can fully support glucose homeostasis (40), making it highly unlikely that an inducible knockout strategy could reduce β-cell mass to the point of glucose intolerance unless the gene is essential for β-cell survival and function; 3) Diabetes pathogenesis likely involves a complex interaction of genetic and environmental effects occurring from birth, making development of frank diabetes in inducible Gata-knockout mice even more unlikely. Further experiments are warranted exploring whether β-cell-specific GATA deletion potentiates or accelerates development of diabetes in established mouse models of ER stress. The inducible deletion strategy used here thus still reveals a specific and significant contribution of GATA proteins to β-cell survival despite coming short of evoking a diabetic phenotype.
To our knowledge, PDX1 is the only other β-cell transcription factor known to regulate β-cell survival and has been shown to do so, in part, by influencing ER homeostasis (11). Interestingly, we show that GATA factors similarly maintain β-cell survival by promoting ER integrity. As secretory cells reported to process up to 1 million insulin molecules per minute, β-cells are uniquely susceptible to malfunctions in protein processing (7). Robust transcriptional control of ER pathways is critical to ensuring β-cell survival; thus it is not surprising that deletion of GATA factors might contribute to β-cell death by modulating ER homeostasis. Notably, the high degree of heterogeneity in ER morphology and modest phenotype following Gata deletion could reflect that GATA factors simply “tip the balance” of ER homeostasis in cells experiencing high baseline secretory loads, thus by no means acting as the sole mediators of ER integrity. At this point it is difficult to determine exactly which gene(s) are responsible for β-cell ER stress. Future studies will be needed to ascertain the precise molecular link(s) in between GATA factors and various ER stress pathways to cause β-cell death.
Increased expression of CHOP, a mediator of ER stress-induced apoptosis, suggests that Gata4- and Gata6-knockout β-cells experience irremediable stress, triggering a self-destructive program. CHOP is normally expressed at very low basal levels and is potently induced in response to acute ER perturbation such as in TG treatment (Supplemental Figure 7). Notably, levels of CHOP expression in our Gata-knockout models are most similar to those after 48 hours of TG treatment, suggesting that Gata-deficient β-cells experience chronic ER stress. Deletion of CHOP partially restored β-cell survival, supporting its suggested role as an effector of apoptosis. This rescue was not completely efficient, consistent with reports citing CHOP as one of several potential mediators of apoptosis in response to unresolvable ER stress (41).
GATA4 has been shown to influence cell survival in several distinct tissue types (42, 43); however, the tissue-specific phenotype we report makes it unlikely that GATA4 influences cell survival in a stereotypic manner. Our transcription factor target analyses suggest that GATA4 exhibits a distinct expression program in pancreatic β-cells and may be the result of cooperativity with FoxA2 (previously known as Hnf3-β), a β-cell-enriched transcription factor. FoxA2 has been shown to coordinate with PDX1 to mediate β-cell-specific expression (44), and FoxA2 and GATA4 functionally interact to activate the glucagon promoter as well as hepatic gene promoters during early development (29, 45). Given that GATA4 is broadly expressed, our observation indicating a β-cell-specific phenotype and expression program has exciting implications for targeting potential diabetes therapies aimed at preserving β-cell mass.
The genetic association of GATA4 and T1DM that we report here, combined with our findings linking GATA factors to ER fidelity and β-cell survival, reveals a previously unexplored role for GATA factors in human pathophysiology as β-cell survival factors. Although our studies in mice suggest that GATA factors are not essential for glucose homeostasis as discussed extensively above, our genetic analyses speak to the importance of this family of transcription factors not only in β-cell survival but in the pathogenesis of human disease. These findings thus argue for the existence of intrinsic β-cell survival factors and moreover depart from a line of thinking that suggests that β-cells are passive victims of immune attack in T1DM. Rather, GATA factors appear to be one of potentially many regulators of β-cell survival that can contribute to type 1 diabetes risk when gone awry.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank D. Martinez, N. Panackal, and S. Agrio of the Childrens Hospital of Philadelphia pathology core for their expert assistance with slides; M. Reasoner for excellent supervision of the Childrens Hospital of Philadelphia laboratory animal facility; E. Rappaport, S. Mahoney, K. Hunter, Z. Zhang, and X. Gai of Childrens Hospital of Philadelphia core facilities for assistance with gene expression studies; R. Meade of the University of Pennsylvania Electron Microscopy Resource; and C. Blalock, K. Rogers, D. Kettlewell, L. Herrera, S. Vale, and S. Ramirez of the Texas Children's Diabetes and Endocrinology Center for their administrative expertise and support.
This study was supported by the National Institutes of Health Grants (1R01DK064101, 1R01AG040110, and P30DK079638), the Juvenile Diabetes Research Foundation, the Commonwealth of Pennsylvania (Center for Excellence in Regenerative Medicine Grant 4100043362), and Robert and Janice McNair Foundation. Microarrays were carried out by the Functional Genomics Core at Childrens Hospital of Philadelphia. We thank the National Institutes of Health-funded University of Pennsylvania Diabetes and Endocrinology Center for the use of the Islet Core (P30-DK19525).
Author contributions: D.J.S. conceived and designed the experiments, performed the experiments, analyzed data, and wrote the manuscript. S.H.L., C.L., J.P.B., H.H., and S.F.G. assisted with performing the experiments. J.A.K conceived and designed the experiments, performed the experiments, analyzed data, and wrote the manuscript. J. A.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: J.A.K. previously served on the scientific advisory board of Johnson & Johnson. No other potential conflicts of interest relevant to this article were reported.
Footnotes
- CHOP
- Gadd153/Ddit3
- ER
- endoplasmic reticulum
- Hnf3
- hepatic nuclear factor 3
- SNP
- single nucleotide polymorphism
- T1DM and T2DM
- type 1 and type 2 diabetes mellitus
- TG
- thapsigargin
- TUNEL
- terminal deoxynucleotide transferase-mediated dUTP nick end labeling
- UPR
- unfolded protein response.
References
- 1. Liadis N, Salmena L, Kwan E, et al. Distinct in vivo roles of caspase-8 in β-cells in physiological and diabetes models. Diabetes. 2007;56(9):2302–2311. [DOI] [PubMed] [Google Scholar]
- 2. Liadis N, Murakami K, Eweida M, et al. Caspase-3-dependent β-cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol Cell Biol. 2005;25(9):3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou YP, Pena JC, Roe MW, et al. Overexpression of Bcl-x(L) in β-cells prevents cell death but impairs mitochondrial signal for insulin secretion. Am J Physiol Endocrinol Metab. 2000;278(2):E340–E351. [DOI] [PubMed] [Google Scholar]
- 4. Federici M, Hribal ML, Ranalli M, et al. The common Arg972 polymorphism in insulin receptor substrate-1 causes apoptosis of human pancreatic islets. FASEB J. 2001;15(1):22–24. [DOI] [PubMed] [Google Scholar]
- 5. Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes. 2005;54(9):2557–2567. [DOI] [PubMed] [Google Scholar]
- 6. Mathis D, Vence L, Benoist C. β-Cell death during progression to diabetes. Nature. 2001;414(6865):792–798. [DOI] [PubMed] [Google Scholar]
- 7. Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev. 2008;29(3):317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eizirik DL, Cnop M. ER stress in pancreatic β cells: the thin red line between adaptation and failure. Sci Signal. 2010;3(110):pe7. [DOI] [PubMed] [Google Scholar]
- 9. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118(10):3378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61(4):818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sachdeva MM, Claiborn KC, Khoo C, et al. Pdx1 (MODY4) regulates pancreatic β cell susceptibility to ER stress. Proc Natl Acad Sci USA. 2009;106(45):19090–19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson JD, Ahmed NT, Luciani DS, et al. Increased islet apoptosis in Pdx1+/− mice. J Clin Invest. 2003;111(8):1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ketola I, Otonkoski T, Pulkkinen MA, et al. Transcription factor GATA-6 is expressed in the endocrine and GATA-4 in the exocrine pancreas. Mol Cell Endocrinol. 2004;226(1–2):51–57. [DOI] [PubMed] [Google Scholar]
- 14. Decker K, Goldman DC, Grasch CL, Sussel L. Gata6 is an important regulator of mouse pancreas development. Dev Biol. 2006;298(2):415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carrasco M, Delgado I, Soria B, Martín F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122(10):3504–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martinelli P, Canamero M, Del Pozo N, Madriles F, Zapata A, Real FX. Gata6 is required for complete acinar differentiation and maintenance of the exocrine pancreas in adult mice. Gut. 2013;62(10):1481–1488. [DOI] [PubMed] [Google Scholar]
- 17. Rodríguez-Seguí S, Akerman I, Ferrer J. GATA believe it: new essential regulators of pancreas development. J Clin Invest. 2012;122(10):3469–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xuan S, Borok MJ, Decker KJ, et al. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122(10):3516–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275(1):235–244. [DOI] [PubMed] [Google Scholar]
- 20. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. [DOI] [PubMed] [Google Scholar]
- 21. Sodhi CP, Li J, Duncan SA. Generation of mice harbouring a conditional loss-of-function allele of Gata6. BMC Dev Biol. 2006;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruzankina Y, Pinzon-Guzman C, Asare A, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1(1):113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rankin MM, Wilbur CJ, Rak K, Shields EJ, Granger A, Kushner JA. β Cells are not generated in pancreatic duct ligation induced injury in adult mice. Diabetes. 2013;62(5):1634–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tuttle AH, Rankin MM, Teta M, et al. Immunofluorescent detection of two thymidine analogues (CldU and IdU) in primary tissue. J Vis Exp. 2010(46).pii 2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radvanyi F, Christgau S, Baekkeskov S, Jolicoeur C, Hanahan D. Pancreatic β cells cultured from individual preneoplastic foci in a multistage tumorigenesis pathway: a potentially general technique for isolating physiologically representative cell lines. Mol Cell Biol. 1993;13(7):4223–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang Y, Bai G, Doliba N, et al. Glucose metabolism and insulin release in mouse β HC9 cells, as model for wild-type pancreatic β-cells. Am J Physiol. 1996;270(5 Pt 1):E846–E857. [DOI] [PubMed] [Google Scholar]
- 27. He LM, Sartori DJ, Teta M, et al. Cyclin D2 protein stability is regulated in pancreatic β-cells. Mol Endocrinol. 2009;23(11):1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kushner JA, Ciemerych MA, Sicinska E, et al. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol. 2005;25(9):3752–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ritz-Laser B, Mamin A, Brun T, Avril I, Schwitzgebel VM, Philippe J. The zinc finger-containing transcription factor Gata-4 is expressed in the developing endocrine pancreas and activates glucagon gene expression. Mol Endocrinol. 2005;19(3):759–770. [DOI] [PubMed] [Google Scholar]
- 30. Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC. Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced β-cell endoplasmic reticulum stress. Diabetes. 2009;58(4):906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci USA. 2004;101(18):6975–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suzuki YJ, Nagase H, Wong CM, et al. Regulation of Bcl-xL expression in lung vascular smooth muscle. Am J Respir Cell Mol Biol. 2007;36(6):678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kyrönlahti A, Rämö M, Tamminen M, et al. GATA-4 regulates Bcl-2 expression in ovarian granulosa cell tumors. Endocrinology. 2008;149(11):5635–5642. [DOI] [PubMed] [Google Scholar]
- 34. Back SH, Scheuner D, Han J, et al. Translation attenuation through eIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in β cells. Cell Metab. 2009;10(1):13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem. 2002;277(27):24390–24398. [DOI] [PubMed] [Google Scholar]
- 36. Jacobsen CM, Mannisto S, Porter-Tinge S, et al. GATA-4:FOG interactions regulate gastric epithelial development in the mouse. Dev Dyn. 2005;234(2):355–362. [DOI] [PubMed] [Google Scholar]
- 37. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bradfield JP, Qu HQ, Wang K, et al. A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS Genet. 2011;7(9):e1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi D, Woo M. Executioners of apoptosis in pancreatic β-cells: not just for cell death. Am J Physiol Endocrinol Metab. 2010;298(4):E735–E741. [DOI] [PubMed] [Google Scholar]
- 40. Rankin MM, Kushner JA. Adaptive β-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58(6):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki YJ, Evans T. Regulation of cardiac myocyte apoptosis by the GATA-4 transcription factor. Life Sci. 2004;74(15):1829–1838. [DOI] [PubMed] [Google Scholar]
- 43. Agnihotri S, Wolf A, Picard D, Hawkins C, Guha A. GATA4 is a regulator of astrocyte cell proliferation and apoptosis in the human and murine central nervous system. Oncogene. 2009;28(34):3033–3046. [DOI] [PubMed] [Google Scholar]
- 44. Marshak S, Benshushan E, Shoshkes M, Havin L, Cerasi E, Melloul D. Functional conservation of regulatory elements in the pdx-1 gene: PDX-1 and hepatocyte nuclear factor 3β transcription factors mediate β-cell-specific expression. Mol Cell Biol. 2000;20(20):7583–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9(2):279–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.