Abstract
Purpose
Ganetespib is a novel inhibitor of the heat shock protein 90 (Hsp90), a chaperone protein critical to tumor growth and proliferation. In this phase II study, we evaluated the activity and tolerability of ganetespib in previously treated patients with non–small cell lung cancer (NSCLC).
Experimental Design
Patients were enrolled into cohort A (mutant EGFR), B (mutant KRAS), or C (no EGFR or KRAS mutations). Patients were treated with 200 mg/m2 ganetespib by intravenous infusion once weekly for 3 weeks followed by 1 week of rest, until disease progression. The primary endpoint was progression-free survival (PFS) at 16 weeks. Secondary endpoints included objective response (ORR), duration of treatment, tolerability, median PFS, overall survival (OS), and correlative studies.
Results
Ninety-nine patients with a median of 2 prior systemic therapies were enrolled; 98 were assigned to cohort A (n = 15), B (n = 17), or C (n = 66), with PFS rates at 16 weeks of 13.3%, 5.9%, and 19.7%, respectively. Four patients (4%) achieved partial response (PR); all had disease that harbored anaplastic lymphoma kinase (ALK) gene rearrangement, retrospectively detected by FISH (n = 1) or PCR-based assays (n = 3), in crizotinib-naïve patients enrolled to cohort C. Eight patients (8.1%) experienced treatment-related serious adverse events (AE); 2 of these (cardiac arrest and renal failure) resulted in death. The most common AEs were diarrhea, fatigue, nausea, and anorexia.
Conclusions
Ganetespib monotherapy showed a manageable side effect profile as well as clinical activity in heavily pretreated patients with advanced NSCLCs, particularly in patients with tumors harboring ALK gene rearrangement.
Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide, with non–small cell lung cancer (NSCLC) representing 80% to 85% of cases (1). For the majority of patients, platinum-based regimens remain the mainstay of treatment with a modest effect on overall survival (OS; refs. 2, 3). Several classes of targeted therapies have been developed in molecularly defined subsets of NSCLCs, most notably those expressing mutant EGFR (4) or re-arranged ALK (5). The EGFR inhibitors gefitinib and erlotinib are currently integrated into the standard of care for the treatment of mutant EGFR disease (6). Crizotinib, an ALK tyrosine kinase inhibitor, was recently granted accelerated approval in the United States for the treatment of patients with ALK-positive NSCLCs. This indication is based on phase I and II studies showing a high objective response rate (ORR) of greater than 50% (7). In a retrospective analysis of nonrandomized ALK-positive patients, crizotinib was associated with improved survival compared with that of crizotinib-untreated ALK-positive controls (8), although prospective data addressing this issue will likely never be available (9).
Heat shock protein 90 (Hsp90) is an attractive therapeutic target, given its ability to inhibit multiple pathways that are biologically relevant in NSCLCs (10). Hsp90 belongs to a class of molecular chaperone proteins that plays a central role in the assembly of multiprotein chaperone complexes and regulates the folding, stability, and function of many client proteins that are oncogenic drivers of lung adenocarcinoma subsets, such as mutant EGFR (11), wild-type c-RAF (12), mutant BRAF (13, 14), wild-type and mutant HER2 (15, 16), as well as the EML4-ALK translocation product (17, 18). Inhibition of Hsp90 depletes these kinases from cancer cells and disrupts signaling pathways critical for proliferation and survival (19). In cell lines and associated xenografts, mutant EGFR and ALK proteins retain sensitivity to Hsp90 inhibition irrespective of secondary gatekeeper mutations that confer resistance to erlotinib or crizotinib, respectively (20–22). KRAS-mutant NSCLC cell lines are also highly sensitive to Hsp90 inhibitors, possibly related to their dependence on c-RAF–mediated signaling (23, 24). Hsp90 inhibitors have induced marked regressions in genetically engineered mouse models driven by mutant EGFR, KRAS, or EML4-ALK; responses in these models are short-lived, although more durable in mice with ALK-rearranged lung cancer (17, 20, 23).
These preclinical observations have prompted the clinical assessment of Hsp90 inhibitors in NSCLCs. In a recent phase II study, 76 patients with metastatic NSCLCs were treated with single-agent retaspimycin hydrochloride (IPI-504). Partial responses were observed in 5 patients, including 1 of 28 with tumor harboring mutant EGFR and 2 of 3 with rearranged ALK. The third patient with ALK-positive disease achieved prolonged disease stability (25).
Ganetespib (STA-9090), 5-[2,4-dihydroxy-5-(1 methylethyl)phenyl]-2,4 dihydro-4-(1-methyl-1H indol-5 yl)-3H-1,2,4 triazole-3-one, is a novel triazolone heterocyclic Hsp90 inhibitor (26). Preclinical studies with this compound revealed potent Hsp90 inhibition and activity against a range of cancer models including lung, prostate, colon, breast, melanoma, and leukemia (27–29). In phase I and II studies, single-agent ganetespib has shown good tolerability, with fatigue and diarrhea as manageable side effects. Importantly, there have been no consistent hepatic or ocular toxicities that currently complicate the development of other agents in this class (30).
This study was undertaken to evaluate the clinical activity of ganetespib monotherapy in previously treated patients with molecularly defined NSCLCs, including those with tumors harboring mutant EGFR, mutant KRAS, or tumors lacking these mutations.
Patients and Methods
Study design
This nonrandomized, open-label multicenter study was conducted in 19 centers in the United States. The primary objective was to assess the effect of ganetespib on progression-free survival (PFS) at 16 weeks in patients with advanced NSCLCs. Secondary objectives were ORR, disease control rate (DCR) at 8 and 16 weeks, median PFS, safety and tolerability, OS, and molecular markers associated with clinical outcome.
The study protocol was approved by the Institutional Review Board at each institution and was conducted according to the recommendations of Good Clinical Practice. The study is registered at www.clinicaltrials.gov (NCT01031225).
Eligibility criteria
Patients were enrolled into one of 3 cohorts: cohort A, mutant EGFR; cohort B, mutant KRAS; and cohort C, no known EGFR or KRAS mutations. Patients’ tumors were prospectively screened for EGFR or KRAS mutation for enrollment into cohort A or B, respectively. Patients with disease without an identified EGFR or KRAS mutation were assigned to cohort C.
Eligible patients had pathologically confirmed stage IIIB/ IV NSCLCs, measurable disease with documented disease progression at baseline, and were ≥18 years of age with Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2. Adequate hematologic, renal, and hepatic function and ventricular ejection fraction ≥50% were required. Patients may have received at least 1 prior therapy, which included an EGFR-tyrosine kinase inhibitor (TKI; cohort A only, unless patients had tumor harboring mutation with known de novo EGFR-TKI resistance) or platinum doublet (cohorts B and C). Clinically and radiologically stable brain metastases were allowed. Exclusion criteria included baseline QTc > 470 msec or known serious cardiac illness. All patients gave written informed consent according to Institutional and Federal Guidelines.
Study treatment
All patients were treated with 200 mg/m2 ganetespib once weekly by intravenous infusion for 3 consecutive weeks followed by a 1-week dose-free interval. Dose delays and reductions were permitted for grade III or IV ganetespib-related toxicities. Treatment with ganetespib continued until disease progression, unacceptable toxicity, or patient consent withdrawal.
Study assessments
Patients’ demographics and medical history were recorded at baseline. Safety assessments were conducted at baseline and weekly during treatment. ECG was conducted at baseline and predose on day 1 of each cycle to evaluate for QTc prolongation. Adverse events (AE) were assessed at baseline and weekly during treatment, and toxicity was graded using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.0 (31).
Clinical activity was assessed by computed tomographic (CT) scans at baseline and every 8 weeks thereafter. Tumor responses were categorized per Response Evaluation Criteria in solid Tumors (RECIST), v1.1 (32), with confirmation of responses conducted at least 4 weeks later.
Correlative biomarker analyses
For prospective assignment to appropriate cohorts, EGFR and KRAS mutational status were determined by direct sequence analysis of cDNA extracted from formalin-fixed, paraffin-embedded archival tumor samples in all patients (Caris Life Sciences).
Additional retrospective biomarker testing included gene mutational analysis, EGFR amplification, and ALK rearrangement, all contingent on remaining tissue availability. Detailed procedures are provided in the Supplementary Methods. Briefly, gene mutational analysis was conducted on DNA extracted from archived tumor samples on the Sequenom Mass ARRAY platform (53 genes; 649 mutations) according to the manufacturer’s protocol; assays were conducted by the Translational Research Laboratory at Oregon Health & Science University (OHSU), Portland, OR. EGFR gene amplification in tissues from cohort C only was determined by FISH using Abbott probes for EGFR (LSI EGFR 7p12, red) with an identifier probe for the chromosome 7 centromeric region (7p11.1-q11.1 Alpha Satellite DNA, green). One hundred interphase cells were scored for each sample. Assays were conducted by the OHSU Research Cytogenetics Laboratory.
At the time the study was conducted, there was no gold-standard test for detection of ALK translocations; therefore, 3 different assays were evaluated in samples from cohort C: (i) break-apart FISH (OHSU Research Cytogenetics Laboratory), (ii) quantitative nuclease protection array (qNPA) conducted by High Throughput Genomics (HTG), which detects mRNA encoding EML4-ALK variants 1, 2, 3a, 3b, 4, 5a, 5b and 6; and (iii) real-time quantitative PCR (RT-qPCR) carried out by Insight Genetics, which detects the presence of any of the 11 different EML4-ALK variants and non-EML4 fusions partners (TFG and KIF5B) that have been reported in NSCLCs.
Statistical analysis
The primary endpoint of the study was PFS rate at 16 weeks defined as the proportion of patients alive and free of disease progression per RECIST. The choice of the PFS rate as the primary endpoint was based on preliminary data from phase I trials with ganetespib, in which prolonged disease stabilization was observed in several patients. Therefore, the use of a standard cytotoxic trial design based on ORR as a primary endpoint was considered inadequate, as it could lead to rejection of a potentially active agent. In heavily treated patients with NSCLCs, PFS is now considered as an optimal surrogate endpoint for assessing the activity of new agents (33). Secondary endpoints were DCR, median PFS, ORR, treatment duration, OS, tolerability, and biomarker analysis. The study was designed as a Simon 2-stage; if ≥2 of 14 patients in each cohort were progression-free at 16 weeks, then the cohort would be expanded to 23 patients. At the end of stage 2, the treatment would be declared to have a substantial activity (≥35%) in a cohort if at least 5 of 23 patients were progression-free at 16 weeks. This design provides 90% statistical power to detect a difference of 25 percentage points (35% vs. 10%) in PFS rate at 16 weeks with a significance level less than 0.1.
As with the other 2 cohorts, cohort C was planned as a 2-stage design. On the basis of the preliminary signal of clinical activity, cohort C was enriched with patients with adenocarcinoma histology. A protocol amendment allowed enrollment of approximately 35 additional patients with adenocarcinoma to cohort C to more accurately determine the PFS rate in this population (with a significance level less than 0.01). Therefore, the total number of patients required in cohort C was 58.
The primary analysis was based on both the intent-to-treat (ITT) population (patients who received at least 1 dose), and the evaluable population (patients who received at least one treatment cycle and had one follow-up scan). The safety population included all patients receiving at least one dose of ganetespib. All analyses were conducted using SAS statistical software, version 9.1 (SAS Institute,).
Results
Patient characteristics
Ninety-nine patients were enrolled between December 2009 and May 2011 and were assigned to cohort A (n =15), B (n = 17), or C (n = 66), with one patient of unknown mutational status not assigned to any cohort. Their baseline characteristics are shown in Table 1. Each cohort required enrollment of 14 patients in stage 1; however, cohorts A and B were overenrolled by 1 and 3 patients, respectively. Similarly, following the stage 2 expansion phase, cohort C enrolled a total of 66 patients, 8 more than required. Overenrolled patients had signed informed consent and were already in screening at the time the cohorts were filled; therefore, it was deemed unethical to not allow their participation. Nine patients did not meet the eligibility criteria due to abnormal baseline serum chemistry (n = 6), insufficient cardiac function (n = 2), or unknown EGFR or KRAS mutational status (n = 1). All 99 patients were included in the ITT analysis for efficacy and safety analysis. The majority of patients were heavily pretreated, with 19 patients (19.2%) receiving at least 3 prior systemic therapies. All patients had documented progressive disease (PD) at baseline. Compared with the other cohorts, patients in the mutant KRAS cohort had the shortest interval since diagnosis of advanced disease and trial enrollment (median, 10.8 months, compared with 33, and 16.7 months in cohorts A and C, respectively; Table 1). The main reasons for treatment discontinuation were disease progression (n = 64, 64.7%), consent withdrawal (n = 9, 9.1%), and investigator’s decision (n = 6, 6.1%).
Table 1.
Patients’ characteristics at baseline
| Number (%) of patients
|
||||
|---|---|---|---|---|
| Cohort A n = 15 (%) | Cohort B n = 17 (%) | Cohort C n = 66 (%) | Totala n = 99 (%) | |
| Age, y | ||||
| Median (range) | 60 (50–79) | 64 (28–76) | 62 (24–82) | 61 (24–82) |
| Gender | ||||
| Male | 5 (33.3) | 4 (23.5) | 38 (57.6) | 47 (47.5) |
| Female | 10 (66.7) | 13 (76.5) | 28 (42.4) | 52 (52.5) |
| Race, n (%) | ||||
| White | 8 (53.3) | 14 (82.4) | 54 (81.8) | 76 (76.8) |
| Black | 0 | 2 (11.8) | 6 (9.1) | 8 (8.1) |
| Other | 7 (46.7) | 1 (5.9) | 6 (9.1) | 15 (15.1) |
| ECOG PS,b n (%) | ||||
| 0 | 6 (40) | 4 (23.5) | 16 (24.2) | 26 (26.3) |
| 1 | 9 (60) | 13 (76.5) | 48 (72.7) | 71 (71.7) |
| Histology, n (%) | ||||
| Squamous | 0 | 2 (11.8) | 5 (7.6) | 7 (7.1) |
| Adenocarcinoma | 15 (100) | 15 (88.2) | 58 (87.9) | 89 (89.9) |
| Large cell | 0 | 0 | 2 (3) | 2 (2) |
| Adenosquamous | 0 | 0 | 0 | 0 |
| NOS | 0 | 0 | 1 (1.5) | 1 (1) |
| Stage at study entry, n (%) | ||||
| IIIb | 2 (13.3) | 0 | 0 | 2 (2) |
| IV | 13 (86.7) | 17 (100) | 66 (100) | 97 (98) |
| Time since diagnosis of advanced NSCLC to consent, mo | ||||
| Median (range) | 33 (5.2–80.4) | 10.8 (0.5–27.3) | 16.7 (3.3–98.3) | 16.7 (0.5–98.3) |
| Prior systemic therapies | ||||
| Median (range) | 2 (1–6) | 2 (1–4) | 2 (1–10) | 2 (1–10) |
Abbreviation: NOS, not otherwise specified.
One enrolled patient had an unknown mutation status and was not assigned to a cohort.
Two patients had missing information.
Clinical activity and biomarker analyses
The planned analysis of activity was conducted when all enrolled patients were followed for at least 16 weeks. Both cohort A (mutant EGFR) and cohort B (mutant KRAS) were terminated following stage 1, due to lack of clinical activity per the protocol prespecified criteria (Table 2). In cohort A, disease stabilization was achieved in 40% of patients; however, the PFS rate at 16 weeks was only 13.3% (2 of 15). In this cohort, all patients but one received and failed prior EGFR-TKI therapy; the EGFR-TKI–naïve patient had tumor harboring exon 20 insertion mutation. All patients had evidence of EGFR mutation; however, as no biopsies had been taken at progression following prior EGFR-TKI treatment, it remains unknown whether their tumors had acquired resistance via secondary EGFR T790M mutation or another mechanism.
Table 2.
Investigator-evaluated assessment of response
| ITT population | Cohort A n = 15 (%) | Cohort B n = 17 (%) | Cohort C n = 66 (%) |
|---|---|---|---|
| Best response,a n (%) | 0 | 0 | 4 (6.1) |
| CR | 0 | 0 | 0 |
| PR | 0 | 0 | 4 (6.1) |
| SD | 6 (40) | 6 (35.3) | 26 (39.4) |
| PD | 7 (46.7) | 7 (41.2) | 26 (39.4) |
| Nonevaluableb | 2 (13.3) | 4 (23.5) | 10 (15.2) |
| DCR (≥8 wk)c | 4 (26.7) | 5 (29.4) | 28 (42.4) |
| PFS rate at 16 wk | 2 (13.3) | 1 (5.9) | 13 (19.7) |
| PFS, median (95% CI), mo | 1.9 (1.6–3.6) | 1.9 (1.6–3.7) | 1.8 (1.8–2.9) |
| OS, median (95% CI), mo | 7.1 (5.2–14.3) | 11.0 (3.9–17.1) | 8.8 (4.4–10.5) |
Initial assessment at 8 weeks from treatment start with confirmation assessment at least 4 weeks later.
Patients discontinuing treatment before the first posttreatment scans were considered nonevaluable. Reasons were: AEs (n = 4); death (n = 3); investigator decision (n = 2), protocol violation (n = 2); symptomatic deterioration (n = 1); and withdrawal of informed consent (n = 4).
DCR: CR and PR and SD ≥ 8 weeks.
In cohort B (mutant KRAS), 47% of patients (8 of 17) had tumor shrinkage (Fig. 1A), although there were no objective responses observed. The 16 week PFS rate was 5.9% (1 of 17; Table 2).
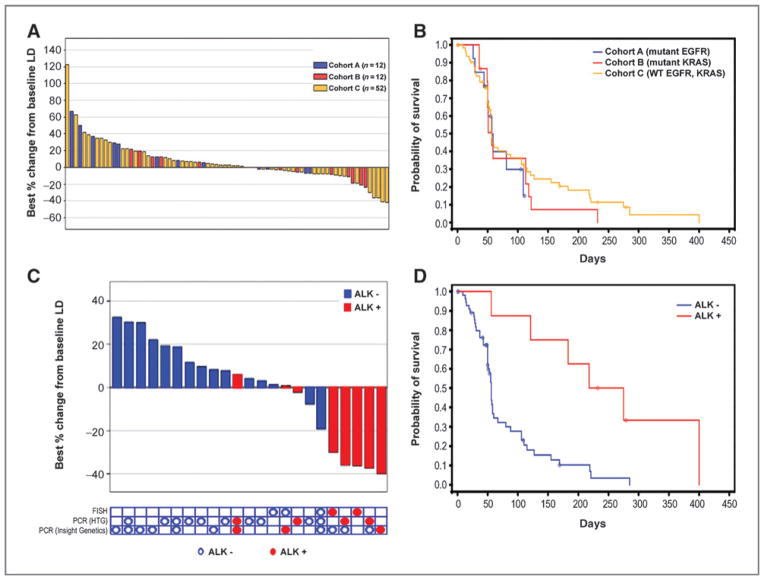
Figure 1.
Clinical activity with ganetespib. A, best response in all cohorts. B, Kaplan–Meier curve for PFS in each cohort. C, changes in tumor size in cohort C patients with (n = 8) and without (n = 15) ALK rearrangement. Of all patients in this cohort, the 23 shown had sufficient tissue for assessing ALK gene rearrangement status. D, Kaplan–Meier curve for PFS in patients with (n = 8) versus without (n = 15) ALK rearrangement.
In cohort C, 2 patients (2 of 14) in stage 1 were progression-free at 16 weeks. Thus, the cohort proceeded to stage 2, after which 5 of 23 patients were progression-free at 16 weeks, before ultimately expanding, following a protocol amendment, to a final total of 66 patients. The PFS rate at 16 weeks was 19.7% (13 of 66; Table 2). Partial response was achieved in 4 patients (6.1%), and all had disease that harbored ALK rearrangement. Of the 66 patients, 20 (30.3%) showed evidence of tumor regression, which included the 4 patients with partial response (Fig. 1A).
Median PFS was 1.9 months [95% confidence interval (CI), 1.6–3.6] for cohort A, 1.9 months (95% CI, 1.6–3.7) for cohort B, and 1.8 months (95% CI, 1.8–2.9) for cohort C (Fig. 1B). Median OS was 7.1 months (95% CI, 5.2–14.3), 11 months (95% CI, 3.9–17.1), and 8.8 months (95% CI, 4.4–10.5) for cohorts A, B and C, respectively (Table 2).
Archival tissue was obtained from 59 of the 99 enrolled patients in all cohorts distributed as follows: cohort A, n =7; cohort B, n = 12; and cohort C, C = 40. Retrospective gene mutational analysis was negative for additional mutations beyond EGFR and KRAS in 30 of the 59 patients, whereas the remaining 29 samples failed processing due to insufficient tumor material. Testing for EGFR amplification was also negative, although EGFR polysomy was noted in 4 patients, all in cohort C.
Of the 40 tumor samples obtained in cohort C, sufficient tissue for retrospective ALK biomarker testing was available from only 23. These 23 patients were tested for the presence of ALK gene rearrangement using the break-apart FISH assay and/or 2 PCR platforms (Fig. 1C). Fifteen of 23 samples had sufficient tumor cells for only one assay, whereas the remaining 8 had enough tumor cells for testing with 2 or 3 assays. Eight of the 23 samples tested showed ALK positivity (FISH, n = 2; PCR, n = 6), all from patients who were crizotinib-naïve. Four of the 8 patients achieved partial response, with treatment duration ranging from 7.4 to 21 months (Fig. 1C, Table 3). Of the remaining 4 ALK-positive patients, 3 patients achieved stable disease (SD) as the best response, with a duration ranging from 121 to 218 days and one patient experienced disease progression (Table 3). Median PFS was 8.1 months, significantly longer than for patients without ALK rearrangement (HR, 0.223; 95% CI, 0.085–0.582; Fig. 1D).
Table 3.
Demographics of patients harboring ALK rearrangement
| Patient ID | Age/gender | Regimen number/treatment | Treatment duration, mo | Best overall response |
|---|---|---|---|---|
| 0660-6021 | 57/M | 1. Carboplatin, pemetrexed | 1.2 | UNK |
| 2. Carboplatin, paclitaxel | 2 | UNK | ||
| 3. Cisplatin, gemcitabine | 1 | UNK | ||
| 4. Ganetespib | 21 (ongoing) | PR | ||
| 0059-6005 | 79/M | 1. Bevacizumab, carboplatin, paclitaxel | 5 | SD |
| 2. Bevacizumab | 19 | SD | ||
| 3. Erlotinib | 10 | SD | ||
| 4. Carboplatin, pemetrexed | 6 | PD | ||
| 5. Ganetespib | 14.8 | PR | ||
| 0654-6006 | 67/M | 1. Bevacizumab, carboplatin, paclitaxel | 2.8 | SD |
| 2. Sunitinib | 1.4 | PD | ||
| 3. Ganetespib | 8.8 | PR | ||
| 0660-6016 | 68/M | 1. Celebrex or placebo, erlotinib | 1.8 | PD |
| 2. Pemetrexed, carboplatin | 2.8 | SD | ||
| 3. Ganetespib | 7.4 | PR | ||
| 0059-6003 | 68/M | 1. Pemetrexed, carboplatin | 3.5 | SD |
| 2. Ganetespib | 11.7 | SD | ||
| 0002-6019 | 70/M | 1. Cisplatin, vinorelbine | 2.8 | SD |
| 2. Pemetrexed | 2.5 | SD | ||
| 3. Ganetespib | 5.8 | SD | ||
| 0002-6016 | 64/F | 1. Pemetrexed, carboplatin | 0.9 | PD |
| 2. Vinorelbine | 0.9 | PD | ||
| 3. Gemcitabine | 4.1 | SD | ||
| 4. Docetaxel | 3.5 | SD | ||
| 5. AZD6244 (investigational) | 4.1 | SD | ||
| 6. Ganetespib | 3.0 | SD | ||
| 0652-6003 | 44/F | 1. Carboplatin, gemcitabine | 2.3 | PD |
| 2. Erlotinib | 2.2 | PD | ||
| 3. Bevacizumab, pemetrexed | 3.9 | PD | ||
| Pemetrexed | 17.7 | PD | ||
| Bevacizumab | 7.6 | PD | ||
| 4. Bevacizumab, erlotinib | 1.4 | PD | ||
| Erlotinib | 0.7 | PD | ||
| 5. Xytotax | 0.7 | PD | ||
| 6. Paclitaxel | 7.0 | PD | ||
| 7. Ganetespib | 1.3 | PD |
Abbreviations: F, female; M, male; UNK, unknown.
There were 2 additional patients enrolled in cohort C with tumors already known to harbor ALK rearrangement who had received prior crizotinib therapy. One patient was treated with crizotinib for 10 months before disease progression. The second patient had a brief, unconfirmed response to crizotinib; subsequent tumor sampling showed a secondary L1152R mutation (34). Both patients showed outright disease progression on ganetespib treatment.
Safety
Twenty-five patients (25.3%) received at least 4 treatment cycles, with 14 (14.1%) remaining on study >6 months, showing the long-term tolerability of ganetespib in some patients. Thirty-four patients (34.3%) experienced serious AEs (SAE), although only in 8 patients (8.1%) were the events considered treatment-related (one each of asthenia, atrial fibrillation, cardiac arrest, diarrhea, fatigue, increased lipase, renal failure, and vomiting). Ten (10.1%) deaths were reported during the study; 2 (2%) were considered treatment-related. One patient with a centrally located squamous cell carcinoma presented with severe hemoptysis and subsequent severe anemia leading to cardiac arrest. The second patient presented with rapidly progressive widespread disease and developed intravascular volume depletion 1 week into treatment, precipitating acute renal failure. Although the primary cause of death was attributed to complications from NSCLCs, the event of acute renal failure was still assessed as possibly related to the study drug. The ganetespib dose was modified in a total of 48 patients (48.5%), mainly due to AEs (n = 30, 30.3%). AEs led to treatment discontinuation in 8 patients (8.1%). The majority of drug-related AEs were grade I and II and the overall incidence of grade III and IV was 29.3%. Gastrointestinal disorders comprised the majority of toxicities reported in nearly all patients (n = 92, 92.9%), including diarrhea (81.8%) and nausea (41.4%; Table 4). Elevated hepatic enzymes were infrequent and generally grade I or II. Fourteen (14.1%), 11 (11.1%), and 10 (10.1%) patients had transient alkaline phosphatase (ALP), ALT, and AST elevation, respectively; of these, 1 (1%) and 3 (3%) patients had grade III ALT and AST elevations that were considered related to ganetespib. Five patients (5.1%) had hyperbilirubinemia, mainly grade 1 (n = 4); 1 event was considered treatment-related. No instances of ganetespib-related visual disturbances were reported.
Table 4.
AEs reported in ≥10% of patients in the safety population, regardless of causality
| Any event | Number (%) of patientsa
|
|
|---|---|---|
| Any grade, n (%) | Grade III and IV, n (%) | |
| 98 (99) | 63 (63.6) | |
| Diarrhea | 81 (81.8) | 8 (8.1) |
| Fatigue | 57 (57.6) | 14 (14.1) |
| Nausea | 41 (41.4) | 0 |
| Decreased appetite | 37 (37.4) | 0 |
| Constipation | 26 (26.3) | 0 |
| Dyspnea | 25 (25.3) | 12 (12.1) |
| Vomiting | 21 (21.2) | 0 |
| Back pain | 20 (20.2) | 4 (4) |
| Cough | 20 (20.2) | 0 |
| Hyponatraemia | 18 (18.2) | 10 (10.1) |
| Weight decreased | 18 (18.2) | 0 |
| Dehydration | 17 (17.2) | 0 |
| Insomnia | 16 (16.2) | 0 |
| Dizziness | 15 (15.2) | 0 |
| ALP elevated | 14 (14.1) | 3 (3) |
| Infusion reactions | 14 (14.1) | 0 |
| Anxiety | 13 (13.1) | 0 |
| Headache | 13 (13.1) | 0 |
| Urinary tract infection | 13 (13.1) | 0 |
| Abdominal pain | 12 (12.1) | 3 (3) |
| ALT elevated | 11 (11.1) | 4 (4) |
| Muscular weakness | 11 (11.1) | 0 |
| Tachycardia | 11 (11.1) | 0 |
| Arthralgia | 10 (10.1) | 2 (2) |
| AST elevated | 10 (10.1) | 5 (5.1) |
| Lipase increased | 9 (9.1) | 5 (5.1) |
A patient may have had more than one event.
Discussion
This multicenter study evaluated the clinical activity and toxicity of ganetespib in molecularly defined cohorts of patients with advanced NSCLCs. Durable objective responses and disease stabilization occurred in the majority of patients with disease harboring ALK gene rearrangement who were crizotinib-naïve. In NSCLCs, ALK rearrangement results in the expression of one of several variants of the EML4-ALK fusion protein, which results in a constitutively active ALK kinase capable of activating downstream signaling cascades that promote cell proliferation and survival (5, 35, 36). In preclinical studies, ALK inhibition has been shown to induce cell death and tumor regression (17, 36, 37). The data from this trial and the recent study of IPI-504 suggest that in addition to direct tyrosine kinase inhibition, ALK can be disabled by Hsp90 inhibition, confirming preclinical predictions (17, 18). The Hsp90-inhibitory activity of ganetespib is further validation for the clinical value of this class of drugs in ALK-positive disease.
The study enrolled 2 patients with acquired crizotinib resistance who did not respond to treatment with ganete-spib; 1 patient had a tumor with a documented secondary mutation (34). In addition to gatekeeper mutations, other ALK-independent mechanisms, including the activation of compensatory signaling pathways, may confer resistance to targeted ALK agents (38). Further work will be necessary to determine whether ganetespib has a role in the treatment of any subsets of crizotinib-resistant disease.
At the time of the retrospective archival tumor analysis, breakapart FISH was not yet considered as the gold standard for detecting ALK rearrangements, which prompted our exploration of other diagnostic technologies. Our results are hypothesis-generating and suggest the use of these tests for detection of ALK rearrangement. Indeed we showed that in 3 of the 4 patients who achieved objective responses, ALK rearrangement was detected by a PCR-based assay. Although FISH is now the most established modality, the assay is costly and technically challenging (39). RT-PCR assays remain under development, and ultimately, these techniques may be combined with immunohistochemistry (IHC) for optimal clinical testing (40). Of note, discordance among multiple assays has been reported, with RT-PCR offering the greatest sensitivity (41). In addition, RT-PCR may facilitate detection of specific EML4-ALK variants that may be important for predicting Hsp90 inhibitor response (42).
Although archival tumor tissue was procured from approximately 60% of the patients in the study, sufficient tissue suitable for retrospective biomarker analysis was present in only around half of these samples. In this analysis, the sensitivity of ALK RT-PCR was critical because archival samples of limited size were subjected to multiple genomic analyses besides those aimed at detecting ALK rearrangement. In several samples, only one ALK assay could be conducted. This represents a limitation of the study and highlights the need for mandating tissue collection in future clinical trials. Furthermore, rebiopsy of patients following disease progression on TKIs to determine mechanisms of acquired resistance will be important to maximize information gained when they are subsequently treated with additional molecularly targeted agents.
In contrast to the promising signal of activity in ALK-positive disease, the study did not meet the protocol criteria for expansion of the initial mutant EGFR and mutant KRAS cohorts. Nearly all patients in the mutant EGFR cohort received prior anti-EGFR therapy, so that secondary EGFR mutations conferring erlotinib resistance or activation of alternative signaling pathways likely occurred. However, preclinical data predicted that EGFRs with secondary mutation as well as other receptor tyrosine kinases activated in erlotinib-resistant cells would be sensitive to degradation after Hsp90 inhibitor exposure (27). Alternatively, induction of small cell histologic changes or epithelial–mesenchymal transition may have been present in some cases (43) and may have contributed to the lack of durable clinical activity seen with ganetespib.
Importantly, preclinical pharmacodynamic modeling with NCI-H1975 (EGFR L858R/T790M) xenografts has shown that that although mutant EGFR is depleted by a single dose of ganetespib, reexpression occurs by 72 hours (44). Therefore, once-weekly dosing may be insufficient to suppress mutant EGFR signaling durably so that apoptosis is induced. Currently, more intense dosing schedules of ganetespib remain under development, including twice-weekly and consecutive day dosing; it will be of interest to determine whether efficacy can be improved against EGFR-mutant disease with alternative administration schedules.
Accumulating evidence suggests that the interaction of client proteins with the Hsp90 chaperone is a multifaceted process, with some kinases forming stable heterocomplexes with the chaperone machinery and others forming more dynamic complexes that are more readily disassembled and in which the client is more modestly ubiquitinated (45). These differences may contribute to the hierarchy of sensitivity of clients to degradation that has been described, with EML4-ALK as exquisitely sensitive, undergoing more rapid and sustained degradation than mutant EGFR following Hsp90 inhibition (18).
Ganetespib also showed only modest clinical activity in mutant KRAS disease, despite the significant sensitivity of KRAS-mutant NSCLC cell lines. Nonetheless, regressions occurred, which is provocative in this population. Interestingly, Hsp90 inhibitors have shown limited efficacy in genetically engineered KRAS-driven models of lung adenocarcinoma (23). RAS-driven cancers are dependent upon tightly regulated levels of reactive oxygen species (ROS; ref. 46). Hsp90 inhibition has been shown to cause production of ROS, producing endoplasmic reticulum (ER) stress. Elevated ROS can be buffered by mTOR-dependent glucose-6-phosphate dehydrogenase activity that promotes accumulation of reduced glutathione. mTOR inhibition has been shown to suppress glutathione production, so that combined inhibition of Hsp90 and mTOR has been shown synergize preclinically, precipitating irresolvable ER stress and catastrophic cellular damage (47). In this regard, a trial combining ganetespib and rapamycin is being planned.
Ganetespib caused toxicities that were primarily of grade I or II severity. Diarrhea was the most commonly reported side effect, consistent with the phase I and II experience (30), and was readily manageable with loperamide or diphenoxylate atropine. Diarrhea has been reported with both geldanamycin and non-geldanamycin Hsp90 inhibitors (48). It is not known whether this is an on-target effect but could possibly be linked to degradation of EGFR in the gut (49). Subsequent studies with ganetespib are incorporating prophylactic management of diarrhea. In contrast to the geldanamycins, severe liver function test abnormalities were uncommon with ganetespib. Importantly, ongoing preclinical evaluations suggest that the physiochemical properties of ganetespib likely result in lower retinal/plasma concentrations and more efficient retinal elimination than other Hsp90 inhibitors, accounting for the lack of ocular toxicity.
In summary, the side effect profile of once-weekly ganetespib observed in phase I trials was confirmed and considered manageable in the advanced NSCLC population. Ganetespib showed encouraging single-agent activity in patients with ALK-rearranged disease, with a response rate of 50%. Of the 4 partial responses, one was ALK-positive as detected by FISH and the remaining 3 were identified by PCR. The FISH assay is currently the gold-standard test; however, PCR may be more sensitive in detecting ALK rearrangement. On the basis of the findings presented here, a phase II study of ganetespib monotherapy in patients with crizotinib-naïve ALK-positive disease has recently been initiated (NCT01562015). This trial will further explore the use of various platforms for ALK testing (IHC, PCR, and FISH) and will prospectively define the activity of ganetespib in this population.
Supplementary Material
Translational Relevance.
Heat shock protein 90 (Hsp90) inhibitors have shown preclinical activity against non–small cell lung cancer (NSCLC) models, including those driven by mutant EGFR, rearranged ALK, or mutant KRAS. Ganetespib is a potent triazolone heterocyclic Hsp90 inhibitor, well-tolerated in phase I studies. This 2-stage phase II study investigated the activity of weekly ganetespib in 3 cohorts of patients with advanced NSCLCs, whose tumors were prospectively genotypically characterized for the presence of EGFR or KRAS mutation or the absence of these mutations. Ganetespib showed activity in the nonmutant EGFR or KRAS cohort, specifically among crizotinib-naïve patients whose tumors harbored ALK rearrangement. Among 8 such patients, there were 4 partial responses and a median progression-free survival of 8.1 months, justifying further study of ganetespib in ALK-rearranged NSCLCs. The initial mutant EGFR and KRAS cohorts did not meet criteria for further expansion. Alternative ganetespib schedules and combinatorial approaches may be required for these NSCLC subsets.
Acknowledgments
The authors thank the patients who participated and their families, as well as co-investigators, research nurses, study coordinators, and operations staff at the participating institutions. They also thank Richard Bates for editorial assistance and preparation of drafts of this manuscript. Kelly Maslin, Joelle Lufkin, Ron Blackman and Jane Kepros, formerly of Synta Pharmaceuticals, provided invaluable input during the inception and conduct of the study.
Grant Support
This study was supported by Synta Pharmaceuticals. K.-K. Wong and G.I. Shapiro were also supported by the Dana-Farber/Harvard Cancer Center Specialized Program for Research Excellence (SPORE) in Lung Cancer NIH grant (P50 CA90578).
Footnotes
Authors’ Contributions
Conception and design: M.A. Socinski, J. Goldman, I. El-Hariry, V. Vukovic, C.P. Belani, S. Ramalingham, F. Teofilovici, G.I. Shapiro
Development of methodology: M.A. Socinski, J. Goldman, I. El-Hariry, V. Vukovic, C.P. Belani
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M.A. Socinski, J. Goldman, K. Koczywas, V. Vukovic, L. Horn, E. Paschold, R. Salgia, H. West, L.V. Sequist, J.R. Brahmer, L.-C. Chen, A.B. Sandler, C.P. Belani, T.R. Webb, H. Harper, M. Huberman, S. Ramalingham, G.I. Shapiro
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M.A. Socinski, J. Goldman, I. El-Hariry, V. Vukovic, L. Horn, J.R. Brahmer, C.P. Belani, S. Ramalingham, F. Teofilovici, W. Guo, G.I. Shapiro
Writing, review, and/or revision of the manuscript: M.A. Socinski, J. Goldman, I. El-Hariry, K. Koczywas, V. Vukovic, L. Horn, R. Salgia, H. West, L. V. Sequist, P. Bonomi, J.R. Brahmer, L.-C. Chen, A.B. Sandler, C.P. Belani, H. Harper, M. Huberman, S. Ramalingham, K.-K. Wong, F. Teofilovici, W. Guo, G.I. Shapiro
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M.A. Socinski, E. Paschold, K.-K. Wong, F. Teofilovici, G.I. Shapiro
Study supervision: M.A. Socinski, J. Goldman, I. El-Hariry, K. Koczywas, V. Vukovic, E. Paschold, F. Teofilovici, G.I. Shapiro
Disclosure of Potential Conflicts of Interest
M.A. Socinski has a commercial research grant and is a consultant/ advisory board member of Synta. I. El-Hariry is employed (other than primary affiliation; e.g., consulting) as a VP of clinical research at Synta Pharmaceuticals. M. Koczywas has honoraria from speakers’ bureau from Pfizer and Genentech and has ownership interest (including patents) in Pfizer. V. Vukovic is employed (other than primary affiliation; e.g., consulting) as a chief medical officer at Synta Pharmaceuticals. L.V. Sequist is a consultant/advisory board member of Clovis Oncology, Boehringer Ingelheim, Merrimack Pharmaceuticals, Daiichi-Sankyo, and GSK. P. Bonomi is a consultant/advisory board member of Synta. J.R. Brahmer is a consultant/advisory board member of Synta. A.B. Sandler has a commercial research grant and is a consultant/advisory board member of Synta. H. Harper has honoraria from speakers’ bureau and is a consultant/advisory board member of Lilly. K.-K. Wong has a commercial research grant from Synta. F. Teofilovici is employed (other than primary affiliation; e.g., consulting) as a senior director of clinical research at Synta Pharmaceuticals. W. Guo is employed (other than primary affiliation; e.g., consulting) as a biostatistician at Synta Pharmaceutical Corp. G.I. Shapiro has a commercial research grant and is a consultant/advisory board member of Synta Pharmaceuticals. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non–small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 3.D’Addario G, Pintilie M, Leighl NB, Feld R, Cernyl T, Shepherd FA. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol. 2005;23:2926–36. doi: 10.1200/JCO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 4.West L, Vidwans SJ, Campbell NP, Shrager J, Simon GR, Bueno R, et al. A novel classification of lung cancer into molecular subtypes. PLoS One. 2012;7:e31906. doi: 10.1371/journal.pone.0031906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Schmid-Bindert G, Zhou C. Erlotinib in the treatment of advanced non-small cell lung cancer: an update for clinicians. Ther Adv Med Oncol. 2012;4:19–29. doi: 10.1177/1758834011427927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran MP. Crizotinib: In locally advanced or metastatic non-small cell lung cancer. Drugs. 2012;72:99–107. doi: 10.2165/11207680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Shimamura T, Shapiro GI. Heat shock protein 90 inhibition in lung cancer. J Thorac Oncol. 2008;3:S152–9. doi: 10.1097/JTO.0b013e318174ea3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimamura T, Lowell AM, Engelman JA, Sahpiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res. 2005;65:6401–8. doi: 10.1158/0008-5472.CAN-05-0933. [DOI] [PubMed] [Google Scholar]
- 12.Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–8. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 13.da Rocha Dias S, Friedlos F, Light Y, Springer C, Workman P, Marais R. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2005;65:10686–91. doi: 10.1158/0008-5472.CAN-05-2632. [DOI] [PubMed] [Google Scholar]
- 14.Grbovic OM, Basso AD, Sawai A, Ye Q, Friedlander P, Solit D, et al. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng FF, Kuduk SD, Chiosis G, Münster PN, Sepp-Lorenzino L, Danishefsky SJ, et al. Identification of a geldanamycin dimer that induces the selective degradation of HER-family tyrosine kinases. Cancer Res. 2000;60:2090–4. [PubMed] [Google Scholar]
- 16.Xu W, Soga S, Beebe K, Lee MJ, Kim YS, Trepel J, et al. Sensitivity of epidermal growth factor receptor and ErbB2 exon 20 insertion mutants to Hsp90 inhibition. Br J Cancer. 2007;97:741–4. doi: 10.1038/sj.bjc.6603950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010;70:9827–36. doi: 10.1158/0008-5472.CAN-10-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011;30:2581–6. doi: 10.1038/onc.2010.625. [DOI] [PubMed] [Google Scholar]
- 19.Neckers L. HSP90 and cancer: Targeting a dynamic chaperone complex. Annals Oncol. 2010;21:ii23. [Google Scholar]
- 20.Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, Borgman CL, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–38. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regales L, Balak MN, Gong Y, Politi K, Sawai A, Le C, et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS One. 2007;2:e810. doi: 10.1371/journal.pone.0000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–40. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sos ML, Michel K, Zander T, Weiss J, Frommolt P, Peifer M, et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J Clin Invest. 2009;119:1727–40. doi: 10.1172/JCI37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blasco RB, Francoz S, Santamaria D, Canamero M, Dubus P, Charron J, et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell. 2011;19:652–63. doi: 10.1016/j.ccr.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non–small-cell lung cancer. J Clin Oncol. 2010;28:1–10. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying W, Du Z, Sun L, Foley KP, Proia DA, Blackman RK, et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther. 2012;11:475–84. doi: 10.1158/1535-7163.MCT-11-0755. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Invest Drugs. 2010;11:1466–76. [PubMed] [Google Scholar]
- 28.Proia DA, Foley KP, Korbut T, Sang J, Smith D, Bates RC, et al. Multifaceted intervention by the Hsp90 inhibitor ganetespib (STA-9090) in cancer cells with activated JAK/STAT signaling. PLoS One. 2011;6:e18552. doi: 10.1371/journal.pone.0018552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S, Zhang C, Shafi AA, Sequeira M, Acquaviva J, Friedland JC, et al. Potent activity of the hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expression. Int J Oncol. 2013;42:35–43. doi: 10.3892/ijo.2012.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi HK, Lee K. Recent updates on the development of ganetespib as a Hsp90 inhibitor. Arch Pharm Res. 2012;35:1855–9. doi: 10.1007/s12272-012-1101-z. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 (CTCAE) Vol. 4.03. Bethesda, MD: US Department of Health And Human Services, National Institutes of Health; 2010. [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Soria JC, Massard C, Le Chevalier T. Should progression-free survival be the primary measure of efficacy for advanced NSCLC therapy? Ann Oncol. 2010;21:2324–32. doi: 10.1093/annonc/mdq204. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–60. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allouche M. ALK is a novel dependence receptor: potential implications in development and cancer. Cell Cycle. 2007;6:1533–8. doi: 10.4161/cc.6.13.4433. [DOI] [PubMed] [Google Scholar]
- 36.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK rearranged lung cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw AT, Solomon B, Kenudson MM. Crizotinib and testing for ALK. J Natl Compr Cancer Netw. 2011;9:1135–41. doi: 10.6004/jnccn.2011.0115. [DOI] [PubMed] [Google Scholar]
- 40.Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, Mark EJ, et al. A novel, highly sensitive antibody allows for routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16:1561–71. doi: 10.1158/1078-0432.CCR-09-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallender ML, Geiersbach KB, Tripp SR, Layfield LJ. Comparison of reverse transcription-polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule-associated proteinlike 4-anaplastic lymphoma kinase fusion-positive non-small cell lung carcinoma. Arch Pathol Lab Med. 2012;136:796–803. doi: 10.5858/arpa.2011-0321-OA. [DOI] [PubMed] [Google Scholar]
- 42.Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18:4682–90. doi: 10.1158/1078-0432.CCR-11-3260. [DOI] [PubMed] [Google Scholar]
- 43.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Trans Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, et al. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18:4973–85. doi: 10.1158/1078-0432.CCR-11-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratt WB, Morishima Y, Peng HM, Osawa Y. Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp Biol Med. 2010;235:278–89. doi: 10.1258/ebm.2009.009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukuyo Y, Inoue M, Nakajima T, Higashikubo R, Horikoshi NT, Hunt C, et al. Oxidative stress plays a critical role in inactivating mutant BRAF by geldanamycin derivatives. Cancer Res. 2008;68:6324–30. doi: 10.1158/0008-5472.CAN-07-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–13. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2011;1823:742–55. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki A, Sekiya S, Gunshima E, Fujii S, Taniguchi H. EGF signaling activates proliferation and blocks apoptosis of mouse and human intestinal stem/progenitor cells in long term monolayer cell culture. Lab Invest. 2010;90:1425–36. doi: 10.1038/labinvest.2010.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.