Abstract
Obstructive sleep apnea (OSA) is frequently associated with obesity and metabolic syndrome. Also frequently associated with metabolic syndrome is type 2 diabetes mellitus (T2DM). Therefore, it is common to find OSA and T2DM together in individuals with metabolic syndrome. Additionally, both OSA and T2DM have a common pathophysiological link with development of insulin resistance. Individuals with severe insulin resistance are likely to have inadequate glycemic control. Long standing poorly controlled T2DM is associated with debilitating microvascular complications such as retinopathy, nephropathy, neuropathy and macrovascular complications such as coronary artery and cerebrovascular disease. There is extensively published literature exploring the cause-effect relationship between OSA and T2DM. In this article we provide an in-depth review of the complex pathophysiological mechanisms linking OSA to T2DM. Specifically, this review focusses on the effect of OSA on the microvascular complications of T2DM such as retinopathy, nephropathy and neuropathy. Additionally, we review the current literature on the effect of continuous positive airway pressure use in individuals with T2DM and OSA.
Keywords: Sleep apnea and diabetes, Obstructive sleep apnea and diabetic complications, Obstructive sleep apnea, Diabetic complications, Sleep related breathing disorder, Diabetes
Core tip: This manuscript addresses the effect of obstructive sleep apnea (OSA) on type 2 diabetes (T2DM) and its associated vascular complications. Specifically, this article provides a comprehensive review of the association between OSA and the microvascular complications of T2DM. Finally, a summary of the effect of the use of continuous positive airway pressure treatment in individuals with OSA and T2DM is reviewed.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep related breathing disorder characterized by collapse of the upper airway leading to cessation of airflow in the setting of continued respiratory effort[1]. The resultant hypoxia leads to frequent arousals causing sleep fragmentation and symptoms of excessive daytime sleepiness. Also, sleep fragmentation increases sympathetic activity, which can increase blood sugar levels by decreasing insulin sensitivity and glucose effectiveness[2].
According to the American Diabetes Association (ADA), diabetes results from a defect in insulin secretion, insulin action or a combination of both[3]. The current global prevalence of diabetes is 135 million and is expected to be 300 million by 2025[4]. Obesity, male gender and older age are well known risk factors for development of OSA and these risk factors are also associated with the increased likelihood of developing type 2 diabetes mellitus (T2DM)[5]. There is a growing body of evidence describing the association between OSA, insulin resistance and the subsequent development of T2DM[6-11]. Also, the importance of effective treatment of OSA in individuals with T2DM has been well studied[12,13]. In spite of this, OSA remains an underdiagnosed and under-treated condition in individuals with T2DM[11-13]. This article provides a concise review of current literature on the relationship between OSA and T2DM, the effect of OSA on the secondary complications of T2DM, and the effect of OSA treatment on outcomes related to T2DM.
OBSTRUCTIVE SLEEP APNEA AND ITS RELATIONSHIP TO GLUCOSE METABOLISM
OSA is known to induce a severe state of insulin resistance (which is a risk factor for cardiovascular disease even in the absence of T2DM), resulting in marked compensatory hyperinsulinemia and thereby increasing the requirement for higher doses of exogenous insulin[5,14]. Two recent studies suggested that sleep disordered breathing is independently associated with glucose intolerance and insulin resistance[12,15]. In this large population based cohort, the investigators found that individuals with OSA were more likely to have lower levels of insulin sensitivity (34% vs 54%, P ≤ 0.0001) and higher levels of fasting insulin production compared to those without OSA. The pathophysiological basis of hyperglycemia in OSA appears to be twofold; hypoxia and sleep fragmentation (Figure 1). The mechanisms involved in the development of hypoxia induced hyperglycemia and insulin resistance have been extensively studied[8-13]. Sympathomimetic hormone (epinephrine, nor-epinephrine and cortisol) levels were noted to be elevated in healthy volunteers subjected to transient hypoxia[16-19]. These studies demonstrate that hypoxia causes a significant elevation in epinephrine levels leading to an increase in hepatic gluconeogenesis and decrease in skeletal muscle reuptake of glucose resulting in hyperglycemia. Additionally, the genesis of metabolic dysfunction in sleep disordered breathing likely involves several distinct but synergistic processes, including activation of the sympathetic nervous system, increase in oxidative stress, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, and low-grade systemic inflammation[9]. Elevation of systemic inflammatory markers such as tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP), fibrinogen and uric acid, all of which could be secondary to the combined interactions of obesity, hyperglycemia and nocturnal hypoxia, are also possible contributing factors[20]. Elevation of these inflammatory markers leads to insulin resistance and impaired glucose utilization[21]. Furthermore, sleep fragmentation for as little as two nights has been shown to decrease insulin sensitivity and impair glucose metabolism[2]. Sleep fragmentation induced hyperglycemia and decreased insulin sensitivity appears to be mediated through alterations in sympathovagal balance, with a shift toward increased sympathetic nervous system activity during sleep and wakefulness[2]. These findings were also specifically studied in women with sleep apnea[15]. Women were noted to have a decrease in insulin sensitivity and beta-cell function, and higher loss of the Homeostasis Model Assessment (HOMA) product[15]. These two abnormalities were associated with poorer glycemic control and a 10% higher hemoglobin A1c (HbA1c) even after adjusting for intensive treatment for T2DM. In fact in this study, women with sleep apnea received more insulin than women without sleep apnea. Therefore, the relationship between OSA as a significant contributing factor to impaired glucose metabolism appears well proven.
Figure 1.
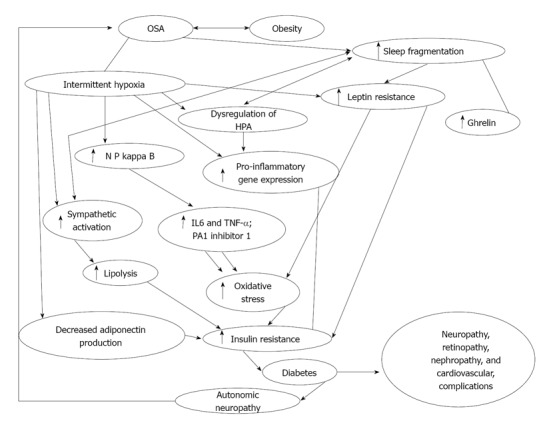
Flow diagram demonstrating interplay between obstructive sleep apnea, intermittent hypoxia, sleep fragmentation and diabetes. OSA: Obstructive sleep apnea; NF-κB: Nuclear factor kappa B; PA1: Plasminogen activator inhibitor 1; HPA: Hypothalamic-pituitary axis; IL: Interleukin; TNF-α: Tumor necrosis factor-α. Modified from Ref [58].
OBSTRUCTIVE SLEEP APNEA AND DIABETES MELLITUS TYPE 2
A vast body of literature has been published establishing the relationship between OSA and T2DM. Cross-sectional estimates from clinic populations and population studies suggest that up to 40% of patients with OSA have diabetes[7,22,23], but the incidence of new diabetes in patients with OSA is not known. Likewise, in patients who are known to have diabetes, the prevalence of OSA may be up to 23%[11], and the prevalence of some form of sleep disordered breathing (SDB) may be as high as 58%[24]. In patients with established T2DM there is a significant relationship between SDB and fasting insulin, glucose, and HbA1c levels, that is independent of obesity as determined by the waist-hip ratio[16]. Although there is now convincing evidence of the association between OSA and decreased insulin sensitivity, the exact pathophysiological mechanism linking OSA as a causative factor of T2DM remains elusive. The converse (T2DM causing OSA) is postulated to involve the dysregulation of the autonomic nervous system leading to SDB. Diabetic autonomic neuropathy likely leads to ventilatory dysfunction through decreased heart rate variability and impaired central control of breathing leading to SDB[25-29]. One study showed that 25% of diabetic individuals with autonomic neuropathy have sleep apnea, a proportion greater than in diabetic subjects without autonomic neuropathy[25]. Based up on the current body of literature it is therefore possible to postulate that there is a strong relationship between T2DM and OSA.
RELATIONSHIP BETWEEN OBSTRUCTIVE SLEEP APNEA AND MICROVASCULAR COMPLICATIONS OF DIABETES MELLITUS
Long standing poorly controlled T2DM is associated with the development of microvascular complications such as retinopathy, nephropathy, neuropathy and macrovascular vascular disease such as coronary artery and cerebrovascular disease. In this section, we review the literature on the association between OSA and the risk of developing T2DM its related microvascular complications. The macrovascular complications of T2DM have been well recognized. OSA leads to increase in insulin resistance and T2DM, which in turn can lead to increase in the inflammatory markers leading to cardiovascular complications. Studies have shown that treatment with continuous positive airway pressure (CPAP) can decrease the levels of IL-6 and CRP, which in turn can lead to decrease in inflammation and reduce vascular complications[6,30]. Several studies have demonstrated an association between OSA and cardiovascular abnormalities[15,31], and improvement with CPAP therapy. Discussing this is beyond the scope of this review. Here we discuss briefly the role of OSA in diabetic retinopathy, neuropathy, nephropathy and insulin resistance and its treatment. The complex interplay of sleep and diabetes demonstrating interplay between OSA, T2DM, intermittent hypoxia, sleep fragmentation and insulin resistance is shown in Figure 1.
DIABETIC RETINOPATHY
The relationship between diabetes mellitus and the development of proliferative retinopathy is well established. Although one of the first studies[32] to assess the potential relationship between OSA and diabetic retinopathy did not find a strong association, other recent studies have found[33-36] that OSA remained an independent predictor of proliferative retinopathy even after adjusting for conventional risk factors and novel biomarkers for diabetic retinopathy. Also, in individuals with OSA, where hypertension and obesity are co-morbid to T2DM, there appears to be an increased risk of proliferative retinopathy[34]. Diabetic retinopathy is mediated by high levels of serum vascular endothelial growth factors and other biomarkers[33,37,38]. Similarly, the increased risk of diabetic retinopathy in OSA appears to be mediated by elevated levels of inflammatory markers, reduced endothelial regulatory function and increased insulin resistance[39,40]. A recent study by Fujita et al[41] noted an increase in the levels of inflammatory markers such as acylation stimulating protein, high sensitive CRP and components of the membrane attack complex (which leads to activation of alternative complement pathway) in obese T2DM patients with retinopathy. As a result, complement mediated activation of inflammation likely leads to acceleration of diabetic microangiopathy leading to development and worsening of diabetic retinopathy. Previous studies[42,43] looking in to complement activation in individuals with OSA have noted increased levels of C3 and a decrease in the levels of IgM and NK cell percentage. All this data strongly points towards a strong relationship between OSA and increased risk of proliferative retinopathy in individuals with OSA and T2DM.
DIABETIC NEPHROPATHY
Diabetes Mellitus is the leading cause of end stage renal disease[44]. Nephropathy in T2DM is postulated to be mediated through an increase in activity of angiotensin II, platelet derived growth factor, and thromboxane. These agents upregulate protein kinase c activity and this leads to activation of transforming growth factor-beta (TGF-β). TGF-β leads to proliferation of extracellular matrix and glomerulosclerosis leading to diabetic nephropathy[45]. Progression of renal failure in the presence of OSA is likely secondary to hypoxia mediated increase in sympathetic activation and inflammatory cytokines[46]. Additionally, a recent study has also shown increased cystatin C levels in individuals with severe OSA[47]. Cystatin C has been recognized as an early biomarker for renal failure development and cardiovascular events[47]. Other studies have shown that the severity of OSA appears to be directly correlated to the degree of loss of renal function[48]. The literature on OSA leading to the progression of diabetic nephropathy is limited. A recent small study evaluated OSA as an independent risk factor for microalbuminuria and did not find any significant relationship[49]. This study consisted of a small sample of patients with moderate to severe OSA (Apnea hypopnea index > 15), and was underpowered to detect a difference. However, given the overall similarity in the pathophysiological mechanisms, it is likely that OSA may contribute to the development of diabetic nephropathy. Future larger population based studies will be necessary to better understand this relationship.
PERIPHERAL NEUROPATHY
Peripheral neuropathy secondary to T2DM is very common, affecting 60%-70% of individuals with diabetes[44]. Severe forms of diabetic neuropathy are a major contributing cause of lower-extremity amputations[44]. The pathophysiologic mechanism of diabetic peripheral neuropathy appears to be complex; involving metabolic and ischemic pathways[50]. Metabolic factors mediated by hyperglycemia lead to abnormal nerve energy transport, impaired axonal transport, increased activity of the sorbitol pathway, non-enzymatic nerve protein glucosylation and abnormal myo-inositol metabolism. The ischemic pathway is mediated through thickening and hyalinization of the microvasculature wall leading to neuronal ischemia[50]. Previous studies have noted the relationship between OSA and peripheral neuropathy and the improvement in peripheral neuropathy with treatment of OSA using CPAP[51-54]. A recent study specifically looking into the relationship between OSA and diabetic peripheral neuropathy found that there was a fourfold increase in the odds of peripheral neuropathy in diabetic patients with OSA compared those without[36]. Also, noted was a significant trend in the prevalence of diabetic peripheral neuropathy with lower levels of oxygen saturations. In the same study, individuals with OSA and diabetic peripheral neuropathy were found to have higher levels of nitrotyrosine and lipid peroxide levels compared to those without OSA. Nitrotyrosine and lipid peroxide leads to nitrosative and oxidative stress that reduces nerve perfusion, resulting in impairment of vascular reactivity of the epineurial arterioles[55]. Overall, there is stronger data now supporting the hypothesis that OSA is an independent risk factor for the development of diabetic peripheral neuropathy. A recent study-linking OSA to diabetic peripheral neuropathy has been postulated suggesting protein kinase, advanced glycation end products, hexosamine and polyol pathway each of those leading to microvascular complications including diabetic peripheral neuropathy[36].
TREATMENT OF OBSTRUCTIVE SLEEP APNEA AND OUTCOMES WITH IMPAIRED GLUCOSE TOLERANCE AND DIABETES MELLITUS
The strong association between insulin resistance and OSA would imply that treatment with CPAP should lead to improved glucose control in patients with T2DM. The effect of CPAP on glucose control has been variable[56]. Review of the literature found that most studies exploring the effect of CPAP on insulin sensitivity show a positive effect[57-65], although other studies have not shown a significant improvement in hyperglycemia or level of insulin resistance[66-69]. The variability in results is likely due to methodological weaknesses such as small sample sizes, lack of adjustment for confounders and absence of polysomnographic evidence to support sleep duration. Other studies have shown that racial or ethnic differences among the study populations could contribute to variability in response to CPAP[70,71]. In one study the response to CPAP was significantly greater if the body mass index (BMI) was less than 30[60]. The likely explanation appears to be that obesity is a more important determinant of insulin resistance than OSA. The less obese the patients are, the greater is the improvement in insulin sensitivity brought about by CPAP treatment[60]. Another study observed a significant decrease in glycated hemoglobin values with CPAP, although there was no significant difference in fasting blood sugars and insulin resistance on a day-to-day basis[68,72]. The likely explanation for this finding is that glycated hemoglobin is a better marker of long term glucose control unlike blood glucose and insulin resistance, which can fluctuate on a day-to-day basis[68]. This suggests that monitoring HbA1c levels as a marker of efficacy of CPAP therapy is likely a better indicator than monitoring indices of insulin resistance or glucose utilization (i.e., HOMA product). A recent randomized control study[64] noted a dose-response effect for both the severity of disease and adherence to CPAP treatment. Insulin sensitivity was significantly better after treatment with CPAP in patients with severe OSA (Apnea hypopnea index ≥ 30) compared to patients with less severe OSA. Also, the same study revealed that each additional hour of active CPAP usage was associated with a significant improvement in insulin sensitivity. The reason for a lack of effect of CPAP on the less severe OSA patients is unknown. In spite of the variability in results of the studies, treatment with CPAP remains an important modality for patients with OSA to improve glucose metabolism. Treatment appears particularly important for patients with severe OSA and subjective symptoms of sleepiness. In light of the current results, clinicians should ensure patients with OSA are effectively treated, particularly with the epidemic of obesity and diabetes.
CONCLUSION
There appears to be an association between OSA and the development of insulin resistance leading to impaired fasting glucose tests and the development of T2DM. Furthermore, data supports the development and progression of complications from long standing T2DM in the setting of inadequately treated OSA. There is an abundance of evidence demonstrating the link between OSA and impaired glucose metabolism. Irrespective of the direction of causality, the association between OSA and T2DM remains irrefutable. Therefore, in spite of the controversial nature of the data regarding the improvement in glycemic control with CPAP therapy, judgment should be reserved until long term rigorously conducted prospective studies can expand knowledge in this area. In the interim, although a strong recommendation for treatment of OSA with CPAP to control diabetes remains controversial, physicians should individualize their decisions based on the particular needs of their patients.
Footnotes
P- Reviewers: Cui WP, Sanchez-MargaletV, Schaffer S S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
References
- 1.Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 2.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30 Suppl 1:S42–S47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 4.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985) 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 6.Harsch IA, Hahn EG, Konturek PC. Insulin resistance and other metabolic aspects of the obstructive sleep apnea syndrome. Med Sci Monit. 2005;11:RA70–RA75. [PubMed] [Google Scholar]
- 7.Meslier N, Gagnadoux F, Giraud P, Person C, Ouksel H, Urban T, Racineux JL. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J. 2003;22:156–160. doi: 10.1183/09031936.03.00089902. [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theorell-Haglöw J, Berne C, Janson C, Svensson M, Lindberg E. Is obstructive sleep apnea associated with the metabolic syndrome and impaired glucose metabolism? Sleep Med. 2006;7:S5. [Google Scholar]
- 11.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61:945–950. doi: 10.1136/thx.2005.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermans MP, Ahn SA, Rousseau MF. Cardiometabolic phenotype and UKPDS risk in male type 2 diabetic patients with obstructive sleep apnoea. Diabetes Metab Syndr. 2009;3:50–54. [Google Scholar]
- 13.Pillai A, Warren G, Gunathilake W, Idris I. Effects of sleep apnea severity on glycemic control in patients with type 2 diabetes prior to continuous positive airway pressure treatment. Diabetes Technol Ther. 2011;13:945–949. doi: 10.1089/dia.2011.0005. [DOI] [PubMed] [Google Scholar]
- 14.Ducimetiere P, Eschwege E, Papoz L, Richard JL, Claude JR, Rosselin G. Relationship of plasma insulin levels to the incidence of myocardial infarction and coronary heart disease mortality in a middle-aged population. Diabetologia. 1980;19:205–210. doi: 10.1007/BF00275270. [DOI] [PubMed] [Google Scholar]
- 15.Hermans MP, Ahn SA, Mahadeb YP, Rousseau MF. Sleep apnoea syndrome and 10-year cardiovascular risk in females with type 2 diabetes: relationship with insulin secretion and insulin resistance. Diabetes Metab Res Rev. 2013;29:227–234. doi: 10.1002/dmrr.2387. [DOI] [PubMed] [Google Scholar]
- 16.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pamidi S, Wroblewski K, Broussard J, Day A, Hanlon EC, Abraham V, Tasali E. Obstructive sleep apnea in young lean men: impact on insulin sensitivity and secretion. Diabetes Care. 2012;35:2384–2389. doi: 10.2337/dc12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, Polotsky VY, O’Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–857. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, Born J, Fehm HL, Peters A. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med. 2004;169:1231–1237. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- 20.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 22.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–1127. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmasry A, Lindberg E, Berne C, Janson C, Gislason T, Awad Tageldin M, Boman G. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med. 2001;249:153–161. doi: 10.1046/j.1365-2796.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 24.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, Ewy GA, Howard BV, Punjabi NM. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 25.Ficker JH, Dertinger SH, Siegfried W, König HJ, Pentz M, Sailer D, Katalinic A, Hahn EG. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur Respir J. 1998;11:14–19. doi: 10.1183/09031936.98.11010014. [DOI] [PubMed] [Google Scholar]
- 26.Ewing DJ, Neilson JM, Shapiro CM, Stewart JA, Reid W. Twenty four hour heart rate variability: effects of posture, sleep, and time of day in healthy controls and comparison with bedside tests of autonomic function in diabetic patients. Br Heart J. 1991;65:239–244. doi: 10.1136/hrt.65.5.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobotka PA, Liss HP, Vinik AI. Impaired hypoxic ventilatory drive in diabetic patients with autonomic neuropathy. J Clin Endocrinol Metab. 1986;62:658–663. doi: 10.1210/jcem-62-4-658. [DOI] [PubMed] [Google Scholar]
- 28.Rees PJ, Prior JG, Cochrane GM, Clark TJ. Sleep apnoea in diabetic patients with autonomic neuropathy. J R Soc Med. 1981;74:192–195. doi: 10.1177/014107688107400306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondini S, Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Ann Neurol. 1985;17:391–395. doi: 10.1002/ana.410170415. [DOI] [PubMed] [Google Scholar]
- 30.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JJ, Ren J. Obstructive sleep apnoea and cardiovascular complications: perception versus knowledge. Clin Exp Pharmacol Physiol. 2012;39:995–1003. doi: 10.1111/1440-1681.12024. [DOI] [PubMed] [Google Scholar]
- 32.Boland LL, Shahar E, Wong TY, Klein R, Punjabi N, Robbins JA, Newman AB. Sleep-disordered breathing is not associated with the presence of retinal microvascular abnormalities: the Sleep Heart Health Study. Sleep. 2004;27:467–473. doi: 10.1093/sleep/27.3.467. [DOI] [PubMed] [Google Scholar]
- 33.Gustavsson C, Agardh E, Bengtsson B, Agardh CD. TNF-α is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J Diabetes Complications. 2008;22:309–316. doi: 10.1016/j.jdiacomp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Shiba T, Takahashi M, Hori Y, Saishin Y, Sato Y, Maeno T. Evaluation of the relationship between background factors and sleep-disordered breathing in patients with proliferative diabetic retinopathy. Jpn J Ophthalmol. 2011;55:638–642. doi: 10.1007/s10384-011-0076-5. [DOI] [PubMed] [Google Scholar]
- 35.Rudrappa S, Warren G, Idris I. Obstructive sleep apnoea is associated with the development and progression of diabetic retinopathy, independent of conventional risk factors and novel biomarkers for diabetic retinopathy. Br J Ophthalmol. 2012;96:1535. doi: 10.1136/bjophthalmol-2012-301991. [DOI] [PubMed] [Google Scholar]
- 36.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, Jose B, Piya MK, Barnett AH, Stevens MJ. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434–441. doi: 10.1164/rccm.201112-2135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacqueminet S, Ben Abdesselam O, Chapman MJ, Nicolay N, Foglietti MJ, Grimaldi A, Beaudeux JL. Elevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathy. Clin Chim Acta. 2006;367:103–107. doi: 10.1016/j.cca.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Myśliwiec M, Balcerska A, Zorena K, Myśliwska J, Lipowski P, Raczyńska K. The role of vascular endothelial growth factor, tumor necrosis factor α and interleukin-6 in pathogenesis of diabetic retinopathy. Diabetes Res Clin Pract. 2008;79:141–146. doi: 10.1016/j.diabres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 40.Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling : association with the severity of apnea-induced hypoxemia during sleep. Chest. 2001;119:1085–1091. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- 41.Fujita T, Hemmi S, Kajiwara M, Yabuki M, Fuke Y, Satomura A, Soma M. Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab Res Rev. 2013;29:220–226. doi: 10.1002/dmrr.2380. [DOI] [PubMed] [Google Scholar]
- 42.Kapsimalis F, Varouchakis G, Manousaki A, Daskas S, Nikita D, Kryger M, Gourgoulianis K. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186:209–217. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 43.Li ZG, Li TP, Ye H, Feng Y, Li DQ. [Immune function changes in patients with obstructive sleep apnea hypopnea syndrome] Nanfang Yike Daxue Xuebao. 2011;31:1003–1005. [PubMed] [Google Scholar]
- 44.Li ZG CDC. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011, Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA. Available from: http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 45.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30:49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 46.Kanbay A, Buyukoglan H, Ozdogan N, Kaya E, Oymak FS, Gulmez I, Demir R, Kokturk O, Covic A. Obstructive sleep apnea syndrome is related to the progression of chronic kidney disease. Int Urol Nephrol. 2012;44:535–539. doi: 10.1007/s11255-011-9927-8. [DOI] [PubMed] [Google Scholar]
- 47.Kato K, Takata Y, Usui Y, Shiina K, Asano K, Hashimura Y, Saruhara H, Nishihata Y, Tomiyama H, Yamashina A. Severe obstructive sleep apnea increases cystatin C in clinically latent renal dysfunction. Respir Med. 2011;105:643–649. doi: 10.1016/j.rmed.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 48.de Oliveira Rodrigues CJ, Marson O, Tufic S, Kohlmann O, Guimarães SM, Togeiro P, Ribeiro AB, Tavares A. Relationship among end-stage renal disease, hypertension, and sleep apnea in nondiabetic dialysis patients. Am J Hypertens. 2005;18:152–157. doi: 10.1016/j.amjhyper.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 49.Buyukaydin B, Akkoyunlu ME, Kazancioglu R, Karakose F, Ozcelik HK, Erkoc R, Kart L. The effect of sleep apnea syndrome on the development of diabetic nephropathy in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;98:140–143. doi: 10.1016/j.diabres.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Lalau JD, Arlot S, Quichaud J. [Pathogenesis of diabetic neuropathies] Ann Med Interne (Paris) 1985;136:486–495. [PubMed] [Google Scholar]
- 51.Mayer P, Dematteis M, Pépin JL, Wuyam B, Veale D, Vila A, Lévy P. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213–219. doi: 10.1164/ajrccm.159.1.9709051. [DOI] [PubMed] [Google Scholar]
- 52.Lüdemann P, Dziewas R, Sörös P, Happe S, Frese A. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70:685–687. doi: 10.1136/jnnp.70.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Seze J. Obstructive sleep apnoea: an underestimated cause of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 2007;78:222. doi: 10.1136/jnnp.2006.107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dziewas R, Schilling M, Engel P, Boentert M, Hor H, Okegwo A, Lüdemann P, Ringelstein EB, Young P. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78:295–297. doi: 10.1136/jnnp.2006.102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obrosova IG, Mabley JG, Zsengellér Z, Charniauskaya T, Abatan OI, Groves JT, Szabó C. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005;19:401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- 56.Surani S, Subramanian S. Effect of continuous positive airway pressure therapy on glucose control. World J Diabetes. 2012;3:65–70. doi: 10.4239/wjd.v3.i4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 58.Czupryniak L, Loba J, Pawlowski M, Nowak D, Bialasiewicz P. Treatment with continuous positive airway pressure may affect blood glucose levels in nondiabetic patients with obstructive sleep apnea syndrome. Sleep. 2005;28:601–603. doi: 10.1093/sleep/28.5.601. [DOI] [PubMed] [Google Scholar]
- 59.Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, Kripke DF, Kline LE. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–542. [PMC free article] [PubMed] [Google Scholar]
- 60.Harsch IA, Schahin SP, Radespiel-Tröger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 61.Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, Budhiraja R, Singer M, Redline S. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 62.Lam JC, Lam B, Yao TJ, Lai AY, Ooi CG, Tam S, Lam KS, Ip MS. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35:138–145. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 63.Shpirer I, Elizur A, Shorer R, Peretz RB, Rabey JM, Khaigrekht M. Hypoxemia correlates with attentional dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2012;16:821–827. doi: 10.1007/s11325-011-0582-1. [DOI] [PubMed] [Google Scholar]
- 64.Bleifeld W. [Fibrinolysis in acute myocardial infarct] Schweiz Med Wochenschr. 1987;117:1641–1647. [PubMed] [Google Scholar]
- 65.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, Yue DK. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–1685. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 66.Stoohs RA, Facchini FS, Philip P, Valencia-Flores M, Guilleminault C. Selected cardiovascular risk factors in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure (n-CPAP) Sleep. 1993;16:S141–S142. doi: 10.1093/sleep/16.suppl_8.s141. [DOI] [PubMed] [Google Scholar]
- 67.Smurra M, Philip P, Taillard J, Guilleminault C, Bioulac B, Gin H. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2:207–213. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 68.Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, Jagia P, Kumar A. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–2286. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 69.Chung S, Yoon IY, Lee CH, Kim JW. The effects of nasal continuous positive airway pressure on vascular functions and serum cardiovascular risk factors in obstructive sleep apnea syndrome. Sleep Breath. 2011;15:71–76. doi: 10.1007/s11325-009-0323-x. [DOI] [PubMed] [Google Scholar]
- 70.Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8:12–17. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 72.Etzioni T, Pillar G. Sleep, sleep apnea, diabetes, and the metabolic syndrome: the role of treatment. Sleep. 2012;35:591–592. doi: 10.5665/sleep.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]


